Abstract
Cadmium (Cd) is a naturally occurring trace metal that is widely considered to be highly toxic to aquatic organisms and a significant health hazard to humans (Amzal et al 2009; Bernhoft 2013; Burger 2008; Satarug et al 2009). The zebrafish (Danio rerio) has been used as a model organism for toxicological studies with Cd (Banni et al 2011; Blechinger et al 2007; Chow et al 2009; Chow et al 2008; Favorito et al 2011; Kusch et al 2007; Matz et al 2007; Wang & Gallagher 2013). We asked what the lasting longitudinal effects would be from short early developmental Cd exposure (between 24-96 hours post-fertilization) in a range that larvae might experience living atop typical Cd-containing surface sediments (0, 0.01, 0.1, 1.0 and 10 μM CdCl2: 1.124, 11.24, 112.4 and 1124 μg Cd/L). The goal of this exposure window was to specifically target secondary neurogenesis, monoaminergic differentiation and cardiovascular development, without affecting earlier patterning processes. Developmental abnormalities in body size and CNS morphology increased with concentration, but were statistically significant only at the highest concentration used (10 μM). Heart rate for Cd-treated larvae increased with concentration, and was significant even at the lowest concentration used (0.01 μM). Longitudinal survival was significantly lower for fish developmentally exposed to the highest concentration. Except for brain weight, overall morphology was not affected by developmental Cd exposure. However, developmental exposure to lower concentrations of Cd (0.01, 0.1, and 1.0 μM) progressively lowered cocaine-induced conditioned place preference (CPP), used to measure function of the reward pathways in the brain. Baseline heart rate was significantly lower in longitudinal fish developmentally exposed to 1.0 μM Cd. Cardiovascular response to isoproterenol, a potent β-adrenergic agonist, in longitudinal adults was also significantly affected by developmental exposure to Cd at low doses (0.01, 0.1 and 1.0 μM). Surviving longitudinal adult fish exposed to the highest concentration of Cd showed normal CPP and cardiovascular physiology. The data imply that even lower exposure concentrations can potentially result in fitness-affecting parameters without affecting survival in a laboratory setting.
Keywords: Zebrafish, Cadmium, Cardiovascular, Behavior, Morphology
Introduction
Cadmium (Cd) is a biologically non-essential trace metal that occurs in soils, sediments and surface waters all over the world. At high concentrations it is highly toxic to aquatic organisms and considered a significant health hazard to humans (Amzal et al 2009; Bernhoft 2013; Burger 2008; Satarug et al 2009). The toxic effects of Cd are not completely understood, but most have been linked to its ability to upset cellular redox balance (Cuypers et al 2010; Nair et al 2013). Cd disrupts electron transport chain function in mitochondria and is thus believed to generate reactive oxygen species (ROS) (Wang et al 2004). Cd also liberates normally bound stores of iron (Fe) and copper (Cu), which in turn accelerate the generation of ROS via the Fenton reaction (Casalino et al 1997; Cuypers et al 2010; Nair et al 2013). Finally, Cd binds to thiol groups in ROS scavenger proteins and antioxidant enzymes, thereby neutralizing their function (Cuypers et al 2010; Jurczuk et al 2004; Nair et al 2013). The secondary effects of the resulting increased ROS production include lipid peroxidation and subsequent damage to other macromolecules including DNA. This damage is believed to play a major role in the carcinogenicity associated with Cd exposure (Hartwig 2010; Satarug et al 2009). The link to mitochondrial function suggests that Cd would be particularly harmful to highly aerobic tissues. Indeed, the kidney, site of most bioaccumulation of Cd, is particularly vulnerable to the effects of the metal (Gobe & Crane 2010). Less is known about the effects on the central nervous system (CNS) and cardiovascular system (CVS). Cd affects redox balance and survival in cultured neurons (Lopez et al 2006) and has been linked to amyloid accumulation and progression of neurodegenerative disorders (Jomova et al 2010; Li et al 2012). Cd has also been linked to the progression of heart disease and to toxicity of cardiomyocytes in vivo and in vitro (Chen et al 2015; Ferramola et al 2012; Limaye & Shaikh 1999), further evidence of the broad impact of this metal.
Because Cd is widely present in top soils and is readily absorbed by plants, the main avenue of non-smoking human exposure is by ingestion of cereals, vegetables, potatoes and rice (Jarup & Akesson 2009; Satarug et al 2009). Sediments in most waterways are also rich in Cd (typically in the range of 0.1 to 0.7 mg/kg dry weight) and the metal enters the food chain via plants and freshwater invertebrates (Cuculic et al 2016; Jacob et al 2013; Li et al 2017; Otter et al 2012). The level currently considered to be safe for human consumption is 7 μg/week/kg body weight (WHO 2004). Most current estimates of average world-wide consumption are between 0.7 and 6.3 μg/week/kg, although some populations, particularly those that eat a lot of rice, are likely to consume much higher levels (WHO 2004). While several studies have shown the negative health impact at levels higher than the WHO standard, values long considered safe have been called into question (Satarug et al 2009; Satarug & Moore 2004). Subtler effects on human health at lower exposure levels are still a matter of debate. Moreover, the ecological impact of Cd on aquatic ecosystems has not been completely assessed. While Cd is common in sediments (Cuculic et al 2016; Jacob et al 2013; Li et al 2017; Otter et al 2012) where it can enter the food chain, relatively little is understood about the impact of this metal at the low levels at which it is typically found in surface sediments and waters (Burger 2008). There have been relatively few controlled laboratory studies linking environmental levels with physiological function, rather than survival and gross morphology.
To date, the zebrafish (Danio rerio) has been used as a model organism for several toxicological studies with Cd (Banni et al 2011; Blechinger et al 2007; Chow et al 2009; Chow et al 2008; Favorito et al 2011; Kusch et al 2007; Matz et al 2007; Wang & Gallagher 2013). The effects of very high acute concentration (100 μM) on early neurogenesis and retinogenesis have been investigated with an emphasis on early neurogenesis effects and morphological abnormalities (Chow et al 2009; Chow et al 2008). In a study looking at longitudinal effects after chronic exposure to lower concentrations of Cd (0.002 - 0.2 μM), it was found that adults displayed an attenuated olfactory-based avoidance response to predator cues, thus linking neurodegenerative effects to behavioral outcomes (Kusch et al 2007). Other studies have examined the bioaccumulation and effects in adult fish after chronic exposure to higher levels of CdCl2, 3.5 to 5 μM (Banni et al 2011; Favorito et al 2011). Thus, exposure to low levels of Cd could have subtle longitudinal effects that might lower fitness without affecting mortality. We asked what the lasting longitudinal effects would be from Cd exposure during a critical developmental window, between 24 and 96 hours post fertilization (hpf), within a range of concentrations that the developing fish might experience living atop Cd-rich surface sediments. In addition to considering longitudinal effects on gross morphology, sex ratio, and survival, we examined more subtle aspects of early exposure on adult heart function and reward-based learning.
Methods
Fish Maintenance
All zebrafish for this study were housed in the facility at the University of North Dakota according to standard IACUC recommendations (Animal Welfare Assurance #A3917-01, protocol 1403-7). Fish were raised in stand-alone fish racks, which included biological, mechanical and charcoal filtration, along with UV sterilization and a daily 10% water change (Aquatic Habitats, Apopka, FL). Fish were fed twice daily, artemia in the morning and pellet in the afternoon. They were kept on a constant light-dark cycle: 14 hours on, 10 hours off. Water on the system was made by dissolving 0.2- 0.3 g/L Instant Ocean (Instant Ocean Spectrum Brands, Blacksburg, VA, USA) and 0.1 g/L sodium bicarbonate in reverse osmosis (RO) water. The water was further buffered with calcium carbonate in the form of crushed coral (Aquatic Habitats, Apopka, FL), such that the final pH was maintained at 7.6-7.8 and conductivity at 800 microsiemens (hereafter called “fish water”). The fish used for these studies was a reporter line that was a generous gift (Goldman et al 2001). This line expresses green fluorescent protein (GFP) driven by the α1-tubulin promoter, which confines expression to the central nervous system (CNS). The GFP expression facilitated vital measurement of larval brain size. Fish heterozygous for the reporter gene were crossed to generate parallel lines of homozygous GFP-positive (GFP+) and homozygous GFP- negative (GFP-) fish. Imaging of larval fish and longitudinal adults was done on the homozygous GFP+ strain. The GFP- line was used for experiments assessing cell death with acridine orange (AO).
Cd treatment paradigm
Cd treatment was performed as shown in Figure 1A. Fish were bred from individual crosses or basket crosses. To target the effects of Cd on neural and cardiac development apart from possible earlier effects on axis and pattern formation, Cd treatment was started at 24 hpf. Cd exposure was stopped before inflation of the swim bladder and the increased swimming behavior higher in the water column. GFP+ sibling embryos were divided into 6 well dishes at a density of 30 individuals per well. A fresh stock solution of 10 mM CdCl2 (Sigma Aldrich, St. Louis MO) dissolved in RO water was made for each application in each experiment. Working solutions of 0.01, 0.1, 1.0, and 10 μM CdCl2 (1.124, 11.24, 112.4 and 1124 μg Cd/L) were made by diluting the 10 mM stock in fish water drawn from the AHAB system (see description of fish water above in Fish Maintenance). After removing most of the water from the wells, 5mls of the appropriate CdCl2 working solution were added to the developing fish. In each experiment there was also a well of control individuals that received no CdCl2. Water and CdCl2 were changed at 48 and 72 hpf. On the fourth day post fertilization (dpf) larvae were rinsed 3 times with fish water and allowed to recover 24 hours before imaging or transferring to the rearing system.
