Abstract
Prenatal exposure to air pollution has been associated with autism spectrum disorder (ASD) risk but no study has examined associations with ASD severity or functioning. Cognitive ability, adaptive functioning, and ASD severity were assessed in 327 children with ASD from the Childhood Autism Risks from Genetics and the Environment study using the Mullen Scales of Early Learning (MSEL), the Vineland Adaptive Behavior Scales (VABS), and the Autism Diagnostic Observation Schedule calibrated severity score. Estimates of nitrogen dioxide (NO2), particulate matter (PM2.5 and PM10), ozone, and near-roadway air pollution were assigned to each trimester of pregnancy and first year of life. Increasing prenatal and first year NO2 exposures were associated with decreased MSEL and VABS scores. Increasing PM10 exposure in the third trimester was paradoxically associated with improved performance on the VABS. ASD severity was not associated with air pollution exposure.
Keywords: autism spectrum disorder, air pollution, Vineland Adaptive Behavioral Scale, Mullen Scales of Early Learning, cognitive impairments
Autism spectrum disorder (ASD) is a neurodevelopmental condition affecting 1 in 68 children in the United States (Christensen et al. 2016). Deficits in communication skills, problems with social interactions, and repetitive behaviors are core characteristics of ASD, but the severity of presentation varies widely per the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-V) (American Psychiatric Association 2013). While the most recent prevalence statistics, published in 2016, reflect diagnostic criteria prior to the adaptation of DSM-V, DSM-IV and DSM-V diagnoses appear comparable suggesting that prevalence is similar across criteria revisions (Huerta et al. 2012; Kim et al. 2014; Mattila et al. 2011). Past research into AD etiology has heavily focused on the role of genetic variation, more recent studies suggest that environmental exposures during gestation and early life may be comparably important (Gaugler et al. 2014; Kim and Leventhal 2015).
Previous research suggests that neurodevelopment is adversely affected by exposure to ambient air pollutants, including particulate matter, polyaromatic hydrocarbons, diesel exhaust, nitrogen dioxide (NO2) and the near-roadway air pollutant mixture (Costa et al. 2015; Genkinger et al. 2015; Jedrychowski et al. 2015; Suades-Gonzalez et al. 2015). A growing body of research has begun to use brain-imaging methods to examine exposure effects directly on brain structure and function, identifying deficits in white matter, altered brain connectivity, and vascularization (Chen et al. 2015; Peterson et al. 2015; Pujol et al. 2016; Wilker et al. 2016). Many studies have specifically examined the relationship between ASD and in utero or early childhood air pollution exposure (Flores-Pajot et al. 2016; Lam et al. 2016). Our own work reported a two-fold increase in the risk of autism among children of mothers in California living within 300m of a freeway during gestation and the first year of life in the Childhood Autism Risks from Genetics and the Environment (CHARGE) case-control study (Volk et al. 2011). In the same subjects, increased residential near-roadway air pollution (NRAP) exposure modeled from a line source dispersion model and regional particulate matter less than 10 microns (PM10) and 2.5 microns in aerodynamic diameter (PM2.5) were also associated with a two-fold increased risk of autism (Volk et al. 2013). More recent studies of criteria and traffic pollutants reported ASD associations with NRAP exposure during pregnancy in Los Angeles (Becerra et al. 2013), prenatal PM2.5 exposure in cases and controls nested in the Nurses Health Study (Raz et al. 2015), prenatal PM10 in a birth records study from North Carolina and Northern California (Kalkbrenner et al. 2015), and with other regional air pollutant exposure, including sulfur dioxide, ozone, carbon monoxide, and nitrogen dioxide, during the first four years of life in Taiwan (Jung et al. 2013). Hazardous air pollutants, including ambient metals, diesel particulate matter, and volatile organic air pollutants, have also been linked to autism in epidemiological studies (Kalkbrenner et al. 2010; Roberts et al. 2013; von Ehrenstein et al. 2014; Windham et al. 2006). Notably, such findings are not supported in the analyses of European birth cohort data (Gong et al. 2016; Guxens et al. 2014).
Although these studies suggest a role for air pollution in the etiology of ASD, to our knowledge no study has examined effects of degree of exposure on functioning among children with ASD or on quantitative ASD measures in children. We further sought to examine specificity of these associations in typically developing controls.
Methods
Description of Study Population
ASD cases from the CHARGE study were used for this analysis. CHARGE is a case-control study of California children with autism, ASD, developmental delay, or typical development, as previously described (Hertz-Picciotto et al. 2006). Eligibility criteria included ages 24–60 months at the time of initial recruitment contact, born in California, lived with at least one biological parent who speaks English or Spanish, and resided within the study’s catchment area of about 20 counties in northern California, the central valley and parts of the Los Angeles metropolitan area. Potential participants were identified through the California Department of Developmental Services (DDS) and its regional centers that coordinate services for children with autism and developmental delay. Additional cases were enrolled based on referrals from health and service providers, including the University of California, Davis MIND Institute.
A total of 446 ASD cases from CHARGE were eligible for study. Scoring methods for the ADOS-CSS for the 87 Southern California participants were not consistent with methods used for the rest of the population; therefore, they were excluded from analysis. We excluded 29 from the analysis due to incomplete air pollution exposure because a residential address was not recorded for each gestational trimester. Phenotype assessment scores were not available for an additional 7. Two subjects were excluded as they did not meet criteria for DSM-V ASD. A final sample of 325 ASD cases born between 1999 and 2007 were included in this analysis. Separate analyses were conducted using data from 227 typically developing controls from CHARGE.
Phenotype Assessment
As part of CHARGE, the Autism Diagnostic Interview-Revised (ADI-R) and the Autism Diagnostic Observation Schedules (ADOS) were used to confirm a DSM-IV-TR autism or ASD diagnosis, as previously described (Hertz-Picciotto et al. 2006). DSM-V criteria was applied upon implementation in 2013 and our analyses include only subjects meeting criteria for both DSM-IV-TR and DSM-V. The ADOS-derived Calculated Severity Score (ADOS-CSS) (Gotham et al. 2009) is a clinician-derived outcome that that is normalized for language ability and age (Gotham et al. 2012; Hus et al. 2012). It is computed from raw scores of the 5 ADOS subscales for study subjects, resulting in a quantitative 10-point severity metric (Gotham et al. 2009).
Additional data were collected to assess cognitive and adaptive function in CHARGE participants. Cognitive ability was assessed using the Mullen Scales of Early Learning (MSEL), which in children 3–60 months of age provides verbal and non-verbal summary scores for four subscales: fine motor, visual reception, expressive language, and receptive language as well as a total composite score (Mullen 1995). The Vineland Adaptive Behavior Scale (VABS) was used to rate adaptive function, indicating how an individual adjusts to daily life activities (Kanne et al. 2011; Sparrow and Cicchetti 1985). The VABS provides subscale scores for adaptive behavior across the domains of socialization, motor skills, daily living skills, and communication, in addition to a composite score. In contrast to the CSS, the MSEL and the VABS are not ASD-specific measures and have general population norms allowing for the assessment of broad cognitive and adaptive behaviors that also may accompany disabilities such as ASD (Kanne et al. 2011; Mullen 1995; Sparrow and Cicchetti 1985). Our analysis used population-normed standardized scores available for the VABS. For the MSEL we calculated the developmental quotient (DQ), the age-equivalent MSEL score divided by the chronological age and multiplied by 100 (Lord et al. 2006; Messinger et al. 2013). This avoided possible floor and ceiling effects in the MSEL distribution, while allowing for examination of metrics similar to IQ (Lord et al. 2006; Munson et al. 2008). We calculated MSEL composite, verbal (calculated from the expressive and receptive language subscales) and non-verbal DQs (calculated from the dependent living and fine motor subscales).
Air Pollution Exposure Assessment
NRAP and criteria air pollutant exposures were assigned based on a parent-reported residential history. In telephone interviews, parents were asked for their residential history (including addresses and dates lived at each location) beginning 3 months before conception and extending to the most recent place of residence. Using this information and the conception date for each child, based on gestational age from ultrasonographic measurements or the date of last menstrual period as determined from prenatal records, air pollution exposure values were assigned for each trimester of pregnancy, and an all pregnancy average (described previously in (Volk et al., 2013). When more than 1 address fell into a time interval, we created a weighted average to reflect the exposure level of the participant across the time of interest, taking into account changes in residence.
Addresses were geo-coded using the Tele Atlas database and software (Tele Atlas, Inc., Boston, CA, www.na.teleatlas.com). The CALINE4 line-source air-quality dispersion model was applied to derive estimates of exposure to the complex NRAP mixture, as previously described (Volk et al. 2013). Briefly, this model uses roadway geometry, link-based traffic volumes, vehicle emission rates, wind speed and direction, atmospheric stability, and mixing heights to estimate average concentrations for the specific locations and time periods examined. Roadway geometry data and annual average daily traffic counts were obtained from Tele Atlas/Geographic Data Technology in 2005, and represent traffic counts collected between 1995 and 2000. Counts were scaled for our specific years of interest based on estimated growth in county average vehicle-miles-traveled. Meteorological data from 56 local monitoring stations were matched to the dates and locations of interest. We used the CALINE4 model to estimate locally varying ambient concentrations of nitrogen oxides NOx contributed by freeways, non-freeways, and all roads located within 5 km of each child’s home. These model-based concentrations should be viewed as an indicator of the estimated near-roadway pollutant mixture rather than of effects of NOx or any other specific pollutant.
