Abstract
Background
Multiple studies demonstrate the benefit of a vegan diet on cardiovascular risk factors when compared to no intervention or usual dietary patterns. The aim of this study is to evaluate the effect of a vegan diet versus the American Heart Association (AHA)-recommended diet on inflammatory and glucometabolic profiles in patients with angiographically defined coronary artery disease (CAD).
Study design
This study is a randomized, open label, blinded end-point trial of 100 patients with CAD as defined by ≥ 50% diameter stenosis in a coronary artery ≥2 mm in diameter on invasive angiography. Participants are randomized to 8 weeks of either a vegan or AHA-recommended diet (March 2014 and February 2017). Participants are provided weekly groceries that adhere to the guidelines of their diet. The primary endpoint is high sensitivity C-reactive concentrations. Secondary endpoints include anthropometric data, other markers of inflammation, lipid parameters, glycemic markers, endothelial function, quality of life data, and assessment of physical activity. Endpoints are measured at each visit (baseline, 4 weeks, and 8 weeks). Dietary adherence is measured by two weekly 24-h dietary recalls, a 4-day food record during the week prior to each visit, and both plasma and urine levels of trimethylamine-N-oxide at each visit.
Conclusion
This study is the first to comprehensively assess multiple indices of inflammation and glucometabolic profile in a rigorously conducted randomized trial of patients with CAD on a vegan versus AHA-recommended diet.
Keywords: Diet intervention, Coronary artery disease, Inflammation, Glucose, Lipids
1. Introduction
Inflammation plays a central role in the development and progression of atherosclerosis [1]. Several studies suggest an associated benefit between a reduction in inflammation via statin or anti-inflammatory therapy and cardiovascular outcomes in patients with coronary artery disease (CAD) [2], [3], [4], [5]. The inflammatory state at the time of coronary revascularization also predicts clinical outcomes. High concentrations of C-reactive protein (CRP) at baseline, in particular, predict a significant increase in adverse cardiovascular outcomes after coronary revascularization [6], [7], [8]. In relatively healthy subjects with elevated concentrations of high sensitivity-CRP (hs-CRP) and normal low-density lipoprotein (LDL) cholesterol, initiation of statin therapy significantly lowers both hs-CRP concentrations and long-term major adverse cardiovascular events [9].
Approximately 30% of the treated stable CAD population has elevated hs-CRP concentrations, highlighting the potential need for alternative strategies to address the residual risk associated with baseline inflammation in patients with CAD [2]. Addressing adverse dietary patterns may allow patients with CAD to positively impact their overall cardiovascular health. The American Heart Association (AHA) has maintained a focus on a “heart healthy” diet that is low in saturated and trans fats and high in fruits and vegetables to reduce the health burden associated with cardiovascular disease and, as such, has been the recommended diet for patients with CAD. There is evidence to suggest that the lowering of low-density lipoprotein (LDL) cholesterol concentrations is responsible for the anti-inflammatory effects noted with statin medications [10]. Given the potential of lowering LDL-cholesterol concentrations from the substitution of animal-based protein with plant-based protein, an antioxidant-rich vegan diet may improve the inflammatory and glucometabolic state better than the AHA-recommended diet in patients with CAD [11].
Although multiple dietary factors influence cardiovascular disease, only a few studies to date examine the effects of overall healthy dietary patterns on cardiovascular disease risk [12], [13], [14], [15]. Even fewer studies compare a particular diet to the standard recommended heart healthy diet in patients with cardiovascular disease, but, instead, utilize an inappropriate control condition (no active intervention, usual dietary patterns) as the comparison group [15], [16], [17], [18].
2. Study aims
The primary aim of this study is to determine the impact of an 8-week vegan diet on inflammation, as measured by hs-CRP concentrations, compared to the AHA-recommended diet in patients with angiographically defined CAD. The secondary aims are to evaluate differential effects of these two diets on other surrogate endpoints that may be affected by diet and impact clinical outcomes, including anthropometric measures, other markers of inflammation, lipid parameters, glycemic markers, endothelial function, quality of life, and physical activity patterns in patients with CAD.
3. Methods
3.1. Organizational structure
This study is a randomized, open-label, blinded end-point trial of patients with angiographically defined CAD at two campuses of the New York University (NYU) School of Medicine, NYU Langone Medical Center and Bellevue Hospital (New York, NY) [19]. The NYU School of Medicine Institutional Review Board, which approved the study, does not require a Data Safety Monitoring Committee for this study given that the potential risks of participation in this study are only those associated with blood collection and breach of confidentiality. The study investigators, Binita Shah, MD, Jonathan Newman, MD, Kathleen Woolf, PhD, RD, and James Slater, MD, oversee all study activities and implementation of the study design. Drs. Shah and Newman review data on a monthly basis to ensure the safe and proper treatment of participants and determine whether there are any substantial deviations from the protocol.
Kathleen Woolf, PhD, RD developed the dietary guidelines, sample 2-week menus, and recipes were developed for both groups. Binita Shah, MD directs blood samples collection, processing, and analysis for soluble adhesion molecules, as well as markers of neutrophil surface activation and monocyte subtypes by flow cytometry in the NYU Smilow Research Building. Stanley L. Hazen, MD, PhD, at the Cleveland Clinic (Cleveland, OH), measures markers of diet adherence (trimethylamine-N-oxide (TMAO), choline, and carnitine) in the plasma and urine.
The Purjes Foundation (Salt Lake City, UT) is the primary sponsor of the trial. The Cleveland HeartLab, Inc (Cleveland, OH) measures standard markers of inflammation (hs-CRP, myeloperoxidase, hemogram with differential), comprehensive lipid profiles, and glucometabolic parameters at no cost. Itamar Medical Ltd (Caesarea, Israel) provides the EndoPat machine to measure endothelial dysfunction. The sponsors did and will not contribute to the study design or data analysis. The trial is registered at clinicaltrials.gov (NCT02135939).
3.2. Timeline
Recruitment began at NYU Langone Medical Center on March 11, 2014 and at Bellevue Hospital Center on November 21, 2014. Although the recruitment period was expected to take 2 years, achievement of the target study population of 100 patients with angiographically defined CAD was completed February 2, 2017, 11 months later than completed.