Figure 1. The experimental design to examine the effects of Cd on early zebrafish development, longitudinal adult behavior, and physiology.
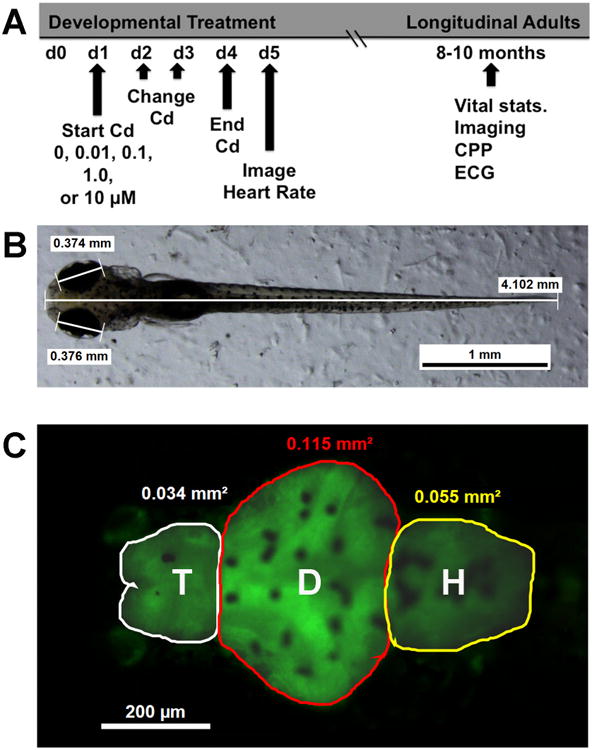
The basic experimental design is shown in 1A. As described in methods, developing zebrafish were exposed to different concentrations of CdCl2 starting at 24 hpf and ending at 96 hpf. After rinsing three times, the larvae were allowed 24 hours of recovery before imaging. Body and eye size were measured (1B). In addition, the areas of brain regions were determined (1C), including what is herein referred to as the telencephalon (T), diencephalon (D), and the hindbrain (H). Larvae from each treatment group were raised to adulthood and assessed for survival, sex, basic morphology, cardiovascular function using an electrocardiogram (ECG) and a conditioned place preference assay (CPP).
All the larvae from each experimental well were transferred to the system and raised for 8-10 months in 3L tanks. The longitudinal raising of fish was done a minimum of 6 times for each Cd treatment group except for those treated with 0 and 10 μM. Low survival of the latter prompted us to increase the n to 10 so there would be more fish for behavioral and cardiovascular testing. We grew a 0 μM control for each of the 4 additional 10 μM Cd fish groups. Not every fish was subjected to every assay described for adult fish in this study. The surviving longitudinal adult fish were tested for behavior or heart rate (see below). They were then sacrificed and assessed for body length, body weight, sex and brain weight. Fish were anesthatized in 40μg/L tricaine (MS-222, Sigma). They were then measured with a mm-scale ruler and weighed. The fish were then quickly decapitated and their heads fixed in 4% paraformaldehyde. The brains were removed from the skulls the next day and weighed. Some brains were also re-fixed, embedded in epon-araldite resin, sectioned and examined for histology essentially as described previously (Gross et al 2005). Because the tissue was fixed in paraformaldehyde, the brain weight reported probably does not reflect the true wet weight. For our purposes this weight provided a relative metric to compare brain size between different treatment groups while providing tissue for histological examination in an ongoing study.
Imaging and measuring brain size and heart rate in 5 dpf larvae
Imaging was done at 5 dpf using a Leica dissecting microscope (M165FC, Leica, Heerbrugg, Switzerland), camera (DFC310FX) (Leica, Heerbrugg, Switzerland), and image analysis software suite (Leica Application Suite version 4.1.0, Leica Microsystems, Heerbrugg, Switzerland). Ten larvae from each condition were immobilized by transferring to 0.2% methylcellulose in fish water. The larvae were then imaged individually, belly down, under bright field illumination at low magnification (between 30–32×) to determine eye size and body length using Leica analysis software (see Figure 1B). The larvae were then imaged at high magnification (120×) under fluorescence illumination to visualize the CNS by GFP expression. The image was later analyzed with Leica software to determine the area of fluorescence as an indicator of brain size. Three brain regions were defined for which the area was measured as shown in Figure 1C. What is referred to as the “telencephalon” included the region between the olfactory epithelium (which we did not measure) and the telencephalic flexure (T in Figure 1C). For convenience in reporting, the “diencephalon” included the optic tectum, midbrain and cerebellum (D in Figure 1C). The “hindbrain” included the rhombencephalon between the cerebellum and the constriction marking the spinal cord (H in Figure 1C). Brains imaged all showed oval profiles for the olfactory epithelium, indicating full extension of the forebrain. The telencephalons of longitudinal adult fish brains were also imaged for GFP+ fish (not shown), but the skull and pigmentation prevented effective imaging of the other brain regions.
AO staining was used to determine if Cd increased cell death in the forebrains of GFP+ larval zebrafish similar to previous work done in the lab (Mersereau et al 2016). Briefly, after the same drug treatment described above, GFP-, 5 larvae at 5 dpf were incubated in 1 g/mL AO (Sigma-Aldrich, St. Louis, MO) dissolved in fish water for 10 min, rinsed three times, and then imaged using a GFP filter at 120×. A depth of focus was chosen such that the surface pigment and olfactory epithelium were clearly in focus then all visible AO+ cells were counted. At least three separate investigators, blind to treatment conditions, counted the AO+ cells.
In a subset of experiments, 5-day old larvae were imaged to determine heart rate. Larval heart rate of Cd-exposed animals was measured as described previously (Mersereau et al 2015). Heart rate was measured on day 5, 24 hours after removing the CdCl2. After imaging for size and brain morphology, larvae were turned on their sides, and allowed to recuperate for 5 minutes. A 12-second video at low resolution and maximal frame rate (69 frame/sec) was shot of the beating heart imaged at high magnification (120×). The videos were then played back in slow motion and the number of beats per minute (bpm) was counted to determine larval heart rate. Counts for all treatment groups were performed by at least three investigators masked to treatment condition.
Conditioned Place Preference
Cocaine-induced conditioned place preference (CPP) was measured essentially as described previously (Darland & Dowling 2001; Darland et al 2012; Mersereau et al 2016). At approximately 8 months of age they were tested for cocaine-induced CPP. Eight months was an empirical threshold determined in previous studies by quantifying the number of fish that froze and thus failed to perform in the CPP test previously (Darland & Dowling 2001; Darland et al 2012; Mersereau et al 2016). Briefly, fish treated with various concentrations of Cd during development and grown up to adulthood, as described earlier. CPP was used to specifically measure function of the reward system of the brain. Ecologically this pathway, governed largely by monoaminergic neurotransmission, may well reflect the learning associated with foraging (Baudonnat et al 2013; Miller et al 2013). Fish were removed from their home tank and housed individually during testing, which took 6 days. On day 1 the fish habituated to the CPP chamber during a 45-minute exposure. The CPP apparatus, described previously (Darland et al 2012; Mersereau et al 2016), was divided into three sections with perforated walls that allowed fish access to all sections and which could be replaced with solid walls for confinement during drug exposure. The two end sections each contained half the volume of water as that in the middle section. On day 2 a baseline preference for each section was recorded during a 10-minute trial. Preference was determined by calculating the percentage of time spent in a given compartment. The fish were then confined in the front compartment for 30 minutes and then confined for 30 minutes in the back compartment. On day 3 the same procedure was repeated except that the confinement order was reversed: fish were initially confined to the back and then later the front. On day 4 fish were tested for their final baseline, after which preference for the two smaller end compartments was averaged (values from days 3 and 4). The fish were then confined to the more preferred compartment without drug for 30 minutes. They were then confined to the least preferred compartment for 30 minutes with cocaine (5mg/L). On day 5 the first CPP was recorded and the conditioning paradigm determined on day 4 was repeated. On day 6 a final CPP was recorded. In our experience, maximal CPP is typically achieved during one of these two trials. Percent change in preference, the metric used to compare the developmental treatment groups, was determined by subtracting the average percent time spent in the least preferred compartment during the baseline trials (measured on days 3 and 4, after conditioning with no drug) from the average of the two CPP trials (measured on days 5 and 6 after conditioning with cocaine).
Electrocardiograms (ECGs) in adult zebrafish
ECGs were recorded as described previously (Mersereau et al 2015). Fish were sedated with 40μg/L tricaine (MS-222, Sigma), placed in a damp sponge belly up and ventilated by a peristaltic pump that continuously perfused the gills and kept the animal sedated. A few ventral scales were removed and two 29-gauge microelectrodes (AD Instruments, Colorado Sprigs, CO) inserted to a depth of approximately 1mm, one in the thorax between the opercula and one in the abdomen, near the anal fins, with the ground electrode placed in the sponge nearby. Electrical signals detected by the electrodes were amplified and translated by a PowerLab data acquisition unit using LabChart 7.2.1 software (AD instruments, Colorado Springs, CO). Recordings were made in the range of 0-10 mV. Digital filters limiting frequency range to 8-40 Hz were applied and an averaging algorithm provided by the software was used to smooth the trace. Once a signal was detected, the pump was turned off to decrease noise and the ECG was recorded for 1 minute. After the baseline recording, the pump was restarted but fish water was mixed with 20 μM isoproterenol, a β-adrenergic agonist, the we have shown elevates heart rate in zebrafish (Mersereau et al 2015). Average heart rate was determined using the LabChart software, which essentially expressed the rate as bpm.