To assess regional pollutants, the EPA’s Air Quality System (AQS) regional air quality measurements of PM2.5, PM10, O3, and NO2, were obtained (https://aqs.epa.gov/aqsweb/documents/data_mart_welcome.html) and monthly data from up to four monitoring stations located within 50 km of each residence were used to assess average exposure for individual time periods based on spatial interpolation of ambient concentrations via inverse distance-squared weighting. If one or more stations were located within 5 km of a residence, only data from those stations were used. For both NRAP and regional pollutants, individual air pollution exposure levels were assigned for each trimester of pregnancy and a whole-pregnancy average, as well as the first year of life (Volk et al. 2011; Volk et al. 2013).
Statistical Analysis
We first examined the relationship between demographic and socioeconomic factors and MSEL, VABS, and CSS scores using univariate tests, including t-test or ANOVA, as appropriate. Linear regression models were used to study the effect of prenatal air pollution exposure on cognitive and adaptive function separately for ASD and typically developing children. The relationship between prenatal air pollution exposure and ASD severity was examined only in ASD cases. We first examined the effect of average exposure during pregnancy, each trimester of pregnancy, and the first year of life in separate models. Next, to try to identify the most relevant time period for each pollutant exposure for which an association was observed, we adjusted each trimester association for other trimesters (e.g. first trimester exposure was adjusted for second trimester exposure) and for first year effects. All regional and near-roadway air pollutants were analyzed as continuous measures and effect estimates were scaled to 2 standard deviations (SDs) of the average distribution of each pollutant during pregnancy, in order to be able to compare health effect sizes across pollutants. The MSEL DQ scores, CSS, and VABS were treated as continuous variables and analyzed using linear regression. The VABS composite score and each of the subscale scores were log transformed, with the exception of the motor skills subset, which satisfied assumptions for linear regression, and results were back-transformed and presented as a percent change in score.
All models were adjusted for potential socio-demographic confounders, including race and ethnicity (Hispanic vs. White, Black/Asian/Other vs. White), parental education (parent with highest of four levels: college degree or higher vs. some high school, high school degree, or some college education), child gender, mother’s smoking during pregnancy (yes or no), mother’s age at delivery (above or below 35 years), referral center (XXX or XXXX) or home ownership (yes or no). Season of conception (Dec-Feb, March-May, June-August, or September-Nov) was also examined as air pollution levels fluctuate across warm (March-August) and cool seasons (September-February) and season of conception has been associated with a slight increase in ASD risk (Zerbo et al. 2011). As a sensitivity analysis we further adjusted models for year of birth and gestational age in weeks. Analyses were conducted using the statistical package R (R version 3.0.1, The R Foundation for Statistical Computing, www.r-project.org). Statistical significance was evaluated at α =0.05.
Results
Among children with ASD, the VABS and MSEL DQ composite scores showed significant differences by race, with white subjects having higher (better functioning) scores, and by parents’ education level, with higher education levels of the parents associated with higher child test scores (Table 1). VABS scores in children with ASD also differed by the mother’s age at delivery, with mothers over 35 having children with higher scores. MSEL DQ composite scores among children with ASD were higher for homeowners than parents who did not own a home. Scores for composite MSEL DQ, VABS, and the CSS did not differ by child gender, maternal smoking during pregnancy, and season of conception. The CSS was comparable across all population characteristics but for Regional Center location, with the Central California centers having lower (better) scores than the other centers. We also examined the correlation between the MSEL DQ, VABS, and CSS scores in children with ASD. There was moderate correlation between the MSEL DQ and VABS composite scores, and little correlation between many of these scores and the ADOS CSS. Subscale and composite scores within each test showed moderate to high correlation. (Supplement Table 1).
Table 1.
Distribution of ASD phenotype scores by sociodemographic characteristics and maternal smoking during pregnancy
| VABS Composite Score |
MSEL Composite DQ Score |
ADOS CSS | ||
|---|---|---|---|---|
| Covariate | n (%) | mean±sd | mean±sd | mean±sd |
| Overall score | 325 (100%) | 64.9 ± 11.9 | 61.5 ± 22.3 | 7.46 ± 1.50 |
| Sex | ||||
| Female | 44 (13.5%) | 62.7 ± 11.8 | 60.5 ± 20.4 | 7.42 ± 1.45 |
| Male | 281 (86.5%) | 65.3 ± 11.9 | 61.7 ± 22.6 | 7.47 ± 1.51 |
| p value* | 0.18 | 0.65 | 0.83 | |
| Center | ||||
| Far North | 137 (42.2%) | 64.2 ± 10.6 | 62.1 ± 22.7 | 7.69 ± 1.50 |
| North Bay | 54 (16.5%) | 65.7 ± 13.7 | 62.2 ± 24.4 | 7.25 ± 1.52 |
| East Bay | 67 (20.5%) | 64.3 ± 11.2 | 60.4 ± 21.6 | 7.58 ± 1.41 |
| Central CA | 67 (20.8%) | 66.6 ± 13.5 | 60.7 ± 21.0 | 7.06 ± 1.49 |
| p value** | 0.55 | 0.95 | 0.02 | |
| Race | ||||
| White | 180 (55.1%) | 67.1 ± 12.1 | 66.1 ± 21.9 | 7.47 ± 1.48 |
| Hispanic | 90 (27.8%) | 62.1 ± 9.85 | 54.7 ± 20.9 | 7.34 ± 1.63 |
| Other | 55 (17.1%) | 62.6 ± 12.9 | 57.3 ± 22.7 | 7.62 ± 1.37 |
| p value** | <0.01 | <0.01 | 0.56 | |
| Education | ||||
| Less than College | 115 (35.5%) | 61.8 ± 10.5 | 55.4 ± 19.0 | 7.40 ± 1.51 |
| College grad | 124 (38.5%) | 65.4 ± 12.2 | 62.2 ± 22.8 | 7.60 ± 1.39 |
| Grad/Prof degree | 86 (26.0%) | 68.5 ± 12.2 | 68.6 ± 23.7 | 7.35 ± 1.64 |
| p value** | <0.01 | <0.01 | 0.44 | |
| Mother’s Age | ||||
| Under 35 | 240 (74.3%) | 64.0 ± 10.6 | 61.0 ± 22.5 | 7.45 ± 1.51 |
| 35+ | 85 (25.7%) | 67.9 ± 14.7 | 62.8 ± 21.9 | 7.49 ± 1.47 |
| p value* | 0.03 | 0.51 | 0.82 | |
| Smoking while pregnant | ||||
| No | 298 (91.7%) | 64.9 ± 12.1 | 61.2 ± 22.7 | 7.43 ± 1.51 |
| Yes | 27 (8.3%) | 65.2 ± 8.7 | 64.5 ± 17.3 | 7.81 ± 1.33 |
| p value* | 0.91 | 0.36 | 0.17 | |
| Season of | ||||
| Conception | ||||
| Warm | 161 (49.5%) | 64.6 ± 11.9 | 61.9 ± 23.3 | 7.47 ± 1.48 |
| Cool | 164 (50.5%) | 65.3 ± 11.9 | 61.1 ± 21.3 | 7.45 ± 1.52 |
| p value* | 0.56 | 0.76 | 0.88 | |
| Home Owner | ||||
| No | 89 (27.5%) | 63.6 ± 11.5 | 57.8 ± 23.3 | 7.57 ± 1.61 |
| Yes | 233 (71.9%) | 65.6 ± 12.0 | 63.2 ± 21.8 | 7.44 ± 1.44 |
| Missing | 3 (0.60%) | 61.0 ± 8.19 | 38.4 ± 12.0 | 6.33 ± 2.52 |
| p value* | 0.34 | 0.03 | 0.33 |
Denotes comparison based on t-test;
Denotes comparison based on ANOVA
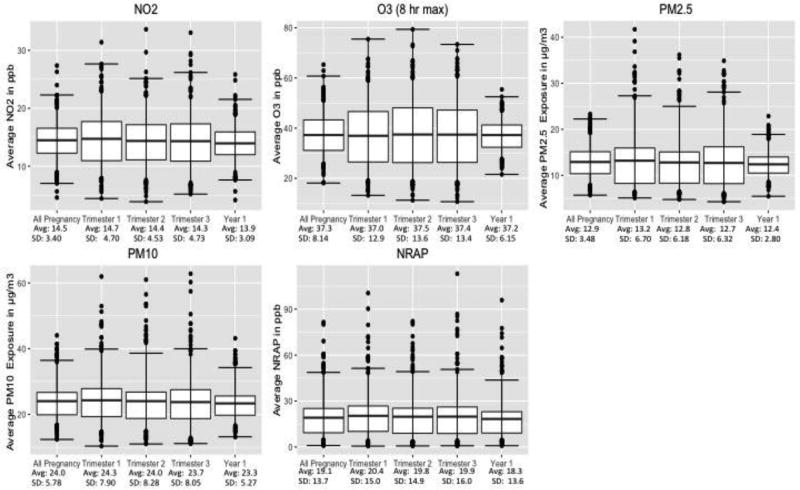
Exposure distributions for NO2, O3, PM2.5, PM10, and NRAP are presented for the entire pregnancy, each trimester and Year 1 of life in Figure 1. The means of each pollutant were similar for all these metrics. Exposures to regional NO2 (all pregnancy mean 14.5 ppb; standard deviation (S.D.) 3.4 ppb) were below the national annual average air quality standard of 53 ppb, with few exceptions. The mean all-pregnancy PM2.5 (pregnancy mean 12.9; S.D. 3.5) exceeded the current national standards of 12 µg/m3). The all-pregnancy PM10 and 8-hour daily maximum O3 averages were 24.0 µg/m3, S.D. 5.8 µg/m3 and 37.3 ppb, S.D. 8.1 ppb. There was a wide range of exposure to the NRAP mixture, for which there also is no standard. Individual air pollutants showed moderate to high correlation across time (Supplement Table 2). During pregnancy and the first year of life PM2.5 and PM10 were highly correlated, while particulate exposure and NO2 showed moderate correlations (Supplement Table 2).
