Abstract
Pregnancy increases women’s nutritional requirements, yet causes aversions to nutritious foods. Most societies further restrict pregnant women’s diet with food taboos. Pregnancy food aversions are theorized to protect mothers and fetuses from teratogens and pathogens or increase dietary diversity in response to resource scarcity. Tests of these hypotheses have had mixed results, perhaps because many studies are in Westernized populations with reliable access to food and low exposure to pathogens. If pregnancy food aversions are adaptations, however, then they likely evolved in environments with uncertain access to food and high exposure to pathogens. Pregnancy food taboos, on the other hand, have been theorized to limit resource consumption, mark social identity, or also protect mothers and fetuses from dangerous foods. There have been few tests of evolutionary theories of culturally transmitted food taboos.
We investigated these and other theories of psychophysiological food aversions and culturally transmitted food taboos among two non-Western populations of pregnant women in Mysore, India, that vary in food insecurity and exposure to infectious disease. The first was a mixed caste rural farming population (N = 72), and the second was the Jenu Kurubas, a resettled population of former hunter-gatherers (N = 30). Women rated their aversions to photos of 31 foods and completed structured interviews that assessed aversions and socially learned avoidances of foods, pathogen exposure, food insecurity, sources of culturally acquired dietary advice, and basic sociodemographic information. Aversions to spicy foods were associated with early trimester and nausea and vomiting, supporting a protective role against plant teratogens. Variation in exposure to pathogens did not explain variation in meat aversions or avoidances, however, raising some doubts about the importance of pathogen avoidance. Aversions to staple foods were common, but were not associated with resource stress, providing mixed support for the role of dietary diversification. Avoided foods outnumbered aversive foods, were believed to be abortifacients or otherwise harmful to the fetus, influenced diet throughout pregnancy, and were largely distinct from aversive foods. These results suggest that aversions target foods with cues of toxicity early in pregnancy, and taboos target suspected abortifacients throughout pregnancy.
1. Introduction
We report a study in two rural Indian populations designed to test several evolutionary hypotheses regarding the function of pregnancy food aversions and culturally transmitted food taboos. Influential theories of dietary shifts in pregnancy propose that because the fetus is particularly vulnerable to developmental disruption during organogenesis, which occurs early in pregnancy, women evolved to experience physiological aversions in the first trimester towards toxic plant foods (Hook, 1978; Profet, 1995). Immunological shifts early in pregnancy that accommodate the developing fetus were thought to increase mothers’ susceptibility to infection, so mothers should also be averse to foods likely to harbor pathogens, such as meat (Fessler, 2002; Flaxman & Sherman, 2000). Food aversions and nausea and vomiting in pregnancy (NVP) were therefore hypothesized to be evolved mechanisms that protect women and fetuses, which is commonly referred to as “the maternal-fetal protection” hypothesis (Patil & Young, 2012; for reviews, see Patil, Abrams, Steinmetz, & Young, 2012).
Although several lines of evidence support the maternal-fetal protection hypothesis, many of these come from studies in high income countries with a low burden of infectious disease (Patil, 2012). Some studies in populations facing resource scarcity, however, have failed to support it. A study in southern Ethiopia for example, found that pregnant women avoided cereals, which were non-toxic staple foods, but craved meat and other livestock products, which were scarce (Demissie, Muroki, & Kogi-Makau, 1998). In Turkana pastoralists, I. L. Pike (2000) found that NVP was associated with adverse health indicators among both mothers and developing fetuses, contrary to the maternal-fetal protection hypothesis. More generally, some studies have found variation in the timing and types of items that women find aversive, or avoid, in pregnancy, not all of which are consistent with maternal-fetal protection (Patil, 2012; A. G. Young & Pike, 2012).
Shifts in dietary preferences might instead be a strategy to diversify nutrient intake for pregnant women with high levels of food insecurity or nutritional deficiencies (Demissie et al., 1998). East African women, for example, have reported aversions towards staple foods, such as maize, and cravings for meat and milk, two foods perceived by women to increase strength, but that are limited due to reduced food availability and low socioeconomic status (A. G. Young & Pike, 2012). South Indian women have reported cravings for pica substances, including mud and chalk, that have questionable health consequences but were directly linked to resource scarcity and psychological distress (Placek & Hagen, 2013).
Culturally transmitted food taboos also shape food choices during pregnancy (e.g., Aunger, 1994; Dentan, 1966; J. Henrich & Henrich, 2010; Placek & Hagen, 2013, 2015). The Semai horticulturalists, for example, avoid unripe fruit in pregnancy because consumption is believed to cause malaria and subsequent fetal death (Dentan, 1966). Aunger (1994) found that for some individuals in the Congo basin, particularly pregnant women, adherence to food taboos reduced caloric intake by up to 9%.
Classic anthropological theory suggests that food taboos could function to protect the environment by limiting resource consumption (Harris, 1998), increase group cohesion by serving as a marker of social identity (Whitehead, 2000), or spread due to symbolic reasoning; e.g. through perceptions of purity and pollution (Douglas, 2003).
Alternatively, food taboos might have culturally evolved to identify dangerous foods. Learning about dangerous foods from parents and other local “experts” reduces costs of individual learning (Aunger, 1994, 2000; Boyd & Richerson, 1985; Boyd, Richerson, & Henrich, 2011; Cashdan, 1994; Cavalli-Sforza & Feldman, 1981; Fessler & Navarrete, 2003; J. Henrich & Boyd, 2002; J. Henrich & Henrich, 2010; Richerson & Boyd, 2005). J. Henrich & Henrich (2010) found that in Fiji, pregnancy and postpartum food taboos targeted toxic marine species, likely to protect mothers, fetuses, and nursing infants from harm. More generally, as similar functionality can evolve genetically or culturally (Boyd & Richerson, 1985), functional hypotheses for food aversions, e.g., increasing dietary diversity, could also apply to food taboos.
Cultural information can be transmitted vertically, from parents to offspring; obliquely, from members of the older generation to members of the younger; and horizontally, among siblings, friends, and other members of the same generation. These modes of transmission are favored by genetic natural selection under different environmental conditions. Vertical transmission is expected for behaviors that impact fertility and are under strong selection in stable environments. Oblique learning, on the other hand, allows more rapid adaptation in variable environments (McElreath & Strimling, 2008). J. Henrich & Henrich (2010) argue for the importance of a prestige bias toward oblique learning, finding that women acquired pregnancy food taboos vertically from mothers and grandmothers, and obliquely from mothers-in-law, elders, and prestigious wise women.
The relationship between pregnancy food aversions and taboos has received relatively little theoretical or empirical attention. If aversions and taboos both function to protect mothers and fetuses from dangerous foods, are these the same foods or different foods? Fessler & Navarrete (2003) propose the socially mediated ingestive conditioning hypothesis, in which aversive reactions of individuals to a particular food, such as meat, are observed by others, who then learn to associate that food with an aversive response, avoiding it themselves. Aversions acquired via socially mediated ingestive conditioning can gain moral weight via various mechanisms (e.g., normative moralization or egocentric empathy; for details, see Fessler & Navarrete 2003), leading to a widespread taboo of that food. Under some scenarios, common aversions might become common taboos; under others, idiosyncratic aversions of a few individuals might become common taboos. The few previous studies found little correspondence between food aversions and food taboos (Aunger, 1994; J. Henrich & Henrich, 2010), raising doubts about scenarios in which common food aversions become common taboos.
During socially mediated ingestive conditioning, individuals associate a food with an aversive reaction (e.g., “papaya made me sick”). As there is no scientific, let alone cultural, consensus on the functions of pregnancy food aversions (if any), food taboos might be accompanied by explanations that have little or nothing to do with their underlying functionality. Indeed, Fessler & Navarrete (2003) suggest that “investigators would do well to pause before assuming that such cultural rationales are the principal factor motivating the generation, acquisition, and perpetuation of attitudes and behaviors – they are as likely, if not more likely, to be justifications rather than causes” (p. 24).
Alternatively, because physiological cues of toxicity, such as bitterness and nausea, do not reliably indicate teratogenicity, women might have evolved to individually and socially learn associations between foods and poor pregnancy outcomes, independent of their own or others’ aversive reactions (Hagen, Roulette, & Sullivan, 2013; Placek & Hagen, 2015), consistent with generic cultural transmission models (e.g., Boyd & Richerson, 1985). Under this hypothesis, individual learners would know why they avoided a food, but might or might not transmit this reason to others (e.g., “do not eat papaya because it causes abortion” vs. “do not eat papaya.”).
1 Study goals and predictions
We investigate four major questions: (1) What is the function of pregnancy food aversions, if any? (2) What is the function of pregnancy food taboos, if any? (3) From whom are pregnancy food taboos acquired? (4) If, as several theorists have suggested, aversions and taboos both function to protect individuals from dangerous foods, are these the same foods or different foods? Because pathogen exposure and constrained access to food are key factors in influential theories of aversions and avoidances, we conducted our study in India, a region of high food insecurity and communicable disease.
Currently, 300 million (30%) of India’s rural population is impoverished and lacks access to sufficient foods, basic health care, and education (“India Food Security Portal,” n.d.). In 2012, 41% of Indian deaths were due to communicable disease (“WHO India,” n.d.). Of those with electricity, power outages occur on a daily basis and last for hours (Wilson, Mignone, & Sinclair, 2014). Hence refrigeration, and thus safe food storage, is often absent or unreliable. Finally, India ranks as one the highest in iron deficiency anemia in the world, with rural pregnant women and children at highest risk (Kalaivani, 2009).
In India, health and illness are framed in terms of humoral theory, in which combinations of five elements in the body --- earth, fire, ether, air, and water --- determines one’s constitution, and thus one’s well-being. Pregnancy is considered a period of increased heat in the body during which women must avoid “hot” foods (“hot” does not refer to spiciness or temperature) and only consume “cooling” foods in order to bring internal balance and thus ensure a successful pregnancy outcome (Nag, 1994; Placek & Hagen, 2015; Van Hollen, 2003).
Placek & Hagen (2015) found that humoral theory had a strong influence on pregnancy diet: South Indian women primarily avoided “hot” foods, mostly fruits but also some meats; often acquired food avoidances via learning; and frequently stated that foods were avoided to prevent fetal or infant harm. Placek & Hagen (2015) also found that pathogen avoidance seemed to best explain avoidance of meat. This study did not systematically distinguish foods that were avoided due to aversive reactions versus those that were avoided due to advice from others, however (instead relying on mothers to make that distinction); did not determine from whom avoidances were learned; and its best-fitting exploratory model of meat aversions used number of household members as an index of pathogen exposure (McDade, Rutherford, Adair, & Kuzawa, 2009), which is an indirect measure at best.
We aimed to improve on Placek & Hagen (2015) by measuring aversive reactions to food photos, creating two separate free-lists of foods that were individually aversive and those that were avoided due to advice from others, determining the sources of dietary advice, the emic reasons for food taboos (“emic”” refers to indigenous concepts; “etic” refers to Western scientific concepts), assessing pathogen exposure with multiple questionnaire items, and including fruits as a priori targets of food taboos.
The word “taboo” derives from the Fijian word “tabu,” which is a culturally transmitted prohibition that, if violated, would bring social or supernatural sanctions. According to local informants at our field site, women who consumed foods they were supposed to avoid would be heavily scolded. Following J. Henrich & Henrich (2010), we therefore operationalize food taboos as food avoidances and will use the terms food taboo and food avoidance interchangeably (for more discussion, see J. Henrich & Henrich, 2010).
