Abstract
Background
The heritable risk for alcohol use disorder (AUD) is expressed partly through alterations in subjective alcohol response. In this study, we investigated the effects of two AUD risk-associated single nucleotide polymorphisms (SNPs), GABRA2 rs279858 and GRIK1 rs2832407, on the subjective response to alcohol administered intravenously to healthy social drinkers in a laboratory setting.
Methods
93 self-identified European American social drinkers underwent three blinded lab sessions in which they received intravenous infusions of ethanol at three target blood alcohol levels (0.00mg%, 40mg%, 100mg%) using a “clamp” procedure. The self-reported Biphasic Alcohol Effects Scale (BAES) stimulation and sedation subscales were the primary outcome measures. We examined the effects of these two genetic variants on subjective response to alcohol.
Results
For the BAES stimulation subscale scores, adjusting for age, baseline scores, and time effects, individuals with two copies of the GABRA2 rs279858 C “risk” allele for AUD exhibited the greatest stimulant responses to high dose alcohol compared to the other risk allele counts (dose-by-allele count interaction effect, p=0.001, post-hoc contrast for C-allele p=0.012). For the BAES sedation subscale scores, adjusting for the same covariates, we detected a dose-by-allele count interaction effect (p=0.0044) such that subjects with two copies of the GRIK1 C “risk” allele reported the greatest sedative response to the higher alcohol dose.
Conclusions
This study suggests that gene variants contributing to the risk for AUD may alter features of the alcohol dose-response relationship in specific ways. GABRA2 rs279858*C enhances stimulant responses to higher levels of alcohol, while the GRIK1 rs2832407*C allele increases sedative responses. In summary, GRIK1 and GABRA2 variants have distinct effects on the dose-related subjective response to intravenous alcohol in humans.
Keywords: Alcohol use disorder, Intravenous ethanol, BAES, GRIK1, GABRA2
INTRODUCTION
The identification of genetic mechanisms influencing alcohol-induced subjective responses may help to guide the development of novel therapeutics for alcohol use disorder (AUD). AUD has a heritability of approximately 50-64% (Verhulst et al., 2015, Kendler, 2001, McGue, 1999). A component of the heritable risk for AUD is expressed as an alteration in the response to alcohol, as individuals with a family history of alcoholism tend to have reduced sedative responses to alcohol (Schuckit, 1994a), although other studies suggest that heightened stimulant responses to ethanol also contribute to the risk for heavy drinking (King et al., 2014, Morean and Corbin, 2010). Further, among people with family histories of AUD, those who have these alterations in ethanol subjective response have an elevated risk of developing AUD (King et al., 2014, Schuckit, 1994b).
Variation in genes influencing the effects of alcohol on the brain may also influence the subjective response to alcohol, and subsequently the propensity for developing AUD (Schuckit et al., 2004, Viken et al., 2003). GABAA receptors are important targets for alcohol in the brain (Krystal et al., 2006). “Extrasynaptic” subtypes of GABAA receptors are direct targets for alcohol, while synaptic GABAA receptors have low sensitivity to alcohol but respond to GABA that may be released during alcohol administration. Rs279858 is a coding SNP in exon 5 that produces a synonymous substitution, but a gene expression study in cultured human neural cells showed that the risk allele “C” was associated with significantly reduced mRNA levels for the α2 subunit of the GABAA receptor (GABRA2), and altered expression of the cluster of four GABAA receptor subunit genes on chromosome 4 (Lieberman et al., 2015). The rs279858*C allele conveys risk for AUD (cf. reviews by Kranzler and Edenberg, 2010, Kumar et al., 2009) and may enhance the stimulant effects or reduce the sedative effects of alcohol in healthy humans (Arias et al., 2014, Kosobud et al., 2015, Pierucci-Lagha et al., 2005, Roh et al., 2011, Uhart et al., 2013), although the findings are somewhat contradictory (Covault et al., 2014).
Ethanol also inhibits NMDA and kainite glutamate receptors (Krystal et al., 2003, Lack et al., 2008). The GRIK1 gene which encodes the ionotropic kainate 1 (GLUK1) subunit of kainite receptors, and specifically its intronic SNP rs2832407, is an AUD risk allele implicated in the antidipsotropic effects of topiramate (Kranzler et al., 2014b, Kranzler and Edenberg, 2010, Kranzler et al., 2009). SNP rs2832407 moderates positive alcohol-related expectancies (Kranzler et al., 2014a), suggesting that it influences alcohol effects. However, it is not yet known whether this SNP has functional effects, whether it alters alcohol response in humans, or whether it is the actual causal variant, i.e., it could be in linkage disequilibrium with an as yet unknown causal variant.
Several different paradigms of ethanol exposure have been developed to measure and elicit different responses in the laboratory, and these include mainly oral alcohol administration paradigms or those where the ethanol is infused intravenously (Plebani et al., 2012, Ray et al., 2010). The intravenous alcohol “clamp” paradigm presents some advantages for studies attempting to examine the association of genetic variation to patterns of subjective alcohol response (O’Connor et al., 1998). By overcoming the variability in oral ethanol absorption, the intravenous ethanol “clamp” paradigm allows one to characterize subjective response to a relatively precisely determined alcohol level, and it enables one to ensure that the target alcohol level will be maintained throughout the assement period (Ramchandani et al., 2006). This likely increases the precision of subjective response measurements that occur during the procedure compared to oral alcohol challenge paradigms, and subjects provide subjective response data at the same alcohol level. Further, the subjective response to ethanol measured with the intravenous method is less contaminated by non-specific neural responses to alcohol-related cues (e.g., seeing a glass of wine) that might confound the signal generated purely through the pharmacologic effects of alcohol (Kareken et al., 2010, Oberlin et al., 2013). The intravenous “clamp” paradigm has been used successfully in the past to demonstrate significant pharmacogenetic differences in the subjective response to alcohol, and also pharmacogentic differences in medication response (Ray et al., 2007, Ray et al., 2013, Ray and Hutchison, 2004). A critique of intravenous ethanol administration paradigms is that they lack the ecological validity of oral administration paradigms such as the “simulated bar” paradigm (Quinn and Fromme, 2016).
