Abstract
Background
Slowed and curved rapid eye movements, saccades, are the well‐known features of progressive supranuclear palsy (PSP). The authors hypothesized that the saccades in PSP not only are slow and curved but also are irregular and have timing deficits.
Methods
This hypothesis was tested in 12 patients with PSP by measuring vertical and horizontal visually guided saccades using a limbus tracker.
Results
Both horizontal and vertical saccades were slow and had irregular trajectory and velocity profiles, but deficits were much more robust in vertical saccades. The irregularity in the saccade velocity was due to premature interruptions that either completely stopped the eyes or moved the eyes at much slower velocity along or in the opposite direction of the ongoing saccade. The direction of the eye's trajectory was often changed after the interruption. A conductance‐based, single‐compartment model of the burst neurons embedded in local feedback circuit for saccade generation was simulated. This model mimicked anatomic and physiologic realism while allowing the liberty to selectively change the activation of individual burst neurons or pause neurons. The PSP saccades were comparable to the simulations during reduced activity of the inhibitory and excitatory burst neurons.
Conclusion
PSP saccades are due to the paucity in burst generation at the excitatory burst neurons and imprecise timing signal from the inhibitory burst neurons. Premature discharge of the inhibitory burst neuron further leads to breaks in the saccade trajectory and maladaptive superior colliculus activity, leading to aberrant saccades that change the intended trajectory of the ongoing saccade.
Keywords: brainstem, eye movement, parkinsonism, superior colliculus
Progressive supranuclear palsy (PSP) is a sporadic neurodegenerative condition related to the abnormal accumulation of tau, the main constituent of the neurofibrillary tangles responsible for neuronal injury.1, 2, 3, 4, 5 Typical clinical features of PSP include early postural instability, axial rigidity, bulbar defects, and supranuclear gaze palsy.6, 7 Prominent autopsy changes in typical forms of PSP are found in basal ganglia of the substantia nigra pars compacta, the subthalamic nucleus, the diencephalon, and the brainstem reticular formation.8, 9
The eye movements can be important biomarkers of PSP. Clinically evident slowing of vertical saccades progresses to vertical gaze limitation, which is a hallmark of PSP.6, 7, 10, 11, 12, 13, 14, 15, 16, 17 Oblique saccades have the prominent curvature with relatively faster horizontal component that distinguishes PSP from other forms of atypical parkinsonism and Parkinson's disease.14 The curved trajectory of vertical saccades in PSP is called “round‐the‐houses sign.”18 Although patients with early PSP present with hypometria of horizontal saccades, the saccades get slower as the disease advances.10
Conventionally, the slowing of saccades in PSP is attributed to the decreases in peak saccade velocity caused by degenerative loss of mesencephalic saccadic burst generators.10 Traditional literature suggested that selective degeneration of the burst neurons responsible for vertical saccades from the rostral interstitial medial longitudinal fasciculus (MLF), while sparing of burst generators in the paramedian pontine reticular formation, leads to isolated vertical saccade slowing.4, 10, 19, 20 Involvement of the omnipause neurons (OPNs) from the pontine reticular formation was considered a less likely etiology, because the dysfunctional OPNs can equally impair both horizontal and vertical saccades.21, 22 We asked whether slowing of saccades occurs due to impaired burst generation caused by an impairment in the function of excitatory burst neurons (EBNs) or by disinhibition from the loss of inhibitory burst neurons (IBNs). The burst neurons provide feedback to the superior colliculus. Therefore, we hypothesized that at least 2 additional phenomena are involved in slowing of saccades. One mechanism includes inappropriately timed interruption of an ongoing saccade followed by a catch‐up saccade. The second mechanism is misdirection of the saccade trajectory and subsequent correction. Delineating the mechanisms of saccade abnormalities in PSP will facilitate development of the reliable and possibly prodromal disease markers that will help in the early differential diagnosis and quantify progression of illness in way not possible with traditional clinical scales.
Patients and Methods
We measured eye movements in 4 age‐matched, healthy controls and 12 patients (6 men and 6 women; age range, 50–74 years) who had a clinical diagnosis of PSP according to criteria defined by Litvan and colleagues.6 In addition, for a comparison of the saccade velocity‐to‐amplitude relationship, we also used normative data values collected from various individuals in our laboratory. History and examination included early falls, axial rigidity, convergence insufficiency, slow vertical saccades, and slow or hypometric horizontal saccades. All participants demonstrated a typical course, with an average time from disease onset of 5.9 ± 3.2 years. They all had limited to no response to levodopa (l‐dopa) and, at the time of testing, 9 patients were on l‐dopa therapy at a mean dose of 605 ± 286 mg per day. The study and consent forms were approved by the Emory Institutional Review Board, and each participant signed an Institutional Review Board‐approved informed consent.
Experimental Setup
We used a limbus tracker to measure horizontal and vertical eye movements (Ober Jazz Novo; Ober Consulting, Poznan, Poland). The apparatus tracked the corneal limbus and the position of both eyes, producing a conjugate vector. The voltages generated by the sensor were digitized with a sampling rate of 1000 Hz. In vivo calibration was performed before each experimental session. The calibration used saccades made to target shifts of known distance. The data were further processed and analyzed with previously published techniques using custom software (Matlab; MathWorks, Natick, MA).23, 24, 25
Experimental Protocol
The participant's head was comfortably restrained with chin and forehead rest. They were asked to look at the target projected straight ahead or at 5, 10, 20, or 30 degrees to the right and left and at 5, 10, and 20 degrees up or down. The eye movements were simultaneously recorded.
Analysis of Saccades
Eye position was differentiated and smoothed with a Savitzky‐Golay filter (polynomial order, 3; frame length, 21) to compute eye velocity. Acceleration was measured by further differentiating and smoothing eye velocity with the same filter. The start of the saccade was determined when the eye position shifted 2 degrees away from the steady baseline after the target shift, and the end was when the eyes reached the new baseline. Breaks in the saccades were determined using the velocity trace. Matlab toolboxes were used for statistical analyses and curve fitting (MathWorks).
