Summary
Heart failure with preserved ejection fraction (HFpEF) is characterized by signs and symptoms of heart failure in the presence of a normal left ventricular ejection fraction. Despite accounting for up to 50% of all clinical presentations of heart failure, the mechanisms implicated in HFpEF are poorly understood, thus precluding effective therapy. The pathophysiological heterogeneity in the HFpEF phenotype also contributes to this disease and likely to the absence of evidence-based therapies. Limited access to human samples and imperfect animal models that completely recapitulate the human HFpEF phenotype have impeded our understanding of the mechanistic underpinnings that exist in this disease. Aging and comorbidities such as atrial fibrillation, hypertension, diabetes and obesity, pulmonary hypertension, and renal dysfunction are highly associated with HFpEF, yet the relationship and contribution between them remains ill-defined. This review discusses some of the distinctive clinical features of HFpEF in association with these comorbidities and highlights the advantages and disadvantage of commonly used murine models used to study the HFpEF phenotype.
Key Words: comorbidities, HFpEF, murine model
Abbreviations and Acronyms: AAC, ascending aortic constriction; BNP, brain natriuretic peptide; DOCA, deoxycorticosterone acetate; EF, ejection fraction; HF, heart failure; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; LV, left ventricular; NaCl, sodium chloride; SAMP, spontaneous senescence prone; SAMR, spontaneous senescence resistant; SHR, spontaneously hypertensive rat; TAC, transverse aortic constriction; ZSF1, Zucker fatty and spontaneously hypertensive heart failure rat
Central Illustration
Understanding HFpEF in Humans: The Reality of HFpEF Today, Pathophysiology, and Dilemmas
Heart failure with preserved ejection fraction (HFpEF): The clinical entity
Heart failure (HF) is characterized by dyspnea at low-normal levels of activity, and fluid and sodium retention. HF involves impaired heart function, and the percent of blood volume ejected with each beat or ejection fraction (EF), has traditionally served as an indicator of pump dysfunction (1). Decades of extensive basic and clinical research has focused on HF that involves impaired left ventricular (LV) systolic function (systolic HF), also now known as HF with reduced ejection fraction (HFrEF). However, nearly one-half of the patients with HF symptoms have a normal/preserved LVEF. Generally, an LVEF ≥50% is used as a threshold for characterizing as preserved LVEF 2, 3. At present, evidence from clinical studies supports the dichotomized distinction that HFpEF and HFrEF are fundamentally and diametrically different with regard to pathophysiology and therapeutic response 4, 5.
HFpEF versus HFrEF: When not only EF matters
HFpEF and HFrEF are fundamentally different beyond segregating and categorizing HF patients into more homogenous groups simply by LVEF. However, this compartmentalization not only allows HF patients with comparable hemodynamics, pathophysiologies, and unique patterns of cardiac and cellular remodeling to be clustered together but also identifies treatment responses in those specific groups (5). HFrEF can be regarded as a cardiac-centric syndrome driven by myocardial cell loss and dysfunction with the heart being subjected to higher wall stress as shown by elevated levels of brain natriuretic peptide (BNP). HFpEF, alternatively, is a systemic syndrome characterized by accumulated risk factors and comorbidities and a noncompliant and stiff heart that is exposed to lower wall stress, reflected in lower BNP levels that, although elevated, are not as high as in HFrEF 6, 7, 8. Therefore, it appears that HFrEF begins from the heart and leads to peripheral changes, whereas HFpEF starts in the periphery and culminates at the heart 4, 9.
Speaking the same language: The challenge for translational HFpEF researchers
Semantics related to HFpEF are a formidable task for translational researchers. Defining various terms, and therefore collecting relevant data, remains a challenge both clinically and in the preclinical area. One of the main sources of confusion lies in the distinction between diastolic dysfunction and HFpEF. These 2 terms have been used interchangeably in both the preclinical and the clinical literature. Diastolic dysfunction was widely and incorrectly touted to be the sine qua non for HFpEF but by itself is not enough to establish it. For example, normal subjects may have diastolic dysfunction, yet have no clinical features of HFpEF 10, 11. Additionally, diastolic dysfunction is a common occurrence in HFrEF (12). Also, use of strain imaging with echocardiography shows subtle abnormalities in systolic function in some HFpEF populations despite preserved global LVEF 13, 14. According to the 2013 American College of Cardiology Foundation (ACCF)/American Heart Association (AHA) guideline for the management of HF (3), neither LV hypertrophy nor diastolic dysfunction is required for diagnosis of HFpEF, whereas in the European HF guidelines relevant structural and/or diastolic dysfunction is a prerequisite (2). HFpEF is also a term, in the sphere of scientific literature, that is virtually nonexistent, given that the U.S. National Library of Medicine’s system of medical subject headings (MeSH) still uses the outmoded terms “HF, systolic” and “HF, diastolic” to index publications. Hence, we prefer the terms HFpEF and HFrEF as they are mutually exclusive, whereas diastolic dysfunction may be present in either systolic HF (HFrEF) or diastolic HF (HFpEF). However, we acknowledge that until universally agreed definitions are adopted, translational research in the field may be hindered.
Modeling HFpEF in the Lab: Different Phenotypes for a Complex Disease
Animal models, as opposed to isolated organ and/or cell preparations, allow examination of physiological effects of cardiac function (15). However, the search for an animal model that resembles the human HFpEF phenotype is akin to “a fishing expedition” and the use and comprehensive characterization of an HFpEF animal model that recapitulates human HFpEF has hampered advancing the understanding of HFpEF. The limited number of truly authentic HFpEF animal models poses a major limitation when investigating new insights into its pathophysiology and in the development of new therapies for HFpEF 16, 17. Moreover, if defining HFpEF in humans evokes controversy (18), it appears that animal models likely follow suit. Animals, unlike humans, cannot report symptoms. LVEF, diastolic dysfunction, and heart structure can be measured directly, but signs must be inferred from animal behavior. Additionally, separate from LVEF, a single measure of systolic function, there are other measures of LV systolic function such as myocardial velocities, strain, and strain rate (measures of myocardial contractility), which may be impaired in HFpEF 13, 14, 19. HFpEF is a complex syndrome where etiologic and pathophysiological paths, by which individual patients develop the disease, are variable (20). Specific comorbidities, as well as aging, are highly associated with the HFpEF phenotype. Given this heterogeneity, it is likely that any animal model only resembles a certain proportion of HFpEF patients. An “ideal” animal model should meet various requirements to mimic human disease, including cardiac, hemodynamic, neurohormonal, and peripheral aberrations commonly seen in HFpEF patients (21). However, similar to humans, a “one-size-fits-all” strategy is unlikely to work in animal models. That being said, the relative importance of comorbidities on the initiation, development, and treatment of HFpEF is unknown (22). A more tailored approach focusing on specific phenotypes is needed in the laboratory to understand the complex interactions underlying this disease. HFpEF in humans is strongly associated with diseases such as hypertension, obesity, and diabetes mellitus 3, 6, which by themselves often occur together as part of the metabolic syndrome. Yet there is much overlap between these comorbidities, and a direct causal relationship between one and the other and HFpEF has not been established. However, if such a causal relationship can be elucidated, a step toward developing targeted therapies might be in sight. A major benefit of an animal model is that it is easier to examine the contribution of each risk factor in isolation without additional comorbidities confounding the findings. Animal models are useful to understand the effect of interventions on the disease process, which in turn helps in the development of therapeutic approaches. Murine models are convenient and inexpensive, particularly in the quest to investigate the molecular basis underlying HFpEF. This is due to the availability of genetically engineered strains and molecular techniques to manipulate sub cellular processes occurring in the heart, as well as the relatively short life span of murine versus larger animal models 23, 24. It is important to stress that although murine models offer easy access to genetic manipulations, extensive characterization of the actual HFpEF phenotype is required to be fully useful for HFpEF studies (25). This being said, specific genetic manipulations may be useful for the development of therapeutics by investigating the specific role of a molecule/factor in the HFpEF syndrome. However, in contrast, specific genetic manipulations that manifest a purported HFpEF phenotype are of little use in understanding common forms of HFpEF or as a disease model.
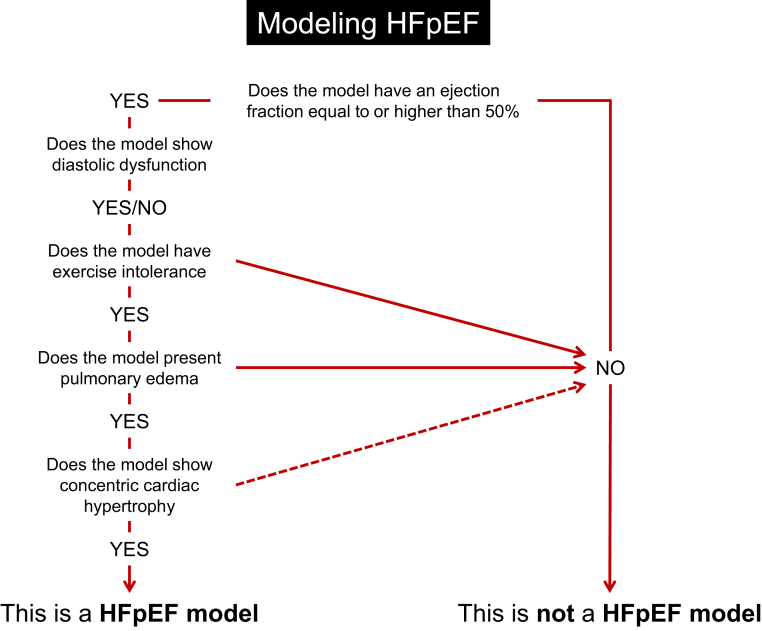
In this review, we consider whether some of the available murine models of cardiac hypertrophy and HF recapitulate human HFpEF disease. Attention has been paid to those phenotypical characteristics that would characterize an “ideal” HFpEF animal model, which are included in Figure 1. Although diastolic dysfunction is certainly part of the compendium of the HFpEF phenotype, it is only 1 piece of the puzzle. It is also necessary to identify models with systemic alterations that are implicated in human HFpEF. These include impairments in peripheral function, such as exercise intolerance, alterations in pulmonary physiology, and cardiac morphology (such as hypertrophy, fibrosis, and capillary rarefaction). We chose to emphasize models that induce HFpEF by the most common comorbidities associated with human HFpEF, although we acknowledge that murine models are less influenced by comorbidities than patients. Table 1 outlines the models included in the present review, divided into aging and comorbidities seen in HFpEF patients (hypertension, obesity and diabetes, atrial fibrillation, pulmonary hypertension, and renal dysfunction).
Figure 1.
Flowchart Identifying Major Features to Fulfill When Modeling HFpEF in Preclinical Studies
Table 1.
Murine Models of HFpEF Classified by Aging and Comorbidities Seen in HFpEF Patients (Hypertension, Obesity and Diabetes, Atrial Fibrillation, Pulmonary Hypertension, and Renal Insufficiency)
| Comorbidity | Animal Models | HFpEF Pathophysiology |
HFpEF | Ref. # | ||||
|---|---|---|---|---|---|---|---|---|
| LV Structure | Systolic Function | Diastolic Function | Pulmonary Congestion | Exercise Intolerance | ||||
| Aging | Fisher 344 rat | Eccentric hypertrophy | Mild dysfunction | Diastolic dysfunction | Yes | Yes | ☹ | 33, 38, 39, 40, 42, 43 |
| SAMP8 mouse | Not described | Preserved to reduced | Diastolic dysfunction | No | Yes | ☹ | 49, 50, 51 | |
| Hypertension | Aldosterone-infused uninephrectomized mouse | Concentric hypertrophy | Preserved | Diastolic dysfunction | Yes | Yes | ☺ | 68, 70, 71, 72, 73, 74 |
| Angiotensin II-infused mouse | Concentric and dilated hypertrophy | Preserved | Diastolic dysfunction | Yes | Yes | ☺ | 16, 81, 82, 84, 85, 86, 89, 90, 91 | |
| Dahl salt-sensitive rat | Concentric to eccentric hypertrophy | Preserved to reduced | Diastolic dysfunction | Yes | Yes | ☹ | 95, 96, 97, 98 | |
| DOCA salt rat | Concentric hypertrophy | Preserved | Diastolic dysfunction | Not described | Not described | ? | 107, 108 | |
| Spontaneously hypertensive rat | Concentric to eccentric hypertrophy | Preserved to reduced | Diastolic dysfunction | Yes | Yes | ☹ | 119, 120, 121, 122 | |
| Transverse aortic constriction-induced pressure overload mouse | Concentric to eccentric hypertrophy | Preserved to reduced | Diastolic dysfunction | Yes | Yes | ☹ | 70, 127, 128, 129 | |
| Obesity and diabetes | db/db mouse | Concentric hypertrophy | Preserved | Diastolic dysfunction | Yes | Yes | ☺ | 152, 155, 159, 160, 161, 162, 163, 164, 165 |
| ob/ob mouse | Concentric hypertrophy | Preserved | Diastolic dysfunction | Not described | +/− | ? | 175, 176, 177, 178, 185 | |
| Streptozotocin-induced diabetic rat | Eccentric hypertrophy or heart atrophy | +/− | Diastolic dysfunction | +/− | Not described | ☹ | 191, 195, 196, 197, 200, 203, 204, 207, 208, 212, 213 | |
| ZSF1rat | Concentric hypertrophy | Preserved | Diastolic dysfunction | Yes | Yes | ☺ | 226, 228, 229, 231 | |
| Atrial fibrillation | Genetic model | None or eccentric hypertrophy | Mild dysfunction | Not described | Not described | Not described | ? | (245) |
| Pulmonary hypertension | Hypoxia-induced pulmonary hypertension | Concentric hypertrophy | Preserved | Not described | Not described | Not described | ? | 267, 268 |
| Renal insufficiency | Subtotal nephrectomy rat | Concentric to eccentric hypertrophy | Preserved to reduced | Diastolic dysfunction | Yes | Yes | ☹ | 275, 277, 278, 279, 281, 282 |
☺ = HFpEF phenotype; ☹ = not a HFpEF phenotype; HFpEF = heart failure with preserved ejection fraction.
Aging HFpEF models
According to the Framingham Heart Study and the Baltimore Longitudinal Study on Aging, there is an increased prevalence of LV hypertrophy with age, even in the absence of clinical hypertension. Diastolic function worsens in the elderly, whereas LVEF is relatively preserved in subjects at rest 26, 27, 28. Aging primarily plays a role in modifying both the passive stiffness of the myocardium and the active diastolic relaxation properties of the cardiomyocyte (28). Alterations in the peripheral vasculature, pulmonary function, oxygen transport, and skeletal muscle function, during aging are also key determinants of exertional fatigue and/or dyspnea in human HFpEF (29).
Mice and rats are commonly used to study aging and age-related diseases (30). Their relatively short life span makes them easier and more cost-effective to study than long-lived animals. Cardiac aging in murine recapitulates many changes observed in humans, with age-dependent increases in LV hypertrophy as well as impaired diastolic function 31, 32. While systolic function declines only slightly with age, the maximal exercise capacity and O2 consumption are significantly reduced (28). Likewise, progressive myocardial degeneration and fibrosis are known to develop in rats with aging (33). Mechanisms implicated in age-associated changes in myocardial structure and function include cardiomyocyte enlargement (34), decrease in cardiomyocyte number due to increased apoptosis and necrosis 35, 36, and compensatory cardiac remodeling with alterations in extracellular matrix composition and cardiac fibroblast number and function (26).
Fischer 344 rat
The Fisher 344 aging rat mirrors the pathophysiology of aging in various organs, including the heart (17). By 28 months to 30 months of age, these rats have cardiac hypertrophy comparable to rats subjected to long-term ascending aortic constriction-induced pressure overload. This cardiac hypertrophy is accompanied by LV chamber dilation, infrequently seen in HFpEF (37), and by extensive interstitial fibrosis with subsequent loss of ventricular cardiomyocytes by 36 months 33, 38. Between 22 months and 30 months of age, the Fischer 344 rat develops mild, progressive LV diastolic dysfunction, with impaired LV relaxation and decreased LV compliance 33, 39, 40, 41, 42. Exercise intolerance is also apparent (43). Pulmonary congestion is evident at 25 months of age, with a modest decline in LV systolic function (43). Although a priori, signs of HF are evident; the development of eccentric hypertrophy and LV dilation makes the Fisher 344 rat limited as an HFpEF model.
Spontaneous senescence prone (SAMP) mouse
The SAMP mouse is a model of spontaneous senescence that shows disorders commonly seen in aging humans, such as neurodegeneration or carcinogenesis 44, 45, 46. The senescence-prone (SAMP) and control senescence-resistant (SAMR) strains were singled out by continuous sister-brother mating (47). The SAMR strains show normal aging characteristics, whereas the SAMP strains show accelerated senescence and age-related pathological phenotypes, similar to aging disorders seen in humans. The aging features in SAMP mice occur at a younger age (10 months to 14 months) compared with normal-aged mice (≥20 months) (48). SAMP8 and SAMR1 are the best-studied strains with respect to cardiovascular disease (49). SAMP8 mice show cardiac dysfunction and adverse cardiac remodeling during senescence (50) with evidence of cardiomyocyte hypertrophy and fibrosis 49, 51. These mice also develop diastolic dysfunction independent of blood pressure changes (49). Systolic function is similar (49) or slightly decreased (51) in SAMP8 compared with SAMR1, but LVEF appears preserved. Evidence suggests that this is a model of diastolic dysfunction; however, no studies have described the presence of HF or HFpEF.