This study evaluated the effects of AUD-risk associated polymorphisms of GABRA2 and GRIK1 on the subjective response to ethanol in healthy human subjects using the intravenous ethanol “clamp” procedure. We hypothesized that these two risk alleles would render differential effects on the subjective response to alcohol in healthy social drinkers.
MATERIALS AND METHODS
Complete details of the intravenous alcohol procedures used were published previously as the participants were recruited in a previous study which had a primary purpose to examine the effects of family history on alcohol response in the lab (Kerfoot et al., 2013). The institutional review boards of the VA Connecticut Healthcare System and Yale University School of Medicine approved this study. All subjects were recruited through advertisements and provided written informed consent after the nature of the procedures had been fully explained.
Study Subjects
Our sample consists of subjects who participated in a parent study of which we included subjects who were of European-American (EA) ancestry and for whom we had DNA available (n=93). Subjects were between the ages of 21 and 30 years old, without DSM-IV psychiatric or substance use disorder diagnoses (excluding alcohol abuse) as measured by the Structured Clinical Interview for DSM-IV (SCID) (First, 2007). Subjects were required to have a negative urine toxicology for drugs of abuse on test days, and could not be alcohol naïve (for more details, see Kerfoot et al., 2013). Women were required to have a negative pregnancy test at screening and on every test day. Those who identified two first or second degree relatives with AUD based on interview were considered to be family history positive. Table 1 shows the characteristics of the study subjects. There were no differences across genotype groups for the two SNP variants of GABRA2 and GRIK1 in terms of age, education, age at first drink, percentage of family history positive subjects, and baseline drinking patterns (all p > 0.05). Other baseline drinking related measures such as alcohol expectancies, self-reported alcohol effects, and alcohol-related problems, did not differ across genotypes (see supplementary table 1).
Table 1.
Demographic characteristics of the study subjects.
| Table 1 | GABRA_rs279858 | GRIK1_rs283247 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Total | CC | CT | TT | p-value | AA | AC | CC | p-value | |
| N (%) | 93 | 17 (18.3%) | 49 (52.7%) | 27 (29.0%) | --- | 7 (7.5%) | 49 (52.7%) | 37 (39.8%) | --- |
| Age (meana±SD) | 24.3±2.6 | 25.1±3.0 | 24.2±2.6 | 23.9±2.2 | 0.35 | 25.0±3.1 | 24.5±2.7 | 23.8±2.3 | 0.35 |
| Sex (Male %) | 51.60% | 53% | 51% | 51.90% | ~1 | 14.30% | 53.10% | 56.80% | 0.13 |
| Education years (mean±SD) | 16.2±1.7 | 16.3±1.5 | 16.1±2.0 | 16.2±1.2 | 0.95 | 16.6±2.2 | 16.0±1.6 | 16.4±1.7 | 0.47 |
| Age First Drink (meana±SD) | 17.7±4.2 | 18.3±2.9 | 17.4±2.0 | 17.9±2.2 | 0.36 | 17.4±1.7 | 17.8±2.3 | 17.7±2.2 | 0.88 |
| Total Drinking Days (in the last 30 days) | 6.0±4.6 | 5.6±3.3 | 5.7±5.0 | 6.8±4.7 | 0.58 | 2.9±1.1 | 6.3±5.1 | 6.2±4.3 | 0.17 |
| Total Number Drinks (in the last 30 days) | 19.6±24.5 | 25.6±43.8 | 15.8±14.1 | 22.7±22.9 | 0.27 | 7.6±4.4 | 17.2±14.9 | 25.1±34.2 | 0.14 |
| Family History Positive (%) | 31.20% | 5 (29.4%) | 16 (32.7%) | 8 (29.6%) | ~1 | 3 (42.9%) | 17 (34.7%) | 9 (24.3%) | 0.42 |
Study Design
This was a randomized, double blind, placebo-controlled, between-subject crossover study. Investigators were kept blind to the randomization scheme which was performed by the research pharmacy service. An ABC scheme was used for randomization to alcohol dose, with the third session (C) kept constant across subjects, the first session (A) randomized with a block procedure, and the second session was the subsequent default alcohol dosage.
All subjects provided blood for DNA extraction and genotyping. Then each subject underwent three test days as described below, corresponding to IV-ethanol administration of three “doses” using the IV “clamp” procedure. The three test days were at least three days apart with administration of either a low dose of ethanol (targeted breathalyzer = 40mg%), a high dose of ethanol (targeted breathalyzer = 100mg%), or placebo. Infusion of ethanol was via a solution of 6% by volume ethanol in 0.9% normal saline solution, delivered by a computerized pump at a fixed level (“clamp”) to achieve a predetermined BrAC.
“Intravenous clamp” Procedure
In the loading phase: The infusion rate was determined using a MatLab® version 6.5 calculation package (The MathWorks Inc., Natick, MA), which generated a linear ascension to target BrAC in 20–30 minutes based on a subject’s individual pharmacokinetic profile based on age, sex, height, and weight. In the plateau phase: The infusion rate was clamped at a rate estimated to maintain a steady-state, and then adjusted as needed to maintain the subject’s BrAC within ±5mg% of target BrAC for 60 minutes. BrAC was measured every 2 minutes during the ascension phase and every 2–8 minutes during steady-state by Alcotest 7410-plus device (Dräger Safety AG & Co. KGaA, Lübeck, Germany).