3.3. Eligibility criteria
The initial target study population was status post percutaneous coronary intervention (PCI). However, due to a delay in recruitment, the inclusion criteria was amended to include any patient with angiographically defined CAD (≥50% lesion in an artery with ≥2 mm caliber), which was approved on October 8, 2014. Specific inclusion and exclusion criteria are summarized in Table 1. Participants have to be at least 3 months post-myocardial infarction, coronary artery bypass graft surgery, or infection to minimize confounders of the primary endpoint, hs-CRP concentration. Every effort is made to include equitable numbers of each sex, and there are no enrollment restrictions based on race and/or ethnic origin. However, given that the study team, particularly the study registered dietitian, can only provide detailed counseling in English or Spanish, individuals who primarily speak a language other than English or Spanish are not recruited for the study. The study does not include any vulnerable subjects.
Table 1.
Inclusion and exclusion criteria for the Effect of a Vegan versus AHA DiEt in Coronary Artery Disease (EVADE CAD) trial.
| Inclusion criteria | Exclusion criteria |
|---|---|
| 1. Angiographically defined coronary artery disease (defined as ≥50% lesion in an artery with ≥2 mm caliber) 2. Speak English or Spanish 3. Age ≥18 years |
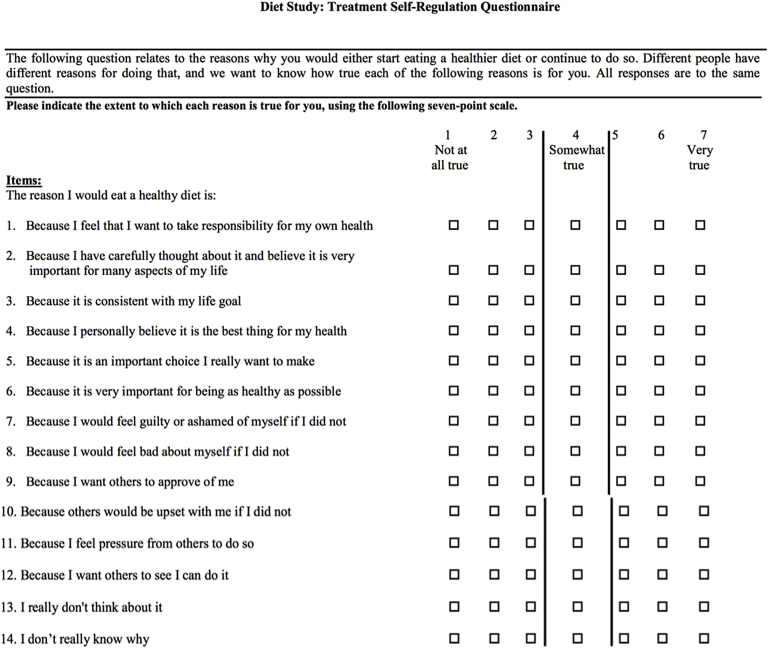
1. History of an eating disorder 2. On a vegetarian or vegan diet 3. Use of steroids or non-steroidal anti-inflammatory medications other than aspirin within 5 half-lives of baseline visit and during the study period 4. History of a myocardial infarction or coronary artery bypass graft surgery within the preceding 3 months 5. Presence of infection within the preceding 3 months 6. Have a planned staged coronary revascularization or other surgical procedure during study period 7. History of colon cleansing (for stool microbiome analysis) 8. Use of probiotics or over the counter supplements other than standard vitamins (for stool microbiome analysis) 9. Pregnant 10. Participating in a competing study 11. Have any condition (e.g., psychiatric illness) or situation that, in the investigator's opinion, may confound the study results, or may interfere significantly with the patient's ability to adhere with study procedures. 12. Score of >4 to any of the amotivational items or if the relative autonomy index (defined as average of answers for the 6 autonomous items – average of answers for the 6 controlled items) is ≤ 0 on the Treatment Self-Regulation Questionnaire (Fig. 1) |
3.4. Screening
All study team members have specific training in the informed consent process. Potential participants who meet inclusion/exclusion criteria are provided with an oral and written description of the project and are asked to demonstrate their understanding of each step by summarizing the study information.
Potential participants who express interest in the study complete a written screening consent that allows the study team to administer a Treatment Self-Regulation Questionnaire, a validated questionnaire that assesses the degree to which one's motivation for a particular behavior is relatively autonomous or self-determined (Fig. 1) [20], [21]. The items on the questionnaire relate to the reasons why an individual would make healthier changes to their diet or continue current habits. Potential participants are excluded if they give an answer of >4 to any of the amotivational items (questions 13 and 14) or if the relative autonomy index (defined as the average of answers for the first 6 autonomous items minus the average of answers for the second 6 controlled items) is < 0 (Fig. 1) [20], [21]. Participants who meet the inclusion criteria based on the Treatment Self-Regulation Questionnaire are provided a 4-day food record and instructions on how to fill out these food records during the week prior to their scheduled baseline visit (Fig. 2).
Fig. 1.
Treatment self-regulation questionnaire. Questions 1 through 6 assess autonomous regulation; questions 7 through 12 assess controlled regulation; and questions 13 through 14 assess amotivation. Patients were excluded if they give an answer of >4 to any of the amotivational items or if the relative autonomy index (defined as average of answers for the first 6 autonomous items – average of answers for the second 6 controlled items) is <0.
Fig. 2.
Four-day food record.
3.5. Baseline visit and randomization
Participants who fill out their 4-day food record are, again, provided an oral description of the project and give written informed consent to participate in the trial at the baseline visit. Only study team members with specific training in the informed consent process obtain this consent, and participants are required to demonstrate their understanding by summarizing the information provided.
Participants then undergo randomization to either a vegan diet or the AHA-recommended diet (based on the AHA Therapeutic Lifestyle Changes (TLC) Diet). Given that weight loss is not a primary goal of the study, participants are not given energy goals. Dietary recommendations for both diets were developed using a 2000 kcal intake. The randomization sequence is computer-generated by the study biostatistician using a 1 to 1 allocation. A member of the cardiovascular clinical research center, who is not a member of the study team, has access to this sequence and provides the dietary assignment via email after the participant provides written consent. All laboratory technicians performing the endpoint assays are blinded to randomization group.
