Abstract
Purpose
Cancer survivors are at greater risk of comorbidities and functional decline due to physiological and psychological stress which can be measured by salivary cortisol. If saliva is used, multiple samples must be collected to accurately quantify long-term stress; however, fingernail (FN) and toenail (TN) clippings offer an opportunity to measure retrospective cortisol levels in a non-invasive manner.
Methods
Three sets of FN and TN clippings were collected at 12-month intervals in conjunction with saliva samples from cancer survivors (n=109) participating in two clinical trials. FN and TN samples were stored at room temperature (RT); a subset underwent additional processing and freezing before analysis. Cortisol levels were determined via enzyme immunoassay, and correlation coefficients were generated to determine overall correspondence of the individual measures.
Results
Matched RT and frozen samples were highly correlated for TN (r=0.95, p=5.44x10−37) and FN (r=0.784, p= 1.05x10−10). Correlations between RT FN and TN were statistically significant (r=0.621, p= 3.61x10−17), as were frozen FN and TN (r=0.310, p=0.0283). RT, but not frozen TN and FN correlated with salivary cortisol (r=0.580, p= 1.65x10−16 and r=0.287, p= 0.00042 for TN and FN, respectively).
Conclusions
FN and TN cortisol levels correlate with salivary cortisol in adult cancer survivors and may offer a less invasive and convenient means for measuring chronic cortisol levels.
Keywords: cancer survivors, cortisol, stress, neoplasms, biomarker
Introduction
Cancer survivors are at increased risk of chronic diseases, functional decline, and psychological disability compared to adults without a history of cancer (1, 2). Fear of recurrence and post-traumatic stress from diagnosis and treatment are prevalent in cancer survivors and have physiological sequelae resulting from chronic stress (3–7). Psychological and physiological stressors induce the production of cortisol, which is a glucocorticoid hormone that helps regulate several biological processes. Importantly, elevated and dysregulated cortisol levels have been associated with increased mortality in survivors of multiple cancer types (8–14).
Cortisol is measured in sera, plasma, urine and saliva; however, these measures are subject to daily fluctuations and only reflect cortisol levels relative to the minutes to hours prior to collection (15). Measuring long-term cortisol entails collecting multiple samples over periods of interest, which increases participant burden and can lead to non-adherence (16). These limitations have prompted the exploration of measuring cortisol levels in other tissues with potential for less rapid turnover, such as the hair (17, 18) and nails (19). Hair cortisol is a reliable measure (17, 18); however, collection can be unacceptable for cultural reasons (18), may be tainted by hair treatments (e.g., bleaching or coloring of hair) (20), and has shown mixed correlations to urine (21) and salivary samples (22). Additionally, the collection of hair samples may be difficult in populations that experience hair loss or baldness, i.e., cancer patients treated with chemotherapy or older adults (23, 24).
Cortisol levels have been evaluated in nails of other mammals (25, 26) and only recently in humans (27). Though growth rates of fingernails (FN) and toenails (TN) differ between individuals, it is estimated that FN grow at 3mm/month and TN at 1mm/month (28). Recent studies suggest FN clippings represent accumulation of cortisol over a 4- to 5-month period (19, 29). Therefore, extracting cortisol from FN and TN clippings may provide a longer-term and more chronic measure of stress, with minimal invasiveness, convenient storage methods with minimal sample degradation, and may be more acceptable than collecting hair samples. Because cancer survivors may experience greater levels of post-treatment stress, this population provides a unique opportunity through which highly varied levels of cortisol could be anticipated (30).
We sought to examine the relationship between nail and salivary cortisol in cancer survivors participating in vegetable gardening interventions over an extended time period of at least 12 months (31). We hypothesized that FN and TN cortisol concentrations would correlate with each other, as well as with salivary cortisol collected at corresponding time points. Additionally, we sought to explore the effects of storage and processing methods on nail cortisol levels.