We specifically tested the following theoretical models of pregnancy food aversions and avoidances that aim to explain (1) which foods are aversive and avoided, and (2) which women will experience aversions and adhere to avoidances. These theories are not mutually exclusive; all could help explain aversions and avoidances.
Maternal-fetal protection
This model posits that in the first trimester of pregnancy foods that pose a high risk of pathogen and toxin ingestion, such as meat and vegetables, will stimulate aversions, nausea, and vomiting, and be more likely to be avoided (Fessler, 2002; Fessler & Navarrete, 2003; Flaxman & Sherman, 2000; Profet, 1995). We therefore tested if early trimester, nausea, and vomiting predicted aversions to, and avoidances of ethnic, strong, and spicy (ESS) foods (spices are often toxic), vegetables, and meat, but not other food categories (e.g., grains, fruits, sweets).
Pathogen avoidance
We added pathogen exposure and disease susceptibility to the previous model to test if these variables predicted aversions to, and avoidances of meat, but not other food categories.
Exploratory Pathogen Avoidance Model
In an exploratory analysis, Placek & Hagen (2015) found that number of household members, a possible index of exposure to pathogens (McDade et al., 2009), and early trimester of pregnancy were the strongest predictors of meat aversions among village women in Tamil Nadu. To confirm this exploratory result, we tested if higher numbers of household members and early trimester of pregnancy predicted aversions to, and avoidances of meat, but not other food categories.
Dietary diversity
Demissie et al. (1998) proposed that, among pregnant women with limited access to food, aversions to staple foods would increase dietary diversity and access to micronutrients, a hypothesis supported by some studies (A. G. Young & Pike, 2012) but not others (Placek & Hagen, 2015). Accordingly, we tested if higher food insecurity predicted aversions to, and avoidances of staple food items (grains, legumes), but not other food categories.
Social transmission model
Food taboos are hypothesized to protect the environment by limiting resource consumption (Harris, 1998), serve as markers of social identity (Whitehead, 2000), or protect individuals from dangerous foods via social rather than individual learning (e.g., J. Henrich & Henrich, 2010). Previous studies found that in some populations pregnancy food taboos functioned to protect mothers and fetuses from dangerous foods, particularly abortifacients (J. Henrich & Henrich, 2010; Placek & Hagen, 2015), which in south India are often fruits (Placek & Hagen, 2015); did not closely correspond to aversions (Aunger, 1994; J. Henrich & Henrich, 2010); and were acquired vertically from mothers and grandmothers, and obliquely from mothers-in-law and wise women (J. Henrich & Henrich, 2010). We therefore investigated (1) the emic function of taboos, (2) if avoided foods were usually fruits, (3) if dietary advice or pressure from others predicted avoidances of food, (4) if the same foods that were aversive were also avoided, and (5) from whom food taboos were acquired.
Sociodemographic model
Some studies found that dietary aversions vary according to age and education (Drewnowski, 1997; Sanjur, 1982). We therefore tested if sociodemographic variables predicted aversions to, and avoidances of any commonly aversive or avoided food categories.
There have been very few studies that systematically compared pregnancy food aversions and avoidances. A final goal of this study was therefore to provide detailed comparisons of their distributions in traditional populations as a foundation for future research.
2 Study Populations
This research took place in Mysore District, Karnataka, India from June to August, 2015. Mysore is located in tropical Southwest India at 12.30° N, 76.65° E., and is about 300 km west of Tiruvannamalai, the site of the research reported in Placek & Hagen (2015). Mysore district has over 900,000 living in the urban area, and over 1.6 million people in rural villages (India, 2011).
2.1 Rural farmers
The “Rural farmer” population comprised ten rural farming villages in Mysore Taluk (a subdistrict of Mysore), which were typical of most of the rural population of Mysore. Rural farmers raise livestock (dairy cattle and poultry) and crops (ragi, millet, pulses, groundnuts, fruits, and vegetables) (Divya & Belagali, 2014). Some rural farmers work their own farms whereas others are low-paid agricultural laborers. There were a total of thirteen castes and subcastes represented in our sample of rural farmers. Main categories included Scheduled Tribes (Nayaka), Scheduled Castes, and several others.
2.2 Jenu Kurubas
The Jenu Kurubas, also referred to as the Kattu Nayaka, are former hunter-gatherers who are honey gatherers by tradition. In 1972, the majority of Jenu Kurubas in Mysore, along with others, were displaced from the forest in the name of development by the Indian government and forced to live in small settlements, apart from other castes and tribal populations (Roy, Hegde, Bhattacharya, Upadhya, & Kholkute, 2015) (the other Nayakas in this study live in mixed-caste farming villages). The Jenu Kurubas primarily work as daily wage agricultural laborers and cultivators, and many are involved in tobacco production. They number around 30,000–35,000 members within the state of Karnataka. Our sample lived in five government-protected hamlets in the eastern section of Mysore district.
Tribal populations in India, such as the Jenu Kurubas, are considered to be the most socially and economically disadvantaged members of society (Vijayalakshmi, 2003). They also differ from other castes in terms of health status, social structure, marital patterns, gender equality, and cultural practices related to maternal health (Prabhakar & Gangadhar, 2011; Vijayalakshmi, 2003). One goal of this study was to further investigate similarities and differences between the Jenu Kurubas and the neighboring rural farmers, as well as contribute to the growing literature on cultural transmission in this group (Demps, Zorondo-Rodriguez, García, & Reyes-García, 2012; Demps, Zorondo-Rodríguez, García, & Reyes-García, 2012).
3 Methods
The study was a cross-sectional design. Pregnant women completed an interviewer-administered questionnaire in the local language of Kannada that asked about physiological aversions and cultural avoidances, modes of acquisition for avoidances, and consequences of consuming the culturally proscribed items.
3.1 Participants
We recruited pregnant women (N = 102). Those from rural farming villages (N = 72) were recruited by female Accredited Social Health Activists (ASHA) and Anganwadi workers. ASHA are trained by the National Rural Health Mission in India to liaise with the public health system, help launch public health programs, and educate women in their communities (Mission, 2014). Anganwadi Centres are run by local workers to improve the nutritional status of women and children. Jenu Kuruba women (N = 30) were recruited by Peer Health Educators trained by the Public Health Research Institute of India (PHRII). Due to the health workers’ level of community integration and knowledge of pregnancy status within their respective communities, this sample is likely representative of the pregnant women who live in these rural regions.
Participants were given a small amount of money in accordance with local norms. The Institutional Review Boards at Washington State University and PHRII in Mysore reviewed and approved this study. Literate women provided written informed consent, and the others provided verbal consent and thumbprints to satisfy PHRII IRB requirements.
3.2 Outcome Variables
Aversions
Rating a fixed list of foods has the advantage that all participants rate all foods, but the disadvantage that the list might omit foods that are important for some participants, whereas free-listed foods will likely include all important foods, but not all participants will rate all foods. We therefore used both techniques.
Participants rated each of a fixed set of 31 photographs of foods that were commonly disliked in pregnancy in this region (Placek & Hagen, 2015) and are also thought to be potentially toxic or pathogenic (e.g. meat, vegetables, ESS foods) (Fessler, 2002; Flaxman & Sherman, 2000; Profet, 1995). They reported their preferences before pregnancy and during pregnancy (two ratings per food), using a 3-point scale: 0=dislike, 1=sometimes like, 2=always like. Independent of the photo rating task, informants provided a potential emic negative consequence of consuming each food for most foods depicted in the photos: “abortion” (miscarriage), “heat”, and “kembara”, a local illness that participants often described as difficulty breathing in infant, or red rashes on the infant’s skin. Some foods, which were compiled in a different South Indian population (Placek & Hagen, 2015), did not have identified negative consequences in these populations.
Participants then free-listed foods that they found “physically aversive,” and described the symptoms caused by each food.
Avoidances
Participants free-listed foods they were avoiding because someone told them to, and then described who told them to avoid each food and the consequences if they consumed it.
We coded all aversive and avoided foods according to Flaxman & Sherman (2000) etic food categories: fruits, meat, non-alcoholic beverages, vegetables, alcoholic beverages, ESS foods, dairy/ice cream, sweets, and grains/starches. In addition, we also distinguished nuts/seeds/legumes; miscellaneous foods such as salt; tobacco; and non-foods (e.g., mud). Each food item listed by each participant was included in only one category.
This coding scheme does not easily accommodate dishes that combine foods from two or more categories. An important example is sambar -- a popular spicy lentil-based vegetable stew in south India -- that is often prepared with chicken, fish, or mutton, and could thus be included in the ESS, meat, nuts/seeds/legumes, or vegetable categories. If a woman explicitly mentioned a meat-based sambar, such as “chicken sambar”, we classified the food as a meat, and if she mentioned “dal sambar” (dal is dried pulse), we classified it as nuts/seeds/legumes. Otherwise, we classified “sambar” as an ESS food. See Placek & Hagen (2015) for more details. For the list of the specific foods assigned to each category, see Table S1.
3.3 Explanatory variables
Each participant completed a structured questionnaire that included the following items designed to test the models described in the Study Goals and Predictions section.
3.3.1 Maternal-fetal protection
Month of pregnancy
Self-reported month of pregnancy.
Nausea or vomiting
Self-reported current presence/absence of nausea or vomiting (either=1; neither=0).
Sanitation
Four-item instrument. Two items were from the hand-washing with soap (HWWS) scale (Curtis, Danquah, & Aunger, 2009): In the past seven days, did you HWWS after using the toilet (0=never, 1=sometimes, 2=always)? and before handling food (0=never, 1=sometimes, 2=always)? (We omitted two items that did not apply to primigravida.) The second two items were perception of drinking water cleanliness (unclean=0, clean=1) and existence of a household toilet (no=0 or yes=1). (All toilets were “squat” style.) The total sanitation score was the sum of Z-scores of the HWWS, clean water, and toilet items.
Perceived susceptibility to disease
Three items from the 7-item perceived infectability measure (Duncan & Schaller, 2009): “In general, I am very susceptible to colds, flu and other infectious diseases;” “My immune system protects me from most illnesses that other people get;” and “I have a history of susceptibility to infectious disease.” (Four items were omitted because they did not translate into Kannada.) The score was the sum of all items, which were on a 3-point scale (0=strongly disagree, 1=sometimes agree, 2=strongly agree).
3.3.2 Exploratory Pathogen Avoidance Model
Trimester
Computed from month of pregnancy (see above).
Household size
Self-reported number living in the household.
3.3.3 Dietary diversity
Food insecurity
The 6-item short-form household-level food insecurity measure, which assesses one’s access to sufficient foods, is reliable and valid (Blumberg, Bialostosky, Hamilton, & Briefel, 1999), has been used in other studies in India (Agarwal et al., 2009; Ghosh-Jerath et al., 2013), and has been used in previous investigations of dietary shifts in pregnancy (Placek & Hagen, 2013, 2015). The 6 items were summed. Higher scores indicate greater food insecurity.
3.3.4 Social Learning Model
Diet advice
“To whom do you go to for advice regarding your diet during pregnancy?” Participants free-listed advisors, and the dietadvice score was the number of advisors.
Diet pressure
“Does anyone pressure you to follow certain guidelines for health during pregnancy? If so, who?” The pressure score was the number of free-listed individuals pressuring each participant.
