Abstract
Background
Visceral leishmaniasis (VL) is a deadly vector-borne disease. Approximately 90% of Indian VL cases occur in Bihar, where the sand fly, Phlebotomus argentipes, is the principal vector. Sand fly control in Bihar consists of indoor residual spraying (IRS), the practice of spraying the inner walls of village dwellings with insecticides. Prior researchers have evaluated success of IRS-control by estimating vector abundance in village houses, but the number of sampling periods (n = 2–3) were minimal, and outdoor-resting P. argentipes were neglected. We describe a large-scale field study, performed in 24 villages within two Bihari districts, during which P. argentipes were collected biweekly over 47-weeks, in cattle enclosures, houses, and outdoors in peri-domestic vegetation. The objectives of this study were to provide updated P. argentipes ecological field data, and determine if program-initiated IRS-treatment had led to noticeable differences in vector abundance.
Principal findings
P. argentipes (n = 126,901) relative abundance was greatest during the summer months (June-August) when minimum temperatures were highest. P. argentipes were most frequently collected from cattle enclosures (~46% total; ~56% blood fed). Many sand flies were found to have taken blood from multiple sources, with ~81% having blood fed on humans and ~60% blood feeding on bovines. Nonparametric statistical tests were determined most appropriate for evaluating IRS-treatment. Differences in P. argentipes abundance in houses, cattle enclosures and vegetation were detected between IRS-treated and untreated villages in only ~9% of evaluation periods occurring during the peak period of human-vector exposure (June-August) and in ~8% of the total observations. No significant differences were detected between the numbers of P. argentipes collected in vegetation close to the experimental villages.
Conclusion
The results of this study provide updated data regarding P. argentipes seasonal abundance, spatial distribution, and host preferances, and suggest vector abundance has not significantly declined in IRS-treated villages. We suggest that IRS be supplemented with vector control strategies targeting exophagic, exophilic P. argentipes, and that disease surveillance be accompanied by rigorous vector population monitoring.
Author summary
Visceral leishmaniasis is a disease caused by a deadly vector-borne parasite (Leishmania donovani) transmitted to man by phlebotomine sand flies. Indoor residual spraying (IRS), performed within village dwellings, is the primary means of sand fly control performed in Bihar, India and more explicit methods of evaluating the success of control are warranted. A field-based study was conducted to collect ecological sand fly data for use in evaluating the effectiveness of IRS in reducing relative sand fly abundance. Results indicate that sand flies blood feed primarily on humans and cattle and are most frequently found within cattle enclosures. Results further suggest IRS-treatment has a limited impact on vector density. Our approach incorporates detailed evaluation of sand fly spatial distribution (cattle enclosures, houses, vegetation), seasonal fluctuations in abundance, host blood meal preferences within Bihari villages, and dates of IRS performed within treated villages. Hence, this study provides an explicit means of monitoring vector populations and evaluating control measures in Bihar.
Introduction
Visceral leishmaniasis (VL), also known as kala-azar, is a parasitic disease transmitted by infected female phlebotomine sand flies. VL is fatal if left untreated, and annually there are an estimated 500,000 human VL cases and 50,000 deaths worldwide, making it the second deadliest parasitic disease surpassed only by malaria [1–2]. The Indian subcontinent accounts for approximately 67% of the reported human cases of VL, the majority of which occur in areas of extreme poverty [3]. Approximately 90% of cases in India occur in Bihar [4]. The known vector of VL in the Indian subcontinent is the sand fly species Phlebotomus argentipes [5–6] which transmits the pathogen (Leishmania donovani) anthroponotically with no known animal reservoir [7]. In 2005, the World Health Organization (WHO) helped the governments of Bangladesh, India, and Nepal to launch a regional elimination initiative to reduce VL cases in the Indian subcontinent to less than one case per 10,000 people in endemic districts by 2015, two of the main strategies being: integrated vector management and effective disease and vector surveillance [8]. Although attempts have been made to control phlebotomine sand fly populations, organization and implementation of effective vector control programs have been hindered largely because of uncertainties surrounding phlebotomine sand fly ecology [9].
Vector ecology
On the Indian subcontinent, researchers have made attempts to answer questions regarding aspects of P. argentipes field ecology, including: seasonality, spatial distribution, and host preference. Researchers have suggested that the abundance of P. argentipes is associated with ecological parameters such as temperature and precipitation [10]. Results of a more recent 12-month study conducted in three Bihari villages in the Saran district, during which 52,653 sand flies were trapped using light traps, suggest P. argentipes numbers are typically highest during the months when evening temperatures are warmest and precipitation is increased (June, July, August) and lowest during the coolest months (January, February, December) [11]. These results further suggested spatial distribution of P. argentipes was not limited to the inside of village dwellings, but also included outlying village vegetation. P. argentipes females are regarded as anautogenous, needing a blood meal to produce each batch of eggs [12], and results of several studies suggest they blood feed almost exclusively on bovines (cattle and domestic buffalo) and humans within rural villages [13–17].
Vector control
Because P. argentipes is believed to be endophilic and endophagic [18], the vector control approach utilized for decades in Bihar has been indoor residual spraying (IRS), a method of applying insecticide to the inner walls of village houses and cattle dwellings. Explicit data addressing the impact of IRS on P. argentipes is limited [19–21] and, therefore, the impact of IRS on field populations is largely unknown.
A cluster randomized trial was conducted by [22] in which IRS was performed in village dwellings in India (DDT), Nepal (alpha-cypermethrin), and Bangladesh (deltamethrin), during which pre-treatment light trap collections, performed over two consecutive nights in November, were compared with post-treatment light trap collections performed over two consecutive nights in April of the following year. One issue with this study design is the lengthy period (~5-months) between IRS treatment and sample collection, during which multiple factors could influence sand fly abundance. The authors mention other caveats as being small sample sizes within sites, limiting the reliability of site-specific analysis, and the fact that the study was conducted under controlled conditions not easily applicable in a national vector control program.
Given the latter limitation, other researchers have collected P. argentipes from treated and untreated households during program-initiated DDT spraying in India [23–26]. While the results of these IRS-evaluation studies are interesting and useful, we would argue that they all share two limitations 1) a minimal number of post-IRS P. argentipes collection periods and 2) a universal neglect of outdoor sand fly population sampling. Post-treatment P. argentipes collections during the previously mentioned studies were typically conducted at ~1-month and ~5-6-months post-treatment. P. argentipes have been collected in large numbers from peri-domestic vegetation [11], and villagers sleep outdoors during warmer months [8] when vector abundance and biting rates are high [27]. Hence the exophilic, exophagic sand fly population should be monitored. Considering the sensitivity of vector abundance to environmental factors such as temperature and precipitation and the tendency P. argentipes to be captured in light traps positioned in vegetation and cattle enclosures [11,28–29], we suggest the current IRS-treatment programs could be better evaluated if P. argentipes were collected at greater frequencies and if trap locations were diversified to include cattle enclosures and outdoor locations such as peri-domestic vegetation.
Objectives
In this paper, we describe a large-scale, 11-month field study, conducted in 24 villages in two VL-endemic districts in Bihar, India, in which sand flies were collected using United States Centers for Disease Control and Prevention (CDC) light traps (Bioquip Products, Rancho Dominguez, CA, USA; John W. Hock Company, Gainesville, FL, USA) during 24 collection periods conducted over 47-weeks. The objectives of this study were to 1) provide updated P. argentipes ecological data within villages in two Bihari districts; and 2) determine if relative P. argentipes abundance in IRS-treated villages was reduced when compared with untreated villages. By utilizing methods previously described by [11] and [17], but using a much larger sample size, field-collected P. argentipes were used to estimate: spatial distribution (cattle enclosures, houses, vegetation), temporal fluctuations in relative abundance, and host preferences of blood fed females. Additionally, we used IRS data for the study villages to compare relative P. argentipes abundance in IRS-treated and untreated villages during the 2016 season. The results of this study will provide 1) useful, current ecological information regarding P. argentipes seasonal abundance, spatial distribution, and host preference in two VL-endemic districts; and 2) an alternative means of evaluating IRS-treatment through biweekly vector monitoring, helping to determine whether integrated methods of vector management should be recommended in Bihar.