MATERIALS AND METHODS
Participants
Adult cancer survivors participating in one of two similarly designed vegetable gardening trials provided written informed consent and agreed to the collection of their TN, FN, and saliva samples at all study visit time points (31). Briefly, both studies utilized a waitlist control design to test the feasibility of one-year home-based vegetable gardening interventions in the greater Birmingham, AL area (n=66) and throughout the state of Alabama (n=43). The larger study was comprised of female breast cancer survivors and the smaller study was comprised of female and male survivors of several cancer types. Data and biospecimens were collected at baseline, 12- and 24-months for the larger study, and at baseline and 12-months for the smaller study.
Sample collection and preparation
Biological specimens were obtained from study participants during annual in-home assessments. Prior to the visit, the participant was instructed not to consume alcohol, caffeine, or nicotine for 12 hours and not to brush their teeth within 45 minutes of saliva collection. 74.9% of assessments and saliva collection occurred between 8 am and 2 pm. At the time of the visit, the participant rinsed their mouth with water 10 minutes prior to saliva collection. Saliva was collected using SalivaBio Oral Swab kits (Salimetrics®, Carlsbad, CA, USA). An oral swab was removed from sealed packaging, placed under the participant’s tongue for 2 minutes, then placed directly into a storage tube, and kept at 4°C until final storage at −80°C.
Participants were instructed to clip FN and TN into pre-labeled bags prior to the assessment. Approximately 1–3 mm of the unattached anterior portion of the nails was clipped using nail clippers. At the 12- and 24-month time points of the larger study, each nail sample was divided into two portions: one for room temperature (RT) storage, and the other for frozen storage (19, 32). Nail samples for frozen storage were vortex-washed twice with 5 ml of isopropanol for one minute. Isopropanol was discarded, and the nails were dried overnight. On the second day, dried nails were stored at −80°C until analysis. RT nail samples were stored in a filing cabinet after collection and held until further analysis. Approximately 20 mg of nails were used for cortisol analysis. All samples were collected from January 2014 to April 2016. Splitting and subsequent freezing of samples began January 2015 and continued for the duration. All samples were analyzed February 2017, hence storage lasted 10–37 months.
Nail processing
For cortisol analysis, FN and TN were processed using a slightly modified procedure as described by Warnock et al. and Meyer et al. (19, 32). All frozen and RT nail samples were washed twice with 2 ml isopropanol and dried overnight. The dried nail samples were added to a preweighed tube containing three 5 mm steel grinding balls (Retsch, Haan, Germany) and ground using a TissueLyser II (Qiagen Venlo, Netherlands) at 30 Hz for 9 min. Methanol (1 ml) was added per 50 mg of powdered nail (w/v) and placed on a rotator for 18 hours at RT to extract the cortisol. Samples were then centrifuged at 10,000 × g for 5 minutes. The supernatant (800 μl/50 mg) was transferred to a clean tube and evaporated under nitrogen gas in a certified fume hood. The evaporated sample was re-suspended in 400 μl of phosphate buffered saline (pH 7.4) per 50 mg of ground sample (w/v).
Cortisol Assays
Storage tubes for saliva were thawed and centrifuged prior to cortisol assay. Cortisol was analyzed using 25 μl of saliva or nail extracts according to the manufacturer's protocol (Salimetrics®, Salivary Cortisol Enzyme Immunoassay Kit, 1–3002, State College, PA). If cortisol readings were outside the range for the standard curve, the samples were either diluted or concentrated and rerun using the same 25 μl volume and within the standard curve. The inter-assay coefficient of variation was 4.7% for the high standard, 14% for the low standard, <12% for control biological replicates (n = 5), and within the manufacturers recommendations. Data were analyzed using StatLIA Enterprise 2.2 software, and are represented as nmol/gram for FN and TN and μg/dL for salivary cortisol levels.
Demographics, diagnoses, and anthropometrics
Participant demographics, cancer type and diagnosis date were obtained from self-report. Height and weight were measured during the in-home assessments using standard procedures; BMI was derived as kg/m2.
Statistical Analysis
Data were analyzed using SPSS Statistics Version 24.0 (IBM Corp, Armonk, NY, USA). Descriptive statistics were obtained, and Pearson correlations were used to determine relationships between cortisol measures at each time point. RT samples were correlated controlling for time point (frozen samples were not processed at all time points and excluded from this analysis). Cortisol data also were log-transformed and analyzed for bivariate and intraclass correlation. Chi-square tests were used to explore differences in high vs. low cortisol levels (high and low quartiles) for the same demographic and anthropometric groups. Additionally, relationships between race, BMI, age, and cancer type were explored using analysis of variance.