Pregnant social partners
Mothers might acquire avoidances from pregnant social partners. We therefore asked, “Are any of your sisters or friends pregnant?” (yes=1, no=0).
We asked each participant if she knew someone who experienced adverse pregnancy outcomes from consuming a particular food, but only 3 did, so we did not analyze this variable further.
3.3.5 Sociodemographic model
Age
Age in years. Because several Jenu Kuruba women did not know their precise age, research assistants estimated them using year of marriage and year of first pregnancy.
Education
Years of education.
Population
Jenu Kurubas=0, Rural farmers=1.
3.4 Analyses
We tested our a priori models as follows: For each scientific food category, we coded each woman as 1 if she was averse to any food in that category, and 0 otherwise. We then used logistic regression to fit each of our models (Table 1) to the presence/absence of an aversion in each of the top three or four most commonly aversive scientific food categories. For each food category, we ranked the seven models using the Akaike information criterion (AIC), corrected for finite sample sizes (AICc), and then report the top-ranked model (Burnham & Anderson, 2003). We conclude that a model is supported if it is the top AIC-ranked model for the target food(s), and unsupported otherwise. We did the same for the common food avoidances. Akaike weights for each model are reported in the supplementary material. We also explored broad patterns of aversions and the consequences of consuming various foods using hierarchical cluster analysis and by plotting our data.
Table 1.
Variables included in each of the 7 logistic regression models used to test our a priori hypotheses of aversions and avoidances. The Target scientific food categories are those that are predicted to be aversive or avoided according to that model.
| Variable | Null | Demographic | Fetal protection | Pathogen avoidance | Exploratory pathogen avoidance | Social learning | Dietary diversity |
|---|---|---|---|---|---|---|---|
| Target scientific food category | Any | Any | ESS, Vegetable, Meat | Meat | Meat | Fruit; most commonly avoided foods | Staple foods (grains, legumes) |
| Population | X | X | X | X | X | X | X |
| Age | X | ||||||
| Education | X | ||||||
| Trimester | X | X | X | ||||
| Nausea/Vomiting | X | X | |||||
| Disease Vuln. | X | ||||||
| Sanitation | X | ||||||
| Food insecurity | X | ||||||
| Diet advice | X | ||||||
| Diet pressure | X | ||||||
| Preg. social partner | X | ||||||
| Household size | X |
Continuous variables were centered at their means and divided by two standard deviations so that regression coefficients represent a 2 SD change, roughly from “low” to “high” values, and are directly comparable to those of binary variables with equal class probabilities, which have a standard deviation of 0.5 (Gelman, 2008). Our main binary variable, Population (Rural farmers vs. Jenu Kurubas), did not have equal numbers of participants in both groups, but its standard deviation was 0.46, which is reasonably close to 0.5. For logistic regression models we report adjusted odds ratios (OR) and Tjur’s coefficient of discrimination (Tjur’s D) (Tjur, 2009). Tjur’s D equals zero when the model does not discriminate between the two classes, and equals one when it discriminates perfectly. We report 95% confidence intervals on all parameters, and chose α = 0.05.
Statistical analyses and document preparation were conducted with R version 3.3.2 (2016-10-31), using the following packages: AICcmodavg (Mazerolle, 2016), binomTools (Christensen & Hansen, 2011), the heatmap function from NMF (Gaujoux & Seoighe, 2010), ggplot2 (Wickham, 2009), and knitr (Xie, 2015).
4 Results
Summary statistics of the explanatory variables for the two populations are in Table 2.
Table 2.
Summary statistics of the explanatory variables by population. Cohen’s d is the effect size of the difference between the two populations; p is the result of a Wilcoxon rank test. Sorted by the absolute value of d. See the Methods section for the definition of each variable.
| Rural farmers | Jenu Kuruba | Effect size | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | N | Min | Max | Mean | SD | N | Min | Max | Mean | SD | d | p |
| Nausea or vomiting (0=No; 1=Yes) | 72 | 0.0 | 1.0 | 0.78 | 0.42 | 30 | 0.0 | 1.00 | 0.77 | 0.43 | 0.027 | 0.91 |
| Proportion of disliked foods (31 photos) | 72 | 0.42 | 0.9 | 0.66 | 0.11 | 29 | 0.29 | 0.94 | 0.68 | 0.14 | −0.120 | 0.46 |
| Age (years) | 72 | 18.00 | 33.0 | 22.00 | 3.10 | 30 | 18.00 | 30.00 | 23.00 | 2.90 | −0.130 | 0.41 |
| Months pregnant | 72 | 3.00 | 9.0 | 6.20 | 1.90 | 30 | 2.50 | 9.00 | 5.80 | 1.70 | 0.220 | 0.27 |
| Household size | 72 | 3.00 | 30.0 | 5.70 | 3.60 | 30 | 3.00 | 14.00 | 6.50 | 2.60 | −0.250 | 0.04 |
| Perceived susceptibility to disease score | 72 | 1.00 | 4.0 | 2.20 | 0.64 | 30 | 1.00 | 4.00 | 2.50 | 0.73 | −0.330 | 0.13 |
| Total dietary pressure | 72 | 0.00 | 3.0 | 0.76 | 0.81 | 30 | 0.00 | 3.00 | 1.10 | 0.96 | −0.400 | 0.10 |
| Parity | 72 | 0.00 | 2.0 | 0.62 | 0.70 | 30 | 0.00 | 2.00 | 0.93 | 0.83 | −0.420 | 0.08 |
| Mean number of free-listed food aversions | 72 | 0.00 | 5.0 | 1.80 | 1.20 | 30 | 0.00 | 4.00 | 0.93 | 1.20 | 0.710 | 1.1e-03 |
| Education (years) | 72 | 0.00 | 15.0 | 8.80 | 3.60 | 30 | 0.00 | 12.00 | 6.00 | 3.70 | 0.780 | 6.4e-04 |
| Sanitation score | 72 | −4.90 | 2.6 | 0.50 | 2.00 | 30 | −4.90 | 2.60 | −1.20 | 1.80 | 0.880 | 8.5e-05 |
| Food insecurity score | 72 | 0.00 | 6.0 | 1.20 | 2.10 | 30 | 0.00 | 6.00 | 3.70 | 2.50 | −1.100 | 1.8e-05 |
| Total dietary advice | 72 | 1.00 | 4.0 | 2.70 | 0.65 | 30 | 0.00 | 4.00 | 1.90 | 0.88 | 1.100 | 9.6e-06 |
| Mean number of free-listed food avoidances | 72 | 0.00 | 7.0 | 4.10 | 1.50 | 30 | 0.00 | 5.00 | 1.30 | 1.40 | 1.900 | 0.0e+00 |
There were small, non-significant differences between the two populations in mean age, months pregnant, experience of nausea or vomiting, and perceived susceptibility to disease, and the Jenu Kurubas had marginally higher parity and dietary pressure than the rural farmers. There were large, highly significant differences in several variables: Rural farmers received more dietary advice from others and avoided more foods, and had more education and better sanitation (and thus lower exposure to pathogens), whereas the Jenu Kurubas had higher levels of food insecurity. In the combined sample, 57% did not have a toilet in the house. Using the established cutoff (food insecurity score ≥ 5 on a 6 point scale; Blumberg et al., 1999), 31.4% of women were experiencing food insecurity with hunger, the most extreme category.
4.1 Food photo aversion ratings
Comparing the pregnancy ratings to the pre-pregnancy ratings, 41 (40%) women reported a shift in preferences for one or more foods, with 24 (24%) reporting a new disliking for one or more foods in pregnancy, and 26 (26%) a new liking for one or more foods in pregnancy. The most common newly disliked foods were fruits (9), which we had predicted for food taboos but not for aversions. ESS foods were the second most common newly disliked foods (6), consistent with the maternal-fetal protection from plant teratogen model. Meats were only the seventh most newly disliked foods, inconsistent with the maternal-fetal protection from pathogen model. The most common newly liked foods were fruits (15) and grains (7).
For any specific scientific food category, the number of participants who newly disliked a food in that category was low, perhaps because these foods were compiled in a different South Indian population (Placek & Hagen, 2015). We therefore restricted further analyses to the pregnancy ratings only, regardless of pre-pregnancy ratings.
We report the top AIC-ranked logistic regression model (Table 3) for the three most commonly aversive scientific food categories: nuts/seeds/legumes, sweets, and grains. For all AICc values and Akaike weights, see Table S2.
Table 3.
Logistic regression models of the three most common aversions in the food photo ratings. Displayed are top AIC-ranked models of each aversion. Coefficients are odds ratios (95% CI). Jenu Kurubas are the base level for Population. The last row indicates if the top-ranked model for that food aversion supports an a priori prediction.
| Variable | Nuts/seeds/legumes | Sweets | Grains |
|---|---|---|---|
| Population (Rural farmer) | 2.72 (1.32, 5.78) | 1.01 (0.4, 2.6) | 0.733 (0.398, 1.35) |
| Food insecurity (centered) | 1.94 (0.986, 3.99) | ||
| Age | 1.07 (0.933, 1.23) | ||
| Education | 0.909 (0.806, 1.02) | ||
| Observations | 102 | 102 | 102 |
| Null deviance (df) | 152 (101) | 140 (101) | 93.9 (101) |
| Residual deviance (df) | 144 (99) | 136 (98) | 92.9 (100) |
| Chisqr | Chisq (2) = 7.98* | Chisq (3) = 4.74 | Chisq (1) = 0.988 |
| AIC | 207.3 | 143.7 | 187 |
| Tjur’s D | 0.039 | 0.046 | 0.0049 |
| Supports a priori prediction | Yes | No | No |
Dietary diversity was the top-ranked model of aversions to a staple food category, nuts/seeds/legumes, as predicted, but it had a very small effect size. The Demographic and Null models best predicted aversions to sweets and grains, respectively, but neither effect size was scientifically meaningful.
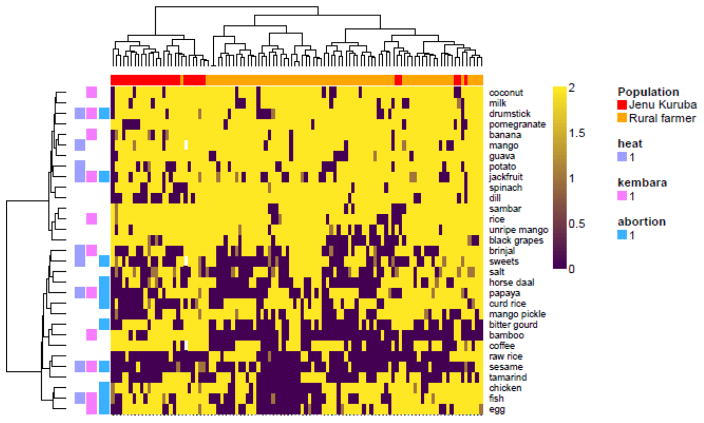
It was possible that food preferences involved groups of foods that cut across our scientific categories. To explore this possibility we used hierarchical cluster analysis, which identified a group of foods that were more often liked (Figure 1, top), and a group of foods that were mostly disliked (Figure 1, bottom). The disliked foods were significantly more often identified as causing abortion than the liked foods (χ2 = 6.23, p = 0.013), consistent with the maternal-fetal protection hypothesis, but there were no significant differences in heat or kembara between the liked and disliked food groups (results not reported). Chicken, fish, and egg (animal products), formed a distinct cluster of unliked foods that were thought to cause abortion, consistent with the pathogen avoidance model.