Experimental Procedures
Subjects were scheduled to receive placebo, low dose ethanol (target BrAC=40mg%), and high dose ethanol (target BrAC=100mg%), on three separate test days, at least three days apart, in a randomized order, under double-blind conditions. In order to maintain the blind, the investigators and research staff administering measures were kept blind to subject conditions, but a study nurse was unblinded to the BrAC and made adjustments to the infusion rate. Prior to each test session, participants were instructed not to consume alcohol or caffeine for 48 hours and were asked to fast overnight. They presented to the Biological Studies Unit of the VA Connecticut Healthcare System, West Haven campus, at approximately 9:00 AM. All subjects were given a standardized breakfast at the beginning of each test day and crackers during the test day, as requested. Prior to testing, subjects underwent urine drug screening for toxicology and breathalyzer screening. Provided that these tests were negative, an IV line was placed. Subjects received infusions of placebo or one of two ethanol doses (low or high) intravenously for approximately 20–30 minutes, until the target BrAC was achieved. Once the BrAC was achieved, (40mg% or 100mg%), it was maintained using the clamp procedure for 60 minutes (O’Connor et al., 1998). Vital signs (heart rate and blood pressure) were measured at regular intervals during each laboratory session (see supplemental figure). Additionally, the respiratory rate was measured 140 minutes prior to the infusion, and 200 minutes after reaching the target BrAC.
We used the Biphasic Alcohol Effects Scale (BAES) (Martin et al., 1993) to measure the subjective alcohol response. Measures of subjective alcohol response were obtained at baseline (140 minutes before the IV infusion), 10 minutes after starting the infusion, immediately upon reaching the target alcohol level, and at 30, 80, 110, 140, and 200 minutes after reaching the target alcohol level.
Genotyping
DNA was extracted from whole blood using the PureGene kit (Gentra Systems, Minneapolis). We genotyped two SNPs, GABRA2 rs279858 and GRIK1 rs2832407, using TaqMan SNP genotyping assays (Life Technologies, Grand Island, New York). All genotypes were obtained in duplicate with consistent results. In our sample, for GABRA2 rs279858 (T/C), the minor allele is “C”, and the minor allele frequency (MAF) is 0.446, while for GRIK1 rs2832407 (C/A), the minor allele is “A”, and the MAF is 0.339. In the reference population of HapMap-CEU, MAF is 0.473 and 0.385 for GABRA2 rs279858 and GRIK1 rs2832407, respectively. Both SNPs are in Hardy Weinberg equilibrium (both p > 0.05).
Data Analysis
Both the BAES stimulation and sedation subscale scores were positively skewed with an excess of zero values. Transformations were unsuccessful in removing the skew and as a result, a repeated measures nonparametric approach (Brunner et al., 2002) was used, where scores are rank ordered and fitted using a linear mixed model with unstructured covariance matrix followed by an ANOVA-type statistic (ATS) that adjusts the p-values. Both time-point and dose were included in the models as categorical variables and entered in the repeated statement with an unstructured block covariance matrix to model the dependence among responses.
Seven time points were included in the analysis relative to the start of IV-ethanol infusions: 10, 30, 60, 110, 140, 170 and 230 min (see Supplementary Figure S1). We also recoded the baseline measure at time point -140 minutes (140 minutes before the IV infusion) as a covariate to control for individual baseline differences. The targeted study variables, the SNP markers, were coded as a count of minor alleles, i.e., a dose effect model. Other covariates included age, time-point, sex, and dose. Sex, dose and the two SNP markers were also exploited to generate and examine six pairwise two-way interaction terms: Sex-by-Dose, Sex-by-GABRA2, Sex-by-GRIK1, Dose-by-GABRA2, Dose-by-GRIK1, GABRA2-by-GRIK1. The three levels of dose (placebo, low and high doses) and the seven time-points were designated as the within-subject repeated variables in the model and both are categorical variables. Significant effects involving either one of the two SNPs were further analyzed through linear contrasts to better understand the nature of the effect. These analyses were conducted using SAS 9.3 (SAS Institute Inc., Cary, NC, USA). Since there were essentially two models used, one for stimulation subscale and one for sedation subscale effects, the alpha significance level was Bonferroni corrected to .025.
RESULTS
Herein we characterized the genetic effects of GABRA2 rs279858 and GRIK1 rs2832407 on the subjective response to alcohol, and discovered that genetic effects of each one of these two SNPs on the subjective response to alcohol were influenced by dose and sex differences. In other words, each SNP appeared to pharmacogenetically moderate the subjective response to alcohol. We summarized these findings in Figure 1 and details are as follows:
Figure 1.
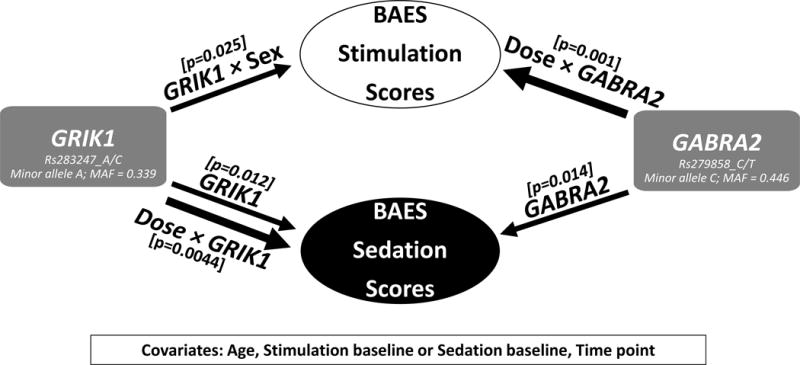
Result summary. BAES, the Biphasic Alcohol Effects Scale. MAF, minor allele frequency. Three interaction effects which reached significance are listed: Dose × GABRA2, Dose × GRIK1 and GRIK1 × Sex representing the Dose-by-GABRA2 SNP interaction, Dose-by-GRIK1 SNP interaction, and Sex-by-GRIK1 SNP interaction.