Statistics
Comparisons between treatment groups were done using one-way ANOVA (Prism 5.0, GraphPad Software Inc., La Jolla, CA, USA). Bonferroni's post-test was used to determine pairwise differences, or Dunnett's post-test was used to compare experimental groups to untreated controls (with α set to 0.05). In some cases, treatment groups were normalized as a percentage of the control group. Percentages were arcsin transformed for statistical analyses but graphed as ratios or percentages as indicated. Similarly, percent survival values were arcsin transformed for statistical comparison, but percent survival is shown in Figure 3. Paired t-tests were used to compare average baseline preference from average post-conditioned preference for individual groups. For change in percent preference comparisons between groups, one-way ANOVA was used as described earlier. These percent values were transformed for statistical purposes, but graphed as change in percent preference. Males and female morphological measurements were done using unpaired, two-tailed, t-tests. Similarly, t-tests were also used to compare heart rates before and after isoproterenol treatment for individual developmental treatment groups. However, the group comparisons in base line heart rate and the percent change in heart rate with isoproterenol treatment were done using one-way ANOVA with Bonferroni's post-test to make pairwise comparisons. Again, the values for isoproterenol's effect on heart rate were graphed as percent change, but the values were transformed for statistical comparison. In the histograms asterisks refer to analysis within a group comparing baseline and treatment, while letters refer to ANOVA analysis between groups.
Figure 3. Cd affects heart development and function.
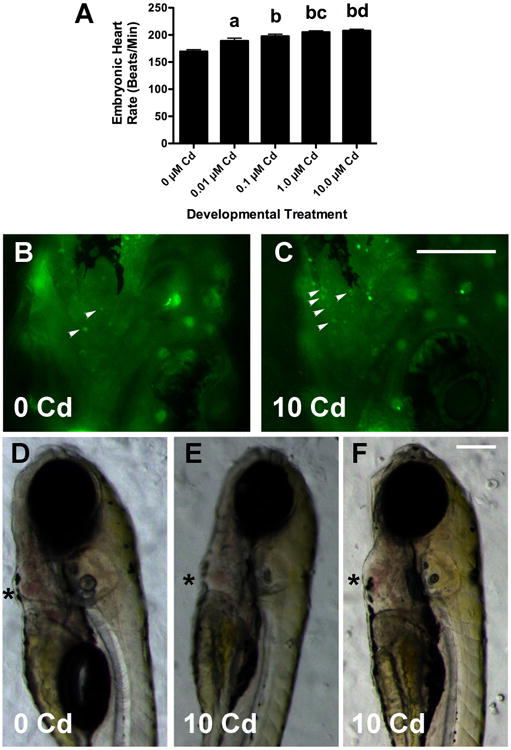
Cd caused a concentration-dependent increase in larval heart rate (3C, data was combined from 3 experiments with a total n = 20 for each Cd group, a: p < 0.01 compared to 0 μM, b: p< 0.001 when compared to 0 μM larvae, c: p < 0.05 when compared to 0.01 μM larvae, and d: p < 0.01 when compared to 0.01 μM larvae). Larvae treated with 10 μM CdCl2 had more AO+ cells (small arrowheads in 2B and 2C) in the heart than untreated larvae. Most 10 μM Cd larvae had hearts that were normal in size and morphology (2D and 2E), but some had an enlarged ventricle and pericardium (3F). The asterisks highlight the ventricles, which showed no obvious defects in looping. There were no discernable differences in morphology or AO+ cells seen in larvae exposed to lower Cd concentrations (not shown). The scale bar for 2B and 2C is 200 μm, and that for 2D-2F is 150 μM.
Results
There were a number of morphological effects of developmental CdCl2 exposure on larval zebrafish (Table 1). Body, eye and brain size were all affected in similar fashion, exemplified by telencephalic area (Figure 2A-C and Table 1). Area measurements revealed that the telencephalon was significantly smaller in fish treated with 10 μM CdCl2, but not at lower concentrations (Table 1 and compare Figure 2A and 2B). Development stage at the time of imaging varied slightly between experiments so the effects of Cd on brain morphology were also calculated as a percentage of control (0 μM CdCl2) for individual experiments (Figure 2C). For the 10 μM CdCl2-treated larvae, the telencephalon, which included the olfactory bulbs, was on average 12% smaller than that of untreated fish in the same experiment (Figure 2C). Table 1 shows that the same pattern of significant reduction in size with 10 μM Cd was seen for body length (dropped by 3.6% when normalized by experiment), diencephalic area (dropped by 7.2% when normalized by experiment), hindbrain area (dropped by 13%, when normalized by experiment), and eye diameter (dropped by 5.1%, when normalized by experiment). For the hindbrain there was also a significant reduction in area with 1 μM CdCl2 (Table 1). However, when values were normalized as a percentage of control for individual experiments, there was no significant effect at this concentration.
Table 1. Cd affects zebrafish larval body, eye and brain size.
| Parameter | 0 μM CdCl2 | 0.01 μM CdCl2 | 0.1 μM CdCl2 | 1.0 μM CdCl2 | 10 μM CdCl2 |
|---|---|---|---|---|---|
| Body Length (mm) | 3.958 ± 0.014 (90) | 3.934 ± 0.017 (34) | 3.925 ± 0.024 (30) | 3.930 ± 0.026 (35) | 3.815 ± 0.017*** (35) |
| Eye Diameter (mm) | 0.336 ± 0.001 (108) | 0.338 ± 0.003 (30) | 0.342 ± 0.002 (30) | 0.334 ± 0.002 (32) | 0.315 ± 0.003*** (30) |
| Telen. Area (mm2) | 0.0305 ± 0.0004 (73) | 0.0319 ± 0.0006 (25) | 0.0300 ± 0.0006 (23) | 0.0293 ± 0.0004 (26) | 0.0264 ± 0.0005*** (28) |
| Dien. Area (mm2) | 0.1178 ± 0.0010 | 0.1183 ± 0.0023 | 0.1186 ± 0.0015 | 0.1188 ± 0.0020 | 0.1107 ± 0.0016** |
| Hind. Area (mm2) | 0.0452 ± 0.0010 | 0.0439 ± 0.0010 | 0.0444 ± 0.0014 | 0.0393 ± 0.0008** | 0.0395 ± 0.0009*** |
| AO+ cells Telen. | 5.2 ± 1.0 (16) | 6.0 ± 0.8 (16) | 9.4 ± 0.6 (16) | 9.0 ± 1.5 (16) | 18.8 ± 3*** (16) |
See Figure 1 and methods for a description of the measurements. The number of individuals measured in given in parentheses and the values are presented ± S.E.M. Values significantly different from untreated controls are in bold and asterisks indicate the p-values from ANOVA (***p < 0.001, **p < 0.01). These values differ from those shown in Figure 2C, which were calculated as a percent control for individual experiments. In this table all the numbers were combined without distinguishing between possible differences in developmental rate between experiments.
Figure 2. Cd decreases brain size and increases the number of acridine orange-positive (AO+) cells in the forebrain of treated larvae.
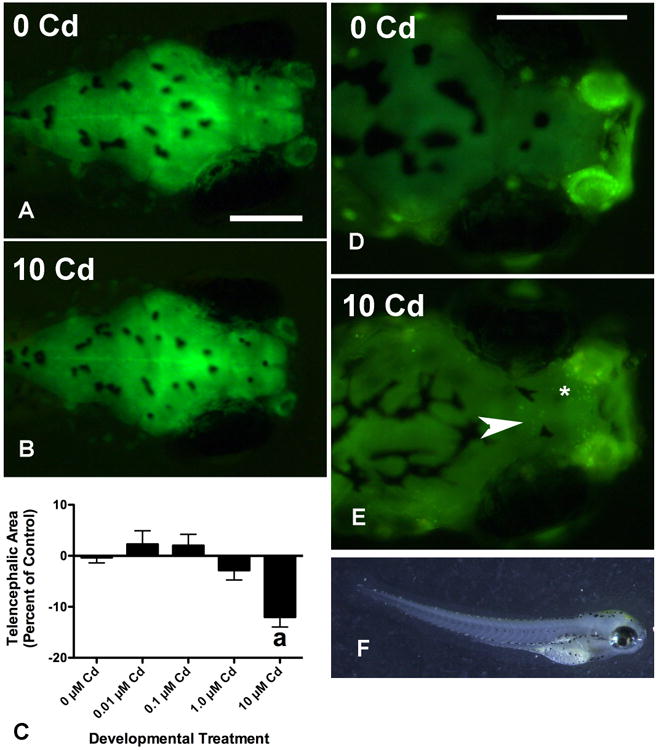
Telencephalic area was significantly reduced in larvae treated with 10 μM CdCl2, which is exemplified by comparing the brain in (2A) with that in (2B). While all three gross brain regions were reduced in size at this concentration, the most significant effect was seen for the telencephalon (2C, a: p < 0.001) and hindbrain (not shown, p < 0.001, see Table 1). The data from 6 experiments is shown in Figure 2C and in each of these the telencephalic area of Cd-treated larvae is expressed as a percentage of that from untreated controls in the same experiment (n = 73 for 0 μM, and n = 23-28 for the other concentrations, for statistical analysis the percentages were arcsin transformed). At least some of this effect was probably due to increased cell death as exemplified by comparing AO staining in panels (2D) and (2E). A significant change in the number of AO+ cells was seen at 10 μM of CdCl2 but not at lower concentrations (Table 1, p < 0.001, n = 16 total for each Cd group from 3 experiments). Most of these cells were localized to the olfactory bulbs (asterisk in 2E), however, many were also found in the middle, ventral telencephalon (arrow in 2E). Many 10 μM Cd larvae had an arched appearance (2F). Scale bars in each panel represent 200 μm and the larva shown in 2F is 3.74 mm long.