Results
Figure 1A–C provides an example of a normal, visually guided saccade measured from a healthy participant. The saccade has high velocity and is uninterrupted, as evident from a single peak of the velocity profile (Fig. 1B). The saccadic pathway follows a straight trajectory, as evident in Figure 1C. Figure 1D–I provides examples of 2 vertical saccades from 1 patient with PSP that were measured during the same experimental session. A small negative velocity deflection at the offset of a saccade in a normal individual depicts physiologic drift. The first example of the vertical saccade illustrates 4 types of interruptions in its trajectory. One type (Fig. 1D, red arrow) completely stops the eye movement. Complete cessation is also evident in the velocity trace (Fig. 1E, red arrow). During second type of interruption of the vertical saccade (Fig. 1D, blue arrow) the eyes do not completely stop but move at much a slower speed compared with the velocity of the preceding or subsequent segment of the saccade (Fig. 1E, blue arrow). The third type of interruption results in slow eye movements in the opposite direction (Fig. 1D, E, green arrows). The second example of a saccade in Figure 1G has fewer interruptions compared with the first example. However, the peak eye velocity is much lower (compare gray arrows in Fig. 1E, H).
Figure 1.
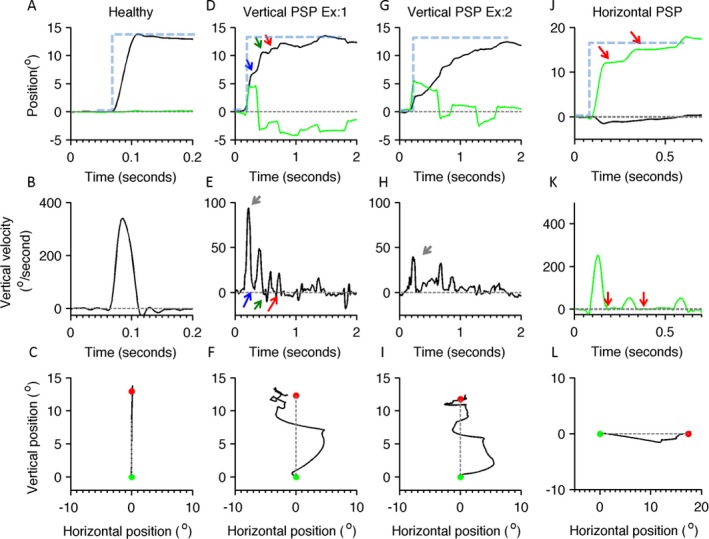
Examples of visually guided saccades from healthy individuals and patients with progressive supranuclear palsy (PSP). In the first row of subplots (A,D,G,J), eye position is plotted on the y‐axis, and the x‐axis depicts the corresponding time in seconds. Black lines indicate the vertical eye position; green traces illustrate the horizontal eye position, gray dashed lines depict the baseline straight‐ahead position, blue dashed lines depict the position of the target (desired eye position), and arrows indicate interruption in ongoing saccades (the blue arrow illustrates 1 type of interruption in which the eyes continue to move at slower velocity during interruption, the green arrow depicts slower eye movement in the opposite direction, and the red arrows illustrate the cessation of eye movement during interruption). A illustrates a normal, visually guided vertical saccade from a healthy individual; D and G depict 2 examples of visually guided vertical saccades from the same individual with PSP; and J depicts eye positions during horizontal saccade. The middle row of subplots (B,E,H,K) depicts eye velocity: B illustrates the eye velocity of a normal, visually guided saccade recorded from the healthy individuals, and E and H depict vertical eye velocity during vertical saccades in PSP. The green line in K illustrates normal horizontal eye velocity during a horizontal saccade in PSP. In these subplots, eye velocity is plotted on the y‐axis, and the x‐axis illustrates the corresponding time. Red arrows indicate an interruption in saccade when the eye velocity was zero, the green arrow indicates when eye moved at slower velocity in the opposite direction, and the blue arrow indicates when eyes moved in the same direction at slower velocity. The bottom row of subplots (C,F,I,L) depicts trajectories of horizontal and vertical saccades: C illustrates a normal saccade from the healthy individual, F and I depict vertical saccade in PSP, and L is a horizontal saccade in PSP. Green dots indicate the start point, red dots indicate the stop point, and gray dashed lines are the desired path of an eye movement. Vertical saccades have a curved and serpentine path, depicting the clinical phenomenon of “round the houses” sign. Similar curvature is present in horizontal saccades as well, but it is much less robust.
These examples of saccades in the patient with PSP are not only slow and interrupted, but they also have curved and irregular trajectories. For instance, in the first example, the initial saccade is upward and to the right (Fig. 1F). After a halt (interruption; not shown in Fig. 1F), the movement crosses the midline but overshoots to the left. After another interruption (not shown in Fig. 1F), the series of subsequent eye movements brings the gaze to the destination (Fig. 1F, red dot). The shape of the trajectory in the example depicted in Figure 1F is curved, suggesting “round‐the‐houses” sign.18 Curved paths are also seen the second example; however, here, the eyes make series of upward‐rightward and upward‐leftward movements to reach the target (Fig. 1I). The trajectory in the example depicted in Figure 1I has the serpentine shape.
Interruptions and curvatures are also present in horizontal saccades (Fig. 1J–L). The horizontal saccade depicted in Figure 1J shows 2 interruptions (Fig. 1J,K, red arrows). During each break, the velocity reaches zero (Fig. 1K, red arrows) and is then followed by a catch‐up saccade, bringing the eyes to their destination. The curvature is also present in the horizontal saccade trajectory. In an illustrated example, for rightward eye movements, the eyes first move to the right and down, and then to the right and up (Fig. 1L). Such interruptions and curvatures of saccades were consistently observed in all patients. Both phenomena are quantified in the sections below.