BAES Stimulation Subscale in Subjective Response to Alcohol Stimulation Effects
After controlling for age, baseline stimulation score, and time point, there were no main effects of genotype for either GABRA2 or GRIK1. There was a significant two-way interaction for Dose-by-GABRA2 (p=0.001) (Table 2A). The Sex-by-GRIK1 (p=0.025) interaction was marginally significant after Bonferroni correction (Type I alpha < 0.025).
Table 2.
Nonparametric mixed-effects model results testing the main effects of dose, sex and the two SNPs in GABRA2 and GRIK1 and their interaction effects on the Biphasic Alcohol Effects Scale (BAES) (A) stimulation subscale and (B) sedation subscale in subjective response to the alcohol. Analysis was adjusted for age, baseline scores, and time-point. Significant effects are in bold and italic font.
| (A) Variable | Number* DF | ATS value** | p-value |
|---|---|---|---|
| Stimulation baseline | 1 | 221.27 | <.0001 |
| Age | 1 | 1.75 | 0.19 |
| Time point | 2.86 | 15.43 | <.0001 |
| Dose | 1.56 | 5.74 | 0.022 |
| Sex | 1 | 0.1 | 0.75 |
| GABRA_count | 1.92 | 0.6 | 0.60 |
| GRIK1_count | 1.21 | 0.22 | 0.62 |
| Dose*Sex | 1.51 | 1.75 | 0.12 |
| Dose*GABRA2_count | 2.76 | 4.95 | 0.001 |
| Dose*GRIK1_count | 2.07 | 0.35 | 0.38 |
| Sex*GABRA2_count | 1.75 | 1.96 | 0.18 |
| Sex*GRIK1_count | 1.18 | 0.67 | 0.025 |
| GABRA2_count*GRIK1_count | 2.6 | 1.58 | 0.002*** |
| (B) Variable | Number DF* | ATS value** | p-value |
|---|---|---|---|
| Sedation baseline | 1 | 82.97 | <.0001 |
| Age | 1 | 6.96 | 0.0083 |
| Time Point | 3.98 | 56.87 | <.0001 |
| Dose | 1.6 | 28.49 | <.0001 |
| Sex | 1 | 1.09 | 0.30 |
| GABRA2_count | 1.91 | 3.48 | 0.014 |
| GRIK1_count | 1.2 | 2.87 | 0.012 |
| Dose*Male | 1.64 | 0.13 | 0.81 |
| Dose*GABRA2_count | 2.99 | 0.57 | 0.30 |
| Dose*GRIK1_count | 2.23 | 1.56 | 0.0044 |
| Sex*GABRA2_count | 1.75 | 1.65 | 0.28 |
| Sex*GRIK1_count | 1.18 | 1.29 | 0.46 |
| GABRA2_count*GRIK1_coun | 2.59 | 2.66 | 0.11 |
Note: Number DF, number of degree of freedom;
ATS, ANOVA-type statistic;
Please refer to the discussion regarding interpretation of this effect modification.
Follow-up contrast analyses revealed 1) the GABRA2 C-allele carriers reported more stimulation than the TT genotype under the high dose compared to the other two doses (p=0.012). In other words, for the Dose-by-GABRA2 interaction, increased stimulation was observed for C-carriers, an AUD risk variant, under high dose compared to those with the “TT” genotype (zero copies of the C-allele) (Figure 2A). For the Sex-by-GRIK1 interaction, the stimulant responses were higher in sequence from zero to one to two copies of the C-allele in males. In other words, there is an allelic dose-response relationship in males, but not in females (p=0.019) (Figure 2B).
Figure 2.
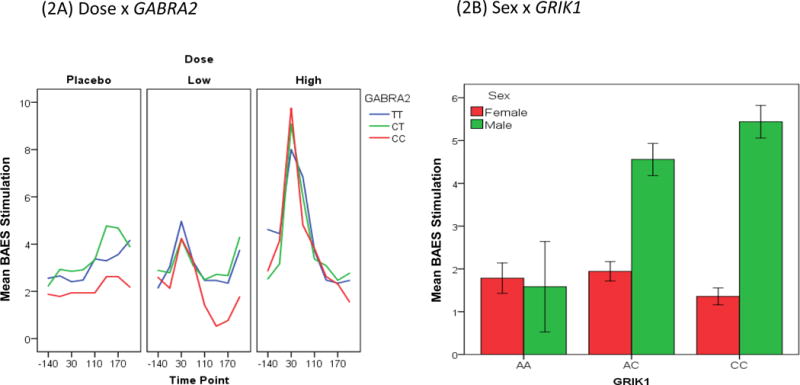
The Biphasic Alcohol Effects Scale (BAES) stimulation score (y axis) response. (2A) Dose-by-GABRA2 SNP interaction. (2B) Sex-by-GRIK1 SNP interaction.
BAES Sedation Subscale in Subjective Response to Alcohol Sedation Effects
After controlling for age, baseline sedation score, and time point, there were significant main effects of genotype; GABRA2 C-allele count (p=0.014) and GRIK1 C allele count (p=0.012) on increased alcohol sedation responses, as well as a significant two-way interaction; Dose-by-GRIK1, between dose and GRIK1 C allele count (p=0.004), (Table 2B). There was no effect of sex or its interactions on the sedation subscale.
