Abstract
Ultrasonography of the lumbosacral and sacrococcygeal spine is described in cats to confirm effective distribution of local anesthetics injected in the sacrococcygeal epidural space. Ultrasound was used to identify the structures of the spinal canal, local anesthetic flow, and to measure the distances between skin and ligamentum flavum.
Résumé
Utilisation de l’échographie pour évaluer les injections épidurales sacrococcygiennes chez les chats. L’échographie de la colonne lombo-sacrée et sacrococcygienne est décrite chez des chats afin de confirmer la distribution efficace des anesthésiques locaux injectés dans l’espace épidural sacrococcygien. L’échographie a été utilisée pour identifier les structures du canal rachidien, l’écoulement local des anesthésiques et mesurer les distances entre la peau et le ligament jaune.
(Traduit par Isabelle Vallières)
Introduction
The spinal cord and dural sac of cats terminate at the 1st and 3rd sacral vertebra, respectively. Therefore, compared to dogs, there is a greater risk of medullar lesion or inadvertent subarachnoid injection when performing epidural anesthesia in cats (1). In order to decrease these risks, sacrococcygeal (SC) epidural anesthesia may be an alternative and a safer technique than lumbosacral (LS) epidural anesthesia in cats (2). Anatomical landmarks may be more difficult to identify in cats because of their small size. It is more difficult, therefore, to properly introduce the needle with only palpation and loss of resistance techniques, so SC epidural anesthesia might not be successful in all cases (3).
Bidimensional ultrasonography, however, may provide precise information about sonoanatomy of LS and SC epidural space in cats (4). The hypothesis of this study, therefore, was that when the ultrasound probe is positioned in the LS space in cats, it is possible to confirm SC epidural injection by the flow of local anesthetic, which produces ventral displacement of the dura mater and enlargement of the LS epidural space. The aim of this study was to describe bidimensional ultrasonography of the LS and SC spine in cats and to investigate whether it is possible to confirm the effectiveness of epidural anesthesia by distribution, observed by LS ultrasound, of the flow of local anesthetic into the LS epidural space after SC epidural injection.
Materials and methods
Twenty-three male and 13 female cats (n = 36), of various breeds, were assigned to abdominal or pelvic surgeries, including castration (n = 18), femur fracture repair (n = 4), cystotomy (n = 11), and penectomy (n = 3). Owner’s consent was obtained and the study was approved by the Institutional Ethical Committee for the Use of Animals in Research. Inclusion criteria were based on preoperative health status evaluated by clinical examination, complete blood (cell) count (CBC), and kidney (urea and creatinine) and liver (alanine transaminase and alkaline phosphatase) biochemical profiles in all cases. Based on preoperative clinical and hematologic evaluation, animals eligible for this study were ASA (American Society of Anesthesiology) I or II, non-aggressive and with a body condition score between 4 and 7 (5).
Cats were sedated with meperidine (Dolosal; Cristália Chemical and Pharmaceutical Products, Itapira, Brazil), 3 mg/kg body weight (BW), IM, xylazine (Xilazin; Syntec, Cotia, Brazil), 0.8 mg/kg BW, IM, and midazolam (Dormire; Cristália Chemical and Pharmaceutical Products), 0.2 mg/kg BW, IM. Anesthesia was induced with propofol (Propovan; Cristália Chemical and Pharmaceutical Products), 4 mg/kg BW, IV, and maintained with isoflurane. Electrocardiography, pulse oximetry, and oscillometric noninvasive blood pressure (numbers 1 to 3 cuff were placed distal to the elbow joint; width of the cuff was 40% of the circumference of the limb) were monitored continuously during all anesthetic procedures and registered every 5 (electrocardiography and pulse oximetry) and 3 min (noninvasive blood pressure).