Once a diet is assigned, the study registered dietitian counsels the participant on the assigned diet (Supplemental appendix 1), providing extensive nutrition education and resources. . Study participants are given measuring cups, measuring spoons, a food scale, a cookbook, handouts from the Nutrition Care Manual® of the Academy of Nutrition and Dietetics (www.eatright.org) (12 pages), a 2-week sample menu with recipes (Supplemental appendix 2), and a large magnet for the participant's refrigerator with summary dietary guidelines. Nutrient analyses for the 2-week sample menus were completed using Food Processor 11.0.137 (ESHA research, Salem, OR). The 2-week sample menus for both diets were developed using a 2000-kcal guideline with 15% calories from protein, 60% from carbohydrates, and 25% from fat. The vegan and AHA diet groups are given the cookbooks Simply Vegan (Baltimore: Vegetarian Resource Group, 2012) and American Heart Association Low-Fat, Low-Cholesterol Cookbook (New York: Clarkson Potter, 2008), respectively. Kathleen Woolf, PhD, RD tested all recipes provided in the 2-week sample menu prior to implementation. Of note, all participants are encouraged and expected to continue to take any medication his or her physician has prescribed.
3.6. Dietary follow-up
Participants are provided $80 worth of groceries per week from a food delivery company that services their area. Participants supplement the $80 per week with their own purchases and are counseled to eat within the recommendations of their assigned diet with food choices and purchases outside of grocery items. The study registered dietitian calls the patient twice weekly on random days throughout the 8 weeks of the study to answer questions, obtain 24-h dietary recalls (twice a week), and obtain food requests from the participant for a grocery order specifically designed for this study (once a week) (Supplemental appendices 3 and 4).
Two follow-up in-person visits are scheduled---one at 4 weeks and one at 8 weeks (interim and final visits, respectively). Participants are instructed to fill out a 4-day food record during the one week prior to each follow-up visit.
Long-term follow-up is assessed by a telephone call at 3 months, 6 months, and yearly for up to 5 years. At each time point, details on interim clinical events (stress test, coronary angiography and/or revascularization, myocardial infarction, development or hospitalization for congestive heart failure, stroke or transient ischemic attack, cardiac arrest, death) are obtained and another 24-h dietary recall is completed. Participants are also asked questions: 1) Are you still following the assigned diet? (If not, are you following any specific diet); 2) How do you rate the healthfulness of you overall diet? (not healthy, somewhat healthy, healthy, very healthy, extremely healthy); and 3) What is you current weight?
3.7. Covariates of interest
Demographic and socioeconomic information are self-reported, and trained study personnel measure vital signs, including height (Seca 216, Model 1814009, Chino, CA, USA), weight (Conair Weight Watchers, Model WW24WN, East Windson, NJ, USA), and waist circumference (Mabis tape measure, Model 35-780-000, Waukegan, IL, USA). Waist circumference is measured at the level of the iliac crest while the participant is in the standing position, and an average of two measures are recorded. An average of three blood pressure measurements taken 1 min apart are recorded while the participant is in the sitting position [22]. Pertinent medical history is obtained from participant interview and review of the electronic medical record. Tobacco use is defined as use of >100 cigarettes or 5 pipes in a lifetime, and current tobacco use is defined as use within the past 6 months. Baseline medications are defined as those used for at least 7 days prior to the baseline visit. Left ventricular ejection fraction is categorized as normal or borderline (≥50%), mildly or moderately reduced (35%–49%), or severely reduced (<35%) and collected from review of noninvasive data or angiography, whichever modality was performed for clinical reasons closest to the time of baseline visit. Finally, a physician investigator determines the indication for coronary angiography and degree of CAD based on review of the invasive coronary angiography report (Table 2).
Table 2.
Covariates of interest in the Effect of a Vegan versus AHA DiEt in Coronary Artery Disease (EVADE CAD) trial.
| Baseline Characteristics | Baseline Characteristics (continued) |
|---|---|
| 1. Demographic data a. Age b. Sex c. Race d. Ethnicity (Hispanic or non-Hispanic) 2. Socioeconomic data a. Highest level of education b. Total household income c. Employment status d. Marital status e. Home zip code 3. Vital signs a. Height b. Weight c. Waist circumference d. Heart rate e. Blood pressure (sitting position; average of 3 measures) 4. Pertinent medical history a. Hypertension b. Hyperlipidemia c. Myocardial infarction d. Percutaneous coronary intervention e. Coronary artery bypass graft f. Congestive heart failure requiring treatment g. Diabetes mellitus h. Peripheral artery disease (known ≥50% or moderate or severe stenosis; history of revascularization) i. Carotid artery disease (known ≥50% or moderate or severe stenosis; or history of revascularization) j. Cerebrovascular disease (clinical history of TIA or stroke) k. Abdominal aneurysm l. Chronic renal dysfunction (creatinine >1.5 mg/dL) m. Inflammatory condition n. Malignancy |
5. Smoking history a. Use of >100 cigarettes or 5 pipes in lifetime b. Use within 6 months (current vs former) 6. Baseline medications (for at least the 7 days prior visit) 7. Left ventricular ejection fraction 8. Indication for coronary angiography 9. Number of coronary arteries diseased (≥50% diameter stenosis in an artery with ≥2 mm caliber; or physiologically significant by fractional flow reserve; or previously revascularized) |
|
4-Week Interim and 8-Week Final Visits | |
| 1. Height, weight, and waist circumference 2. Smoking status 3. Changes in medications from baseline and interim use of antibiotics 4. Interim events a. Emergency room visit with or without observation or inpatient admission b. Heart attack (details of cardiac biomarkers collected if answered affirmatively) c. Stroke or mini-stroke d. Hospitalization for heart failure e. Resuscitated cardiac arrest f. Cardiac procedure (including coronary angiography and/or coronary revascularization) g. Outpatient clinic visits h. Cardiac rehabilitation visits i. Death | |
|
Long-Term Follow-Up Calls | |
| 1. Patient-reported weight 2. Changes in cardiac medications from last contact 3. Interim events (same as above) |
At the 4-week interim and 8-week final visits, trained study personnel again measure height, weight, waist circumference, and blood pressure per protocol, and participants are asked about current smoking status and changes in medications from the prior visit.
