Abstract
Paediatric palliative care (PPC) endeavours to alleviate the suffering and improve the quality of life of children with serious illnesses and their families. In the past two decades since WHO defined PPC and called for its inclusion in paediatric oncology care, rigorous investigation has provided important insights. For example, the first decade of research focused on end-of-life experiences of the child and the family, underscoring the high prevalence of symptom burden, the barriers to parent–provider concordance with regards to prognosis, as well as the need for bereavement supports. The second decade expanded PPC oncology investigation to include the entire cancer continuum and the voices of patients. Other studies identified the need for support of parents, siblings, and racial and ethnic minority groups. Promising interventions designed to improve outcomes were tested in randomised clinical trials. Future research will build on these findings and pose novel questions about how to continue to reduce the burdens of paediatric cancer.
Introduction
Survival outcomes for children with cancer have changed extensively over the past several decades, mostly because of rigorous collaborative research. Only 58% of children diagnosed between 1975 and 1979 survived their disease, compared with 83% of those diagnosed between 2000 and 2009.1 Nevertheless the lived experience of children with cancer and their families has been consistent, in that the disease and its treatment continue to cause physical and psychosocial suffering.1 Cancer remains a life-changing diagnosis with substantial prognostic uncertainty and caregiving demands; thus, its effect on child and family life can be extensive and long-lasting.2
Paediatric palliative care (PPC) aims to alleviate patient and family suffering via “the active total care of a child’s body, mind, and spirit”.3 With the understanding that PPC might improve the experiences of children with cancer, in 1998, WHO suggested that PPC should be integrated into standard paediatric oncology care.4 The organisation noted that successful PPC would begin when a child is diagnosed, continue regardless of treatment choices or survival outcomes, and include a multidisciplinary approach and the provision of support to the family. In 2000, the American Academy of Pediatrics (AAP) also recommended integrated palliative care for children with serious illnesses.5 Research in PPC oncology increased substantially over the nearly two decades since the WHO and AAP recommendations.6 In anticipation of the third decade of research, we reviewed the literature to describe key historical work that informed clinical care and PPC oncology research (appendix) and to highlight important opportunities for future investigation.
The first decade of PPC oncology research (2000–09)
PPC oncology investigation in the early 2000s tended to focus on the end-of-life period, including predominantly descriptive studies of patient symptoms, parent–provider communication and decision making, and bereavement outcomes.
Symptoms and suffering
In 2000, a study7 of 103 bereaved parents of children with cancer suggested that 89% of parents believed that their child suffered “a lot” from at least one symptom during their last month of life, and 51% believed that their children suffered from at least three concurrent symptoms. Individual symptom-directed therapies were successful in the treatment of fewer than 30% of cases. In 2006, results from two additional studies8,9 confirmed a high symptom burden at the end of life. First, in a population-based survey of 449 Swedish bereaved parents,8 symptoms with a moderate-to-high impact on child wellbeing included fatigue (86%), reduced mobility (76%), pain (73%), and anorexia (71%). Second, clinical nurse specialists described symptoms of 164 children over a median of 34 (range 0–354) days before the child’s death.9 In that time, suffering from pain increased from 71% to 92%. Findings from these studies suggested that opportunities exist to improve the management of end-of-life symptoms. However, only 10% of 228 paediatric oncologists surveyed by Hilden and colleagues10 in 1998 had formal training in end-of-life care. Almost all paediatric oncologists felt competent in pain management, but nearly half felt anxious in managing other difficult symptoms. The absence of an accessible PPC team was considered a hindrance in the provision of good end-of-life care. A similar survey11 of 632 paediatric oncologists in 2006 found that 85% and 67% respectively, felt comfortable in managing end-of-life pain and psychological issues—those who had formal training and more than 10 years of experience were more comfortable with this management.
Communication about prognosis
An early identified obstacle in PPC oncology was that parents and physicians might have had unrealistic prognostic expectations. In the 1998 survey of paediatric oncologists, a “family’s unrealistic expectations” was considered the greatest hindrance to the provision of good end-of-life care.10 Among the bereaved parents of children with cancer, evidence suggested that a delay existed in the understanding of prognosis between physicians and parents12—physicians recognised that a child had no realistic chance for cure approximately 200 days before his or her death, whereas parents conceded the same 100 days later. Only 49% of parents attributed their understanding to discussions with the medical team, whereas 30% attributed their understanding to a perceived change in their child and 9% to a feeling. Differences in the understanding of prognosis between parents and physicians were smaller among more educated parents than less educated parents and when psychosocial clinicians were involved in the end-of-life care of the child.
Later studies explored the parental preferences regarding the delivery of prognostic information. In a cross-sectional survey of 194 non-bereaved parents of children with cancer,13 87% wanted as much information as possible about the prognosis of their child, and 86% wanted the information expressed numerically (ie, chance of survival). More than a third of parents wanted more information than they received, and most parents felt that the prognostic information helped them to maintain hope, even when the prognosis was poor. Although parents tended to be more optimistic than physicians regarding the likelihood of cure,14 the prognostic understanding of parents was associated with several factors (table 1). Parents and physicians were most likely to agree about the likelihood of cure when physicians were confident in their knowledge and when parents adopted their preferred decision-making role.14 Concurrent chemotherapy and inadequate previous explicit information about the child’s incurable disease were both associated with short parental awareness (ie, <24 h) of the impending death of the child.15 Parents of children whose last cancer-directed treatment was haemopoietic cell transplantation (HCT) recognised the poor prospect of cure of their child 16 days before death, compared with 84 days among those parents whose children received other cancer-directed therapies.16 Unsurprisingly, parents whose children last received HCT reported feeling less prepared for the death of their child. Finally, parents were more able to accept that their child’s cancer was incurable if they felt prognostic information was delivered appropriately, and had others with whom to share the information.17
Table 1.
Modifiable factors associated with parental prognostic understanding at the end of a child’s life
| Evidence-based examples | Considerations for clinicians | |
|---|---|---|
| Role of medical staff | Numeric estimate of prognosis (ie, percentage chance of survival or cure);13 physician confidence regarding cure likelihood;14 statements regarding possible death or abbreviated life expectancy;15 and preferred role in decision making14 | Include numeric assessment of prognosis during discussions about diagnosis, treatment, and goals of care; sharing of clinical perspective and expertise regarding probable outcomes might help families to understand prognosis; consider explicit discussions of life expectancy or the possibility of death; and explore parent and family decision-making practices and preferences |
| Communication within the family | Understanding of prognosis is discussed with partner or other family members14,16 | Encourage parents to discuss prognostic understanding with partners or other loved ones |
| Structure of medical team | Psychosocial clinician involvement12 | Actively include multidisciplinary team members in the child’s care |
| Medical care | Concurrent chemotherapy15,16 | Routinely explore goals of cancer-directed therapies; and consider cessation of chemotherapy if it does not relieve symptoms or meet other patient or family goals |
Findings from additional studies identified the important consequences of physician–parent or parent–parent agreement regarding cure likelihood or goals of care. When both parties acknowledged the impending death of the child for more than 50 days, parents were more likely to report high-quality end-of-life care, and both were more likely to report goals of lessening the child’s suffering, than parties who did not recognise the child’s impending death.12 Conversely, when parental dyads disagreed about the goals of the end-of-life care of their child, each was more likely to report that the child was suffering than were couples who agreed on the goal of care.18
Decision making
Investigations aiming to understand end-of-life treatment decisions and goals of care were largely qualitative.19–22 In a prospective multisite ethnography19 of 34 parents of children with recurrent cancer and an estimated likelihood of cure of less than 30%, no parent initiated discontinuation of cancer-directed therapies. Instead, 82% of parents continued to pursue these therapies, including via second opinions when local oncologists declined further treatment. Cancer-directed and symptom-directed therapies were not considered mutually exclusive—all parents endorsed concurrent supportive care.