Materials and methods
Study area
The study was conducted in the Saran and Muzaffarpur districts of Bihar, India. Saran and Muzaffarpur are adjacent districts located ~30 km northwest (25.8560° N, 84.8568° E) ~60 km north (26.121736° N, 85.373700° E) of Patna, respectively. Summers are warm with maximum temperatures often ranging from 35–40°C. The winter months (December-February) are typically much cooler [11]. The rainy season typically occurs from July-September and April is generally the driest month.
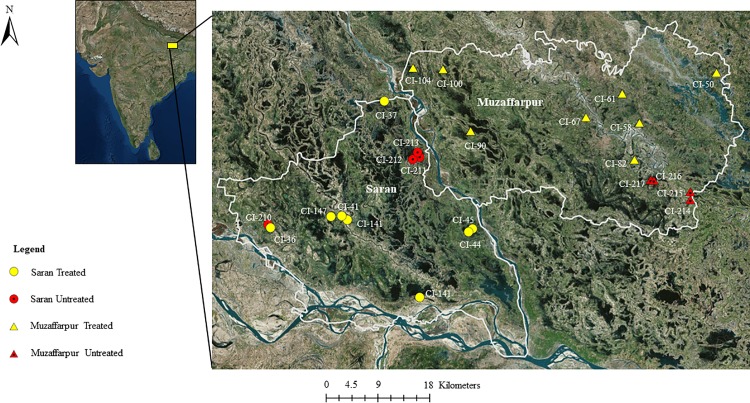
All villages were part of a large-scale VL-incidence survey performed in 60 villages within each district (n = 120) in 2015. At the beginning of 2016, 12 villages in each district were selected for sand fly collection (n = 24) (Fig 1) with the aim of collecting sand flies from February-December. Prior to study initiation, it was discovered that two rounds of IRS application had been performed within several villages in both districts in 2015 and that application would be repeated in 2016. To evaluate the impact of IRS on vector abundance, we selected 8 IRS-treated villages and 4 untreated villages within each district (n = 16; n = 8) for CDC light trap collection. The main criteria for village selection were a population of >1,000 villagers, confirmed cases of VL spanning 2013–2015, and IRS-status (treated or untreated). All villages shared similar bioclimatic and agricultural characteristics. Livestock ownership was common with cows (Bos taurus, Bos indicus), domestic buffalo (Bubalus bubalus), and domestic goats (Capra aegagrus hircus) prevalent in each village. Dwellings were constructed primarily of thatch, mud, brick, and/or concrete, consisting mainly of human houses and cattle enclosures (cattle sheds, houses cohabitated by humans and bovine). At night, livestock were typically tethered to stakes, and were kept in rooms within cattle enclosures or outdoors adjacent to human dwellings and/or cattle enclosures.
Fig 1. Map of 24 study villages in two VL-endemic districts in Bihar, India.
Map was generated in ArcGISusing ArcMap with a World Imagery base layer (Sources: Esri, DigitalGlobe, GeoEye, i-cubed, USDA FSA, USGS, AEX, Getmapping, Aerogrid, IGN, IGP, swisstopo, and the GIS User Community).
Local weather data including daily temperature (°C), relative humidity (%), and precipitation (mm) (February 10-December 31, 2016) were collected from the closest, attainable weather monitoring station, located at Jay Prakash Narayan International Airport (VEPT) in Patna [30].
Sand fly collection
CDC light traps were used to collect sand flies in study villages. Twelve (12) CDC light traps were set, in fixed locations, within each of the 24 study villages (n = 288 trap-nights per collection week) biweekly from February 10-December 29, 2016 (24 collection periods). During each collection period, CDC light trapping was performed over two consecutive nights with 12 villages being sampled on night-1 and the remainder on night-2. Collection was not conducted in January because adult sand flies are typically not active during this period [11]. CDC traps were set in randomly selected homes (n = 4), cattle enclosures (cattle sheds or dwellings cohabitated by bovines and humans) (n = 4), and outdoors in peri-domestic village vegetation (n = 4) to better estimate P. argentipes spatial distribution. Traps were identified by a unique code and waypoints taken with a handheld GPS (Garmin Etrex 30, Olathe, KS, USA). Traps were positioned with the fan ~1 m above the ground [11]. In peri-domestic vegetation, CDC traps were fitted with protective lids to shield the mechanical components from falling debris or rain. Traps were set at ~18:00 and removed at ~06:00 the following morning. Trap catches were individually numbered, transported to the laboratory in Patna, and frozen at -20°C until further processing. Because dry-ice was not available within the state of Bihar, we were unable to compare the efficiency CDC light traps with CO2 traps. Permission was received from village residents prior to conducting light trapping.
Sand fly counts and identification
Individual CDC light trap catches were uniquely numbered and identified by trap location and date of capture. Captured sand flies were separated from other arthropods, counted, identified morphologically by species, sexed, and females confirmed as unfed, blood fed, or gravid. If sand fly species was uncertain, specimens were placed under a dissecting microscope and identified morphologically by observing the male genitalia and the female spermatheca, the latter which required dissection [31].
Molecular species confirmation of sand flies was performed routinely on a random sample of specimens from each village. Whole sand fly DNA was extracted individually according to manufacturing instructions using the DNAzol reagent (ThermoFisher Scientific, Waltham, MA, USA). Individual sand fly DNA was amplified using forward primer 5’ -TCG AAT CTA TGG GTG GT-3’ and reverse primer 5’- CAC AAT CCC AAC CAC GAA G-3’ for the 18S rRNA target gene. Restriction endonuclease digestion was performed on the PCR product of 18S r RNA using HAE II and HAE III enzymes. Banding pattern after Gel electrophoresis confirmed the identification of P. argentipes [32].
Sand fly blood meal analysis
After completing counts and identification, blood fed female P. argentipes were separated and placed into dry 1.5 ml centrifuge tubes and stored at -20°C. Sand fly heads were removed from the bodies prior to analysis. DNA was then extracted from the abdomen and thorax of blood fed sand flies. The cytochrome b gene region (344 bp conserved mitochondria gene) was amplified using bio-tinilated universal primers designed earlier by [33]. Cow blood was used as positive control and sterile water as negative control. The amplified products were used in reverse line blotting hybridization as probes, followed by chromogenic detection. Immobilization, hybridization and detection were done according to methods of [33].
Previously developed source-specific probes for human, cow, buffalo, goat, and chicken were used to analyze the blood meal contents for the blood fed sand flies collected during this study. These techniques allowed for detecting multiple host species in a single P. argentipes blood meal. These procedures are described in detail by [17,33].
Sand fly spatial distribution
P. argentipes successfully analyzed by cytochrome b amplification and reverse line blotting were categorized by blood meal content (human, bovine, goat, etc.) and trap placement (cattle enclosure, house, vegetation). A Pearson’s chi square (X2) test was used to estimate dependence of blood meal content on the spatial distribution of blood fed P. argentipes (trap placement) (p = 0.05).
P. argentipes distribution was further evaluated by comparing the relative abundance of all P. argentipes within trap placements. Differences between and within trap catches in cattle enclosures, houses, and vegetation in Muzaffarpur, Saran, and cumulative districts were assessed using Analysis of Variance (ANOVA) (p = 0.05) followed by Tukey’s W procedure (p = 0.05).