Results
One hundred nine cancer survivors provided biological samples over the course of the two-year period (Table 1). Participants were primarily female and had been diagnosed with and treated previously for breast cancer. Mean age of participants at baseline was 64 years, and average BMI was 29.7 kg/m2. Participants were 5.9 ± 6.2 years post-diagnosis at baseline. Participants in the larger study population were younger (age 60.2 ± 11.1 years vs. 70.3 ± 8.0 years), more racially diverse (27% black vs 2% black), and only female survivors of breast cancer; 31% of participants in the smaller study were male. Years since diagnosis and BMI did not differ between groups.
Table 1.
Baseline characteristics of study participants (N=109)
| n (%) | |
|---|---|
| Age (mean, SD) | 64.1, 9.7 |
| 40–49 | 7 (6%) |
| 50–59 | 21 (19%) |
| 60–69 | 54 (50%) |
| 70+ | 27 (25%) |
| Body Mass Index (mean, SD) | 29.2 (5.8) |
| Normal weight | 26 (24%) |
| Overweight | 40 (37%) |
| Obese | 43 (39%) |
| Sex | |
| Female | 95 (87%) |
| Male | 14 (13%) |
| Race | |
| Non-Hispanic Black | 16 (15%) |
| Non-Hispanic White | 93 (85%) |
| Comorbidities (Mean, SD) | 2.3 (1.1) |
| 0–1 | 26 (24%) |
| 2–3 | 35 (32%) |
| 4–5 | 22 (20%) |
| 6 + | 20 (18%) |
| Cancer Type | |
| Breast | 90 (83%) |
| Prostate | 5 (5%) |
| Others* | 14 (12%) |
| Cancer Treatment Type | |
| Chemotherapy | 61 (56%) |
| Hormone Therapy | 47 (43% |
| Surgery | 101 (93% |
| Radiation | 62 (57%) |
| Years since diagnosis (mean, SD) | 6.2 (6.3) |
| < 2 years | 21 (19%) |
| 2 < 4 years | 27 (25%) |
| 4 < 6 years | 21 (19%) |
| 6 + years | 26 (24%) |
| unknown | 14 (13%) |
Other cancers include bladder, colon, kidney, lung, lymphoma, multiple myeloma, pancreatic, parotid, thyroid, and tongue
Ninety-six TN samples and 89 FN samples were submitted that were divided for both frozen and RT processing. 85 participants provided both TN and FN samples adequate for divided processing. Of the frozen samples, 13 TN (13.5%) and 29 FN (32.6%) had insufficient mass (<10 mg) for analysis; of the RT samples, 14 TN (14.6%) and 19 FN (21.3%) had insufficient mass for analysis.
A total of 143 TN and 143 FN samples were submitted by study participants that were not divided for frozen and RT storage; a total of 13 TN and 18 FN were not submitted by participants (refusal due to short nails or fear of inducing injury). Of those submitted, 14 TN (9.8%) and 28 FN (19.6%) had insufficient mass for processing. Additionally, samples with nail polish that could not be removed resulted in implausibly high cortisol levels that were greater than two standard deviations from the mean and were not included in analyses (n=12).
Mean cortisol levels for frozen samples were 0.0152 ± 0.0394 nmol/g (median 0.0049, 10th–90th percentile 0.0019–0.0250 nmol/g) and 0.0290 ± 0.0624 nmol/g (median 0.0084, 10th–90th percentile 0.0021–0.00622 nmol/g) for TN (n=79) and FN (n=55), respectively. RT TN (n=200) and FN (n=174) cortisol levels were 0.0607 ± 0.3004 nmol/g (median 0.0062, 10th–90th percentile 0.0013–0.0916 nmol/g) and 0.0870 ± 0.3319 nmol/g (median 0.0097, 10th–90th percentile 0.0037–0.1196 nmol/g), respectively. Differences between RT and frozen samples were not significant (paired sample t-tests, p=0.152 and p=0.744 for TN and FN, respectively). Mean salivary cortisol (n=226) was 0.2894 ± 0.5683 μg/dL (median 0.1630, 10th–90th percentile 0.0589–0.4633 μg/dL) for all samples.