AO staining suggested that at least some of the change in telencephalic size after 10 μM CdCl2 treatment was due to cell death (Figure 2D and 2E, Table 1). Fish treated with 10 μM CdCl2 averaged 3.7 times as many AO positive cells as untreated fish (Table 1). Most of the AO positive cells were localized in the olfactory bulbs (asterisk in Figure 2E), similar to what has been reported by others (Blechinger et al 2007; Kusch et al 2007; Wang & Gallagher 2013). However, many fish had a cluster of AO+ cells centrally and ventrally located in the telencephalon as well (arrow in Figure 2E). There was no significant difference in AO+ cells seen between untreated fish and fish treated at lower concentrations (Table 1), and there was no difference in AO+ cells in other brain regions at any concentration of Cd (data not shown). There was considerable cell death evident in the olfactory epithelium at 10 μM, but not at lower concentrations. The background fluorescence of the olfactory epithelium made this difficult to quantify in this study. However, others have extensively documented Cd effects on neurotoxicity in the zebrafish olfactory system (Blechinger et al 2007; Kusch et al 2007; Wang & Gallagher 2013).
Several other morphological and physiological parameters were affected by CdCl2 exposure, although most differences were only significant at the highest treatment concentration (10 μM). Eye diameter decreased by 5% at the highest concentration and was significantly smaller than that of untreated controls (Table 1). Body length was also significantly decreased by 3.6% in fish treated with 10 μM CdCl2 (Table 1). Nearly 15% of the fish treated at this concentration also displayed a characteristic arch (shown in Figure 2F), similar to that described by others (Hallare et al 2005). These larvae were also imaged in profile, measured with a segmental line function, and compared to untreated larvae to insure that the decrease in size was not due to an effect of imaging angle and body curvature. Body curvature did not alter results significantly (10 μM fish measured in profile were 4% smaller than untreated controls). Larval heart rate was significantly elevated in fish treated with CdCl2, and increased progressively with concentration (Figure 3A). All four Cd treatment groups showed significantly higher heart rates relative to untreated control larvae (0 μM). The larvae treated with 1.0 μM and 10 μM Cd Cl2 had heart rates also significantly higher than those treated with 0.01 μM Cd (p < 0.05 and p < 0.01 respectively). Larvae treated with 10 μM Cd showed a striking increase above untreated larvae in the number of AO positive cells in the heart (Figure 3B and 3C). Since the dying cells were tightly clustered in the Cd-treated larvae it was difficult to get precise counts at this level of resolution. The significantly elevated heart rate was not associated with morphological abnormalities (Figure 3D-F). While some larvae treated with 10 μM CdCl2 showed hypertrophy of the ventricle and pericardium, there was no obvious abnormal looping (asterisk in Figure 3F). Also obvious from Figure 3 is that swim bladders did not inflate for most larvae treated with 10 μM CdCl2. Nevertheless, very little difference in early mortality was seen before the larvae were transferred to the main system for rearing.
Longitudinal adult survival, however, was significantly impacted by developmental exposure to 10 μM CdCl2, but not to lower concentrations (Figure 4A, p < 0.01). We also did four experiments (120 larvae total) using this exposure paradigm at 100 μM CdCl2, but all the larvae died by day 5. Body length and weight differed between groups, but obviously survival rate, and thus rearing density, affected individual growth. Fish treated with 10 μM CdCl2 during development were typically larger that those treated with other concentrations, probably because they had much lower survival and were thus raised at lower density. With this in mind we made several morphological measurements to see if ratios could be used to determine whether brain and eye size were specifically affected by developmental Cd exposure.
Figure 4. Developmental Cd exposure affects survival and brain size in longitudinal adult fish.
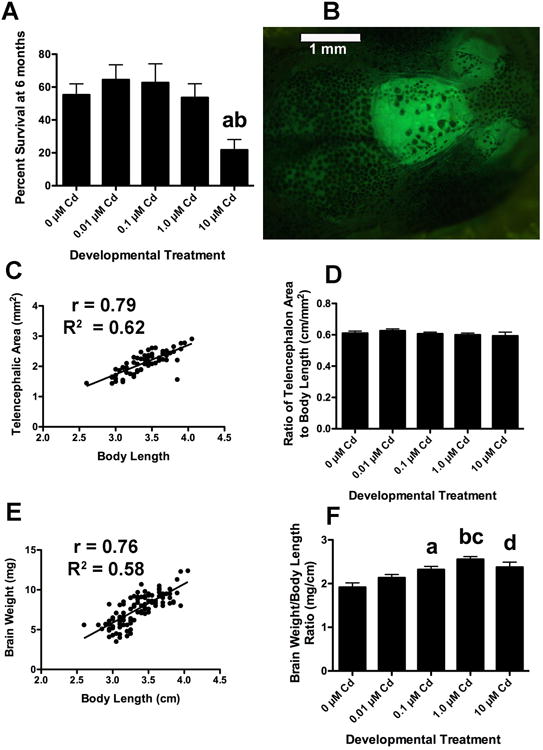
Fish treated with 10 μM CdCl2 during development showed significantly lower survival at 6 months than untreated controls (4A). This experiment was carried out a minimum of 6 times for each treatment group (a: p < 0.05 when compared to the 0 μM, 0.1 μM and 1.0 μM groups and b: p < 0.01 compared to the 0.01 μM group). The telencephalons of longitudinal adult GFP+ fish were imaged and the area measured as described in methods (4B). Body length and telencephalic area are strongly correlated (3C, p < 0.0001, n = 152). The telencephalon to body length ratio (TA/BL) did not vary significantly between the different longitudinal treatment groups (4D this experiment was done 3 times with an n = 35 for each Cd group, except the 10 μM Cd, which had much lower survival and an n = 10, p < 0.52). However, the shape of the concentration response curve was similar to that for survival (4A) and larval telencephalic area (2C), with a slight, but statistically insignificant, increase at the lower concentrations (0.01 μM), and a decrease at higher concentrations (1.0 and 10 μM). Brain weight was strongly correlated with body length when all groups were considered together (3E, p < 0.0001, n = 103). The brain weight to body length ratio differed between CdCl2 treatment groups. Fish treated with 0.1 μM, 1.0 μM and 10 μM CdCl2 during development had proportionally heavier brains than untreated controls (n = 21-24 for each Cd group except the 10 μM group, which had an n =12, a: p < 0.01 compared to the 0 μM group, b: p < 0.001 compared to the 0 μM group, c: p < 0.01 when compared to the 0.01 μM group and d: p < 0.05 compared to the 1.0 μM fish).
As expected, body length and body weight strongly correlated (Pearson r = 0.93, R2 = 0.86 and p < 0.0001). Other morphological measurements strongly correlated with both body length and body weight. However, since body length consistently showed slightly higher coefficients and thus provided the more reliable normalization factor, we report the correlation with body length here. Transparency of the skull above the telencephalon allowed imaging of this brain region and thus morphological measurement of area (Figure 3B). Telencephalic area also strongly correlated with body length (Pearson r = 0.79, R2 = 0.62 and p < 0.0001). Eye diameter was strongly correlated with body length (Pearson r = 0.82, R2 = 0.67 and p < 0.0001), as was brain weight (Figure 3E: Pearson r = 0.76, R2 =0.58 and p < 0.0001).
We compared males and females for all morphological measurements (Table 2). In all cases, and at all concentrations of CdCl2 treatment, females were significantly larger than males. Females were longer than males (3.45 ± 0.04 cm vs. 3.25 ± 0.05 cm, p = 0.0011) and weighed more (0.385 ± 0.016 g vs. 0.281 ± 0.013 g, p < 0.0001). These results were comparable to our previous studies (Mersereau et al 2016). As expected, females also had larger eye diameters (2.48 ± 0.21 mm vs. 2.34 ± 0.21 mm, p = 0.0025), heavier brains (8.2 ± 0.3 vs. 7.4 ± 0.2, p = 0.0342), and larger telencephalons (2.24 ± 0.06 mm2 vs. 1.99 ± 0.07 mm2, p = 0.012). Interestingly, when normalized to body length (or body weight), eye and brain size were nearly identical between the sexes (Table 2). We thus used these ratios to compare the impact of developmental CdCl2 exposure on longitudinal adults. We also looked at ratios of brain region areas to body length in 5 dpf larvae and found the correlation significant, but not as robust as that seen for adults (Pearson r = 0.42, R2 = 0.18 and p = 0.0009). We, therefore, reported the data without normalizing to body length (Table 1 and Figure 2).
Table 2. Comparing adult male and female zebrafish body dimensions.