Sand fly seasonal fluctuations
To estimate relative P. argentipes seasonal fluctuations in abundance, we compared overall P. argentipes seasonal abundance in Muzaffarpur and Saran and compared seasonal abundance in cattle enclosures, houses, and vegetation within each district. More specifically, we compared the mean P. argentipes per trap-night per month (n = 11) and per collection period (n = 24) within districts and trap placements. A nonparametric Sign test (p = 0.05) was used to estimate whether changes in monthly and biweekly abundance were significantly different between districts and between trap placements within districts. That is, whether P. argentipes abundance tended to increase and decrease in relative unison throughout the calendar year.
Comparing IRS-treated with untreated villages
The specific 2016 IRS spray dates for each village were provided by CARE India (Patna, Bihar) (S1 Table), written on the walls of the village dwellings by the applicators, and were also confirmed by the home owners. The inner walls of houses and cattle enclosures in IRS-treated villages had been sprayed with two rounds of alpha-cypermethrin (5%) wettable powder (WP) at a rate of 25mg/m2, and the dates of application varied on a village-to-village basis. The first round of IRS application ranged from April 1–May 30 in Muzaffarpur and April 6-June 2 in Saran (S1 Table). The second round of IRS application ranged from August 20-November 14 and September 15-November 28 in Muzzaffarpur and Saran, respectively. All IRS-treated study villages received two rounds of IRS application in 2015. None of the untreated study villages had received IRS-treatment in over three years. In response to insecticide resistance, alpha-cypermethrin (a synthetic pyrethroid) has replaced DDT in 15 Bihari districts [34].
Because CDC light trap collections were performed during on-going program-initiated IRS-treatment, we were unable to collect pre-treatment (baseline) data. We first compared the mean P. argentipes per trap-night per collection period (±SE) in IRS-treated and untreated villages within each district. We were specifically interested in differences occurring during the months with the highest relative vector abundance (June-August) and, hence, whether the first round of IRS application may have influenced P. argentipes abundance during this period of peak human-vector exposure. Nonparametric methods deemed sufficient for most skewed analyses [35], were considered most appropriate to analyze differences between IRS-treated and untreated villages. Hence, a Wilcoxon rank sum test was used to estimate differences in relative abundance of P. argentipes in IRS-treated and untreated villages (p = 0.05) by district. One of the villages in Saran (CI-210), initially untreated during the first round in 2016, received IRS application during the second round (September 21), due to newly reported cases within the village, and therefore was excluded from the statistical analysis after September. We additionally excluded February and December from statistical analysis due to low vector abundance.
Results
April and December on average had the high and low maximum temperatures with means of 40.6°C and 21.6°C, respectively (S2 Table). The highest mean minimum temperatures were recorded in June, July and August. July was the rainiest month, with ~202.9 mm precipitation being recorded and nearly all precipitation (>99%) was recorded May-October. Maximum humidity was generally high (>80%) with the low point being in April (59.6%). Minimum humidity ranged from 16.9% in April to 69.1% in September.
Sand fly collection, counts and identification
During 24 collection periods, occurring over 47-weeks (6,349 trap-nights), a total of 155,908 sand flies were captured, counted, and identified (S3 Table), of which 126,901 were P. argentipes (Males = 76,904; unfed females = 44,133; blood fed females = 2,299; gravid (no blood seen) females = 3,565) (Table 1). Individual trap-night yields ranged from 0–3,248 P. argentipes. A total of 76,516 and 50,385 P. argentipes were caught in CDC light traps in villages in Muzaffarpur and Saran, respectively. Twenty-four thousand two hundred eighty (24,280) Sergentomyia spp. and 1,477 P. papatasi were also collected.
Table 1. Summary of the total number of P. argentipes collected from houses (H), cattle enclosures (CE), and peri-domestic vegetation (V) between February 10-December 29, 2016.
| District | Location | Phlebotomus argentipes | ||||
|---|---|---|---|---|---|---|
| M | F (UF) | F (BF) | F (G) | Total | ||
| Muzaffarpur | H | 15052 | 10176 | 482 | 678 | 26388 |
| CE | 21391 | 11729 | 634 | 920 | 34674 | |
| V | 9346 | 5531 | 176 | 401 | 15454 | |
| Total | 45789 | 27436 | 1292 | 1999 | 76516 | |
| Saran | H | 7221 | 4137 | 160 | 434 | 11952 |
| CE | 15252 | 7221 | 679 | 808 | 23960 | |
| V | 8642 | 5339 | 168 | 324 | 14473 | |
| Total | 31115 | 16697 | 1007 | 1566 | 50385 | |
M = Male, F = Female, (UF) = Unfed, (BF) = Blood fed, (G) = Gravid
In total, 38,583 and 30,236 female sand flies were identified by dissection and PCR analysis, respectively (S1 Fig, S2 Fig). Three thousand two hundred twenty-four (3,224) female Grassomyia indica were also identified. Twenty six (26) females could not be identified.
Blood meal analysis
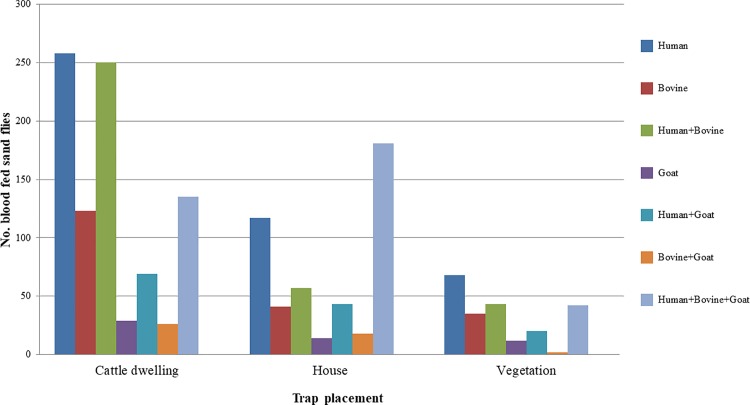
Of the 2,299 blood fed P. argentipes collected, a total of 1,583 were successfully analyzed using cytochrome b PCR and reverse-line blot analysis (Fig 2). Blood meal content consisted of human, bovine, and goat blood, exclusively. Approximately 60% P. argentipes blood meals were positive for more than one host species. Of all blood fed P. argentipes successfully analyzed, the majority were positive for human (~81%) and/or bovine (~60%) blood.
Fig 2. Blood meal sources and trap placement of P. argentipes successfully analyzed using reverse-line blotting.
Spatial distribution
Blood meal status was dependent on trap placement (X2; p<0.0001), primarily in cattle enclosures. Overall, blood fed female P. argentipes were most frequently collected in cattle enclosures (~56%) followed by houses (~30%), and vegetation (~14%) (Fig 2). As would be expected, more P. argentipes containing bovine blood meals were collected in cattle enclosures (~56%) than in houses (~31%) or vegetation (~13%). But more P. argentipes containing human blood meals were also collected in cattle enclosures (~55%) than in houses (~31%). This greater dependence on cattle enclosures was observed in all P. argentipes blood meal content types except for mixed human, bovine and goat blood meals, which were collected primarily from houses (~50%), followed by cattle enclosures (~38%) and vegetation (~12%).
The overall P. argentipes spatial distribution was relatively similar to that of blood fed females, with more being collected from cattle enclosures (~46%) followed by houses (~30%) and outlying vegetation (~24%) (Table 1). The mean P. argentipes per trap-night were determined to be significantly different between trap placements in Muzaffarpur (ANOVA; p<0.0001), in Saran (ANOVA; p = 0.0018), and in cumulative districts (ANOVA; p<0.0001). Differences within trap placements were estimated between cattle enclosures and vegetation in Muzaffarpur (Tukey’s W; p<0.0001), in Saran (Tukey’s W; p = 0.0234), and in cumulative districts (Tukey’s W; p<0.0001); between cattle enclosures and houses in Saran (Tukeys W; p = 0.0020) and in cumulative districts (Tukey’s W; p = 0.0015); and between houses and vegetation in Muzaffarpur (Tukey’s W; p = 0.0397).