For GABRA2-rs279858, increased sedation was observed for C-carriers compared to those with the TT genotype, i.e., zero copies of the AUD-risk C-allele (Figure 3A). For GRIK1-rs2832407, subjects with the CC genotype had higher sedation compared to those with AC and AA genotypes (Figure 3B). Further, the GRIK1 variant interacted with ethanol dose; those with the CC genotype had the most sedation compared to other genotypes, and had increasing sedation with increasing ethanol dose (Figure 3C). Follow-up contrasts revealed; 1) no difference between GRIK1 allele counts at the placebo dose, but at low and high doses respectively, subjects with two copies reported more sedation than subjects with zero or one copy (p=.005), and; 2) subjects with two copies of GRIK1 reported more sedation than subjects with zero or one copy (p=.010).
Figure 3.
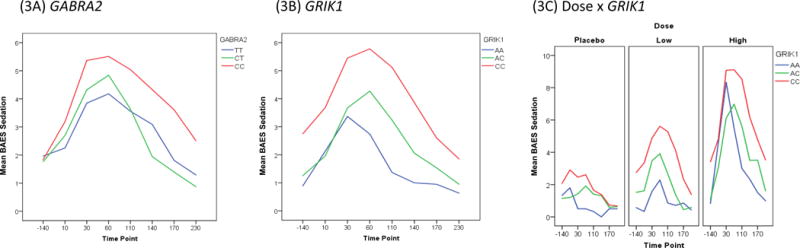
The Biphasic Alcohol Effects Scale (BAES) sedation score (y axis) response is shown over time (x axis) for both genetic variants. (3A) GABRA2 main effect. (3B) GRIK1 main effect. (3C) The interaction of dose and GRIK1 genotype broken down by dose of ethanol, with the BAES sedation score (y axis) shown over time (x axis).
DISCUSSION
This study investigated the effects of two AUD risk-associated SNPs, GABRA2 rs279858 and GRIK1 rs2832407, on the subjective response to alcohol and found that each one of the two SNPs in GABRA2 and GRIK1 appeared to pharmacogenetically moderate the subjective response to alcohol, measured by the BAES in healthy EA social drinkers. Our findings suggest that these SNPs might contribute to the risk of developing AUD by altering the rewarding and reinforcing effects of alcohol. These results provide evidence that the two risk alleles were differentially associated with the stimulant and sedative effects of alcohol, suggesting that there might be dissociable features of the genetics and neurobiology of the subjective response to alcohol in humans. The effects of the risk-associated alleles were distinct, and both enhanced the subjective response to ethanol. Thus, when our current results are added to the existing literature on rs279858, and the subjective response to alcohol viewed as a three-factor construct described by Ray et al., (2009), a somewhat consistent pattern of effects emerges with the C-allele being associated with increased positively reinforcing properties.
The results for GABRA2 rs279858 in this study are consistent with previous findings from an oral alcohol challenge paradigm in which the AUD-risk associated C-carriers experienced increased stimulation and euphoriant effects of alcohol. Similar to Arias et al., (2014), in this study there was greater stimulation with alcohol administration for C-allele carriers compared to the TT genotype. As noted earlier, alcohol, at the doses studied here, probably does not act directly at synaptic GABAA receptors bearing the α2 subunit, although it might stimulate these receptors indirectly by evoking GABA release (Krystal et al., 2006).
With regard to GABRA2 SNP effects on the BAES sedation subscale, there was no pharmacogenetic effect on sedation response, but the significant main effect suggests a possible behavioral effect not related to alcohol dose. Subjects with two AUD risk C-alleles (CC) reported greater sedation than those that are T allele carriers, even with the placebo dose of alcohol.
While GRIK1 did not influence the subjective stimulant response among the study subjects as a whole, the GRIK1 SNP showed a marginally significant (p=.025) interaction with sex on the stimulant response. The allelic dose-response relationship of increasing alcohol stimulant responses (i.e., CC > AC > AA) only occurred in the male participants, but not females. This subjective response was not related to the dose of alcohol received and might reflect differences in excitability based on genotype and sex.
In terms of sedative responses, the GRIK1 AUD-risk associated C-allele was associated with a greater sedative response to ethanol, which was most pronounced for those with the CC genotype. The GRIK1 findings are novel and potentially relevant given the possible contribution of GRIK1 rs2832407 to AUD risk and a variable topiramate treatment response in AUD.
Limitations of the study include that it was conducted in healthy social drinkers, and some AUD-related pharmacogenetic effects may not be represented in this sample. The average age of the subjects (24.3 years) is beyond the peak age of onset for AUD, which may limit the generalizability of the findings, though the average-aged subject was still within a reasonable window of risk for developing the disorder (Grant et al., 2004). Additionally, only EA subjects were included, and thus we might not be able to generalize the findings to other populations. EA subjects were identified by self-reported race, with no genome-wide genotypes available to implement a more sophisticated adjustment for population stratification. The study’s repeated measures design, which included eight repeated measures per subject for each of the three test days, resulted in reasonable statistical power (Bakeman, 2005) despite the small sample size. This study is nevertheless one of the largest to date to evaluate these SNPs using the intravenous ethanol clamp procedure. Of note, there was a significant GRIK1-by-GABRA2 modification effect, though two of the GRIK1-by-GABRA2 genotype combinations had very small sample sizes (N=1 and 2), and likely overestimated effect sizes (Gelman and Carlin, 2014). Thus we exercised caution and limited the report only to significant single-gene (single-variant) effects.
At least part of the findings in the subjective response to alcohol herein might be due to ethanol’s physiological effects (i.e., on heart rate, blood pressure), an open question which is beyond the scope of this study. Also, the present study does not explain how these genetic variations cause changes in subjective response to alcohol from a neurobiological standpoint, and this is a possible direction for future studies.