Results from similar studies21–23 suggested that families found it difficult when choosing between cancer-directed therapy or supportive care. In a study22 of 58 families who recently decided to enrol the child on a phase 1 investigational trial, adopt an order to limit resuscitation, or transition to terminal (comfort-directed) care, the 31 families (53%) who enrolled on phase 1 trials felt compelled to continue cancer-directed therapy rather than focus on the quality of life of the child. In another study23 of 77 parents of children with no reasonable chance of cure, more than half preferred to continue cancer-directed therapy rather than supportive care when a discrete choice was given between the two. Factors that influenced the decision making of parents included the wishes of the child,20,22 staff recommendations,20,23 perceptions of quality of life,20–23 hope,23 and their own ideologies of good parenting.20 Good parenting was defined by most parents (89%) as incorporation of the best interest of the child, consideration of how to be present and supportive (48%), and ensuring that the child felt loved (42%).21 Only 9% of the parents considered “making my child healthy” in their definition of good parenting. Although physician recommendations for end-of-life care reflected patient and family preferences, physicians also commonly incorporated estimates of the survival, comorbidities, and suffering of the child.20,23 Likewise, when the quality of end-of-life care was considered, physicians prioritised pain management and the time spent in hospital, whereas parents prioritised communication practices.24 Few studies directly assessed the perspectives of patients, despite findings that most children were aware of their cancer diagnosis and prognosis and the possibility of death from their disease.25 Among 20 patients (aged 10–20 years) who participated in end-of-life conversations, qualitative analyses suggested that all patients could identify death as a consequence.20 When given a hypothetical end-of-life scenario about a child with cancer, adolescent cancer survivors (n=83) were more likely than their age-matched healthy peers (n=1769) to consider that non-treatment and supportive care decisions are appropriate.26
Bereavement outcomes
Findings from descriptive studies of bereaved parents of children with cancer suggested that these parents were at high risk of poor outcomes, including impaired mental, social, and physical health, as well as early mortality.27–29 Although some of these outcomes improved over time, others continued for decades.30 Hence, investigators endeavoured to identify specific factors of the child’s medical experience that could inform anticipatory guidance or intervention. For example, one study31 found that treatment experiences and adverse events as early as the time of cancer diagnosis were associated with post-traumatic stress reported by parents. Results from two additional studies32,33 suggested that high intensity therapies such as HCT were associated with risks of prolonged grief, anxiety, depression, and poor quality of life in parents.
Specific end-of-life experiences were also associated with poor parental outcomes. Complicated grief, anxiety, depression, and stress were more frequent in parents whose child died in the hospital than in parents whose child died at home.32,34 When parents perceived the quality of life of the child to be inferior—for example, when the child was thought to be anxious or when staff were deemed poorly responsive to the needs of the child—parents more frequently experienced prolonged grief, stress, and guilt, higher psychological distress, and poorer quality of life.35–37 Conversely, parent outcomes were improved if they had someone with whom to share their concerns, felt prepared for the death of their child, and were present during their child’s last living moments.35,37
Parental regret was also a topic of investigation. Among 51 bereaved parents whose child received cancer-directed therapy after the parent recognised that no realistic chance of cure existed, 20% still reported the goal of therapy was to cure the cancer, and 20% reported the goal was to lessen suffering.38 When parents were asked what their goals of therapy should have been, only 12% stated to cure and up to 43% stated to lessen suffering. Parents who believed that their child had suffered from cancer-directed therapy were less likely to recommend it to future families than parents who did not believe that their child suffered. Among parents who did or did not talk directly to their child about his or her impending death, none of the 147 parents who discussed it regretted it, but 69 (27%) of the 258 parents who did not do so regretted the missed opportunity.39 The odds of parental regret increased if parents sensed that the child was aware of his or her imminent death, or if the child was an adolescent or a young adult. Parents did not, however, regret their participation in PPC research.40 After participation in a population-based study querying the end-of-life care of children, 423 (94%) of 449 parents found the investigation valuable, and 285 (57%) were positively affected.
Current era of PPC oncology research (2010 to present)
As the investigation of PPC oncology evolved, experts suggested that it should expand to include the whole trajectory of illness41–43 (figure 1), and that health-care professionals involved in comprehensive oncology care should recognise the physical and psychosocial domains of overall patient and family wellbeing.44–47 To meet these suggestions, clinical palliative care providers were categorised on the basis of their roles and skillsets.48 Primary PPC was defined as care provided by the primary medical (eg, paediatric oncology) team, including standard management of physical and emotional symptoms, discussions of prognosis, goals of care, suffering, and advance care planning. Specialty PPC encompassed more complex pain and symptom management, expert communication assistance, and, when needed, conflict resolution.48 PPC oncology investigation subsequently broadened its focus to include the entire cancer continuum (beginning at diagnosis), and to fill gaps in knowledge regarding: the use of PPC service; the perspectives of patients; the impact of cancer on the family; cultural and other disparities; and PPC interventions.
Figure 1.
Conceptual model of palliative care integration across the cancer continuum for patients who do not or who do survive. Adapted from Liben and colleagues.43
Perceptions of PPC and its clinical use
Public perceptions of palliative care might impede its implementation. For example, in a 2011 poll of 800 adults (including parents) from the USA, only 36% approved of palliative care.49 After these participants were informed that palliative care focused on the relief of symptoms, pain, and stress, with a goal to improve the quality of life for patients and families, approval ratings of palliative care rose to 60%. 92% of respondents said that they would recommend palliative care for a loved one and 94% said it should be available for patients of all ages. In a convenience sample of 129 parent–child dyads treated at a large children’s research hospital surveyed between 2011 and 2015, 98% of patients and 70% of parents had never heard of palliative care.50 After explanation that palliative care was defined as a service provided by “experts in treating symptoms and improving quality of life”, more than 50% of participants said that they would want PPC from the time of diagnosis and thereafter.
Additional investigations suggested other barriers to implementation. Although 58–66% of Children’s Oncology Group centres had PPC teams, their services varied.11,51 In a retrospective study52 of 75 bereaved parents and their 48 paediatric oncologists, 20 elements of PPC were identified as highly valuable by both groups, but only three were consistently accessible. Commonly inaccessible elements included parent preparation for medical end-of-life care and sibling support. Parents were more likely than physicians to value religious or spiritual support and cancer-directed therapy during the last month of their child’s life.
End-of-life experiences of children with cancer might also be shifting. In a large retrospective study53 of 815 children with cancer, those who died after 2004 were more likely to have been in an intensive care unit, mechanically ventilated, or to have died in the hospital than those who died between 2000 and 2004. Taken together, these experiences underscore the need to understand, standardise, and integrate evolving PPC roles and services in paediatric oncology care.
Inclusion of the voices of patients
Historical reliance on the reporting of child symptoms by parents or staff was problematic not only because many symptoms are subjective (eg, pain, fatigue, and emotional wellbeing), but also because surrogate assessments tended to be inaccurate.54–58 In order to understand symptom burdens and health-related quality of life from the perspectives of children with advanced cancer (defined as progressive, recurrent, or non-responsive disease), investigators in the Paediatric Quality of Life and Symptoms Technology (PediQUEST) study59,60 prospectively surveyed 104 children from three large children’s hospitals as often as once per week. Over a 9 month period, 920 surveys were collected, including 73 surveys of 25 children during their last 3 months of life. The most common symptoms reported were pain (48%), fatigue (46%), drowsiness (39%), and irritability (37%). Most child reports suggested that children had high levels of distress caused by their symptoms.59 Recent disease progression or moderate-to-high intensity cancer-directed therapies were associated with increased total, physical, and psychological symptom burdens. Patients reported a median of three concurrent distressing symptoms per survey; 73%, 35%, and 12% of patients reported at least two, five, and nine concurrent symptoms, respectively.60 13 symptoms were independently associated with reductions in patient-reported health-related quality of life, which was further reduced when concurrent symptoms existed.