Sand fly seasonal fluctuations
Monthly
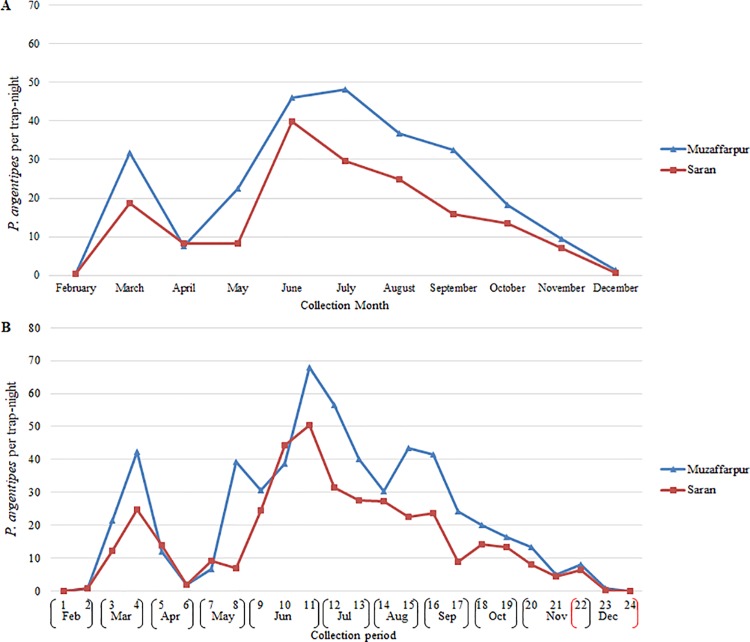
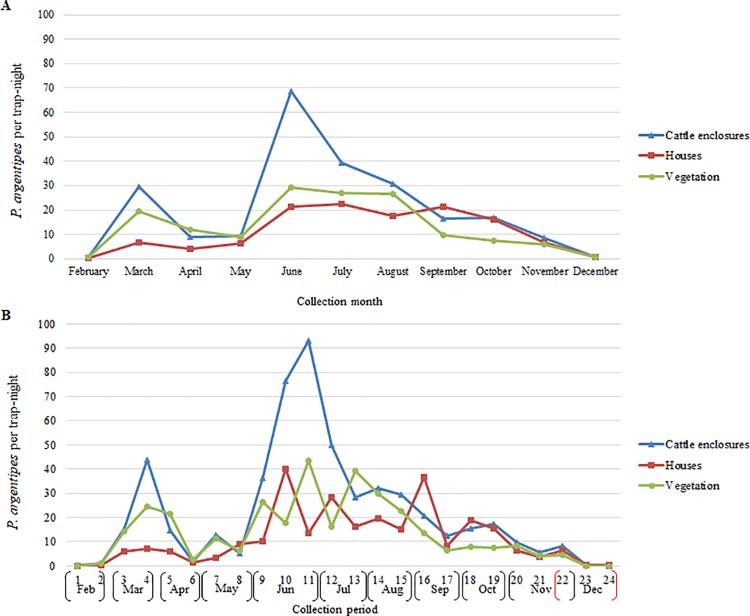
Results of CDC light trapping in Muzaffarpur and Saran suggested similar fluctuations in the monthly relative abundance of adult P. argentipes (Fig 3A). The first peak in adult P. argentipes occurred in March followed by a sharp decrease in April. Relative abundance was highest during the summer months (June, July, August) and lowest in February and December. Differences in general trends in monthly relative abundance of P. argentipes in Muzaffarpur and Saran, were nearly significant (Sign test; p = 0.0654).
Fig 3.
P. argentipes per trap-night per month in Muzaffarpur and Saran by a) month and b) trapping period.
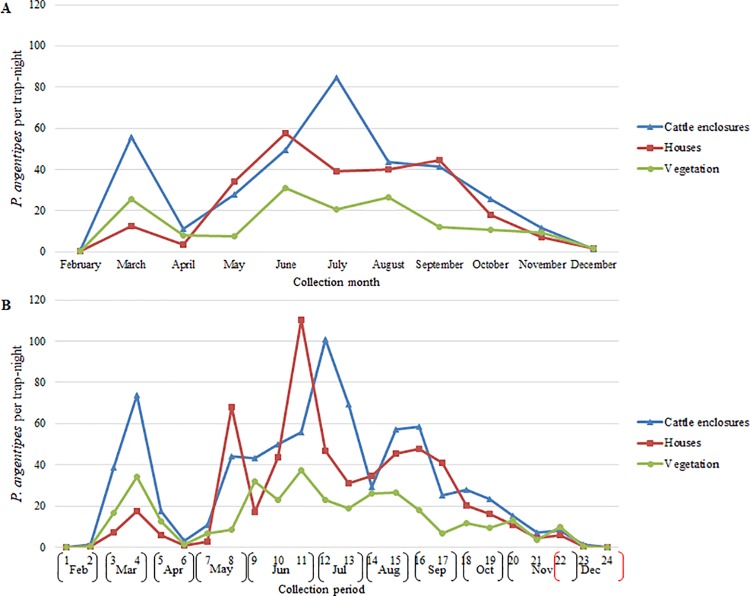
The mean number of P. argentipes per trap-night was greatest in traps positioned in cattle enclosures in July (84.7) and June (68.7) in Muzaffarpur and Saran, respectively (Figs 4A and 5A). The largest peaks in the mean P. argentipes per trap-night occurred in June in houses and vegetation in Muzaffarpur (57.4, 30.9) and Saran (22.4, 29.3). P. argentipes numbers were lowest in February and December for all trap location types in both districts. Differences in monthly fluctuations in relative abundance were most apparent when comparing cattle enclosures with vegetation (Sign test; p = 0.0117), and no significant differences were determined when comparing cattle enclosures to houses or vegetation to houses.
Fig 4.
P. argentipes per trap-night in cattle enclosures, houses and vegetation in Muzaffarpur by a) month and b) trapping period.
Fig 5.
P. argentipes per trap-night in cattle enclosures, houses and vegetation in Saran by a) month and b) trapping period.
Biweekly
Results suggested that fluctuations in biweekly P. argentipes relative abundance were less comparable. In both districts, the first peak occurred during collection period-4 in March and the largest peak occurred during collection period-11 in June (Fig 3B). Numbers declined noticeably during collection period-6 in April. However, villages in Muzaffarpur on average experienced a greater number of P. argentipes peaks (collection periods-4,8,11,15) than villages in Saran (collection periods-4,11). General trends in biweekly relative abundance within Muzaffarpur and Saran were determined to be significantly different (Sign test; p = 0.0066).
The seasonal abundance observed in cattle sheds appeared to be noticeably different from that of houses and vegetation. The largest peaks in mean P. argentipes per trap-night by trap placement by trapping period were recorded during collection period-11 (June) in houses (110.4) and cattle enclosures (93.2) in Muzaffarpur and Saran, respectively (Figs 4B and 5B). In Muzaffarpur and Saran, significant differences were determined between cattle enclosures and houses (Sign test; p = 0.0066, p = 0.0015) and between cattle enclosures and vegetation (Sign test; p<0.0001, p = 0.0015). In Muzaffarpur and Saran, no significant differences were detected between houses and vegetation (Sign test; p = 0.8388).