Figure 1.
Pollutant exposure distribution for each trimester, the entire pregnancy and for the first year of life.
Center line is mean, boxes represent IQR, and bars are the 95% CI
All-pregnancy and first year exposure associations
None of the scores on cognitive or adaptive scales was associated with overall prenatal or year 1 exposures to O2, PM2.5, PM10 or NRAP exposures. However, higher average regional NO2 exposure during pregnancy and the first year of life was associated with decreases in both adaptive functioning and cognitive scores after adjustment for covariates (Table 2). During pregnancy, there was a 11.0% (95% CI: −17.0%, −1.45%) decrease in VABS composite score per 2 SD increase in NO2 (6.82 ppb). NO2 was also associated with decreased performance on the communication (−13.3% [95% CI: −23.7%, −2.71%]) and socialization (−9.60% [95%CI: −18.6%, −0.43%]) subscale scores. Increased NO2 exposure was associated with decreased MSEL composite DQ performance (−7.47 points [(95%CI: −12.7, −2.21]) and on both MSEL DQ subscales: −9.72 points for the verbal subscale (95% CI: −15.7, −3.72), and −5.21 points for receptive language (95% CI: −10.3, −0.12). Associations of cognitive and adaptive functioning with first year NO2 were consistently larger than with average pregnancy exposures, and additional associations that were statistically significant for first year exposure included VABS dependent living (−10.6% [-19.6%, −1.38%]). We did not find an association of any pollutant exposure with the CSS (Table 2). Further adjustment for gestational age in weeks and year of birth did not change results (Supplement Table 3).
Table 2.
Adjusteda Association of Air Pollutant Exposure During Entire Pregnancy and Year 1 of Life with VABS, MSEL, and CSS Scores
| Time | Score | Air Pollutant Exposureb (N=325) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||
| NO2 | 95% CI | O3 | 95% CI | PM10 | 95% CI | PM2.5 | 95% CI | NRAP | 95% CI | ||
| Prenatal | VABS Composite Score | −11.0% | (−17.0%, −1.45%)* | −0.91% | (−8.74%, 6.98%) | 4.41% | (−8.13%, 17.1%) | −4.49% | (−14.9%, 6.12%) | −0.48% | (−9.26%, 8.32%) |
| VABS Communication Subscale | −13.3% | (−23.7%, −2.71%)* | 0.23% | (−8.75%, 9.28%) | 4.61% | (−9.75%, 19.1%) | −4.24% | (−16.2%, 7.93%) | −1.25% | (−11.3%, 8.83%) | |
| VABS Dependent Living Subscale | −7.60% | (−15.8%, 0.69%) | −1.91% | (−8.87%, 5.08%) | 3.93% | (−7.21%, 15.2%) | −1.31% | (−10.7%, 8.15%) | 1.13% | (−6.66%, 8.95%) | |
| VABS Socialization Subscale | −9.60% | (−18.6%, −0.43%)* | 1.60% | (−6.13%, 9.38%) | 4.64% | (−7.71%, 17.1%) | −4.12% | (−14.4%, 6.33%) | −2.71% | (−11.3%, 5.94%) | |
| VABS Motor Skills Subscale | −3.49 | (−7.64, 0.65) | −0.91 | (−4.38, 2.56) | 1.61 | (−3.94, 7.15) | −2.35 | (−7.02, 2.33) | 0.70 | (−3.18, 4.58) | |
| MSEL Composite DQ | −7.47 | (−12.7, −2.21)* | −0.06 | (−2.78, 2.66) | 0.47 | (−3.67, 4.62) | −4.30 | (−10.1, 1.53) | −0.68 | (−1.91, 0.56) | |
| MSEL Verbal DQ | −9.72 | (−15.7, −3.72)* | 0.18 | (−4.90, 5.27) | −0.95 | (−9.07, 7.18) | −6.08 | (−12.9, 0.74) | −4.60 | (−10.3, 1.06) | |
| MSEL Non Verbal DQ | −5.21 | (−10.3, −0.12)* | −0.39 | (−4.66, 3.88) | 2.56 | (−4.25, 9.38) | −2.72 | (−8.47, 3.03) | −0.81 | (−5.58, 3.96) | |
| ADOS CSS | 0.15 | (−0.23, 0.52) | −0.39 | (−4.66, 3.88) | 0.22 | (−0.27, 0.72) | 0.05 | (−0.36, 0.47) | 0.11 | (−0.23, 0.46) | |
|
|
|||||||||||
| Year 1 | VABS composite score | −14.6% | (−20.9%, −3.71%)* | 0.91% | (−11.4%, 13.4%) | −3.75% | (−18.7%, 11.3%) | −8.74% | (−22.7%, 5.48%) | 1.50% | (−7.48%, 10.5%) |
| VABS Communication Subscale | −18.0% | (−29.5%, −6.38%)* | 5.14% | (−9.02%, 19.5%) | −7.14% | (−24.1%, 10.1%) | −10.69% | (−26.6%, 5.55%) | 0.95% | (−9.32%, 11.3%) | |
| VABS Dependent Living Subscale | −10.6% | (−19.6%, −1.38%)* | −1.54% | (−12.5%, 9.51%) | −7.14% | (−20.3%, 6.20%) | −10.04% | (−22.4%, 2.53%) | 3.01% | (−4.96%, 11.0%) | |
| VABS Socialization Subscale | −11.7% | (−21.7%, −1.51%)* | 3.86% | (−8.32%, 16.2%) | 5.57% | (−9.20%, 20.5%) | 2.89% | (−11.1%, 17.1%) | −3.62% | (−12.4%, 5.22%) | |
| VABS Motor Skills Subscale | −4.45 | (−9.06, 0.16) | −0.82 | (−6.29, 4.65) | −0.15 | (−6.79, 6.49) | −3.39 | (−9.68, 2.90) | 1.90 | (−2.06, 5.86) | |
| MSEL Composite DQ | −9.62 | (−15.4, −3.81)* | 1.43 | (−2.85, 5.71) | −1.12 | (−6.08, 3.83) | −3.68 | (−11.5, 4.17) | −0.49 | (−1.76, 0.77) | |
| MSEL Verbal DQ | −11.8 | (−18.4, −5.12)* | 3.36 | (−4.62, 11.4) | −4.41 | (−14.1, 5.27) | −6.74 | (−15.9, 2.42) | −2.67 | (−8.46, 3.11) | |
| MSEL Non Verbal DQ | −7.48 | (−13.1, −1.84)* | 1.30 | (−5.42, 8.03) | 0.58 | (−7.59, 8.74) | −0.79 | (−8.53, 6.96) | −1.28 | (−6.16, 3.59) | |
| ADOS CSS | 0.26 | (−0.15, 0.67) | −0.13 | (−0.61, 0.36) | −0.09 | (−0.68, 0.50) | −0.05 | (−0.61, 0.51) | 0.17 | (−0.18, 0.53) | |
All models were adjusted for sex, max education in the home, referral center, race, mother’s age, prenatal smoking, season of conception and home ownership
Pollutant effects were scaled to 2 SD of the distribution of the pregnancy average exposure: 6.82 ppb for NO2, 11.1 ppb for O3, 11.6 µg/m3 for PM10, 6.97 µg/m3 for PM2.5, and 27.3 ppb for NRAP
p<0.05
Trimester-specific exposure associations
As for the entire pregnancy exposure, only NO2 exposure showed trimester-specific associations with the outcomes of interest. Specifically, NO2 exposure in the first trimester was associated with decreased VABS composite, communication and socialization scores, and with decreased MSEL composite and verbal DQ scores. (See Table 3). Increasing NO2 exposure in the second and third trimesters was also associated with reduced adaptive and cognition function, but the associations were weaker than with first trimester or year 1 exposure, and associations with third trimester exposure were not statistically significant. Third, but neither first nor second, trimester PM10 was, paradoxically, associated with improved composite and the socialization subscale VABS scores. No other trimester-specific pollutant exposures were associated with any outcomes.
Table 3.
