Abstract
Introduction
The evidence linking dietary intake with diabetic retinopathy (DR) is growing but unclear. We conducted a systematic review of the association between dietary intake and DR.
Methods
We systematically searched PubMed, Embase, Medline, and the Cochrane Central register of controlled trials, for publications between January 1967 and January 2017 using standardized criteria for diet and DR. Interventional and observational studies investigating micro- and macro-nutrient intakes; food and beverage consumptions; and dietary patterns were included. Study quality was evaluated using a modified Newcastle-Ottawa scale for observational studies, and the Cochrane collaboration tool for interventional studies.
Results
Of 4265 titles initially identified, 31 studies (3 interventional, 28 Observational) were retained. Higher intakes of dietary fibre, oily fish, and greater adherence to a Mediterranean diet were protective of DR. Conversely, high total caloric intake was associated with higher risk of DR. No significant associations of carbohydrate, vitamin D, and sodium intake with DR were found. Associations of antioxidants, fatty acids, proteins and alcohol with DR remain equivocal.
Conclusions
Dietary fibre, oily fish, a Mediterranean diet and a reduced caloric intake are associated with lower risk of DR. Longitudinal data and interventional models are warranted to confirm our findings and better inform clinical guidelines.
Introduction
Diabetic retinopathy (DR) is a major microvascular complication of diabetes and a leading cause of vision loss and blindness globally[1]. Nearly all patients with type 1 diabetes and >60% of patients with type 2 diabetes will have some form of DR within 20 years of developing diabetes[2, 3]. With the rapidly increasing prevalence of diabetes worldwide, the prevention and management of DR has become a crucial public health concern[4].
Optimal nutrition forms a crucial component of overall diabetes care[5, 6]. While comprehensive dietary guidelines for overall diabetes management have been developed, these guidelines do not extend specifically to the prevention and management of DR[7, 8]. As such, DR-specific dietary recommendations for patients with diabetes at risk of development or progression of DR are not available. Several studies have explored the association between various dietary components and DR; they include micronutrients (e.g. antioxidants, sodium, vitamin D)[9–11], macronutrients (e.g. carbohydrates, proteins, fats)[12–14], food groups and beverages (e.g. fruit and vegetables, fish, coffee, tea)[15–18], as well as broader dietary patterns and characteristics (e.g. Mediterranean [Med] diet, total caloric intake)[10, 19]. However, findings remain inconclusive, and current evidence do not inform the specific dietary components which are likely to reduce (or increase) the risk of DR.
There are also few comprehensive reviews on diet and DR, with existing reviews mostly either focused on a specific nutrient or food group (e.g. alcohol, micronutrients)[20–22] or providing only a summary of the potential of the diet to influence DR pathogenic mechanisms. To our knowledge, there are no comprehensive review of the entire spectrum of dietary components and their association or effects on DR as a clinical outcome[23]. To address this major clinical gap, we performed a systematic review on the associations between dietary intake and DR, with the primary goal of providing a comprehensive assessment of the existing knowledge on the topic. We also identified key knowledge gaps and suggest future research directions.
Methodology
Literature search
No existing protocol exists for this systematic review. We performed a systemic review and comprehensive literature search using four databases (PubMed, Embase, Medline and the Cochrane Central Register of Controlled Trials), with a date range of January 1967 to January 2017 with no language restrictions. The databases were systematically searched using a combination of the following keywords: Diet OR Dietary factors OR Dietary Intake OR intake OR Consumption OR food OR nutrition OR dietary protein OR antioxidant OR Nutrient OR Fibre OR carbohydrate OR fat OR fatty acid OR glycemic food OR vegetables OR Fruit OR vitamin OR caffeine OR fish OR alcohol OR calorie OR caloric OR Mediterranean AND Diabetic Retinopathy OR Diabetic Complications OR Microvascular Complications OR Diabetic Macular Edema.
During preliminary searches, search keywords were initially based on similar reviews[24–27], which used broader generic dietary terms such as “diet OR dietary factors OR dietary intake OR consumption”. Furthermore, for an improved search comprehensiveness, additional specific dietary terms (such as “fibre” or “antioxidants”) based on areas our preliminary search found evidence of prior research, were included. This process continued until a search saturation point was found; i.e. the point at which additional terms showed no improvement in our search result. Relevant references identified from the bibliographies of pertinent articles or review papers were also retrieved.