Quantitative Characteristics of Saccade Interruptions
We measured the ratio of the eye velocity during the interrupted segment and the peak velocity of the preceding saccade segment to quantitatively characterize the interruptions. The ratio would be zero in instances where the break led to a complete cessation of the eye movement. A nonzero value of the ratio suggested slow eye movement during the interruption. The positive value of the ratio meant that the slow eye movement was in the same direction as the ongoing saccade, whereas the negative value suggested slow eye movements in the opposite direction. In most instances, the eyes did not completely stop during the interrupted segment. Instead, they slowly moved in the direction of an ongoing saccade or in the opposite direction. We chose to compute ratios instead of using absolute eye velocity during interruption for 2 reasons: (1) Eye velocities are already slow in patients with PSP; therefore, it might be difficult to distinguish whether there was a further reduction in eye velocity during interruption. (2) The ratio would provide a robust measure of the change in eye velocity during interruption compared with an ongoing saccade. Figure 2 provides a summary of the ratios. Each histogram in Figure 2A,C depicts the summary from an individual patient of vertical and horizontal saccades, respectively. The histograms represent ratios that, in most instances, had nonzero values and spanned in positive and negative directions in all patients. The histograms in Figure 2B,D summarize the ratios from all patients. The dashed lines in Figure 2B,D graphically illustrate the mean value of the ratios. The range of values encompassing 66% of the area under the curve was from −0.42 to 0.05 degrees for vertical saccades and from −0.24 to −0.006 degrees for horizontal saccades. The mean vertical ratio was 0.01 ± 0.014 incidences, and the mean horizontal ratio was 0.01 ± 0.2 incidences. A comparison of the distribution of ratios from horizontal and vertical saccades revealed statistical significance (Kolmogorov‐Smirnov test; P < 0.001).
Figure 2.
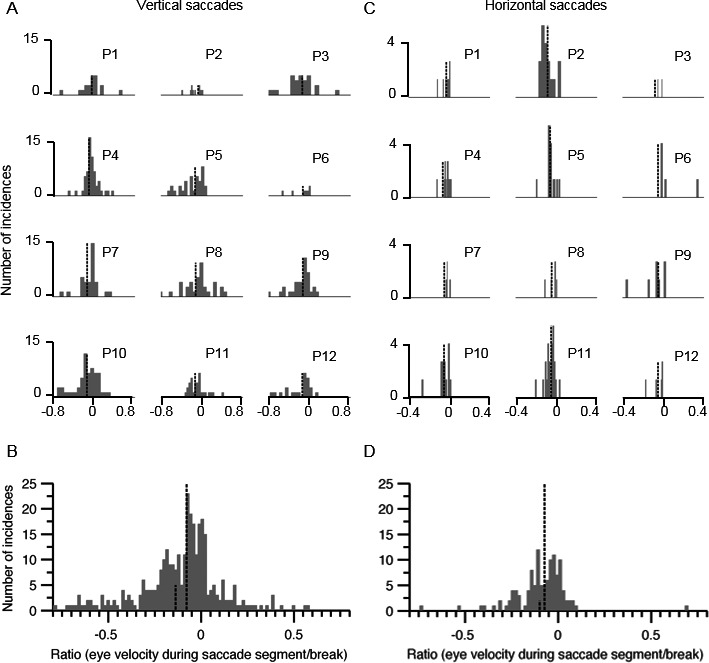
Summary of eye velocity ratios during interruption and saccade segment of interrupted horizontal and vertical saccades in progressive supranuclear palsy (PSP). The left column (A,B) depicts vertical saccades, and the right column (C,D) illustrates horizontal saccades. The histograms in A and C depict individual Patients 1 through 12 (P1–P12). In B and D, charts provide a cumulative summary of ratios from all participants. Ratios near zero suggest that eye movements paused completely during interruption, ratios with a positive value indicate slow eye movement in the same direction as saccades, and negative ratios suggest slow eye movement in the opposite direction. The x‐axis depicts bins of ratio, and the y‐axis indicates the number of incidences in the given bin. Tall and short vertical dashed lines indicate mean and median values, respectively.
Quantitative Characteristics of Saccade Curvature
We also measured changes in the direction of saccades after the interruption to quantify curvatures and irregularity. We computed differences in the direction of the trajectories of saccades before and after the interruption. Figure 3 provides a summary of the differences in the direction of the trajectories of saccade segments before and after the interruption. Each histogram in Figure 3A,C illustrates 1 individual; Figure 3A depicts vertical saccades, and horizontal saccades are represented in Figure 3C. The mean of the difference in the direction of the trajectory was a nonzero value in all participants. Figure 3B,D provides summaries of all saccades from all participants. The range of values encompassing 66% area under the curve was from −141.1 to 63.1 degrees for vertical saccades and from −18.3 to 14.7 degrees for horizontal saccades. The mean value of changes in the vertical saccade trajectory was −10.5 ± 83.8 degrees, and it was −0.73 ± 19.9 degrees for the horizontal saccade trajectory. A comparison of the histograms representing the distribution of directional changes in saccade trajectories revealed statistical significance (Kolmogorov‐Smirnov test; P < 0.001). These results suggest that interrupted saccades in patients with PSP were invariably misdirected and had to make multiple changes in trajectory to reach the target of interest.
Figure 3.
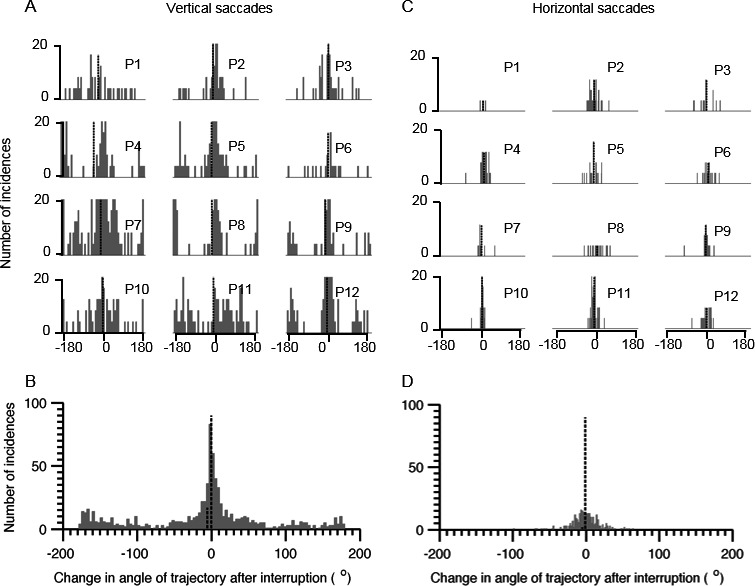
Summary of changes in the angle of trajectory after interruption of horizontal and vertical saccades in progressive supranuclear palsy PSP). The left column (A,B) depicts vertical saccades, and the right column (C,D) illustrates horizontal saccades. Histograms in A and C depict individual patients labeled from Patient 1 (P1) through P12. The histograms in B and D provide a cumulative summary of changes in the trajectory angle from all participants. Angle of zero degrees suggests no change in saccades trajectory after the interruption, and nonzero values suggest a change in the trajectory. The x‐axis depicts bins of trajectory angles, and the y‐axis indicates the number of incidences in the given bin. Tall and short vertical dashed lines indicate mean and median values, respectively.