Comparing IRS-treated with untreated villages
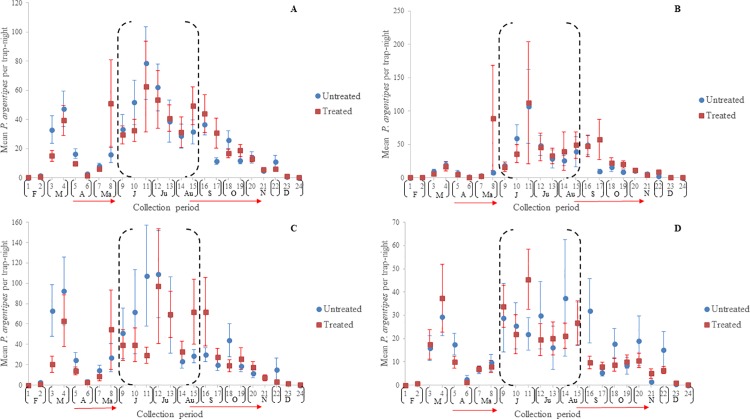
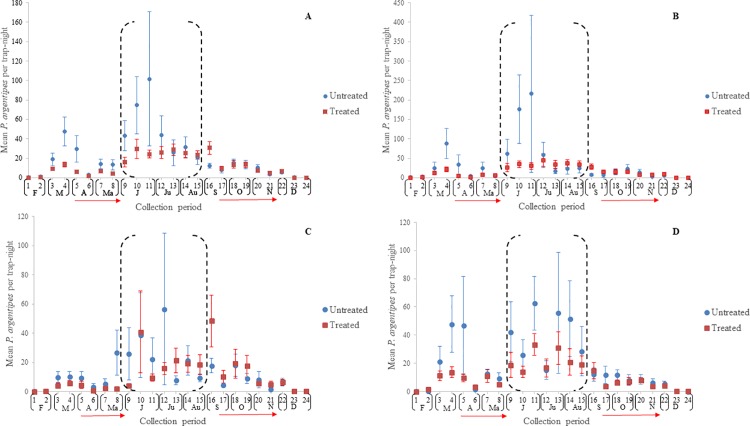
The mean number of P. argentipes per trap-night per collection period in Muzaffarpur and Saran are indicated in Fig 6 and Fig 7, respectively. As noted, the first round of IRS applications was conducted between early April and late May to early June in both districts. Results of collections suggest that IRS-treatment failed to prevent the P. argentipes abundance in IRS-treated villages from increasing post-treatment June-August. Additionally, they indicate that if P. argentipes abundance was decreased in IRS-treated villages, the decrease was only temporary.
Fig 6.
Mean P. argentipes per trap-night in IRS-treated and untreated villages in Muzaffarpur in a) pooled locations, b) cattle enclosures, c) houses, and d) vegetation. Vertical lines indicate the ±standard error (SE) and horizontal red arrows the range in 1st and 2nd round IRS spray dates. The black-dashed brackets (June-August) indicate the months of highest risk of human-vector exposure.
Fig 7.
Mean P. argentipes per trap-night in IRS-treated and untreated villages in Saran in a) pooled locations, b) cattle enclosures, c) houses, and d) vegetation. Vertical lines indicate the ±standard error (SE) and horizontal red arrows the range in 1st and 2nd round IRS spray dates. The black-dashed brackets (June-August) indicate the months of highest risk of human-vector exposure.
In Muzaffarpur, the relative P. argentipes abundance per trapping period in IRS-treated and untreated villages were similar (Fig 6A). The most notable difference occurred during collection period-11 (June) in houses where the mean P. argentipes per trap-night in IRS-treated villages was markedly lower (Fig 6C). However, the mean was comparable to the untreated villages during collection period-12.
In Saran, the mean number of P. argentipes in cattle enclosures suggested differences in P. argentipes abundance occurred in June (collection periods-9, 10, 11) (Fig 7B) but standard error was sizable because of outliers in one village (CI-210), particularly during collection period-11, and differences were less evident from collection period-12 onwards. Noticeable differences between P. argentipes abundance in IRS treated and untreated villages did not occur after the second round of IRS.
Nonparametric means of statistical analysis were used to estimate differences in P. argentipes abundance in IRS-treated and untreated villages because of the large standard errors reported. In total, of 40 cumulative evaluation periods (20 collection periods x 2 districts) and 120 trap placement evaluation periods (20 collection periods x 3 trap placements x 2 districts), significant differences were detected for 8 periods (20%) and 10 periods (~8%), respectively (S4 Table). Of 56 evaluation periods occurring June-August, statistical differences were detected in 5 periods (~9%), 4 occurring in June and 1 in July. Significant differences were most frequently detected during analysis of cumulative trap placements (n = 8) followed by houses (n = 5) cattle enclosures (n = 4) and vegetation (n = 1).
Discussion
The results of this study 1) provide ecological data regarding P. argentipes monthly relative abundance, spatial distribution, and host preferance comparable to previous research; and 2) suggest relative P. argentipes abundance within IRS-treated and untreated villages do not differ significantly. To our knowledge, this is the most extensive entomology-based study, with insights into IRS-performance, conducted in Bihar, India.
Monthly P. argentipes relative abundance was comparable to results presented by [11], being highest in June, July and August and lowest in winter. P. argentipes spatial distribution was similar in that specimens were more frequently collected in cattle enclosures than other areas. Additionally, blood meal analysis suggested P. argentipes fed primarly on bovines and humans as reported by [17], but suggested a slight increase in the proportion taking full or partial blood meals from bovines (~60%: current; ~50%: previous).
The results of this study do not indicate that P. argentipes abundance in IRS-treated villages significantly differs from untreated villages in Muzaffarpur or Saran. Additionally, the application of IRS did not appear to prevent P. argentipes from increasing June-August. The lack of difference between IRS-treated and untreated villages is noteworthy, considering a recent mathematical, epidemiological model predicted a >67% reduction in P. argentipes abundance would be necessary to remove VL from a population [36]. [22] reported a 72% reduction in P. argentipes abundance resulting from IRS. However, these results are limited temporally, given that post-treatment collections were performed only twice in April, during which results of the current study and those of previous researchers [11,37] suggest sand fly abundance in Bihar declines naturally.
The efficacy of IRS is largely dependent on sand flies being endophilic [38] which should help explain why little difference was observed in P. argentipes abundance in vegetation. The presence of sand flies outdoors during this study and past studies [11,28,29] suggests a high percentage of the sand fly population may not come into contact with IRS-treated surfaces. One hundred and seventy three (173) P. argentipes collected in vegetation during the current study were confirmed to have taken a full or partial blood meal from humans. Considering the vaste majority of village households have members who sleep outdoors [8, 39], particularly during the warmer summer months, a proportion of P. argentipes may be able to feed and potentially transmit VL without ever entering a village dwelling. The increased likelihood of sleeping outdoors June-August, in conjunction with the greater vector density observed during this period in the current study, suggests villagers are placed at high risk of exposure to exophagic and exophilic P. argentipes.
For evaluation of IRS against exophilic, endophagic sand flies, the mortality rates of the insects entering sprayed households would be of interest. Researchers conducting a recent study in the Judean Desert of Jerusalem [40] observed that the number of sand flies captured in CO2 traps entering an experimental home (EH) with treated and untreated surfaces didn’t differ significantly, but noted that the mortality rate on deltamethrin-treated surfaces was higher when compared with untreated control (~69%, ~23%). While this would be useful information to obtain for the current study, the vaste number of insect species within CDC light traps, the process of collecting the large quantity of traps set per collection night (n = 144), and hot Indian temperatures made estimating sand fly mortality infeasible. However, the mean number of blood fed P. argentipes collected in CDC light traps positioned in homes in IRS-treated villages (n = 14.1) was comparable to untreated (n = 12), suggesting limited effect of IRS.
Although nonparametric statistical methods were used to account for outliers, the importance of these outliers and their potential implications in medical entomology and vector ecology should not be ignored. For this reason, we report the arithmetic means as well. A single house in one IRS-treated village (CI-58) in Muzaffarpur yielded 2,308 P. argentipes in a single trap (May 19), 33 days after being sprayed with alpha-cypermethrin. Another home located within the same village yielded 2,941 P. argentipes on June 30. In total, of six individual trap-nights which each yielded >1,000 P. argentipes, all were positioned within dwellings, and five were located in IRS-treated villages in Muzaffarpur. The sixth trap, which yielded 3,248 P. argentipes, was located in an untreated cattle enclosure in Saran (CI-210). It is unusual that dwellings in the latter village were not sprayed, given it was <1 km from an IRS-treated village (Fig 1), and interesting in that new cases were reported in 2016, leading to the village being sprayed on September 21 (S1 Table). The ability of some Phlebotomus spp. to fly distances >1–2 km [41–42], in addition to potential movement of VL-infected persons between villages, suggests that villages in close proximity to those with current VL cases should also be treated.