Study selection
Using our search strategy, 4265 titles were initially identified. Two authors (MW and RM) assessed the titles independently according to predefined inclusion criteria. Studies were then systematically excluded after detailed examination, if the title and abstract were not relevant. The full-text articles of studies deemed potentially relevant were also obtained, particularly if there was insufficient information within the abstract for exclusion.
Inclusion criteria
Our eligibility criteria were based on the PICOS (participants, intervention, comparability, outcomes, study design) framework recommended by the PRISMA guidelines[28].
Study Type. We included both interventional (randomized controlled trials, and post-hoc analyses of interventional studies) and observational studies (cross-sectional, case-control and prospective).
Participants. Studies involved on human participants with type 1 diabetes, type 2 diabetes, or both.
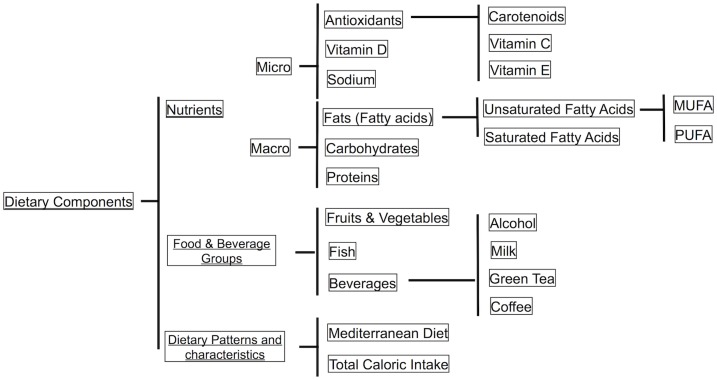
Exposures (interventions). Exposures had to measure a form of dietary intake, either through standard dietary methods (validated food frequency questionnaire, 24 hr- dietary recall, dietary history etc.), general interviewer-administered questionnaires or estimations from biomarker levels (such studies had to use biomarkers as a means to estimate dietary intake levels; before using these final estimated dietary intake levels as the main exposure). Dietary intake included the consumption of specific foods and beverages, the intake of micro/macronutrients, and adherence to meal patterns (Fig 1). Selected studies also had to specify how dietary intake was measured and quantified.
Outcomes. Outcomes were the prevalence, incidence or progression of DR or diabetic macula edema (DME). We accepted studies using different DR assessment methods, including but not limited to: fundus photography with or without mydriatic eyedrops; fundoscopy; direct or indirect ophthalmoscopy; and fluorescein angiography. We also included studies using different scales to grade DR severity, including but not limited to the Early Treatment Diabetic Retinopathy Study (ETDRS) scale[29] or the International Classification system of DR[30].
Fig 1. Overview of dietary components assessed in the studies included in our systematic review.
Exclusion criteria
The following types of papers were excluded:
Reviews
Studies on animals, and in-vitro / in-vivo studies.
Studies on a non-diabetic population, including participants with impaired glucose tolerance (IGT) or pre-diabetes.
Studies not defining the exposure or outcome variables
Studies measuring biomarkers in serum, blood, or urine without any link to dietary intake.
Exposures which involved multi-formulaic supplements (supplements which comprise of multiple different types of nutrients)
Studies only measuring outcomes of “retinal changes”, “visual acuity” or “microvascular complications” without specific reference to DR/DME.
Articles with insufficient data to draw conclusions. This included any form of data insufficiency which did not enable us to draw conclusions from/evaluate the study, (e.g. lack of exposure/outcome definitions, or lack of statistical analysis)
Data extraction
A standardized data extraction form based on the “Strengthening the Reporting of Observational Studies in Epidemiology” (STROBE) statement[31] was used to extract the following relevant data from each included article: authors, year, study design, sample size, population characteristics, age of participants, dietary components, method of dietary assessment, DR outcome type, method of DR diagnosis, DR categorization, adjustment for confounders used in analysis, statistical methods used, and summary of key findings. Data extraction was done by one author (MW) and vetted by another (RM). Any potential disagreements were resolved through consulting the corresponding author (EL).