Other investigations queried the perspectives and experiences of adolescent and young adult patients regarding their communication, coping, and health behaviours during the cancer experience. Among these patients, sexual activity and substance use were common, and perhaps clinically under-recognised.61 These patients considered their physical symptoms to be the greatest burden of their cancer, but also identified benefits from their experience with cancer such as strengthened relationships and new life perspectives.62 Many adolescents and young adults used coping resources such as social support, positive reframing, and stress management.63 Furthermore, they wanted to be included in early and ongoing discussions of prognosis and medical decisions64,65 and had distinct preferences on how and by whom this information should be delivered.66 Following advance care planning conversations about possible poor outcomes, 24% of patients reported feeling sad, but 71% said that the conversation was worthwhile and 91% found it helpful.67
Cancer and the family
Investigations have also included siblings and parents. For example, in a dual-centre survey68 of 58 bereaved siblings of children with cancer, respondents reported that their anxiety, depression, and substance use increased in the first year following the death of their sibling. Those who felt dissatisfied with the end-of-life communication, who were poorly prepared for the death of their sibling, or who had not had a chance to say goodbye reported higher ongoing distress and lower current social support. Almost all participants were still affected by their loss, and half said the experience influenced their educational or vocational goals. Two additional studies described external perceptions of bereaved sibling adjustment. First, among 36 mothers and 24 fathers of 39 bereaved siblings, 69% reported that the sibling had changed with respect to their personality, school or work behaviours, life perspectives, or engagement in activities.69 Nearly half of these parents reported changes in bereaved siblings’ intrafamily and peer relationships. Second, school teachers of 105 bereaved siblings compared their behaviours with age-matched peers.70 Bereaved children in elementary school were viewed as more sensitive or isolated than their peers, whereas bereaved children in high school were perceived as leaders or more popular than their peers.
Results from the PediQUEST study identified important factors of the prognostic understanding and psychological wellbeing of parents. First, although no differences existed in the survival between patients with haematological, central nervous system, or other solid tumour malignancies, 76% of parents of children with haematological malignancies believed that their child would be cured compared with 29% and 34% in the other groups, respectively.71 Second, over half of parents reported having high psychological distress, with one in seven having distress serious enough to impair their ability to care for the patient or other children in the home, or both.72 Distress of parents was alleviated when their prognostic understanding was aligned with the goals of care (eg, parents who understood that their child might not survive reported a corresponding aim to reduce suffering). By contrast, distress was higher in parents when they believed that their child was suffering or when they perceived an economic hardship caused by the cancer experience.
Economic hardship caused by paediatric cancer has only been described in this current era. In 2011, a retrospective, cross-sectional study73 of 141 American and 89 Australian bereaved parents suggested that 24% and 39%, respectively, experienced a “great deal” of hardship as a result of the child’s cancer. In the PediQUEST study, 94% of parents reported work disruptions and 42% reported at least one parent quitting his or her job.74 Findings from a prospective study75 of families of children treated at a single institution with high psychosocial support showed that 25% of families lost more than 40% of their household income secondary to treatment-related work disruptions during the first 6 months of therapy, and 29% experienced food, energy, or housing insecurities.
Socioeconomic and cultural disparities
Evidence of financial hardship highlights important susceptible populations within paediatric oncology. For example, among 575 children treated for acute lymphoblastic leukaemia within a large cooperative group, those from neighbourhoods with high poverty had an overall survival of 85% compared with 92% in children from neighbourhoods with low poverty.76 Although no differences existed in the cumulative incidence of relapse, 92% of relapses in high-poverty areas were early, and therefore associated with poorer prognosis, compared with 48% of relapses elsewhere.
Geography is not the only contributor to health disparities. Poor outcomes among racial and ethnic minority populations are well described and probably reflect a combination of differences in disease biology in addition to cultural and economic factors of health-care access, health literacy, and adherence.77 In a study of 310 English-speaking parents and 56 Spanish-speaking parents with limited English proficiency, the latter were more likely to have quit or changed jobs because of the child’s cancer and were less likely to correctly recognise whether the child was on a clinical trial.78 A third of Spanish-speaking parents believed that the care of their children would have been better if they were English speaking.
Investigations also explored the associations between race or ethnicity and end-of-life experiences. At two different referral centres, race was not associated with the provision of end-of-life health services including PPC consultation, do not resuscitate orders, time between do not resuscitate order and death, timing and number of end-of-life discussions, and hospice referrals.79,80 By contrast, findings from a third centre suggested that race or ethnicity was associated with hospice enrolment after adjustment for payer status, diagnosis, and religion.81 In no study was race or ethnicity associated with the death of a patient while receiving hospice care; a significant proportion of parents withdrew their child from hospice before his or her death, regardless of racial or ethnic background.82 Nevertheless, the location of death might be associated with racial differences. In a large population-based study,82 non-Hispanic children were more likely to die at home than Hispanic children, regardless of their type of cancer.
Little is known about PPC in the global community. A systematic review83 of 30 PPC programmes in 21 low-income and middle-income countries identified gaps in needed services and infrastructure such as national health systems and specialised PPC education—opioids were unavailable in 14 (67%) of the 21 representative nations. Results from another systematic literature review84 of cross-cultural perspectives suggested variable cultural influences on end-of-life philosophies and experiences, including decision making, communication, suffering, and location of death.
Interventions
The past decade of PPC oncology research has culminated in the design and testing of interventions. Investigators of three studies85–87 considered specialty PPC as a separate intervention, and compared children’s end-of-life experiences with or without it. First, among 114 children who died at a children’s hospital (not all from cancer), inpatient PPC consultation was associated with higher frequencies of pain assessment and management, increased use of integrative medicine, fewer diagnostic or invasive procedures in the last 48 h of life, and more orders to limit resuscitation than those who did not receive inpatient PPC consultation.85 Among bereaved parents of children with cancer treated at a second centre, PPC team involvement was associated with increased documentation about the medical aspects of the dying process and possibility of death, including with the child, when appropriate.86 Finally, among children receiving HCT at a third centre, 97% of families who received specialty PPC discussed the prognosis of the child compared with 83% of families who did not receive PPC.87 These discussions occurred earlier with PPC than without PPC (8 days vs 2 days before the death of the child), and children were less likely to receive intense end-of-life interventions such as intubation or cardiopulmonary resuscitation.
Primary palliative oncology interventions might be conceptualised in many ways, including standardised clinical and psychosocial resources directed at patient and family supportive care,88–90 communication tools to assist with advance care planning,91 as well as in-training for staff to develop basic PPC competencies.92,93 Few interventions have been labelled as PPC interventions, even if they intend to alleviate suffering and improve quality of life. Even fewer interventions have been rigorously developed, tested, and disseminated in the scientific literature. Here, we focus on four interventions that have been tested for efficacy in randomised controlled trials (table 2).
Table 2.
Primary palliative oncology interventions tested in randomised controlled trials
| Intervention design | Target population | Key trial findings | Clinical implications | |
|---|---|---|---|---|
| PediQUEST94 | ePROs reported back to clinicians and parents (vs not reported back) | Children with advanced cancer (n=104) | Helped >90% of parents to understand how the child is feeling and >60% of health-care providers to identify psychosocial symptoms; was associated with positive but non-statistically significant improvements in symptom burden and quality of life; and improved quality of life among patients ≥8 years of age who survived >20 weeks | ePROs might improve clinical care, and patient and family experiences, and might be most effective for children old enough to understand the language used in ePROs |
| Family-centred advanced care planning67 | Staged survey, interview, and completion of advance directive (vs brochure only) | AYAs with cancer and their parents (n=60 dyads) | Increased concordance between AYAs and their parents regarding hypothetical future decision making and informed AYAs regarding end-of-life decisions | Early facilitated conversations about hypothetical situations are feasible and might facilitate later AYA–parent decision making |
| Therapeutic music video95 | Six structured music therapy sessions designed to encourage active engagement, reflection, and coping (vs audiobook only) | AYAs receiving haematopoietic cell transplantation (n=113) | Increased AYAs’ “courageous” coping, social integration, and family environment and was associated with positive but non-statistically significant effects in spiritual perspective and self-transcendence | Creative (eg, art and music) therapy might facilitate ability of AYAs to cope and improve outcomes |
| Bright IDEAS96 | Eight sessions of problem-solving skills training (vs standard, non-directive supportive care) | Parents of children with cancer (n=309 mothers) | Increased problem-solving skills, improved mood, and decreased symptoms of post-traumatic stress and depression | Early skills-based training might improve and sustain wellbeing of parents |
PediQUEST=paediatric quality of life and symptoms technology. ePROs=electronic patient-reported outcomes. AYA=adolescent and young adult.