3.8. Endpoints of interest
For each visit (baseline, 4-week interim, and 8-week final), participants are instructed to 1) drink plenty of fluids on the day before their appointment; 2) not consume any food after midnight the night before the visit (water is allowed); and 3) wear loose-fitting, comfortable clothing. Blood is collected using standard peripheral venipuncture techniques with a 21-guage needle. Up to 45 cc of blood is collected in the following order: 3.2% (0.109 mol L−1) sodium citrate tubes (BD Vacutainer, Franklin Lakes, New Jersey), serum separator tubes (BD Vacutainer, Franklin Lakes, New Jersey), 5.4-mg K2 EDTA tubes (BD Vacutainer, Franklin Lakes, New Jersey), and a Z serum separator clot activator lipotube (Greiner Bio-One, Monroe, North Carolina). Citrate- and EDTA-anticoagulated bloods designated for plasma collection are centrifuged within 15 min of collection at 2500g for 10 min. Blood in the serum separator tubes and the lipotube are allowed to clot for 30 min at room temperature prior to centrifugation at 2500g for 10 min. Plasma and serum aliquots are stored at −80 °C until analysis. Good clinical practices are followed to certify proper storage and daily and long-term quality control of reagents, instruments, and technique. Endpoints are shown in Table 3.
Table 3.
Endpoints of interest in the Effect of a Vegan versus AHA DiEt in Coronary Artery Disease (EVADE CAD) trial.
| Primary Endpoint |
| High sensitivity C-reactive protein |
| Secondary Endpoints |
| Anthropometric data Weight Body mass index Waist circumference Waist circumference to height ratio |
| Other markers of inflammation Myeloperoxidase White blood cell count Neutrophil/lymphocyte ratio Neutrophil-surface expression of L-selectin and the β2 integrin CD11b Soluble L-selectin and E-selectin Soluble intercellular adhesion molecule and vascular cell adhesion protein Soluble neutrophil gelatinase-associated lipocalin Monocyte subtypes Oxidized LDL Urine F2-isoprostane/creatinine |
| Lipid parameters Total cholesterol Low-density lipoprotein (LDL) cholesterol (calculated) Direct high-density lipoprotein (HDL) cholesterol Triglycerides Non-HDL cholesterol LDL, small LDL, and HDL particle numbers Large very low-density lipoprotein (VLDL) and large HDL LDL, VLDL, and HDL size Lp-PLA2 Lipoprotein insulin resistance index |
| Glycemic markers Fasting blood glucose Blood insulin Hemoglobin A1c |
| Other Index of endothelial function (EndoPAT, Itamar Medical Ltd, Caesarea, Israel) Quality of life (EuroQol 5 dimensions questionnaire, health evaluation on a visual analogue scale) Physical Activity (International Physical Activity questionnaire) |
| Clinical outcomes Major adverse cardiovascular events (MACE) (a composite of all-cause mortality, non-fatal myocardial infarction, non-fatal stroke, and repeat coronary revascularization) Individual components of MACE |
3.9. Primary endpoint
The primary endpoint is hs-CRP concentration. Serum samples for hs-CRP are shipped on the same day of collection at 2 °C–8 °C to the Cleveland HeartLab, Inc (Cleveland, OH) for analysis.
3.10. Secondary endpoints
Secondary endpoints include anthropometric measures, other markers of inflammation, lipid parameters, glycemic markers, endothelial function, quality of life, and physical activity patterns.
Myeloperoxidase is measured from EDTA plasma, and white blood cell count with differential is measured from EDTA whole blood. Surface markers of neutrophil activity (L-selectin and CD11b) and monocyte subtypes are measured using citrate-anticoagulated blood via an Accuri C6 flow cytometer (BD Biosciences, Franklin Lakes, NJ). For neutrophil analyses, whole blood is stained with anti-L-selectin (CD62L) and anti-β2 integrin (CD11b) antibodies (BD Biosciences, Franklin Lakes, NJ) within 15 min of collection. The neutrophil population is identified by their characteristic forward-sidelight scatter characteristics. To assess monocyte subtypes, formaldehyde-fixed whole blood is stained with CD86, CD14, and CD16 (BD Biosciences, Franklin Lakes, NJ). Monocytes are identified as CD86+ and have characteristic forward-side light scatter properties. All antibodies are directly conjugated and IgG antibodies are used as controls for all flow cytometry analyses. All other inflammatory markers (such as soluble selectin and integrin molecules) will be measured in triplicate on stored plasma at the end of the study using commercially available multiplex assays.
Lipid profiles are measured using nuclear magnetic resonance spectroscopy. The lipid profiles include measures of total cholesterol; LDL cholesterol (calculated); high-density lipoprotein (HDL) cholesterol; triglycerides; LDL, small LDL, and HDL particle numbers; large very low-density lipoprotein (VLDL) and large HDL; LDL, VLDL, and HDL size; and lipoprotein insulin resistance index.
Glycemic markers, such as blood glucose and insulin, are measured from serum, while hemoglobin A1c is measured from EDTA whole blood.
Endothelium-mediated changes in vascular tone are measured using the EndoPat device. Biosensors are placed on fingertips, and endothelium-mediated changes in arterial tone are elicited by a downstream hyperemic response induced by a standard 5-min occlusion of the brachial artery. Measurements from the contralateral arm are used to control for concurrent non-endothelial dependent changes in vascular tone, and the ratio of the two measurements result in an index of endothelial function. An index score of <1.67 is considered abnormal [23].
Quality of life is assessed using the EuroQol five dimensions questionnaire, which is comprised of a health state description and an evaluation component. On the questionnaire, health status is evaluated in five areas (mobility, self-care, usual activities, pain/discomfort, and anxiety/depression), and each area is rated on a three-level scale (1-no problems; 2-having some problems; 3-having extreme problems). The self-reported data are then summarized using the United States preference-weighted index score [24]. Health evaluation is assessed using a visual analogue scale, a 20 cm vertical scale that ranges from 0 to 100 (“worst” to “best imaginable health state”), and reflects the participant's overall health on the day of the visit.
Physical activity is assessed using the International Physical Activity Questionnaire – short form (IPAQ-SF) [25]. Participants complete the questionnaire at the baseline, 4-week interim, and 8-week final visits. Briefly, the IPAQ-SF is a short, well-validated instrument used to assess a typical week of physical activity [25]. The IPAQ-SF asks participants to report frequency (days per week) and time spent engaging in activities that have been performed for at least 10 min in the past 7 days at three different levels of intensity (“walking”, “moderate”, and “vigorous”). Examples for each intensity level are provided and include leisure, work, household, and transport-related activities. The physical activity data are scored following established methods and reported as a continuous measure in the metabolic equivalent of task-minutes per week.