In order to normalize for differential survival and resulting clutch size of Cd-treated fish, various measurements were made and expressed as ratios of body length. This table shows that although males and female fish differed in size, certain morphometric ratios were consistent. In addition to compensating for clutch size, this normalization also allowed for comparative analysis without separating the sexes.
| Sex | L (cm) | W (g) | ED (mm) | TA (mm2) | BW (mg) | W/L | ED/L | TA/L | BW/L |
|---|---|---|---|---|---|---|---|---|---|
| M (44) | 3.25 ± 0.05 | 0.28 ± 0.01 | 2.34 ± 0.04 | 1.99 ± 0.07 | 7.4 ± 0.3 | 0.085 ±0.003 | 0.72 ± 0.01 | 0.60 ± 0.01 | 2.27 ± 0.07 |
| F (59) | 3.45 ± 0.04 | 0.39 ± 0.02 | 2.48 ± 0.03 | 2.24 ± 0.06 | 8.1 ± 0.2 | 0.109 ±0.004 | 0.72 ± 0.01 | 0.60 ± 0.01 | 2.35 ± 0.05 |
| p | 0.001 | 0.0001 | 0.0025 | 0.012 | 0.03 | 0.0001 | 0.69 | 0.71 | 0.36 |
The values were similar regardless of whether we grouped fish from all treatment groups or just untreated fish. Abbreviations are included for males (M), females (F), body length (L), body weight (W), eye diameter (ED), telencephalic area (TA), and brain weight (BW). Bold values are statistically significant, with p-values listed resulting from two-tailed t-tests comparing males and females. The numbers of measured fish are given in parentheses under M and F and the values include ± SEM.
Very few longitudinal effects of developmental Cd exposure were seen in the morphology of adult fish. The highest concentration of CdCl2 produced a modest increase in the ratio of body weight to body length (data not shown when compared to untreated control fish). This was probably related to the fact that most 10 μM Cd groups were smaller in number; the fish tended to put on extra weight. Sex ratio also did not vary between developmental treatment groups; on average the longitudinal groups were 44% male (p < 0.967 for comparisons between treatment groups). When normalized to body length, eye diameter (p = 0.64) and telencephalic area (Figure 4D, p = 0.37) did not vary significantly between treatment groups. However, the concentration response curve of the telencephalic area to body length ratio (Figure 4D) was similar in shape to the change in larval telencephalic area (Figure 2C) and also to survival of the longitudinal groups (Figure 4A). That is, fish showed a slight, but statistically insignificant, increase in survival and telencephalic area after developmental exposure to low Cd concentrations (0.01 μM) but a decrease after developmental exposure to high concentrations (10 μM) when compared to untreated controls. In contrast, the ratio of brain weight to body length did vary significantly (p < 0.0001) between groups (Figure 4F). The difference depended on concentration, with 1 μM developmental exposure having the greatest effect (p < 0.001, when compared to untreated control fish) and a lesser effect seen with 0.1 μM (p < 0.01) and 10 μM (p < 0.05). Interestingly, this Cd concentration effect on brain weight/body length ratio did inversely correspond somewhat to that seen for CPP behavior.
Changes in longitudinal behavior and baseline heart rate were seen in fish treated with CdCl2 during early development. We used a classic conditioning paradigm, cocaine induced place preference assay (CPP), to assess monoaminergic, reward-based learning. Fish not treated with cocaine showed no significant increase in preference above baseline (Unt in Figure 5A). Since the response of the untreated fish from all developmental exposure groups did not differ from one another (p < 0.44), they are combined for convenience in Figure 5A (Unt). Fish not treated with CdCl2 as larvae (0 μM Cd in Figure 5A) and fish treated with 10 μM Cd showed a significant increase in side preference after cocaine conditioning (** p < 0.01 by paired t-test in Figure 5A). The magnitude of the responses for the 0 μM and 10 μM groups, a change in percent preference of 13.1 and 11.2 respectively, were comparable to what we have reported for CPP before using 5 mg/L cocaine (Darland & Dowling 2001; Darland et al 2012; Mersereau et al 2016). The degree of the change in preference for the 0 μM fish was significantly higher than that of fish not treated with cocaine (a: p < 0.01 compared to Unt in Figure 5A). The adult CPP behavior dropped progressively with developmental exposure to CdCl2 except at the highest concentration, 10 μM. Fish exposed to 0.1 μM and 1.0 μM CdCl2 as larvae showed significantly lower CPP than 0 μM fish (b: p < 0.01 and c: p < 0.001). Finally, the 1 μM Cd group showed significantly lower CPP than that of the 10 μM Cd fish (c: also represents p < 0.05 for this comparison in Figure 5A).
Figure 5. Developmental Cd exposure affects CPP behavior and heart rate in longitudinal adult fish.
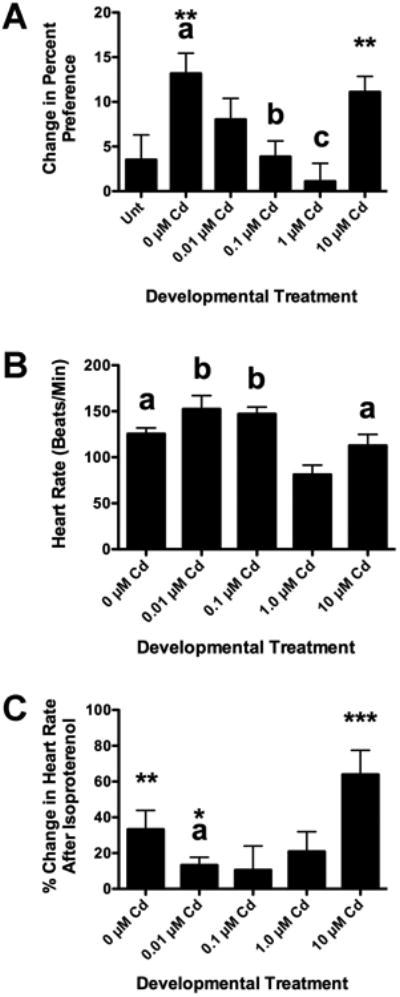
Fish not treated with CdCl2 during early development (0 μM Cd) and fish treated with 10 μM CdCl2 display normal CPP in response to cocaine (**p < 0.01 when baseline trials are compared to post-conditioning trials with cocaine). The response of the 0 μM group is significantly higher than that of fish not conditioned with cocaine (5A Unt, a: p < 0.05). Developmental exposure to CdCl2 progressively attenuated adult CPP, except at the highest concentration, 10 μM (5A b: p < 0.01 when compared to 0 μM Cd, c: p < 0.001 when compared to 0 μM CdCl2 and p < 0.05 when compared to 10 μM Cd fish). This experiment was repeated 3 times, by different sets of investigators with a minimum total n = 30 for each group. Longitudinal adult fish were also tested for heart rate by measuring their ECG (Figure 5B). Adult fish treated with 1.0 μM CdCl2 during development had a significantly lower baseline heart rate than fish from all other treatment groups (5B, a: p < 0.01 and b: p < 0.001 when compared to the 1 μM Cd fish). After determination of baseline heart rate, the same fish were tested for their cardiovascular response to isoproterenol, a potent β-adrenergic agonist. Fish not developmentally exposed to Cd (0 μM Cd) showed a significant response to the isoproterenol (** p = 0.01, when isoproterenol is compared to baseline using a paired t-test), as did the fish treated with 0.01 μM and 10 μM during development (*p < 0.05 and *** p < 0.001). In addition, fish treated with 0.01 μM CdCl2 during development showed a significantly lower response to isoproterenol than either the 0 μM and 10 μM groups (a: p < 0.05 as determined by ANOVA of arcsin transformed values). This experiment was repeated twice, with ECG recordings by two sets of investigators with a minimum n = 8 for each Cd group.
Longitudinal adult baseline heart rate was affected by developmental CdCl2 exposure (Figure 5B). Fish untreated during development showed a baseline heart rate of 126.3 ± 7.0 bpm, similar to what we have described before (Mersereau et al 2015). Fish exposed to 0.01 μM and 0.1 μM CdCl2 during development showed a 15% increase in average baseline heart rate, but this proved not to be statistically significant when compared to the 0 μM adults. Baseline heart rate was significantly lower for the 1 μM Cd treatment group compared to all other groups (Figure 5B). Developmental treatment with 1.0 μM CdCl2 resulted in an average heart rate 42% lower than 0 μM adults (p < 0.05) and 50% lower that that seen for 0.01 μM and 0.1 μM CdCl2 exposure (p < 0.001). As seen with CPP behavior, the fish that survived developmental treatment with 10 μM had an average heart rate similar to that of the 0 μM fish and significantly higher than the 1.0 μM fish (112.8 ± 13.5 bpm, p < 0.05).
We also treated fish from each developmental Cd treatment group with isoproterenol, a β-adrenergic agonist, to simulate the cardiovascular response to stress, and provide a scope of cardiac performance (Figure 5C). Isoproterenol significantly increased the heart rate by 33.3% above baseline for the 0 μM Cd fish (p = 0.004, using a paired t-test), which was a similar change to what we have previously reported (Mersereau et al 2015). Isoproterenol also significantly raised heart rate above baseline for the 0.01 μM, 10 μM Cd treatment groups (p = 0.03 and p = 0.0001 respectively). However, isoproterenol did not significantly change heart rate for the 0.1 and 1.0 μM groups (p = 0.57 and 0.18 respectively). While isoproterenol significantly changed heart rate in 0.01 μM fish, this change was significantly smaller than that seen for 0 μM and 10 μM fish (p < 0.05). Finally, The 1 μM Cd fish, which had the lowest average baseline heart rate, also had significantly lower heart rates than all other groups after isoproterenol treatment (data not shown, p < 0.01 compared to 0, 0.01, and 1.0 μM Cd groups; p < 0.001 compared to 10 μM Cd fish). The differing responses to isoproterenol suggest that there are long-term effects on cardiovascular function resulting from developmental Cd exposure, even at concentrations as low as 0.01 μM.