Study quality evaluation
The quality of observational studies was assessed using a modified version of the Newcastle Ottawa Scale (NOS), a validated tool for evaluating observational study designs[32]. Originally designed to assess prospective and case-control studies, an adapted version of the NOS was used in the current study for the assessment of cross-sectional studies[33, 34]. The NOS uses three main bias-reducing criteria to award up to a maximum of 9 stars: (a) the selection and representativeness of the participants (maximum of 4 stars), (b) the comparability of groups (maximum of 2 stars), and (c) the ascertainment of exposure (for case-control) or outcome (for prospective and cross-sectional) (maximum of 3 stars). We also gave studies an additional star if they assessed dietary intake using validated dietary measurement tools, such as validated FFQs, or 24hr dietary record by dietician interviews, or if they estimated dietary intake from biomarker levels. Following previous reviews, studies assigned 0–4, 5–7, and ≥8 stars were considered as low, medium and high quality respectively[35–37].
For the evaluation of interventional studies (RCT), the Cochrane Collaboration Risk of Bias Tool was used, which measures risk of bias through seven criteria; sequence generation, allocation concealment, blinding of participants, masking of outcome assessment, incomplete outcome data, selective outcome reporting, and other sources of bias. Each criterion is individually graded according to whether it is deemed to have a high, low or unclear risk of bias. Studies which had a low risk of bias for all key domains were considered to be at an overall low risk of bias, studies with low or unclear risk of bias for all key domains were considered to be at a medium risk of bias, and studies with high risk of bias for one or more key domains were considered at an overall high risk of bias[38].
Results
Description of studies
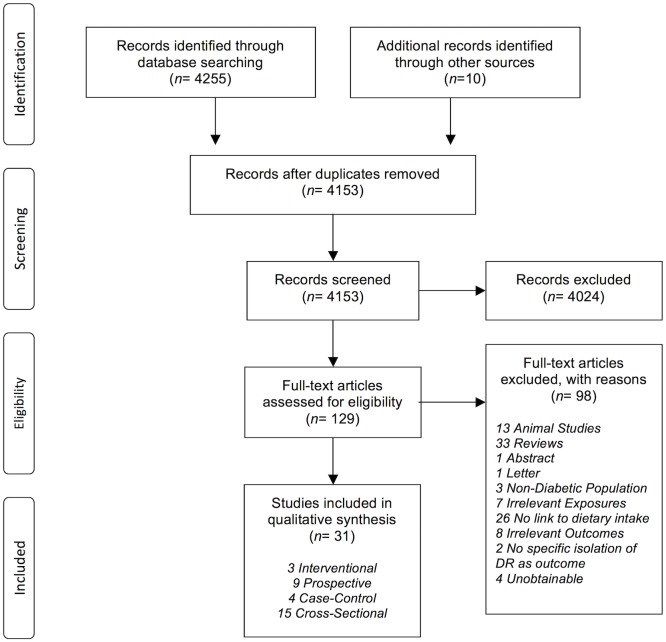
Of 4266 titles screened, 129 abstracts were extracted for detailed evaluation, of which 31 papers adhered to our inclusion criteria (Fig 2). They comprised of 3 interventional (RCTs) and 28 observational studies (9 prospective, 4 case-control, and 15 cross-sectional)
Fig 2. PRISMA Flow Diagram: Selection of included studies.
Measurement of exposures and outcomes
For measurement of dietary intake in observational studies, most studies (n = 17) used standard dietary methods, including 24-hour recall (n = 2), food frequency questionnaires-FFQ (n = 14)[9–12, 15, 16, 18, 39–45] or 3-D food records (n = 1). Some studies used a general interviewer-administered questionnaire (n = 10)[17, 46–54], while one study estimated dietary sodium intake from urinary excretion levels. Studies determined DR outcomes through fundus photographs (n = 18), ophthalmologist examination (n = 6), direct ophthalmoscopy (n = 2) or linkage to patient’s previous medical/clinical/hospital records (n = 2) (Table 1).