Hypertension-induced HFpEF models
Systemic hypertension is the single most important comorbidity seen in HFpEF, with a prevalence of 60% to 89% reported from large controlled trials, epidemiological studies, and HF registries (3). Elevated blood pressure is a major determinant of LV structural alterations. The relationship between systemic hypertension and LV hypertrophy is well known, with elevated blood pressure in midlife correlating with LV hypertrophy in later life (52). Increased blood pressure induces cardiomyocyte and fibroblast changes and accelerates cardiac remodeling. Moreover, hypertension results in vascular changes such as endothelial dysfunction, reduced coronary reserve blood flow, and diminished capillary density, all of which lead to reduced oxygen delivery. Systemic hypertension also results in arterial stiffness, which imposes a disproportionate load on the heart, leading to ventricular-vascular uncoupling and afterload mismatch (53). These changes lead to impaired systolic and diastolic function (15). Although blood pressure reduction in patients with diastolic dysfunction is an effective approach to improving echocardiographic parameters 54, 55, there is no clear evidence that blood pressure reduction in HFpEF improves outcomes (56). Additionally, although hypertension is a major risk factor for HFpEF, “management” of hypertension (which encompasses diagnosis and blood pressure treatment and control) has not decreased the incidence or prevalence of HFpEF 57, 58, 59, 60, 61.
Murine models of hypertensive HFpEF have many of the features of human HFpEF, as shown in Table 2. There are a wide range of models, and most mimic some aspects of the relevant human HFpEF disease, but no single model recapitulates all, although some more than others. A major deficiency in these models is that they do not reproduce the slow onset seen in patients with hypertension that lead to HFpEF development (62).
Table 2.
Murine Models: Pathophysiology of Hypertension-Associated HFpEF Based on the Existing Literature
| Findings in Hypertension-Associated Human HFpEF (15) | Aldosterone- Infused Mouse | Angiotensin II–Infused Mouse | Dahl Salt-Sensitive Rat | DOCA Salt Rat | Transverse Aortic Constriction Mouse |
|---|---|---|---|---|---|
| Elevated blood pressure | Yes | Yes | Yes | Yes | Yes |
| Fatigue/exercise intolerance | Yes | N/A | Yes | N/A | Yes |
| Pulmonary congestion | Yes | N/A | Yes | N/A | Yes |
| Concentric hypertrophy or increased LV mass | Yes | Yes | Yes | Yes | Yes |
| Impaired active relaxation | Yes | Yes | Yes | Yes | Yes |
| Impaired passive filling | Yes | Yes | Yes | Yes | Yes |
| Enlarged left atrium | N/A | N/A | Yes | N/A | Yes |
| Cardiomyocyte hypertrophy | Yes | Yes | Yes | Yes | Yes |
| Myocardial fibrosis | Yes | Yes | Yes | Yes | Yes |
| Decreased intra-myocardial capillary density | N/A | Yes | Yes | Yes | N/A |
| Increased biomarkers such as NT-proBNP, BNP, and troponin | Yes | Yes | Yes | Yes | Yes |
BNP = brain natriuretic peptide; LV = left ventricular; N/A = not available; NT-proBNP = N-terminal pro–brain natriuretic peptide; other abbreviation as in Table 1.
Aldosterone-infused and unilateral nephrectomized mouse
Pioneering studies more than 2 decades ago reported that the combination of uninephrectomy, aldosterone infusion, and 1% sodium chloride (NaCl) administration in rats induced blood pressure elevation, cardiac hypertrophy, and fibrosis 63, 64, 65. This effect was also described in mice (66). Circulating aldosterone levels in this HFpEF model are ∼6.0 to 7.5 ng/ml 67, 68, comparable to circulating levels of aldosterone seen in human acute HF (mean of 18 ng/ml) (69). Mice subjected to uninephrectomy and aldosterone infusion for 4 weeks, accompanied by 1% NaCl intake, develop HFpEF with moderate hypertension, concentric LV hypertrophy, pulmonary congestion, and echocardiographic evidence of diastolic dysfunction while maintaining a normal/preserved LVEF 68, 70, 71. These mice also show exercise impairment (72). At the molecular level, LV tissue from these mice show an increase in natriuretic peptides, cardiac size, (73), as well as an increase in the expression of the titin transcript variants n2ba and n2b (74). Titin plays a pivotal role in diastolic stiffness in HFpEF 75, 76 along with the extracellular matrix (77). In addition to increased myocardial oxidative stress, there is altered expression of cardiomyocyte proteins that are involved in the mobilization of calcium during the excitation-contraction coupling process in the heart. Sarcoplasmic reticulum Ca(2+)–adenosine triphosphatase expression is decreased, as are protein kinase A (PKA)-dependent and Ca2+/calmodulin-dependent protein kinase II (CAMKII)-dependent phosphorylation of phospholamban (68). Interestingly, in this model, serum creatinine is mildly increased, along with an increase in glomerular size and urine albuminuria, indicating impaired renal function (67), which is seen in human HFpEF. Thus, this model recapitulates the clinical HFpEF phenotype and also features molecular changes that have been described clinically 75, 78, 79, 80.
Angiotensin II–infused mouse
Administration of angiotensin II for a variable timeframe (1 to 8 weeks) in mice leads to cardiac hypertrophy and remodeling, both in the presence 81, 82, 83, 84, 85 and absence 16, 86 of hypertension, suggesting that cardiac remodeling under angiotensin II infusion is due to blood pressure–dependent and –independent factors. It is apparent that the observed elevation in blood pressure is dependent on the dose of angiotensin II used. However, the relevance of supraphysiological circulating angiotensin II levels in human disease has been questioned. In addition, although it is difficult to extrapolate from studies in different species, any potential comparison is complicated by the fact that the majority of angiotensin II–infusion animal studies have not measured or do not report circulating blood levels (87). Moreover, cardiac hypertrophy appears strain-specific. Whereas C57BL/6J mice develop compensatory concentric hypertrophy and fibrosis in response to angiotensin II (88), Balb/c mice show severe LV chamber dilatation (89), which is infrequently seen in HFpEF and more characteristic of a dilated cardiomyopathy. Although, the angiotensin II-infused mouse model demonstrates diastolic dysfunction 81, 84, 90, 91, it has shown both a lack of change in systolic function 16, 86 as well as a decrease in LVEF (92) and these discrepant findings are likely dose-dependent. Pulmonary congestion (89), as well as exercise intolerance, are evident and seem to be related to angiotensin II–induced skeletal muscle abnormalities, including impaired mitochondrial function and skeletal muscle atrophy (93). In summary, if strain and dosage are optimized to mirror the human HFpEF phenotype, angiotensin II infusion appears to be a relevant HFpEF model. This corroborates, once again, the importance of the experimental design when performing preclinical studies in the field.
Dahl salt-sensitive rat
This mutant strain of the Sprague-Dawley rat is characterized by increased salt sensitivity and is only genetically distinguishable from the Dahl salt-resistant rat by a polymorphism in the renin gene (94). When placed on a high-salt diet (8%) at 6 weeks to 8 weeks of age, the Dahl salt-sensitive rats develop a steep elevation in blood pressure and progressive concentric LV hypertrophy, precipitating HFpEF at approximately 14 to 19 weeks 95, 96, 97. Signs of HF include tachypnea, labored breathing, inactivity, and postmortem evidence of pulmonary edema and ascites 95, 98. However, with continued exposure to a high-salt diet (∼26 weeks) or when diet is introduced at an older age, these animals develop HFrEF 23, 95. Thus, this model is used to study the transition from compensated cardiac hypertrophy to HFrEF 23, 95, something uncommonly seen in human HFpEF 99, 100. Dahl salt-sensitive rats also have renal dysfunction and metabolic disturbances, including insulin resistance and dyslipidemia (17). A relative strength of the Dahl salt-sensitive rat is that its cardiac phenotype is due to salt and water retention, quite reflective of the clinical situation 101, 102. The demographics and comorbidities commonly found in HFpEF are strongly associated with blood pressure salt sensitivity. Similarly, sodium restriction in salt-sensitive hypertensive HFpEF patients reduces systemic blood pressure, arterial stiffness, and oxidative stress (102). However, this model lacks clinical applicability as an HFpEF model because very high systolic blood pressure values (>175 mm Hg) develop 95, 103, compared with humans, where often only moderate hypertension is seen (15). In addition, this HFpEF model will progress to HFrEF with continued salt administration.
Deoxycorticosterone acetate (DOCA)–salt rat and mouse
The DOCA-salt hypertensive rat model was first described in 1969 (104). DOCA is administrated by intraperitoneal injections or subcutaneous pellet implantation for 4 weeks, accompanied by unilateral nephrectomy (1 week before DOCA, at 6 to 10 weeks of age) and 1% NaCl drinking water 105, 106. This combination of procedures causes hypertension, cardiac hypertrophy, and perivascular fibrosis (107). Information regarding HFpEF development in this model is limited, but concentric hypertrophy accompanied by severe restrictive diastolic dysfunction in the presence of a preserved LVEF occurs 28 days after DOCA (108). More recently, DOCA has been used in mice. The DOCA-salt mouse develops cardiac hypertrophy, whereas blood pressure is either unchanged or only mildly increased 109, 110. Thus, it is unclear whether it is a hypertension-HFpEF model. Cardiac hypertrophy associates with increased fibrosis and vascular rarefaction (110). Increased myocardial oxidative stress, nitric oxide synthase-dependent superoxide production, and reduced nitric oxide production are also seen (111). DOCA-salt mice show diastolic dysfunction with preserved systolic function 109, 111, 112 accompanied by exercise intolerance, but without pulmonary congestion or HF (113).
Spontaneously hypertensive rat (SHR)
This inbred strain is pre-disposed to hypertension, with evidence of pre-hypertensive, developing, and sustained phases, similar to essential hypertension in humans 114, 115, 116. The SHR cardiovascular literature is vast, and its resemblance to the human condition and the relative lack of individual variation in this strain makes this an attractive model 17, 117. It develops compensated concentric LV hypertrophy while preserving contractility during the first year of age but thereafter develops overt HFrEF characterized by LV dilatation with diastolic dysfunction 118, 119, 120 which is uncommonly seen in human HFpEF 99, 100. By 18 months to 24 months of age, more than one-half of the SHRs develop cardiac decompensation, with tachypnea and severely decreased LVEF (121). However, some colonies of SHR lack this decline in LVEF at 20 months, despite the evidence of diastolic dysfunction, concentric remodeling, and lung congestion (122). Abnormalities in myocardial mechanics, such as strain and myocardial velocities, occur together with T-tubule disorganization and impaired Ca(2+) cycling in the hypertensive SHR, which occur before the development of cardiac fibrosis, cardiac dysfunction, and HF (19). Although HF in SHR occurs around 2 years of age, mimicking some aspects of the human disease where HFpEF is highly prevalent in the aging population (21), this is a time-consuming and therefore expensive model. Importantly, despite the lengthy duration required for HF development, data regarding the presence of preserved LVEF in this model are limited and confusing, making it less attractive for HFpEF research. Basic researchers should systematically determine which specific SHR colonies are more likely to develop HFpEF and use those animals for study. Importantly, the transition in SHR from compensated cardiac hypertrophy to HFrEF failure limits its use as an HFpEF model. As discussed earlier, the transition from HFpEF to HFrEF (by dropping LVEF) is uncommon in human HFpEF.
Thoracic aortic constriction-induced pressure overload in mouse
Aortic constriction is a well-established surgical procedure for the induction of LV chronic pressure overload. It was first described in 1991 (123) and since then has been widely used to investigate cardiac hypertrophy. In this model, a band is placed around the aorta of a young mouse and, as the mouse grows, the band becomes obstructive, resulting in cardiac hypertrophy, with increased cardiomyocyte size and collagen volume fraction 124, 125. Thoracic aortic constriction can either be close to the origin of the aorta (ascending aortic constriction, AAC) or in the aortic arch between the brachiocephalic and the left carotid arteries (transverse aortic constriction, TAC) (23). AAC is technically more challenging and generally used to study acute effects of early pressure overload, independent of hypertension, because mice do not show an increase in blood pressure. Moreover, diastolic dysfunction is secondary to pressure overload and dependent on the severity and the rapid onset of the constriction. As such, AAC represents more closely a model of aortic valve stenosis rather than a model of HFpEF, where a clinical scenario of acutely increased afterload is not commonly observed 16, 17; that is, aortic stenosis is an uncommon cause of HFpEF and may proceed directly to HFrEF. In contrast, TAC results in a slightly more gradual rise of LV pressure and progression toward cardiac hypertrophy and HF 23, 126. In the preclinical literature, TAC is generally used as a model of diastolic dysfunction. Both AAC and TAC are characterized by an initial compensatory phase, with concentric LV hypertrophy, followed by cardiac chamber enlargement and eccentric LV remodeling, resulting in further deterioration of the LV systolic function, including a decline in LVEF (126). There is procedural variability depending on the severity of constriction of the banding, the age of the mouse at the time of the surgery, and, more importantly, the length of follow-up. In general, AAC and TAC both result in an acute imposition of LV pressure load. Thus, signs of HF, including LV concentric hypertrophy, diastolic dysfunction, and lung congestion are evident after 2 to 3 weeks of constriction 70, 127. It then progresses to HFrEF, with LV chamber dilatation and impaired systolic function after ≥4 weeks 128, 129. However, the progression from HFpEF to HFrEF is atypical in humans 99, 100, 103. In response to pressure overload, the heart undergoes remodeling at the structural, cellular, and molecular level. The hallmark of this remodeling includes increased collagen deposition resulting in a stiff myocardium and recapitulation of the fetal gene program such as increased expression of the β-isoform of myosin heavy chain (130). Moreover, similar to the aldosterone-infused model, pressure overload decreases sarcoplasmic reticulum Ca(2+)–adenosine triphosphatase expression and downregulation of phospholamban expression as early as 2 weeks post-TAC (131).
Metabolic phenotype: Obesity and diabetes models
Large outcome trials and registries reveal that being overweight or obese is a major risk factor for HFpEF 6, 132. Obesity induces significant structural changes in the LV (133), and patients with HFpEF are significantly more likely to be obese. A body mass index >35 kg/m2 is associated with increased mortality and hospitalization rates (134). There are multiple mechanisms whereby obesity could contribute to HFpEF. Increased adiposity promotes inflammation, insulin resistance, and dyslipidemia and also impairs arterial, skeletal muscle, and physical function 135, 136, 137, 138, all of which are abnormal in patients with HFpEF 1, 9, 135, 139. Until recently, sparse attention has been paid to increased adiposity and obesity in HFpEF (139), with no clinical trials addressing the role of obesity in HFpEF. Weight loss after bariatric surgery is reported to decrease cardiac hypertrophy and LV filling pressures and to improve diastolic dysfunction (140). However, this strategy has not been specifically studied in the HFpEF population 141, 142.
Diabetes is also commonly seen in HFpEF (143). Although its presence is associated with a poor prognosis in HF, regardless of LVEF, the relative risk of cardiovascular death or HF hospitalization conferred by diabetes is greater in HFpEF than in HFrEF (144). There are multiple mechanisms by which diabetes could perpetuate HFpEF. Systemic insulin resistance and hyperglycemia trigger cardiac insulin resistance and neurohormonal, sympathetic, and cytokine imbalance in the heart 145, 146. This, in turn, might induce cardiac remodeling processes such as cardiomyocyte hypertrophy, interstitial fibrosis, and collagen and titin modifications, leading to further cell damage and deterioration of diastolic and systolic function 147, 148. Peripheral mechanisms may also mediate the deleterious effect of diabetes in HFpEF. Prolonged hyperglycemia and hypo- or hyperinsulinemia contribute to skeletal muscle dysfunction with changes in glucose use and capillary density (149). These may all limit exercise capacity, one of the earliest symptoms of HFpEF.
Murine models of obesity and diabetes–induced HFpEF have several limitations compared with the human phenotype. First, preclinical models usually present with fulminant and uncontrolled hyperglycemia or insulin resistance, which does not mimic the clinical situation in HFpEF (150). Studies performed before the onset of diabetes most likely reflect changes due to obesity and insulin resistance, and studies performed after the onset of diabetes reflect the added effects of hyperglycemia of variable duration because insulin resistance and diabetes develop at different stages (23). Lastly, although several models showed the effect of diabetes per se, in the absence of obesity, on the onset of HFpEF, there is sparse information regarding an obesity model alone that does not have insulin resistance or diabetes (which are cofounders in an obesity model), which therefore limits investigating the exclusive role of increased adiposity in HFpEF. Table 3 shows the major HFpEF features seen in the following murine models.
Table 3.