Correlations for all samples from all time points are reported in Table 2. Matched RT and frozen samples were highly correlated for TN (r=0.950) and FN (r=0.784). RT TN and RT FN samples were significantly correlated (r=0.621), as were frozen TN and frozen FN samples (r=0.310). RT, but not frozen TN and FN samples correlated with salivary cortisol (r=0.580 and r=0.287 for RT TN and FN, respectively). Data for each time point indicate concordance for saliva, TN and FN samples, with correlations of RT TN and saliva having the largest statistical significance (Table 2).
Table 2.
Correlates of cortisol measures in cancer survivors
| All time points | ||||
|---|---|---|---|---|
| FN Frozen | TN Room Temp | FN Room Temp | Saliva | |
| TN Frozen | 0.310 | 0.95 | 0.736 | −0.004 |
| p | 0.02827 | 5.44E-37 | 1.05E-10 | 0.97656 |
| n | 50 | 72 | 56 | 67 |
| FN Frozen | - | 0.352 | 0.784 | −0.061 |
| p | 0.01299 | 6.29E-12 | 0.68503 | |
| n | 49 | 52 | 46 | |
| TN Room Temp | - | 0.621 | 0.580 | |
| p | 3.61E-17 | 1.65E-16 | ||
| n | 148 | 168 | ||
| FN Room Temp | - | 0.287 | ||
| p | 0.00042 | |||
| n | 147 | |||
| Baseline | ||||
| FN Room Temp | Saliva | |||
| TN Room Temp | 0.801 | 0.557 | ||
| p | 4.60E-14 | 3.85E-06 | ||
| n | 58 | 60 | ||
| FN Room Temp | - | 0.445 | ||
| p | 0.001 | |||
| n | 51 | |||
| 12 months | ||||
| FN | TN Room Temp | TN Room Temp | Saliva | |
| Frozen | ||||
| TN Frozen | 0.676 | 0.820 | 0.321 | 0.120 |
| p | 0.0001 | 5.31E-10 | 0.078 | 0.485 |
| n | 27 | 37 | 31 | 36 |
| FN Frozen | - | 0.844 | 0.599 | −0.015 |
| p | 3.16E-08 | 4.70E-04 | 0.941 | |
| n | 27 | 30 | 25 | |
| TN Room Temp | - | −0.013 | 0.677 | |
| p | 0.92 | 1.65E-10 | ||
| n | 61 | 69 | ||
| FN Room Temp | - | 0.288 | ||
| p | 0.02 | |||
| n | 65 | |||
| 24 months | ||||
| FN | TN Room Temp | FN Room Temp | Saliva | |
| Frozen | ||||
| TN Frozen | 0.264 | 0.956 | 0.750 | −0.021 |
| p | 0.203 | 1.21E-19 | 7.91E-07 | 0.903 |
| n | 25 | 36 | 32 | 35 |
| FN Frozen | - | 0.303 | 0.798 | −0.081 |
| p | 0.16 | 1.74E-06 | 0.707 | |
| n | 23 | 25 | 24 | |
| TN Room Temp | - | 0.791 | −0.003 | |
| p | 6.99E-08 | 0.983 | ||
| n | 32 | 40 | ||
| FN Room Temp | - | 0.004 | ||
| p | 0.982 | |||
| n | 35 | |||
TN, toenail; FN, fingernail
Partial correlations for RT samples are included in Supplementary Table 1. Associations between all samples were attenuated when controlling for time of collection, with only TN and FN remaining significantly correlated. Log-transformation of RT data only slightly decreased significant associations at each time point when compared to non-transformed data (Supplementary Table 2). Intraclass correlation coefficients for the RT and salivary cortisol measures indicate high reliability for TN and FN, and only moderate reliability for salivary cortisol.(33)
Post-hoc analyses indicate cortisol levels did not differ between race, gender, age group, years since diagnosis, or BMI category. Because TN cortisol measures had the greatest standard deviation, the highest and lowest quartiles of baseline TN cortisol were compared and no age or BMI differences were found.