Table 1. Characteristics of studies (n = 31).
| Author, year | Sample Size | Diabetes | Age | Dietary Component | Dietary Assessment | DR outcome | Method of Diagnosing DR | DR Classification | Quality |
|---|---|---|---|---|---|---|---|---|---|
| Interventional Studies (n = 3) | |||||||||
| Houtsmuller, 1979 | 96 | Any Diabetes | n.a. | Saturated Fat vs Unsaturated Fat | n.a. | Progression & incidence | Fundus Photography | None, NPDR, PDR, PRP | High Bias |
| Howard-Williams, 1985 | 149 | Any diabetes | <66 | Saturated Fat vs Unsaturated Fat | n.a. | Incidence | Ophthalmologist Examination | None, Retinopathy | High Bias |
| Diaz-Lopez, 2015 | 3614 | T2DM | 55–80 | Med Diet | n.a. | Incidence | Ophthalmologist Examination | None, NPDR, PDR | Moderate Bias |
| Prospective Studies (n = 9) | |||||||||
| Young, 1984 | 296 | Any Diabetes | 20–59 | Alcohol | Self Report in general questionnaire | Incidence | Direct Ophthalmoscopy | Modified ETDRS | 8 |
| Moss, 1993 | Young: 439 Older: 478 |
Any Diabetes | 21–94 | Alcohol | Self Report in general questionnaire | Incidence & progression | Fundus Photography | Modified ETDRS | 9 |
| Roy, 2010 | 469 | T1DM | NR* | MUFA, PUFA, Oleic Acid, Protein, Dietary Fibre, carbohydrates, sodium, high caloric | Validated FFQ | Progression & Incidence | Fundus Photography | Modified ETDRS | 9 |
| Cundiff, 2005 | 1412 | T1DM | 13–39 | MUFA, PUFA, Carbohydrates, Protein, Dietary Fibre, Sodium, Alcohol, High Calories | Dietary History Interview | Progression | Fundus Photography | Modified ETDRS | 8 |
| Lee, 2010 | 1239 | T2DM | 55–81 | Alcohol | Self Report in general questionnaire | Progression | Fundus Photography | Modified ETDRS | 9 |
| Tanaka, 2013 | 978 | T2DM | 40–70 | Fruit & vegetables, Vitamin C, Vitamin E, Carotenoids | Validated FFQ + 24 Hr Dietary Recall | Incidence | Ophthalmologist Examination | International Classification System | 10 |
| Horikawa, 2014 | 978 | T2DM | 40–70 | Sodium | Validated FFQ | Progression& incidence | Ophthalmologist Examination | International Classification System | 10 |
| Horikawa, 2017 | 978 | T2DM | 40–70 | Carbohydrates | Validated FFQ | Progression & Incidence | Ophthalmologist Examination | International Classification System | 10 |
| Sala-Vila, 2016 | 3482 | T2DM | 55–80 | PUFA (LCw3) & Oily Fish | Validated FFQ | Incidence | Clinical and Hospital Records | None, NPDR, PDR | 9 |
| Case-Control Studies (n = 4) | |||||||||
| Giuffre, 2004 | Cse = 45 Ctr: 87 |
Any Diabetes | >40 | Alcohol | Self Report in general questionnaire | Prevalence | Direct Opthalmoscopy + Fundus Photography | ETDRS | 7 |
| Ma, 2014 | Cse: 100 Ctr: 100 |
T2DM | >18 | Green Tea | Questionnaire on tea consumption | Prevalence | Fundus Photography | ETDRS | 8 |
| Alcubierre, 2015 | Cse: 139 Ctr: 144 |
T2DM | NR | Vitamin D, calcium | Validated FFQ | Prevalence | Ophthalmologist Examination | International Classification System | 8 |
| Alcubierre, 2016 | Cse: 146 Ctr: 148 |
T2DM | 40–75 | MUFA, PUFA, Oleic Acid, Carbohydrates, Protein, Dietary Fibre, | Validated FFQ | Prevalence | Ophthalmologist Examination | International Classification System | 10 |