If vector density in Bihar has not decreased significantly in villages treated with IRS, it would suggest that the decline in reported VL cases [43] in Bihar could be related to other factors. Results of several studies indicate a repeating periodicity of VL in which peaks in cases occur every 10–20 years [44]. In the Goalpara District, Assam, five historical epidemic peaks were described by [45] occuring in 1885, 1897, 1913, 1925, and 1944. The three previous VL peaks in India have occured in 1978, 1992, and 2007 [46], a trend seen also in Bihar [47]. Researchers have suggested uncertainty as to whether the recent reduction in VL cases is a result of limited IRS efficacy or the natural VL cycle in south Asia [26], but given the abundance of P.argentipes in Bihar, the redundant periodicity of VL incidence, and the time since the most recent VL peak in Bihar, it is not unreasonable to suggest that the latter hypothesis may be correct. Other issues such as underreporting could be a factor, as many researchers believe the reported cases to be a gross underestimation of the true number [48–50]. Reported cases have been estimated to represent as low as 5–8% the total cases occuring in Bihar [51]. House to house surveys conducted in 14 villages in Bihar suggested only 12.3% of VL cases were officially reported [48].
Our results suggest that the current vector control strategy in Bihar may benefit from the integration of another vector control strategy aimed at targeting outdoor P. argentipes. One such vector control strategy to consider would be the treating of village vegetation with toxic sugar baits [52–53] an approach that exploits male and female Phlebotomus spp. sand flies tendency to feed on sugar from plants. Although this approach has not been pursued in Bihar, previous studies report a possible association between P. argentipes and Palmyra palm trees (Borassus flabellifer) [28–29] and banana plants (Musa acuminata) [29,54], suggesting these plant species may be candidates for sugar bait application.
The association of P. argentipes with cattle enclosures and bovine blood meals observed during this study suggests treating bovines systemically with approved drugs may also be a promising approach. Laboratory studies performed in Bihar confirm that endectocides administered systemically to lesser-bandicoot rats (Bandicota bengalensis) and roof rats (Rattus rattus) were highly efficacious against adult and larval P. argentipes [55]. A pen study during which fipronil was orally administered to cattle indicated near 100% mortality of adult female P. argentipes blood feeding on cattle 21 days after a single oral dose and 100% mortality of P. argentipes larvae feeding on feces collected from the same animals 21 days post-exposure [56]. The oviposition sites of P. argentipes are largely unknown, but when eggs, larvae, and/or pupae are found in Bihari villages, they are often found in proximity to cattle sheds [57–59], perhaps because the feces provide nutrition for developing larvae, which if confirmed would validate fipronil as a potential larvacide. We recommend the oviposition behavior of P. argentipes be explored at great length in future studies, as knowledge regarding larval habitat would greatly improve vector control schemes.
Conclusion
We have collected abundant, contemporary ecological data from similar geographical locations in Bihar, which are suggested to produce more precise epidemiological models [60]. Our study is primarily an entomological field evaluation and in future studies the methods we implemented should be modified to include a baseline collection period and should be coupled with explicit epidemiological surveillance. Ecologically, our results suggest P. argentipes to 1) feed opportunistically on humans and bovines, 2) show a preference for cattle enclosures, and 3) be present outdoors in village vegetation. Because the majority of Bihari villagers sleep outdoors during periods when vector abundance is high, it is likely that many P. argentipes feed exophagically. This theory is supported by the lack of difference in P. argentipes abundance within IRS-treated and untreated villages, by previous field observations and blood meal analysis [11,17], and through observing P. argentipes infesting cattle during peak biting periods. Logically, IRS can only be efficacious in reducing endophagic vectors, which reinforces a need for supplemental vector control practices to reduce outdoor feeding populations. Because P. argentipes feeds heavily on bovines, endectocide-treated cattle may provide an appropriate means of reducing vectors unexposed to IRS-treated dwellings. By considering a complimentary form of vector control to better target exophagic, exophilic P. argentipes and by conducting explicit, frequent P. argentipes collection in combination with active and passive case detection, we could expand upon both integrated vector management and disease-vector surveilance, two of the main VL-reduction strategies discussed by the WHO [8]. As a result, we should be able to better protect outdoor-sleeping villagers and better estimate the sustainability of VL-reduction through program-initiated vector control.
Supporting information
(XLSX)
(DOCX)
(DOCX)
(DOCX)
(XLSX)
Microscopic images demonstrating the differences in appearance of a) male genitalia; and b) female spermatheca of Phlebotomus argenitpes, P. papatasi, and Sergentomyia babu, the three most common sand fly species collected in CDC light traps February 10-December 29, 2016.
(DOCX)
(DOCX)
Acknowledgments
We would like to thank CARE India (Patna, Bihar) for generously providing IRS data pertaining to our test villages in Muzaffarpur and Saran. We also would like to thank the village elders and residents who granted us permission to conduct the work and who provided us with much appreciated assistance when needed.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was funded by the Bill and Melinda Gates Foundation Grant No. 5112. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Desjeux P. The increase in risk factors for leishmaniasis worldwide. Trans R Soc Trop Med Hyg. 2001; 95: 239–243. [DOI] [PubMed] [Google Scholar]
- 2.Desjeux P. Worldwide increasing risk factors for leishmaniasis. Med Microbiol Immunol. 2001; 190: 77–79. [DOI] [PubMed] [Google Scholar]
- 3.Chappuis F, Sundar S, Hailu A, Ghalib H, Rijal S, et al. Visceral leishmaniasis: what are the needs for diagnosis, treatment and control? Nat Rev Microbiol. 2007; 5: 873–882. doi: 10.1038/nrmicro1748 [DOI] [PubMed] [Google Scholar]
- 4.Singh RK, Pandey HP, Sundar S. Visceral leishmaniasis (kala-azar): challenges ahead. Indian J Med Res. 2006; 123: 331–344. [PubMed] [Google Scholar]