Saccade Amplitude‐to‐Velocity Relationship
Next, we assessed the amplitude‐to‐velocity relationship (main sequence) of vertical and horizontal saccades (Fig. 4A,B). There were 2 components to this analysis, as schematized in Figure 4B (inset). In the first component, we compared desired saccade amplitude (“Ad” in Fig. 4B, inset; dots in Fig. 4A,B) with the peak velocity (Vmax) during the entire gaze shift. The unique aspect of this analysis was that it investigated whether an “interrupted” saccade is comprised of multiple, sequential, “normal” saccades or is a single, pathologically interrupted saccade. If these were multiple, sequential, small saccades with normal kinematic properties, then we would expect a normal amplitude‐to‐velocity relationship when comparing the velocity with the amplitude of each saccade segment. However, in that case, the main sequence of peak velocity (which can be any saccade segment) to the desired amplitude would be abnormal, because a segmented saccade is scaled for smaller amplitude. In contrast, if the saccade is “normal” in size but interrupted, then we would find increased velocity of a saccade segment for the amplitude of the given segment; however, there will be normal peak velocity for the desired amplitude. Finally, if the saccades are slow and interrupted, then we expected to observe slowing in both comparisons. The analysis compared the amplitude of segmented saccade (“A1” in Fig. 4B, inset; open symbols in Fig. 4A,B) with the corresponding velocity (V1). Figure 4A,B depicts the summary of the main sequence from 12 patients with PSP. Colored dots in Figure 4A depict the main sequence of the desired saccade amplitude, with each color indicating 1 individual. Open symbols in Figure 4A illustrate the main sequence of the segmented portions of the saccades, and black dashed lines represent the normative range. In all instances, the solid data points depicting the main sequence of desired saccades fall below the normative range, whereas open symbols are below the normative values in Patients 1, 2, 7, 8, 10, and 11. We then performed 2 parts of main sequence analysis for horizontal saccades. A summary of this analysis is depicted in Figure 4B. The main sequence comparison of a desired saccade (comparison of the peak saccade velocity with the amplitude to the desired saccade) is illustrated by colored dots, and such symbols in all except Patients 1, 4, and 9 fell below the lower range of normal. The open symbols depicting the comparison of the velocity of saccade segment with the amplitude of each segment fell within the normal range (between the black dashed lines) in all patients (Fig. 4B).
Figure 4.
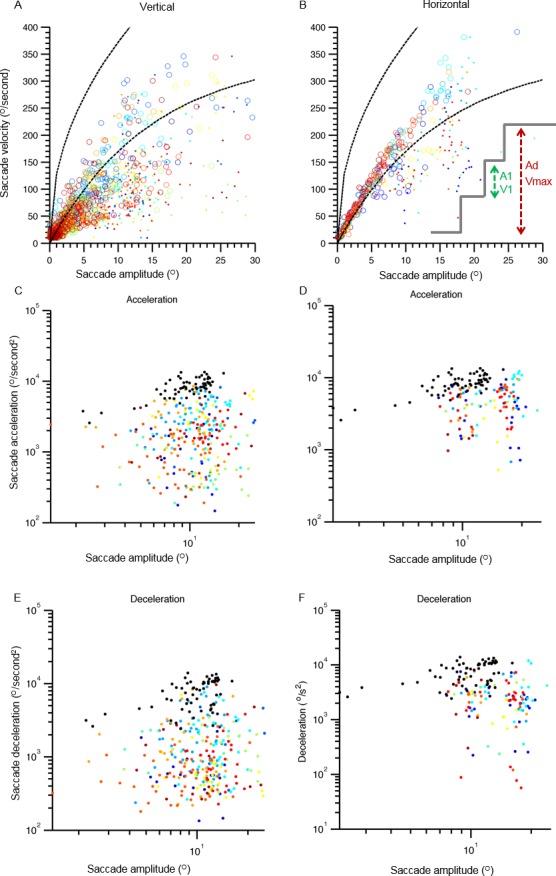
Comparison of saccade velocity and corresponding amplitude in main sequence analysis for vertical (A) and horizontal (B) saccades. Saccade velocity is plotted on the y‐axis, and the x‐axis depicts saccade amplitude. Each color depicts 1 individual, and each symbol depicts 1 saccade. Solid symbols depict the relationship of peak saccade velocity with the desired amplitude of saccade, and open symbols show the relationship between the amplitude of the broken segment of the saccade and the corresponding velocity. Dashed black lines indicate the normative range. (C,D) Saccade acceleration and the corresponding amplitude for vertical and horizontal saccades are compared. (E,F) A similar comparison for saccade deceleration is depicted. Acceleration or deceleration is plotted on y‐axis, and the corresponding amplitude is plotted on the x‐axis. Each color depicts 1 individual, and each symbol is 1 saccade. Black symbols represent data from normal individuals. The inset in B schematizes the analysis scheme. Green arrows depict a segmented saccade, A1 is the amplitude of segmented saccade, and V1 is corresponding velocity. The red arrows indicate the desired saccade amplitude (Ad). Vmax is the maximal velocity during entire saccade.