Finally, rate of major adverse cardiovascular events (a composite of all-cause mortality, non-fatal myocardial infarction, non-fatal stroke, and repeat coronary revascularization) are assessed. Participants are asked about interim clinical events (emergency room visit, hospital observation or inpatient stay, admission to an extended care facility, cardiac rehabilitation, stress test, coronary angiography and/or revascularization, myocardial infarction, development or hospitalization for congestive heart failure, stroke or transient ischemic attack, cardiac arrest, death). If a clinical event does occur outside the NYU system, participants are asked for authorization to obtain details of this event from the outside physician or facility if their NYU physician did not already do so. Information regarding interim clinical events is also captured on long-term telephone follow-ups.
All secondary endpoints are measured at NYU School of Medicine, with the exception of myeloperoxidase, white blood cell count with differential, lipid profiles, and glycemic markers, which are shipped on the same day of collection at 2 °C–8 °C to the Cleveland HeartLab, Inc (Cleveland, OH) for analysis.
3.11. Measures of dietary adherence
We developed a novel assessment tool to evaluate adherence to the animal protein recommendations for each diet. Adherence is assessed each week using the information collected during the two 24-h dietary recalls. Participants on the vegan diet receive 1 point for abstinence from each of the following: 1) meat/poultry/eggs, 2) dairy, 3) seafood. A score of ≥5 of 6 is considered adherent for that week. Participants on the AHA diet receive 1 point for consumption of each of the following: 1) ≤5 oz of animal protein/day, 2) only low-fat/fat-free dairy, 3) fish ≥2 times/week. A score of ≥4 of 5 is considered adherent for that week. Participants are determined to be adherent at the 4-week interim and 8-week final follow-ups if they are adherent for ≥2 of 3 weeks evaluated between visits. As a secondary measure of dietary adherence, MyPlate servings of fruits, vegetables, and whole grains intake will be assessed in each diet group.
Participants are told that adherence to their assigned diet will also be measured via blood and urine, but no further details are provided. Both plasma and spot urine trimethylamine-N-oxide (TMAO) concentrations, an oxidized metabolite of choline, are measured in all participants from aliquots stored at −80 °C. l-carnitine is an abundant nutrient in red meat and contains a structure similar to choline [26]. These markers will be used as objective evidence of adherence.
3.12. Data storage and confidentiality
All participant study data are kept strictly confidential and managed electronically with REDCap, a secure web-based application for building and managing online databases [27]. The program provides automated export of data to common statistical packages (e.g. SPSS, R), and branching logic, file uploading, and calculated fields. Hard copies of data are secured in a locked cabinet in a locked office on NYU School of Medicine property. All biological specimens are encoded with a study number that had no patient identifier information.
Food Processor 11.0.137 (ESHA research, Salem, OR) is used to enter the 24-h dietary recalls and food records for each participant. De-identified dietary intake data are obtained for24 days as per the following schedule: 4-day food record from the week prior to the baseline visit and at weeks 4 and 8; and twice a week 24-h dietary recalls during weeks 1 through 3 and weeks 5 through 7. The nutrient variables of interest include: energy (kcal), protein (g), carbohydrates (g), dietary fiber (g), soluble fiber (g), fat (g), saturated fat (g), trans fatty acids (g), cholesterol (mg), omega 3 fatty acids (g), sodium (mg), and proportion of recommendations met for MyPlate.gov food groups (grain, vegetable, fruit, dairy, protein).
3.13. Statistical analysis
Demographic and baseline characteristics will be presented. Summary statistics (number, mean, standard deviation, median, interquartile range) will be calculated for continuous variables. Frequency and percentages will be calculated for categorical variables. Normal assumptions will be checked using quantile-quantile plots and Shapiro-Wilks test. Non-parametric alternatives and transformations will be considered.
Baseline variables will be compared between the two dietary groups with t-test for normally distributed continuous variables, Mann-Whitney-Wilcoxon for skewed continuous variables, and tests of proportions for categorical variables. Changes in endpoints over time within and between groups will be compared using a linear mixed effect model to assess the outcome over covariates including collection time point (baseline, 4-week interim, and 8-week final), type of diet, and the interaction term between these two variables. The interaction term will assess whether the changes in outcome over time differ between treatment groups. Although the anticipated rate of missing data is anticipated to be low, appropriate statistical methods (e.g. last observation forward, multiple imputation) will be used based upon the recommendations of the study biostatistician. Statistical significance for the primary endpoint will be tested using a 2-sided alpha level of 0.05. The primary analysis will be conducted as intention-to-treat. Secondary analyses will include a per-protocol evaluation and a sensitivity analysis will be conducted for different thresholds of adherence as defined by the adherence tool and metabolites.
3.14. Sample size calculation
Preliminary data from our cardiac catheterization laboratory demonstrated a mean hs-CRP concentration of 2.07 mg/L ± 0.57 mg/L in patients with CAD. Based on an estimated decrease in mean hs-CRP concentration by 20% with the vegan diet as compared to the AHA diet, and a 2-sided 2 sample t-test, the number of participants needed in each group to achieve 80% power at the 0.05 significance level was estimated to be 30 [16]. We approximated that up to 40% of the participants enrolled may have low hs-CRP concentrations at baseline (floor effect) or drop out, and, thus, increased the sample size in each group to 50. Finally, approximately 1/3 of participants who signed screening consent were expected to not show up to their baseline visit prior to randomization. Therefore, IRB approval was granted for the consent of 150 screened participants to reach our goal of 100 randomized participants.
4. Discussion
Patients with CAD require evidenced-based strategies to reduce inflammation and improve clinical outcomes. Lifestyle interventions, including diet, may be an important approach to address the risks associated with inflammation and impact cardiovascular health. Although epidemiological research supports the relationship between “heart healthy” dietary patterns and vegan diets on inflammatory markers, well-designed randomized clinical trials are limited and the results are mixed [28], [29], [30], [31]. Furthermore, the study participants included in previous research may have been free from cardiovascular disease. We present the first study to date to comprehensively assess the impact of a vegan diet compared to the currently recommended AHA diet on multiple indices of inflammation and glycometabolic risk in a randomized clinical trial of patients with angiographically defined CAD.