Adjusteda Associations of Trimester-specific Air Pollutant Exposure with VABS, MSEL, and CSS Scores
| Prenatal Air Pollutant Exposureb (N=325) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| NO2 | 95% CI | O3 | 95% CI | PM10 | 95% CI | PM2.5 | 95% CI | NRAP | 95% CI | ||
| First Trimester | VABS Composite score | −7.56% | (−11.9%, −0.80%)* | −2.19% | (−6.85%, 2.49%) | −0.80% | (−8.26%, 6.71%) | −0.35% | (−5.17%, 4.51%) | −0.96% | (−9.03%, 7.12%) |
| VABS Communication Subscale | −8.57% | (−16.0%, −1.01%)* | −1.78% | (−7.12%, 3.58%) | 0.49% | (−8.05%, 9.10%) | −0.03% | (−5.55%, 5.53%) | −1.87% | (−11.1%, 7.38%) | |
| VABS Dependent Living Subscale | −5.09% | (−11.0%, 0.81%) | −2.03% | (−6.17%, 2.12%) | −1.82% | (−8.44%, 4.84%) | −0.07% | (−4.35%, 4.24%) | −0.26% | (−7.42%, 6.92%) | |
| VABS Socialization Subscale | −8.40% | (−14.8%, −1.92%)* | −0.36% | (−4.96%, 4.26%) | −1.11% | (−8.45%, 6.28%) | −1.57% | (−6.30%, 3.20%) | −4.40% | (−12.3%, 3.53%) | |
| VABS Motor Skills Subscale | −2.21 | (−5.16, 0.73) | −1.06 | (−3.13, 1.00) | −0.37 | (−3.67, 2.93) | −0.22 | (−2.36, 1.91) | 1.25 | (−2.31, 4.81) | |
| MSEL Composite DQ | −4.92 | (−8.65, −1.19)* | −0.32 | (−1.94, 1.29) | −0.67 | (−3.14, 1.79) | −1.37 | (−4.03, 1.29) | −0.49 | (−1.63, 0.64) | |
| MSEL Verbal DQ | −6.30 | (−10.6, −2.04)* | 0.08 | (−2.94, 3.10) | −1.61 | (−6.43, 3.21) | −1.99 | (−5.10, 1.12) | −3.82 | (−9.01, 1.36) | |
| MSEL Non Verbal DQ | −3.53 | (−7.15, 0.08) | −1.13 | (−3.67, 1.41) | −0.70 | (−4.76, 3.36) | −0.81 | (−3.44, 1.81) | −0.12 | (−4.50, 4.26) | |
| ADOS CSS | −0.03 | (−0.29, 0.24) | 0.04 | (−0.14, 0.23) | −0.11 | (−0.41, 0.18) | −0.06 | (−0.25, 0.13) | 0.07 | (−0.25, 0.39) | |
| Second Trimester | VABS Composite score | −7.93% | (−13.1%, −0.23%)* | −1.31% | (−5.54%, 2.94%) | −2.58% | (−10.7%, 5.56%) | −2.17% | (−8.23%, 3.93%) | 1.44% | (−6.65%, 9.55%) |
| VABS Communication Subscale | −8.43% | (−17.1%, 0.35%) | −0.55% | (−5.40%, 4.31%) | −2.05% | (−11.3%, 7.28%) | −1.47% | (−8.40%, 5.54%) | 1.78% | (−7.47%, 11.1%) | |
| VABS Dependent Living Subscale | −5.83% | (−12.6%, 1.00%) | −1.22% | (−4.98%, 2.55%) | 0.74% | (−6.46%, 7.99%) | −0.65% | (−6.04%, 4.79%) | 2.03% | (−5.15%, 9.22%) | |
| VABS Socialization Subscale | −8.05% | (−15.5%, −0.52%)* | −0.17% | (−4.34%, 4.02%) | −3.62% | (−11.6%, 4.37%) | −2.39% | (−8.34%, 3.62%) | −0.91% | (−8.86%, 7.06%) | |
| VABS Motor Skills Subscale | −2.40 | (−5.81, 1.01) | −0.84 | (−2.71, 1.03) | −0.64 | (−4.23, 2.95) | −0.83 | (−3.52, 1.86) | 1.12 | (−2.44, 4.69) | |
| MSEL Composite DQ | −4.94 | (−9.27, −0.61)* | −0.20 | (−1.67, 1.26) | −0.71 | (−3.39, 1.97) | −1.08 | (−4.43, 2.28) | −0.29 | (−1.43, 0.84) | |
| MSEL Verbal DQ | −6.15 | (−11.1, −1.20)* | −0.34 | (−3.08, 2.39) | −1.89 | (−7.12, 3.35) | −1.45 | (−5.38, 2.48) | −2.21 | (−7.42, 2.99) | |
| MSEL Non Verbal DQ | −3.73 | (−7.92, 0.46) | −0.32 | (−2.62, 1.98) | −0.55 | (−4.96, 3.86) | −0.75 | (−4.06, 2.56) | −0.14 | (−4.53, 4.25) | |
| ADOS CSS | 0.11 | (−0.19, 0.42) | 0.11 | (−0.06, 0.28) | 0.21 | (−0.11, 0.53) | 0.02 | (−0.22, 0.26) | 0.03 | (−0.29, 0.35) | |
| Third Trimester | VABS Composite score | −1.76% | (−6.93%, 4.01%) | 3.60% | (−1.24%, 8.46%) | 8.24% | (1.31%, 15.2%)* | −0.91% | (−5.96%, 4.18%) | 0.88% | (−6.81%, 8.60%) |
| VABS Communication Subscale | −3.29% | (−10.7%, 4.15%) | 3.41% | (−2.13%, 8.98%) | 7.36% | (−0.60%, 15.4%) | −1.19% | (−6.97%, 4.64%) | −0.17% | (−8.97%, 8.66%) | |
| VABS Dependent Living Subscale | −1.62% | (−7.36%, 4.17%) | 2.15% | (−2.15%, 6.47%) | 5.26% | (−0.91%, 11.5%) | −0.78% | (−5.28%, 3.74%) | 2.06% | (−4.78%, 8.91%) | |
| VABS Socialization Subscale | 1.48% | (−4.90%, 7.91%) | 2.84% | (−1.92%, 7.62%) | 10.1% | (3.31%, 16.9%)* | 1.12% | (−3.87%, 6.14%) | −0.82% | (−8.38%, 6.76%) | |
| VABS Motor Skills Subscale | −0.92 | (−3.78, 1.95) | 1.37 | (−0.77, 3.50) | 2.56 | (−0.50, 5.62) | −0.71 | (−2.95, 1.53) | 1.03 | (−2.37, 4.43) | |
| MSEL Composite DQ | −1.81 | (−5.46, 1.84) | 0.53 | (−1.14, 2.21) | 1.70 | (−0.59, 3.98) | −0.40 | (−3.20, 2.39) | −0.60 | (−1.68, 0.48) | |
| MSEL Verbal DQ | −2.59 | (−6.76, 1.59) | 0.35 | (−2.77, 3.47) | 2.28 | (−2.19, 6.76) | −0.43 | (−3.70, 2.84) | −2.74 | (−7.69, 2.21) | |
| MSEL Non Verbal DQ | −1.03 | (−4.56, 2.50) | 1.40 | (−1.23, 4.02) | 3.52 | (−0.24, 7.28) | −0.40 | (−3.15, 2.36) | −2.10 | (−6.27, 2.08) | |
| ADOS CSS | 0.15 | (−0.10, 0.41) | −0.06 | (−0.25, 0.13) | 0.17 | (−0.11, 0.44) | 0.12 | (−0.08, 0.32) | 0.18 | (−0.12, 0.49) | |
All models were adjusted for sex, max education in the home, referral center, race, mother’s age, prenatal smoking, season of conception and home ownership
Pollutant effects were scaled to 2 SD of the distribution of the pregnancy average exposure: 6.82 ppb for NO2, 11.1 ppb for O3, 11.6 µg/m3 for PM10, 6.97 µg/m3 for PM2.5, and 27.3 ppb for NRAP
p<0.05
Correlations of NO2 exposures across developmental windows of exposure from trimester 1 to year 1 of life were between 0.46 and 0.66 (Supplemental Table 4), with the exception of 1st trimester with year 1 exposures (rho= −0.03). Thus, the seasonal variations were sufficient to allow for modeling effects of two exposure windows in a single model. For NO2 exposures that were statistically significant (trimesters 1, 2 and year 1 exposures), we fitted models with two periods of exposure, in an attempt to identify an unconfounded likely developmental window for NO2 effects. The diagonal (shaded) in Table 4 represents the unadjusted trimester- and year 1-specific effect for each outcome (from Tables 2 and 3) that can be compared to effects after adjustment for exposure in another period (estimates shown across the same row). The association with each outcome, adjusted for another window of exposure, is presented in unshaded cells across the row corresponding to the trimester of interest. For example, the first trimester association of NO2 with decreased VABS composite score (−7.56%; 95% CI: −11.9%, −0.80%) was attenuated after adjustment for second trimester exposure (−5.38%; 95% CI: −11.7%, 2.75%) or after adjustment for year 1 exposure (−3.26%; 95% CI: −9.50%, 4.07%). Notably, associations with year 1 exposure remained statistically significant after adjusting for other time points In these models, year 1 effects were generally larger than in unadjusted models, albeit with wider confidence intervals, and the associations remained significant for the VABS composite, communication, and dependent living scores, and all MSEL DQ scores. First-trimester NO2 associations with VABS and MSEL scores were no longer significant after adjustment for second trimester NO2 exposure. Adjustment for third-trimester exposure had little effect on the pattern of associations of other exposures (results not shown).
Table 4.