Discussion
To our knowledge, this is the first study in cancer survivors, to report a positive and significant correlation between TN, FN and salivary cortisol levels. Additionally, we found that compared to RT storage, washing and freezing samples did not significantly alter cortisol levels within nail samples. However, RT nail samples were more strongly correlated with salivary cortisol, and TN samples were most reliably associated with salivary cortisol across time points.
Collecting sufficient samples, however, was problematic in this study. For samples that were not divided into RT and frozen storage analysis, 11.5% of samples were not of sufficient mass for processing. Future studies should provide participants with a visual depicting an adequate sample of clippings as well as give the patients adequate notice to allow sufficient growth of their nails prior to collection.
The subsample of TN and FN that were subsequently frozen resulted in lower cortisol concentrations, though the differences did not reach statistical significance. It is possible that the additional processing (propanol washing and drying) drew off additional amounts of cortisol on the surface of the clippings. Though this cannot be determined from our results, future studies should ensure that all samples receive identical processing, including number of washes.
Correlations at each time point indicated that the associations between nail and salivary cortisol were consistent and reliable. However, the lack of significant associations at the 24 month time point may be due to the decrease in sample size, as only one study collected samples at this time. This may also be due in part to the larger study sample having an age range of 44 years compared to 31 in the smaller study.
Previous reports correlating hair cortisol to age (34) and BMI (35) in general populations were not supported by the results of our study, either because we assessed cortisol in nails (not hair) or because our population of cancer survivors differed from these healthy populations. Additionally, cortisol levels did not differ significantly between time points. This does raise questions as to the utility of nail cortisol as a sensitive indicator of stress in lifestyle interventions. Because the samples were taken at 12-month intervals, this study does not negate the possibility that nail cortisol levels fluctuate throughout the year (36), so further investigation is warranted.
In humans, only three other studies have been published analyzing FN cortisol levels. Most recently, cortisol levels measured in FN, TN and facial hair samples from 19 young men were collected in 3-month intervals from college-age males and significant correlations were observed between FN and TN (r=0.610, p<0.001), with each also correlating with facial hair (27). One pilot study assessed FN cortisol levels in students before taking final exams, and again five months later. Cortisol was slightly higher (p=0.256) in the post-exam samples; however, there was no control group and other biomarkers were not assessed (19). A two-part study investigated the relationships between hair, FN, and salivary cortisol (29). The first part of the study compared FN cortisol compared hair and nail cortisol samples in men (n=58) and found a moderate correlation (r=0.430; p<0.05). The second part compared monthly FN cortisol to a retrospective salivary cortisol value (four samples in one day) and found the strongest correlations at four months (p<0.05) and five months (P<0.01) prior (29). These results suggest that nail cortisol may be both a retrospective or chronic measure of cortisol depending on the depth (transverse area) of the nail sample (See Figure 1).
Figure 1.
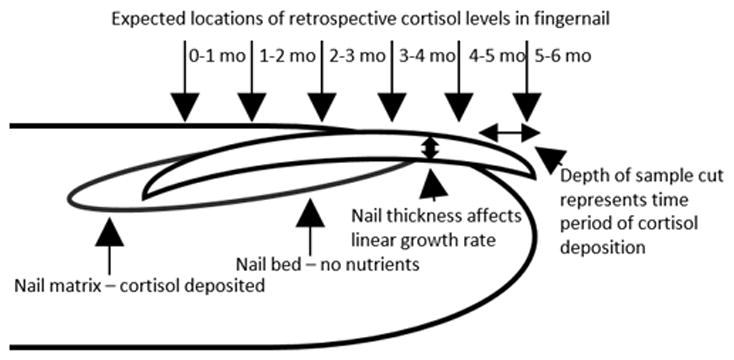
The fingernail grows from the nail matrix, where cortisol and other hormones are deposited into the growing nail. The average growth rate of fingernails is 3mm per month, however the growth rate can vary depending on nail thickness, and overall nail length can vary between individuals. A nail sample that has greater transverse mass, or depth, will represent a more longitudinal cortisol exposure.