Figure 1.
Heatmap of ratings of photos of foods (rows) by each participant (columns). Rows and columns clustered using the Euclidean metric and the Ward agglomeration algorithm. Do not like: 0 (dark purple); Sometimes like: 1 (dark yellow); Always like: 2 (bright yellow). Columns annotated by population. Jenu Kurubas: red. Rural farmers: orange. Rows annotated with the emic potential negative consequences of eating that food during pregnancy. Not all foods had identified negative consequences. White cells indicate missing data. The top cluster of foods were generally liked, and the bottom were generally disliked. The bottom cluster included significantly more foods thought to cause abortion (see text).
The cluster analysis also revealed that the two populations have distinct dietary preferences. The left cluster was mostly Jenu Kuruba women, and the right cluster was mostly rural farmer women. Jenu Kurubas mostly liked bitter gourd and bamboo whereas rural farmers mostly did not. Jenu Kurubas also mostly disliked dill whereas the rural farmer women liked it (Figure 1).
4.2 Free-listed aversions
Participants free-listed 156 aversive foods, 47 of which were unique. Grains (primarily rice, a staple) were the most common aversion (29% of participants), consistent with the dietary diversity model, followed by nuts/seeds/legumes (23%). Aversions to ESS foods (16%), primarily sambar, were also common, consistent with the fetal protection model, as were aversions to meat (16%) consistent with pathogen avoidance.
We used AICc to rank our seven logistic regression models (Table 1) for the presence/absence of aversions to the top four food categories. The Maternal-Fetal Protection model was the highest-ranked model of ESS foods, as predicted, and was also the highest ranked model of nuts/seeds/legumes, contrary to predictions; both models had small-to-modest effect sizes. Grain aversions were best predicted by the Exploratory pathogen avoidance model, contrary to predictions, and meat by the Null model, but the effect sizes were not scientifically or statistically significant for either model. See Table 4.
Table 4.
Top AIC-ranked logistic regression models of the four most common aversions among the free-listed foods. Coefficients are odds ratios (95% CI). Jenu Kurubas are the base level for Population; no nausea or vomiting is the base level for nausea or vomiting. The last row indicates if the top-ranked model for that food aversion supports an a priori prediction.
| Variable | ESS | Grains | Meat | Nuts/seeds/legumes |
|---|---|---|---|---|
| Population (Rural farmers) | 0.76 (0.219, 2.81) | 0.952 (0.365, 2.62) | 1.98 (0.58, 9.16) | 14.2 (2.67, 264) |
| Trimester | 0.194 (0.0736, 0.445) | 0.815 (0.434, 1.53) | 1.01 (0.485, 2.17) | |
| Nausea or vomiting | 2.51 (0.559, 18.2) | 4.67e7 (4.97e-24, NA) | ||
| Household size (centered) | 3.73 (1.33, 13.7) | |||
| Observations | 102 | 102 | 102 | 102 |
| Null deviance (df) | 88.6 (101) | 124 (101) | 88.6 (101) | 109 (101) |
| Residual deviance (df) | 70.6 (98) | 116 (98) | 87.5 (100) | 83.3 (98) |
| Chisqr | Chisq (3) = 18*** | Chisq (3) = 7.53 | Chisq (1) = 1.12 | Chisq (3) = 25.6*** |
| AIC | 78.59 | 124.1 | 91.51 | 91.27 |
| Tjur’s D | 0.23 | 0.079 | 0.01 | 0.2 |
| Supports a priori prediction | Yes | No | No | No |
4.3 Free-listed food avoidances
Participants avoided 333 foods, 55 of which were unique. Fruits (primarily papaya and jackfruit) were the most commonly avoided food category (70%), the pattern found by Placek & Hagen (2015), followed by vegetables (52%), nuts/seeds/legumes (41%), and meat (41%).
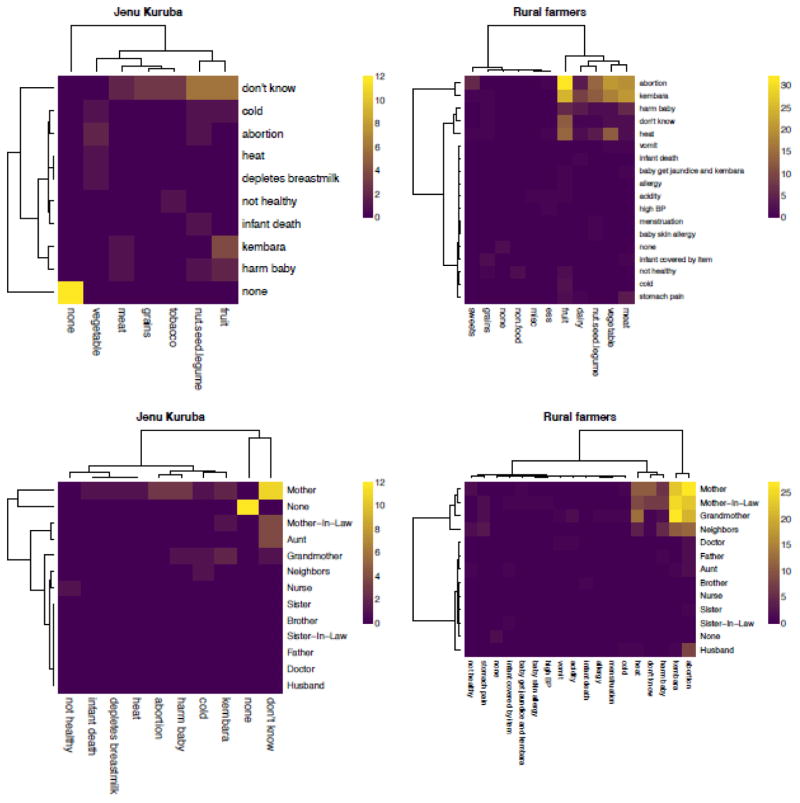
All self-reported reasons for avoiding foods involved negative health outcomes, primarily abortion or kembara, which were frequently linked with fruit; harm to baby and “heat” were important secondary concerns. “Don’t know” was also a common response, especially among the Jenu Kurubas, who also often did not report avoiding any foods based on advice from others, which might indicate some form of individual learning. No reasons for avoiding foods involved resource conservation, protecting the environment, or identity. See Figure 2, top row.
Figure 2.
Top row: Heatmaps showing the consequences of eating avoided foods, by population. Bottom row: Sources of advice about negative consequences of eating avoided foods, by population. The color of each cell represents the count of participant responses for that combination of row and column variables (cross-tabulations). Row and column clusters were computed with the Euclidean metric and the Ward agglomeration algorithm.
The Social Transmission model was the highest ranked model of fruit avoidances, as predicted, with a small-to-modest effect size. None of the other models supported our predictions: Vegetable avoidances were best predicted by the Dietary Diversity model, with a statistically significant effect of modest size; meat avoidances were best predicted by the Null model (population only), with a statistically significant but small effect; and nuts/seeds/legumes were best predicted Null model, but the effect was small and not statistically significant. See Table 5. For all AICc values and Akaike weights, see Table S3.
Table 5.
Top AIC-ranked logistic regression models of the four most common avoidances among the free-listed foods. Coefficients are odds ratios (95% CI). Jenu Kurubas are the base level for Population; None is the base level for Pregnant social partners. The last row indicates if the top-ranked model for that food avoidance supports an a priori prediction.
| Variable | Fruit | Nuts/seeds/legumes | Vegetables | Meat |
|---|---|---|---|---|
| Population (Rural farmers) | 4.81 (1.65, 14.5) | 1.97 (0.814, 5.08) | 27.9 (7.14, 159) | 7.26 (2.52, 26.5) |
| Total diet advice (centered) | 5.2 (1.78, 17) | |||
| Total pressure (centered) | 0.877 (0.326, 2.42) | |||
| Pregnant social partners (yes) | 0.786 (0.241, 2.69) | |||
| Food insecurity (centered) | 3.03 (0.943, 13.1) | |||
| Observations | 102 | 102 | 102 | 102 |
| Null deviance (df) | 125 (101) | 138 (101) | 141 (101) | 138 (101) |
| Residual deviance (df) | 95 (97) | 136 (100) | 110 (99) | 123 (100) |
| Chisqr | Chisq (4) = 30.3*** | Chisq (1) = 2.24 | Chisq (2) = 30.9*** | Chisq (1) = 15.1*** |
| AIC | 105 | 140 | 116.3 | 127.2 |
| Tjur’s D | 0.29 | 0.021 | 0.27 | 0.13 |
| Supports a priori prediction | Yes | No | No | No |
4.3.1 Sources of food avoidances
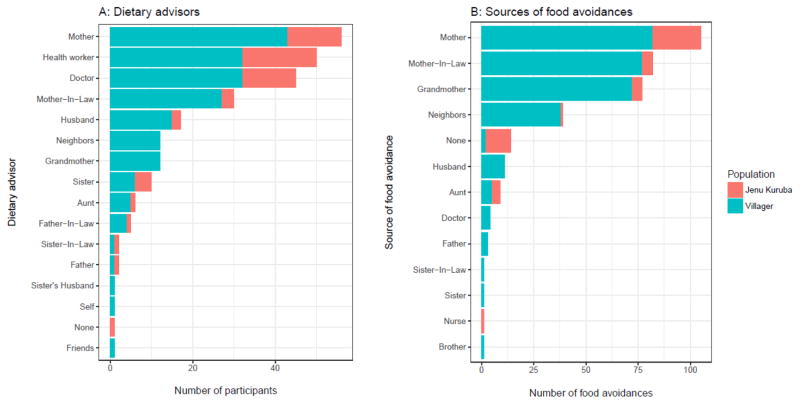
Women listed all individuals that gave them dietary advice during pregnancy (M = 2.5, SD = 0.8), who we termed “dietary advisors.” The top four most frequently mentioned advisors were mothers, health workers, doctors, and mothers-in-law, who together accounted for 61% of all nominated advisors. For each free-listed avoided food we then asked participants who, if anyone, told them to avoid that food. Mothers, grandmothers, and mothers-in-law were the primary sources of advice against eating specific avoided foods, with abortion and kembara the main reasons among rural farmers (Figure 2, bottom row). These female relatives were responsible for 75.9% of all food avoidances. Combined, all family members were responsible for 83.3% of food avoidances.
Participants did not free-list any dietary advisor that was the equivalent of the “wise women” reported by J. Henrich & Henrich (2010), but doctors, nurses, and health workers are plausibly interpreted as prestigious, knowledgeable sources of dietary information. Combined, these latter sources were responsible for 1.4% of food avoidances. See Figure 3.
Figure 3.
A: Dietary advisors (free-listed). B: The number of food avoidances acquired from each source (free-listed).
We coded dietary advice from fathers, mothers, and grandmothers as vertical transmission; from siblings, siblings-in-law, husbands and friends as horizontal transmission; from parents-in-law and aunts/uncles as oblique transmission; and from everyone else, such as neighbors, health-workers and others of indeterminate age, as horizontal/oblique transmission. Then, across all participants, we computed the percent of “Dietary advisors” and “Food avoidances” that were vertical, horizontal, oblique, horizontal/oblique, or none (i.e., personal experience only). See Figure S1.
The main difference between populations was the importance of personal experience (the “none” category), which was the source of 24% of Jenu Kuruba food avoidances, but only about 1% of Rural farmer food avoidances. See Figure 3 and Figure S1.