In summary, we found confirmatory evidence that GABRA2 rs279858*C is associated with an altered subjective response to alcohol, possibly explaining its association to an increased risk for developing AUD. GRIK1 rs2832407*C also appears to have a pharmacogenetic interaction with alcohol resulting in an altered subjective response that may convey an increased AUD risk with the C-allele. Further study of the effects of these variants on alcohol-related phenotypes and behaviors may reveal critical information about the pathophysiology of AUD, perhaps leading to better treatments.
Supplementary Material
Acknowledgments
The authors acknowledge the important contributions of Angelina Genovese, R.N.C., M.B.A., Elizabeth O’Donnell, R.N., Michelle Lynn SanPedro, R.N., Willie Ford of the Neurobiological Studies Unit of the VA Connecticut Healthcare System, West Haven Campus, West Haven, CT; and Ann Marie Lacobelle, MS, for technical assistance. In addition, the authors acknowledge support for this work from the Department of Veterans Affairs (National Center for PTSD Research; VA Medical Research Program (MERIT review grant to JG); VA CT MIRECC), and National Institute on Alcohol Abuse and Alcoholism (Center for the Translational Neuroscience of Alcoholism - 2P50-AA012870-07); NIAAA R01 AA017535; and NIDA R01 DA12690 and K01 DA24758. We also thank Brian Pittman, M.S., for consultation regarding the statistical model.
Footnotes
DR. BAO ZHU YANG (Orcid ID : 0000-0001-8506-6946)
CONFLICT OF INTEREST
Dr Petrakis has served as a consultant for Alkermes over the past year.
Dr. Krystal:
Consultant
Note: – The Individual Consultant Agreements listed below are less than $5,000 per year
AMGEN, AstraZeneca Pharmaceuticals, Biogen, Idec, MA, Biomedisyn Corporation, Forum Pharmaceuticals, Janssen Research & Development, Otsuka America Pharmaceutical, Inc., Sunovion Pharmaceuticals, Inc., Takeda Industries, Taisho Pharmaceutical Co., Ltd, Scientific Advisory Board, Biohaven Pharmaceuticals, Blackthorn Therapeutics, Inc., Lohocla Research Corporation, Luc Therapeutics, Inc., Pfizer Pharmaceuticals, TRImaran Pharma
Stock
Biohaven Pharmaceuticals Medical Sciences
Stock Options
Blackthorn Therapeutics, Inc.
Luc Therapeutics, Inc.
Income Greater than $10,000
Editorial Board
Editor - Biological Psychiatry
Employment:
Yale University School of Medicine
VA CT Healthcare System
Patents and Inventions
1) Seibyl JP, Krystal JH, Charney DS. Dopamine and noradrenergic reuptake inhibitors in treatment of schizophrenia. US Patent #:5,447,948. September 5, 1995
2) Vladimir, Coric, Krystal, John H, Sanacora, Gerard – Glutamate Modulating Agents in the Treatment of Mental Disorders US Patent No. 8,778,979 B2 Patent Issue Date: July 15, 2014.
3) Charney D, Krystal JH, Manji H, Matthew S, Zarate C., - Intranasal Administration of Ketamine to Treat Depression United States Application No. 14/197,767 filed on March 5, 2014; United States application or PCT International application No. 14/306,382 filed on June 17, 2014
4) Arias A, Petrakis I, Krystal JH. – Composition and methods to treat addiction. Provisional Use Patent Application no.61/973/961. April 2, 2014. Filed by Yale University Office of Cooperative Research.
5): Chekroud, A., Gueorguieva, R., & Krystal, JH. “Treatment Selection for Major Depressive Disorder” [filing date 3rd June 2016, USPTO docket number Y0087.70116US00]. Provisional patent submission by Yale University
NON Federal Research Support
AstraZeneca Pharmaceuticals provides the drug, Saracatinib, for research related to NIAAA grant “Center for Translational Neuroscience of Alcoholism [CTNA-4]
Pfizer Pharmaceuticals provides an investigational drug, PF-03463275, for research related to
NIH grant “Translational Neuroscience Optimization of GlyT1 Inhibitor”
References
- Arias AJ, Covault J, Feinn R, Pond T, Yang BZ, Ge W, Oncken C, Kranzler HR. A GABRA2 variant is associated with increased stimulation and ‘high’ following alcohol administration. Alcohol Alcohol. 2014;49:1–9. doi: 10.1093/alcalc/agt163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakeman R. Recommended effect size statistics for repeated measures designs. Behavior research methods. 2005;37:379–84. doi: 10.3758/bf03192707. [DOI] [PubMed] [Google Scholar]
- Brunner E, Domhof S, Langer F. Nonparametric analysis of longitudinal data in factorial experiments. J. Wiley; 2002. [Google Scholar]
- Covault J, Pond T, Feinn R, Arias AJ, Oncken C, Kranzler HR. Dutasteride reduces alcohol’s sedative effects in men in a human laboratory setting and reduces drinking in the natural environment. Psychopharmacology (Berl) 2014;231:3609–18. doi: 10.1007/s00213-014-3487-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Williams Janet BW, Spitzer Robert L, Gibbon Miriam. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Clinical Trials Version (SCID-CT) New York, New York: Biometrics Research, New York State Psychiatric Institute; 2007. [Google Scholar]
- Gelman A, Carlin J. Beyond Power Calculations: Assessing Type S (Sign) and Type M (Magnitude) Errors. Perspectives on psychological science : a journal of the Association for Psychological Science. 2014;9:641–51. doi: 10.1177/1745691614551642. [DOI] [PubMed] [Google Scholar]