Future studies should control for nail growth rate by having participants trim nails at regular intervals (weekly or monthly) and depths to better assess the window of time at which cortisol was deposited from the nail matrix (37). Furthermore, subjective stress measures should be employed at corresponding intervals to allow for correlation of self-reported and physiological markers of stress. Another factor that has been observed is the effect of taxane chemotherapy on nail development (38); this synopsis of case reports indicates nail growth rate may be affected at least intermittently by damage to the nail matrix and epithelium. While some patients experience long-term nail abnormalities, the rate and persistence of nail perturbance due to taxane treatment has not been determined. Nonetheless, type of chemotherapy should be obtained when studying biological specimens in this population.
One limitation of this study was the loss of adequate sample mass due to our goal to evaluate separate processing/storage techniques, i.e., RT vs. frozen. Additionally, the use of only one salivary cortisol sample at each time point is a major limitation, though most samples were collected after morning cortisol peaks would have subsided. Also, type of chemotherapy was not obtained from participants, limiting our ability to observe effects of different treatments on cortisol levels. Despite these limitations, the collection, processing and analytical methods herein are beneficial to investigators seeking to study cortisol in cancer survivors. Given that FN and TN cortisol values were consistent for participants over time, further investigation is warranted. Nonetheless, the current study is the first to investigate TN cortisol levels and first to report congruence between FN, TN, and salivary cortisol. Nail clippings are a promising specimen for analysis and may offer a means to measure chronic cortisol levels in cancer survivors.
Supplementary Material
Acknowledgments
Funding: National Cancer Institute R21 CA182508, P30 CA13148, and R25 CA047888; UAB Comprehensive Cancer Center (P30AR050948); Center for Clinical Translational Science (UL1TR001417), UAB Diabetes Research Center (P30 DK079626), and American Institute for Cancer Research: Diana Dyer Cancer Survivors’ Nutrition Research Endowment.
Footnotes
The authors declare no potential conflicts of interest.
Compliance with Ethical Standards
Conflict of Interest: The authors declare that they have no conflict of interest Protection of human subjects and informed consent: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Written informed consent was obtained from all individual participants included in the study.
References
- 1.Hewitt M, Rowland JH, Yancik R. Cancer survivors in the United States: age, health, and disability. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2003;58:M82–M91. doi: 10.1093/gerona/58.1.m82. [DOI] [PubMed] [Google Scholar]
- 2.Berry NM, Miller MD, Woodman RJ, et al. Differences in chronic conditions and lifestyle behaviour between people with a history of cancer and matched controls. Med J Aust. 2014;201:96–100. doi: 10.5694/mja13.10701. [DOI] [PubMed] [Google Scholar]
- 3.Simard S, Thewes B, Humphris G, et al. Fear of cancer recurrence in adult cancer survivors: a systematic review of quantitative studies. Journal of cancer survivorship : research and practice. 2013;7:300–22. doi: 10.1007/s11764-013-0272-z. [DOI] [PubMed] [Google Scholar]
- 4.Swartzman S, Booth JN, Munro A, Sani F. Posttraumatic stress disorder after cancer diagnosis in adults: A meta-analysis. Depression and anxiety. 2017;34:327–39. doi: 10.1002/da.22542. [DOI] [PubMed] [Google Scholar]