Murine Models: Pathophysiology of Obesity and Diabetes-Associated HFpEF Based on the Existing Literature
| Findings in Obesity and Diabetes-Associated Human HFpEF 53, 139 | db/db Mouse | ob/ob Mouse | Streptozotocin- Induced Diabetic Rat | ZFS1 Rat |
|---|---|---|---|---|
| Obesity | Yes | Yes | No | Yes |
| Insulin resistance or diabetes | Yes | Yes | Yes | Yes |
| Fatigue/exercise intolerance | Yes | +/− | N/A | Yes |
| Edema | Yes | N/A | +/− | Yes |
| Concentric hypertrophy or increased LV mass | Yes | Yes | +/− | Yes |
| Arterial stiffness | Yes | Yes | Yes | Yes |
| Disturbed ventricular-arterial coupling | Yes | N/A | Yes | N/A |
| Cardiomyocyte hypertrophy | Yes | Yes | Yes | Yes |
| Myocardial fibrosis | Yes | Yes | Yes | Yes |
| Decreased intra-myocardial capillary density | Yes | +/− | Yes | No |
| Natriuretic peptides levels | ↓ | =/↓ | ↑ | N/A |
| Systemic inflammation | Yes | N/A | Yes | N/A |
db/db mouse
The db/db leptin receptor-deficient mouse has a point mutation in the diabetes (db) gene encoding the leptin receptor, which spontaneously causes morbid obesity accompanied by severe hyperglycemia secondary to type 2 diabetes 151, 152. The progression of diabetes in the hyperleptinemic db/db mice, with initial insulin resistance followed by an insulin secretion defect, is similar to the pathogenesis of type 2 diabetes in humans (153). Thus, this model is valuable in exploring the combined contribution of obesity and type 2 diabetes to HFpEF, which is representative of this particular HFpEF phenotype (16). db/db mice show an inflammatory, systemic cytokine fingerprint characterized by increased proinflammatory cytokines in early disease (8 weeks) but lower levels of a similar proinflammatory profile at older ages (28 weeks) (154). Despite the presence of both hyperinsulinemia and hyperleptinemia, mice do not initially show cardiac hypertrophy 152, 155, 156, 157, but it eventually develops at older ages 158, 159, 160. At the histological level, these mice hearts have enlarged cardiomyocytes (158), evidence of fibrosis (161), and capillary rarefaction. There is also diastolic dysfunction with increasing age, whereas systolic function remains preserved even at 6 months of age 152, 158, 162, 163. Diabetic db/db mice show abnormal ventricular-arterial coupling due to decreased vascular compliance and increased LV stiffness. The latter is associated with increased titin-isoform N2B expression (152). Exercise intolerance parallels diastolic dysfunction and is severely reduced by 12 weeks of age (164), with evidence of pulmonary congestion (165). Natriuretic peptide levels are also reduced (166), similar to the human obesity phenotype. Obese HF patients often have low circulating natriuretic peptides levels for a given degree of HF (167). In conclusion, the db/db mice appear to represent the obese/metabolic HFpEF phenotype, with evidence of HF, whereas LVEF is preserved. Importantly, the reduced levels of natriuretic peptides make this model even more attractive because this is a specific feature seen in obese HFpEF patients.
ob/ob mouse
The ob/ob leptin-deficient mouse spontaneously develops obesity and type 2 diabetes secondary to hyperphagia, hyperglycemia, and hyperinsulinemia 168, 169. These mice are indistinguishable from their lean littermates (ob/+) at birth, but within 2 weeks gain weight and are hyperinsulinemic. By 4 weeks of age there is also marked hyperglycemia. Blood glucose rises to reach a peak after 3 to 5 months, accompanied by accelerated food intake and rapid growth 169, 170, 171, 172. Thereafter, it decreases and eventually normalizes in older mice, although they remain insulin resistant 170, 173, 174. These mice develop myocardial hypertrophy, with increased cardiomyocyte size 175, 176, 177 in parallel with diastolic dysfunction, but without changes in systolic function (178). Compared with ob/+ mice, variable arterial blood pressure is observed 176, 179, 180. There is also cardiac fibrosis (181) and increased arterial stiffness due to a destabilized elastic fiber network and increased elastolytic activity (182). Natriuretic peptide cardiac expression is reportedly unchanged or slightly reduced compared with control ob/+ mice 178, 181, 183, 184. Moreover, although ob/ob mice are less active than their control, this is attributed to excess body weight rather than to their cardiac phenotype (185). Survival is decreased in ob/ob mice compared with ob/+ mice (186), and it appears that they die before cardiac hypertrophy progresses to overt HF (177). The main disadvantages of this model are 2-fold. First, at least some of the cardiac changes observed appear to be secondary to the loss of leptin-mediated signaling (187). The aberrations seen in ventricular cardiomyocytes from ob/ob mice, such as cellular hypertrophy, depressed peak cell shortening, reduced maximal velocities of shortening/re-lengthening, and prolonged duration of re-lengthening, are reversed by recombinant leptin treatment 158, 176. Additionally, obese humans with leptin deficiency are uncommon, so the ob/ob mice do not mimic the human phenotype 169, 188.
Streptozotocin-induced diabetic rat
Streptozotocin administration in rats was first described in 1963 (189). Rapid β-cell degranulation and necrosis occurs 7 to 10 h after injection, and causes hyperinsulinemia and hypoglycemia. After longer periods (28 days), prolonged hyperglycemia and a reduction in pancreatic insulin levels ensues (190). Streptozotocin induces either type 1 or type 2 diabetes, depending on the dosage and duration of administration. Doses of 50 to 65 mg/kg lead to hyperglycemia without ketosis, and insulin administration is not required. However, at higher doses (75 mg/kg and greater) spontaneous ketosis and death occurs within days if insulin is not administered (191). Streptozotocin-injected rats exhibit many of the cardiovascular complications found in humans with both type 1 and type 2 diabetes. They have increased levels of cardiac and circulating natriuretic peptides 192, 193 and inflammatory markers, such as interleukin 1, interleukin 6, and intracellular adhesion molecule 1 (194). Blood pressure remains within normal ranges, indicating that hyperglycemia alone may be sufficient to account for the observed changes seen in the heart in this model (17). This includes eccentric cardiac hypertrophy, gradual diastolic dysfunction with impaired contractile performance, and increased ventricular stiffness 191, 195, 196, 197. Fibrosis and a titin-isoform pattern switch is observed 198, 199, 200, correlating with increased ventricular stiffness (201). Streptozotocin-induced diabetic rats also show altered systolic load of the left ventricle coupled to the arterial system and decreased systemic arterial compliance (202). Although relative lung weights are reported to be increased (203), this is more likely due to a decrease in body weight than to the presence of lung congestion per se, as other studies showed no differences in lung weights relative to tibia length or wet-to-dry lung ratio 204, 205. Cardiac atrophy is also described, with chronic apoptotic cardiomyocyte loss and inadequate reactive hypertrophy 206, 207, 208. There is also significantly reduced cardiac capillary density (209). A major disadvantage of using the streptozotocin-induced diabetes model in cardiovascular and HFpEF studies is that some of the purported cardiac effects are partly attributed to the direct toxic actions of streptozotocin itself (210). More importantly, LVEF values in this model are variable, with some studies showing a preserved LVEF 200, 211, 212, 213, whereas others show a reduction in LVEF during diabetes development (214). This discrepancy could be due to the severity of diabetes or the methods used to evaluate cardiac function (215).
Zucker rat
In 1961, a mutation designated “fatty” appeared spontaneously in a group of outbred rats giving rise to the Zucker fatty rat model (216). This mutation occurs at the leptin receptor gene, resulting in decreased affinity of leptin for its receptor and, consequently, causing hyperphagia and obesity (217). These fatty rats, although insulin resistant, did not become diabetic, but later inbreeding using obese and diabetic Zucker rats resulted in the Zucker diabetic fatty rat model (218). Both these models develop mild hypertension and cardiac alterations such as LV hypertrophy and early diastolic and moderated systolic dysfunction 17, 219, 220, 221, 222, with preserved LVEF (223). There is also cardiomyocyte hypertrophy, vascular rarefaction, and fibrosis 220, 222, 223, 224. However, to our knowledge, lung congestion or other signs of HFpEF have not been described.
Zucker fatty and spontaneously hypertensive HF rat
The diabetic Zucker fatty spontaneously hypertensive heart failure F1 hybrid (ZSF1) model was developed by crossing rat strains with 2 different leptin receptor mutations (fa and facp), the lean female Zucker diabetic fatty rat (+/fa) and the lean male spontaneously hypertensive HF rat (+/facp), derived from the obese SHR carrying the corpulent facp gene 225, 226, 227. ZSF1 rats develop HFpEF between 10 and 20 weeks of age. The obese animals die at an early age (∼12 months) with symptoms of end-stage renal failure, accompanied by marked cardiac hypertrophy (227). There is hypertension, concentric LV remodeling, progressive diastolic dysfunction, preserved LVEF, lung congestion, and increased arterial stiffness, all followed by insulin resistance, glycosuria, and proteinuria 228, 229, 230. Additional studies showed impaired exercise tolerance in ZSF1 rats, reinforcing the potential usefulness of this model to understand HFpEF pathophysiology and for therapeutic insights (231). At the cellular level, there is cardiomyocyte hypertrophy (228). Fibrosis develops later and seems to be induced by a hyperproliferative vascular reaction, which leads to replacement fibrosis of functional vascular structures (229). Interestingly, ZSF1 rats show increased myocardial stiffness, largely due to increased cardiomyocyte stiffness resulting from N2B titin isoform hypophosphorylation (228). This is similar to human HFpEF in which both increased cardiac expression (232) and hypophosphorylation (233) of the stiff N2B titin isoform are described. The use of SU5416 (a vascular endothelial growth factor receptor blocker) in ZSF1 rats induces a “2-hit” model of pulmonary hypertension plus metabolically impairment in HFpEF. There is increased right ventricular systolic pressure, elevated pulmonary vascular resistance, and pulmonary vascular proliferative remodeling (234). Notably, the ZSF1 represents a good model of HFpEF, but in addition to the obesity/metabolic phenotype, it presents other comorbidities such as hypertension or chronic kidney disease, which may be argued to mimic the HFpEF patient in many scenarios. However, if the aim is to discern the contributions of obesity/metabolic phenotype per se, then these additional comorbidities would confound the findings. It might be a good model to study combinations of comorbidities in advanced studies.
Atrial fibrillation
Atrial fibrillation, the most common arrhythmia encountered in clinical practice, is highly associated with an increased risk of HF, stroke, and overall mortality (235). Epidemiological studies show a close relationship between HFpEF and atrial fibrillation, including shared risk factors, such as older age and hypertension (236). Diagnosis of HFpEF in the setting of atrial fibrillation is challenging because of overlapping symptoms, such as dyspnea, fatigue, and impaired exercise tolerance, and comparable echocardiograph parameters, which may include diastolic dysfunction (237) and enlarged left atrial size. Additionally, it is often unclear which is the inciting event, HFpEF or atrial fibrillation, and which of these 2 events should be considered the cause and which the effect.
Historically, cardiac arrhythmias were studied in large mammals such as the goat, pig, or dog, because their hearts were more akin to the human heart than rabbits or rodents (25). Early studies in dogs 238, 239 showed that the severity of cardiac dysfunction caused by atrial pacing directly correlates with the rate of pacing. This induces an end-stage dilated cardiomyopathy and an HFrEF phenotype, with a significant decrease in both systolic and diastolic function. There was no increase in LV mass nor collagen content (21).
Although it was believed that arrhythmias did not occur in mice due to their lack of critical cardiac mass (240), this has been shown to be incorrect (241). Studying atrial fibrillation is difficult compared with other HFpEF comorbidities. Numerous murine models are reported to harbor atrial arrhythmias, and in vivo electrophysiological techniques 242, 243, 244 are well established. Transgenic mouse models showing the development of atrial fibrillation, secondary to alterations in major regulatory pathways of cell functions, have been broadly reviewed (245). In these, atrial fibrillation is often associated with alterations in a substrate for atrial conduction, electrophysiological abnormalities that accelerate atrial repolarization, or triggered activity and focal discharges (246). However, to our knowledge, although some of these models show dilated cardiomyopathy or hypertrophic cardiomyopathy, information regarding HF status is lacking, and none have examined the presence of HFpEF in the setting of atrial fibrillation.
Pulmonary hypertension
Pulmonary hypertension is a known complication of any disease that elevates LV filling pressure (247), including LV systolic (248) and diastolic dysfunction (249). For reasons that are yet uncertain, a subset of patients with HFpEF go on to develop pulmonary hypertension (250). The clinical pulmonary hypertension phenotype associated with HFpEF varies and may be seen in acute or chronic HF. However, when pulmonary hypertension is evident in chronic HF, its clinical significance is tightly linked to increased disease severity and adverse outcomes (251).
Pulmonary hypertension in animal models may not fully represent the clinical observations. It has been argued that the severity of pulmonary hypertension in vivo poorly mimics the observed human phenotype (252). Preclinical models of pulmonary hypertension may reflect milder forms of human pulmonary hypertension, a stage that is often missed at the time of diagnosis (253), and thus do not represent the later, more severe, disease (252). There are strain issues with murine models which recapitulate the human phenotype of metabolic syndrome and pulmonary hypertension-associated HFpEF. The latter is only seen in select mouse strains (284). Nevertheless, animal models have undergone major development and improvement over the years, and pulmonary hypertension models are more common nowadays, although, as noted, the pulmonary changes observed are not completely comparable between rodents and humans (254).
Hypoxia-induced pulmonary hypertensive rat and mouse
Chronic hypoxia is observed in chronic obstructive pulmonary disease, interstitial lung disease, sleep apnea, and exposure to high altitudes in humans and is thought to play a crucial role in the development of pulmonary hypertension and severe HF 252, 255. Similarly, in the research laboratory, chronic hypoxia also leads to pulmonary hypertension (255). Typically, it is induced by placing the rodent in a hypobaric chamber (either 10% fraction of inspired oxygen or hypobaric pressure of 380 mm Hg, which is equivalent to one-half that at sea level) for 3 to 4 weeks 256, 257, 258, 259. This model is useful because it is predictable and reproducible within a selected animal strain. There is also variability in the responses to chronic hypoxia between species (260). Hypoxia in rats produces characteristic morphological changes in the pulmonary vasculature, including an extension of smooth muscle into more peripheral pulmonary arteries and reduction in their number (261). Nevertheless, exposure of mice to chronic hypoxia conditions, although causing an elevation in pulmonary artery pressure, is associated with less vascular remodeling than in rats 262, 263, 264. Although the mechanisms of hypoxia-induced vascular remodeling are partially understood, the complex obliterate lesions found in human patients with severe primary pulmonary hypertension do not develop in this rodent model (265). Instead of these obliterate lesions in the pulmonary vasculature of humans with left-sided heart disease, HF and pulmonary hypertension (266), there is evidence of alveolar septa thickening, cellular proliferation, and collagen deposition (234). It has been described that chronic hypoxia causes right ventricle 258, 259 and LV 267, 268 hypertrophy in rats/mice, but to our knowledge there are no in vivo studies of HFpEF induced by chronic hypoxia, although this might be an applicable model of pulmonary hypertension-associated HFpEF 62, 260.
Renal dysfunction phenotype
HFpEF and renal dysfunction are mutually promoting (6). Abnormalities in the kidney’s ability to maintain sodium and fluid balance may precede the development of HFpEF. Evidences suggest a putative role for the kidney in HFpEF as a potential starting point in patients vulnerable to the deleterious effect of excessive salt and water retention, such as in hypertension (269). Conversely, the detrimental impact of HFpEF on renal function might be explained by the effect of increased filling pressures, typical for HFpEF, entailing both a relative decreased cardiac output and increased renal congestion. This, together with an impaired ventricular-vascular coupling, can lead to reduced renal blood flow and renal dysfunction 270, 271.
Subtotal nephrectomy rat and mouse
Often termed 5/6 nephrectomy, it is the most established model of progressive renal failure with loss of renal mass. Although commonly performed in the rat, a similar mouse model exists but appears to be less reliable and more strain dependent 272, 273. The model is based on the reduction of renal mass with a uninephrectomy and resection of the poles of the contralateral kidney (approximately 50%) 1 to 2 weeks later (274). Rather than mimicking a renal disease per se, subtotal nephrectomy parallels the consequences of reducing functional nephron number. In this model, LV hypertrophy is a consistent feature (275) and resembles that seen in early chronic kidney disease in humans (276). The presence of LV dilatation is variable (273), and cardiac systolic function is generally maintained, with preserved LVEF. Early diastolic dysfunction is seen with a commensurate increase in cardiomyocyte cross-sectional area 277, 278, 279. Other documented features of this model include increased myocardial artery wall thickness, decreased capillary density (280), interstitial fibrosis 277, 279, fluid congestion (281), and exercise intolerance (282). However, to our knowledge, it has not been used as a model of HFpEF associated renal disease and its significance in modeling HFpEF is unknown.
Murine Models: A Funhouse Mirror?
Relevant preclinical models for HFpEF will provide mechanistic information to understand the contribution of comorbidities that are highly associated with this disease. Additionally, these models will provide insights into pathways relevant to disease development, progression, and potential implications in therapeutics. As expected, an animal model cannot completely mimic the human disease, and limitations regarding their applicability are obvious, partly because human HFpEF is so heterogeneous and encompasses a large spectrum of symptoms, signs, and disease presentation (62). It is therefore important for the translational researcher to carefully select which comorbidity or combination thereof, leading to this heterogeneous HFpEF syndrome, should be included in the experimental design. A general limitation of most HFpEF murine models is the sudden onset of the HF due to a surgical or drug intervention, whereas human HFpEF is generally thought to develop progressively over months to years (23). Moreover, substantial differences exist between rodents and human cardiac features, such as its small size and higher heart rates, which sometimes limit diastolic function measurements, especially when noninvasive techniques, such as echocardiography, are used 103, 283. It should also, be emphasized that many murine models of purported HFpEF progress to HFrEF within a variable amount of time, suggesting that in these models, HFpEF is a merely a temporary step to the development to HFrEF, which is rare in human HFpEF 100, 103.