- Grant BF, Dawson DA, Stinson FS, Chou SP, Dufour MC, Pickering RP. The 12-month prevalence and trends in DSM-IV alcohol abuse and dependence: United States, 1991–1992 and 2001–2002. Drug Alcohol Depend. 2004;74:223–34. doi: 10.1016/j.drugalcdep.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Ittiwut C, Yang BZ, Kranzler HR, Anton RF, Hirunsatit R, Weiss RD, Covault J, Farrer LA, Gelernter J. GABRG1 and GABRA2 Variation Associated with Alcohol Dependence in African Americans. Alcohol Clin Exp Res. 2011;2011:1530–0277. doi: 10.1111/j.1530-0277.2011.01637.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kareken DA, Bragulat V, Dzemidzic M, Cox C, Talavage T, Davidson D, O’connor SJ. Family history of alcoholism mediates the frontal response to alcoholic drink odors and alcohol in at-risk drinkers. NeuroImage. 2010;50:267–76. doi: 10.1016/j.neuroimage.2009.11.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS. Twin studies of psychiatric illness: an update. Arch Gen Psychiatry. 2001;58:1005–14. doi: 10.1001/archpsyc.58.11.1005. [DOI] [PubMed] [Google Scholar]
- Kerfoot K, Pittman B, Ralevski E, Limoncelli D, Koretski J, Newcomb J, Arias AJ, Petrakis IL. Effects of family history of alcohol dependence on the subjective response to alcohol using the intravenous alcohol clamp. Alcohol Clin Exp Res. 2013;37:2011–8. doi: 10.1111/acer.12199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King AC, De Wit H, Mcnamara PJ, Cao D. Rewarding, stimulant, and sedative alcohol responses and relationship to future binge drinking. Arch Gen Psychiatry. 2011;68:389–99. doi: 10.1001/archgenpsychiatry.2011.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King AC, Mcnamara PJ, Hasin DS, Cao D. Alcohol challenge responses predict future alcohol use disorder symptoms: a 6-year prospective study. Biol Psychiatry. 2014;75:798–806. doi: 10.1016/j.biopsych.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosobud AE, Wetherill L, Plawecki MH, Kareken DA, Liang T, Nurnberger JL, Windisch K, Xuei X, Edenberg HJ, Foroud TM, O’connor SJ. Adaptation of Subjective Responses to Alcohol is Affected by an Interaction of GABRA2 Genotype and Recent Drinking. Alcohol Clin Exp Res. 2015;39:1148–57. doi: 10.1111/acer.12749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kranzler HR, Armeli S, Feinn R, Tennen H, Gelernter J, Covault J. GRIK1 genotype moderates topiramate’s effects on daily drinking level, expectations of alcohol’s positive effects and desire to drink. The international journal of neuropsychopharmacology/official scientific journal of the Collegium Internationale Neuropsychopharmacologicum (CINP) 2014a;17:1549–56. doi: 10.1017/S1461145714000510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kranzler HR, Covault J, Feinn R, Armeli S, Tennen H, Arias AJ, Gelernter J, Pond T, Oncken C, Kampman KM. Topiramate treatment for heavy drinkers: moderation by a GRIK1 polymorphism. Am J Psychiatry. 2014b;171:445–52. doi: 10.1176/appi.ajp.2013.13081014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kranzler HR, Edenberg HJ. Pharmacogenetics of alcohol and alcohol dependence treatment. Current pharmaceutical design. 2010;16:2141–8. doi: 10.2174/138161210791516387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kranzler HR, Gelernter J, Anton RF, Arias AJ, Herman A, Zhao H, Burian L, Covault J. Association of markers in the 3′ region of the GluR5 kainate receptor subunit gene to alcohol dependence. Alcohol Clin Exp Res. 2009;33:925–30. doi: 10.1111/j.1530-0277.2009.00913.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krystal JH, Petrakis IL, Mason G, Trevisan L, D’souza DC. N-methyl-D-aspartate glutamate receptors and alcoholism: reward, dependence, treatment, and vulnerability. Pharmacol Ther. 2003;99:79–94. doi: 10.1016/s0163-7258(03)00054-8. [DOI] [PubMed] [Google Scholar]
- Krystal JH, Staley J, Mason G, Petrakis IL, Kaufman J, Harris RA, Gelernter J, Lappalainen J. Gamma-aminobutyric acid type A receptors and alcoholism: intoxication, dependence, vulnerability, and treatment. Arch Gen Psychiatry. 2006;63:957–68. doi: 10.1001/archpsyc.63.9.957. [DOI] [PubMed] [Google Scholar]
- Kumar S, Porcu P, Werner DF, Matthews DB, Diaz-Granados JL, Helfand RS, Morrow AL. The role of GABA(A) receptors in the acute and chronic effects of ethanol: a decade of progress. Psychopharmacology (Berl) 2009;205:529–64. doi: 10.1007/s00213-009-1562-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lack AK, Ariwodola OJ, Chappell AM, Weiner JL, Mccool BA. Ethanol inhibition of kainate receptor-mediated excitatory neurotransmission in the rat basolateral nucleus of the amygdala. Neuropharmacology. 2008;55:661–8. doi: 10.1016/j.neuropharm.2008.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman R, Kranzler HR, Joshi P, Shin DG, Covault J. GABRA2 Alcohol Dependence Risk Allele is Associated with Reduced Expression of Chromosome 4p12 GABAA Subunit Genes in Human Neural Cultures. Alcohol Clin Exp Res. 2015;39:1654–64. doi: 10.1111/acer.12807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin CS, Earleywine M, Musty RE, Perrine MW, Swift RM. Development and validation of the Biphasic Alcohol Effects Scale. Alcohol Clin Exp Res. 1993;17:140–6. doi: 10.1111/j.1530-0277.1993.tb00739.x. [DOI] [PubMed] [Google Scholar]