| Cross-Sectional Studies (n = 15) | |||||||||
| Roy, 1989 | 34 | Any Diabetes | NR | MUFA, PUFA, Carbohydrates, Protein, Dietary Fibre | 3-d Food Record | Prevalence | Fundus Photography | Modified Airlie House Classification | 5 |
| Moss, 1992 | Young: 891 Older: 987 |
Any Diabetes | 2–96 | Alcohol | Self Report in general questionnaire | Prevalence | Fundus Photography | Modified Airlie House | 9 |
| Mayer-Davis, 1998 | 387 | T2DM | 20–74 | Vitamin C, E & Beta-Carotene | 24 Hr Dietary Recall | Prevalence | Dilated Fundus Photography | Modified Airlie House Criteria | 9 |
| Millen, 2004 | 1353 | Any Diabetes | 45–65 | Vitamin C & E | Validated FFQ | Prevalence | Non-Dilated Fundus Photography | Modified Airlie House | 8 |
| Beulens, 2008 | 1857 | T1DM | 15–60 | Alcohol | Self Report in general questionnaire | Prevalence | Dilated Fundus Photography | None, background, proliferative | 10 |
| Ganesan, 2012 | 1261 | Any Diabetes | >40 | Dietary Fibre | Validated Fibre Questionnaire | Prevalence | Dilated Fundus Photography | Modified ETDRS | 10 |
| Harjutsalo, 2013 | 3608 | T1DM | NR | Alcohol | Self Report in general questionnaire | Prevalence | History of Laser Photocoagulation | Severe DR Vs None | 8 |
| Lugo-Radillo, 2013 | 88 | Any Diabetes | NR | Fruit & Vegetables | Oral Questionnaire on F&V Consumption | Prevalence | Ophthalmologist Examination | International Classification System | 5 |
| Mahoney, 2014 | 155 | Any Diabetes | >40 | Fruit & Vegetables | Validated FFQ | Prevalence | Undilated Fundus Photography | ETDRS | 8 |
| Engelen, 2014 | 1880 | T1DM | 15–60 | Sodium | Estimated from Urinary Sodium Excretion | Prevalence | Fundus Photography | None, NPDR, PDR | 7 |
| Kumari, 2014 | 353 | Any Diabetes | 21–95 | Coffee | Questionnaire on coffee consumption | Prevalence | Dilated Fundus Photography | Modified Airlie House Classification | 8 |
| Sasaki, 2015 | 379 | Any Diabetes | >18 | Vitamin C, E, B-Carotene, MUFA, PUFA, carbohydrates, protein | Validated FFQ | Prevalence | Fundus Photography | Modified ETDRS | 8 |
| Fenwick, 2015 | 395 | T2DM | >18 | Alcohol | Validated FFQ | Prevalence | Undilated Fundus Photography | ETDRS | 10 |
| Millen, 2016 | 1305 | Any Diabetes | 45–65 | Vitamin D, Fish, Milk | Validated FFQ | Prevalence | Fundus Photography | Modified Airlie House | 9 |
| Sahli, 2016 | 1430 | Any Diabetes | 45–65 | Carotenoids (Lutein) | Validated FFQ | Prevalence | Non-Dilated Fundus Photography | ETDRS | 9 |
Methodological quality
Of 28 observational studies, the majority had high NOS scores, with 25 classified as “high quality” (>8 stars) and 3 classified as “moderate quality” (5–7 stars). Of the 3 interventional studies, 2 and 1 had a high-risk and medium risk of bias, respectively (Table 1).
Associations between micronutrient intake and DR
Antioxidants
Carotenoids, Vitamin C and Vitamin E are common antioxidants, and their associations with DR are reflected in Table 2.
Table 2. Dietary intake of micro-nutrients and DR.