The use of murine models is an extremely valuable tool in understanding the pathophysiology of HFpEF. These models are essential for understanding the molecular alterations underlying the development of the disease, as they allow the identification of molecular pathways that may be causative or contribute to the pathophysiology and progression of HFpEF 23, 103. However, it is essential for these models to be validated on the basis of reliability and reproducibility in multiple experimental settings, including time-course analysis. Attention should be also paid to the background strain of the model, as different stimuli may elicit a variable response depending on the strain (284). It has been suggested that similar to human HFpEF, the use of outbred murine colonies could contribute to a better experimental setting because, in contrast, the use of inbred strains represents limited genetic diversity and might not reflect the responses generated in a diverse human population (285).
Importantly, HFpEF models must be carefully characterized to ensure they have features consistent with human HFpEF (Figure 1). It is likely that some murine models in the present review do have HFpEF, but have not been described. Conversely, others touted as HFpEF models do not present the real HF phenotype. Similarly, in the preclinical literature diastolic dysfunction has often been used incorrectly as interchangeable with the term diastolic heart failure and thus thought to represent HFpEF.
Lastly, although recent technological advances in echocardiography, imaging, and micromanometer conductance catheters have greatly improved the assessment of cardiac function in rodents (23), they cannot be the only method for HFpEF diagnosis, as they may not reflect a real HF syndrome. For this reason, the presence of HF in vivo should be more accurately detected by determining exercise intolerance (17). The recommended method for post-mortem identification of HFpEF is determining pulmonary congestion, which could be assessed by examining either the ratio of lung weight to tibia length or the wet-to-dry lung ratio (17).
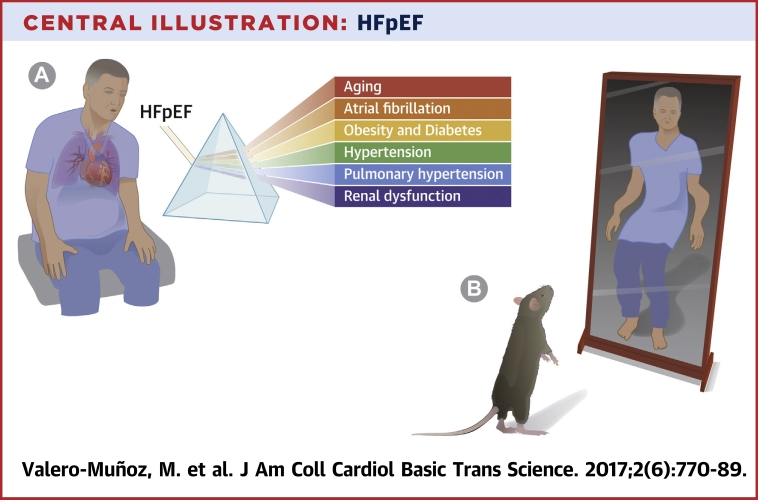
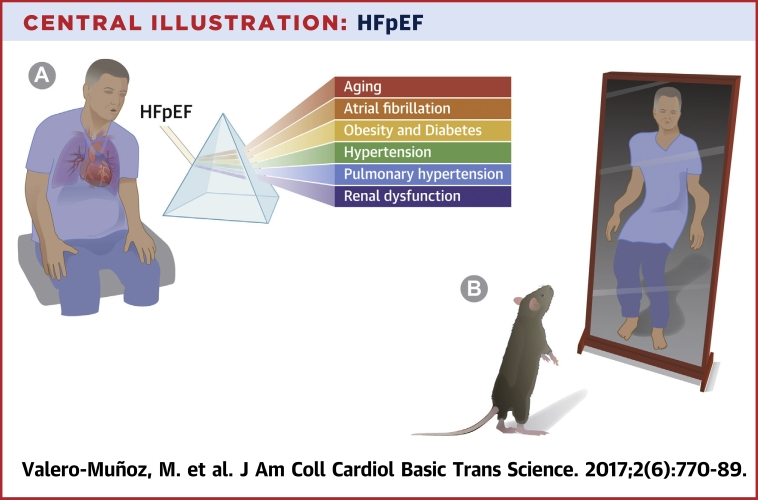
As with all fishing expeditions, we attempt to gather facts about HFpEF using relevant preclinical models that accurately recapitulate the complexities of human HFpEF disease and define specific phenotypes to discover novel targets, ultimately for the development of effective therapeutics (Central Illustration).
Central Illustration.
HFpEF
(A) Modeling heart failure with preserved ejection fraction (HFpEF) in the lab with major comorbidities and conditions highly associated with human HFpEF. (B) However, murine models touted as HFpEF models are often lost in translation when applied to the clinical situation.
Footnotes
Dr. Sam has received a grant from the National Institutes of Health (HL117153). Dr. Valero-Muñoz has received a grant from the American Heart Association (17POST33660439). Dr. Backman has reported that he has no relationships relevant to the content of this paper to report.
All authors attest they are in compliance with human studies committees and animal welfare regulations of the authors' institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACC: Basic to Translational Scienceauthor instructions page.
References
- 1.Sharma K., Kass D.A. Heart failure with preserved ejection fraction: mechanisms, clinical features, and therapies. Circ Res. 2014;115:79–96. doi: 10.1161/CIRCRESAHA.115.302922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ponikowski P., Voors A.A., Anker S.D. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2016;18:891–975. doi: 10.1002/ejhf.592. [DOI] [PubMed] [Google Scholar]
- 3.Yancy C.W., Jessup M., Bozkurt B. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. J Am Coll Cardiol. 2013;62:e147–e239. doi: 10.1016/j.jacc.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 4.Ferrari R., Bohm M., Cleland J.G. Heart failure with preserved ejection fraction: uncertainties and dilemmas. Eur J Heart Fail. 2015;17:665–671. doi: 10.1002/ejhf.304. [DOI] [PubMed] [Google Scholar]
- 5.Borlaug B.A. Defining HFpEF: where do we draw the line? Eur Heart J. 2016;37:463–465. doi: 10.1093/eurheartj/ehv561. [DOI] [PubMed] [Google Scholar]
- 6.Shah S.J., Kitzman D.W., Borlaug B.A. Phenotype-specific treatment of heart failure with preserved ejection fraction: a multiorgan roadmap. Circulation. 2016;134:73–90. doi: 10.1161/CIRCULATIONAHA.116.021884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brouwers F.P., de Boer R.A., van der Harst P. Incidence and epidemiology of new onset heart failure with preserved vs. reduced ejection fraction in a community-based cohort: 11-year follow-up of PREVEND. Eur Heart J. 2013;34:1424–1431. doi: 10.1093/eurheartj/eht066. [DOI] [PubMed] [Google Scholar]
- 8.Borlaug B.A. Heart failure with preserved and reduced ejection fraction: different risk profiles for different diseases. Eur Heart J. 2013;34:1393–1395. doi: 10.1093/eurheartj/eht117. [DOI] [PubMed] [Google Scholar]
- 9.Paulus W.J., Tschope C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol. 2013;62:263–271. doi: 10.1016/j.jacc.2013.02.092. [DOI] [PubMed] [Google Scholar]
- 10.Wan S.H., Vogel M.W., Chen H.H. Pre-clinical diastolic dysfunction. J Am Coll Cardiol. 2014;63:407–416. doi: 10.1016/j.jacc.2013.10.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kane G.C., Karon B.L., Mahoney D.W. Progression of left ventricular diastolic dysfunction and risk of heart failure. JAMA. 2011;306:856–863. doi: 10.1001/jama.2011.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Packer M. Abnormalities of diastolic function as a potential cause of exercise intolerance in chronic heart failure. Circulation. 1990;81:III78–III86. [PubMed] [Google Scholar]
- 13.Kraigher-Krainer E., Shah A.M., Gupta D.K. Impaired systolic function by strain imaging in heart failure with preserved ejection fraction. J Am Coll Cardiol. 2014;63:447–456. doi: 10.1016/j.jacc.2013.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DeVore A.D., McNulty S., Alenezi F. Impaired left ventricular global longitudinal strain in patients with heart failure with preserved ejection fraction: insights from the RELAX trial. Eur J Heart Fail. 2017;19:893–900. doi: 10.1002/ejhf.754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Houser S.R., Margulies K.B., Murphy A.M. Animal models of heart failure: a scientific statement from the American Heart Association. Circ Res. 2012;111:131–150. doi: 10.1161/RES.0b013e3182582523. [DOI] [PubMed] [Google Scholar]
- 16.Regan J.A., Mauro A.G., Carbone S. A mouse model of heart failure with preserved ejection fraction due to chronic infusion of a low subpressor dose of angiotensin II. Am J Physiol Heart Circ Physiol. 2015;309:H771–H778. doi: 10.1152/ajpheart.00282.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Horgan S., Watson C., Glezeva N., Baugh J. Murine models of diastolic dysfunction and heart failure with preserved ejection fraction. J Card Fail. 2014;20:984–995. doi: 10.1016/j.cardfail.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 18.Konstam M.A., Abboud F.M. Ejection fraction: misunderstood and overrated (changing the paradigm in categorizing heart failure) Circulation. 2017;135:717–719. doi: 10.1161/CIRCULATIONAHA.116.025795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shah S.J., Aistrup G.L., Gupta D.K. Ultrastructural and cellular basis for the development of abnormal myocardial mechanics during the transition from hypertension to heart failure. Am J Physiol Heart Circ Physiol. 2014;306:H88–H100. doi: 10.1152/ajpheart.00642.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shah S.J. Matchmaking for the optimization of clinical trials of heart failure with preserved ejection fraction: no laughing matter. J Am Coll Cardiol. 2013;62:1339–1342. doi: 10.1016/j.jacc.2013.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Halapas A., Papalois A., Stauropoulou A. In vivo models for heart failure research. In Vivo. 2008;22:767–780. [PubMed] [Google Scholar]
- 22.Breckenridge R. Heart failure and mouse models. Dis Model Mech. 2010;3:138–143. doi: 10.1242/dmm.005017. [DOI] [PubMed] [Google Scholar]
- 23.Gomes A.C., Falcao-Pires I., Pires A.L., Bras-Silva C., Leite-Moreira A.F. Rodent models of heart failure: an updated review. Heart Fail Rev. 2013;18:219–249. doi: 10.1007/s10741-012-9305-3. [DOI] [PubMed] [Google Scholar]
- 24.Rai V., Sharma P., Agrawal S., Agrawal D.K. Relevance of mouse models of cardiac fibrosis and hypertrophy in cardiac research. Mol Cell Biochem. 2017;424:123–145. doi: 10.1007/s11010-016-2849-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Russell J.C., Proctor S.D. Small animal models of cardiovascular disease: tools for the study of the roles of metabolic syndrome, dyslipidemia, and atherosclerosis. Cardiovasc Pathol. 2006;15:318–330. doi: 10.1016/j.carpath.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 26.Lakatta E.G. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part III: cellular and molecular clues to heart and arterial aging. Circulation. 2003;107:490–497. doi: 10.1161/01.cir.0000048894.99865.02. [DOI] [PubMed] [Google Scholar]
- 27.Lakatta E.G., Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: part II: the aging heart in health: links to heart disease. Circulation. 2003;107:346–354. doi: 10.1161/01.cir.0000048893.62841.f7. [DOI] [PubMed] [Google Scholar]
- 28.Dai D.F., Rabinovitch P.S. Cardiac aging in mice and humans: the role of mitochondrial oxidative stress. Trends Cardiovasc Med. 2009;19:213–220. doi: 10.1016/j.tcm.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kaila K., Haykowsky M.J., Thompson R.B., Paterson D.I. Heart failure with preserved ejection fraction in the elderly: scope of the problem. Heart Fail Rev. 2012;17:555–562. doi: 10.1007/s10741-011-9273-z. [DOI] [PubMed] [Google Scholar]
- 30.Mitchell S.J., Scheibye-Knudsen M., Longo D.L., de Cabo R. Animal models of aging research: implications for human aging and age-related diseases. Annu Rev Anim Biosci. 2015;3:283–303. doi: 10.1146/annurev-animal-022114-110829. [DOI] [PubMed] [Google Scholar]
- 31.Loffredo F.S., Nikolova A.P., Pancoast J.R., Lee R.T. Heart failure with preserved ejection fraction: molecular pathways of the aging myocardium. Circ Res. 2014;115:97–107. doi: 10.1161/CIRCRESAHA.115.302929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Redfield M.M., Jacobsen S.J., Borlaug B.A., Rodeheffer R.J., Kass D.A. Age- and gender-related ventricular-vascular stiffening: a community-based study. Circulation. 2005;112:2254–2262. doi: 10.1161/CIRCULATIONAHA.105.541078. [DOI] [PubMed] [Google Scholar]
- 33.Walker E.M., Jr., Nillas M.S., Mangiarua E.I. Age-associated changes in hearts of male Fischer 344/Brown Norway F1 rats. Ann Clin Lab Sci. 2006;36:427–438. [PubMed] [Google Scholar]
- 34.Fraticelli A., Josephson R., Danziger R., Lakatta E., Spurgeon H. Morphological and contractile characteristics of rat cardiac myocytes from maturation to senescence. Am J Physiol. 1989;257:H259–H265. doi: 10.1152/ajpheart.1989.257.1.H259. [DOI] [PubMed] [Google Scholar]
- 35.Goldspink D.F., Burniston J.G., Tan L.B. Cardiomyocyte death and the ageing and failing heart. Exp Physiol. 2003;88:447–458. doi: 10.1113/eph8802549. [DOI] [PubMed] [Google Scholar]
- 36.Olivetti G., Melissari M., Capasso J.M., Anversa P. Cardiomyopathy of the aging human heart. Myocyte loss and reactive cellular hypertrophy. Circ Res. 1991;68:1560–1568. doi: 10.1161/01.res.68.6.1560. [DOI] [PubMed] [Google Scholar]
- 37.McMurray J.J., Adamopoulos S., Anker S.D. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2012;33:1787–1847. doi: 10.1093/eurheartj/ehs104. [DOI] [PubMed] [Google Scholar]
- 38.Boluyt M.O., Devor S.T., Opiteck J.A., White T.P. Age effects on the adaptive response of the female rat heart following aortic constriction. J Gerontol A Biol Sci Med Sci. 2000;55:B307–B314. doi: 10.1093/gerona/55.6.b307. [DOI] [PubMed] [Google Scholar]
- 39.Boluyt M.O., Converso K., Hwang H.S., Mikkor A., Russell M.W. Echocardiographic assessment of age-associated changes in systolic and diastolic function of the female F344 rat heart. J Appl Physiol. 2004;96:822–828. doi: 10.1152/japplphysiol.01026.2003. [DOI] [PubMed] [Google Scholar]
- 40.Brenner D.A., Apstein C.S., Saupe K.W. Exercise training attenuates age-associated diastolic dysfunction in rats. Circulation. 2001;104:221–226. doi: 10.1161/01.cir.104.2.221. [DOI] [PubMed] [Google Scholar]
- 41.Varma N., Eberli F.R., Apstein C.S. Increased diastolic chamber stiffness during demand ischemia: response to quick length change differentiates rigor-activated from calcium-activated tension. Circulation. 2000;101:2185–2192. doi: 10.1161/01.cir.101.18.2185. [DOI] [PubMed] [Google Scholar]
- 42.Pacher P., Mabley J.G., Liaudet L. Left ventricular pressure-volume relationship in a rat model of advanced aging-associated heart failure. Am J Physiol Heart Circ Physiol. 2004;287:H2132–H2137. doi: 10.1152/ajpheart.00405.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Choi S.Y., Chang H.J., Choi S.I. Long-term exercise training attenuates age-related diastolic dysfunction: association of myocardial collagen cross-linking. J Korean Med Sci. 2009;24:32–39. doi: 10.3346/jkms.2009.24.1.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hosokawa M., Abe T., Higuchi K. Management and design of the maintenance of SAM mouse strains: an animal model for accelerated senescence and age-associated disorders. Exp Gerontol. 1997;32:111–116. doi: 10.1016/s0531-5565(96)00078-2. [DOI] [PubMed] [Google Scholar]
- 45.Takeda T. Senescence-accelerated mouse (SAM): a biogerontological resource in aging research. Neurobiol Aging. 1999;20:105–110. doi: 10.1016/s0197-4580(99)00008-1. [DOI] [PubMed] [Google Scholar]
- 46.Takeda T., Matsushita T., Kurozumi M., Takemura K., Higuchi K., Hosokawa M. Pathobiology of the senescence-accelerated mouse (SAM) Exp Gerontol. 1997;32:117–127. doi: 10.1016/s0531-5565(96)00068-x. [DOI] [PubMed] [Google Scholar]
- 47.Takeda T., Hosokawa M., Takeshita S. A new murine model of accelerated senescence. Mech Ageing Dev. 1981;17:183–194. doi: 10.1016/0047-6374(81)90084-1. [DOI] [PubMed] [Google Scholar]
- 48.Takeda T. Senescence-accelerated mouse (SAM) with special references to neurodegeneration models, SAMP8 and SAMP10 mice. Neurochem Res. 2009;34:639–659. doi: 10.1007/s11064-009-9922-y. [DOI] [PubMed] [Google Scholar]
- 49.Reed A.L., Tanaka A., Sorescu D. Diastolic dysfunction is associated with cardiac fibrosis in the senescence-accelerated mouse. Am J Physiol Heart Circ Physiol. 2011;301:H824–H831. doi: 10.1152/ajpheart.00407.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Karuppagounder V., Arumugam S., Babu S.S. The senescence accelerated mouse prone 8 (SAMP8): a novel murine model for cardiac aging. Ageing Res Rev. 2016:35–291. doi: 10.1016/j.arr.2016.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sreedhar R., Giridharan V.V., Arumugam S. Role of MAPK-mediated endoplasmic reticulum stress signaling in the heart during aging in senescence-accelerated prone mice. Biofactors. 2016;42:368–375. doi: 10.1002/biof.1280. [DOI] [PubMed] [Google Scholar]