Discussion
This study differs from other Cd studies in zebrafish by the combination of concentrations used, the developmental window of exposure, and the longitudinal parameters examined (Blechinger et al 2007; Chow et al 2009; Chow et al 2008; Kusch et al 2007; Matz et al 2007; Wang & Gallagher 2013). First, we used a range of concentrations that allowed survival and longitudinal studies on behavior and heart rate (larvae treated with 100 μM, 11240 μg Cd/L, all died using this paradigm). Second, administration of Cd began at 24 hpf, after axis formation and the onset of primary neurogenesis and monoaminergic cell development (Wullimann 2009; Wullimann & Rink 2002). This time point also occurs after the heart has begun to form although definitive looping is not yet complete (Straudt & Stainier 2012). We also cut off Cd treatment at 4 dpf, just before inflation of swim bladder, and increased time spent higher in the water column. This developmental step would perhaps lower sediment exposure for the larval fish. We examined different aspects of physiology and behavior. Given the suspected mechanism of action, Cd would be expected to affect particularly aerobic tissues like the CNS, kidney and heart. A study on the developmental and longitudinal effects of Cd exposure on heart rate has not been reported in zebrafish. Finally, we looked for longitudinal effects of developmental Cd exposure on conditioned learning behavior, which has not been reported previously in zebrafish.
Dramatic effects of Cd exposure were seen in larvae treated at the highest concentrations. Body length, brain size, and eye size were all decreased in a similar manner, with the most significant effects seen with treatment of 10 μM CdCl2. Previous studies have shown a decrease in neurogenesis and retinogenesis with Cd treatment, though these studies were conducted at a higher concentration (100 μM), with shorter duration, and earlier exposure onset to target early CNS patterning and gene expression events (Chow et al 2009; Chow et al 2008). We report here a significant increase in neural cell death as indicated by AO staining. Cd has been reported to stimulate apoptosis in cultured neurons, a process linked to the destruction of mitochondria and ROS generation (Jomova et al 2010; Lopez et al 2006). The eye and olfactory epithelium were also particularly susceptible to Cd treatment, which has been previously reported by others (Blechinger et al 2007; Chow et al 2009; Kusch et al 2007; Wang & Gallagher 2013). Given the dramatic effects of Cd on neural development reported here and by others, longitudinal effects on survival and behavior would certainly be expected.
In fact, survival was affected significantly, but only at the highest concentration of CdCl2. The decline in mortality occurred after placement of the treated 5-dpf larvae on the rearing system. When ratios of telencephalic area to body length and eye diameter to body length were compared between longitudinal adults from developmental Cd-treated groups, no significant differences were seen. However, the ratio of brain weight to body length did significantly vary between groups, though not in the way expected. Given the reported effects on neurogenesis and our observations on cell death in Cd-treated larvae, smaller longitudinal adult brains might be expected, but this was not the case. Adult brain weights were proportionally higher in fish exposed to 0.1 μM, 1.0 μM CdCl2 and 10 μM CdCl2 during development. In another study in which adult fish were exposed to high concentrations of Cd showed a decrease in organization, possibly the cellular or synaptic arrangements, of the optic tectum (Favorito et al 2011). In the present study zebrafish were treated during early development, but it is possible that the organization in the adult fish was similarly changed in organization, which in turn somehow affected tissue weight. Alternatively, an injury response caused by developmental Cd exposure could have reprogrammed the neural stem cells to make more neurons. We are currently exploring these possibilities by conducting detailed histological and immunohistological analyses of the Cd-treated brains. While the mechanism driving the differences in brain weight has not been found yet, the relative changes did inversely correspond to the effect on CPP behavior (Compare Figure 4F with 5A).
We sought to test if exposure to lower levels of CdCl2 might have more subtle effects on neural organization that might be reflected by altered behavior. We looked at cocaine-induced CPP as a measure of conditioned learning involving the dopaminergic reward pathways of the adult zebrafish brain, believed to extend from the caudal diencephalon to the dorsal area of the ventral telencephalon (Vd) (Darland et al 2012; Wullimann & Rink 2002). Whether CdCl2 led to specific lesions in the dopaminergic reward pathway is an area we are currently investigating. Interestingly AO staining of 10μM Cd treated larvae often showed a pocket of cell death in the medial telencephalon, consistent with the Vd (arrow in Figure 2E). Rats exposed in utero to CdCl2 have also been reported to display lower cocaine-induced CPP as well as lower cocaine self-administration, another assay that measures reward-based learning (Cardon et al 2004; Smith & Nation 2003). In the present study we show that CPP in zebrafish was decreased in a concentration dependent fashion, except at the highest Cd-treatment concentration tested (10 μM), which displayed a normal response. This concentration response curve was inverted relative to brain weight and somewhat similar to results looking at adult heart rate.
Given its impact on mitochondrial function and ROS generation, Cd would be expected to affect development and function of the heart. However, little has been reported looking at the cardiac effects of developmental exposure to Cd. We report an increase in baseline heart rate of Cd-treated larval zebrafish. A similar result was reported for Japanese medaka (Oryzias latipes) exposed to Cd-spiked sediments during early development (Barjhoux et al 2016). In that study, a concentration dependent increase in heart rate by 6 dpf was reported and followed by a progressive drop in heart rate by 7dpf. There were also significant effects on looping induced by Cd in the heart. In the present study we observed an increased number of AO+ cells in the hearts of larvae treated with 10 μM CdCl2. In many of the 10 μM Cd-treated larvae that were arched, the pericardium and ventricle were also enlarged, but we did not see evidence of abnormal looping as was reported in the medaka study. Some studies have shown toxic effects of Cd on cultured cardiomyocytes and in whole animals (Chen et al 2015; Ferramola et al 2012). The increased cell death might lower pumping efficiency, and the resulting drop in peripheral blood pressure could induce a compensatory baroreflex in heart rate. Alternatively, others have proposed that metal exposure might induce a stress response that progressively raises the heart rate (Barjhoux et al 2016). In longitudinal adults, the only fish whose base line heart rates were affected significantly by Cd were those treated with 1.0 μM. These fish could represent a population in which permanent damage was done to the heart, or perhaps central sympathetic tone was permanently affected in the larvae. We favor the latter explanation given the decided lack of isoproterenol effect on 0.01, 0.1 and 1.0 μM Cd-treated fish. We are currently investigating the pharmacological and histology properties of the Cd-treated cardiovascular system to find the likely cause for the deficit in function. While the adults exposed to 10 μM CdCl2 appeared normal in heart rate, brain weight and CPP behavior, it is likely that this is because most of the fish were severely affected but died. It is, therefore, a reasonable hypothesis that early treatment with 10 μM CdCl2 selected for particularly robust, or Cd-resistant individuals. We are currently testing these hypotheses.
A question not often considered in these types of studies is ecological relevance of the Cd concentrations used. Most heavy metals in freshwater ecosystems complex with organics and are sequestered into the sediments. A recent survey of sediments taken from the Northern Plains in the United States showed that the surface sediments of potholes, rivers and lakes averaged 0.38 mg of Cd per kg dry weight (Jacob et al 2013). Similar levels have been reported in other surveys conducted in the Southeastern United States, China and Central Europe (Cuculic et al 2016; Li et al 2017; Otter et al 2012), although these can be affected dramatically by anthropogenic activity, particularly mining and agriculture (Audry et al 2004; O'Neill et al 2015). Much less has been reported on surface waters since these tend to have levels below detection limits. However, general detectable concentrations reported are in the range of 0.05 to 0.65 μg/L (Cremazy et al 2015; Linnik et al 2015; Zhang et al 2016). These reported Cd concentrations are far lower than those used in our study (1.124 to 1124 μg/L). However, the bioavailability of heavy metals in an aquatic system is a dynamic process that has not been fully explored (Burger 2008).
In some preliminary investigations looking at Cd bioaccumulation in larvae, we have seen a range of 1.02-5.69 mg Cd/kg dry weight in larvae treated with concentrations between 0.1 and 10 μM CdCl2. These results are similar to those described in other studies looking at developmental and adult Cd exposure in zebrafish (Banni et al 2011; Favorito et al 2011; Matz et al 2007). Japanese medaka exposed to Cd-spiked sediments over the same approximate developmental time course as ours showed similar Cd bioaccumulation (Barjhoux et al 2016). These bioaccumulation levels are also similar to those quantified for bass found in waterways with moderate to levels of Cd in sediments, but not in surface waters (Andres et al 2000; Otter et al 2012). Fish are thought to take up Cd by using divalent cation pumps and channels localized to the gills and specific areas of the surface integument as well as by ingestion of sedimentary particles (Burger 2008; Tjalve & J. 1999; Wicklund Glynn et al 1994). Uptake by developing larvae sitting on the surface of the sediment would be expected to increase the local solubilization of Cd sequestered in the sediments of the microenvironment by mass action. Order of magnitude differences in concentration of the microenvironment are therefore not impossible. Also, any local surge in anthropogenic activity could easily increase the bioavailability of Cd. In fact, other studies consider the range we have used in the current study to be ecologically relevant (Pereira et al 2016). This validates our model system and experimental approach, particularly given the observed differences in heart rate and behavior at lower concentrations.