Our study design incorporates several unique and innovative elements to address the research questions. First, this study compares the impact of a vegan diet and the AHA diet on multiple indices of inflammation and glycometabolic risk. All participants receive random assignment to an intervention with the potential to improve health. Thus, the study design calls for as much attention to the control group (AHA) as the comparator group (vegan). Participants are counseled equally on their respective dietary guidelines and complete twice weekly 24-h dietary recalls that allow for equal amount of contact with the research dietitian. The intervention groups are also provided with the same resources: provision of a starter kit that includes a food scale, measuring cups, measuring spoons, cookbook, two-week sample menu with recipes, nutrition education materials summarizing the diet (12 pages), and handouts on serving size and instructions on how to read nutrition labels, as well as weekly groceries. The only difference in these resources is the substitution of animal-based protein in the AHA group for plant-based protein in the vegan group.
Second, we provide groceries and dietary counseling for all study participants. Participants in this study receive $80 in weekly groceries as part of the study design. During a weekly phone call from the study dietitian, participants complete a grocery order for food items to be delivered to their home from a grocery delivery service. Participants are able to select items from a list of foods/ingredients (Supplemental appendix 4) that support the dietary recommendations of their intervention assignment. Participants are also provided with a two-week sample menu that incorporates the foods/ingredients on the grocery order (Supplemental appendix 2). Most nutrition clinical studies utilize nutrition education, cooking classes, educational resources, and individual/group counseling as part of the study design when educating research participants on their respective intervention assignment. However, providing whole foods/ingredients to research participants may enhance adherence to the dietary guidelines and is an innovative aspect of this study. During the 15 one-on-one educational sessions with the study research dietitian, the research participants learn how to incorporate these foods/ingredients into healthy meals, which may continue once the study has ended. Other studies have provided some food items to research participants, such as meal replacement products, frozen food entrees, and margarine [32], [33]. However, increasing data suggest a failure of translating research findings into clinical practice, particularly those related to adherence to dietary advice [34]. Our study provides study participants with the education and tools necessary to prepare healthy meals that follow the dietary recommendations of their intervention assignment and sustain the behavior. This, together with the twice a week telephone calls from the study registered dietitian, may beneficially impact dietary adherence [35].
Third, this study incorporates detailed scripts and guidelines, allowing the intervention materials to be disseminated consistently by the study registered dietitian. A counseling guide was developed to encourage a similar discussion between the study registered dietitian and study participants randomly assigned to each intervention group. Furthermore, a script was created for the 24-h dietary recalls so that the initiation of the conversation, questions asked, and guidance for participants were similar for participants assigned to the same intervention group.
Fourth, the multiple measures of adherence are another central aspect of the study's design. These measures include dietary intake (animal protein recommendation for each diet; MyPlate servings of fruits, vegetables, and whole grains) and both plasma and urine TMAO concentrations. Although TMAO concentrations are only detected after the consumption of red meat, participants were only told that there was a marker in the blood and urine that allowed investigators to determine dietary adherence [26]. A unique study-specific scoring system was also developed, as described above, to quantitate level of dietary adherence using similar criteria in each group.
There are several limitations to the current study. First, the use of a Treatment Self-Regulation Questionnaire may limit generalizability of the study results. Given our relatively small sample size, we need to effectively enroll potentially adherent participants, allowing us to better examine the impact of the intervention. Second, although the study registered dietitian consistently encourages intake of whole food choices at each of the 15 educational one-on-one sessions, convenience items are allowed to promote adherence to the diets in the real-world setting. Third, the study nutrition education materials are based on the TLC Program, guidelines that were recommended by the AHA at the time the intervention materials were developed. Although dietary recommendations continue to evolve, the TLC Program is consistent with the current AHA guidelines.
Finally, irrespective of the primary findings, this randomized blinded-endpoint trial will be of scientific importance as a large repository of biological specimens with a comprehensive analysis of inflammatory markers, lipid profiles, and glucometabolic parameters in conjunction with detailed dietary reports in patients with CAD. Thus, the biologic repository can allow for exploration of the effects of different dietary strategies on additional markers of cardiometabolic health. In addition, the results of this study may provide preliminary data for a larger randomized trial evaluating the effects of the two dietary strategies on traditional clinical cardiovascular outcomes in patients with CAD. Alternatively, if significant reductions in inflammatory or glucometabolic profiles are noted in both groups, data from the current study may be used to support the evaluation of a rigorous approach to dietary counseling on long-term cardiovascular events in patients with established CAD.
In summary, the EVADE CAD study is a unique and innovative randomized clinical trial comparing the effectiveness of an 8-week vegan diet to the AHA-recommended diet on hs-CRP concentrations in patients with angiographically defined CAD. The study results may provide important information for a larger outcomes-based trial evaluating dietary strategies to decrease inflammation and improve glucometabolic profiles in patients with CAD.
Acknowledgements
Binita Shah was supported in part by the National Center for Advancing Translational Sciences (NYU CTSA UL1TR000038) and New York State (Empire Clinical Research Investigator Program) in 2015 and the Biomedical Laboratory Research & Development Service of the VA Office of Research and Development (iK2CX001074) in 2016. Dr. Newman was partially funded by the National Heart, Lung, and Blood Institute (NHBLI) of the National Institute of Health (NIH, K23HL125991) and the American Heart Association Mentored Clinical and Population Research Award (15MCPRP24480132). Statistical support was provided in part by the New York University School of Medicine Cardiovascular Outcomes Group.
We would like to acknowledge the contributions of Bryan Velez de Villa, BS; Erini Farid; Christine A. Berthoumieux, BA; Elissa Driggin, BS; Melissa Goldman, MA; and Tamsin Shephard, BS to the data collection and electronic entry for this study.
Footnotes
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.conctc.2017.09.003.