The sole PPC oncology intervention indexed in PubMed is PediQUEST.94 Designed as a digital platform for collection of electronic patient-reported outcomes (ePROs), investigators hypothesised that sharing of child PediQUEST reports with parents and healthcare providers would facilitate the recognition and treatment of symptoms, thereby alleviating distress and improving quality of life. In a multisite trial,94 children with advanced cancer prospectively completed ePROs; reports of patients who were randomly assigned to PediQUEST were shared with staff and parents. 104 children completed 920 ePROs over 9 months of follow-up.94 More than 90% of parents whose children completed PediQUEST reports thought it helped them to understand how their child was feeling. Most clinicians stated that these reports provided new information about the psychosocial symptoms of the child. Although significant improvements in quality-of-life scores were seen only among patients aged 8 years and older, non-statistically significant improvements in symptom scores and quality of life were seen in all age groups. Conclusions were that the intervention had potential but could be strengthened by exploring ways to better capture the voices of younger children or by providing tools for clinicians to respond to identified symptoms.94
Other interventions have targeted communication and coping processes. For example, the family-centred advance care planning intervention includes three 60-min sessions for adolescents and young adults with cancer and their surrogate decision makers. The first session includes a survey to assess values; the second is a structured interview to discuss prognostic understanding, hypothetical negative outcomes, and corresponding treatment preferences; and the third session is joint completion of an advance directive.67,97 In a single-site randomised trial of 60 adolescents and young adults with cancer and their parents, dyads who participated in the intervention were more likely to agree on the end-of-life preferences than those who didn’t participate.67 The intervention was not associated with increased depression, nor did it impair quality of life; rather, recipients of family-centred advance care planning reported lower anxiety and higher spiritual wellbeing than those who did not receive this intervention. 97 Conclusions were that discussions about end of life were feasible and valuable to patients and families early in the cancer experience. Future research would need to confirm the same conclusion in adolescents and young adults with advanced cancer.67
An additional intervention was a therapeutic music video for adolescents and young adults.95 A series of six structured music therapy sessions enabled adolescents and young adults to design and produce a music video. Concurrent activities targeted the development of protective coping factors, including spirituality, social integration, family support, and meaning-making. In a multisite randomised trial of 113 adolescents and young adults receiving HCT, recipients of therapeutic music video therapy reported improved social support, family environment, and “courageous coping”95—notably, the latter two terms were not clearly defined. Future access to therapeutic music video therapy could be limited by the fact that this therapy required formal training of staff and that music therapists are not widely available. Conclusions were that therapeutic music video or other psychosocial interventions were feasible and potentially impactful among adolescents and young adults receiving high-risk therapies like HCT.
The Bright IDEAS problem-solving skills training intervention was tested in parents of children with cancer.96 Problem-solving skills training includes eight structured sessions designed for parents without clinical psychopathology. In a multisite randomised trial of 309 mothers of children with newly diagnosed cancer,96 compared with standard, non-directive supportive care, problem-solving skills training was associated with notable improvements in the mood and symptoms of anxiety and post-traumatic stress in mothers. Although the intervention also required intensive training of staff, investigators proposed dissemination strategies to enable broad access to intervention materials.
The next generation of PPC oncology investigation
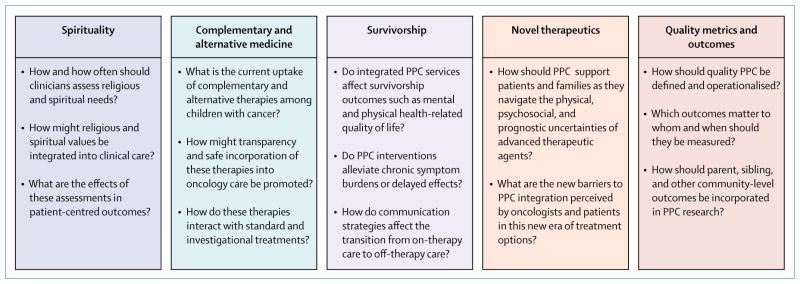
PPC oncology investigation has grown extensively in a short period of time. Recent recommendations have called for continued investigation of physical and psychosocial care (including symptom management and psychosocial supports), communication and decision making, as well as health systems (care coordination and access to services).98,99 Additional areas of research might include questions about ethics (eg, risk-to-benefit considerations for surgical biopsy before and after experimental therapies), health policy (eg, the rationale for concurrent cancer-directed and hospice care), and research methods (eg, systematic integration of patient-reported outcomes in clinical oncology research), as well as further trials assessing the effect of standardised primary PPC interventions and subspecialised PPC teams in the care of children with advanced cancer. We propose five additional, novel domains for future research (figure 2), which are founded on the experiences of children with cancer and their families and represent previously understudied but important aspects of their wellbeing.
Figure 2. Proposed novel domains and unanswered questions for the next generation of paediatric palliative care oncology investigation.
PPC=paediatric palliative care.
Spirituality
PPC definitions and guidelines all recognise the role of spirituality in overall patient and family wellbeing.2,3,43 Indeed, most parents report that religion, spirituality, and life philosophy are important determinants of their values and medical decisions.100 Little is known about how to assess or support the religious and spiritual needs of children with cancer and their families. Nurses caring for children with serious illness have observed that poor spiritual coping strategies (eg, feeling angry with God and feelings of blame and regret) might represent a potentially unmet need that contributes to patient and family suffering.101 Similarly, fewer than half of parents of children with serious illness believe that their spiritual needs have been met, and those whose needs are addressed perceive higher levels of overall support from their medical team.102 Future investigations might want to evaluate how best to assess and fill this important gap in patient-centred care.
Complementary and alternative medicine
Integrative medicine, such as herbal and nutritional treatments, acupuncture, and hypnosis, is garnering public interest. Up to 84% of parents report using an unconventional therapy and fewer than half share this information with their physicians.103–105 Importantly, experimentation with complementary and alternative therapies might increase when formal treatment options become scarce.103 Investigations are needed to understand the barriers to sharing of this information with medical teams, methods to facilitate its inclusion in oncology care, as well as the interactions between complementary, alternative, and experimental therapies.
Survivorship
The numerous late physical and psychosocial effects of cancer treatment among children and their families have been well described. PPC research methods might provide a unique opportunity to improve these outcomes. 41–43 Future investigations might include efforts to alleviate symptoms in survivors, develop communication standards about transitions from cancer-directed therapy to survivorship care, as well as interventions designed to improve the quality of life of patients suffering from chronic sequelae of cancer therapy (eg, disability or graft-versus-host disease).
Novel therapeutics
While PPC oncology investigation has been making major advancements, so too have breakthrough therapies such as immunotherapy.106 For example, up to 90% of children with previously refractory and incurable acute lymphoblastic leukaemia achieve remission with chimeric antigen receptor T-cell therapy.107 For this reason, more patients might seek out and receive this or another highly intensive experimental treatment. Although a substantial subset of these patients might ultimately experience a relapse and die, immunotherapy has changed the paradigm of treatment options and associated prognostication. 108 Furthermore, partly because of their novelty, late effects and toxicities from chimeric antigen receptor T-cell therapy and other targeted therapeutics are poorly understood.109 Future investigations should explore the barriers to and optimal integration of PPC for these patients and families.
Quality metrics and outcomes
Whereas medical oncologists caring for adults have published guidelines about quality metrics of palliative care delivery,110 no such metrics exist for paediatrics. A recent systematic review111 of PPC outcomes concluded that there were no ideal outcome assessment measures for PPC that consistently captured its impact or value. Furthermore, translation of adult-centred measures to paediatric populations is problematic; many adult metrics (eg, assigning of durable power of attorney) are irrelevant in paediatrics, and child and family experiences and psychosocial needs are distinct. Therefore standardised metrics that reflect paediatric priorities and experiences are needed.
Evidence-based clinical implications
PPC oncology is about provision of support to children with cancer and their families to alleviate suffering and improve their quality of life. This concept is not novel for oncology clinicians; most clinicians regularly practice primary PPC when they weigh treatment decisions and toxicities, provide symptom management and anticipatory guidance, engage in difficult conversations about diagnosis or prognosis, and consider the psychosocial needs of the family. These practices might be improved by weighing the wealth of evidence that has been generated over the past few decades. We have learned that children with cancer have high burdens of symptoms and corresponding suffering, and that early and ongoing communication about prognosis and goals of care is a crucial factor to ensure patient and family wellbeing. We have determined that parents and families also suffer during the cancer experience, economic hardship is prevalent, and racial and ethnic disparities influence cancer experiences and outcomes.