4.4 Comparing free-listed aversions and avoidances
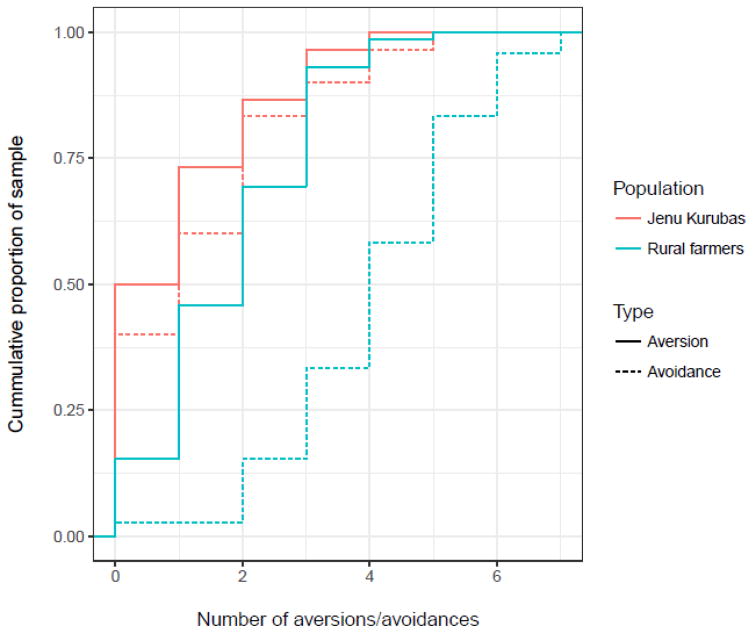
Participants avoided more than twice as many foods as they found aversive (333 vs. 156). Although the distributions of number of aversions were similar in each population (Figure 4), 15 (50%) of the 30 Jenu Kuruba women were averse to at least one food, whereas 61 (85%) of the 72 rural farmer women were averse to at least one food, a significant difference (χ2 = 13.4, 3 = 2.5 × 10−4).
Figure 4.
Cumulative distributions of numbers of aversions and avoidances by population.
Among the Jenu Kurubas, the distribution of number of avoidances was similar to that of aversions. Among the rural farmer women, however, there were many more avoidances (Figure 4), indicating an important difference in this culturally transmitted repertoire. Specifically, 18 (60%) Jenu Kurubas avoided at least one food, whereas 70 (97%) rural farmers avoided at least one food, a significant difference ((χ2 = 24.8, 3 = 6.4 × 10−7).
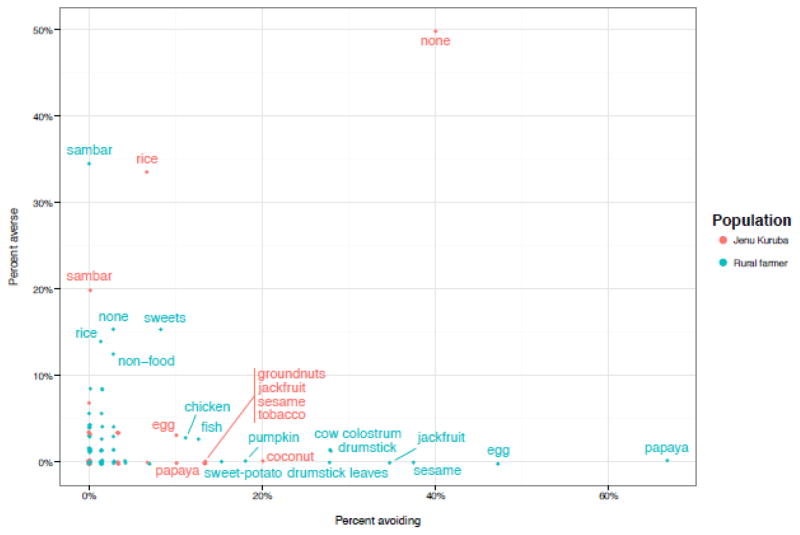
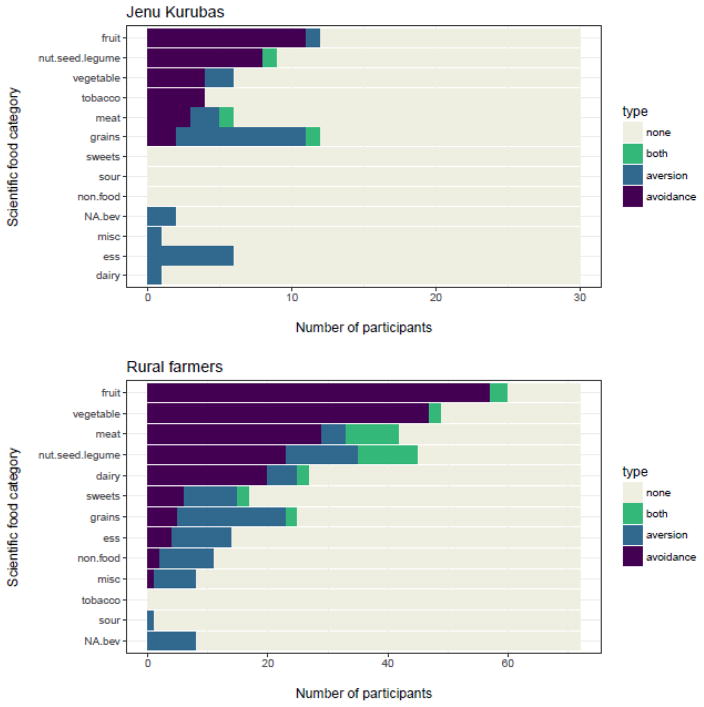
Aversive foods were rarely avoided, and avoided foods were rarely aversive (Figure 5), and few women were both averse to, and avoidant of, foods in the same scientific category (green bars, Figure 6). Note that most women were not averse to, and did not avoid, most food categories (Figure 6), and if they did avoid foods in a category, such as fruits, it was only one or two foods in the category and not all foods. For a list of specific foods avoided in each category, see Table S1.
Figure 5.
Free-listed food aversions vs. avoidances: x- and y-values were the percent of each population (Jenu Kurubas vs. Rural farmers) avoiding, or averse to, each food. Food labels displayed for foods that were avoided by, or aversive to, at least 10% of women in each population. “None” indicates the proportion of women in each population that either did not avoid, or were not averse to, any food.
Figure 6.
Distribution of avoidances and aversions by scientific food category and population. Sorted by the number of avoidances in each scientific category.
Finally, whereas the number of aversions decreased by month of pregnancy, the number of avoidances increased (Table S4 and Figure S2).
5 Discussion
This study examined both pregnancy aversions and avoidances in two neighboring but culturally distinct populations that experienced considerably greater resource stress and infectious disease burden than typically found in high income populations. The Jenu Kuruba women reported higher food insecurity and less education than rural farmer women, however, worse sanitation, fewer food avoidances, and less dietary advice.
The clearest support emerged for the theories proposing that aversions and avoidances both protect fetuses and mothers from foods high in potentially teratogenic plant secondary compounds, yet aversive and avoided foods were largely distinct. There was weaker and less consistent support for theories emphasizing protection from meat-borne pathogens and dietary diversification.
5.1 The function(s) of pregnancy food aversions
We tested 7 a priori models of the presence/absence of aversions and avoidances of foods grouped into 11 scientific categories (e.g., meat, vegetables).
5.1.1 Maternal-fetal protection
In the food photo rating study, the most common foods that were newly disliked in pregnancy were ESS foods (usually sambar, a spicy vegetable stew), as predicted, and fruits, similar to Placek & Hagen (2015). Although fruits are not typically viewed as containing high levels of plant secondary compounds, many do, even when ripe, and many contain latexes, which are potent allergens and can cause anaphylaxis during pregnancy (Cipollini & Levey, 1997; Placek & Hagen, 2015). In addition, compared to the “liked” food cluster (Figure 1, top), the “unliked” food cluster (Figure 1, bottom) contained significantly more foods thought to cause abortion.
Contrary to the maternal-fetal protection model, the top three aversions in the food photos (not necessarily new aversions) were grains, nuts/seeds/legumes, and sweets, which are either non-meat staple foods or do not contain teratogens (sweets), and none were well predicted by any of our logistic regression models.
For the free-listed aversions, the maternal-fetal protection logistic regression model was the highest AIC-ranked model of ESS foods (Table 4), as predicted, and also of nuts, seeds, and legumes, with moderate effect sizes (Tjur’s D = 0.23 and 0.2, respectively). Although nuts/seeds/legumes were not an a priori food target for fetal protection, seeds and nuts are plant reproductive organs that are often chemically defended (Zangerl & Bazzaz, 1992). The latter result therefore provides some exploratory (not confirmatory) support for the maternal-fetal protection hypothesis.
Taken together, these results support teratogen avoidance as one function of pregnancy food aversions (Profet, 1995), similar to other recent studies (McKerracher, Collard, & Henrich, 2016; Mckerracher, Collard, & Henrich, 2015).
5.1.2 Pathogen avoidance
In our sample of women, 57% of whom did not have a household toilet (indicating low levels of sanitation and high pathogen exposure), meats formed a distinct cluster in the food photo ratings (and all were thought to cause abortion), and were among the top four aversive free-listed foods, consistent with the pathogen avoidance model (Flaxman & Sherman, 2000; Fessler, 2002). Only 16 (16%) were averse to meat, however, and meat aversions were not well predicted by any of our logistic regression models (Table 4), including our pathogen avoidance model, which assessed sanitation and perceived vulnerability to disease. We also did not replicate our previous finding that trimester and household size predicted meat aversions (Placek & Hagen, 2015).
This mixed support for the pathogen avoidance model could partly be due to the high rate of vegetarianism in India (Flood, 1996), including in our rural farmer population, or inability to afford meat, as in the Jenu Kurubas. If women are already less likely to consume meat prior to becoming pregnant, then pregnancy meat aversions might be unnecessary to protect the fetus. Our sample was also biased toward women in later pregnancy, whereas meat aversions are expected earlier in pregnancy.
5.1.3 Dietary diversity
In our sample, 31.4% of the women were experiencing food insecurity with hunger and the staple food category grains (primarily rice) was among the top aversive categories in both the food photos and free-listed foods, as predicted by the dietary diversity model, and a pattern seen in other populations (Steinmetz, Abrams, & Young, 2012; A. G. Young & Pike, 2012). Dietary diversity was also the top AIC-ranked model of aversion to nuts, seeds, and legumes in the food photos, as predicted by this model, but the effect size was too small to be scientifically significant (Table 3).
Aversions to staple foods could support the idea that pregnancy food aversions function to increase dietary diversity (Coronios-Vargas, Toma, Tuveson, & Schutz, 1992), but these aversions were not associated with food insecurity, contrary to predictions. Alternatively, because grain dust can contain pesticides and mycotoxins, which can lead to early labor in pregnancy and other adverse health outcomes (Douwes, Thorne, Pearce, & Heederik, 2003; Kristensen, Irgens, Anderson, Bye, & Sundheim, 1997), this pattern could support Profet’s (1995) theory that pregnancy aversions protect against plant teratogens. In summary, our study provided mixed support for the dietary diversification model.
5.1.4 Sociodemographics
Our sociodemographic model, comprising population, age, and education, was the highest AIC-ranked model of aversion to sweets in the food photos, but the effect size was small and not scientifically significant (Table 3). Our study therefore did not find support for the role of these sociodemographic variables in aversions to any food category, contrary to some previous studies (Drewnowski, 1997; Sanjur, 1982).