Cats were positioned in sternal recumbency with the hind limbs flexed cranially only during epidural space ultrasound scanning and puncture. Lumbosacral and SC regions were clipped and sterile lidocaine gel was applied at both spaces. Ultrasonography was performed with a linear transducer (13-6 MHz, Sonosite MicroMaxx; Sonosite, Bothell, Washington, USA) at both the LS and SC regions, using transverse, sagittal, and parasagittal axis approaches (4). By palpation of the iliac crests, the ultrasound probe was positioned at the sagittal plane in the LS region for identification of structures. The sacral crests were identified, in the sagittal approach, as a continuous hyperechoic wavy horizontal line that produced ventral acoustic shadowing. When the transducer was slid cranially, at the parasagittal approach, the LS space was identified by observing an acoustic anechoic window, caudal to another hyperechoic horizontal line, which formed a ventral acoustic shadowing identified as the L7 lamina. A double hyperechoic horizontal line located at the top of the interlaminar space between L7 and S1 and that did not produce a ventral acoustic shadowing was identified as the LF/dura mater complex. Two less echogenic parallel longitudinal lines, located ventrally to the LF/dura mater complex, were identified as pia mater and cauda equina. At this position the distance between the skin and ligamentum flavum (LF) was measured using ultrasound software. A parasagittal approach was taken to improve visualization of the contents inside the vertebral canal and, in a continuous act, the probe was rotated 90°, for a transverse approach. The neuraxial midline was determined by observing the hyperechoic structure at the top of the image, as the L7 spinal process. When the beam was aligned with the interlaminar space, the probe was tilted cephalad to a 70° angle relative to the spine to improve the visualization of the vertebral canal. At this approach, the distance between the skin and LF was identified and measured again. The probe was returned to the sagittal position and slid caudally and, after observing the sacral crests, another acoustic anechoic window was visualized distally, corresponding to the SC intervertebral space. The same procedure performed for the LS space was repeated at the SC space, including measurement of the distance between the skin and LF. A mark was made with a pen on the skin at the exact puncture site to assist insertion of the needle.
After complete ultrasound scanning and measuring, epidural anesthesia was performed under aseptic conditions based on palpation of anatomical SC landmarks (blind technique). A 22-gauge, 50-mm Tuohy needle (Tuohy BD; Becton Dickinson Surgical Industries, São Paulo — SP — Brazil) was inserted, at an angle of 70° to 80° caudal to the skin, at the midline of the SC space. When bone, such as the vertebral lamina or the coccygeal spinal process was contacted, the needle was withdrawn and redirected cranially and advanced until a popping sensation was felt, suggesting piercing of the LF at the intervertebral space. Based on the expected duration of the surgery, a total volume of 0.3 mL/kg BW of either 0.5% bupivacaine (Neocaína; Cristália Chemical and Pharmaceutical Products) or 2% lidocaine (Xylestesin; Cristália Chemical and Pharmaceutical Products) was injected. After a negative aspiration test, the transducer was positioned at the LS in transverse approach (Figure 1B) and 0.1 to 0.2 mL of local anesthetic was injected. Then, the total volume was injected continuously over 60 s. The depth of insertion of the needle at the SC space, measured from the tip of needle to a mark drawn on the needle with a pen at the skin level, was recorded for all animals. Both the injection test and total volume injected were observed in real time by ultrasonography, according to epidural space enlargement produced by the local anesthetic flow.
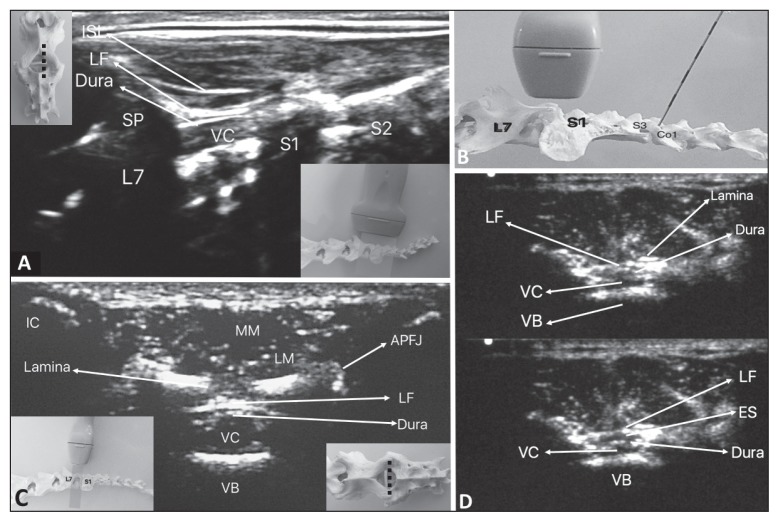
Figure 1.