Future investigations should focus on rigorously building the existing evidence base regarding identification and management of physical and psychosocial symptoms, communication, decision making, end-of-life care, and bereavement support, and use and delivery of health services. Novel areas of investigation include the domains of spirituality, complementary and alternative therapies, advanced therapeutics, survivorship, and quality improvement. Together, these efforts have great potential to alleviate the burdens of cancer on children and their families.
Supplementary Material
Key messages.
Paediatric palliative care (PPC) research in children with cancer has increased substantially.
Children with advanced cancer commonly have multiple physical and psychosocial symptoms, which not only reduce their quality of life, but also contribute to parent distress. Integration of specialty palliative care teams and patient-reported outcomes in clinical settings might improve symptom recognition and management.
Early, open, and ongoing discussion of prognosis, family values, and perspectives is associated with parent understanding of prognosis, corresponding goal concordant care, child wellbeing at the end of life, and parent wellbeing during bereavement.
Paediatric cancer can have a lasting impact on the whole family, including high financial burdens. Comparatively little is known about the corresponding sociodemographic health disparities in the use of PPC health service and cultural differences in end-of-life preferences.
Additional gaps in the scientific literature include the roles of religion and spirituality in family-centred paediatric oncology care, use of complementary and alternative medicine and how best to integrate it, incorporation of PPC principles into survivorship care, the role of PPC in the era of promising novel therapeutics, as well as standardisation of PPC quality metrics.
Search strategy and selection criteria.
We searched PubMed for papers published between 1974 and April 30, 2017, using the following terms: “pediatric”, “palliative care”, and “oncology”. Articles were also identified through searches of the authors’ own files, reference lists of selected key papers, and through solicitation of opinion from members of the Pediatric Palliative Care Research Network. Only papers published in English were reviewed. The final reference list was selected on the basis of originality and reference to the broad scope of this Review. See appendix for additional information.
Acknowledgments
ARR was supported by the grant NIH/NCATS KL2 TR000421. The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the US National Institutes of Health.
Footnotes
See Online for appendix
Contributors
ARR drafted the Review. ARR and JW contributed to and approved the final version.
Declarations of interests
We declare no competing interests.
References
- 1.American Cancer Society. [accessed April 24, 2017];Cancer treatment and survivorship: facts and figures. 2015 https://old.cancer.org/acs/groups/content/@research/documents/document/acspc-042801.pdf.
- 2.Waldman E, Wolfe J. Palliative care for children with cancer. Nat Rev Clin Oncol. 2013;10:100–07. doi: 10.1038/nrclinonc.2012.238. [DOI] [PubMed] [Google Scholar]
- 3.WHO. [accessed April 24, 2017];WHO definition of palliative care. 1998 http://www.who.int/cancer/palliative/definition/en/
- 4.WHO. Cancer pain relief and palliative care in children with cancer. Geneva: World Health Organization Press; 1998. [Google Scholar]
- 5.American Academy of Pediatrics. Committee on bioethics and committee on hospital care. Palliative care for children. Pediatrics. 2000;106:351–57. [PubMed] [Google Scholar]
- 6.US National Library of Medicine. [accessed April 24, 2017]; https://www.ncbi.nlm.nih.gov/pubmed/?term=pediatic+palliative+care+oncology.
- 7.Wolfe J, Grier HE, Klar N, et al. Symptoms and suffering at the end of life in children with cancer. N Engl J Med. 2000;342:326–33. doi: 10.1056/NEJM200002033420506. [DOI] [PubMed] [Google Scholar]
- 8.Jalmsell L, Kreicbergs U, Onelov E, Steineck G, Henter JI. Symptoms affecting children with malignancies during the last month of life: a nationwide follow-up. Pediatrics. 2006;117:1314–20. doi: 10.1542/peds.2005-1479. [DOI] [PubMed] [Google Scholar]
- 9.Goldman A, Hewitt M, Collins GS, Childs M, Hain R United Kingdom Children’s Cancer Study Group/Paediatric Oncology Nurses’ Forum Palliative Care Working Group. Symptoms in children/young people with progressive malignant disease: United Kingdom Children’s Cancer Study Group/Paediatric Oncology Nurses Forum survey. Pediatrics. 2006;117:e1179–86. doi: 10.1542/peds.2005-0683. [DOI] [PubMed] [Google Scholar]
- 10.Hilden JM, Emanuel EJ, Fairclough DL, et al. Attitudes and practices among pediatric oncologists regarding end-of-life care: results of the 1998 American Society of Clinical Oncology survey. J Clin Oncol. 2001;19:205–12. doi: 10.1200/JCO.2001.19.1.205. [DOI] [PubMed] [Google Scholar]
- 11.Fowler K, Poehling K, Billheimer D, et al. Hospice referral practices for children with cancer: a survey of pediatric oncologists. J Clin Oncol. 2006;24:1099–104. doi: 10.1200/JCO.2005.02.6591. [DOI] [PubMed] [Google Scholar]
- 12.Wolfe J, Klar N, Grier HE, et al. Understanding of prognosis among parents of children who died of cancer: impact on treatment goals and integration of palliative care. JAMA. 2000;284:2469–75. doi: 10.1001/jama.284.19.2469. [DOI] [PubMed] [Google Scholar]
- 13.Mack JW, Wolfe J, Grier HE, Cleary PD, Weeks JC. Communication about prognosis between parents and physicians of children with cancer: parent preferences and the impact of prognostic information. J Clin Oncol. 2006;24:5265–70. doi: 10.1200/JCO.2006.06.5326. [DOI] [PubMed] [Google Scholar]
- 14.Mack JW, Cook EF, Wolfe J, Grier HE, Cleary PD, Weeks JC. Understanding of prognosis among parents of children with cancer: parental optimism and the parent-physician interaction. J Clin Oncol. 2007;25:1357–62. doi: 10.1200/JCO.2006.08.3170. [DOI] [PubMed] [Google Scholar]
- 15.Valdimarsdottir U, Kreicbergs U, Hauksdottir A, et al. Parents’ intellectual and emotional awareness of their child’s impending death to cancer: a population-based long-term follow-up study. Lancet Oncol. 2007;8:706–14. doi: 10.1016/S1470-2045(07)70209-7. [DOI] [PubMed] [Google Scholar]
- 16.Ullrich CK, Dussel V, Hilden JM, Sheaffer JW, Lehmann L, Wolfe J. End-of-life experience of children undergoing stem cell transplantation for malignancy: parent and provider perspectives and patterns of care. Blood. 2010;115:3879–85. doi: 10.1182/blood-2009-10-250225. [DOI] [PubMed] [Google Scholar]
- 17.Lannen P, Wolfe J, Mack J, Onelov E, Nyberg U, Kreicbergs U. Absorbing information about a child’s incurable cancer. Oncology. 2010;78:259–66. doi: 10.1159/000315732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Edwards KE, Neville BA, Cook EF, Jr, Aldridge SH, Dussel V, Wolfe J. Understanding of prognosis and goals of care among couples whose child died of cancer. J Clin Oncol. 2008;26:1310–15. doi: 10.1200/JCO.2007.13.4056. [DOI] [PubMed] [Google Scholar]
- 19.Bluebond-Langner M, Belasco JB, Goldman A, Belasco C. Understanding parents’ approaches to care and treatment of children with cancer when standard therapy has failed. J Clin Oncol. 2007;25:2414–19. doi: 10.1200/JCO.2006.08.7759. [DOI] [PubMed] [Google Scholar]