5.2 The function of pregnancy food taboos
Considering the theoretical and empirical attention paid to pregnancy aversions in most studies, striking results of our study included that (1) participants, especially rural farmers, reported more than twice as many food avoidances as food aversions (Figure 4); (2) avoidances shaped diet throughout pregnancy (Figure S2); (3) fruits were most avoided (Figures 2 & 6); (4) avoided foods were largely distinct from aversive foods (Figure 5); and (5) the emic function of pregnancy food avoidances was almost always to protect the fetus from harm, with abortion and kembara the most frequently listed types of fetal harm, and “heat” also common (Figure 2). An important caveat is that some mothers, especially among the Jenu Kurubas, could not identify a function for a particular avoidance, or claimed there was none (Figure 2).
As predicted, social learning was the top AIC-ranked model of fruits (Table 5), the most frequently avoided food category (70% of women avoided fruit). Papaya, the primary avoided fruit (and food) in this study (Figure 5), is a known abortifacient that is widely used to terminate pregnancy across South and Southeast Asia (Anuar, Zahari, Taib, & Rahman, 2008; Boer & Cotingting, 2014; Odirichukwu, 2015), and is also widely avoided in pregnancy (Nag, 1994; Placek & Hagen, 2015; Van Hollen, 2003). Jackfruit, avoided by many women in this study, is also linked to abortion (Morton, 1987; Visaria, Ramachandran, Ganatra, & Kalyanwala, 2004). Vegetables and nuts/seeds/legumes, which often contain plant teratogens, were also frequently avoided (Figure 6).
Overall, our results strongly support the basic social transmission model of food taboos that emphasizes avoidance of dangerous foods (Aunger, 1994; Boyd & Richerson, 1985; Cashdan, 1994; Fessler & Navarrete, 2003), especially during pregnancy (e.g., J. Henrich & Henrich, 2010). None of the emic reasons (Figure 2) were consistent with limiting resource consumption to protect the environment (Harris, 1998) marking social identity (Whitehead, 2000), or any other function.
Meat avoidances were common (41% of women avoided meat), supporting the argument that meat is “good to taboo” (Fessler & Navarrete, 2003), but were not associated with pathogen exposure. Instead, they were best explained by the Null model (population only; Table 5), although the Akaike weight for the Fetal protection model (sans pathogen exposure variables) was nearly as large (Table S3). In either case, the effect size was small. Eggs were the most avoided meat (Figure 5), probably reflecting concerns over Salmonella contamination, which is common in South India (Suresh, Hatha, Sreenivasan, Sangeetha, & Lashmanaperumalsamy, 2006). Fessler & Navarrete (2003) also noted that food taboos might be manipulated to benefit certain group members at the expense of others. Pregnant and nursing women have increased caloric requirements. Conceivably, pregnancy meat taboos might restrict access to this valuable resource by some pregnant women, to the benefit of other group members. See the supplementary information for a brief exploratory test of this hypothesis.
Dietary diversity was also the top AIC-ranked model of avoidance of vegetables, with a moderate effect size (Table 5), but vegetables were not an a priori target food for this model: this category contained a staple food -- potatoes -- but also several non-staple foods that would contribute to dietary diversity (only 1 woman was averse to potatoes, and 2 avoided them).
Although the distribution of numbers of aversions was similar in both populations, the rural farmers had many more avoidances (Figure 4). Higher food insecurity might have predisposed Jenu Kurubas to avoid fewer foods, but their food insecurity scores did not significantly correlate with the number of avoidances (r = 0.18, p = 0.34). Given the relatively short amount of time the Jenu Kurubas have spent in their settlements, it is possible that they have not culturally evolved the repertoire of avoidances that rural farmers have.
5.2.1 Sources of food taboos
Immediate family members were responsible for 83.3% of all specific dietary avoidances, with most, 75.9%, acquired vertically from mothers and grandmothers, and obliquely from mothers-in-law, relatives with a high degree of relatedness to the infant. Although mothers did not free-list any equivalent of older “wise” women outside the family, doctors and health workers were frequently listed as general dietary advisors, consistent with a prestige bias, yet combined they were only responsible for 1.4% of specific food avoidances, contrary to a prestige bias. See Figure 3.
This heavy reliance on familial sources of dietary advice was strikingly similar to the pattern found by J. Henrich & Henrich (2010), who nevertheless argued for the important influence of prestigious, knowledgeable older women outside the immediate family. J. Henrich & Henrich (2010) explain this discrepancy as follows: when the cultural evolutionary process is at or near equilibrium, mothers should learn from easily accessible “low cost” family members who have an incentive to help kin, turning to outside sources when far from equilibrium. The Jenu Kurubas, however, are arguably further from equilibrium than rural farmers, yet relied even more heavily on their mothers (Figure 2). In addition, our informants stated that women in our study populations would be expected to follow advice from family members not because they would be more convenient but because they would be more trusted than non-family members.
Mothers-in-law were more influential among rural farmers than Jenu Kurubas (Figure 2 and Figure 3). Rural farmers are traditionally patrilineal and patrilocal, with arranged marriages (Suchitra & Swaminathan, n.d.). In patrilocal Indian societies, mothers-in-law are known to play an influential role in women’s health and reproductive decision making (Chandran, Tharyan, Muliyil, & Abraham, 2002; Char, Saavala, & Kulmala, 2010). The Jenu Kurubas have “love marriages” and commonly elope, however, and although this tribe is patrilineal, they are neolocal. Neolocality might explain the reduced influence of mothers-in-law among the Jenu Kurubas.
For a brief discussion of the relative importance of vertical, oblique, and horizontal transmission, see the supplementary material.
In summary, our results provided only mixed support for a prestige bias. Future research on pregnancy dietary advisors should investigate the role of biological relatedness to the infant, trust, and if mothers are selecting among their relatives based on perceived expertise.
5.3 The relationship between aversions and avoidances
Individual women rarely reported aversions and avoidances of the same specific foods (Figure 5), and even infrequently reported aversions and avoidances of the same scientific food categories (green bars in Figure 6). In part, this is because most women reported only 1–2 aversive foods (Figure 4). Aunger (1994) identified 15 types of food taboos among ethnic groups living in the Ituri Forest, only three of which involved personal avoidances due to aversive reactions to the food or other idiosyncratic reasons. J. Henrich & Henrich (2010) found no connection between pregnancy food taboos and pregnancy aversions. Thus, despite evidence presented here that aversions and avoidances both protect mothers and fetuses from plant teratogens, aversive and avoided foods seem to be largely distinct. These results cast doubt on scenarios in which common aversions become common avoidances, at least in these populations.
It is possible that more idiosyncratic aversions could culturally evolve into common avoidances via various mechanisms (Fessler & Navarrete, 2003). However, most rural farmer women identified harm to the fetus or infant as the reason for a food avoidance, as did a number of Jenu Kuruba women. For example, although no woman free-listed an aversion to papaya, 51 women avoided it, all but 6 of whom identified abortion or other fetal harm as the reason. Fetal harm could be a post hoc justification (Fessler & Navarrete, 2003), but we think it is more consistent with generic individual and social learning models (e.g., Boyd & Richerson, 1985) in which individuals learn an association between consumption of a particular food and a poor pregnancy outcome (e.g., “papaya caused my miscarriage”) and then transmit this information to others, independent of psychophysiological food aversions.
Humans have numerous adaptations to detect toxins, such as bitter taste receptors, nausea, and vomiting. Some substances that are highly toxic are not teratogenic, however, some that have low toxicity are potent teratogens, and some that are teratogenic in one species are not teratogenic in other species. Thalidomide, for example, which belongs to the same chemical family as natural plant glutarimide alkaloids, is tasteless, has low toxicity, is not teratogenic in rodents, and is antiemetic, so it was widely prescribed to pregnant women to treat nausea and vomiting. Tragically, thalidomide turned out to be a potent human teratogen that caused severe birth defects in thousands of children (R. C. Gupta, 2017).
There might therefore have been a selection pressure for adaptations to learn associations between consuming certain foods and poor pregnancy outcomes, independent of bitterness, nausea, and other cues of toxicity (for more discussion, see Hagen et al., 2013; Hagen & Sullivan, forthcoming; Placek & Hagen, 2015). Such learned associations would presumably be accurate only if the poor pregnancy outcome occurred relatively soon after consumption of a particular food (e.g., within a few days). Socially transmitted warnings about such dangerous foods might or might not include the reason (e.g., “do not eat papaya because it causes miscarriage” vs. “do not eat papaya”). These warnings gain moral weight, we propose, because older women with a direct fitness interest in a good pregnancy outcome, such as mothers, grandmothers, and mothers-in-law, enforce them.
In our view, the rural farmer results are more parsimoniously explained by individually and socially learned associations with poor pregnancy outcomes than by theories that root food avoidances in learned associations with aversive reactions. This is not so different from the US and other populations in which pregnant women are advised to avoid dangerous foods that might not be aversive, such as fish with high levels of methylmercury, a neurotoxin that can disrupt neural development at very low doses (Mahaffey et al., 2011).
Other results are more consistent with Fessler & Navarrete (2003). Many Jenu Kuruba women could not provide a reason for an avoidance, for example (Figure 2), and the “unliked” food cluster (Figure 1) contained taboo foods that were thought to cause abortion, suggesting a link between aversive and taboo foods. (There were also some discrepancies between the food photo ratings and the free-listed aversions, with some women not liking a food but not free-listing it as aversive. This is probably because in the food photo task women only indicated if they liked or disliked a food, and disliking might not involve a physical aversion, whereas in the free-listed aversion task women only included foods that were physically aversive.) It is likely that aversive reactions and learned associations with poor pregnancy outcomes each play a role in the origins and cultural evolution of pregnancy food taboos.
5.4 Limitations
This study was cross-sectional so it is impossible to know how individual women’s dietary preferences varied over the course of pregnancy. The sample was not a probability sample, and therefore might not accurately represent dietary choices of pregnant women in these populations. Effect sizes for top-ranked models were modest, and the sample size was also relatively small, which would limit our power to detect smaller effects. Women might also have responded in ways they deemed more socially acceptable, which would also introduce bias. Social learning is complex and women might have had a difficult time recalling exactly how they acquired information on diet. Future research could include interviews with “teachers” to help corroborate findings and learn more about the consequences of consuming taboo food items. In addition, women were asked about aversions prior to avoidances, perhaps making them feel like they needed to give distinct responses for each question. However, women were told that it is okay to mention similar items for both questions. Although our categorization of the various sambar dishes did not influence our primary conclusions regarding the importance of the social transmission of food avoidances, it undoubtedly influenced the relative importance of aversions of ESS foods vs. meats. If we had instead classified all sambars as ESS foods, for instance, this would have reduced the frequency of meat aversions. It is possible that our measures of pathogen exposure, based on self-report, did not accurately reflect actual pathogen exposure. Finally, other than papaya, we do not have evidence that avoided foods are in fact harmful to the fetus and/or mother.
6 Concluding remarks
The most common newly disliked foods in pregnancy were plant foods with high levels of defensive chemicals, and aversions to them were associated with early trimester, nausea, and vomiting. Staple foods like rice were also aversive to some women, however, a pattern seen in populations, like ours, with high levels of food insecurity (Steinmetz et al., 2012; A. G. Young & Pike, 2012), and which deserves further investigation, perhaps as a strategy to increase dietary diversity. On the whole, though, our results best support the hypothesis that psychophysiological aversions function, in part, to protect fetuses from plant teratogens (Hook, 1978; Profet, 1995).