A — Ultrasonographic view of the lumbosacral (LS) space in parasagittal approach in the cat; B — Positioning of ultrasound transducer at the LS space and needle at the sacrococcygeal (SC) space; C — Ultrasonographic view of the LS space in transverse approach in the cat; D — Sonoanatomy of the LS space in transverse plane — note that, in the lower image, there is an increase in the LS epidural space enlarged by SC epidural injection of local anesthetic (LA).
ISL — Interspinous ligament; LF — ligamentum flavum; VC — vertebral canal; VB — vertebral body; ES — epidural space; SP — Spinal process; L7 — 7th lumbar vertebra; S1, S2, and S3 — 1st, 2nd, and 3rd sacral vertebrae; Co1 — 1st coccygeal vertebra; IC — Iliac crest; LM — longissimus muscle; MM — multifidus muscle; APFJ — articular process/facet joint.
Anal sphincter tonus and patellar and hind limb withdrawal reflexes were evaluated both before and after tracheal intubation, and at 5 and 10 min after epidural injection. Effective epidural anesthesia was confirmed by presence of withdraw reflex in thoracic limbs and absence of podal reflex in pelvic limbs after epidural injection and at the end of surgery during recovery. At the end of surgeries, the trachea was extubated, yohimbine (Yohimbine-DrogaVet, Santos, Brazil), 0.1 mg/kg BW, IM, was administered and the effectiveness of epidural anesthesia was evaluated again. Trans-operative analgesia was evaluated continuously and considered adequate when heart rate and arterial blood pressure were not increased above 20% compared to pre-surgical values. In cases in which epidural anesthesia was not successful, analgesia was provided with fentanyl (Fentanest; Cristália Chemical and Pharmaceutical Products), 5 μg/kg BW, IV. Cardiorespiratory stability and no requirement for intraoperative analgesia further confirmed effectiveness of epidural anesthesia.
Animals were evaluated for 48 h after surgery by the same person who administered the anesthetic. Evaluation was done every hour in the first 8 h, then every 6 h thereafter for the first day, and every 12 h for the second day. Assessments included inspection of the surgical wound, dressing changes, and signs of pain. Analgesia was scored using the visual analog scale (VAS) at 1, 6, 24, and 48 h after surgery. The animal’s behavior (activity, mental status, posture, and vocalization) was observed in the boarding cattery. The value corresponding to the degree of pain was ticked on the line with 100 mm length, in which the left extreme was considered as no pain and the right extreme as the worst possible pain.
All animals received oral tramadol (Tramadon; Cristália Chemical and Pharmaceutical Products), 2 mg/kg BW, q8h and meloxicam (Maxican 0.2%; Ouro Fino Animal Health, Osasco, Brazil), 0.1 mg/kg, q24h for 3 d for postoperative analgesia.
Statistical analysis
Distances between the skin and LF at the LS and at the SC spaces for each probe position were compared by a paired t-test. Visual analogue scale (VAS) scores were compared by analysis of variance (ANOVA) followed by Tukey’s test. Data are expressed as mean ± SD. Differences were considered significant when P < 0.05.
Results
The mean weight of the animals was 2.95 ± 0.77 kg, mean age was 4.25 ± 3.86 y, and the mean duration of surgeries was 45.5 ± 19 min. Sonoanatomy of the LS space is illustrated in Figure 1(A and C). The mean distances (cm) from the skin to the LF at the LS and SC spaces, using the parasagittal and transverse axis approaches were, 0.93 ± 0.14 and 0.94 ± 0.13, respectively (P = 0.67). The mean distances (cm) between the LF and skin at the SC space were 0.56 ± 0.1 at the sagittal approach and 0.53 ± 0.1 at the transverse approach (P = 0.16).