Effects of Trimester 1 and 2 and Year 1 NO2 exposures adjusted for each othera
| Time Point | Adjustmentb | ||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Trimester 1 | Trimester 2 | Year 1 | |||||
| betac | 95% CI | betac | 95% CI | betac | 95% CI | ||
|
|
|||||||
| VABS Composite Score | Trimester 1 | −7.56% | (−11.9%, −0.80%)* | −5.38% | (−11.7%, 2.75%) | −3.26% | (−9.50%, 4.07%) |
| Trimester 2 | −3.97% | (−10.6%, 3.95%) | −7.93% | (−13.1%, −0.23%)* | 0.44% | (−9.31%, 10.2%) | |
| Year 1 | −11.8% | (−16.6%, −3.18%)* | −15.1% | (−22.2%, −3.09%)* | −14.6% | (−20.9%, −3.71%)* | |
|
| |||||||
| VABS Communication Subscale | Trimester 1 | −8.57% | (−16.0%, −1.01%)* | −6.63% | (−16.4%, 3.26%) | −2.90% | (−12.0%, 6.36%) |
| Trimester 2 | −3.55% | (−13.3%, 6.38%) | −8.43% | (−17.1%, 0.35%) | 3.66% | (−9.49%, 17.1%) | |
| Year 1 | −15.5% | (−24.5%, −6.40%)* | −21.6% | (−34.3%, −8.71%)* | −18.0% | (−29.5%, −6.38%)* | |
|
| |||||||
| VABS Dependent Living Subscale | Trimester 1 | −5.09% | (−11.0%, 0.81%) | −3.17% | (−10.8%, 4.55%) | −1.82% | (−8.98%, 5.41%) |
| Trimester 2 | −3.50% | (−11.1%, 4.21%) | −5.83% | (−12.6%, 1.00%) | 0.05% | (−10.2%, 10.4%) | |
| Year 1 | −8.95% | (−16.0%, −1.80%)* | −10.6% | (−20.7%, −0.38%)* | −10.6% | (−19.6%, −1.38%)* | |
|
| |||||||
| VABS Socialization Subscale | Trimester 1 | −8.40% | (−14.8%, −1.92%)* | −6.70% | (−15.1%, 1.76%) | −6.16% | (−14.0%, 1.78%) |
| Trimester 2 | −3.11% - | (−11.5%, 5.40%) | −8.05% | (−15.5%, −0.52%)* | −3.62% | (−14.9%, 7.84%) | |
| Year 1 | 6.19% | (−14.0%, 1.75%) | −8.0% | (−19.3%, 3.34%) | −11.7% | (−21.7%, −1.51%)* | |
|
| |||||||
| VABS Motor Skills Subscale | Trimester 1 | −2.21 | (−5.16, 0.73) | −1.51 | (−5.34, 2.33) | −0.88 | (−4.47, 2.71) |
| Trimester 2 | −1.28 | (−5.12, 2.55) | −2.40 | (−5.81, 1.01) | 0.14 | (−5.00, 5.27) | |
| Year 1 | −3.66 | (−7.25, −0.07)* | −4.59 | (−9.73, 0.55) | −4.45 | (−9.06, 0.16) | |
|
| |||||||
| MSEL DQ composite | Trimester 1 | −4.92 | (−8.65, −1.19)* | −3.70 | (−8.56, 1.15) | −2.09 | (−6.61, 2.44) |
| Trimester 2 | −2.20 | (−7.06, 2.65) | −4.94 | (−9.27, −0.61)* | 0.85 | (−5.63, 7.33) | |
| Year 1 | −7.75 | (−12.3, −3.22)* | −10.49 | (−17.0, −4.01)* | −9.62 | (−15.4, −3.81)* | |
|
| |||||||
| MSEL DQ verbal | Trimester 1 | −6.30 | (−10.6, −2.04)* | −4.91 | (−10.5, 0.63) | −2.99 | (−8.15, 2.18) |
| Trimester 2 | −2.52 | (−8.06, 3.02) | −6.15 | (−11.1, −1.20)* | 0.78 | (−6.62, 8.18) | |
| Year 1 | −9.08 | (−14.3, −3.92)* | −12.6 | (−20.0, −5.15)* | −11.8 | (−18.4, −5.12)* | |
|
| |||||||
| MSEL DQ non verbal | Trimester 1 | −3.53 | (−7.15, 0.08) | −2.49 | (−7.20, 2.21) | −1.19 | (−5.59, 3.20) |
| Trimester 2 | −1.88 | (−6.59, 2.82) | −3.73 | (−7.92, 0.46) | 0.92 | (−5.37, 7.20) | |
| Year 1 | −6.41 | (−10.8, −2.02)* | −8.42 | (−14.7, −2.13)* | −7.48 | (−13.1, −1.84)* | |
|
| |||||||
| ADOS CSS | Trimester 1 | −0.03 | (−0.29, 0.24) | −0.15 | (−0.49, 0.19) | −0.18 | (−0.50, 0.14) |
| Trimester 2 | 0.22 | (−0.12, 0.57) | 0.11 | (−0.19, 0.42) | −0.07 | (−0.53, 0.39) | |
| Year 1 | 0.42 | (0.10, 0.74) | 0.33 | (−0.13, 0.79) | 0.26 | (−0.15, 0.67) | |
Shaded diagonal represents the main effect for each time point. Each time point (row) was then adjusted for an additional time point (column) for each test score
All models were adjusted for sex, max education in the home, referral center, race, mother’s age, prenatal smoking, season of conception and home ownership
Pollutant effects were scaled to 6.82 ppb for NO2 (2 SD of the distribution of the pregnancy average exposure)
p<0.05
Comparison of findings in typically developing controls
To improve understanding of these findings we examined these same relationships among typically developing subjects. We found that the VABS composite score was higher in girls than in boys (Supplemental Table 5). The MSEL DQ shows significant differences by gender, maternal education, and homeownership, with higher scores seen in girls, children of college graduate mothers, and homeowners. Children from the East Bay regional center performed better than those from the other regional centers of the MSEL DQ. We did not find any associations between prenatal air pollution exposure and VABS or MSEL scores among typically developing children in adjusted models (Supplemental Table 6). First year of life exposures to NO2 and ozone were associated with improved performance on the VABS dependent living and motor skills subscales, and MSEL non-verbal DQ.
Discussion
Among children with ASD, NO2 exposures during pregnancy and the first year of life were associated with increased impairment in cognitive and adaptive skills. Associations with decreased language, communication, adaptive abilities, and fine motor skills were strongest during the first trimester of gestation and the first year of life. Notably the strongest associations were found for first year of life, suggesting the importance of early postnatal air pollution exposure on course of ASD. We did not observe associations between prenatal air pollution and cognitive and adaptive behavior in typically developing subjects, suggesting that the observed effects may not reflect on broad neurodevelopment and be limited to ASD or other neurodevelopmental disorders.
Several air pollutants have been previously linked to ASD diagnosis (Becerra et al. 2013; Raz et al. 2015; Roberts et al. 2013; Volk et al. 2011; Volk et al. 2013) and to neurological and cognitive impairment in other populations of children (Morales et al. 2009; Perera et al. 2013; Wang et al. 2009). NO2 has also been associated with cognitive and verbal deficits among children in several studies (Guxens et al. 2012a; Guxens et al. 2012b; Morales et al. 2009; Porta et al. 2015; Vrijheid et al. 2012; Wang et al. 2009; Yorifuji et al. 2015). However, to our knowledge the effect of air pollution on cognitive and adaptive deficits among children diagnosed with ASD has not been previously studied.
NO2 is both a regional pollutant and a commonly measured proxy for NRAP, because NO2 has marked spatial variation in close proximity to major roadways (Zhu et al. 2002). Several studies have found associations between NO2 and neurocognitive outcomes in children in settings in which NO2 may have been a marker for the NRAP mixture (Guxens et al. 2012b; Guxens and Sunyer 2012; Porta et al. 2015; Wang et al. 2009; Yorifuji et al. 2015). For example, prenatal exposure to traffic-related NO2 in Japan was associated with subsequent decreased verbal ability, psychomotor ability and inability to express emotions in children aged 2.5 to 5.5 years (Yorifuji et al. 2015), and in an Italian birth cohort with subsequent decreased verbal cognitive development at seven years old (Porta et al. 2015). NO2, as a proxy for NRAP, has also been associated with ASD (Becerra et al. 2013; Jung et al. 2013; Volk et al. 2013), including in the CHARGE study (Volk et al 2013). However, in this analysis focused on children with ASD, we did not observe associations of cognitive or adaptive deficits with NRAP, which is likely to have been a better marker of the near-roadway mixture than NO2 estimated from the regional monitoring network. NO2 may also have been a proxy for some other unmeasured pollutant exposure. Diesel engines are a major source for NO2 in urban areas, and diesel exhaust particulate has been shown to produce subsequent neurological deficits, including ASD-like phenotypes and altered behavior and learning in rodents (Costa et al. 2014; Ema et al. 2013; Suzuki et al. 2010).