- 52.Ghosh A.K., Hardy R.J., Francis D.P. Midlife blood pressure change and left ventricular mass and remodelling in older age in the 1946 British Birth Cohort Study. Eur Heart J. 2014;35:3287–3295. doi: 10.1093/eurheartj/ehu389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Samson R., Jaiswal A., Ennezat P.V., Cassidy M., Le Jemtel T.H. Clinical phenotypes in heart failure with preserved ejection fraction. J Am Heart Assoc. 2016;5 doi: 10.1161/JAHA.115.002477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Solomon S.D., Janardhanan R., Verma A. Effect of angiotensin receptor blockade and antihypertensive drugs on diastolic function in patients with hypertension and diastolic dysfunction: a randomised trial. Lancet. 2007;369:2079–2087. doi: 10.1016/S0140-6736(07)60980-5. [DOI] [PubMed] [Google Scholar]
- 55.Solomon S.D., Verma A., Desai A. Effect of intensive versus standard blood pressure lowering on diastolic function in patients with uncontrolled hypertension and diastolic dysfunction. Hypertension. 2010;55:241–248. doi: 10.1161/HYPERTENSIONAHA.109.138529. [DOI] [PubMed] [Google Scholar]
- 56.Adams V., Alves M., Fischer T. High-intensity interval training attenuates endothelial dysfunction in a Dahl salt-sensitive rat model of heart failure with preserved ejection fraction. J Appl Physiol. 2015;119:745–752. doi: 10.1152/japplphysiol.01123.2014. [DOI] [PubMed] [Google Scholar]
- 57.Davis B.R., Kostis J.B., Simpson L.M. Heart failure with preserved and reduced left ventricular ejection fraction in the antihypertensive and lipid-lowering treatment to prevent heart attack trial. Circulation. 2008;118:2259–2267. doi: 10.1161/CIRCULATIONAHA.107.762229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Benjamin E.J., Blaha M.J., Chiuve S.E. Heart Disease and Stroke Statistics—2017 Update: a report From the American Heart Association. Circulation. 2017;135:e146–e603. doi: 10.1161/CIR.0000000000000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lloyd-Jones D.M., Hong Y., Labarthe D. Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association's strategic Impact Goal through 2020 and beyond. Circulation. 2010;121:586–613. doi: 10.1161/CIRCULATIONAHA.109.192703. [DOI] [PubMed] [Google Scholar]
- 60.Persell S.D. Prevalence of resistant hypertension in the United States, 2003–2008. Hypertension. 2011;57:1076–1080. doi: 10.1161/HYPERTENSIONAHA.111.170308. [DOI] [PubMed] [Google Scholar]
- 61.Heidenreich P.A., Albert N.M., Allen L.A. Forecasting the impact of heart failure in the United States: a policy statement from the American Heart Association. Circ Heart Fail. 2013;6:606–619. doi: 10.1161/HHF.0b013e318291329a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Doggrell S.A., Brown L. Rat models of hypertension, cardiac hypertrophy and failure. Cardiovasc Res. 1998;39:89–105. doi: 10.1016/s0008-6363(98)00076-5. [DOI] [PubMed] [Google Scholar]
- 63.Brilla C.G., Weber K.T. Mineralocorticoid excess, dietary sodium, and myocardial fibrosis. J Lab Clin Med. 1992;120:893–901. [PubMed] [Google Scholar]
- 64.Brilla C.G., Weber K.T. Reactive and reparative myocardial fibrosis in arterial hypertension in the rat. Cardiovasc Res. 1992;26:671–677. doi: 10.1093/cvr/26.7.671. [DOI] [PubMed] [Google Scholar]
- 65.Young M., Fullerton M., Dilley R., Funder J. Mineralocorticoids, hypertension, and cardiac fibrosis. J Clin Invest. 1994;93:2578–2583. doi: 10.1172/JCI117269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sam F., Xie Z., Ooi H. Mice lacking osteopontin exhibit increased left ventricular dilation and reduced fibrosis after aldosterone infusion. Am J Hypertens. 2004;17:188–193. doi: 10.1016/j.amjhyper.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 67.Sam F., Duhaney T.A., Sato K. Adiponectin deficiency, diastolic dysfunction, and diastolic heart failure. Endocrinology. 2010;151:322–331. doi: 10.1210/en.2009-0806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tanaka K., Wilson R.M., Essick E.E. Effects of adiponectin on calcium-handling proteins in heart failure with preserved ejection fraction. Circ Heart Fail. 2014;7:976–985. doi: 10.1161/CIRCHEARTFAILURE.114.001279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Girerd N., Pang P.S., Swedberg K. Serum aldosterone is associated with mortality and re-hospitalization in patients with reduced ejection fraction hospitalized for acute heart failure: analysis from the EVEREST trial. Eur J Heart Fail. 2013;15:1228–1235. doi: 10.1093/eurjhf/hft100. [DOI] [PubMed] [Google Scholar]
- 70.Valero-Munoz M., Li S., Wilson R.M. Heart failure with preserved ejection fraction induces beiging in adipose tissue. Circ Heart Fail. 2016;9 doi: 10.1161/CIRCHEARTFAILURE.115.002724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Garcia A.G., Wilson R.M., Heo J. Interferon-gamma ablation exacerbates myocardial hypertrophy in diastolic heart failure. Am J Physiol Heart Circ Physiol. 2012;303:H587–H596. doi: 10.1152/ajpheart.00298.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wilson R.M., De Silva D.S., Sato K., Izumiya Y., Sam F. Effects of fixed-dose isosorbide dinitrate/hydralazine on diastolic function and exercise capacity in hypertension-induced diastolic heart failure. Hypertension. 2009;54:583–590. doi: 10.1161/HYPERTENSIONAHA.109.134932. [DOI] [PubMed] [Google Scholar]
- 73.Tanaka K., Valero-Munoz M., Wilson R.M. Follistatin like 1 regulates hypertrophy in heart failure with preserved ejection fraction. J Am Coll Cardiol Basic Trans Sci. 2016;1:207–221. doi: 10.1016/j.jacbts.2016.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Valero-Munoz M., Li S., Wilson R.M., Boldbaatar B., Iglarz M., Sam F. Dual endothelin-A/endothelin-B receptor blockade and cardiac remodeling in heart failure with preserved ejection fraction. Circ Heart Fail. 2016;9 doi: 10.1161/CIRCHEARTFAILURE.116.003381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zile M.R., Baicu C.F., Ikonomidis J.S. Myocardial stiffness in patients with heart failure and a preserved ejection fraction: contributions of collagen and titin. Circulation. 2015;131:1247–1259. doi: 10.1161/CIRCULATIONAHA.114.013215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hamdani N., Paulus W.J. Myocardial titin and collagen in cardiac diastolic dysfunction: partners in crime. Circulation. 2013;128:5–8. doi: 10.1161/CIRCULATIONAHA.113.003437. [DOI] [PubMed] [Google Scholar]
- 77.Collier P., Watson C.J., van Es M.H. Getting to the heart of cardiac remodeling; how collagen subtypes may contribute to phenotype. J Mol Cell Cardiol. 2012;52:148–153. doi: 10.1016/j.yjmcc.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 78.Borbely A., van der Velden J., Papp Z. Cardiomyocyte stiffness in diastolic heart failure. Circulation. 2005;111:774–781. doi: 10.1161/01.CIR.0000155257.33485.6D. [DOI] [PubMed] [Google Scholar]
- 79.Mohammed S.F., Hussain S., Mirzoyev S.A., Edwards W.D., Maleszewski J.J., Redfield M.M. Coronary microvascular rarefaction and myocardial fibrosis in heart failure with preserved ejection fraction. Circulation. 2015;131:550–559. doi: 10.1161/CIRCULATIONAHA.114.009625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.van Heerebeek L., Hamdani N., Falcao-Pires I. Low myocardial protein kinase G activity in heart failure with preserved ejection fraction. Circulation. 2012;126:830–839. doi: 10.1161/CIRCULATIONAHA.111.076075. [DOI] [PubMed] [Google Scholar]
- 81.Becher P.M., Lindner D., Miteva K. Role of heart rate reduction in the prevention of experimental heart failure: comparison between If-channel blockade and beta-receptor blockade. Hypertension. 2012;59:949–957. doi: 10.1161/HYPERTENSIONAHA.111.183913. [DOI] [PubMed] [Google Scholar]
- 82.Murdoch C.E., Chaubey S., Zeng L. Endothelial NADPH oxidase-2 promotes interstitial cardiac fibrosis and diastolic dysfunction through proinflammatory effects and endothelial-mesenchymal transition. J Am Coll Cardiol. 2014;63:2734–2741. doi: 10.1016/j.jacc.2014.02.572. [DOI] [PubMed] [Google Scholar]
- 83.Glenn D.J., Cardema M.C., Ni W. Cardiac steatosis potentiates angiotensin II effects in the heart. Am J Physiol Heart Circ Physiol. 2015;308:H339–H350. doi: 10.1152/ajpheart.00742.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ichihara S., Senbonmatsu T., Price E., Jr., Ichiki T., Gaffney F.A., Inagami T. Angiotensin II type 2 receptor is essential for left ventricular hypertrophy and cardiac fibrosis in chronic angiotensin II-induced hypertension. Circulation. 2001;104:346–351. doi: 10.1161/01.cir.104.3.346. [DOI] [PubMed] [Google Scholar]
- 85.Shen Y., Cheng F., Sharma M. Granzyme B deficiency protects against angiotensin ii-induced cardiac fibrosis. Am J Pathol. 2016;186:87–100. doi: 10.1016/j.ajpath.2015.09.010. [DOI] [PubMed] [Google Scholar]
- 86.Matsumoto E., Sasaki S., Kinoshita H. Angiotensin II-induced cardiac hypertrophy and fibrosis are promoted in mice lacking Fgf16. Genes Cells. 2013;18:544–553. doi: 10.1111/gtc.12055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gomolak J.R., Didion S.P. Angiotensin II-induced endothelial dysfunction is temporally linked with increases in interleukin-6 and vascular macrophage accumulation. Front Physiol. 2014;5:396. doi: 10.3389/fphys.2014.00396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Essick E.E., Wilson R.M., Pimentel D.R. Adiponectin modulates oxidative stress-induced autophagy in cardiomyocytes. PLoS ONE. 2013;8 doi: 10.1371/journal.pone.0068697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Peng H., Yang X.P., Carretero O.A. Angiotensin II-induced dilated cardiomyopathy in Balb/c but not C57BL/6J mice. Exp Physiol. 2011;96:756–764. doi: 10.1113/expphysiol.2011.057612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Xu Z., Okamoto H., Akino M., Onozuka H., Matsui Y., Tsutsui H. Pravastatin attenuates left ventricular remodeling and diastolic dysfunction in angiotensin II-induced hypertensive mice. J Cardiovasc Pharmacol. 2008;51:62–70. doi: 10.1097/FJC.0b013e31815bb629. [DOI] [PubMed] [Google Scholar]
- 91.Mori J., Alrob O.A., Wagg C.S., Harris R.A., Lopaschuk G.D., Oudit G.Y. ANG II causes insulin resistance and induces cardiac metabolic switch and inefficiency: a critical role of PDK4. Am J Physiol Heart Circ Physiol. 2013;304:H1103–H1113. doi: 10.1152/ajpheart.00636.2012. [DOI] [PubMed] [Google Scholar]
- 92.Westermann D., Becher P.M., Lindner D. Selective PDE5A inhibition with sildenafil rescues left ventricular dysfunction, inflammatory immune response and cardiac remodeling in angiotensin II-induced heart failure in vivo. Basic Res Cardiol. 2012;107:308. doi: 10.1007/s00395-012-0308-y. [DOI] [PubMed] [Google Scholar]
- 93.Kadoguchi T., Kinugawa S., Takada S. Angiotensin II can directly induce mitochondrial dysfunction, decrease oxidative fibre number and induce atrophy in mouse hindlimb skeletal muscle. Exp Physiol. 2015;100:312–322. doi: 10.1113/expphysiol.2014.084095. [DOI] [PubMed] [Google Scholar]
- 94.Rapp J.P., Wang S.M., Dene H. A genetic polymorphism in the renin gene of Dahl rats cosegregates with blood pressure. Science. 1989;243:542–544. doi: 10.1126/science.2563177. [DOI] [PubMed] [Google Scholar]
- 95.Doi R., Masuyama T., Yamamoto K. Development of different phenotypes of hypertensive heart failure: systolic versus diastolic failure in Dahl salt-sensitive rats. J Hypertens. 2000;18:111–120. doi: 10.1097/00004872-200018010-00016. [DOI] [PubMed] [Google Scholar]
- 96.Inoko M., Kihara Y., Morii I., Fujiwara H., Sasayama S. Transition from compensatory hypertrophy to dilated, failing left ventricles in Dahl salt-sensitive rats. Am J Physiol. 1994;267:H2471–H2482. doi: 10.1152/ajpheart.1994.267.6.H2471. [DOI] [PubMed] [Google Scholar]
- 97.Kamimura D., Ohtani T., Sakata Y. Ca2+ entry mode of Na+/Ca2+ exchanger as a new therapeutic target for heart failure with preserved ejection fraction. Eur Heart J. 2012;33:1408–1416. doi: 10.1093/eurheartj/ehr106. [DOI] [PubMed] [Google Scholar]
- 98.Omori Y., Ohtani T., Sakata Y. L-Carnitine prevents the development of ventricular fibrosis and heart failure with preserved ejection fraction in hypertensive heart disease. J Hypertens. 2012;30:1834–1844. doi: 10.1097/HJH.0b013e3283569c5a. [DOI] [PubMed] [Google Scholar]
- 99.Abbate A., Arena R., Abouzaki N. Heart failure with preserved ejection fraction: refocusing on diastole. Int J Cardiol. 2015;179:430–440. doi: 10.1016/j.ijcard.2014.11.106. [DOI] [PubMed] [Google Scholar]
- 100.Dunlay S.M., Roger V.L., Weston S.A., Jiang R., Redfield M.M. Longitudinal changes in ejection fraction in heart failure patients with preserved and reduced ejection fraction. Circ Heart Fail. 2012;5:720–726. doi: 10.1161/CIRCHEARTFAILURE.111.966366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Weinberger M.H. Salt sensitivity of blood pressure in humans. Hypertension. 1996;27:481–490. doi: 10.1161/01.hyp.27.3.481. [DOI] [PubMed] [Google Scholar]
- 102.Hummel S.L., Seymour E.M., Brook R.D. Low-sodium dietary approaches to stop hypertension diet reduces blood pressure, arterial stiffness, and oxidative stress in hypertensive heart failure with preserved ejection fraction. Hypertension. 2012;60:1200–1206. doi: 10.1161/HYPERTENSIONAHA.112.202705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Conceicao G., Heinonen I., Lourenco A.P., Duncker D.J., Falcao-Pires I. Animal models of heart failure with preserved ejection fraction. Neth Heart J. 2016;24:275–286. doi: 10.1007/s12471-016-0815-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Willard P.W. A model for evaluation of thiazide-induced hypotension. J Pharm Pharmacol. 1969;21:406–408. doi: 10.1111/j.2042-7158.1969.tb08280.x. [DOI] [PubMed] [Google Scholar]
- 105.Ogata T., Miyauchi T., Sakai S., Takanashi M., Irukayama-Tomobe Y., Yamaguchi I. Myocardial fibrosis and diastolic dysfunction in deoxycorticosterone acetate-salt hypertensive rats is ameliorated by the peroxisome proliferator-activated receptor-alpha activator fenofibrate, partly by suppressing inflammatory responses associated with the nuclear factor-kappa-B pathway. J Am Coll Cardiol. 2004;43:1481–1488. doi: 10.1016/j.jacc.2003.11.043. [DOI] [PubMed] [Google Scholar]
- 106.Matsumura Y., Kuro T., Konishi F., Takaoka M., Gariepy C.E., Yanagisawa M. Enhanced blood pressure sensitivity to DOCA-salt treatment in endothelin ET(B) receptor–deficient rats. Br J Pharmacol. 2000;129:1060–1062. doi: 10.1038/sj.bjp.0703157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Grobe J.L., Mecca A.P., Mao H., Katovich M.J. Chronic angiotensin-(1-7) prevents cardiac fibrosis in DOCA-salt model of hypertension. Am J Physiol Heart Circ Physiol. 2006;290:H2417–H2423. doi: 10.1152/ajpheart.01170.2005. [DOI] [PubMed] [Google Scholar]