In 6 of 36 cats (17%), although injection of 0.1 to 0.2 mL of LA was not forceful; the flow of LA was not observed by LS ultrasound. After repositioning the needle in these 6 cats, LA was observed flowing through the LS canal by injecting 0.1 mL of LA at the transverse approach, then injecting the total volume. Only when flow of LA was confirmed, ventral displacement of the dorsal dura mater and cranial distribution of the LA, produced by injection, using the transverse axis approach (Figure 1D) was observed. This procedure confirmed epidural injection in all animals, supported by the fact that no transoperative rescue analgesia was required. Added to this, no resistance to injection was observed in any patient.
The depth of insertion of the needle for epidural anesthesia was approximately 0.6 cm at the SC space and the distance between skin and LF, measured by ultrasound software, was 1 cm for the LS space.
Anal sphincter tonus, hind limb reflexes, and forelimb withdrawal reflex were present before epidural anesthesia, both before and after tracheal intubation. Forelimb withdrawal reflex was present, and anal sphincter tonus and hind limb reflexes were absent in all animals 10 min after epidural anesthesia, and at the end of surgery, after tracheal extubation. This finding, combined with the fact that no animal required trans-anesthetic analgesic rescue, confirmed that epidural anesthesia was effective in all cases. Recovery was quiet and without complications.
Mean VAS highest values were observed about 1 h after surgery when cats were placed in the boarding cattery (32 ± 8 mm). The VAS scores were reduced significantly (P < 0.001) to 20 ± 9 mm about 6 h after surgery and were 3 ± 6 mm both at 24 and 48 h after surgery (P < 0.001 compared to 1 and 6 h after surgery).
Discussion
Sacrococcygeal epidural anesthesia is an effective technique in cats (2); however, it is difficult to accurately define the depth of insertion of the needle when the blind technique is used. The “pop” sensation when the needle pierces the LF in small animals is subjective (6), so the epidural anesthesia can fail. Assessment of epidural space may be confirmed by the hanging drop technique in dogs (6); however, it is possible that underlying tissues or LF plug the spinal needle during attempts at insertion of the needle. This might produce a “false negative” response with this method, even when using a Tuohy stylet needle. In order to avoid this potential problem, it is recommended that the tip of the needle be positioned as close as possible to the LF before removal of the stylet (7).
Pre-puncture measurement of the distance from the skin to LF makes the epidural technique safer and easier and the appropriate needle may be selected (8). Our results showed that the epidural space is shallow in cats. The finding that it was possible to properly identify anatomical landmarks at the LS and SC regions shows that high-frequency linear ultrasound transducers (13-6 MHz) can help to confirm SC epidural injection in cats. Both sagittal and transverse approaches were precise and showed the same distance observed from the skin to the LF, at both the LS and SC spaces.
When the needle is inserted, it is usually difficult to determine if the needle is touching the vertebral lamina or the floor of the spinal canal. These findings reinforce the need to confirm the correct placement of the needle into the epidural space, to prevent tissue damage and to avoid failure of the technique.
In this study, anesthetic flow into the LS epidural space was clearly observed by ultrasound, even after the injection of small volumes (0.1 to 0.2 mL). When flow is not observed the needle may be repositioned to guarantee an epidural block. Another finding that confirmed the correct placement of the needle was the ventral displacement of dura mater after injection of LA into the epidural space; this corroborates the results of a recent study and shows the utility of ultrasound to confirm correct epidural injection (4). The enlargement of the epidural space after LA injection was most likely due to the injection of LA in the epidural space. Considering that there is no dural sac at the SC space, subarachnoid injection would not be possible.
Although the puncture site for epidural injection was the SC space, the LS space was chosen as the optimal acoustic window to observe LA flow, because the small size of cats does not allow positioning of the transducer and injecting the anesthetic at the same intervertebral space (10). Otero et al (4) reported that the color doppler ultrasound can differentiate epidural from intrathecal injections, when the LS space is accessed, making the technique safer in cats. Risk of intrathecal injection is reduced when SC epidural is conducted (4).