| Author, year | Association | Study Design | Quality | Dietary Factor | Sample Size | DR outcome type | Confounders adjusted for | Statistical methods | Main Findings |
|---|---|---|---|---|---|---|---|---|---|
| Antioxidants | |||||||||
| Carotenoids | |||||||||
| Tanaka, 2013 | Protective | Prospective | 10 | Carotenoids | 978 | Incidence | Age, sex, BMI, HbA1c, duration of diabetes, treatment by insulin, treatment by oral hypoglycemic agents without insulin, systolic blood pressure, LDL Cholesterol, HDL cholesterol, triglycerides, smoking, alcohol, physical activity, total energy intake, proportions of dietary protein, fat, carbohydrate, saturated fatty acids, n-6 PUGA and n-3 PUFA, cholesterol & sodium | Multivariate Cox Regression | Highest Intake Quartile (Q4) vs lowest Intake Quartile (Q1), HR: 0.52 (0.33–0.81) |
| Mayer-Davis, 1998 | NS | Cross Sectional | 9 | Carotenoids (B-Carotene) | 387 | Prevalence | Age, duration of diabetes, ethnicity, glycosylated hemoglobin, hypertension, caloric intake, gender & insulin use. | Multivariable logistic regression | No significant associations with DR (Data not reported) |
| Sahli, 2016 | NS | Cross Sectional | 9 | Carotenoids (Lutein) | 1430 | Prevalence | HbA1c, blood pressure, duration of diabetes, race, total energy consumption & study center | Multivariable logistic regression | Intake Q3 vs Q1, OR: 1.54 (0.96–2.47) Intake Q4 vs Q1, OR: 1.41 (0.87–2.28) |
| Sasaki, 2015 | NS | Cross Sectional | 8 | Carotenoids (B-Carotene) | 379 | Prevalence | Energy Intake | Data not reported | No significant associations with DR (Data not reported) |
| Vitamin C | |||||||||
| Tanaka, 2013 | Protective | Prospective | 10 | Vitamin C | 978 | Incidence | Age, sex, BMI, HbA1c, duration of diabetes, treatment by insulin, treatment by oral hypoglycemic agents without insulin, systolic blood pressure, LDL Cholesterol, HDL cholesterol, triglycerides, smoking, alcohol, physical activity, total energy intake, proportions of dietary protein, fat, carbohydrate, saturated fatty acids, n-6 PUGA and n-3 PUFA, cholesterol & sodium | Multivariate Cox Regression | Intake Q4 vs Q1, HR: 0.61 (0.39–0.96) |
| Mayer-Davis, 1998 | Risk | Cross Sectional | 9 | Vitamin C | 387 | Prevalence | Age, duration of diabetes, ethnicity, glycosylated hemoglobin, hypertension, caloric intake, gender & insulin use. | Multivariable logistic regression | Intake 9th Decile vs 1st Quintile, OR: 2.21 (p = 0.011) |
| Millen, 2004 | NS | Cross Sectional | 8 | Vitamin C | 1353 | Prevalence | Total energy intake, race, duration of diabetes, serum glucose, hypertension, BMI, waist-hip ratio, smoking, alcohol, drinking status, plasma triacylglycerol, plasma cholesterol, hematocrit value, prevalent coronary heart disease, diabetes treatment group, &use of oral hypoglycemic agents or use of insulin | Multivariable logistic regression | Intake Q4 vs Q1, OR: 1.4 (0.8–2.4) |
| Sasaki, 2015 | NS | Cross Sectional | 8 | Vitamin C | 379 | Prevalence | Energy Intake | Data not reported | No significant associations with DR (Data not reported) |
| Vitamin E | |||||||||
| Mayer-Davis, 1998 | Risk (in insulin non-taking subjects) | Cross Sectional | 9 | Vitamin E | 387 | Prevalence | Age, duration of diabetes, ethnicity, glycosylated hemoglobin, hypertension, caloric intake, gender & insulin use. | Multivariable logistic regression |
Insulin Subjects: No Association Non-Insulin taking Subjects: Intake 10th Decile vs 1st Quintile, OR: 3.79 (p<0.02) |
| Tanaka, 2013 | NS | Prospective | 10 | Vitamin E | 978 | Incidence | Age, sex, BMI, HbA1c, duration of diabetes, treatment by insulin, treatment by oral hypoglycemic agents without insulin, systolic blood pressure, LDL Cholesterol, HDL cholesterol, triglycerides, smoking, alcohol, physical activity, total energy intake, proportions of dietary protein, fat, carbohydrate, saturated fatty acids, n-6 PUGA and n-3 PUFA, cholesterol & sodium | Multivariate Cox Regression | Intake Q4 vs Q1, HR: 0.84 (0.51–1,40) |