The ecological impact of environmental Cd exposure in most waterways where the metal is at low concentration is a question of some debate (Burger 2008). In the present study we show a decrease in baseline heart rate and CPP behavior at a Cd concentration that has no effect on survival in the laboratory. While we still need to ascertain the cause of the lowered heart rate, lower cardiac performance would be expected to affect fitness of the individual. Similarly the altered CPP behavior suggests an effect on monoaminergic reward systems of the brain, which are important neural substrates governing foraging behavior (Baudonnat et al 2013; Miller et al 2013). Cd-exposed individuals might be expected to forage less effectively, thereby lowering fitness. In another study, olfactory-based predator avoidance behavior in zebrafish was completely eliminated in longitudinal adult zebrafish raised from clutches exposed to 20 μg/L Cd (0.2 μM) for 50 days (Kusch et al 2007). The subtle effects seen in the laboratory fish might become much more important in a natural population faced with conditions of scarcity, competition, and predation. Future studies will be aimed at exploring the impact of low Cd exposure on these subtle aspects of fitness.
Highlights.
Developmental exposure to 10 μM Cd significantly decreased body, eye, and brain size of 5 day-old larvae treated between 24 and 96 hours post-fertilization. Lower concentrations (0.01, 0.1, and 1.0 μM) had no effect.
Developmental exposure to these same doses of Cd progressively increased larval heart rate in 5 day-old larvae treated between 24 and 96 hours post-fertilization.
Longitudinal survival of fish treated with Cd during development was unaffected except at the highest concentration used (10 μM)
Morphologically, longitudinal adult fish did not vary significantly in any way except for brain weight.
Cocaine-induced conditioned place preference (CPP) was used to measure function of the monoaminergic reward systems in the brains of adult fish developmentally exposed to Cd. Developmental Cd exposure progressively decreased CPP response, except for fish that survived the highest dose (10 μM), which behaved normally.
Base line heart rate was significantly lower in fish treated with 1.0 μM Cd during development.
Cardiovascular response to isoproterenol was significantly reduced in fish treated with 0.01, 0.1 and 1.0 μM Cd, but not fish that survived the 10 μM developmental treatment.
Collectively, the data suggest that even low exposure to Cd can influence individual fitness without necessarily affecting survival or gross morphology in a laboratory setting.
Acknowledgments
Technical support over the years: by Elvira Tkach, Matthew Berosik, Kayla Yarusso, Brandon One Feather, Geoff Schaubhut, Kayla Nelson, and Beau R. Burkholder. We would also like to acknowledge Professor David T. Pierce and Yuqiang Wang from the Chemistry Department of UND for help with preliminary assessment of Cd bioaccumulation in zebrafish.
Grant support: We are grateful for funding support by a University of North Dakota (UND) faculty start-up package including North Dakota EPSCoR, College of Arts and Sciences, Vice President of Research, UND Biology Department and the Department of Pathology at the UND School of Medicine and Health Sciences. This work was also supported by National Science Foundation (NSF) REU Site Grant 0851869 and by NIH Grant P20GM103442/P20RR016471. This publication was also made possible by grant number U261HS0045-04-01 from the Indian Health Service (IHS), with the support of the NIH. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the IHS or the NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amzal B, Julin B, Vahter M, Wolk A, Johanson G, Akesson A. Population toxicokinetic modeling of cadmium for health risk assessment. Environmental Health Perspectives. 2009;117:1293–301. doi: 10.1289/ehp.0800317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andres S, Ribeyre F, Tourencq JN, Boudou A. Interspecific comparison of cadmium and zinc contamination in the organs of four fish species along a polymetallic pollution gradient (Lot River, France) Science of the Total Environment. 2000;248:11–25. doi: 10.1016/s0048-9697(99)00477-5. [DOI] [PubMed] [Google Scholar]
- Audry S, Schafer J, Blanc G, Jouanneau L. Fifty-year sedimentary record of heavy metal pollution (Cd, Zn, Cu, Pb) in the Lot River reservoirs (France) Environ Pollution. 2004;132:413–26. doi: 10.1016/j.envpol.2004.05.025. [DOI] [PubMed] [Google Scholar]
- Banni M, Chouchene L, Said K, Kerkeni A, Messaoudi I. Mechanisms underlying the protective effect of zinc and selenium against cadmium-induced oxidative stress in zebrafish. Biometals. 2011;24:981–92. doi: 10.1007/s10534-011-9456-z. [DOI] [PubMed] [Google Scholar]
- Barjhoux I, Gonzalez P, Baudrimont M, Cachot J. Molecular and phenotypic responses of Japanese medaka (Oryzias latipes) early life stages to environmental concentrations of cadmium in sediment. Environ Sci Pollut Res. 2016;23:17969–81. doi: 10.1007/s11356-016-6995-4. [DOI] [PubMed] [Google Scholar]
- Baudonnat M, Huber A, David V, Walton ME. Heads for learning, tails for memory: reward, reinforcement and a role of dopamine in determining behavioral relevance across multiple timescales. Frontiers in Neuroscience. 2013;7:1–14. doi: 10.3389/fnins.2013.00175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernhoft RA. Cadmium toxicity and treatment. The Scientific World Journal. 2013;2013:1–7. doi: 10.1155/2013/394652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blechinger SR, Kusch RC, Haugo K, Matz C, Chivers DP, Krone PH. Brief embryonic cadmium exposure induces a stress response and cell death in the developing olfactory system followed by long-term olfactory deficits in juvenile zebrafish. Toxicology and Applied Pharmacology. 2007;224:72–80. doi: 10.1016/j.taap.2007.06.025. [DOI] [PubMed] [Google Scholar]
- Burger J. Assessment and management of risk to wildlife from cadmium. Science of the Total Environment. 2008;389:37–45. doi: 10.1016/j.scitotenv.2007.08.037. [DOI] [PubMed] [Google Scholar]
- Cardon AL, Rocha A, Valles R, Bratton GR, Nation JR. Exposure to cadmium during gestation and lactation decreases cocaine self-administration in rats. NeuroToxicology. 2004;25:869–75. doi: 10.1016/j.neuro.2004.01.001. [DOI] [PubMed] [Google Scholar]
- Casalino E, Sblano C, Landriscina C. Enzyme activity alteration by cadmium administration to rats: the possibility of iron involvement in lipid peroxidation. Arch Biochem Biophys. 1997;346:171–9. doi: 10.1006/abbi.1997.0197. [DOI] [PubMed] [Google Scholar]
- Chen Cy, Zhang Sl, Lui Zy, Tian Y, Sun Q. Cadmium toxicity induces ER stress and apoptosis via impairing energy homeostasis in cardiomyocytes. Bioscience Reports. 2015;35:e00214. doi: 10.1042/BSR20140170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow ESH, Hui MNY, Cheng CW, Cheng SH. Cadmium affects retinogenesis during zebrafish embryonic development. Toxicology and Applied Pharmacology. 2009;235:68–76. doi: 10.1016/j.taap.2008.11.013. [DOI] [PubMed] [Google Scholar]
- Chow ESH, Hui MNY, Lin CC, Cheng SH. Cadmium inhibits neurogenesis in zebrafish embryonic brain development. Aquatic Toxicology. 2008;87:157–69. doi: 10.1016/j.aquatox.2008.01.019. [DOI] [PubMed] [Google Scholar]
- Cremazy A, Leclair S, Mueller KK, Vigneault B, Campbell PGC, Fortin C. Development of an in situ ion-exchange technique for the determination of free Cd, Co, Ni, and Zn concentrations in freshwaters. Aquat Geochem. 2015;21:259–79. [Google Scholar]
- Cuculic V, Franciskovic-Bilinski S, Billinski H, Maldini K, Tomas D, Tomasic N. Multi-methodological approach to evaluate trace elements and major components in wetland system with subsaline and freshwater characteristics. Environ Earth Sci. 2016;75 [Google Scholar]
- Cuypers A, Plusquin M, Remans T, Josefczak M, Keunen E, et al. Cadmium stress: an oxidative challenge. Biometals. 2010;23:927–40. doi: 10.1007/s10534-010-9329-x. [DOI] [PubMed] [Google Scholar]
- Darland T, Dowling JE. Behavioral screening for cocaine sensitivity in mutagenized zebrafish. PNAS. 2001;98:11691–6. doi: 10.1073/pnas.191380698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darland T, Mauch JT, Meier EM, Hagan SJ, Dowling JE, Darland DC. Sulpiride, but not SCH23390, modifies cocaine-induced conditioned place preference and expression of tyrosine hydroxylase and elongation factor 1a in zebrafish. Pharmacology, Biochemistry and Behavior. 2012;103:157–67. doi: 10.1016/j.pbb.2012.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favorito R, Chiarelli G, Grimaldi MC, De Bronis S, Lancieri M, Ferrandino I. Bioaccumulation of cadmium and its cytotoxic effect on zebrafish brain. Chemistry and Ecology. 2011;27:39–46. [Google Scholar]