We quantified the main sequence by measuring the goodness of fit to the equation: V = K * AL. In this equation, V is peak saccade velocity, A is the saccade amplitude, and 2 parameters, K and L, determine the amplitude dependence of the saccade velocity. This analysis was separately done for 2 types of comparisons of the main sequence for vertical and horizontal saccades. Table S1 depicts the summary of results (see online supporting information). We observed a weak correlation between the values of parameters K and L for the main sequence relationship of desired as well as interrupted saccades in the horizontal and vertical directions and the duration of PSP. For vertical saccades, there was a weak relationship between the disease duration and the value of parameter K for both components of analyses (desired saccade component, r2 = 0.04; segmented saccade component, r2 = 0.3). The parameter L weakly correlated with disease duration in case of both desired and interrupted saccade (desired, r2 = 0.4; interrupted, r2 = 0.36). There was no correlation between disease duration and the parameters K and L for either components of the analyses in horizontal saccades (K: desired saccade, r2 = 0.007; segmented saccade, r2 = 0.04; L: desired saccade, r2 = 0.004; interrupted horizontal saccade, r2 = 0.0002). These results suggest that disease duration has no influence on the velocity of the interrupted saccade, suggesting that saccadic abnormalities are independent of disease progression, as viewed by the traditional clinical rating scale.
Amplitude to Acceleration Comparison
In subsequent analyses, we compared amplitude dependence of peak vertical and horizontal saccade acceleration. As depicted in Figure 4C, all data points (colored symbols) representing such a relationship for vertical saccade in patients with PSP fell below the normative values (Fig. 4, black symbols). Figure 4D illustrates the dependence of peak acceleration on horizontal saccade amplitude. All colored symbols depicting horizontal saccades in patients with PSP fell below the normative value (Fig. 4, black symbols). Figure 4E,F illustrates the relationship of peak deceleration with vertical and horizontal saccade amplitude, respectively. In both comparisons, the data points depicting saccades from patients with PSP fell below the normative values illustrated by black data points. These results depict a reduction in the saccade acceleration and deceleration in horizontal and vertical axes.
Discussion
Slowing and curved trajectories are known features of saccades in PSP.6, 14, 18 We discovered that PSP saccades are not only slow and curved, but they are often interrupted and have irregular trajectories. We also found that kinematic properties of abnormal saccades do not correlate with the duration or severity of PSP. The latter discovery challenged predictions practiced in conventional clinical rating scales for PSP. Various phenomena can explain the abnormal features of visually guided saccades in PSP. We hypothesize that saccade slowing in PSP is due to the involvement of IBNs and EBNs, and it also affects the burst generator feedback system involving the superior colliculus. We predict a maladaptive firing in superior colliculus activity, leading to an abnormally directed saccade trajectory. It is also possible that, with further progression of the disease, there is involvement of the OPNs, leading to further impairment in saccade generation. We observed an overlap between all 3 phenomena in our patients with PSP.
Impaired Function of Burst Generators in PSP
Rapid shifts in eye movements rely on abrupt increases in the excitability of the burst neuron due to sudden cessation of inhibition, i.e., the postinhibitory rebound (PIR).22, 26, 27, 28, 29 Two sources of sustained inhibitory influence are modulated through glycinergic inputs. The sustained activity of OPNs, 1 of the inhibitory sources, ceases at the time of desired saccade onset, leading to an abrupt increase in EBN firing due to PIR.22, 26, 27, 28, 29 Simultaneously, the activity of ipsilateral IBNs inhibits contralateral EBNs, hence preventing the activation of antagonistic eye muscles. However, malfunction of OPNs is less likely to cause saccade slowing, because it equally affects horizontal and vertical saccades, whereas patients with PSP have prominent involvement of vertical saccades. Here, we investigated whether impaired inhibition through the IBNs or decreased excitation of EBNs caused slowing of the saccade.
To test the influence of OPNs, IBNs, and EBNs in the makings of slow and irregular saccades, we simulated a conductance‐based, single‐compartment neuromimetic model of the burst generators within a local feedback loop model of saccades27 and compared the outcome with data from our patients with PSP. The yellow box in Figure 5 (top) depicts the architecture of the conductance‐based model of saccade generation. The technical details of this model were previously published.27, 28
Figure 5.
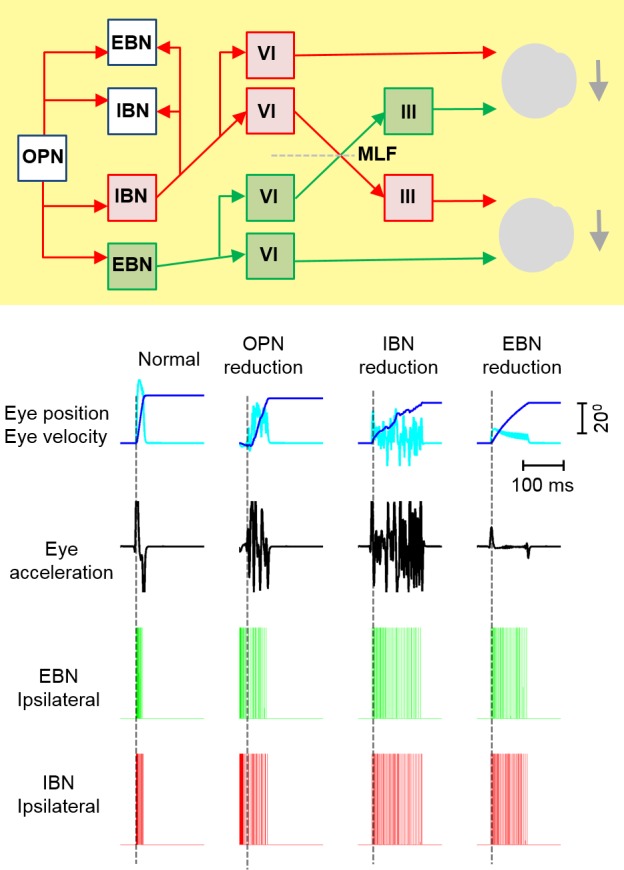
(Top) Architecture of the neuromimetic model for saccade generation (yellow box). Each unit, labeled EBN (excitatory burst neuron) and IBN (inhibitory burst neuron), is comprised of membrane kinematics, as reported by Miura and Optican 200627 and Shaikh et al., 2007.28 The red arrows depicts inhibitory (glycinergic) connections, and the green arrow indicates excitatory connections. OPN indicates omnipause neuron; VI, abducens nucleus; III, oculomotor nucleus; MLF, medial longitudinal fasciculus. (Bottom) A simulation of the model in normal individuals is depicted in the column headed “Normal.” Dark‐blue lines indicate a simulated saccade position, light‐blue lines indicate saccade velocity, black traces depict eye acceleration, green lines are spikes of ipsilateral EBNs, and red spikes are ipsilateral IBNs. Dashed vertical lines depict the onset of saccade. It is normal for EBNs and IBNs to be inactive and to abruptly burst at the onset of saccade. The second column, “OPN reduction,” depicts the slowing of saccade velocity due to reducing OPN activity in the model. In addition, there is lack of abrupt change in EBN or IBN discharge. The third column, “IBM reduction,” depicts the model simulation of slow and decreased acceleration of a saccade when IBN activity is reduced in the model. Saccades also have an irregular trajectory (dark‐blue line). The neural spike rate of IBNs and EBNs abruptly increases, but there is much less dense spike activity, suggesting weak neural burst. Such abrupt firing but weak burst is also present when the EBN gain was reduced (EBN reduction), resultant simulated saccades were slow, and it had reduced acceleration, but the trajectory was not irregular.