| Millen, 2004 | NS | Cross Sectional | 8 | Vitamin E | 1353 | Prevalence | Total energy intake, race, duration of diabetes, serum glucose, hypertension, BMI, waist-hip ratio, smoking, alcohol, drinking status, plasma triacylglycerol, plasma cholesterol, hematocrit value, prevalent coronary heart disease, diabetes treatment group & use of oral hypoglycemic agents or use of insulin | Multivariable logistic regression | Intake Q4 vs Q1, OR: 1.4 (0.8–2.3) |
| Sasaki, 2015 | NS | Cross Sectional | 8 | Vitamin E | 379 | Prevalence | Energy Intake | Data not reported | No significant associations with DR (Data not reported) |
| Vitamin D | |||||||||
| Millen, 2016 | NS | Cross-Sectional | 9 | Vitamin D | 1305 | Prevalence | Race, duration of diabetes, HbA1c & hypertension | Multivariate Logistic Regression | Intake Q4 Vs Q1, OR: 1.20 (0.76–1.89) |
| Alcubierre, 2015 | NS | Case-Control | 8 | Vitamin D | Case: 139 Ctrl: 144 |
Prevalence | NIL | Chi-Squared | No significant associations with DR (p = 0.93) |
| Calcium | |||||||||
| Alcubierre, 2015 | NS | Case-Control | 8 | Calcium | Case: 139 Ctrl: 144 |
Prevalence | NIL | Chi-Squared | No significant associations with DR (p = 0.65) |
| Sodium | |||||||||
| Roy, 2010 | Risk (For DME) NS for DR |
Prospective | 10 | Sodium | 469 | Progression & Incidence | Total caloric intake, age, sex, physical exercise, glycated hemoglobin, oleic acid intake, protein intake, carbohydrate intake & hypertension | Multivariable Logistic Regression | No significant associations with DR For DME, Intake Q4 Vs Q1, OR: 1.43 (1.10–1.86) |
| Horikawa, 2014 | NS | Prospective | 10 | Sodium | 978 | Progression& incidence | Age, Sex, BMI, HbA1c, diabtes duration, LDL cholesterol, HDL cholesterol, log-transformed triglycerides, insulin treatment, treatment by lipid-lowering agents, current smoking, alcohol intake, energy intake, sodium intake & physical activity | Multivariable Cox Regression | Intake Q4 Vs Q1, HR: 1.10 (0.75–1.61) |
| Cundiff, 2005 | NS | Prospective | 8 | Sodium | 1412 | Progression | Energy Intake | Spearman Correlation | Sodium in mg/kcal against DR progression rate, r = 0.02 (p = 0.47) |
| Engelen, 2014 | NS | Cross-Sectional | 7 | Sodium | 1880 | Prevalence | Age, sex, BMI, smoking, urinary potassium excretion, antihypertensive medication, total energy intake, physical activity, sat fat intake, protein intake, fibre intake & alcohol intake | Multivariable Logistic Regression | Per 1g/day increase in dietary salt intake, OR: 1.00 (0.96–1.04) |
Carotenoids—Using a prospective design, Tanaka and associates[15] found carotenoids to be protective of incident DR using a multivariate cox regression analysis (4th (highest) intake quartile [Q4] vs. 1st (lowest) intake quartile [Q1], Hazard Ratio [HR]: 0.52, 95% confident interval [CI]: 0.33–0.81). On the other hand, the other three cross-sectional studies reported non-significant associations between carotenoids and DR[12, 44, 55].
Vitamin C—Similarly, Tanaka and associates[15] reported a protective relationship between Vitamin C intake and incident DR (Q4 vs. Q1, HR, 95% CI: 0.61, 0.39–0.96), in contrast to a cross-sectional study by Mayer-Davis and colleagues[55] that reported a risk association between vitamin C intake and prevalent DR (9th decile vs. 1st quintile, Odds Ratio [OR]: 2.21, p = 0.01). The remaining two other cross-sectional studies found non-significant relationships between Vitamin C intake and DR[11, 12].
Vitamin E—Mayer-Davis and colleagues’ cross-sectional investigation[55] found a risk association between Vitamin E and prevalent DR (10th decile vs 1st quintile, OR: 3.79, p<0.002), but only within non-insulin taking patients. All remaining studies (two prospective, one cross-sectional) reported no significant associations. [11, 12, 15].
Overall, the associations between these common antioxidants and DR remain inconsistent.
Vitamin D
The only two studies[9, 18] that examined the association between dietary vitamin D intake and DR did not find any significant associations. (Table 2).