Culturally transmitted food avoidances in pregnancy have been studied much less than food aversions, yet in our study, one of the few to systematically compare them, avoidances outnumbered aversions by more than two-to-one, influenced diet throughout pregnancy, not just in early pregnancy, and their emic function was overwhelmingly to prevent abortion (miscarriage) or other harm to the fetus. Avoidances were largely acquired from mothers, grandmothers, and mothers-in-law, individuals with a direct fitness interest in the infant, and personal experience. Fruits, the most avoided category of foods, included papaya, a known abortifacient, and were best predicted by our social transmission model. Although aversions and avoidances both appeared to protect mothers and fetuses from plant teratogens, they involved almost completely different foods.
In a rural tropical region with a higher burden of infectious disease than most populations in high-income countries, meat avoidances were not uncommon, but neither avoidances nor aversions to meat were associated with exposure to pathogens. Additional dimensions of sanitation, like access to refrigeration, should be assessed, and additional hypotheses for meat taboos should be considered, such as intragroup competition for resources.
Taken together, the results of J. Henrich & Henrich (2010), whose study was conducted in Fiji, and our results suggest that there might be two systems that protect fetus and infants from dangerous foods: aversions to foods that provide cues of toxicity (and perhaps pathogenicity) early in pregnancy, and culturally acquired avoidances of potential abortifacients throughout pregnancy. Future research should investigate if taboo foods are actually harmful to the fetus or mother and the extent to which they are grounded in learned associations with aversive reactions or poor pregnancy outcomes, or both.
Supplementary Material
Acknowledgments
Many thanks to the staff at the Public Health Research Institute of India, particularly Fazila and Lakshman, for assistance in data collection; Courtney Meehan, Rob Quinlan, Andrea Wiley, and two anonymous reviewers for numerous helpful comments; and the pregnant women in our two populations who participated in our study.
Funding: This work was supported by the Washington State University Meyer Award and Washington State University Vancouver mini-grant.
Footnotes
8 Data Availability
The data associated with this research are available at doi: 10.5281/zenodo.836844.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agarwal S, Sethi V, Gupta P, Jha M, Agnihotri A, Nord M. Experiential household food insecurity in an urban underserved slum of North India. Food Security. 2009;1(3):239–250. http://doi.org/10.1007/s12571-009-0034-y. [Google Scholar]
- Alexander RD. The evolution of social behavior. Annual Review of Ecology and Systematics. 1974:325–383. [Google Scholar]
- Anuar NS, Zahari SS, Taib IA, Rahman MT. Effect of green and ripe Carica papaya epicarp extracts on wound healing and during pregnancy. Food and Chemical Toxicology. 2008;46(7):2384–2389. doi: 10.1016/j.fct.2008.03.025. http://doi.org/10.1016/j.fct.2008.03.025. [DOI] [PubMed] [Google Scholar]
- Aunger R. Are food avoidances maladaptive in the Ituri Forest of Zaire? Journal of Anthropological Research. 1994:277–310. [Google Scholar]
- Aunger R. The life history of culture learning in a face-to-face society. Ethos. 2000;28(3):445–481. http://doi.org/10.1525/eth.2000.28.3.445. [Google Scholar]
- Blumberg SJ, Bialostosky K, Hamilton WL, Briefel RR. The effectiveness of a short form of the Household Food Security Scale. American Journal of Public Health. 1999;89(8):1231–1234. doi: 10.2105/ajph.89.8.1231. Retrieved from http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1508674/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Boer HJ, Cotingting C. Medicinal plants for women’s healthcare in southeast Asia: A meta-analysis of their traditional use, chemical constituents, and pharmacology. Journal of Ethnopharmacology. 2014;151(2):747–767. doi: 10.1016/j.jep.2013.11.030. http://doi.org/10.1016/j.jep.2013.11.030. [DOI] [PubMed] [Google Scholar]
- Boyd R, Richerson PJ. Culture and the evolutionary process. University of Chicago Press; 1985. [Google Scholar]
- Boyd R, Richerson PJ, Henrich J. The cultural niche: Why social learning is essential for human adaptation. Proceedings of the National Academy of Sciences. 2011;108(Supplement 2):10918–10925. doi: 10.1073/pnas.1100290108. http://doi.org/10.1073/pnas.1100290108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnham KP, Anderson DR. Model selection and multimodel inference: A practical information-theoretic approach. Springer; 2003. [Google Scholar]
- Cashdan E. A sensitive period for learning about food. Human Nature. 1994;5(3):279–291. doi: 10.1007/BF02692155. http://doi.org/10.1007/BF02692155. [DOI] [PubMed] [Google Scholar]
- Cavalli-Sforza LL, Feldman MW. Cultural Transmission and Evolution: A Quantitative Approach. Princeton University Press; 1981. [PubMed] [Google Scholar]
- Chandran M, Tharyan P, Muliyil J, Abraham S. Post-partum depression in a cohort of women from a rural area of Tamil Nadu, India. The British Journal of Psychiatry. 2002;181(6):499–504. doi: 10.1192/bjp.181.6.499. http://doi.org/10.1192/bjp.181.6.499. [DOI] [PubMed] [Google Scholar]
- Char A, Saavala M, Kulmala T. Influence of mothers-in-law on young couples’ family planning decisions in rural India - Reproductive Health Matters. Reproductive Health Matters. 2010;18(35):154–162. doi: 10.1016/S0968-8080(10)35497-8. Retrieved from http://www.rhm-elsevier.com/article/S0968-8080(10)35497-8/abstract?cc=y= [DOI] [PubMed] [Google Scholar]
- Christensen RHB, Hansen MK. BinomTools: Performing diagnostics on binomial regression models. 2011 Retrieved from https://CRAN.R-project.org/package=binomTools.
- Cipollini ML, Levey DJ. Secondary metabolites of fleshy vertebrate-dispersed fruits: Adaptive hypotheses and implications for seed dispersal. The American Naturalist. 1997;150(3):346–372. doi: 10.1086/286069. http://doi.org/10.1086/286069. [DOI] [PubMed] [Google Scholar]
- Coronios-Vargas M, Toma RB, Tuveson RV, Schutz IM. Cultural influences on food cravings and aversions during pregnancy. Ecology of Food and Nutrition. 1992;27(1):43–49. http://doi.org/10.1080/03670244.1992.9991224. [Google Scholar]
- Curtis VA, Danquah LO, Aunger R. Planned, motivated and habitual hygiene behaviour: An eleven country review. Health Education Research. 2009;24(4):655–673. doi: 10.1093/her/cyp002. http://doi.org/10.1093/her/cyp002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demissie T, Muroki NM, Kogi-Makau W. Food aversions and cravings during pregnancy: Prevalence and significance for maternal nutrition in Ethiopia. Food & Nutrition Bulletin. 1998;19(1):20–26. [Google Scholar]
- Demps K, Zorondo-Rodriguez F, García C, Reyes-García V. The selective persistence of local ecological knowledge: Honey collecting with the Jenu Kuruba in South India. Human Ecology. 2012;40(3):427–434. [Google Scholar]
- Demps K, Zorondo-Rodríguez F, García C, Reyes-García V. Social learning across the life cycle: Cultural knowledge acquisition for honey collection among the Jenu Kuruba, India. Evolution and Human Behavior. 2012;33(5):460–470. [Google Scholar]
- Dentan RK. Some Senoi Semai dietary restrictions: A study of food behaviour in a Malayan hill tribe. Yale University; 1966. Retrieved from https://scholar.google.com/scholar.bib?q=info:SRmXuh6qQZ0J:scholar.google.com/&output=citation&hl=en&ct=citation&cd=0. [Google Scholar]
- Divya J, Belagali S. Quantification of chemical fertilizers residues in soil and water samples collected around agricultural areas of Mysore taluk, Karnataka, India. International Journal of Current Engineering and Technology. 2014;4(3):1314–1323. Retrieved from http://inpressco.com/wp-content/uploads/2014/05/Paper211314-1323.pdf. [Google Scholar]
- Douglas PM. Purity and Danger: An Analysis of Concepts of Pollution and Taboo. Routledge; 2003. [Google Scholar]
- Douwes J, Thorne P, Pearce N, Heederik D. Bioaerosol Health Effects and Exposure Assessment: Progress and Prospects. Annals of Occupational Hygiene. 2003;47(3):187–200. doi: 10.1093/annhyg/meg032. http://doi.org/10.1093/annhyg/meg032. [DOI] [PubMed] [Google Scholar]
- Drewnowski A. Taste Preferences and Food Intake. Annual Review of Nutrition. 1997;17(1):237–253. doi: 10.1146/annurev.nutr.17.1.237. http://doi.org/10.1146/annurev.nutr.17.1.237. [DOI] [PubMed] [Google Scholar]
- Duncan L, Schaller M. Perceived vulnerability to disease: Development and validation of a 15-item self-report instrument. Personality and Individual Differences. 2009;47:541–546. http://doi.org/10.1016/j.paid.2009.05.001. [Google Scholar]
- Fessler DMT. Reproductive Immunosuppression and Diet: An Evolutionary Perspective on Pregnancy Sickness and Meat Consumption. Current Anthropology. 2002;43(1):19–61. doi: 10.1086/324128. http://doi.org/10.1086/324128. [DOI] [PubMed] [Google Scholar]
- Fessler DMT, Navarrete CD. Meat Is Good to Taboo: Dietary Proscriptions as a Product of the Interaction of Psychological Mechanisms and Social Processes. Journal of Cognition and Culture. 2003;3(1):1–40. http://doi.org/10.1163/156853703321598563. [Google Scholar]
- Flaxman SM, Sherman PW. Morning Sickness: A Mechanism for Protecting Mother and Embryo. The Quarterly Review of Biology. 2000;75(2):113–148. doi: 10.1086/393377. Retrieved from http://www.jstor.org/stable/2664252. [DOI] [PubMed] [Google Scholar]
- Flood GD. An Introduction to Hinduism. Cambridge University Press; 1996. [Google Scholar]
- Gaujoux R, Seoighe C. A flexible r package for nonnegative matrix factorization. BMC Bioinformatics. 2010;11(1):1. doi: 10.1186/1471-2105-11-367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelman A. Scaling regression inputs by dividing by two standard deviations. Statistics in Medicine. 2008;27(15):2865–2873. doi: 10.1002/sim.3107. Retrieved from http://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.138.3252&rep=rep1&type=pdf. [DOI] [PubMed] [Google Scholar]
- Ghosh-Jerath S, Singh A, Bhattacharya A, Ray S, Yunus S, Zodpey SP. Dimensions of nutritional vulnerability: Assessment of women and children in Sahariya tribal community of Madhya Pradesh in India. Indian Journal of Public Health. 2013;57(4):260. doi: 10.4103/0019-557X.123268. http://doi.org/10.4103/0019-557X.123268. [DOI] [PubMed] [Google Scholar]
- Gupta RC. Reproductive and developmental toxicology. Academic Press; 2017. [Google Scholar]
- Hagen EH, Sullivan RJ. The evolutionary significance of drug toxicity over reward. In: Ahmed S, Pickard H, editors. Routledge handbook of philosophy and science of addiction. Routledge; n.d. [Google Scholar]
- Hagen EH, Roulette CJ, Sullivan RJ. Explaining human recreational use of “pesticides”: The neurotoxin regulation model of substance use vs. the hijack model and implications for age and sex differences in drug consumption. Frontiers in Psychiatry. 2013;4:142. doi: 10.3389/fpsyt.2013.00142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris M. Good to Eat: Riddles of Food and Culture. Waveland Press; 1998. [Google Scholar]
- Henrich J, Boyd R. On modeling cognition and culture: Why cultural evolution does not require replication of representations. Journal of Cognition and Culture. 2002;2(2):87–112. Retrieved from http://booksandjournals.brillonline.com/content/journals/10.1163/156853702320281836. [Google Scholar]
- Henrich J, Henrich N. The evolution of cultural adaptations: Fijian food taboos protect against dangerous marine toxins. Proceedings of the Royal Society of London B: Biological Sciences. 2010;277(1701):3715–3724. doi: 10.1098/rspb.2010.1191. http://doi.org/10.1098/rspb.2010.1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hook EB. Dietary cravings and aversions during pregnancy. The American Journal of Clinical Nutrition. 1978;31(8):1355–1362. doi: 10.1093/ajcn/31.8.1355. Retrieved from http://ajcn.nutrition.org/content/31/8/1355. [DOI] [PubMed] [Google Scholar]
- India Food Security Portal. n.d Retrieved from http://www.foodsecurityportal.org/india/resources.