- 108.Allan A., Fenning A., Levick S., Hoey A., Brown L. Reversal of cardiac dysfunction by selective ET-A receptor antagonism. Br J Pharmacol. 2005;146:846–853. doi: 10.1038/sj.bjp.0706384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lovelock J.D., Monasky M.M., Jeong E.M. Ranolazine improves cardiac diastolic dysfunction through modulation of myofilament calcium sensitivity. Circ Res. 2012;110:841–850. doi: 10.1161/CIRCRESAHA.111.258251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Mohammed S.F., Ohtani T., Korinek J. Mineralocorticoid accelerates transition to heart failure with preserved ejection fraction via “nongenomic effects.”. Circulation. 2010;122:370–378. doi: 10.1161/CIRCULATIONAHA.109.915215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Silberman G.A., Fan T.H., Liu H. Uncoupled cardiac nitric oxide synthase mediates diastolic dysfunction. Circulation. 2010;121:519–528. doi: 10.1161/CIRCULATIONAHA.109.883777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Jeong E.M., Monasky M.M., Gu L. Tetrahydrobiopterin improves diastolic dysfunction by reversing changes in myofilament properties. J Mol Cell Cardiol. 2013;56:44–54. doi: 10.1016/j.yjmcc.2012.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Bowen T.S., Eisenkolb S., Drobner J. High-intensity interval training prevents oxidant-mediated diaphragm muscle weakness in hypertensive mice. FASEB J. 2017;31:60–71. doi: 10.1096/fj.201600672R. [DOI] [PubMed] [Google Scholar]
- 114.Okamoto K. Spontaneous hypertension in rats. Int Rev Exp Pathol. 1969;7:227–270. [PubMed] [Google Scholar]
- 115.Okamoto K., AOKI K. Development of a strain of spontaneously hypertensive rats. Jpn Circ J. 1963;27:282–293. doi: 10.1253/jcj.27.282. [DOI] [PubMed] [Google Scholar]
- 116.Folkow B. Early structural changes in hypertension: pathophysiology and clinical consequences. J Cardiovasc Pharmacol. 1993;22(suppl 1):S1–S6. [PubMed] [Google Scholar]
- 117.Lindpaintner K., Kreutz R., Ganten D. Genetic variation in hypertensive and “control” strains. What are we controlling for anyway? Hypertension. 1992;19:428–430. doi: 10.1161/01.hyp.19.5.428. [DOI] [PubMed] [Google Scholar]
- 118.Heyen J.R., Blasi E.R., Nikula K. Structural, functional, and molecular characterization of the SHHF model of heart failure. Am J Physiol Heart Circ Physiol. 2002;283:H1775–H1784. doi: 10.1152/ajpheart.00305.2002. [DOI] [PubMed] [Google Scholar]
- 119.Pfeffer J.M., Pfeffer M.A., Fishbein M.C., Frohlich E.D. Cardiac function and morphology with aging in the spontaneously hypertensive rat. Am J Physiol. 1979;237:H461–H468. doi: 10.1152/ajpheart.1979.237.4.H461. [DOI] [PubMed] [Google Scholar]
- 120.Damatto R.L., Martinez P.F., Lima A.R. Heart failure-induced skeletal myopathy in spontaneously hypertensive rats. Int J Cardiol. 2013;167:698–703. doi: 10.1016/j.ijcard.2012.03.063. [DOI] [PubMed] [Google Scholar]
- 121.Bing O.H., Brooks W.W., Robinson K.G. The spontaneously hypertensive rat as a model of the transition from compensated left ventricular hypertrophy to failure. J Mol Cell Cardiol. 1995;27:383–396. doi: 10.1016/s0022-2828(08)80035-1. [DOI] [PubMed] [Google Scholar]
- 122.Kuoppala A., Shiota N., Lindstedt K.A. Expression of bradykinin receptors in the left ventricles of rats with pressure overload hypertrophy and heart failure. J Hypertens. 2003;21:1729–1736. doi: 10.1097/00004872-200309000-00023. [DOI] [PubMed] [Google Scholar]
- 123.Rockman H.A., Ross R.S., Harris A.N. Segregation of atrial-specific and inducible expression of an atrial natriuretic factor transgene in an in vivo murine model of cardiac hypertrophy. Proc Natl Acad Sci U S A. 1991;88:8277–8281. doi: 10.1073/pnas.88.18.8277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Beetz N., Rommel C., Schnick T. Ablation of biglycan attenuates cardiac hypertrophy and fibrosis after left ventricular pressure overload. J Mol Cell Cardiol. 2016;101:145–155. doi: 10.1016/j.yjmcc.2016.10.011. [DOI] [PubMed] [Google Scholar]
- 125.Shimano M., Ouchi N., Nakamura K. Cardiac myocyte-specific ablation of follistatin-like 3 attenuates stress-induced myocardial hypertrophy. J Biol Chem. 2011;286:9840–9848. doi: 10.1074/jbc.M110.197079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Boluyt M.O., Robinson K.G., Meredith A.L. Heart failure after long-term supravalvular aortic constriction in rats. Am J Hypertens. 2005;18:202–212. doi: 10.1016/j.amjhyper.2004.08.034. [DOI] [PubMed] [Google Scholar]
- 127.Liao Y., Takashima S., Maeda N. Exacerbation of heart failure in adiponectin-deficient mice due to impaired regulation of AMPK and glucose metabolism. Cardiovasc Res. 2005;67:705–713. doi: 10.1016/j.cardiores.2005.04.018. [DOI] [PubMed] [Google Scholar]
- 128.Knight W.E., Chen S., Zhang Y. PDE1C deficiency antagonizes pathological cardiac remodeling and dysfunction. Proc Natl Acad Sci U S A. 2016;113(45):E7116–E7125. doi: 10.1073/pnas.1607728113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ma J., Luo T., Zeng Z. Histone deacetylase inhibitor phenylbutyrate exaggerates heart failure in pressure overloaded mice independently of HDAC inhibition. Sci Rep. 2016;6:34036. doi: 10.1038/srep34036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Perrino C., Naga Prasad S.V., Mao L. Intermittent pressure overload triggers hypertrophy-independent cardiac dysfunction and vascular rarefaction. J Clin Invest. 2006;116:1547–1560. doi: 10.1172/JCI25397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Chen H., Hwang H., McKee L.A. Temporal and morphological impact of pressure overload in transgenic FHC mice. Front Physiol. 2013;4:205. doi: 10.3389/fphys.2013.00205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Lavie C.J., Milani R.V., Ventura H.O. Obesity and cardiovascular disease: risk factor, paradox, and impact of weight loss. J Am Coll Cardiol. 2009;53:1925–1932. doi: 10.1016/j.jacc.2008.12.068. [DOI] [PubMed] [Google Scholar]
- 133.Turkbey E.B., McClelland R.L., Kronmal R.A. The impact of obesity on the left ventricle: the Multi-Ethnic Study of Atherosclerosis (MESA) J Am Coll Cardiol Img. 2010;3:266–274. doi: 10.1016/j.jcmg.2009.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Haass M., Kitzman D.W., Anand I.S. Body mass index and adverse cardiovascular outcomes in heart failure patients with preserved ejection fraction: results from the Irbesartan in Heart Failure with Preserved Ejection Fraction (I-PRESERVE) trial. Circ Heart Fail. 2011;4:324–331. doi: 10.1161/CIRCHEARTFAILURE.110.959890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Haykowsky M.J., Kouba E.J., Brubaker P.H., Nicklas B.J., Eggebeen J., Kitzman D.W. Skeletal muscle composition and its relation to exercise intolerance in older patients with heart failure and preserved ejection fraction. Am J Cardiol. 2014;113:1211–1216. doi: 10.1016/j.amjcard.2013.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Beavers K.M., Beavers D.P., Houston D.K. Associations between body composition and gait-speed decline: results from the Health, Aging, and Body Composition study. Am J Clin Nutr. 2013;97:552–560. doi: 10.3945/ajcn.112.047860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Normandin E., Houston D.K., Nicklas B.J. Caloric restriction for treatment of geriatric obesity: do the benefits outweigh the risks? Curr Nutr Rep. 2015;4:143–155. doi: 10.1007/s13668-015-0123-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Katzel L.I., Bleecker E.R., Colman E.G., Rogus E.M., Sorkin J.D., Goldberg A.P. Effects of weight loss vs aerobic exercise training on risk factors for coronary disease in healthy, obese, middle-aged and older men. A randomized controlled trial. JAMA. 1995;274:1915–1921. doi: 10.1001/jama.1995.03530240025035. [DOI] [PubMed] [Google Scholar]
- 139.Kitzman D.W., Shah S.J. The HFpEF obesity phenotype: the elephant in the room. J Am Coll Cardiol. 2016;68:200–203. doi: 10.1016/j.jacc.2016.05.019. [DOI] [PubMed] [Google Scholar]
- 140.de las Fuentes L., Waggoner A.D., Mohammed B.S. Effect of moderate diet-induced weight loss and weight regain on cardiovascular structure and function. J Am Coll Cardiol. 2009;54:2376–2381. doi: 10.1016/j.jacc.2009.07.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Ikonomidis I., Mazarakis A., Papadopoulos C. Weight loss after bariatric surgery improves aortic elastic properties and left ventricular function in individuals with morbid obesity: a 3-year follow-up study. J Hypertens. 2007;25:439–447. doi: 10.1097/HJH.0b013e3280115bfb. [DOI] [PubMed] [Google Scholar]
- 142.Nanayakkara S., Kaye D.M. Management of heart failure with preserved ejection fraction: a review. Clin Ther. 2015;37:2186–2198. doi: 10.1016/j.clinthera.2015.08.005. [DOI] [PubMed] [Google Scholar]
- 143.Triposkiadis F., Giamouzis G., Parissis J. Reframing the association and significance of co-morbidities in heart failure. Eur J Heart Fail. 2016;18:744–758. doi: 10.1002/ejhf.600. [DOI] [PubMed] [Google Scholar]
- 144.MacDonald M.R., Petrie M.C., Varyani F. Impact of diabetes on outcomes in patients with low and preserved ejection fraction heart failure: an analysis of the Candesartan in Heart failure: Assessment of Reduction in Mortality and morbidity (CHARM) programme. Eur Heart J. 2008;29:1377–1385. doi: 10.1093/eurheartj/ehn153. [DOI] [PubMed] [Google Scholar]
- 145.von Bibra H., Paulus W., St John Sutton M. Cardiometabolic syndrome and increased risk of heart failure. Curr Heart Fail Rep. 2016;13:219–229. doi: 10.1007/s11897-016-0298-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Boudina S., Abel E.D. Diabetic cardiomyopathy revisited. Circulation. 2007;115:3213–3223. doi: 10.1161/CIRCULATIONAHA.106.679597. [DOI] [PubMed] [Google Scholar]
- 147.Mentz R.J., Kelly J.P., von Lueder T.G. Noncardiac comorbidities in heart failure with reduced versus preserved ejection fraction. J Am Coll Cardiol. 2014;64:2281–2293. doi: 10.1016/j.jacc.2014.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Aroor A.R., Mandavia C.H., Sowers J.R. Insulin resistance and heart failure: molecular mechanisms. Heart Fail Clin. 2012;8:609–617. doi: 10.1016/j.hfc.2012.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Scherbakov N., Bauer M., Sandek A. Insulin resistance in heart failure: differences between patients with reduced and preserved left ventricular ejection fraction. Eur J Heart Fail. 2015;17:1015–1021. doi: 10.1002/ejhf.317. [DOI] [PubMed] [Google Scholar]
- 150.Konduracka E., Gackowski A., Rostoff P., Galicka-Latala D., Frasik W., Piwowarska W. Diabetes-specific cardiomyopathy in type 1 diabetes mellitus: no evidence for its occurrence in the era of intensive insulin therapy. Eur Heart J. 2007;28:2465–2471. doi: 10.1093/eurheartj/ehm361. [DOI] [PubMed] [Google Scholar]
- 151.Chen H., Charlat O., Tartaglia L.A. Evidence that the diabetes gene encodes the leptin receptor: identification of a mutation in the leptin receptor gene in db/db mice. Cell. 1996;84:491–495. doi: 10.1016/s0092-8674(00)81294-5. [DOI] [PubMed] [Google Scholar]
- 152.Reil J.C., Hohl M., Reil G.H. Heart rate reduction by If-inhibition improves vascular stiffness and left ventricular systolic and diastolic function in a mouse model of heart failure with preserved ejection fraction. Eur Heart J. 2013;34:2839–2849. doi: 10.1093/eurheartj/ehs218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Cavaghan M.K., Ehrmann D.A., Polonsky K.S. Interactions between insulin resistance and insulin secretion in the development of glucose intolerance. J Clin Invest. 2000;106:329–333. doi: 10.1172/JCI10761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Cucak H., Grunnet L.G., Rosendahl A. Accumulation of M1-like macrophages in type 2 diabetic islets is followed by a systemic shift in macrophage polarization. J Leukoc Biol. 2014;95:149–160. doi: 10.1189/jlb.0213075. [DOI] [PubMed] [Google Scholar]
- 155.Belke D.D., Larsen T.S., Gibbs E.M., Severson D.L. Altered metabolism causes cardiac dysfunction in perfused hearts from diabetic (db/db) mice. Am J Physiol Endocrinol Metab. 2000;279:E1104–E1113. doi: 10.1152/ajpendo.2000.279.5.E1104. [DOI] [PubMed] [Google Scholar]
- 156.Semeniuk L.M., Kryski A.J., Severson D.L. Echocardiographic assessment of cardiac function in diabetic db/db and transgenic db/db-hGLUT4 mice. Am J Physiol Heart Circ Physiol. 2002;283:H976–H982. doi: 10.1152/ajpheart.00088.2002. [DOI] [PubMed] [Google Scholar]
- 157.Aasum E., Hafstad A.D., Severson D.L., Larsen T.S. Age-dependent changes in metabolism, contractile function, and ischemic sensitivity in hearts from db/db mice. Diabetes. 2003;52:434–441. doi: 10.2337/diabetes.52.2.434. [DOI] [PubMed] [Google Scholar]
- 158.Barouch L.A., Berkowitz D.E., Harrison R.W., O'Donnell C.P., Hare J.M. Disruption of leptin signaling contributes to cardiac hypertrophy independently of body weight in mice. Circulation. 2003;108:754–759. doi: 10.1161/01.CIR.0000083716.82622.FD. [DOI] [PubMed] [Google Scholar]
- 159.Van den Bergh A., Flameng W., Herijgers P. Type II diabetic mice exhibit contractile dysfunction but maintain cardiac output by favourable loading conditions. Eur J Heart Fail. 2006;8:777–783. doi: 10.1016/j.ejheart.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 160.Giacomelli F., Wiener J. Primary myocardial disease in the diabetic mouse. An ultrastructural study. Lab Invest. 1979;40:460–473. [PubMed] [Google Scholar]
- 161.Plante E., Menaouar A., Danalache B.A., Broderick T.L., Jankowski M., Gutkowska J. Treatment with brain natriuretic peptide prevents the development of cardiac dysfunction in obese diabetic db/db mice. Diabetologia. 2014;57:1257–1267. doi: 10.1007/s00125-014-3201-4. [DOI] [PubMed] [Google Scholar]
- 162.Mori J., Patel V.B., Abo Alrob O. Angiotensin 1-7 ameliorates diabetic cardiomyopathy and diastolic dysfunction in db/db mice by reducing lipotoxicity and inflammation. Circ Heart Fail. 2014;7:327–339. doi: 10.1161/CIRCHEARTFAILURE.113.000672. [DOI] [PubMed] [Google Scholar]
- 163.Hamdani N., Hervent A.S., Vandekerckhove L. Left ventricular diastolic dysfunction and myocardial stiffness in diabetic mice is attenuated by inhibition of dipeptidyl peptidase 4. Cardiovasc Res. 2014;104:423–431. doi: 10.1093/cvr/cvu223. [DOI] [PubMed] [Google Scholar]
- 164.Ostler J.E., Maurya S.K., Dials J. Effects of insulin resistance on skeletal muscle growth and exercise capacity in type 2 diabetic mouse models. Am J Physiol Endocrinol Metab. 2014;306:E592–E605. doi: 10.1152/ajpendo.00277.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Papinska A.M., Soto M., Meeks C.J., Rodgers K.E. Long-term administration of angiotensin (1-7) prevents heart and lung dysfunction in a mouse model of type 2 diabetes (db/db) by reducing oxidative stress, inflammation and pathological remodeling. Pharmacol Res. 2016;107:372–380. doi: 10.1016/j.phrs.2016.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Broderick T.L., Jankowski M., Wang D., Danalache B.A., Parrott C.R., Gutkowska J. Downregulation in GATA4 and downstream structural and contractile genes in the db/db mouse heart. ISRN Endocrinol. 2012;2012:736860. doi: 10.5402/2012/736860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Wang T.J., Larson M.G., Levy D. Impact of obesity on plasma natriuretic peptide levels. Circulation. 2004;109:594–600. doi: 10.1161/01.CIR.0000112582.16683.EA. [DOI] [PubMed] [Google Scholar]