- 20.Hinds PS, Drew D, Oakes LL, et al. End-of-life care preferences of pediatric patients with cancer. J Clin Oncol. 2005;23:9146–54. doi: 10.1200/JCO.2005.10.538. [DOI] [PubMed] [Google Scholar]
- 21.Hinds PS, Oakes LL, Hicks J, et al. “Trying to be a good parent” as defined by interviews with parents who made phase I, terminal care, and resuscitation decisions for their children. J Clin Oncol. 2009;27:5979–85. doi: 10.1200/JCO.2008.20.0204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maurer SH, Hinds PS, Spunt SL, Furman WL, Kane JR, Baker JN. Decision making by parents of children with incurable cancer who opt for enrollment on a phase I trial compared with choosing a do not resuscitate/terminal care option. J Clin Oncol. 2010;28:3292–98. doi: 10.1200/JCO.2009.26.6502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tomlinson D, Bartels U, Gammon J, et al. Chemotherapy versus supportive care alone in pediatric palliative care for cancer: comparing the preferences of parents and health care professionals. CMAJ. 2011;183:E1252–58. doi: 10.1503/cmaj.110392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mack JW, Hilden JM, Watterson J, et al. Parent and physician perspectives on quality of care at the end of life in children with cancer. J Clin Oncol. 2005;23:9155–61. doi: 10.1200/JCO.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 25.Bluebond-Langner M. The private worlds of dying children. Princeton: Princeton University Press; 1980. [Google Scholar]
- 26.Pousset G, Bilsen J, De Wilde J, et al. Attitudes of adolescent cancer survivors toward end-of-life decisions for minors. Pediatrics. 2009;124:e1142–48. doi: 10.1542/peds.2009-0621. [DOI] [PubMed] [Google Scholar]
- 27.Rosenberg AR, Baker KS, Syrjala K, Wolfe J. Systematic review of psychosocial morbidities among bereaved parents of children with cancer. Pediatr Blood Cancer. 2012;58:503–12. doi: 10.1002/pbc.23386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li J, Precht DH, Mortensen PB, Olsen J. Mortality in parents after death of a child in Denmark: a nationwide follow-up study. Lancet. 2003;361:363–67. doi: 10.1016/S0140-6736(03)12387-2. [DOI] [PubMed] [Google Scholar]
- 29.Middleton W, Raphael B, Burnett P, Martinek N. A longitudinal study comparing bereavement phenomena in recently bereaved spouses, adult children and parents. Aust N Z J Psychiatry. 1998;32:235–41. doi: 10.3109/00048679809062734. [DOI] [PubMed] [Google Scholar]
- 30.Kreicbergs U, Valdimarsdottir U, Onelov E, Henter JI, Steineck G. Anxiety and depression in parents 4–9 years after the loss of a child owing to a malignancy: a population-based follow-up. Psychol Med. 2004;34:1431–41. doi: 10.1017/s0033291704002740. [DOI] [PubMed] [Google Scholar]
- 31.Lindahl Norberg A, Poder U, von Essen L. Early avoidance of disease- and treatment-related distress predicts post-traumatic stress in parents of children with cancer. Eur J Oncol Nurs. 2011;15:80–84. doi: 10.1016/j.ejon.2010.05.009. [DOI] [PubMed] [Google Scholar]
- 32.Drew D, Goodenough B, Maurice L, Foreman T, Willis L. Parental grieving after a child dies from cancer: is stress from stem cell transplant a factor? Int J Palliat Nurs. 2005;11:266–73. doi: 10.12968/ijpn.2005.11.6.18293. [DOI] [PubMed] [Google Scholar]
- 33.Jalmsell L, Onelov E, Steineck G, Henter JI, Kreicbergs U. Hematopoietic stem cell transplantation in children with cancer and the risk of long-term psychological morbidity in the bereaved parents. Bone Marrow Transplant. 2011;46:1063–70. doi: 10.1038/bmt.2010.287. [DOI] [PubMed] [Google Scholar]
- 34.Goodenough B, Drew D, Higgins S, Trethewie S. Bereavement outcomes for parents who lose a child to cancer: are place of death and sex of parent associated with differences in psychological functioning? Psychooncology. 2004;13:779–91. doi: 10.1002/pon.795. [DOI] [PubMed] [Google Scholar]
- 35.Surkan PJ, Kreicbergs U, Valdimarsdottir U, et al. Perceptions of inadequate health care and feelings of guilt in parents after the death of a child to a malignancy: a population-based long-term follow-up. J Palliat Med. 2006;9:317–31. doi: 10.1089/jpm.2006.9.317. [DOI] [PubMed] [Google Scholar]
- 36.Jalmsell L, Kreicbergs U, Onelov E, Steineck G, Henter JI. Anxiety is contagious-symptoms of anxiety in the terminally ill child affect long-term psychological well-being in bereaved parents. Pediatr Blood Cancer. 2010;54:751–57. doi: 10.1002/pbc.22418. [DOI] [PubMed] [Google Scholar]
- 37.McCarthy MC, Clarke NE, Ting CL, Conroy R, Anderson VA, Heath JA. Prevalence and predictors of parental grief and depression after the death of a child from cancer. J Palliat Med. 2010;13:1321–26. doi: 10.1089/jpm.2010.0037. [DOI] [PubMed] [Google Scholar]
- 38.Mack JW, Joffe S, Hilden JM, et al. Parents’ views of cancer-directed therapy for children with no realistic chance for cure. J Clin Oncol. 2008;26:4759–64. doi: 10.1200/JCO.2007.15.6059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kreicbergs U, Valdimarsdottir U, Onelov E, Henter JI, Steineck G. Talking about death with children who have severe malignant disease. N Engl J Med. 2004;351:1175–86. doi: 10.1056/NEJMoa040366. [DOI] [PubMed] [Google Scholar]
- 40.Kreicbergs U, Valdimarsdottir U, Steineck G, Henter JI. A population-based nationwide study of parents’ perceptions of a questionnaire on their child’s death due to cancer. Lancet. 2004;364:787–89. doi: 10.1016/S0140-6736(04)16939-0. [DOI] [PubMed] [Google Scholar]
- 41.Himelstein BP, Hilden JM, Boldt AM, Weissman D. Pediatric palliative care. N Engl J Med. 2004;350:1752–62. doi: 10.1056/NEJMra030334. [DOI] [PubMed] [Google Scholar]
- 42.Harris MB. Palliative care in children with cancer: which child and when? J Natl Cancer Inst Monogr. 2004:144–49. doi: 10.1093/jncimonographs/lgh007. [DOI] [PubMed] [Google Scholar]
- 43.Liben S, Papadatou D, Wolfe J. Paediatric palliative care: challenges and emerging ideas. Lancet. 2008;371:852–64. doi: 10.1016/S0140-6736(07)61203-3. [DOI] [PubMed] [Google Scholar]
- 44.Institute of Medicine. [accessed July 5, 2017];Cancer care for the whole patient: meeting psychosocial needs. 2007 https://www.nap.edu/catalog/11993/cancer-care-for-the-whole-patient-meeting-psychosocial-healthneeds.
- 45.Jacobsen PB, Wagner LI. A new quality standard: the integration of psychosocial care into routine cancer care. J Clin Oncol. 2012;30:1154–59. doi: 10.1200/JCO.2011.39.5046. [DOI] [PubMed] [Google Scholar]
- 46.Jacobsen PB, Holland JC, Steensma DP. Caring for the whole patient: the science of psychosocial care. J Clin Oncol. 2012;30:1151–53. doi: 10.1200/JCO.2011.41.4078. [DOI] [PubMed] [Google Scholar]
- 47.Wein S, Pery S, Zer A. Role of palliative care in adolescent and young adult oncology. J Clin Oncol. 2010;28:4819–24. doi: 10.1200/JCO.2009.22.4543. [DOI] [PubMed] [Google Scholar]
- 48.Quill TE, Abernethy AP. Generalist plus specialist palliative care—creating a more sustainable model. N Engl J Med. 2013;368:1173–75. doi: 10.1056/NEJMp1215620. [DOI] [PubMed] [Google Scholar]
- 49.Center for the Advancement of Palliative Care. [accessed March 31, 2017];Public opinion research on palliative care. 2011 https://media.capc.org/filer_public/18/ab/18ab708c-f835-4380-921d-fbf729702e36/2011-public-opinion-research-on-palliative-care.pdf.