The normal membrane properties and profile of ion channel expression in the model simulated a normal saccade, as depicted in the first column from the left in Figure 5, bottom. Dark‐blue and light‐blue traces in the first row in Figure 5 (bottom) depict normal eye position and eye velocity, respectively; whereas black traces in the second row depict the biphasic acceleration and deceleration profile during simulated saccades. Such kinematic properties of a normal saccade are supported by temporally precise activation of IBNs and EBNs (Fig. 5, bottom; green and red traces, respectively).
The second column from the left in Figure 5 (bottom) depicts an example of a simulated saccade when inhibition to the burst neurons was reduced (as expected from reduced function of OPNs). The simulated saccade was irregular (Fig. 5, bottom; dark‐blue and light‐blue traces), and there was a reduction in peak velocity (light‐blue trace), peak acceleration (black trace), and maximal deceleration (black trace). Disruption of the firing synchrony and absence of an abrupt change in the firing rate suggest a lack of PIR. Simulated features matched the characteristics of saccades in PSP. As previously suggested, the OPN‐mediated mechanism of a slow saccade is less likely, because the pause neurons equally affected horizontal and vertical saccades.10 We also observed more substantial impairment of vertical saccade generation.
The third column from the left in Figure 5 (bottom) illustrates an example of saccade generated when the inhibitory influence of IBNs was reduced. The resultant saccade had reduced peak velocity (Fig. 5, bottom; light‐blue trace) and irregular trajectory (dark‐blue trace), but there was no reduction in peak acceleration or deceleration (black traces). There was an abrupt increase in neural firing, but the density of neural discharge was reduced, suggesting weak PIR. The features of such simulated saccades are comparable to those of visually guided saccades in PSP, with the exception of a lack of change in peak acceleration.
The fourth column from the left in Figure 5 (bottom) depicts an example of simulated saccade when the EBN gain was reduced. The resultant saccade was slow, with reduced acceleration and deceleration; however, unlike PSP, the trajectory was regular. The simulated neural response had PIR, but it was weak, as suggested by the lack of burst in neural spikes.
These simulations suggest that prominently slow and irregular vertical saccades are consistent with combined involvement of the EBNs and IBNs. The deficits are not caused by OPN involvement, at least early in the disease course.10 However, progressive OPN degeneration in advanced disease will have a synergetic effect on saccade slowing. These predictions of our analysis of saccades and the neuromimetic model are consistent with previous histopathologic reports.9, 20 A few investigations have documented the involvement of mesencephalic reticular formation in PSP.4, 6, 7, 19, 30 Hence, greater slowing of vertical saccades in PSP is likely to be consistent with decreased function of EBNs and IBNs in the rostral interstitial MLF; however, as disease progresses, there is additional involvement of the pause neurons, causing a further deficit in horizontal burst neurons and enhancing the slowness of vertical saccades. Indeed, a study measuring cell counts from the raphe interpositus (the anatomic location of OPNs) revealed evidence of neuronal loss.20
We also suggest that reversal in saccade direction can be induced by poorly synchronized IBNs and inadequate inhibition imposed by these neurons. The latter phenomenon can lead to weak PIR and the emergence of oscillations. Although such oscillations are observed in patients, they are much less robust compared with the model simulation. We anticipate 2 potential causes for this finding: (1) The model is simulated with 1 set of neurons; whereas, in a real biologic system (such as in patients with PSP), the response is further shaped by an ensemble firing response from a group of neurons; and this activity is further smoothened by elastic plant dynamics, hence causing robustness of the oscillations. (2) When reduced activity of IBNs is combined with that of EBNs and OPNs, the latter as part of broader degenerative involvement, we would expect less robust oscillations but pronounced slowing.
Superior Colliculus Activation and Maladaptive Changes in Saccade Trajectory
Hypothetically, directional changes in the saccade trajectory can be described by stimulation of the superior colliculus fixation zone during the ongoing saccade.31, 32, 33, 34 Our findings resembled the saccades that were interrupted in primates by selective stimulation of the fixation zone of the superior colliculus.31 The stimulation of the rostral pole of the superior colliculus in nonhuman primates causes significant deviation in the trajectory of the ongoing saccade. The random trajectory of the redirected saccades in patients with PSP leads to curvature of the ongoing vertical saccade and suggests premature activation of the fixation zone of the superior colliculus in these patients. One hypothesis is that the IBNs responsible for the vertical saccades are the first affected in PSP. Because of the degenerative insult, the burst neurons discharge spontaneously and out of synchronization (Fig. 5, simulations). Such a discharge pattern of the IBNs during the ongoing saccade is not only inferred as a signal to pause the OPNs, but it is also forwarded in the feedback circuit to the superior colliculus.35 The consequence is undesired activation of the collicular fixation zone, leading to redirected saccades in an arbitrary direction. Such a maladaptive process involving the circuit of abnormally firing IBNs and maladaptively compensating superior colliculus results in slow, interrupted, and curved saccades in PSP.