A separate literature has begun to support the hypothesis that small particles that have both traffic and regional sources, including PM2.5 and even small ultrafine particles (UFP) less than 100 nm in aerodynamic diameter, have an effect on the brain. Both UFP mass and particle number have recently been recognized to have a substantial regional component due to secondary photooxidation (Singh et al. 2006). UFP, which may be translocated into the systemic circulation through the lung (Kreyling et al. 2004) and which has been shown postnatally also to be translocated directly into the brain through the olfactory bulb, have been associated with neuroinflammation and oxidative stress in the brain (Block and Calderon-Garciduenas 2009; Lucchini et al. 2012). In a mouse model exposure to UFP during gestation and early life at concentrations within the range of urban exposure caused a pattern of developmental neurotoxicity consistent with what might be expected in ASD, including its greater impact in males (Allen et al. 2014; Allen et al. 2015). Only recently have methods emerged for assignment of UFP in population studies, and were not available at the time of our exposure assignments (Hu et al. 2015).
It is also possible that NO2 itself was responsible for associations observed. Indoor NO2 from gas appliances, which is not a surrogate for pollutants from other ambient sources, has been associated with deficits in cognitive function in children with early life exposure (Morales et al. 2009; Vrijheid et al. 2012). NO2 is a powerful oxidant that generates free radicals causing oxidative stress and inflammatory damage to the lungs when inhaled (Riva et al. 2011), and because it is relatively water insoluble, the dose of NO2 to the distal airways and alveoli relative to ambient concentrations may be larger than some other reactive pollutants, such as O3. Associated systemic oxidative stress and inflammation may affect other organs including the brain (Morgan et al. 2011). In animal studies inhalation of NO2 caused widespread damage to the brain including neuronal damage in the hippocampus (Li and Xin 2013) and depletion of lipids in the cerebral hemispheres, cerebellum, and mid brain (Farahani and Hasan 1990; Farahani and Hasan 1992). Inhalation of NO2 may cause damage to the brain through mitochondrial damage (Yan et al. 2015), which can increase the generation of reactive oxygen species in the brain (Calabrese et al. 2005; Li and Xin 2013) and induce an altered signaling cascade of brain function, including energy production, cell metabolism, and apoptosis triggering, which could affect brain development (Yan et al. 2015). Additionally, mitochondrial dysfunction has also been implicated in ASD (Correia et al. 2006; Giulivi et al. 2010; Goh et al. 2014; Pons et al. 2004; Rossignol and Frye 2012; Rossignol and Frye 2014; Weissman et al. 2008), and co-occurring mitochondrial disorders are significantly higher in ASD subjects than the general population (Correia et al. 2006; Oliveira et al. 2005; Rossignol and Frye 2012; Rossignol and Frye 2014). Despite this rationale, no study to date has examined the effect of the air pollutant mix on neurodevelopment with the goal of identifying the sole causal compound underlying these observed relationships. Therefore, we cannot conclude that any one pollutant, for example NO2, is responsible and it may be the mix of pollutants, for which NO2 is a marker, truly responsible for inducing systemic effects which effect the developing brain.
Recent studies have examined ASD risk with respect to trimester-specific windows of susceptibility to prenatal air pollution exposure and have even suggested causal models consistent with a third trimester association that has been observed in some studies (Raz et al. 2015; Weisskopf et al. 2015). In contrast, we found that NO2 exposure early in pregnancy and during the first year of life may be more important when examining deficits in cognitive and adaptive behaviors among autistic children. Our results suggest, in fact, that early life exposure may be more important for broad cognitive impairment and are somewhat consistent with recent reports of postnatal PAH exposure effects on verbal IQ (Jedrychowski et al. 2015). Further study of associations of developmental windows of exposure with autism risk and autism phenotype which extend into childhood may provide insight into both the shared effects of air pollution exposure on neurodevelopment more broadly and into distinct mechanistic factors that may differentiate these general cognitive and adaptive deficits from risk of ASD.
Recent epidemiological studies of children and animal toxicological studies indicate that early life PM2.5 exposure produces diffuse neurodevelopmental damage (Costa et al. 2014; Costa et al. 2015), including associations with autism (Raz et al. 2015; Volk et al. 2013). We observed no associations of cognitive, adaptive or autism severity scores with PM2.5. However, the extent of deficits among children with autism that we studied here is a different outcome from the risk of autism. Given the associations identified with composite MSEL and VABS scores it is not surprising that we find further association with MSEL and VABS subscales. While additional evaluation is needed these results may provide additional insight into specific cognitive domains and processes affected by air pollution exposure, balancing the statistical cost of additional analytic comparisons. Future research might address language formation or executive function, drawing on associations with communication subscales, to further evaluate the underpinnings on these results. In addition, we unexpectedly found an association between third trimester PM10 and better adaptive scores among children with ASD. These associations were significant in models adjusted for PM10 exposure in other trimesters and for NO2 exposure during the first or second trimester or during the first year of life (results not shown), and were robust to adjustment for season. Because there were no associations with PM2.5, if the associations with third trimester PM10 are causal, they likely reflect an effect of the coarse PM fraction containing bioaerosols and other components present in PM10 but not present in PM2.5. Additionally, these findings appear to be isolated (limited to one subscale and one composite) and are likely a result of random fluctuation or residual confounding, rather than a true association. Further investigation is needed to see if these observations are reproducible.
Although we found associations of NO2 with broad markers of neurodevelopmental delay in children with ASD, no significant associations with the ADOS CSS, indicative of ASD severity, were identified. In our study the CSS was only weakly correlated with the adaptive and cognitive outcomes, consistent with another recent report (Thurm et al. 2015). Therefore, it is possible that prenatal pollution exposure may broadly affect cognitive and adaptive function phenotypes in children with autism but not the severity of the disorder. Investigation of other measures of ASD-related quantitative traits, not currently available in CHARGE, could help to corroborate these results and may also show stronger correlations with measures of cognitive and adaptive function. We also speculate that additional factors, such as genetic susceptibility, may interact with air pollution to increase risk of ASD in pathways that do not contribute to disorder severity.
The higher cognitive and adaptive scores in white children and in children of older parents and better educated parents may reflect true variation in disease phenotype among all children with ASD, but also could reflect better ascertainment of children with milder impairment of these domains in families with these characteristics that likely also correlate with access to medical care and to state disability services. Because air pollution has been found to be higher on average in low income, minority areas, and because the ASD children in this study whose parents had lower education or were Hispanic or non-white scored lower on both cognitive and adaptive scales, confounding from socioeconomic status would tend to produce a bias away from the null. However, we adjusted all analyses for parental education, race/ethnicity of the child, and home ownership, a proxy for wealth. The possibility of residual confounding of the findings for NO2 remains, either due to our categories not fully capturing the variation of SES or resulting from other related factors such as nutrition or quality of parent-child interactions. Other possible limitations to this study include the limited geographic coverage of the population that may affect generalizability of results and the potential bias due to inaccurate recall of past residential addresses or measurement error inherent in the reliance on a dispersed monitoring network for assigning personal indoor residential exposure where children spend most of their time during gestation and early life (Sheppard et al. 2012). This bias might have been non-differential with respect to the outcomes, leading to an underestimate of the strength of observed effects (Dominici et al. 2000); it is possible, however, that children in some areas or population groups (e.g., rural communities, Hispanic families) are spending more time outdoors or are from more transient families, potentially giving rise to differential measurement error. MSEL and VABS measurements are normalized to typically developing children.
In conclusion, we showed that prenatal NO2 exposure was associated with cognitive and adaptive deficits in a sample of children with ASD. Further study of effects of air pollution and other environmental exposures on these outcomes may provide both new opportunities for understanding the determinants of phenotypic characteristics of ASD and new targets for prevention.
Supplementary Material
Acknowledgments
Compliance with Ethical Standards:
This study was funded by the National Institute of Environmental Health Sciences (ES013678, ES019002, ES015359, ES11269), the National Institute of Mental Health (MH073124), the Eunice Kennedy Shriver National Institute of Child Health and Human Development (HD079125), and the Environmental Protection Agency (829388, 833292). All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and within the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants in the study.
Funding: Grant sponsors: National Institute of Environmental Health Sciences of the National Institutes of Health: Grant numbers: T32ES013678; ES019002, ES007048, ES013578, P01-ES11269, R01-ES015359, P30-ES023513, ES016535, ES022845, MH089430; and ES011627; EPA Star-R; 823392, 833292; 83544101; MIND Institute matching funds and pilot grant program.
Financial Disclosures:
Fred Lurmann is employed by Sonoma Technology Inc., Petaluma, CA. Rob McConnell and Fred Lurmann have received support from an air quality violations settlement agreement between the South Coast Air Quality Management District, a California state regulatory agency, and BP. Drs. Volk and Eckel received travel funds from Autism Speaks to present at an academic conference.
Footnotes
Conflict of Interest: The other authors declare no competing financial interests.