- India, C. of. Census of India: Census Data Online. 2011 Retrieved from http://censusindia.gov.in/2011-common/censusdataonline.html?drpQuick=&drpQuickSelect=&q=jenukuruba.
- Kalaivani K. Prevalence & consequences of anaemia in pregnancy. Indian J Med Res. 2009;130(5):627–633. Retrieved from http://icmr.nic.in/ijmr/2009/november/1125.pdf. [PubMed] [Google Scholar]
- Kristensen P, Irgens LM, Anderson A, Bye AS, Sundheim L. Gestational Age, Birth Weight, and Perinatal Death among Births to Norwegian Farmers, 1967–1991. American Journal of Epidemiology. 1997;146(4):329–338. doi: 10.1093/oxfordjournals.aje.a009274. Retrieved from http://aje.oxfordjournals.org/content/146/4/329. [DOI] [PubMed] [Google Scholar]
- Mahaffey KR, Sunderland EM, Chan HM, Choi AL, Grandjean P, … Marien K, et al. Balancing the benefits of n-3 polyunsaturated fatty acids and the risks of methylmercury exposure from fish consumption. Nutrition Reviews. 2011;69(9):493–508. doi: 10.1111/j.1753-4887.2011.00415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazerolle MJ. AICcmodavg: Model selection and multimodel inference based on (q)AIC(c) 2016 Retrieved from https://cran.r-project.org/package=AICcmodavg.
- McDade TW, Rutherford JN, Adair L, Kuzawa C. Population differences in associations between C-reactive protein concentration and adiposity: Comparison of young adults in the Philippines and the United States. The American Journal of Clinical Nutrition. 2009;89(4):1237–1245. doi: 10.3945/ajcn.2008.27080. http://doi.org/10.3945/ajcn.2008.27080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McElreath R, Strimling P. When Natural Selection Favors Imitation of Parents. Current Anthropology. 2008;49(2):307–316. http://doi.org/10.1086/524364. [Google Scholar]
- McKerracher L, Collard M, Henrich J. Food aversions and cravings during pregnancy on yasawa island, fiji. Human Nature. 2016:1–20. doi: 10.1007/s12110-016-9262-y. [DOI] [PubMed] [Google Scholar]
- Mckerracher L, Collard M, Henrich J. The expression and adaptive significance of pregnancy-related nausea, vomiting, and aversions on yasawa island, fiji. Evolution and Human Behavior. 2015;36(2):95–102. [Google Scholar]
- Mission NH. About ASHA - Governnment of India. 2014 Retrieved from http://nrhm.gov.in/communitisation/asha/about-asha.html.
- Morton JF. Fruits of warm climates. Creative Resources Systems, Inc; 1987. [Google Scholar]
- Nag M. Beliefs and Practices about Food during Pregnancy: Implications for Maternal Nutrition. Economic and Political Weekly. 1994;29(37):2427–2438. Retrieved from http://www.jstor.org/stable/4401755. [Google Scholar]
- Odirichukwu E. Effects of Aqueous Methanolic Extract of Unripe Carica papaya fruit on Midterm Pregnancy in Wistar Albino Rats. Journal of Veterinary Advances. 2015;5(5):1. http://doi.org/10.5455/jva.20150523091721. [Google Scholar]
- Paige K, Paige JM. The politics of reproductive ritual. Univ of California Press; 1981. [Google Scholar]
- Patil CL. Appetite Sensations in Pregnancy among Agropastoral Women in Rural Tanzania. Ecology of Food and Nutrition. 2012;51(5):431–443. doi: 10.1080/03670244.2012.696012. http://doi.org/10.1080/03670244.2012.696012. [DOI] [PubMed] [Google Scholar]
- Patil CL, Young SL. Biocultural considerations of food cravings and aversions: An introduction. Ecology of Food and Nutrition. 2012;51(5):365–373. doi: 10.1080/03670244.2012.696007. [DOI] [PubMed] [Google Scholar]
- Patil CL, Abrams ET, Steinmetz AR, Young SL. Appetite Sensations and Nausea and Vomiting in Pregnancy: An Overview of the Explanations. Ecology of Food and Nutrition. 2012;51(5):394–417. doi: 10.1080/03670244.2012.696010. http://doi.org/10.1080/03670244.2012.696010. [DOI] [PubMed] [Google Scholar]
- Pike IL. The nutritional consequences of pregnancy sickness. Human Nature. 2000;11(3):207–232. doi: 10.1007/s12110-000-1011-5. [DOI] [PubMed] [Google Scholar]
- Placek CD, Hagen EH. A test of three hypotheses of pica and amylophagy among pregnant women in Tamil Nadu, India. American Journal of Human Biology. 2013;25(6):803–813. doi: 10.1002/ajhb.22456. http://doi.org/10.1002/ajhb.22456. [DOI] [PubMed] [Google Scholar]
- Placek CD, Hagen EH. Fetal protection: The roles of social learning and innate food aversions in South India. Human Nature. 2015;26(3):255–276. doi: 10.1007/s12110-015-9239-2. http://doi.org/10.1007/s12110-015-9239-2. [DOI] [PubMed] [Google Scholar]
- Prabhakar SJ, Gangadhar MR. Dietary status among Jenu Kuruba and Yerava tribal children of Mysore district, Karnataka. Anthropologist. 2011;13(2):159–162. Retrieved from http://www.krepublishers.com/02-Journals/T-Anth/Anth-13-0-000-11-Web/Anth-13-2-000-11-Abst-Pdf/Anth-13-2-159-11-633-Prabhakar-S-C-J/Anth-13-2-159-11-633-Prabhakar-S-C-J-Tt.pdf. [Google Scholar]
- Profet M. Pregnancy sickness as adaptation: A deterrent to maternal ingestion of teratogens. Barkow Jerome H, Cosmides Leda, Tooby John., editors. The Adapted Mind. 1995:327–366. Retrieved from https://books.google.com/books?hl=en&lr=&id=SxX4gRzOS6oC&oi=fnd&pg=PA327&dq=profet+++aversions&ots=Bh1s1L0TAO&sig=EZqszob-CWmMrjXFb3QRashWzlA.
- Richerson PJ, Boyd R. Not by genes alone. Chicago: University of Chicago Press; 2005. Retrieved from https://scholar.google.com/scholar.bib?q=info:CvyW52iq4W4J:scholar.google.com/&output=citation&hl=en&ct=citation&cd=1. [Google Scholar]
- Roy S, Hegde H, Bhattacharya D, Upadhya V, Kholkute S. Tribes in Karnataka: Status of health research. 2015;141(5):673–687. doi: 10.4103/0971-5916.159586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanjur D. Social and cultural perspectives in nutrition. Prentice-Hall; 1982. Retrieved from http://agris.fao.org/agris-search/search.do?recordID=US8238081. [Google Scholar]
- Steinmetz AR, Abrams ET, Young SL. Patterns of nausea, vomiting, aversions, and cravings during pregnancy on Pemba Island, Zanzibar, Tanzania. Ecology of Food and Nutrition. 2012;51(5):418–430. doi: 10.1080/03670244.2012.696011. [DOI] [PubMed] [Google Scholar]
- Suchitra JY, Swaminathan H. Asset Acquisition among Matrilineal and Patrilineal Communities: A Case Study of Coastal Karnataka. n.d Retrieved from http://www.genderassetgap.org/sites/default/files/WP3.pdf.
- Suresh T, Hatha A, Sreenivasan D, Sangeetha N, Lashmanaperumalsamy P. Prevalence and antimicrobial resistance of Salmonella enteritidis and other salmonellas in the eggs and egg-storing trays from retails markets of Coimbatore, South India. Food Microbiology. 2006;23(3):294–299. doi: 10.1016/j.fm.2005.04.001. http://doi.org/https://doi.org/10.1016/j.fm.2005.04.001. [DOI] [PubMed] [Google Scholar]
- Tjur T. Coefficients of Determination in Logistic Regression Models—A New Proposal: The Coefficient of Discrimination. The American Statistician. 2009;63(4):366–372. http://doi.org/10.1198/tast.2009.08210. [Google Scholar]
- Van Hollen CC. Birth on the Threshold: Childbirth and Modernity in South India. University of California Press; 2003. [Google Scholar]
- Vijayalakshmi V. Scheduled tribes and gender: Development perceptions from Karnataka. Bangalore: Institute for Social; Economic Change; 2003. [Google Scholar]
- Visaria L, Ramachandran V, Ganatra B, Kalyanwala S. Abortion in India: Emerging issues from qualitative studies. Economic and Political Weekly. 2004:5044–5052. [Google Scholar]
- Whitehead H. Food Rules: Hunting, Sharing, and Tabooing Game in Papua New Guinea. University of Michigan Press; 2000. [Google Scholar]
- WHO India. WHO. n.d Retrieved from http://www.who.int/countries/ind/en/
- Wickham H. ggplot2: Elegant graphics for data analysis. Springer-Verlag; New York: 2009. Retrieved from http://ggplot2.org. [Google Scholar]
- Wilson BK, Mignone JJ, Sinclair AJ. Contextual influences on the sustainability of prospective livelihood diversification initiatives in farm villages in the Karnataka semiarid dryland region of India. Development Studies Research. 2014;1(1):368–381. http://doi.org/10.1080/21665095.2014.988361. [Google Scholar]
- Xie Y. Dynamic documents with r and knitr. Vol. 29. CRC Press; 2015. [Google Scholar]
- Young AG, Pike IL. A Biocultural Framework for Examining Maternal Cravings and Aversions among Pastoral Women in East Africa. Ecology of Food and Nutrition. 2012;51(5):444–462. doi: 10.1080/03670244.2012.696013. http://doi.org/10.1080/03670244.2012.696013. [DOI] [PubMed] [Google Scholar]
- Zangerl AR, Bazzaz FA. Fritz r, simms e, editors, plant resistance to herbivores and pathogens: Ecology, evolution, and genetics. University of Chicago Press; 1992. Theory and pattern in plant defense allocation; pp. 363–391. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.