- 50.Levine DR, Mandrell BN, Sykes A, et al. Patients’ and parents’ needs, attitudes, and perceptions about early palliative care integration in pediatric oncology. JAMA Oncol. 2017 doi: 10.1001/jamaoncol.2017.0368. published online March 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Johnston DL, Nagel K, Friedman DL, Meza JL, Hurwitz CA, Friebert S. Availability and use of palliative care and end-of-life services for pediatric oncology patients. J Clin Oncol. 2008;26:4646–50. doi: 10.1200/JCO.2008.16.1562. [DOI] [PubMed] [Google Scholar]
- 52.Kassam A, Skiadaresis J, Habib S, Alexander S, Wolfe J. Moving toward quality palliative cancer care: parent and clinician perspectives on gaps between what matters and what is accessible. J Clin Oncol. 2013;31:910–15. doi: 10.1200/JCO.2012.44.8936. [DOI] [PubMed] [Google Scholar]
- 53.Kassam A, Sutradhar R, Widger K, et al. Predictors of and trends in high-intensity end-of-life care among children with cancer: a population-based study using health services data. J Clin Oncol. 2017;35:236–42. doi: 10.1200/JCO.2016.68.8283. [DOI] [PubMed] [Google Scholar]
- 54.Klaassen RJ, Barr RD, Hughes J, et al. Nurses provide valuable proxy assessment of the health-related quality of life of children with Hodgkin disease. Cancer. 2010;116:1602–07. doi: 10.1002/cncr.24888. [DOI] [PubMed] [Google Scholar]
- 55.Hockenberry MJ, Hinds PS, Barrera P, et al. Three instruments to assess fatigue in children with cancer: the child, parent and staff perspectives. J Pain Symptom Manage. 2003;25:319–28. doi: 10.1016/s0885-3924(02)00680-2. [DOI] [PubMed] [Google Scholar]
- 56.Glaser AW, Davies K, Walker D, Brazier D. Influence of proxy respondents and mode of administration on health status assessment following central nervous system tumours in childhood. Qual Life Res. 1997;6:43–53. doi: 10.1023/a:1026465411669. [DOI] [PubMed] [Google Scholar]
- 57.Parsons SK, Barlow SE, Levy SL, Supran SE, Kaplan SH. Health-related quality of life in pediatric bone marrow transplant survivors: according to whom? Int J Cancer Suppl. 1999;12:46–51. doi: 10.1002/(sici)1097-0215(1999)83:12+<46::aid-ijc9>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 58.Basch E, Deal AM, Kris MG, et al. Symptom monitoring with patient-reported outcomes during routine cancer treatment: a randomized controlled trial. J Clin Oncol. 2016;34:557–65. doi: 10.1200/JCO.2015.63.0830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wolfe J, Orellana L, Ullrich C, et al. Symptoms and distress in children with advanced cancer: prospective patient-reported outcomes from the PediQUEST study. J Clin Oncol. 2015;33:1928–35. doi: 10.1200/JCO.2014.59.1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rosenberg AR, Orellana L, Ullrich C, et al. Quality of life in children with advanced cancer: a report from the PediQUEST study. J Pain Symptom Manage. 2016;52:243–53. doi: 10.1016/j.jpainsymman.2016.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rosenberg AR, Bona K, Ketterl T, Wharton CM, Wolfe J, Baker KS. Intimacy, substance use, and communication needs during cancer therapy: a report from the “resilience in adolescents and young adults” study. J Adolesc Health. 2017;60:93–99. doi: 10.1016/j.jadohealth.2016.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Straehla JP, Barton KS, Yi-Frazier JP, et al. The benefits and burdens of cancer: a prospective longitudinal cohort study of adolescents and young adults. J Palliat Med. 2017;20:494–501. doi: 10.1089/jpm.2016.0369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rosenberg AR, Yi-Frazier JP, Wharton C, Gordon K, Jones B. Contributors and inhibitors of resilience among adolescents and young adults with cancer. J Adolesc Young Adult Oncol. 2014;3:185–93. doi: 10.1089/jayao.2014.0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wiener L, Zadeh S, Battles H, et al. Allowing adolescents and young adults to plan their end-of-life care. Pediatrics. 2012;130:897–905. doi: 10.1542/peds.2012-0663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jacobs S, Perez J, Cheng YI, Sill A, Wang J, Lyon ME. Adolescent end of life preferences and congruence with their parents’ preferences: results of a survey of adolescents with cancer. Pediatr Blood Cancer. 2015;62:710–14. doi: 10.1002/pbc.25358. [DOI] [PubMed] [Google Scholar]
- 66.Kelly KP, Mowbray C, Pyke-Grimm K, Hinds PS. Identifying a conceptual shift in child and adolescent-reported treatment decision making: “Having a say, as I need at this time”. Pediatr Blood Cancer. 2017 doi: 10.1002/pbc.26262. published online Nov 5. [DOI] [PubMed] [Google Scholar]
- 67.Lyon ME, Jacobs S, Briggs L, Cheng YI, Wang J. Family-centered advance care planning for teens with cancer. JAMA Pediatr. 2013;167:460–67. doi: 10.1001/jamapediatrics.2013.943. [DOI] [PubMed] [Google Scholar]
- 68.Rosenberg AR, Postier A, Osenga K, et al. Long-term psychosocial outcomes among bereaved siblings of children with cancer. J Pain Symptom Manage. 2015;49:55–65. doi: 10.1016/j.jpainsymman.2014.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Foster TL, Gilmer MJ, Vannatta K, et al. Changes in siblings after the death of a child from cancer. Cancer Nurs. 2012;35:347–54. doi: 10.1097/NCC.0b013e3182365646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gerhardt CA, Fairclough DL, Grossenbacher JC, et al. Peer relationships of bereaved siblings and comparison classmates after a child’s death from cancer. J Pediatr Psychol. 2012;37:209–19. doi: 10.1093/jpepsy/jsr082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rosenberg AR, Orellana L, Kang TI, et al. Differences in parent-provider concordance regarding prognosis and goals of care among children with advanced cancer. J Clin Oncol. 2014;32:3005–11. doi: 10.1200/JCO.2014.55.4659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rosenberg AR, Dussel V, Kang T, et al. Psychological distress in parents of children with advanced cancer. JAMA Pediatr. 2013;167:537–43. doi: 10.1001/jamapediatrics.2013.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dussel V, Bona K, Heath JA, Hilden JM, Weeks JC, Wolfe J. Unmeasured costs of a child’s death: perceived financial burden, work disruptions, and economic coping strategies used by American and Australian families who lost children to cancer. J Clin Oncol. 2011;29:1007–13. doi: 10.1200/JCO.2009.27.8960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bona K, Dussel V, Orellana L, et al. Economic impact of advanced pediatric cancer on families. J Pain Symptom Manage. 2014;47:594–603. doi: 10.1016/j.jpainsymman.2013.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bona K, London WB, Guo D, Frank DA, Wolfe J. Trajectory of material hardship and income poverty in families of children undergoing chemotherapy: a prospective cohort study. Pediatr Blood Cancer. 2016;63:105–111. doi: 10.1002/pbc.25762. [DOI] [PubMed] [Google Scholar]
- 76.Bona K, Blonquist TM, Neuberg DS, Silverman LB, Wolfe J. Impact of socioeconomic status on timing of relapse and overall survival for children treated on Dana-Farber Cancer Institute ALL consortium protocols (2000–2010) Pediatr Blood Cancer. 2016;63:1012–18. doi: 10.1002/pbc.25928. [DOI] [PubMed] [Google Scholar]
- 77.Bhatia S. Disparities in cancer outcomes: lessons learned from children with cancer. Pediatr Blood Cancer. 2011;56:994–1002. doi: 10.1002/pbc.23078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zamora ER, Kaul S, Kirchhoff AC, et al. The impact of language barriers and immigration status on the care experience for Spanish-speaking caregivers of patients with pediatric cancer. Pediatr Blood Cancer. 2016;63:2173–80. doi: 10.1002/pbc.26150. [DOI] [PubMed] [Google Scholar]
- 79.Baker JN, Rai S, Liu W, et al. Race does not influence do-not-resuscitate status or the number or timing of end-of-life care discussions at a pediatric oncology referral center. J Palliat Med. 2009;12:71–76. doi: 10.1089/jpm.2008.0172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Brock KE, Steineck A, Twist CJ. Trends in end-of-life care in pediatric hematology, oncology, and stem cell transplant patients. Pediatr Blood Cancer. 2016;63:516–22. doi: 10.1002/pbc.25822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Thienprayoon R, Lee SC, Leonard D, Winick N. Racial and ethnic differences in hospice enrollment among children with cancer. Pediatr Blood Cancer. 2013;60:1662–66. doi: 10.1002/pbc.24590. [DOI] [PubMed] [Google Scholar]