- Ferramola ML, Diaz MFFP, Honore SM, Sanchez SS, Anton RI, et al. Cadmium-induced oxidative stress and histological damage in the myocardium. Effects of a soy-based diet. Toxicology and Applied Pharmacology. 2012;265:380–9. doi: 10.1016/j.taap.2012.09.009. [DOI] [PubMed] [Google Scholar]
- Gobe G, Crane D. Mitochondria, reactive oxygen species and cadmium toxicity in the kidney. Toxicology Letters. 2010;198:49–55. doi: 10.1016/j.toxlet.2010.04.013. [DOI] [PubMed] [Google Scholar]
- Goldman D, Hankin M, Li Z, Dai X, Ding J. Transgenic zebrafish for studying nervous system development and regeneration. Transgenic Research. 2001;10:21–33. doi: 10.1023/a:1008998832552. [DOI] [PubMed] [Google Scholar]
- Gross JM, Perkin BD, Amsterdam A, Egana A, Darland T, et al. Identification of zebrafish insertional mutants with defects in visual system development and function. Genetics. 2005;170:245–61. doi: 10.1534/genetics.104.039727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallare AV, Schirling M, Luckenback T, Kohler HR, Triebskorn R. Combined effects of temperature and cadmium on developmental parameters and biomarker responses in zebrafish (Danio rerio) embryos. Journal of Thermal Biology. 2005;30:7–17. [Google Scholar]
- Hartwig A. Mechanisms in cadmium-induced carcinogenicity:recent insights. Biometals. 2010;23:951–60. doi: 10.1007/s10534-010-9330-4. [DOI] [PubMed] [Google Scholar]
- Jacob DL, Yellick AA, Kissoon LTT, Asgary A, Wijeyaratne DN, et al. Cadmium and associated metals in soils and sediments of wetlands across the Northern Plains, USA. Environmental Pollution. 2013;178:211–9. doi: 10.1016/j.envpol.2013.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarup L, Akesson A. Current status of cadmium as an environmental health problem. Toxicology and Applied Pharmacology. 2009;238:201–8. doi: 10.1016/j.taap.2009.04.020. [DOI] [PubMed] [Google Scholar]
- Jomova K, Vondrakova D, Lawson M, Valko M. Metals, oxidative stress and neurodegenerative disorders. Mol Cell Biochem. 2010;345:91–104. doi: 10.1007/s11010-010-0563-x. [DOI] [PubMed] [Google Scholar]
- Jurczuk M, Brzoska MM, Moniuszko-Jakoniuk J, Galazyn-Sidorczuk M, Kulikowska-Karpinska E. Antioxidant enzymes activity and lipid peroxidation in liver and kidney of rats exposed to cadmium and ethanol. Food and Chemical Toxicology. 2004;42:429–38. doi: 10.1016/j.fct.2003.10.005. [DOI] [PubMed] [Google Scholar]
- Kusch RC, Krone PH, Chivers DP. Chronic exposure to low concentrations of waterborne cadmium during embryonic and larval development results in long-term hindrance of antipredator behavior in zebrafish. Environmental Toxicology and Chemistry. 2007;27:705–10. doi: 10.1897/07-273.1. [DOI] [PubMed] [Google Scholar]
- Li N, Tian Y, Zhang J, Zuo W, Zhan W, Zhang J. Heavy metal contamination status and source appointment in sediments of Songhua River Harbin region, Northeast China. Environ Sci Pollut Res. 2017;24:3214–25. doi: 10.1007/s11356-016-7132-0. [DOI] [PubMed] [Google Scholar]
- Li X, LV Y, Yu S, Zhao H, Tao L. The effect of cadmium on Aβ levels in APP/PS1 transgenic mice. Experimental and Therapeutic Medicine. 2012;4:125–30. doi: 10.3892/etm.2012.562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limaye DA, Shaikh ZA. Cytotoxicity of Cadmium and Characteristics of its Transport in Cardiomyocytes. Toxicology and Applied Pharmacology. 1999;154:59–66. doi: 10.1006/taap.1998.8575. [DOI] [PubMed] [Google Scholar]
- Linnik PN, Zhezherya VA, Linnik RP, Ignatenko II, Zubenko IB. Metals in surface water of Ukraine: the migration forms, feature of distribution between the abiotic component of aquatic ecosystems, and potential bioavailability. Russian Journal of General Chemistry. 2015;85:2965–84. [Google Scholar]
- Lopez E, Arce C, Oset-Gasque MJ, Canadas S, Gonzalez MP. Cadmium induces reactive oxygen species generation and lipid peroxidation in cortical neurons in culture. Free Radical Biology & Medicine. 2006;40:940–51. doi: 10.1016/j.freeradbiomed.2005.10.062. [DOI] [PubMed] [Google Scholar]
- Matz CJ, Treble RG, Krone PH. Accumulation and elimination of cadmium in larval stage zebrafish following acute exposure. Ecotoxicology and Environmental Safety. 2007;66:44–8. doi: 10.1016/j.ecoenv.2005.11.001. [DOI] [PubMed] [Google Scholar]
- Mersereau EJ, Boyle CA, Poitra SL, Espinoza A, Seiler J, et al. Longitudinal effects of embryonic exposure to cocaine on morphology, cardiovascular physiology, and behavior in zebrafish. International Journal of Molecular Sciences. 2016;17:847. doi: 10.3390/ijms17060847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mersereau EJ, Poitra SL, Espinoza A, Crossley DA, II, Darland T. The effects of cocaine on heart rate and electrocardiogram in zebrafish (Danio rerio) Comparative Biochemistry and Physiology, Part C Toxicology Pharmacology. 2015:172–173. 1–6. doi: 10.1016/j.cbpc.2015.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MA, Thome A, Cowen SL. Intersection of effort and risk: ethological and neurobiological perspectives. Frontiers in Neuroscience. 2013;7:1–11. doi: 10.3389/fnins.2013.00208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair AR, DeGheselle O, Smeets K, Van Kerkhove E, Cuypers A. Cadmium-induced pathologies: where is the oxidative balance lost (or not)? International Journal of Molecular Sciences. 2013;14:6116–43. doi: 10.3390/ijms14036116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill A, Phillips DH, Bowen J, Sen Gupta B. Science of the Total Environment. 2015. Contaminants in surface water and sediments near the Tynagh silver mine site, County Galway, Ireland; pp. 512–513.pp. 261–72. [DOI] [PubMed] [Google Scholar]
- Otter RR, Schreiber EA, van den Hurk P, Klaine SJ. Assessment of heavy metal and PAH exposure in large mouth bass (Micropterus salmoides) in the Reedy River Watershed, South Carolina, a multi-season assessment of metallothionein and bile fluorescence. Environmental Toxicology and Chemistry. 2012;31:2763–70. doi: 10.1002/etc.2000. [DOI] [PubMed] [Google Scholar]
- Pereira LS, Ribas JLC, Vicari T, Silva SB, Stival J, et al. Effects of ecologically relevant concentrations of cadmium in a freshwater fish. Ectotoxicology and Environmental Safety. 2016;130:29–36. doi: 10.1016/j.ecoenv.2016.03.046. [DOI] [PubMed] [Google Scholar]
- Satarug S, Garrett SH, Sens MA, Sens DA. Cadmium, environmental exposure and health outcomes. Environmental Health Perspectives. 2009 doi: 10.1289/ehp.0901234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satarug S, Moore MR. Adverse health effects of chronic exposure to low-level cadmium in foodstuffs and cigarette smoke. Environ Health Perspect. 2004;112:1099–103. doi: 10.1289/ehp.6751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KR, Nation JR. Developmental exposure to cadmium alters responsiveness to cocaine in the rat. Drug and Alcohol Dependence. 2003;72:1–11. doi: 10.1016/s0376-8716(03)00170-4. [DOI] [PubMed] [Google Scholar]
- Straudt D, Stainier D. Uncovering the molecular and cellular mechanisms of heart development using the zebrafish. Annu Rev Genet. 2012;46:397–418. doi: 10.1146/annurev-genet-110711-155646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjalve H, H J. Uptake of metals in the brain via olfactory pathways. Neurotoxicology. 1999;20:181–96. [PubMed] [Google Scholar]
- Wang L, Gallagher EP. Role of nrf2 antioxidant defense in mitigating cadmium-induced oxidative stress in the olfactory system of the zebrafish. Toxicology and Applied Pharmacology. 2013;266:177–86. doi: 10.1016/j.taap.2012.11.010. [DOI] [PubMed] [Google Scholar]
- Wang Y, Fang J, Leonard SS, Rao KMK. Cd inhibits the electron transfer chain and induces reactive oxygen species. Free Radic Biol Med. 2004;36:1434–43. doi: 10.1016/j.freeradbiomed.2004.03.010. [DOI] [PubMed] [Google Scholar]
- WHO. 61st report evaluation of certain food additives and contaminants. United Nations; Geneva: 2004. [Google Scholar]
- Wicklund Glynn A, Norrgren L, Mussener A. Differences in uptake of inorganic mercury and cadmium in the gills of zebrafish, Brachydanio rerio. Aquat Toxicol. 1994;30:13–26. [Google Scholar]
- Wullimann MF. Secondary neurogenesis and telencephalic organization in zebrafish and mice: a brief review. Integrative Zoology. 2009;4:123–33. doi: 10.1111/j.1749-4877.2008.00140.x. [DOI] [PubMed] [Google Scholar]
- Wullimann MF, Rink E. The teleostean forebrain: A comparative and developmental view based on early proliferation, Pax6 activity and catecholaminergic organization. Brain Research Bulletin. 2002;57:363–70. doi: 10.1016/s0361-9230(01)00666-9. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Wang JJ, Ali A, DeLaune RD. Heavy metal distribution and water quality characterization of water bodies in Louisianna's Lake Pontchartrain Basin, USA. Environ Monitor Assess. 2016;188:628. doi: 10.1007/s10661-016-5639-y. [DOI] [PubMed] [Google Scholar]