- 5.Swaminath C. S., Shortt H. E., & Anderson L. A. P. Transmission of Indian kala-azar to man by the bites of Phlebotomus argentipes, Ann. and Brun. Indian J Med Res. 1942; 123(3), C473. [PubMed] [Google Scholar]
- 6.Dinesh DS, Kar SK, Kishore K, Palit A, Verma N, et al. Screening sandflies for natural infection with Leishmania donovani, using a non-radioactive probe based on the total DNA of the parasite. Ann Trop Med Parasitol. 2000; 94: 447–451. [DOI] [PubMed] [Google Scholar]
- 7.Guerin PJ, Olliaro P, Sundar S, Boelaert M, Croft SL, et al. Visceral leishmaniasis: current status of control, diagnosis, and treatment, and a proposed research and development agenda. Lancet Infect Dis. 2002; 2: 494–501. [DOI] [PubMed] [Google Scholar]
- 8.WHO Regional Office for South-East Asia. Regional strategic framework for elimination of Kala-azar from the south-east Asia region World Health Organization; 2012. Available from: http://www.searo.who.int/entity/vector_borne_tropical_diseases/documents/SEA-CD-239/en/ [Google Scholar]
- 9.Warburg A, Faiman R. Research priorities for the control of phlebotomine sand flies. J Vector Ecol. 2011; 36 Suppl 1: 10–16. [DOI] [PubMed] [Google Scholar]
- 10.Ghosh K, Mukhopadhyay J, Desai MM, Senroy S, Bhattacharya A. Population ecology of Phlebotomus argentipes (Diptera: Psychodidae) in West Bengal, India. J Med Entomol. 1999; 36: 588–594. [DOI] [PubMed] [Google Scholar]
- 11.Poché D, Garlapati R, Ingenloff K, Remmers J, Poché R. Bionomics of phlebotomine sand flies from three villages in Bihar, India. J Vector Ecol. 2011; 36 Suppl 1: S106–117. [DOI] [PubMed] [Google Scholar]
- 12.Dinesh DS, Kumar V, Kesari S, Kumar AJ, Das P. Is Phlebotomus argentipes Annandale and Brunetti (Diptera: Psychodidae) autogenous? J Vector Borne Dis. 2008; 45: 174–175. [PubMed] [Google Scholar]
- 13.Mukhopadhyay AK, Chakravarty AK. Bloodmeal preference of Phlebotomus argentipes & Ph. papatasi of north Bihar, India. Indian J Med Res. 1987; 86: 475–480. [PubMed] [Google Scholar]
- 14.Ghosh KN, Bhattacharya A, Ghosh TN. Blood meal analysis of Phlebotomus argentipes in eight districts of West Bengal. J Commun Dis. 1990; 22: 67–71. [PubMed] [Google Scholar]
- 15.Basak B, Kundu M, Tandon N. Observation on host preference of Phlebotomus argentipes in district South-24-Parganas, West Bengal, India. J Commun Dis. 1995; 27: 122–123. [PubMed] [Google Scholar]
- 16.Palit A, Bhattacharya SK, Kundu SN. Host preference of Phlebotomus argentipes and Phlebotomus papatasi in different biotopes of West Bengal, India. Intl J Environ Health Res. 2005; 15: 449–454 [DOI] [PubMed] [Google Scholar]
- 17.Garlapati RB, Abbasi I, Warburg A, Poché D, Poché R. Identification of bloodmeals in wild caught blood fed Phlebotomus argentipes (Diptera: Psychodidae) using cytochrome b PCR and reverse line blotting in Bihar, India. J Med Entomol. 2012; 49: 515–521. [DOI] [PubMed] [Google Scholar]
- 18.Gidwani K, Picado A, Rijal S, Singh SP, Roy L, Volfova V, et al. Serological markers of sand fly exposure to evaluate insecticidal nets against visceral leishmaniasis in India and Nepal: a cluster-randomized trial. PLoS Negl Trop Dis. 2011; 5(9): e1296 doi: 10.1371/journal.pntd.0001296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Joshi RD, Rai RN. Impact of DDT spraying on populations of Phlebotomus argentipes and P. papatasi in Varanasi district, Uttar Pradesh. J Commun Dis. 1994; 26(1): 56–58. [PubMed] [Google Scholar]
- 20.Kaul SM, Sharma RS, Dey KP, Rai RN, Verghese T. Impact of DDT indoor residual spraying on Phlebotomus argentipes in a kala-azar endemic village in eastern Uttar Pradesh. Bull World Health Organ. 1994; 72(1):79–81. [PMC free article] [PubMed] [Google Scholar]
- 21.Mukhopadhyay AK, Hati AK, Chakraborty S, Saxena NB. Effect of DDT on Phlebotomus sandflies in Kala-Azar endemic foci in West Bengal. J Commun Dis. 1996; 28(3):171–175. [PubMed] [Google Scholar]
- 22.Joshi AB, Das ML, Akhter S, Chowdhury R, Mondal D, Kumar V, et al. Chemical and environmental vector control as a contribution to the elimination of visceral leishmaniasis on the Indian subcontinent: cluster randomized controlled trials in Bangladesh, India and Nepal. BMC Med. 2009; 7(1): 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kumar V, Kesari S, Dinesh DS, Tiwari A, Kumar AJ, Kumar R, et al. A report on the indoor residual spraying (IRS) in the control of Phlebotomus argentipes, the vector of visceral leishmaniasis in Bihar (India): an initiative towards total elimination targeting 2015 (Series-1). J Vector Borne Dis. 2009; 46(3): 225–229. [PubMed] [Google Scholar]
- 24.Huda MM, Mondal D, Kumar V, Das P, Sharma SN, Das ML, et al. Toolkit for monitoring and evaluation of indoor residual spraying for visceral leishmaniasis control in the Indian subcontinent: application and results. J Trop Med 2011: doi: 10.1155/2011/876742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chowdhury R, Huda MM, Kumar V, Das P, Joshi AB, Banjara MR, et al. The Indian and Nepalese programmes of indoor residual spraying for the elimination of visceral leishmaniasis: performance and effectiveness. Ann Trop Med Parasitol. 2011; 105(1): 31–35. doi: 10.1179/136485911X12899838683124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Coleman M, Foster GM, Deb R, Singh RP, Ismail HM, Shivam P. DDT-based indoor residual spraying suboptimal for visceral leishmaniasis elimination in India. Proc Natl Acad Sci. 2015; 112(28): 8573–8578. doi: 10.1073/pnas.1507782112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dinesh DS, Ranjan A, Palit A, Kishore K, Kar SK. Seasonal and nocturnal landing/biting behaviour of Phlebotomus argentipes (Diptera: Psychodidae). Annals of Tropical Medicine & Parasitology. 2001. March 1;95(2):197–202. [DOI] [PubMed] [Google Scholar]
- 28.Poché RM, Garlapati R, Elnaiem D-EA, Perry D, Poché D. The role of Palmyra palm trees (Borassus flabellifer) and sand fly distribution in northeastern India. J Vector Ecol. 2012; 37: 148–153. doi: 10.1111/j.1948-7134.2012.00211.x [DOI] [PubMed] [Google Scholar]
- 29.Poché DM, Poché RM, Mukherjee S, Franckowiak GA, Briley LN, Somers DJ, et al. Phlebotomine sandfly ecology on the Indian subcontinent: does village vegetation play a role in sandfly distribution in Bihar, India? Med Vet Entomol. 2017; doi: 10.1111/mve.12224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weather Underground. Patna India: Weather History for VEPT. The Weather Company, LLC. 2017. Available from: https://www.wunderground.com/history/airport/VEPT/2016/2/1/MonthlyHistory.html?req_city=Patna&req_statename=India&reqdb.zip=&reqdb.magic=&reqdb.wmo.