Some limitations of this study are that technical knowledge and practice are necessary to perform ultrasound and this technique is not a real-time ultrasound-guided epidural, as described in humans (9,10). As this is a preliminary study, the most difficult individuals in which to perform epidural administration of anesthetic were excluded. Obese animals were excluded because the aim was to first describe and standardize the technique in animals in normal body condition. Thin animals were excluded due to the poor contact between the skin and the transducer, because bones would be more prominent, making it difficult for coaptation between the skin and transducer. Aggressive animals were excluded because it would be difficult to perform postoperative evaluation in these animals when they awake from anesthesia. Therefore the technique should be tested in obese and thin animals before being established in clinical practice in the future. Yohimbine was used as an antagonist to provide a quick anesthetic recovery.
The most important limitation was the absence of a control group by injecting LA outside the epidural space. However the absence of dura mater displacement and enlargement of the epidural space in the 6 cats in which LA flow was not observed and the finding that epidural anesthesia was effective in all cases suggest that observation of LA flowing in the epidural space guarantees that the technique is correctly performed.
Bidimensional ultrasonography precisely identified the anatomy of LS and SC epidural spaces in cats. When the ultrasound probe is positioned at the transverse approach at the LS space, the anesthetic flow and epidural space enlargement produced by SC epidural injection of LA can be observed, thereby confirming epidural SC injection in real time. CVJ
Footnotes
Use of this article is limited to a single copy for personal study. Anyone interested in obtaining reprints should contact the CVMA office (hbroughton@cvma-acmv.org) for additional copies or permission to use this material elsewhere.
References
- 1.Maierl J, Liebich HG. Investigations on the postnatal development of the macroscopic proportions and the topographic anatomy of the feline spinal cord. Anat Histol Embryol. 1998;27:375–379. doi: 10.1111/j.1439-0264.1998.tb00210.x. [DOI] [PubMed] [Google Scholar]
- 2.O’Hearn AK, Wright BD. Coccygeal epidural with local anesthetic for catheterization and pain management in the treatment of feline urethral obstruction. J Vet Emerg Crit Care. 2011;21:50–52. doi: 10.1111/j.1476-4431.2010.00609.x. [DOI] [PubMed] [Google Scholar]
- 3.Otero PE, Verdier N, Zaccagnini AS, Fuensalida SE, Tarragona L, Portela DA. The use of a nerve stimulation test to confirm sacrococcygeal epidural needle placement in cats. Vet Anaesth Analg. 2015;42:115–118. doi: 10.1111/vaa.12173. [DOI] [PubMed] [Google Scholar]
- 4.Otero PE, Verdier N, Zaccagnini AS, et al. Sonographic evaluation of epidural and intrathecal injections in cats. Vet Anaesth Analg. 2016;43:652–661. doi: 10.1111/vaa.12361. [DOI] [PubMed] [Google Scholar]
- 5.Laflamme D. Development and validation of a body condition score system for cats: A clinical tool. Feline Pract. 1997;5:13–18. [Google Scholar]
- 6.Valverde A. Epidural analgesia and anesthesia in dogs and cats. Vet Clin Small Anim. 2008;38:1205–1230. doi: 10.1016/j.cvsm.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 7.Naganobu K, Hagio M. The effect of body position on the ‘hanging drop’ method for identifying the extradural space in anaesthetized dogs. Vet Anaesth Analg. 2007;34:59–62. doi: 10.1111/j.1467-2995.2006.00290.x. [DOI] [PubMed] [Google Scholar]
- 8.Kil HK, Cho JE, Kim WO, Koo BN, Han SW, Kim JY. Prepuncture ultrasound-measured distance: An accurate reflection of epidural depth in infants and small children. Reg Anesth Pain Med. 2007;32:102–106. doi: 10.1016/j.rapm.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 9.Gregori T, Viscasillas J, Benigni L. Ultrasonographic anatomy of the sacrococcygeal region and ultrasound-guided epidural injection at the sacrococcygeal space in dogs. Vet Rec. 2014;175:68. doi: 10.1136/vr.102453. [DOI] [PubMed] [Google Scholar]
- 10.Karmakar MK, Li X, Ho AMH, Kwok WH, Chui PT. Real-time ultrasound-guided paramedian epidural access: Evaluation of a novel in-plane technique. Br J Anaesth. 2009;102:845–854. doi: 10.1093/bja/aep079. [DOI] [PubMed] [Google Scholar]