References
- Allen JL, et al. Early postnatal exposure to ultrafine particulate matter air pollution: persistent ventriculomegaly, neurochemical disruption, and glial activation preferentially in male mice. Environ Health Perspect. 2014;122:939–45. doi: 10.1289/ehp.1307984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen JL, et al. Developmental neurotoxicity of inhaled ambient ultrafine particle air pollution: Parallels with neuropathological and behavioral features of autism and other neurodevelopmental disorders. Neurotoxicology. 2015 doi: 10.1016/j.neuro.2015.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Fifth. Arlington, VA: American Psychiatric Publishing; 2013. Text Revision. [Google Scholar]
- Becerra TA, Wilhelm M, Olsen J, Cockburn M, Ritz B. Ambient air pollution and autism in Los Angeles county, California. Environ Health Perspect. 2013;121:380–6. doi: 10.1289/ehp.1205827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block ML, Calderon-Garciduenas L. Air pollution: mechanisms of neuroinflammation and CNS disease. Trends Neurosci. 2009;32:506–16. doi: 10.1016/j.tins.2009.05.009. doi:S0166-2236(09)00128-3 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabrese V, et al. Oxidative stress, mitochondrial dysfunction and cellular stress response in Friedreich’s ataxia. J Neurol Sci. 2005;233:145–62. doi: 10.1016/j.jns.2005.03.012. [DOI] [PubMed] [Google Scholar]
- Chen JC, et al. Ambient air pollution and neurotoxicity on brain structure: Evidence from women’s health initiative memory study. Ann Neurol. 2015;78:466–76. doi: 10.1002/ana.24460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen DL, et al. Prevalence and Characteristics of Autism Spectrum Disorder Among Children Aged 8 Years--Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2012. MMWR Surveill Summ. 2016;65:1–23. doi: 10.15585/mmwr.ss6503a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correia C, et al. Brief report: High frequency of biochemical markers for mitochondrial dysfunction in autism: no association with the mitochondrial aspartate/glutamate carrier SLC25A12 gene. J Autism Dev Disord. 2006;36:1137–40. doi: 10.1007/s10803-006-0138-6. [DOI] [PubMed] [Google Scholar]
- Costa LG, Cole TB, Coburn J, Chang YC, Dao K, Roque P. Neurotoxicants are in the air: convergence of human, animal, and in vitro studies on the effects of air pollution on the brain. Biomed Res Int. 2014;2014:736385. doi: 10.1155/2014/736385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa LG, Cole TB, Coburn J, Chang YC, Dao K, Roqué PJ. Neurotoxicity of traffic-related air pollution. Neurotoxicology. 2015 doi: 10.1016/j.neuro.2015.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominici F, Zeger SL, Samet JM. A measurement error model for time-series studies of air pollution and mortality. Biostatistics. 2000;1:157–75. doi: 10.1093/biostatistics/1.2.157. [DOI] [PubMed] [Google Scholar]
- Ema M, Naya M, Horimoto M, Kato H. Developmental toxicity of diesel exhaust: a review of studies in experimental animals. Reprod Toxicol. 2013;42:1–17. doi: 10.1016/j.reprotox.2013.06.074. [DOI] [PubMed] [Google Scholar]
- Farahani H, Hasan M. Effect of NO2 on lipids and lipid peroxidation in the CNS of the guinea-pig. Pharmacol Toxicol. 1990;66:146–9. doi: 10.1111/j.1600-0773.1990.tb00722.x. [DOI] [PubMed] [Google Scholar]
- Farahani H, Hasan M. Nitrogen dioxide induced changes in level of free fatty acids, triglyceride, esterified fatty acid, ganglioside and lipase activity in the guinea pig brain. J Environ Sci Health B. 1992;27:53–71. doi: 10.1080/03601239209372767. [DOI] [PubMed] [Google Scholar]
- Flores-Pajot MC, Ofner M, Do MT, Lavigne E, Villeneuve PJ. Childhood autism spectrum disorders and exposure to nitrogen dioxide, and particulate matter air pollution: A review and meta-analysis. Environ Res. 2016;151:763–776. doi: 10.1016/j.envres.2016.07.030. [DOI] [PubMed] [Google Scholar]
- Gaugler T, et al. Most genetic risk for autism resides with common variation. Nat Genet. 2014;46:881–5. doi: 10.1038/ng.3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genkinger JM, et al. Prenatal polycyclic aromatic hydrocarbon (PAH) exposure, antioxidant levels and behavioral development of children ages 6–9. Environ Res. 2015;140:136–44. doi: 10.1016/j.envres.2015.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giulivi C, et al. Mitochondrial dysfunction in autism. JAMA. 2010;304:2389–96. doi: 10.1001/jama.2010.1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goh S, Dong Z, Zhang Y, DiMauro S, Peterson BS. Mitochondrial dysfunction as a neurobiological subtype of autism spectrum disorder: evidence from brain imaging. JAMA Psychiatry. 2014;71:665–71. doi: 10.1001/jamapsychiatry.2014.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong T, et al. Perinatal Exposure to Traffic-Related Air Pollution and Autism Spectrum Disorders. Environ Health Perspect. 2016 doi: 10.1289/EHP118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotham K, Pickles A, Lord C. Standardizing ADOS scores for a measure of severity in autism spectrum disorders. J Autism Dev Disord. 2009;39:693–705. doi: 10.1007/s10803-008-0674-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotham K, Pickles A, Lord C. Trajectories of autism severity in children using standardized ADOS scores. Pediatrics. 2012;130:e1278–84. doi: 10.1542/peds.2011-3668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guxens M, et al. Prenatal exposure to residential air pollution and infant mental development: modulation by antioxidants and detoxification factors. Environ Health Perspect. 2012a;120:144–9. doi: 10.1289/ehp.1103469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guxens M, et al. Prenatal exposure to residential air pollution and infant mental development: modulation by antioxidants and detoxification factors. Environ Health Perspect. 2012b;120:144–9. doi: 10.1289/ehp.1103469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guxens M, et al. Air pollution during pregnancy and childhood cognitive and psychomotor development: six European birth cohorts. Epidemiology. 2014;25:636–47. doi: 10.1097/EDE.0000000000000133. [DOI] [PubMed] [Google Scholar]
- Guxens M, Sunyer J. A review of epidemiological studies on neuropsychological effects of air pollution. Swiss Med Wkly. 2012;141:w13322. doi: 10.4414/smw.2011.13322. [DOI] [PubMed] [Google Scholar]
- Hertz-Picciotto I, Croen LA, Hansen R, Jones CR, van de Water J, Pessah IN. The CHARGE study: an epidemiologic investigation of genetic and environmental factors contributing to autism. Environ Health Perspect. 2006; 114:1119–25. doi: 10.1289/ehp.8483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J, Zhang H, Ying Q, Chen S, Vandenberghe F, Kleeman MJ. Long-term particulate matter modeling for health effect studies in California - Part 1: Model performance on temporal and spatial variations. Atmos Chem Phys. 2015:3445–3461. [Google Scholar]
- Huerta M, Bishop SL, Duncan A, Hus V, Lord C. Application of DSM-5 criteria for autism spectrum disorder to three samples of children with DSM-IV diagnoses of pervasive developmental disorders. Am J Psychiatry. 2012;169:1056–64. doi: 10.1176/appi.ajp.2012.12020276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hus V, Gotham K, Lord C. Standardizing ADOS Domain Scores: Separating Severity of Social Affect and Restricted and Repetitive Behaviors. J. Autism Dev Disord. 2012 doi: 10.1007/s10803-012-1719-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jedrychowski WA, et al. Prenatal exposure to polycyclic aromatic hydrocarbons and cognitive dysfunction in children. Environ Sci Pollut Res Int. 2015;22:3631–9. doi: 10.1007/s11356-014-3627-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung CR, Lin YT, Hwang BF. Air pollution and newly diagnostic autism spectrum disorders: a population-based cohort study in Taiwan. PLoS One. 2013;8:e75510. doi: 10.1371/journal.pone.0075510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalkbrenner AE, Daniels JL, Chen JC, Poole C, Emch M, Morrissey J. Perinatal exposure to hazardous air pollutants and autism spectrum disorders at age 8. Epidemiology. 2010;21:631–41. doi: 10.1097/EDE.0b013e3181e65d76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalkbrenner AE, et al. Particulate matter exposure, prenatal and postnatal windows of susceptibility, and autism spectrum disorders. Epidemiology. 2015;26:30–42. doi: 10.1097/EDE.0000000000000173. [DOI] [PubMed] [Google Scholar]
- Kanne SM, Gerber AJ, Quirmbach LM, Sparrow SS, Cicchetti DV, Saulnier CA. The role of adaptive behavior in autism spectrum disorders: implications for functional outcome. J Autism Dev Disord. 2011;41:1007–18. doi: 10.1007/s10803-010-1126-4. [DOI] [PubMed] [Google Scholar]
- Kim YS, Fombonne E, Koh YJ, Kim SJ, Cheon KA, Leventhal BL. A comparison of DSM-IV pervasive developmental disorder and DSM-5 autism spectrum disorder prevalence in an epidemiologic sample. J Am Acad Child Adolesc Psychiatry. 2014;53:500–8. doi: 10.1016/j.jaac.2013.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YS, Leventhal BL. Genetic epidemiology and insights into interactive genetic and environmental effects in autism spectrum disorders. Biol Psychiatry. 2015;77:66–74. doi: 10.1016/j.biopsych.2014.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreyling WG, Semmler M, Möller W. Dosimetry and toxicology of ultrafine particles. J Aerosol Med. 2004;17:140–52. doi: 10.1089/0894268041457147. [DOI] [PubMed] [Google Scholar]