- Mcgue M. The Behavioral Genetics of Alcoholism. Current Directions in Psychological Science. 1999;8:109–115. [Google Scholar]
- Morean ME, Corbin WR. Subjective response to alcohol: a critical review of the literature. Alcohol Clin Exp Res. 2010;34:385–95. doi: 10.1111/j.1530-0277.2009.01103.x. [DOI] [PubMed] [Google Scholar]
- O’connor S, Morzorati S, Christian J, Li TK. Clamping breath alcohol concentration reduces experimental variance: application to the study of acute tolerance to alcohol and alcohol elimination rate. Alcohol Clin Exp Res. 1998;22:202–10. [PubMed] [Google Scholar]
- Oberlin BG, Dzemidzic M, Tran SM, Soeurt CM, Albrecht DS, Yoder KK, Kareken DA. Beer flavor provokes striatal dopamine release in male drinkers: mediation by family history of alcoholism. Neuropsychopharmacology. 2013;38:1617–24. doi: 10.1038/npp.2013.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierucci-Lagha A, Covault J, Feinn R, Nellissery M, Hernandez-Avila C, Oncken C, Morrow AL, Kranzler HR. GABRA2 alleles moderate the subjective effects of alcohol, which are attenuated by finasteride. Neuropsychopharmacology. 2005;30:1193–203. doi: 10.1038/sj.npp.1300688. [DOI] [PubMed] [Google Scholar]
- Plebani JG, Ray LA, Morean ME, Corbin WR, Mackillop J, Amlung M, King AC. Human laboratory paradigms in alcohol research. Alcohol Clin Exp Res. 2012;36:972–83. doi: 10.1111/j.1530-0277.2011.01704.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn PD, Fromme K. Individual differences in subjective alcohol responses and alcohol-related disinhibition. Experimental and clinical psychopharmacology. 2016;24:90–9. doi: 10.1037/pha0000065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramchandani VA, O’connor S, Neumark Y, Zimmermann US, Morzorati SL, Wit H. The alcohol clamp: applications, challenges, and new directions–an RSA 2004 symposium summary. Alcoholism: Clinical and Experimental Research. 2006;30:155–164. doi: 10.1111/j.1530-0277.2006.00017.x. [DOI] [PubMed] [Google Scholar]
- Ray LA, Bujarski S, Mackillop J, Courtney KE, Monti PM, Miotto K. Subjective response to alcohol among alcohol-dependent individuals: effects of the mu-opioid receptor (OPRM1) gene and alcoholism severity. Alcohol Clin Exp Res. 2013;371(Suppl):E116–24. doi: 10.1111/j.1530-0277.2012.01916.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray LA, Hutchison KE. A polymorphism of the mu-opioid receptor gene (OPRM1) and sensitivity to the effects of alcohol in humans. Alcohol Clin Exp Res. 2004;28:1789–95. doi: 10.1097/01.alc.0000148114.34000.b9. [DOI] [PubMed] [Google Scholar]
- Ray LA, Mackillop J, Leventhal A, Hutchison KE. Catching the alcohol buzz: an examination of the latent factor structure of subjective intoxication. Alcohol Clin Exp Res. 2009;33:2154–61. doi: 10.1111/j.1530-0277.2009.01053.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray LA, Mackillop J, Monti PM. Subjective responses to alcohol consumption as endophenotypes: advancing behavioral genetics in etiological and treatment models of alcoholism. Substance use & misuse. 2010;45:1742–65. doi: 10.3109/10826084.2010.482427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray LA, Miranda R, Jr, Kahler CW, Leventhal AM, Monti PM, Swift R, Hutchison KE. Pharmacological effects of naltrexone and intravenous alcohol on craving for cigarettes among light smokers: a pilot study. Psychopharmacology (Berl) 2007;193:449–56. doi: 10.1007/s00213-007-0794-z. [DOI] [PubMed] [Google Scholar]
- Roh S, Matsushita S, Hara S, Maesato H, Matsui T, Suzuki G, Miyakawa T, Ramchandani VA, Li TK, Higuchi S. Role of GABRA2 in moderating subjective responses to alcohol. Alcohol Clin Exp Res. 2011;35:400–7. doi: 10.1111/j.1530-0277.2010.01357.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuckit MA. The 1994 Isaacson Award Lecture: a prospective study of sons of alcoholics. Alcohol and alcoholism (Oxford, Oxfordshire). Supplement. 1994a;2:1–6. [PubMed] [Google Scholar]
- Schuckit MA. Low level of response to alcohol as a predictor of future alcoholism. Am J Psychiatry. 1994b;151:184–9. doi: 10.1176/ajp.151.2.184. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Smith TL, Kalmijn J. The search for genes contributing to the low level of response to alcohol: patterns of findings across studies. Alcohol Clin Exp Res. 2004;28:1449–58. doi: 10.1097/01.alc.0000141637.01925.f6. [DOI] [PubMed] [Google Scholar]
- Uhart M, Weerts EM, Mccaul ME, Guo X, Yan X, Kranzler HR, Li N, Wand GS. GABRA2 markers moderate the subjective effects of alcohol. Addict Biol. 2013;18:357–69. doi: 10.1111/j.1369-1600.2012.00457.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhulst B, Neale MC, Kendler KS. The heritability of alcohol use disorders: a meta-analysis of twin and adoption studies. Psychological medicine. 2015;45:1061–72. doi: 10.1017/S0033291714002165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viken RJ, Rose RJ, Morzorati SL, Christian JC, Li TK. Subjective intoxication in response to alcohol challenge: heritability and covariation with personality, breath alcohol level, and drinking history. Alcohol Clin Exp Res. 2003;27:795–803. doi: 10.1097/01.ALC.0000067974.41160.95. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