- 168.Ingalls A.M., Dickie M.M., Snell G.D. Obese, a new mutation in the house mouse. J Hered. 1950;41:317–318. doi: 10.1093/oxfordjournals.jhered.a106073. [DOI] [PubMed] [Google Scholar]
- 169.Lindstrom P. The physiology of obese-hyperglycemic mice [ob/ob mice] ScientificWorldJournal. 2007;7:666–685. doi: 10.1100/tsw.2007.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Westman S. Development of the obese-hyperglycaemic syndrome in mice. Diabetologia. 1968;4:141–149. doi: 10.1007/BF01219435. [DOI] [PubMed] [Google Scholar]
- 171.Edvell A., Lindstrom P. Development of insulin secretory function in young obese hyperglycemic mice (Umeå ob/ob) Metabolism. 1995;44:906–913. doi: 10.1016/0026-0495(95)90244-9. [DOI] [PubMed] [Google Scholar]
- 172.Edvell A., Lindstrom P. Initiation of increased pancreatic islet growth in young normoglycemic mice (Umeå +/?) Endocrinology. 1999;140:778–783. doi: 10.1210/endo.140.2.6514. [DOI] [PubMed] [Google Scholar]
- 173.Herberg L., Major E., Hennigs U., Gruneklee D., Freytag G., Gries F.A. Differences in the development of the obese-hyperglycemic syndrome in obob and NZO mice. Diabetologia. 1970;6:292–299. doi: 10.1007/BF01212241. [DOI] [PubMed] [Google Scholar]
- 174.Danielsson A., Hellman B., Taljedal I.B. Glucose tolerance in the period preceding the appearance of the manifest obese-hyperglycemic syndrome in mice. Acta Physiol Scand. 1968;72:81–84. doi: 10.1111/j.1748-1716.1968.tb03829.x. [DOI] [PubMed] [Google Scholar]
- 175.Petriz B.A., Cunha V.N., Villeth G.R. Effects of acute exercise over heart proteome from monogenic obese (ob/ob) mice. J Cell Physiol. 2013;228:824–834. doi: 10.1002/jcp.24231. [DOI] [PubMed] [Google Scholar]
- 176.Dong F., Zhang X., Yang X. Impaired cardiac contractile function in ventricular myocytes from leptin-deficient ob/ob obese mice. J Endocrinol. 2006;188:25–36. doi: 10.1677/joe.1.06241. [DOI] [PubMed] [Google Scholar]
- 177.Trivedi P., Yang R., Barouch L.A. Decreased p110alpha catalytic activity accompanies increased myocyte apoptosis and cardiac hypertrophy in leptin deficient ob/ob mice. Cell Cycle. 2008;7:560–565. doi: 10.4161/cc.7.5.5529. [DOI] [PubMed] [Google Scholar]
- 178.Christoffersen C., Bollano E., Lindegaard M.L. Cardiac lipid accumulation associated with diastolic dysfunction in obese mice. Endocrinology. 2003;144:3483–3490. doi: 10.1210/en.2003-0242. [DOI] [PubMed] [Google Scholar]
- 179.Mark A.L., Shaffer R.A., Correia M.L., Morgan D.A., Sigmund C.D., Haynes W.G. Contrasting blood pressure effects of obesity in leptin-deficient ob/ob mice and agouti yellow obese mice. J Hypertens. 1999;17:1949–1953. doi: 10.1097/00004872-199917121-00026. [DOI] [PubMed] [Google Scholar]
- 180.Swoap S.J. Altered leptin signaling is sufficient, but not required, for hypotension associated with caloric restriction. Am J Physiol Heart Circ Physiol. 2001;281:H2473–H2479. doi: 10.1152/ajpheart.2001.281.6.H2473. [DOI] [PubMed] [Google Scholar]
- 181.Manolescu D.C., Jankowski M., Danalache B.A. All-trans retinoic acid stimulates gene expression of the cardioprotective natriuretic peptide system and prevents fibrosis and apoptosis in cardiomyocytes of obese ob/ob mice. Appl Physiol Nutr Metab. 2014;39:1127–1136. doi: 10.1139/apnm-2014-0005. [DOI] [PubMed] [Google Scholar]
- 182.Chen J.Y., Tsai P.J., Tai H.C. Increased aortic stiffness and attenuated lysyl oxidase activity in obesity. Arterioscler Thromb Vasc Biol. 2013;33:839–846. doi: 10.1161/ATVBAHA.112.300036. [DOI] [PubMed] [Google Scholar]
- 183.Christoffersen C., Bartels E.D., Nielsen L.B. Heart specific up-regulation of genes for B-type and C-type natriuretic peptide receptors in diabetic mice. Eur J Clin Invest. 2006;36:69–75. doi: 10.1111/j.1365-2362.2006.01596.x. [DOI] [PubMed] [Google Scholar]
- 184.Broderick T.L., Wang D., Jankowski M., Gutkowska J. Unexpected effects of voluntary exercise training on natriuretic peptide and receptor mRNA expression in the ob/ob mouse heart. Regul Pept. 2014;188:52–59. doi: 10.1016/j.regpep.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 185.Pelleymounter M.A., Cullen M.J., Baker M.B. Effects of the obese gene product on body weight regulation in ob/ob mice. Science. 1995;269:540–543. doi: 10.1126/science.7624776. [DOI] [PubMed] [Google Scholar]
- 186.Barouch L.A., Gao D., Chen L. Cardiac myocyte apoptosis is associated with increased DNA damage and decreased survival in murine models of obesity. Circ Res. 2006;98:119–124. doi: 10.1161/01.RES.0000199348.10580.1d. [DOI] [PubMed] [Google Scholar]
- 187.Abel E.D., Litwin S.E., Sweeney G. Cardiac remodeling in obesity. Physiol Rev. 2008;88:389–419. doi: 10.1152/physrev.00017.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Clement K. Genetics of human obesity. C R Biol. 2006;329:608–622. doi: 10.1016/j.crvi.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 189.Rakieten N., Rakieten M.L., Nadkarni M.R. Studies on the diabetogenic action of streptozotocin (NSC-37917) Cancer Chemother Rep. 1963;29:91–98. [PubMed] [Google Scholar]
- 190.Junod A., Lambert A.E., Orci L., Pictet R., Gonet A.E., Renold A.E. Studies of the diabetogenic action of streptozotocin. Proc Soc Exp Biol Med. 1967;126:201–205. doi: 10.3181/00379727-126-32401. [DOI] [PubMed] [Google Scholar]
- 191.Wei M., Ong L., Smith M.T. The streptozotocin-diabetic rat as a model of the chronic complications of human diabetes. Heart Lung Circ. 2003;12:44–50. doi: 10.1046/j.1444-2892.2003.00160.x. [DOI] [PubMed] [Google Scholar]
- 192.Obineche E., Chandranath I., Adeghate E., Benedict S., Fahim M., Adem A. Alterations in atrial natriuretic peptide and its receptor levels in long-term, streptozotocin-induced, diabetes in rats. Ann N Y Acad Sci. 2006;1084:223–234. doi: 10.1196/annals.1372.025. [DOI] [PubMed] [Google Scholar]
- 193.Saklani R., Gupta S.K., Mohanty I.R., Kumar B., Srivastava S., Mathur R. Cardioprotective effects of rutin via alteration in TNF-alpha, CRP, and BNP levels coupled with antioxidant effect in STZ-induced diabetic rats. Mol Cell Biochem. 2016;420:65–72. doi: 10.1007/s11010-016-2767-1. [DOI] [PubMed] [Google Scholar]
- 194.Wang Y.H., Liu Y.H., He G.R., Lv Y., Du G.H. Esculin improves dyslipidemia, inflammation and renal damage in streptozotocin-induced diabetic rats. BMC Complement Altern Med. 2015;15:402. doi: 10.1186/s12906-015-0817-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Fein F.S., Kornstein L.B., Strobeck J.E., Capasso J.M., Sonnenblick E.H. Altered myocardial mechanics in diabetic rats. Circ Res. 1980;47:922–933. doi: 10.1161/01.res.47.6.922. [DOI] [PubMed] [Google Scholar]
- 196.Carbonell L.F., Salom M.G., Garcia-Estan J., Salazar F.J., Ubeda M., Quesada T. Hemodynamic alterations in chronically conscious unrestrained diabetic rats. Am J Physiol. 1987;252:H900–H905. doi: 10.1152/ajpheart.1987.252.5.H900. [DOI] [PubMed] [Google Scholar]
- 197.Mihm M.J., Seifert J.L., Coyle C.M., Bauer J.A. Diabetes related cardiomyopathy time dependent echocardiographic evaluation in an experimental rat model. Life Sci. 2001;69:527–542. doi: 10.1016/s0024-3205(01)01141-9. [DOI] [PubMed] [Google Scholar]
- 198.Miric G., Dallemagne C., Endre Z., Margolin S., Taylor S.M., Brown L. Reversal of cardiac and renal fibrosis by pirfenidone and spironolactone in streptozotocin-diabetic rats. Br J Pharmacol. 2001;133:687–694. doi: 10.1038/sj.bjp.0704131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Zhou H., Li Y.J., Wang M. Involvement of RhoA/ROCK in myocardial fibrosis in a rat model of type 2 diabetes. Acta Pharmacol Sin. 2011;32:999–1008. doi: 10.1038/aps.2011.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Zheng H., Pu S.Y., Fan X.F. Treatment with angiotensin-(1-9) alleviates the cardiomyopathy in streptozotocin-induced diabetic rats. Biochem Pharmacol. 2015;95:38–45. doi: 10.1016/j.bcp.2015.03.009. [DOI] [PubMed] [Google Scholar]
- 201.Kruger M., Babicz K., von Frieling-Salewsky M., Linke W.A. Insulin signaling regulates cardiac titin properties in heart development and diabetic cardiomyopathy. J Mol Cell Cardiol. 2010;48:910–916. doi: 10.1016/j.yjmcc.2010.02.012. [DOI] [PubMed] [Google Scholar]
- 202.Satheesan S., Figarola J.L., Dabbs T., Rahbar S., Ermel R. Effects of a new advanced glycation inhibitor, LR-90, on mitigating arterial stiffening and improving arterial elasticity and compliance in a diabetic rat model: aortic impedance analysis. Br J Pharmacol. 2014;171:3103–3114. doi: 10.1111/bph.12656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Falcao-Pires I., Palladini G., Goncalves N. Distinct mechanisms for diastolic dysfunction in diabetes mellitus and chronic pressure-overload. Basic Res Cardiol. 2011;106:801–814. doi: 10.1007/s00395-011-0184-x. [DOI] [PubMed] [Google Scholar]
- 204.Hung C.H., Tzeng J.I., Chang C.N., Chen Y.W., Cho C.Y., Wang J.J. Treadmill exercise preconditioning attenuates lung damage caused by systemic endotoxemia in type 1 diabetic rats. J Diabetes Res. 2013;2013:527090. doi: 10.1155/2013/527090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Connelly K.A., Kelly D.J., Zhang Y. Functional, structural and molecular aspects of diastolic heart failure in the diabetic (mRen-2)27 rat. Cardiovasc Res. 2007;76:280–291. doi: 10.1016/j.cardiores.2007.06.022. [DOI] [PubMed] [Google Scholar]
- 206.Poornima I.G., Parikh P., Shannon R.P. Diabetic cardiomyopathy: the search for a unifying hypothesis. Circ Res. 2006;98:596–605. doi: 10.1161/01.RES.0000207406.94146.c2. [DOI] [PubMed] [Google Scholar]
- 207.Fiordaliso F., Li B., Latini R. Myocyte death in streptozotocin-induced diabetes in rats in angiotensin II- dependent. Lab Invest. 2000;80:513–527. doi: 10.1038/labinvest.3780057. [DOI] [PubMed] [Google Scholar]
- 208.Depre C., Young M.E., Ying J. Streptozotocin-induced changes in cardiac gene expression in the absence of severe contractile dysfunction. J Mol Cell Cardiol. 2000;32:985–996. doi: 10.1006/jmcc.2000.1139. [DOI] [PubMed] [Google Scholar]
- 209.Lu J., Yao Y.Y., Dai Q.M. Erythropoietin attenuates cardiac dysfunction by increasing myocardial angiogenesis and inhibiting interstitial fibrosis in diabetic rats. Cardiovasc Diabetol. 2012;11:105. doi: 10.1186/1475-2840-11-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Salem K.A., Kosanovic M., Qureshi A., Ljubisavljevic M., Howarth F.C. The direct effects of streptozotocin and alloxan on contractile function in rat heart. Pharmacol Res. 2009;59:235–241. doi: 10.1016/j.phrs.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 211.Lacombe V.A., Viatchenko-Karpinski S., Terentyev D. Mechanisms of impaired calcium handling underlying subclinical diastolic dysfunction in diabetes. Am J Physiol Regul Integr Comp Physiol. 2007;293:R1787–R1797. doi: 10.1152/ajpregu.00059.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Joffe I.I., Travers K.E., Perreault-Micale C.L. Abnormal cardiac function in the streptozotocin-induced non-insulin-dependent diabetic rat: noninvasive assessment with doppler echocardiography and contribution of the nitric oxide pathway. J Am Coll Cardiol. 1999;34:2111–2119. doi: 10.1016/s0735-1097(99)00436-2. [DOI] [PubMed] [Google Scholar]
- 213.Akula A., Kota M.K., Gopisetty S.G. Biochemical, histological and echocardiographic changes during experimental cardiomyopathy in STZ-induced diabetic rats. Pharmacol Res. 2003;48:429–435. doi: 10.1016/s1043-6618(03)00191-9. [DOI] [PubMed] [Google Scholar]
- 214.Nagueh S.F. Dobutamine echocardiography versus nuclear cardiac imaging for evaluation of myocardial viability. Curr Opin Cardiol. 1997;12:547–552. doi: 10.1097/00001573-199711000-00008. [DOI] [PubMed] [Google Scholar]
- 215.An D., Rodrigues B. Role of changes in cardiac metabolism in development of diabetic cardiomyopathy. Am J Physiol Heart Circ Physiol. 2006;291:H1489–H1506. doi: 10.1152/ajpheart.00278.2006. [DOI] [PubMed] [Google Scholar]
- 216.Zucker L.M., Zucker T.F. Fatty, a new mutation in the rats. J Heredity. 1961;52:275–278. [Google Scholar]
- 217.Bray G.A., York D.A. Studies on food intake of genetically obese rats. Am J Physiol. 1972;223:176–179. doi: 10.1152/ajplegacy.1972.223.1.176. [DOI] [PubMed] [Google Scholar]
- 218.Velez M., Kohli S., Sabbah H.N. Animal models of insulin resistance and heart failure. Heart Fail Rev. 2014;19:1–13. doi: 10.1007/s10741-013-9387-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Ren J., Sowers J.R., Walsh M.F., Brown R.A. Reduced contractile response to insulin and IGF-I in ventricular myocytes from genetically obese Zucker rats. Am J Physiol Heart Circ Physiol. 2000;279:H1708–H1714. doi: 10.1152/ajpheart.2000.279.4.H1708. [DOI] [PubMed] [Google Scholar]
- 220.Conti M., Renaud I.M., Poirier B. High levels of myocardial antioxidant defense in aging nondiabetic normotensive Zucker obese rats. Am J Physiol Regul Integr Comp Physiol. 2004;286:R793–R800. doi: 10.1152/ajpregu.00521.2002. [DOI] [PubMed] [Google Scholar]
- 221.Wang P., Lloyd S.G., Zeng H., Bonen A., Chatham J.C. Impact of altered substrate utilization on cardiac function in isolated hearts from Zucker diabetic fatty rats. Am J Physiol Heart Circ Physiol. 2005;288:H2102–H2110. doi: 10.1152/ajpheart.00935.2004. [DOI] [PubMed] [Google Scholar]
- 222.van den Brom C.E., Bosmans J.W., Vlasblom R. Diabetic cardiomyopathy in Zucker diabetic fatty rats: the forgotten right ventricle. Cardiovasc Diabetol. 2010;9:25. doi: 10.1186/1475-2840-9-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Fredersdorf S., Thumann C., Ulucan C. Myocardial hypertrophy and enhanced left ventricular contractility in Zucker diabetic fatty rats. Cardiovasc Pathol. 2004;13:11–19. doi: 10.1016/S1054-8807(03)00109-1. [DOI] [PubMed] [Google Scholar]
- 224.Toblli J.E., Cao G., DeRosa G., Di G.F., Forcada P. Angiotensin-converting enzyme inhibition and angiogenesis in myocardium of obese Zucker rats. Am J Hypertens. 2004;17:172–180. doi: 10.1016/j.amjhyper.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 225.Tofovic S.P., Jackson E.K. Rat models of the metabolic syndrome. Methods Mol Med. 2003;86:29–46. doi: 10.1385/1-59259-392-5:29. [DOI] [PubMed] [Google Scholar]
- 226.Tofovic S.P., Kusaka H., Kost C.K., Jr., Bastacky S. Renal function and structure in diabetic, hypertensive, obese ZDFxSHHF-hybrid rats. Ren Fail. 2000;22:387–406. doi: 10.1081/jdi-100100882. [DOI] [PubMed] [Google Scholar]