- Lam J, et al. A Systematic Review and Meta-Analysis of Multiple Airborne Pollutants and Autism Spectrum Disorder. PLoS One. 2016;11:e0161851. doi: 10.1371/journal.pone.0161851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Xin X. Nitrogen dioxide (NO(2)) pollution as a potential risk factor for developing vascular dementia and its synaptic mechanisms. Chemosphere. 2013;92:52–8. doi: 10.1016/j.chemosphere.2013.02.061. [DOI] [PubMed] [Google Scholar]
- Lord C, Risi S, DiLavore PS, Shulman C, Thurm A, Pickles A. Autism from 2 to 9 years of age. Arch Gen Psychiatry. 2006;63:694–701. doi: 10.1001/archpsyc.63.6.694. [DOI] [PubMed] [Google Scholar]
- Lucchini RG, Dorman DC, Elder A, Veronesi B. Neurological impacts from inhalation of pollutants and the nose-brain connection. Neurotoxicology. 2012;33:838–41. doi: 10.1016/j.neuro.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattila ML, et al. Autism spectrum disorders according to DSM-IV-TR and comparison with DSM-5 draft criteria: an epidemiological study. J Am Acad Child Adolesc Psychiatry. 2011;50:583–592. e11. doi: 10.1016/j.jaac.2011.04.001. [DOI] [PubMed] [Google Scholar]
- Messinger D, et al. Beyond autism: a baby siblings research consortium study of high-risk children at three years of age. J Am Acad Child Adolesc Psychiatry. 2013;52:300–308. e1. doi: 10.1016/j.jaac.2012.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales E, et al. Association of early-life exposure to household gas appliances and indoor nitrogen dioxide with cognition and attention behavior in preschoolers. Am J Epidemiol. 2009;169:1327–36. doi: 10.1093/aje/kwp067. doi:kwp067 [pii] [DOI] [PubMed] [Google Scholar]
- Morgan TE, et al. Glutamatergic neurons in rodent models respond to nanoscale particulate urban air pollutants in vivo and in vitro. Environ Health Perspect. 2011;119:1003–9. doi: 10.1289/ehp.1002973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullen . Mullen Scales of Early Learning. Circle Pines, MN: American Guidance Services Inc.; 1995. [Google Scholar]
- Munson J, et al. Evidence for latent classes of IQ in young children with autism spectrum disorder. Am J Ment Retard. 2008;113:439–52. doi: 10.1352/2008.113:439-452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira G, et al. Mitochondrial dysfunction in autism spectrum disorders: a population-based study. Dev Med Child Neurol. 2005;47:185–9. doi: 10.1017/s0012162205000332. [DOI] [PubMed] [Google Scholar]
- Perera FP, et al. Prenatal exposure to air pollution, maternal psychological distress, and child behavior. Pediatrics. 2013;132:e1284–94. doi: 10.1542/peds.2012-3844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson BS, et al. Effects of prenatal exposure to air pollutants (polycyclic aromatic hydrocarbons) on the development of brain white matter, cognition, and behavior in later childhood. JAMA Psychiatry. 2015;72:531–40. doi: 10.1001/jamapsychiatry.2015.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pons R, et al. Mitochondrial DNA abnormalities and autistic spectrum disorders. J Pediatr. 2004;144:81–5. doi: 10.1016/j.jpeds.2003.10.023. [DOI] [PubMed] [Google Scholar]
- Porta D, et al. Air pollution and cognitive development at age seven in a prospective Italian birth cohort. Epidemiology. 2015 doi: 10.1097/EDE.0000000000000405. [DOI] [PubMed] [Google Scholar]
- Pujol J, et al. Traffic pollution exposure is associated with altered brain connectivity in school children. Neuroimage. 2016;129:175–84. doi: 10.1016/j.neuroimage.2016.01.036. [DOI] [PubMed] [Google Scholar]
- Raz R, et al. Autism spectrum disorder and particulate matter air pollution before, during, and after pregnancy: a nested case-control analysis within the Nurses’ Health Study II Cohort. Environ Health Perspect. 2015;123:264–70. doi: 10.1289/ehp.1408133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riva DR, et al. Low dose of fine particulate matter (PM2.5) can induce acute oxidative stress, inflammation and pulmonary impairment in healthy mice. Inhal Toxicol. 2011;23:257–67. doi: 10.3109/08958378.2011.566290. [DOI] [PubMed] [Google Scholar]
- Roberts AL, et al. Perinatal Air Pollutant Exposures and Autism Spectrum Disorder in the Children of Nurses’ Health Study II Participants. Environ Health Perspect. 2013;121:978–84. doi: 10.1289/ehp.1206187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossignol DA, Frye RE. Mitochondrial dysfunction in autism spectrum disorders: a systematic review and meta-analysis. Mol Psychiatry. 2012;17:290–314. doi: 10.1038/mp.2010.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossignol DA, Frye RE. Evidence linking oxidative stress, mitochondrial dysfunction, and inflammation in the brain of individuals with autism. Front Physiol. 2014;5:150. doi: 10.3389/fphys.2014.00150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheppard L, et al. Confounding and exposure measurement error in air pollution epidemiology. Air Qual Atmos Health. 2012;5:203–216. doi: 10.1007/s11869-011-0140-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh M, Phuleria HC, Bowers K, Sioutas C. Seasonal and spatial trends in particle number concentrations and size distributions at the children’s health study sites in Southern California. J Expo Sci Environ Epidemiol. 2006;16:3–18. doi: 10.1038/sj.jea.7500432. [DOI] [PubMed] [Google Scholar]
- Sparrow SS, Cicchetti DV. Diagnostic uses of the Vineland Adaptive Behavior Scales. J Pediatr Psychol. 1985;10:215–25. doi: 10.1093/jpepsy/10.2.215. [DOI] [PubMed] [Google Scholar]
- Suades-Gonzalez E, Gascon M, Guxens M, Sunyer J. Air Pollution and Neuropsychological Development: A Review of the Latest Evidence. Endocrinology. 2015;156:3473–82. doi: 10.1210/en.2015-1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki T, et al. In utero exposure to a low concentration of diesel exhaust affects spontaneous locomotor activity and monoaminergic system in male mice. Part Fibre Toxicol. 2010;7:7. doi: 10.1186/1743-8977-7-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurm A, Manwaring SS, Swineford L, Farmer C. Longitudinal study of symptom severity and language in minimally verbal children with autism. J Child Psychol Psychiatry. 2015;56:97–104. doi: 10.1111/jcpp.12285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volk HE, Hertz-Picciotto I, Delwiche L, Lurmann F, McConnell R. Residential Proximity to Freeways and Autism in the CHARGE Study. Environ Health Perspect. 2011;119:873–7. doi: 10.1289/ehp.1002835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volk HE, Lurmann F, Penfold B, Hertz-Picciotto I, McConnell R. Traffic-related air pollution, particulate matter, and autism. JAMA Psychiatry. 2013;70:71–7. doi: 10.1001/jamapsychiatry.2013.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Ehrenstein OS, Aralis H, Cockburn M, Ritz B. In Utero Exposure to Toxic Air Pollutants and Risk of Childhood Autism. Epidemiology. 2014 doi: 10.1097/EDE.0000000000000150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrijheid M, et al. Indoor air pollution from gas cooking and infant neurodevelopment. Epidemiology. 2012;23:23–32. doi: 10.1097/EDE.0b013e31823a4023. [DOI] [PubMed] [Google Scholar]
- Wang S, Zhang J, Zeng X, Zeng Y, Chen S. Association of traffic-related air pollution with children’s neurobehavioral functions in Quanzhou, China. Environ Health Perspect. 2009;117:1612–8. doi: 10.1289/ehp.0800023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisskopf MG, Kioumourtzoglou MA, Roberts AL. Air Pollution and Autism Spectrum Disorders: Causal or Confounded? Current environmental health reports. 2015;2:430–9. doi: 10.1007/s40572-015-0073-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman JR, et al. Mitochondrial disease in autism spectrum disorder patients: a cohort analysis. PLoS One. 2008;3:e3815. doi: 10.1371/journal.pone.0003815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilker EH, et al. Fine Particulate Matter, Residential Proximity to Major Roads, and Markers of Small Vessel Disease in a Memory Study Population. Journal of Alzheimer’s disease : JAD. 2016;53:1315–23. doi: 10.3233/JAD-151143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windham GC, Zhang L, Gunier R, Croen LA, Grether JK. Autism spectrum disorders in relation to distribution of hazardous air pollutants in the san francisco bay area. Environ Health Perspect. 2006;114:1438–44. doi: 10.1289/ehp.9120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan W, Ji X, Shi J, Li G, Sang N. Acute nitrogen dioxide inhalation induces mitochondrial dysfunction in rat brain. Environ Res. 2015;138:416–24. doi: 10.1016/j.envres.2015.02.022. [DOI] [PubMed] [Google Scholar]
- Yorifuji T, Kashima S, Higa Diez M, Kado Y, Sanada S, Doi H. Prenatal Exposure to Traffic-related Air Pollution and Child Behavioral Development Milestone Delays in Japan. Epidemiology. 2015 doi: 10.1097/EDE.0000000000000361. [DOI] [PubMed] [Google Scholar]
- Zerbo O, Iosif AM, Delwiche L, Walker C, Hertz-Picciotto I. Month of conception and risk of autism. Epidemiology. 2011;22:469–75. doi: 10.1097/EDE.0b013e31821d0b53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Hinds WC, Kim S, Sioutas C. Concentration and size distribution of ultrafine particles near a major highway. J Air Waste Manag Assoc. 2002;52:1032–42. doi: 10.1080/10473289.2002.10470842. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.