- 5.Cordova MJ, Riba MB, Spiegel D. Post-traumatic stress disorder and cancer. The Lancet Psychiatry. 2017 doi: 10.1016/S2215-0366(17)30014-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abbey G, Thompson SB, Hickish T, Heathcote D. A meta-analysis of prevalence rates and moderating factors for cancer-related post-traumatic stress disorder. Psycho-Oncology. 2015;24:371–81. doi: 10.1002/pon.3654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Simard S, Savard J. Screening and comorbidity of clinical levels of fear of cancer recurrence. Journal of Cancer Survivorship. 2015;9:481–91. doi: 10.1007/s11764-015-0424-4. [DOI] [PubMed] [Google Scholar]
- 8.Sephton SE, Sapolsky RM, Kraemer HC, Spiegel D. Diurnal cortisol rhythm as a predictor of breast cancer survival. Journal of the National Cancer Institute. 2000;92:994–1000. doi: 10.1093/jnci/92.12.994. [DOI] [PubMed] [Google Scholar]
- 9.Debono M, Bradburn M, Bull M, Harrison B, Ross RJ, Newell-Price J. Cortisol as a marker for increased mortality in patients with incidental adrenocortical adenomas. The Journal of clinical endocrinology and metabolism. 2014;99:4462–70. doi: 10.1210/jc.2014-3007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim HM, Ha KS, Hwang IC, Ahn HY, Youn CH. Random Serum Cortisol as a Predictor for Survival of Terminally Ill Patients With Cancer: A Preliminary Study. The American journal of hospice & palliative care. 2016;33:281–5. doi: 10.1177/1049909114563065. [DOI] [PubMed] [Google Scholar]
- 11.Schrepf A, Thaker PH, Goodheart MJ, et al. Diurnal cortisol and survival in epithelial ovarian cancer. Psychoneuroendocrinology. 2015;53:256–67. doi: 10.1016/j.psyneuen.2015.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sephton SE, Lush E, Dedert EA, et al. Diurnal cortisol rhythm as a predictor of lung cancer survival. Brain, behavior, and immunity. 2013;30(Suppl):S163–70. doi: 10.1016/j.bbi.2012.07.019. [DOI] [PubMed] [Google Scholar]
- 13.Diaz M, Aldridge-Gerry A, Spiegel D. Posttraumatic growth and diurnal cortisol slope among women with metastatic breast cancer. Psychoneuroendocrinology. 2014;44:83–7. doi: 10.1016/j.psyneuen.2014.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cohen L, Cole SW, Sood AK, et al. Depressive symptoms and cortisol rhythmicity predict survival in patients with renal cell carcinoma: role of inflammatory signaling. PloS one. 2012;7:e42324. doi: 10.1371/journal.pone.0042324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Trifonova ST, Gantenbein M, Turner JD, Muller CP. The use of saliva for assessment of cortisol pulsatile secretion by deconvolution analysis. Psychoneuroendocrinology. 2013;38:1090–101. doi: 10.1016/j.psyneuen.2012.10.016. [DOI] [PubMed] [Google Scholar]
- 16.Kudielka BM, Broderick JE, Kirschbaum C. Compliance with saliva sampling protocols: electronic monitoring reveals invalid cortisol daytime profiles in noncompliant subjects. Psychosomatic medicine. 2003;65:313–9. doi: 10.1097/01.psy.0000058374.50240.bf. [DOI] [PubMed] [Google Scholar]
- 17.O'Brien KM, Tronick EZ, Moore CL. Relationship between hair cortisol and perceived chronic stress in a diverse sample. Stress and health : journal of the International Society for the Investigation of Stress. 2013;29:337–44. doi: 10.1002/smi.2475. [DOI] [PubMed] [Google Scholar]
- 18.Russell E, Koren G, Rieder M, Van Uum S. Hair cortisol as a biological marker of chronic stress: current status, future directions and unanswered questions. Psychoneuroendocrinology. 2012;37:589–601. doi: 10.1016/j.psyneuen.2011.09.009. [DOI] [PubMed] [Google Scholar]
- 19.Warnock F, McElwee K, Seo RJ, et al. Measuring cortisol and DHEA in fingernails: a pilot study. Neuropsychiatr Dis Treat. 2010;6:1–7. [PMC free article] [PubMed] [Google Scholar]
- 20.Wennig R. Potential problems with the interpretation of hair analysis results. Forensic science international. 2000;107:5–12. doi: 10.1016/s0379-0738(99)00146-2. [DOI] [PubMed] [Google Scholar]
- 21.Davenport MD, Tiefenbacher S, Lutz CK, Novak MA, Meyer JS. Analysis of endogenous cortisol concentrations in the hair of rhesus macaques. General and comparative endocrinology. 2006;147:255–61. doi: 10.1016/j.ygcen.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 22.Sauve B, Koren G, Walsh G, Tokmakejian S, Van Uum SH. Measurement of cortisol in human hair as a biomarker of systemic exposure. Clinical and investigative medicine. Medecine clinique et experimentale. 2007;30:E183–91. doi: 10.25011/cim.v30i5.2894. [DOI] [PubMed] [Google Scholar]