Appendix A. Supplementary data
The following are the supplementary data related to this article:
References
- 1.Packard R.R., Libby P. Inflammation in atherosclerosis: from vascular biology to biomarker discovery and risk prediction. Clin. Chem. 2008;54:24–38. doi: 10.1373/clinchem.2007.097360. [DOI] [PubMed] [Google Scholar]
- 2.Nidorf M., Thompson P.L. Effect of colchicine (0.5 mg twice daily) on high-sensitivity C-reactive protein independent of aspirin and atorvastatin in participants with stable coronary artery disease. Am. J. Cardiol. 2007;99:805–807. doi: 10.1016/j.amjcard.2006.10.039. [DOI] [PubMed] [Google Scholar]
- 3.Nidorf S.M., Eikelboom J.W., Budgeon C.A. Low-dose colchicine for secondary prevention of cardiovascular disease. J. Am. Coll. Cardiol. 2013;61:404–410. doi: 10.1016/j.jacc.2012.10.027. [DOI] [PubMed] [Google Scholar]
- 4.Patti G., Massimo C., Pasceri V., Colonna D., Nusca A., Miglionico M., D'Ambrosio A., Covino E., Di Sciascio G. Protection from procedural myocardial injury by atorvastatin is associated with lower levels of adhesion molecules after percutaneous coronary intervention: results from the ARMYDA-CAMs substudy. J. Am. Coll. Cardiol. 2006;48:1560–1566. doi: 10.1016/j.jacc.2006.06.061. [DOI] [PubMed] [Google Scholar]
- 5.Pasceri V., Patti G., Nusca A., Pristipino C., Richichi G., Di Sciascio G. Randomized trial of atorvastatin for reduction of myocardial damage during coronary intervention: results from the ARMYDA study. Circulation. 2004;110:674–678. doi: 10.1161/01.CIR.0000137828.06205.87. [DOI] [PubMed] [Google Scholar]
- 6.Kwaijtaal M., van Diest R., Bar F.W., van der Ven A.J., Bruggeman C.A., de Baets M.H., Appels A. Inflammatory markers predict late cardiac events in participants who are exhausted after percutnaeous coronary intervention. Atherosclerosis. 2005;182:341–348. doi: 10.1016/j.atherosclerosis.2005.02.022. [DOI] [PubMed] [Google Scholar]
- 7.Buffon A., Liuzzo G., Biascucci L.M., Pasqualetti P., Ramazzotti V., Rebuzzi A.G., Crea F., Maseri A. Preprocedural serum levels of C-reactive protein predict early complications and late restenosis after coronary angioplasty. J. Am. Coll. Cardiol. 1999;34:1512–1521. doi: 10.1016/s0735-1097(99)00348-4. [DOI] [PubMed] [Google Scholar]
- 8.Walter D.H., Fichtlscherer S., Sellwig M., Auch-Schwelk W., Schachinger V., Zeiher A.M. Preprocedural C-reactive protein and cardiovascular events after coronary stent implantation. J. Am. Coll. Cardiol. 2001;37:839–846. doi: 10.1016/s0735-1097(00)01193-1. [DOI] [PubMed] [Google Scholar]
- 9.Ridker P.M., Danielson E., Fonseca F.A., Genest J., Gotto A.M., Jr., Kastelein J.J., Koenig W., Libby P., Lorenzatti A.J., MacFadyen J.G., Nordestgaard B.G., Shepherd J., Willerson J.T., Glynn R.J. JUPITER Study Group. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N. Engl. J. Med. 2008;359:2195–2207. doi: 10.1056/NEJMoa0807646. [DOI] [PubMed] [Google Scholar]
- 10.Oesterle A., Laufs U., Liao J.K. Pleiotropic effects of statins on the cardiovascular system. Circ. Res. 2017;120:229–243. doi: 10.1161/CIRCRESAHA.116.308537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.xxx.
- 12.Appel L.J., Moore T.J., Obarzanek E., Vollmer W.M., Svetkey L.P., Sacks F.M., Bray G.A., Vogt T.M., Cutler J.A., Windhauser M.M., Lin P.H., Karanja N. A clinical trial of the effects of dietary patterns on blood pressure. DASH Collaborative Research Group. N. Engl. J. Med. 1997;336:1117–1124. doi: 10.1056/NEJM199704173361601. [DOI] [PubMed] [Google Scholar]
- 13.Appel L.J., Sacks F.M., Carey V.J., Obarzanek E., Swain J.F., Miller E.R., 3rd, Conlin P.R., Erlinger T.P., Rosner B.A., Laranjo N.M., Charleston J., McCarron P., Bishop L.M. OmniHeart Collaborative Research Group. Effects of protein, monounsaturated fat, and carbohydrate intake on blood pressure and serum lipids: results of the OmniHeart randomized trial. JAMA. 2005;294:2455–2464. doi: 10.1001/jama.294.19.2455. [DOI] [PubMed] [Google Scholar]
- 14.Howard B.V., Van Horn L., Hsia J., Manson J.E., Stefanick M.L., Wassertheil-Smoller S., Kuller L.H., LaCroix A.Z., Langer R.D., Lasser N.L., Lewis C.E., Limacher M.C., Margolis K.L., Mysiw W.J., Ockene J.K., Parker L.M., Perri M.G., Phillips L., Prentice R.L., Robbins J., Rossouw J.E., Sarto G.E., Schatz I.J., Snetselaar L.G., Stevens V.J., Tinker L.F., Trevisan M., Vitolins M.Z., Anderson G.L., Assaf A.R., Bassford T., Beresford S.A., Black H.R., Brunner R.L., Brzyski R.G., Caan B., Chlebowski R.T., Gass M., Granek I., Greenland P., Hays J., Heber D., Heiss G., Hendrix S.L., Hubbell F.A., Johnson K.C., Kotchen J.M. Low-fat dietary pattern and risk of cardiovascular disease: the Women's health initiative randomized controlled dietary modification trial. JAMA. 2006;295:655–666. doi: 10.1001/jama.295.6.655. [DOI] [PubMed] [Google Scholar]
- 15.Estruch R., Ros E., Salas-Salvadó J., Covas M.I., Corella D., Arós F., Gómez-Gracia E., Ruiz-Gutiérrez V., Fiol M., Lapetra J., Lamuela-Raventos R.M., Serra-Majem L., Pintó X., Basora J., Muñoz M.A., Sorlí J.V., Martínez J.A., Martínez-González M.A. PREDIMED Study Investigators. Primary prevention of cardiovascular disease with a Mediterranean diet. N. Engl. J. Med. 2013;368:1279–1290. [Google Scholar]
- 16.Dod H.S., Bhardwaj R., Sajja V., Weidner G., Hobbs G.R., Konat G.W., Manivannan S., Gharib W., Warden B.E., Nanda N.C., Beto R.J., Ornish D., Jain A.C. Effect of intensive lifestyle changes on endothelial function and on inflammatory markers of atherosclerosis. Am. J. Cardiol. 2010;105:362–367. doi: 10.1016/j.amjcard.2009.09.038. [DOI] [PubMed] [Google Scholar]