We emphasize that the saccades in PSP are interrupted but not hypometric. There is a fundamental difference between hypometria and interrupted saccades. Inappropriate scaling of saccades, often a result of cerebellar deficits, causes hypometria. On the contrary, we propose that interruptions in patients with PSP are caused by abnormal function of the pontine‐mesencephalic saccade generation network and a subsequent maladaptive process at the level of the tectum.
We used a corneal curvature tracker, which measures the position of both eyes and yields the conjugate eye position vector. Therefore, it can be speculated that the observed irregularity in eye position during saccade is the product of disconjugacy between 2 eyes. This possibility is unlikely for 2 compelling reasons: (1) saccades in PSP are not disconjugate,10, 11, 14, 15, 36, 37 and (2) the previous studies using gold‐standard, search‐coil techniques and measuring the position of only 1 eye also revealed such irregularities in vertical saccade trajectories (see Fig. 1A,B,D in Schneider et al.15 and Fig. 1 in Bhidayasiri et al.10). Those previous studies were aimed at entirely different experimental questions and did not mention or analyze irregularities or curvatures in the saccade trajectory. We conclude that curvatures and irregularities are the physiologic phenomenon.
In summary, our results suggest that slow eye movements in PSP are not an exclusive consequence of the decreased saccadic velocity but also are caused by the curved and irregular trajectory and interruptions in ongoing saccade. We also observed that saccade curvature was not merely due to mismatch in the velocity of horizontal and vertical saccade components (horizontal being faster) but also to the aberrant activation of saccades in a random trajectory after an interruption. We further propose that irregularities and slowing could be caused by impaired function of EBNs and IBNs; whereas impaired function and lack of IBNs’ timing leads to maladaptive process involving premature activation of the superior colliculus.
Author Roles: 1. Research Project: A. Conception, B. Organization, C. Execution; 2. Statistical Analysis: A. Design, B. Execution, C. Review and Critique; 3. Manuscript Preparation: A. Writing the First Draft, B. Review and Critique.
A.G.S.: 1A, 2B, 2C, 3A, 3B
S.A.F.: 1B, 3B
J.L.J.: 1B, 3B
Disclosures
Ethical Compliance Statement: We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this work is consistent with those guidelines.
Funding Sources and Conflict of Interest: Aasef G. Shaikh was supported by a Dystonia Medical Research Foundation Clinical Fellowship Award and a Dystonia Coalition/Dystonia Medical Research Foundation Career Development Award (NIH U54 TR001456). Stewart A. Factor reports honoraria from Neurocrine, Lundbeck, Auspex/Teva, Avanir, Cynapsus Therapeutics, Adamas, and UCB, grants from Ipsen, Medtronics, Teva, US World Meds, Pharm‐Olam, Cynapsus Therapeutics, Solstice, Vaccinex, the CHDI Foundation, the Michael J. Fox Foundation, and the National Institutes of Health; and royalties from Demos, Blackwell Futura (for textbooks), and UpToDate. Jorge L. Juncos reports support from the National Institutes of Health, the National Institute of Child Health and Development, the Michael J. Fox Foundation, Adamas, and World Meds and support for research through Emory University.
Financial Disclosures for the previous 12 months: The authors report no sources of funding and no conflicts of interest.
Supporting information
Table S1. Summary of results.
Relevant disclosures and conflicts of interest are listed at the end of this article.
Supporting information may be found in the online version of this article
References
- 1. Armstrong RA, Cairns NJ. Spatial patterns of the tau pathology in progressive supranuclear palsy. Neurol Sci 2013;34:337–344. [DOI] [PubMed] [Google Scholar]
- 2. Cervos‐Navarro J, Schumacher K. Neurofibrillary pathology in progressive supranuclear palsy (PSP). J Neural Transm Suppl 1994;42: 153–164. [DOI] [PubMed] [Google Scholar]
- 3. Dickson DW, Rademakers R, Hutton ML. Progressive supranuclear palsy: pathology and genetics. Brain Pathol 2007;17:74–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Morris HR, Wood NW, Lees AJ. Progressive supranuclear palsy (Steele‐Richardson‐Olszewski disease). Postgrad Med J 1999;75:579–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Probst A, Langui D, Lautenschlager C, Ulrich J, Brion JP, Anderton BH. Progressive supranuclear palsy: extensive neuropil threads in addition to neurofibrillary tangles. Very similar antigenicity of subcortical neuronal pathology in progressive supranuclear palsy and Alzheimer's disease. Acta Neuropathol 1988;77:61–68. [DOI] [PubMed] [Google Scholar]
- 6. Litvan I, Agid Y, Calne D, et al. Clinical research criteria for the diagnosis of progressive supranuclear palsy (Steele‐Richardson‐Olszewski syndrome): report of the NINDS‐SPSP international workshop. Neurology 1996;47:1–9. [DOI] [PubMed] [Google Scholar]
- 7. Litvan I, Dickson DW, Buttner‐Ennever JA, et al. Research goals in progressive supranuclear palsy. First International Brainstorming Conference on PSP. Mov Disord 2000;15:446–458. [PubMed] [Google Scholar]
- 8. Halliday GM, Hardman CD, Cordato NJ, Hely MA, Morris JG. A role for the substantia nigra pars reticulata in the gaze palsy of progressive supranuclear palsy. Brain 2000;123(Pt 4):724–732. [DOI] [PubMed] [Google Scholar]
- 9. Juncos JL, Hirsch EC, Malessa S, Duyckaerts C, Hersh LB, Agid Y. Mesencephalic cholinergic nuclei in progressive supranuclear palsy. Neurology 1991;41:25–30. [DOI] [PubMed] [Google Scholar]
- 10. Bhidayasiri R, Riley DE, Somers JT, Lerner AJ, Buttner‐Ennever JA, Leigh RJ. Pathophysiology of slow vertical saccades in progressive supranuclear palsy. Neurology 2001;57:2070–2077. [DOI] [PubMed] [Google Scholar]