- 23.Lemieux J, Maunsell E, Provencher L. Chemotherapy-induced alopecia and effects on quality of life among women with breast cancer: a literature review. Psycho-oncology. 2008;17:317. doi: 10.1002/pon.1245. [DOI] [PubMed] [Google Scholar]
- 24.Trüeb RM. Aging of hair. Journal of Cosmetic Dermatology. 2005;4:60–72. doi: 10.1111/j.1473-2165.2005.40203.x. [DOI] [PubMed] [Google Scholar]
- 25.Mack Z, Fokidis HB. A novel method for assessing chronic cortisol concentrations in dogs using the nail as a source. Domestic Animal Endocrinology. 2017;59:53–7. doi: 10.1016/j.domaniend.2016.11.003. [DOI] [PubMed] [Google Scholar]
- 26.Veronesi MC, Comin A, Meloni T, Faustini M, Rota A, Prandi A. Coat and claws as new matrices for noninvasive long-term cortisol assessment in dogs from birth up to 30 days of age. Theriogenology. 2015;84:791–6. doi: 10.1016/j.theriogenology.2015.05.013. [DOI] [PubMed] [Google Scholar]
- 27.Nejad J, Ghaseminezhad M, Sung K, Hoseinzadeh F, Cabibi J. A Cortisol Study; Facial Hair and Nails. J Steroids Horm Sci. 2016;7:2. [Google Scholar]
- 28.de Berker DA, Andre J, Baran R. Nail biology and nail science. International journal of cosmetic science. 2007;29:241–75. doi: 10.1111/j.1467-2494.2007.00372.x. [DOI] [PubMed] [Google Scholar]
- 29.Izawa S, Miki K, Tsuchiya M, et al. Cortisol level measurements in fingernails as a retrospective index of hormone production. Psychoneuroendocrinology. 2015;54:24–30. doi: 10.1016/j.psyneuen.2015.01.015. [DOI] [PubMed] [Google Scholar]
- 30.Zeitzer JM, Nouriani B, Neri E, Spiegel D. Correspondence of Plasma and Salivary Cortisol Patterns in Women with Breast Cancer. Neuroendocrinology. 2014;100:153–61. doi: 10.1159/000367925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cases MG, Frugé AD, De Los Santos JF, et al. Detailed methods of two home-based vegetable gardening intervention trials to improve diet, physical activity, and quality of life in two different populations of cancer survivors. Contemporary Clinical Trials. 2016;50:201–12. doi: 10.1016/j.cct.2016.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Meyer J, Novak M, Hamel A, Rosenberg K. Extraction and analysis of cortisol from human and monkey hair. Journal of visualized experiments: JoVE. 2014 doi: 10.3791/50882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kotsopoulos J, Tworoger SS, Campos H, et al. Reproducibility of Plasma, Red Blood Cell, and Urine Biomarkers among Premenopausal and Postmenopausal Women from the Nurses' Health Studies. Cancer Epidemiology Biomarkers & Prevention. 2010;19:938–46. doi: 10.1158/1055-9965.EPI-09-1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Feller S, Vigl M, Bergmann MM, Boeing H, Kirschbaum C, Stalder T. Predictors of hair cortisol concentrations in older adults. Psychoneuroendocrinology. 2014;39:132–40. doi: 10.1016/j.psyneuen.2013.10.007. [DOI] [PubMed] [Google Scholar]
- 35.Jackson SE, Kirschbaum C, Steptoe A. Hair cortisol and adiposity in a population-based sample of 2,527 men and women aged 54 to 87 years. Obesity. 2017;25:539–44. doi: 10.1002/oby.21733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Persson R, Garde AH, Hansen AM, et al. Seasonal variation in human salivary cortisol concentration. Chronobiology international. 2008;25:923–37. doi: 10.1080/07420520802553648. [DOI] [PubMed] [Google Scholar]
- 37.Gilchrist ML, Buxton LHD. The relation of finger-nail growth to nutritional status. Journal of Anatomy. 1939;73:575–82. [PMC free article] [PubMed] [Google Scholar]
- 38.Minisini AM, Tosti A, Sobrero AF, et al. Taxane-induced nail changes: incidence, clinical presentation and outcome. Annals of Oncology. 2003;14:333–7. doi: 10.1093/annonc/mdg050. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