- 17.Ornish D., Scherwitz L.W., Doody R.S., Kesten D., McLanahan S.M., Brown S.E., DePuey E., Sonnemaker R., Haynes C., Lester J., McAllister G.K., Hall R.J., Burdine J.A., Gotto A.M., Jr. Effects of stress management training and dietary changes in treating ischemic heart disease. JAMA. 1983;249:54–59. [PubMed] [Google Scholar]
- 18.Ornish D., Brown S.E., Scherwitz L.W., Billings J.H., Armstrong W.T., Ports T.A., McLanahan S.M., Kirkeeide R.L., Brand R.J., Gould K.L. Can lifestyle changes reverse coronary heart disease? The Lifestyle Heart Trial. Lancet. 1990;336:129–133. doi: 10.1016/0140-6736(90)91656-u. [DOI] [PubMed] [Google Scholar]
- 19.Hansson L., Hedner T., Dahlöf B. Prospective randomized open blinded end-point (PROBE) study. A novel design for intervention trials. Prospective Randomized Open Blinded End-Point. Blood Press. 1992;1:113–119. doi: 10.3109/08037059209077502. [DOI] [PubMed] [Google Scholar]
- 20.Ryan R.M., Connell J.P. Perceived locus of causality and internalization: examining reasons for acting in two domains. J. Personal. Soc. Psychol. 1989;57:749–761. doi: 10.1037//0022-3514.57.5.749. [DOI] [PubMed] [Google Scholar]
- 21.Levesque C.S., Williams G.C., Elliot D., Pickering M.A., Bodenhamer B., Finley P.J. Validating the theoretical structure of the Treatment Self-Regulation Questionnaire (TSRQ) across three different health behaviors. Health Educ. Res. 2007;22:691–702. doi: 10.1093/her/cyl148. [DOI] [PubMed] [Google Scholar]
- 22.Pickering T.G., Hall J.E., Appel L.J., Falkner B.E., Graves J., Hill M.N., Jones D.W., Kurtz T., Sheps S.G., Roccella E.J. Recommendations for blood pressure measurement in humans and experimental animals: part 1: blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Circulation. 2005;111:697–716. doi: 10.1161/01.CIR.0000154900.76284.F6. [DOI] [PubMed] [Google Scholar]
- 23.Bonetti P.O., Pumper G.M., Higano S.T., Holmes D.R., Jr., Kuvin J.T., Lerman A. Noninvasive identification of patients with early coronary atherosclerosis by assessment of digital reactive hyperemia. J. Am. Coll. Cardiol. 2004;44:2137–2141. doi: 10.1016/j.jacc.2004.08.062. [DOI] [PubMed] [Google Scholar]
- 24.Rabin R., de Charro F. EQ-5D: a measure of health status from the EuroQol Group. Ann. Med. 2001;33:337–343. doi: 10.3109/07853890109002087. [DOI] [PubMed] [Google Scholar]
- 25.Craig C.L., Marshall A.L., Sjöström M., Bauman A.E., Booth M.L., Ainsworth B.E., Pratt M., Ekelund U., Yngve A., Sallis J.F., Oja P. International physical activity questionnaire: 12-country reliability and validity. Med. Sci. Sports Exerc. 2003;35:1381–1395. doi: 10.1249/01.MSS.0000078924.61453.FB. [DOI] [PubMed] [Google Scholar]
- 26.Koeth R.A., Wang Z., Levison B.S. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat. Med. 2013;19:576–585. doi: 10.1038/nm.3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harris P.A., Taylor R., Thielke R., Payne J., Gonzalez N., Conde J.G. Research electronic data capture (REDCap)-A metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inf. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jiao J., Xu J.Y., Zhang W., Han S., Qin L.Q. Effect of dietary fiber on circulating C-reactive protein in overweight and obese adults: a meta-analysis of randomized controlled trials. Int. J. Food Sci. Nutr. 2015;66:114–119. doi: 10.3109/09637486.2014.959898. [DOI] [PubMed] [Google Scholar]
- 29.Gopinath B., Buyken A.E., Flood V.M., Empson M., Rochtchina E., Mitchell P. Consumption of polyunsaturated fatty acids, fish, and nuts and risk of inflammatory disease mortality. Am. J. Clin. Nutr. 2011;93:1073–1079. doi: 10.3945/ajcn.110.009977. [DOI] [PubMed] [Google Scholar]
- 30.Oliveira A., Rodríguez-Artalejo F., Lopes C. The association of fruits, vegetables, antioxidant vitamins and fibre intake with high-sensitivity C-reactive protein: sex and body mass index interactions. Eur. J. Clin. Nutr. 2009;63:1345–1352. doi: 10.1038/ejcn.2009.61. [DOI] [PubMed] [Google Scholar]
- 31.Corley J., Kyle J.A., Starr J.M., McNeill G., Deary I.J. Dietary factors and biomarkers of systemic inflammation in older people: the Lothian Birth Cohort 1936. Br. J. Nutr. 2015;114:1088–1098. doi: 10.1017/S000711451500210X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ryan D.H., Espeland M.A., Foster G.D., Haffner S.M., Hubbard V.S., Johnson K.C., Kahn S.E., Knowler W.C., Yanovski S.Z. Look AHEAD Research Group. Look AHEAD (Action for Health in Diabetes): design and methods for a clinical trial of weight loss for the prevention of cardiovascular disease in type 2 diabetes. Control Clin. Trials. 2003;24:610–618. doi: 10.1016/s0197-2456(03)00064-3. [DOI] [PubMed] [Google Scholar]
- 33.de Lorgeril M., Renaud S., Mamelle N., Salen P., Martin J.L., Monjaud I., Guidollet J., Touboul P., Delaye J. Mediterranean alpha-linolenic acid-rich diet in secondary prevention of coronary heart disease. Lancet. 1994 Jun 11;343:1454–1459. doi: 10.1016/s0140-6736(94)92580-1. [DOI] [PubMed] [Google Scholar]
- 34.Desroches S., Lapointe A., Ratte S., Gravel K., Legare F., Thirsk J. Interventions to enhance adherence to dietary advice for preventing and managing chronic diseases in adults: a study protocol. BMC Public Health. 2011;11:111. doi: 10.1186/1471-2458-11-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Desroches S., Lapointe A., Ratte S., Gravel K., Legare F., Turcotte S. Interventions to enhance adherence to dietary advice for preventing and managing chronic diseases in adults. Cochrane Database Syst. Rev. 2013 doi: 10.1002/14651858.CD008722.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.