- 11. Hardwick A, Rucker JC, Cohen ML, et al. Evolution of oculomotor and clinical findings in autopsy‐proven Richardson syndrome. Neurology 2009;73:2122–2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Joshi AC, Riley DE, Mustari MJ, Cohen ML, Leigh RJ. Selective defects of visual tracking in progressive supranuclear palsy (PSP): implications for mechanisms of motion vision. Vision Res 2010;50:761–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kuniyoshi S, Riley DE, Zee DS, Reich SG, Whitney C, Leigh RJ. Distinguishing progressive supranuclear palsy from other forms of Parkinson's disease: evaluation of new signs. Ann N Y Acad Sci 2002;956:484–486. [DOI] [PubMed] [Google Scholar]
- 14. Rottach KG, Riley DE, DiScenna AO, Zivotofsky AZ, Leigh RJ. Dynamic properties of horizontal and vertical eye movements in parkinsonian syndromes. Ann Neurol 1996;39:368–377. [DOI] [PubMed] [Google Scholar]
- 15. Schneider R, Chen AL, King SA, et al. Influence of orbital eye position on vertical saccades in progressive supranuclear palsy. Ann N Y Acad Sci 2011;1233:64–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chen AL, Riley DE, King SA, et al. The disturbance of gaze in progressive supranuclear palsy: implications for pathogenesis [serial online]. Front Neurol 2010;1:147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Otero‐Millan J, Serra A, Leigh RJ, Troncoso XG, Macknik SL, Martinez‐Conde S. Distinctive features of saccadic intrusions and microsaccades in progressive supranuclear palsy. J Neurosci 2011;31:4379–4387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Quinn N. The, “round the houses” sign in progressive supranuclear palsy [letter]. Ann Neurol 1996;40:951. [DOI] [PubMed] [Google Scholar]
- 19. Collins SJ, Ahlskog JE, Parisi JE, Maraganore DM. Progressive supranuclear palsy: neuropathologically based diagnostic clinical criteria. J Neurol Neurosurg Psychiatry 1995;58:167–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Revesz T, Sangha H, Daniel SE. The nucleus raphe interpositus in the Steele‐Richardson‐Olszewski syndrome (progressive supranuclear palsy). Brain 1996;119(Pt 4):1137–1143. [DOI] [PubMed] [Google Scholar]
- 21. Soetedjo R, Kaneko CR, Fuchs AF. Evidence that the superior colliculus participates in the feedback control of saccadic eye movements. J Neurophysiol 2002;87:679–695. [DOI] [PubMed] [Google Scholar]
- 22. Shaikh AG, Wong AL, Optican LM, Miura K, Solomon D, Zee DS. Sustained eye closure slows saccades. Vision Res 2010;50:1665–1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ghasia FF, Shaikh AG, Jacobs J, Walker MF. Cross‐coupled eye movement supports neural origin of pattern strabismus. Invest Ophthalmol Vis Sci 2015;56:2855–2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ghasia FF, Wilmot G, Ahmed A, Shaikh AG. Strabismus and micro‐opsoclonus in Machado‐Joseph disease. Cerebellum 2016;15:491–497. [DOI] [PubMed] [Google Scholar]
- 25. Shaikh AG, Ghasia FF. Misdirected horizontal saccades in pan‐cerebellar atrophy. J Neurol Sci 2015;355(1–2):125–130. [DOI] [PubMed] [Google Scholar]
- 26. Enderle JD, Engelken EJ. Simulation of oculomotor post‐inhibitory rebound burst firing using a Hodgkin‐Huxley model of a neuron. Biomed Sci Instrum 1995;31:53–58. [PubMed] [Google Scholar]
- 27. Miura K, Optican LM. Membrane channel properties of premotor excitatory burst neurons may underlie saccade slowing after lesions of omnipause neurons. J Comput Neurosci 2006;20:25–41. [DOI] [PubMed] [Google Scholar]
- 28. Shaikh AG, Miura K, Optican LM, Ramat S, Leigh RJ, Zee DS. A new familial disease of saccadic oscillations and limb tremor provides clues to mechanisms of common tremor disorders. Brain 2007;130(Pt 11):3020–3031. [DOI] [PubMed] [Google Scholar]
- 29. Shaikh AG, Ramat S, Optican LM, Miura K, Leigh RJ, Zee DS. Saccadic burst cell membrane dysfunction is responsible for saccadic oscillations. J Neuroophthalmol 2008;28:329–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Steele JC, Richardson JC, Olszewski J. Progressive supranuclear palsy. A heterogeneous degeneration involving the brain stem, basal ganglia and cerebellum with vertical gaze and pseudobulbar palsy, nuchal dystonia and dementia. Arch Neurol 1964;10:333–359. [DOI] [PubMed] [Google Scholar]
- 31. Gandhi NJ, Keller EL. Comparison of saccades perturbed by stimulation of the rostral superior colliculus, the caudal superior colliculus, and the omnipause neuron region. J Neurophysiol 1999;82:3236–3253. [DOI] [PubMed] [Google Scholar]
- 32. Munoz DP, Waitzman DM, Wurtz RH. Activity of neurons in monkey superior colliculus during interrupted saccades. J Neurophysiol 1996;75:2562–2580. [DOI] [PubMed] [Google Scholar]
- 33. Munoz DP, Wurtz RH. Fixation cells in monkey superior colliculus. II. Reversible activation and deactivation. J Neurophysiol 1993;70:576–589. [DOI] [PubMed] [Google Scholar]
- 34. Munoz DP, Wurtz RH. Fixation cells in monkey superior colliculus. I. Characteristics of cell discharge. J Neurophysiol 1993;70:559–575. [DOI] [PubMed] [Google Scholar]
- 35. Waitzman DM, Ma TP, Optican LM, Wurtz RH. Superior colliculus neurons mediate the dynamic characteristics of saccades. J Neurophysiol 1991;66:1716–1737. [DOI] [PubMed] [Google Scholar]
- 36. Otero‐Millan J, Schneider R, Leigh RJ, Macknik SL, Martinez‐Conde S. Saccades during attempted fixation in parkinsonian disorders and recessive ataxia: from microsaccades to square‐wave jerks [serial online]. PLoS ONE 2013;8:e58535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Garbutt S, Riley DE, Kumar AN, Han Y, Harwood MR, Leigh RJ. Abnormalities of optokinetic nystagmus in progressive supranuclear palsy. J Neurol Neurosurg Psychiatry 2004;75:1386–1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Summary of results.