- 31.Ilango K. Phlebotomine sandfly reproduction: fine structure and function of the spermathecae Doctoral Dissertation, London School of Hygiene & Tropical Medicine. 1995. Available from: http://researchonline.lshtm.ac.uk/1416608/1/481220.pdf
- 32.Surendran SN, Karunaratne SP, Adams Z, Hemingway J, Hawkes NJ. Molecular and biochemical characterization of a sand fly population from Sri Lanka: evidence for insecticide resistance due to altered esterases and insensitive acetylcholinesterase. Bull Entomol Res. 2005; 95(4): 371–380. [DOI] [PubMed] [Google Scholar]
- 33.Abbasi I, Cunio R, Warburg A. Identification of blood meals imbibed by Phlebotomine sand flies using cytochrome b PCR and reverse line blotting. Vector Borne Zoonotic Dis 9: 79–86. doi: 10.1089/vbz.2008.0064 [DOI] [PubMed] [Google Scholar]
- 34.Dhariwal AC, Gupta D, Roy N, Raina VK. Operational guidelines on kala-azar (Visceral leishmaniasis) elimination in India-2015. National Vector Borne Disease Control Programme. 2015. Available from: http://www.nvbdcp.gov.in/Doc/opertional-guideline-KA-2015.pdf. [Google Scholar]
- 35.Alexander N, 2012. Analysis of parasite and other skewed counts. Trop Med Int Health. 2009; 17(6): 684–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stauch A, Duerr HP, Picado A, Ostyn B, Sundar S, Rijal S., et al. Model-based investigations of different vector-related intervention strategies to eliminate visceral leishmaniasis on the Indian subcontinent. PLoS Negl Trop Dis 2014; 8: e2810 doi: 10.1371/journal.pntd.0002810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Poché DM, Grant WE and Wang HH. Visceral leishmaniasis on the Indian subcontinent: modelling the dynamic relationship between vector control schemes and vector life cycles. PLoS Negl Trop Dis. 2016; 10(8): e0004868 doi: 10.1371/journal.pntd.0004868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.WHO World Health Organization. Indoor Residual Spraying: an Operation Manual for Indoor Residual Spraying (IRS) for Malaria Transmission Control and Elimination, Second Edition WHO, Geneva, Switzerland: 2015. [Google Scholar]
- 39.Perry D, Dixon K, Garlapati R, Gendernalik A, Poché D, Poché R. (2013) Visceral leishmaniasis prevalence and associated risk factors in the Saran district of Bihar, India, from 2009 to July of 2011. Am J Trop Med Hyg. 2012; 88: 778–784. doi: 10.4269/ajtmh.12-0442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kirstein OD, Faiman R, Knigin A, Gueta H, Stone A, Warburg A. Studies on the behaviour and control of phlebotomine sandflies using experimental houses. Medical and Veterinary Entomology. 2017. August 3. [DOI] [PubMed] [Google Scholar]
- 41.Killick-Kendrick R, Rioux JA, Bailly M, Guy MW, Wilkes TJ, Guy FM, et al. Ecology of leishmaniasis in the south of France. 20. Dispersal of Phlebotomus ariasi Tonnoir, 1921 as a factor in the spread of visceral leishmaniasis in the Cevennes. Annales de parasitologie humaine et comparee. 1983; 59(6): 555–572. [PubMed] [Google Scholar]
- 42.Orshan L, Elbaz S, Ben-Ari Y, Akad F, Afik O, Ben-Avi I, et al. Distribution and dispersal of Phlebotomus papatasi (Diptera: Psychodidae) in a zoonotic cutaneous leishmaniasis focus, the Northern Negev, Israel. PLoS Negl Trop Dis. 2016; 10(7): e0004819 doi: 10.1371/journal.pntd.0004819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.National Vector Borne Disease Programme. Kala-azar cases and deaths in the country since 2010. Government of India. Available from: http://www.nvbdcp.gov.in/ka-cd.html.
- 44.Dye C, Wolpert DM. Earthquakes, influenza and cycles of Indian kala-azar. Trans R Soc Trop Med Hyg. 1988; 82(6): 843–850. [DOI] [PubMed] [Google Scholar]
- 45.Ashford R, Bettini S. Ecology and epidemiology: Old World The Leishmaniasis in Biology and Medicine. Peters W, Kilick-Kendrick R. (editors). London: Academic Press; 1987. pp. 365–424. [Google Scholar]
- 46.Muniaraj M. The lost hope of elimination of Kala-azar (visceral leishmaniasis) by 2010 and cyclic occurrence of its outbreak in India, blame falls on vector control practices or co-infection with human immunodeficiency virus or therapeutic modalities? Trop Parasitol. 2014; 4(1): 10–19. doi: 10.4103/2229-5070.129143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Malaviya P, Picado A, Singh SP, Hasker E, Singh RP, Boelaert M, et al. Visceral leishmaniasis in Muzaffarpur district, Bihar, India from 1990 to 2008. PLoS One. 2011; 6(3): e14751 doi: 10.1371/journal.pone.0014751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Singh SP, Reddy DCS., Rai M, Sundar S. Serious underreporting of visceral leishmaniasis through passive case reporting in Bihar, India. Trop Med Int Health. 2006; 11(6): 899–905. doi: 10.1111/j.1365-3156.2006.01647.x [DOI] [PubMed] [Google Scholar]
- 49.Boelaert M, Meheus P, Sanchez A, Singh SP, Vanlerberghe V, Picado A, et al. The poorest of the poor: a poverty appraisal of households affected by visceral leishmaniasis in Bihar, India. Trop Med Int Health. 2009; 14(6): 639–644. doi: 10.1111/j.1365-3156.2009.02279.x [DOI] [PubMed] [Google Scholar]
- 50.Singh V, Ranjan A, K. Topno R, Verma R, Siddique N, Ravidas V.N., et al. Short Report: Estimation of Under-Reporting of Visceral Leishmaniasis Cases in Bihar, India. Am J Trop Med Hyg. 2010; 82(1): 9–11. doi: 10.4269/ajtmh.2010.09-0235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mubayi A, Castillo-Chavez C, Chowell G, Kribs-Zaleta C, Ali Siddiqui N, et al. Transmission dynamics and underreporting of Kala-azar in the Indian state of Bihar. J Theor Biol. 2010; 262(1): 177–185. doi: 10.1016/j.jtbi.2009.09.012 [DOI] [PubMed] [Google Scholar]
- 52.Schlein Y, Müller GC. Experimental control of Phlebotomus papatasi by spraying attractive toxic sugar bait (ATSB) on vegetation. Trans R Soc Trop Med Hyg. 2010; 104(12): 766–771. doi: 10.1016/j.trstmh.2010.08.014 [DOI] [PubMed] [Google Scholar]
- 53.Qualls WA, Müller GC, Khallaayoune K, Revay EE, Zhioua E, Kravchenko VD, et al. Control of sand flies with attractive toxic sugar baits (ATSB) and potential impact on non-target organisms in Morocco. Parasit Vector. 2015; 8(1): 87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chowdhury R, Kumar V, Mondal D, Das ML, Das P, Dash AP, Kroeger A. Implication of vector characteristics of Phlebotomus argentipes in the kala-azar elimination programme in the Indian sub-continent. Pathog Glob Health. 2016; 110(3): 87–96. doi: 10.1080/20477724.2016.1180775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ingenloff K, Garlapati R, Poché DM, Singh MI, Remmers JL, Poché DM. Feed-through insecticides for the control of the sand fly Phlebotomus argentipes. Med Vet Entomol. 2013; 27(1): 10–18. doi: 10.1111/j.1365-2915.2012.00995.x [DOI] [PubMed] [Google Scholar]
- 56.Poché RM, Garlapati R, Singh MI, Poché DM. Evaluation of fipronil oral dosing to cattle for control of adult and larval sand flies under controlled conditions. J Med Entomol. 2013; 50(4): 833–837. [PubMed] [Google Scholar]
- 57.Ghosh KN, Bhattacharya A. Breeding places of Phlebotomus argentipes Annandale and Brunetti (Diptera: Psychodidae) in West Bengal, India. Parassitologia. 1991; 33 Suppl: 267–272. [PubMed] [Google Scholar]
- 58.Kundu M, Basak B, Tandon N. A simple technique for detection and isolation of Phlebotomous argentipes larvae from soil samples. J Commun Dis. 1995; 27(1): 58–59. [PubMed] [Google Scholar]
- 59.Singh R, Lal S, Saxena VK. Breeding ecology of visceral leishmaniasis vector sandfly in Bihar state of India. Acta Trop. 2008; 107(2): 117–120. doi: 10.1016/j.actatropica.2008.04.025 [DOI] [PubMed] [Google Scholar]
- 60.Cameron MM, Acosta-Serrano A, Bern C, Boelaert M, Den Boer M, Burza S, et al. Understanding the transmission dynamics of Leishmania donovani to provide robust evidence for interventions to eliminate visceral leishmaniasis in Bihar, India. Parasit Vectors. 2016; 9(1): doi: 10.1186/s13071-016-1309-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
(DOCX)
(DOCX)
(DOCX)
(XLSX)
Microscopic images demonstrating the differences in appearance of a) male genitalia; and b) female spermatheca of Phlebotomus argenitpes, P. papatasi, and Sergentomyia babu, the three most common sand fly species collected in CDC light traps February 10-December 29, 2016.
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.