- 227.Griffin K.A., Abu-Naser M., Abu-Amarah I., Picken M., Williamson G.A., Bidani A.K. Dynamic blood pressure load and nephropathy in the ZSF1 (fa/fa cp) model of type 2 diabetes. Am J Physiol Renal Physiol. 2007;293:F1605–F1613. doi: 10.1152/ajprenal.00511.2006. [DOI] [PubMed] [Google Scholar]
- 228.Hamdani N., Franssen C., Lourenco A. Myocardial titin hypophosphorylation importantly contributes to heart failure with preserved ejection fraction in a rat metabolic risk model. Circ Heart Fail. 2013;6:1239–1249. doi: 10.1161/CIRCHEARTFAILURE.113.000539. [DOI] [PubMed] [Google Scholar]
- 229.van Dijk C.G., Oosterhuis N.R., Xu Y.J. Distinct endothelial cell responses in the heart and kidney microvasculature characterize the progression of heart failure with preserved ejection fraction in the obese ZSF1 rat with cardiorenal metabolic syndrome. Circ Heart Fail. 2016;9 doi: 10.1161/CIRCHEARTFAILURE.115.002760. [DOI] [PubMed] [Google Scholar]
- 230.Mohanan A., Gupta R., Dubey A. TRC120038, a novel dual AT(1)/ET(A) receptor blocker for control of hypertension, diabetic nephropathy, and cardiomyopathy in ob-ZSF1 Rats. Int J Hypertens. 2011;2011:751513. doi: 10.4061/2011/751513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Leite S., Oliveira-Pinto J., Tavares-Silva M. Echocardiography and invasive hemodynamics during stress testing for diagnosis of heart failure with preserved ejection fraction: an experimental study. Am J Physiol Heart Circ Physiol. 2015;308:H1556–H1563. doi: 10.1152/ajpheart.00076.2015. [DOI] [PubMed] [Google Scholar]
- 232.van Heerebeek L., Borbely A., Niessen H.W. Myocardial structure and function differ in systolic and diastolic heart failure. Circulation. 2006;113:1966–1973. doi: 10.1161/CIRCULATIONAHA.105.587519. [DOI] [PubMed] [Google Scholar]
- 233.Borbely A., Falcao-Pires I., van Heerebeek L. Hypophosphorylation of the Stiff N2B titin isoform raises cardiomyocyte resting tension in failing human myocardium. Circ Res. 2009;104:780–786. doi: 10.1161/CIRCRESAHA.108.193326. [DOI] [PubMed] [Google Scholar]
- 234.Lai Y.C., Tabima D.M., Dube J.J. SIRT3-AMP-activated protein kinase activation by nitrite and metformin improves hyperglycemia and normalizes pulmonary hypertension associated with heart failure with preserved ejection fraction. Circulation. 2016;133:717–731. doi: 10.1161/CIRCULATIONAHA.115.018935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Fuster V., Ryden L.E., Cannom D.S. ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association task force on practice guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Revise the 2001 Guidelines for the Management of Patients With Atrial Fibrillation): developed in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. Circulation. 2006;114:e257–e354. doi: 10.1161/CIRCULATIONAHA.106.177292. [DOI] [PubMed] [Google Scholar]
- 236.Vermond R.A., Geelhoed B., Verweij N. Incidence of atrial fibrillation and relationship with cardiovascular events, heart failure, and mortality: a community-based study from the Netherlands. J Am Coll Cardiol. 2015;66:1000–1007. doi: 10.1016/j.jacc.2015.06.1314. [DOI] [PubMed] [Google Scholar]
- 237.Kotecha D., Lam C.S., van Veldhuisen D.J., Van Gelder I.C., Voors A.A., Rienstra M. Heart failure with preserved ejection fraction and atrial fibrillation: vicious twins. J Am Coll Cardiol. 2016;68:2217–2228. doi: 10.1016/j.jacc.2016.08.048. [DOI] [PubMed] [Google Scholar]
- 238.Armstrong P.W., Stopps T.P., Ford S.E., de Bold A.J. Rapid ventricular pacing in the dog: pathophysiologic studies of heart failure. Circulation. 1986;74:1075–1084. doi: 10.1161/01.cir.74.5.1075. [DOI] [PubMed] [Google Scholar]
- 239.Wilson J.R., Douglas P., Hickey W.F. Experimental congestive heart failure produced by rapid ventricular pacing in the dog: cardiac effects. Circulation. 1987;75:857–867. doi: 10.1161/01.cir.75.4.857. [DOI] [PubMed] [Google Scholar]
- 240.Janse M.J., Rosen M.R. History of arrhythmias. Handb Exp Pharmacol. 2006:1–39. doi: 10.1007/3-540-29715-4_1. [DOI] [PubMed] [Google Scholar]
- 241.Vaidya D., Morley G.E., Samie F.H., Jalife J. Reentry and fibrillation in the mouse heart. A challenge to the critical mass hypothesis. Circ Res. 1999;85:174–181. doi: 10.1161/01.res.85.2.174. [DOI] [PubMed] [Google Scholar]
- 242.Berul C.I., Aronovitz M.J., Wang P.J., Mendelsohn M.E. In vivo cardiac electrophysiology studies in the mouse. Circulation. 1996;94:2641–2648. doi: 10.1161/01.cir.94.10.2641. [DOI] [PubMed] [Google Scholar]
- 243.Hagendorff A., Schumacher B., Kirchhoff S., Luderitz B., Willecke K. Conduction disturbances and increased atrial vulnerability in Connexin40-deficient mice analyzed by transesophageal stimulation. Circulation. 1999;99:1508–1515. doi: 10.1161/01.cir.99.11.1508. [DOI] [PubMed] [Google Scholar]
- 244.Schrickel J.W., Bielik H., Yang A. Induction of atrial fibrillation in mice by rapid transesophageal atrial pacing. Basic Res Cardiol. 2002;97:452–460. doi: 10.1007/s003950200052. [DOI] [PubMed] [Google Scholar]
- 245.Riley G., Syeda F., Kirchhof P., Fabritz L. An introduction to murine models of atrial fibrillation. Front Physiol. 2012;3:296. doi: 10.3389/fphys.2012.00296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Nishida K., Michael G., Dobrev D., Nattel S. Animal models for atrial fibrillation: clinical insights and scientific opportunities. Europace. 2010;12:160–172. doi: 10.1093/europace/eup328. [DOI] [PubMed] [Google Scholar]
- 247.Thenappan T., Shah S.J., Gomberg-Maitland M. Clinical characteristics of pulmonary hypertension in patients with heart failure and preserved ejection fraction. Circ Heart Fail. 2011;4:257–265. doi: 10.1161/CIRCHEARTFAILURE.110.958801. [DOI] [PubMed] [Google Scholar]
- 248.Ghio S., Gavazzi A., Campana C. Independent and additive prognostic value of right ventricular systolic function and pulmonary artery pressure in patients with chronic heart failure. J Am Coll Cardiol. 2001;37:183–188. doi: 10.1016/s0735-1097(00)01102-5. [DOI] [PubMed] [Google Scholar]
- 249.Kessler K.M., Willens H.J., Mallon S.M. Diastolic left ventricular dysfunction leading to severe reversible pulmonary hypertension. Am Heart J. 1993;126:234–235. doi: 10.1016/s0002-8703(07)80038-x. [DOI] [PubMed] [Google Scholar]
- 250.Waxman A.B. Pulmonary hypertension in heart failure with preserved ejection fraction: a target for therapy? Circulation. 2011;124:133–135. doi: 10.1161/CIRCULATIONAHA.111.038885. [DOI] [PubMed] [Google Scholar]
- 251.Guazzi M. Pulmonary hypertension in heart failure preserved ejection fraction: prevalence, pathophysiology, and clinical perspectives. Circ Heart Fail. 2014;7:367–377. doi: 10.1161/CIRCHEARTFAILURE.113.000823. [DOI] [PubMed] [Google Scholar]
- 252.Maarman G., Lecour S., Butrous G., Thienemann F., Sliwa K. A comprehensive review: the evolution of animal models in pulmonary hypertension research; are we there yet? Pulm Circ. 2013;3:739–756. doi: 10.1086/674770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Benisty J.I. Cardiology patient page. Pulmonary hypertension. Circulation. 2002;106:e192–e194. doi: 10.1161/01.cir.0000042762.47822.fe. [DOI] [PubMed] [Google Scholar]
- 254.Heath D. The rat is a poor animal model for the study of human pulmonary hypertension. Cardioscience. 1992;3:1–6. [PubMed] [Google Scholar]
- 255.Hislop A., Reid L. New findings in pulmonary arteries of rats with hypoxia-induced pulmonary hypertension. Br J Exp Pathol. 1976;57:542–554. [PMC free article] [PubMed] [Google Scholar]
- 256.Michelakis E.D., McMurtry M.S., Wu X.C. Dichloroacetate, a metabolic modulator, prevents and reverses chronic hypoxic pulmonary hypertension in rats: role of increased expression and activity of voltage-gated potassium channels. Circulation. 2002;105:244–250. doi: 10.1161/hc0202.101974. [DOI] [PubMed] [Google Scholar]
- 257.Muramatsu M., Tyler R.C., Gutkowska J. Atrial natriuretic peptide accounts for increased cGMP in hypoxia-induced hypertensive rat lungs. Am J Physiol. 1997;272:L1126–L1132. doi: 10.1152/ajplung.1997.272.6.L1126. [DOI] [PubMed] [Google Scholar]
- 258.Choudhary G., Troncales F., Martin D., Harrington E.O., Klinger J.R. Bosentan attenuates right ventricular hypertrophy and fibrosis in normobaric hypoxia model of pulmonary hypertension. J Heart Lung Transplant. 2011;30:827–833. doi: 10.1016/j.healun.2011.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Abud E.M., Maylor J., Undem C. Digoxin inhibits development of hypoxic pulmonary hypertension in mice. Proc Natl Acad Sci U S A. 2012;109:1239–1244. doi: 10.1073/pnas.1120385109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Stenmark K.R., Meyrick B., Galie N., Mooi W.J., McMurtry I.F. Animal models of pulmonary arterial hypertension: the hope for etiological discovery and pharmacological cure. Am J Physiol Lung Cell Mol Physiol. 2009;297:L1013–L1032. doi: 10.1152/ajplung.00217.2009. [DOI] [PubMed] [Google Scholar]
- 261.Rabinovitch M., Gamble W., Nadas A.S., Miettinen O.S., Reid L. Rat pulmonary circulation after chronic hypoxia: hemodynamic and structural features. Am J Physiol. 1979;236:H818–H827. doi: 10.1152/ajpheart.1979.236.6.H818. [DOI] [PubMed] [Google Scholar]
- 262.Dempsey E.C., Wick M.J., Karoor V. Neprilysin null mice develop exaggerated pulmonary vascular remodeling in response to chronic hypoxia. Am J Pathol. 2009;174:782–796. doi: 10.2353/ajpath.2009.080345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Nozik-Grayck E., Suliman H.B., Majka S. Lung EC-SOD overexpression attenuates hypoxic induction of Egr-1 and chronic hypoxic pulmonary vascular remodeling. Am J Physiol Lung Cell Mol Physiol. 2008;295:L422–L430. doi: 10.1152/ajplung.90293.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Steiner M.K., Syrkina O.L., Kolliputi N., Mark E.J., Hales C.A., Waxman A.B. Interleukin-6 overexpression induces pulmonary hypertension. Circ Res. 2009;104:236–244. doi: 10.1161/CIRCRESAHA.108.182014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Voelkel N.F., Tuder R.M. Hypoxia-induced pulmonary vascular remodeling: a model for what human disease? J Clin Invest. 2000;106:733–738. doi: 10.1172/JCI11144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Kulik T.J. Pulmonary hypertension caused by pulmonary venous hypertension. Pulm Circ. 2014;4:581–595. doi: 10.1086/678471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Genovese A., Latte S., Bozzaotre M., Chiariello M. Response of the left ventricular connective tissue to hypoxia. Res Exp Med (Berl) 1983;183:111–115. doi: 10.1007/BF01851776. [DOI] [PubMed] [Google Scholar]
- 268.Nakada Y., Canseco D.C., Thet S. Hypoxia induces heart regeneration in adult mice. Nature. 2017;541:222–227. doi: 10.1038/nature20173. [DOI] [PubMed] [Google Scholar]
- 269.Fang J.C. Heart failure with preserved ejection fraction: a kidney disorder? Circulation. 2016;134:435–437. doi: 10.1161/CIRCULATIONAHA.116.022249. [DOI] [PubMed] [Google Scholar]
- 270.Ter Maaten J.M., Damman K., Verhaar M.C. Connecting heart failure with preserved ejection fraction and renal dysfunction: the role of endothelial dysfunction and inflammation. Eur J Heart Fail. 2016;18:588–598. doi: 10.1002/ejhf.497. [DOI] [PubMed] [Google Scholar]
- 271.Ter Maaten J.M., Voors A.A. Renal dysfunction in heart failure with a preserved ejection fraction: cause or consequence? Eur J Heart Fail. 2016;18:113–114. doi: 10.1002/ejhf.461. [DOI] [PubMed] [Google Scholar]
- 272.Hewitson T.D., Holt S.G., Smith E.R. Animal models to study links between cardiovascular disease and renal failure and their relevance to human pathology. Front Immunol. 2015;6:465. doi: 10.3389/fimmu.2015.00465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Bongartz L.G., Braam B., Gaillard C.A. Target organ cross talk in cardiorenal syndrome: animal models. Am J Physiol Renal Physiol. 2012;303:F1253–F1263. doi: 10.1152/ajprenal.00392.2012. [DOI] [PubMed] [Google Scholar]
- 274.Hewitson T.D., Ono T., Becker G.J. Small animal models of kidney disease: a review. Methods Mol Biol. 2009;466:41–57. doi: 10.1007/978-1-59745-352-3_4. [DOI] [PubMed] [Google Scholar]
- 275.Rambausek M., Ritz E., Mall G., Mehls O., Katus H. Myocardial hypertrophy in rats with renal insufficiency. Kidney Int. 1985;28:775–782. doi: 10.1038/ki.1985.197. [DOI] [PubMed] [Google Scholar]
- 276.Stefanski A., Schmidt K.G., Waldherr R., Ritz E. Early increase in blood pressure and diastolic left ventricular malfunction in patients with glomerulonephritis. Kidney Int. 1996;50:1321–1326. doi: 10.1038/ki.1996.444. [DOI] [PubMed] [Google Scholar]
- 277.Lekawanvijit S., Kompa A.R., Manabe M. Chronic kidney disease-induced cardiac fibrosis is ameliorated by reducing circulating levels of a non-dialysable uremic toxin, indoxyl sulfate. PLoS ONE. 2012;7 doi: 10.1371/journal.pone.0041281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Suzuki H., Schaefer L., Ling H. Prevention of cardiac hypertrophy in experimental chronic renal failure by long-term ACE inhibitor administration: potential role of lysosomal proteinases. Am J Nephrol. 1995;15:129–136. doi: 10.1159/000168817. [DOI] [PubMed] [Google Scholar]
- 279.Kennedy D.J., Vetteth S., Periyasamy S.M. Central role for the cardiotonic steroid marinobufagenin in the pathogenesis of experimental uremic cardiomyopathy. Hypertension. 2006;47:488–495. doi: 10.1161/01.HYP.0000202594.82271.92. [DOI] [PubMed] [Google Scholar]
- 280.Amann K., Wiest G., Zimmer G., Gretz N., Ritz E., Mall G. Reduced capillary density in the myocardium of uremic rats—a stereological study. Kidney Int. 1992;42:1079–1085. doi: 10.1038/ki.1992.390. [DOI] [PubMed] [Google Scholar]
- 281.Bongartz L.G., Braam B., Verhaar M.C. Transient nitric oxide reduction induces permanent cardiac systolic dysfunction and worsens kidney damage in rats with chronic kidney disease. Am J Physiol Regul Integr Comp Physiol. 2010;298:R815–R823. doi: 10.1152/ajpregu.00727.2009. [DOI] [PubMed] [Google Scholar]
- 282.Tamaki M., Miyashita K., Wakino S., Mitsuishi M., Hayashi K., Itoh H. Chronic kidney disease reduces muscle mitochondria and exercise endurance and its exacerbation by dietary protein through inactivation of pyruvate dehydrogenase. Kidney Int. 2014;85:1330–1339. doi: 10.1038/ki.2013.473. [DOI] [PubMed] [Google Scholar]
- 283.Becher P.M., Jugdutt B.I., Baugh J., Schmack B. Experimental heart failure models and their pathophysiological characterization. Biomed Res Int. 2016;2016:2538263. doi: 10.1155/2016/2538263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Meng Q., Lai Y.C., Kelly N.J. Development of a mouse model of metabolic syndrome, pulmonary hypertension, and heart failure with preserved ejection fraction. Am J Respir Cell Mol Biol. 2017;56:497–505. doi: 10.1165/rcmb.2016-0177OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Justice M.J., Dhillon P. Using the mouse to model human disease: increasing validity and reproducibility. Dis Model Mech. 2016;9:101–103. doi: 10.1242/dmm.024547. [DOI] [PMC free article] [PubMed] [Google Scholar]