- 82.Cawkwell PB, Gardner SL, Weitzman M. Persistent racial and ethnic differences in location of death for children with cancer. Pediatr Blood Cancer. 2015;62:1403–08. doi: 10.1002/pbc.25479. [DOI] [PubMed] [Google Scholar]
- 83.Caruso Brown AE, Howard SC, Baker JN, Ribeiro RC, Lam CG. Reported availability and gaps of pediatric palliative care in low- and middle-income countries: a systematic review of published data. J Palliat Med. 2014;17:1369–83. doi: 10.1089/jpm.2014.0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wiener L, McConnell DG, Latella L, Ludi E. Cultural and religious considerations in pediatric palliative care. Palliat Support Care. 2013;11:47–67. doi: 10.1017/S1478951511001027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Osenga K, Postier A, Dreyfus J, Foster L, Teeple W, Friedrichsdorf SJ. A comparison of circumstances at the end of life in a hospital setting for children with palliative care involvement versus those without. J Pain Symptom Manage. 2016;52:673–80. doi: 10.1016/j.jpainsymman.2016.05.024. [DOI] [PubMed] [Google Scholar]
- 86.Kassam A, Skiadaresis J, Alexander S, Wolfe J. Differences in end-of-life communication for children with advanced cancer who were referred to a palliative care team. Pediatr Blood Cancer. 2015;62:1409–13. doi: 10.1002/pbc.25530. [DOI] [PubMed] [Google Scholar]
- 87.Ullrich CK, Lehmann L, London WB, et al. End-of-life care patterns associated with pediatric palliative care among children who underwent hematopoietic stem cell transplant. Biol Blood Marrow Transplant. 2016;22:1049–55. doi: 10.1016/j.bbmt.2016.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Weaver MS, Heinze KE, Kelly KP, et al. Palliative care as a standard of care in pediatric oncology. Pediatr Blood Cancer. 2015;62:S829–33. doi: 10.1002/pbc.25695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lichtenthal WG, Sweeney CR, Roberts KE, et al. Bereavement follow-up after the death of a child as a standard of care in pediatric oncology. Pediatr Blood Cancer. 2015;62:S834–69. doi: 10.1002/pbc.25700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kearney JA, Salley CG, Muriel AC. Standards of psychosocial care for parents of children with cancer. Pediatr Blood Cancer. 2015;62:S632–83. doi: 10.1002/pbc.25761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zadeh S, Pao M, Wiener L. Opening end-of-life discussions: how to introduce VOICING My CHOiCES™, an advance care planning guide for adolescents and young adults. Palliat Support Care. 2015;13:591–99. doi: 10.1017/S1478951514000054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Widger K, Friedrichsdorf S, Wolfe J, et al. Protocol: evaluating the impact of a nation-wide train-the-trainer educational initiative to enhance the quality of palliative care for children with cancer. BMC Palliat Care. 2016;15:12. doi: 10.1186/s12904-016-0085-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Brock KE, Cohen HJ, Sourkes BM, Good JJ, Halamek LP. Training pediatric fellows in palliative care: a pilot comparison of simulation training and didactic education. J Palliat Med. 2017 doi: 10.1089/jpm.2016.0556. published online April 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wolfe J, Orellana L, Cook EF, et al. Improving the care of children with advanced cancer by using an electronic patient-reported feedback intervention: results from the PediQUEST randomized controlled trial. J Clin Oncol. 2014;32:1119–26. doi: 10.1200/JCO.2013.51.5981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Robb SL, Burns DS, Stegenga KA, et al. Randomized clinical trial of therapeutic music video intervention for resilience outcomes in adolescents/young adults undergoing hematopoietic stem cell transplant: a report from the Children’s Oncology Group. Cancer. 2014;120:909–17. doi: 10.1002/cncr.28355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sahler OJ, Dolgin MJ, Phipps S, et al. Specificity of problem-solving skills training in mothers of children newly diagnosed with cancer: results of a multisite randomized clinical trial. J Clin Oncol. 2013;31:1329–35. doi: 10.1200/JCO.2011.39.1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lyon ME, Jacobs S, Briggs L, Cheng YI, Wang J. A longitudinal, randomized, controlled trial of advance care planning for teens with cancer: anxiety, depression, quality of life, advance directives, spirituality. J Adolesc Health. 2014;54:710–17. doi: 10.1016/j.jadohealth.2013.10.206. [DOI] [PubMed] [Google Scholar]
- 98.Baker JN, Levine DR, Hinds PS, et al. Research priorities in pediatric palliative care. J Pediatr. 2015;167:467–70. e3. doi: 10.1016/j.jpeds.2015.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Miller EG, Levy C, Linebarger JS, Klick JC, Carter BS. Pediatric palliative care: current evidence and evidence gaps. J Pediatr. 2015;166:1536–40. e1. doi: 10.1016/j.jpeds.2015.02.019. [DOI] [PubMed] [Google Scholar]
- 100.Hexem KR, Mollen CJ, Carroll K, Lanctot DA, Feudtner C. How parents of children receiving pediatric palliative care use religion, spirituality, or life philosophy in tough times. J Palliat Med. 2011;14:39–44. doi: 10.1089/jpm.2010.0256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ferrell B, Wittenberg E, Battista V, Walker G. Exploring the spiritual needs of families with seriously ill children. Int J Palliat Nurs. 2016;22:388–94. doi: 10.12968/ijpn.2016.22.8.388. [DOI] [PubMed] [Google Scholar]
- 102.Kelly JA, May CS, Maurer SH. Assessment of the spiritual needs of primary caregivers of children with life-limiting illnesses is valuable yet inconsistently performed in the hospital. J Palliat Med. 2016;19:763–66. doi: 10.1089/jpm.2015.0509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sencer SF, Kelly KM. Complementary and alternative therapies in pediatric oncology. Pediatr Clin North Am. 2007;54:1043–60. xiii. doi: 10.1016/j.pcl.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 104.Kelly KM, Jacobson JS, Kennedy DD, Braudt SM, Mallick M, Weiner MA. Use of unconventional therapies by children with cancer at an urban medical center. J Pediatr Hematol Oncol. 2000;22:412–16. doi: 10.1097/00043426-200009000-00005. [DOI] [PubMed] [Google Scholar]
- 105.Kelly KM. Complementary and alternative medical therapies for children with cancer. Eur J Cancer. 2004;40:2041–46. doi: 10.1016/j.ejca.2004.05.012. [DOI] [PubMed] [Google Scholar]
- 106.Mackall CL, Merchant MS, Fry TJ. Immune-based therapies for childhood cancer. Nat Rev Clin Oncol. 2014;11:693–703. doi: 10.1038/nrclinonc.2014.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Maude SL, Frey N, Shaw PA, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med. 2014;371:1507–17. doi: 10.1056/NEJMoa1407222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Temel JS, Shaw AT, Greer JA. Challenge of prognostic uncertainty in the modern era of cancer therapeutics. J Clin Oncol. 2016;34:3605–08. doi: 10.1200/JCO.2016.67.8573. [DOI] [PubMed] [Google Scholar]
- 109.Mody RJ, Prensner JR, Everett J, Parsons DW, Chinnaiyan AM. Precision medicine in pediatric oncology: lessons learned and next steps. Pediatr Blood Cancer. 2017;64:e26288. doi: 10.1002/pbc.26288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Bickel KE, McNiff K, Buss MK, et al. Defining high-quality palliative care in oncology practice: an American Society of Clinical Oncology/American Academy of Hospice and Palliative Medicine Guidance Statement. J Oncol Pract. 2016;12:e828–38. doi: 10.1200/JOP.2016.010686. [DOI] [PubMed] [Google Scholar]
- 111.Coombes LH, Wiseman T, Lucas G, Sangha A, Murtagh FE. Health-related quality-of-life outcome measures in paediatric palliative care: a systematic review of psychometric properties and feasibility of use. Palliat Med. 2016;30:935–49. doi: 10.1177/0269216316649155. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.