Sodium
The evidence overwhelmingly suggests (n = 4) that sodium intake is not associated with DR[10, 13, 42, 56] (Table 2). However, one study reported a risk association between sodium intake and DME progression[10] (Q4 vs. Q1, OR, 95% CI: 1.43, 1.10–1.86).
Associations between macronutrient intake and DR
Mono-Unsaturated Fatty Acids (MUFA)
Alcubierre and associates,[39] using a case-control design, reported a protective association between MUFA intake and DR (high MUFA intake vs. low MUFA intake, OR, 95% CI: 0.42, 0.18–0.97). In contrast, a prospective study by Cundiff and colleagues[13] reported a risk relationship between MUFA intake and DR progression, but did not adjust for important confounders such as duration of diabetes, HbA1c or diabetes treatment. The remaining majority of studies (two cross-sectional and one prospective) found no significant relationships between MUFA intake and DR[10, 12, 14](Table 3). Two studies that further analyzed the effects of Oleic acid (a specific MUFA) on DR also reported contrasting results[10, 39].
Table 3. Dietary intake of macro-nutrients and DR.
| Author, year | Association | Study Design | Quality | Dietary Factor | Sample Size | DR outcome type | Confounders adjusted for | Statistical methods | Main Findings | |
|---|---|---|---|---|---|---|---|---|---|---|
| Dietary Fats / lipids | ||||||||||
| Mono-Unsaturated Fatty Acids (MUFA) | ||||||||||
| Alcubierre, 2016 | Protective | Case-Control | 10 | MUFA | Case: 146 Ctrl: 148 |
Prevalence | Age, gender, diabetes duration, energy intake, educational level, physical activity, waist circumference, systolic BP, HDL cholesterol & diabetes treatment | Multivariable Logistic Regression | High MUFA consumption vs Low MUFA consumption, OR: 0.42 (0.18–0.97) | |
| Cundiff, 2005 | Risk | Prospective | 8 | MUFA | 1412 | Progression | Energy Intake | Spearman Correlation | MUFA in %/kcal against DR progression rate, r = 0.12 (p = 0.001) | |
| Roy, 2010 | NS | Prospective | 9 | MUFA | 469 | Progression & Incidence | Total caloric intake, total fat, sat fat, oleic acid, linoleic acid, protein, fiber, cholesterol & sodium intakes | Multivariable Logistic Regression | No significant associations with DR (Data not reported) | |
| Sasaki, 2015 | NS | Cross Sectional | 10 | MUFA | 379 | Prevalence | Age, gender, HBA1C, mean arterial pressure & diabetes duration | Multivariable logistic regression models | Per 10 energy-adjusted g/d increase, OR: 1.19 (0.74–1.92) | |
| Roy, 1989 | NS | Cross-Sectional | 5 | MUFA | 34 | Prevalence | Energy Intake | t-test | No significant associations with DR (Data not reported) | |
| Poly-Unsaturated Fatty Acids (PUFA) | ||||||||||
| Sala-Vila, 2016 | Protective | Prospective | 9 | PUFA (LCw3) | 3482 | Incidence | Age, gender, BMI, intervention group, yeasr after diagnosis of diabetes, use of insulin, use of oral hypoglycemic agents, smoking, systolic BP, hypertension, physical activity, adherence to meddiet. | Cox Proportional Hazard Model | >500mg/d Vs <500mg/d, HR: 0.52 (0.31–0.88) | |
| Sasaki, 2015 | Protective for well controlled diabetics | Cross Sectional | 10 | PUFA | 379 | Prevalence | Age, gender, HBA1C, mean arterial pressure & diabetes duration | Multivariable logistic regression models |
All subjects: Per 10 energy-adjusted g/d increase, OR: 0.67 (0.37–1.20) Well controlled Diabetics: Per 10 energy-adjusted g/d increase, OR: 0.18 (0.06–0.59) |
|
| Cundiff, 2005 | Risk | Prospective | 8 | PUFA | 1412 | Progression | Energy Intake | Spearman Correlation | PUFA in %/kcal against DR progression rate, r = 0.09 (r = 0.004) | |
| Roy, 2010 | NS | Prospective | 9 | PUFA | 469 | Progression & Incidence | Total caloric intake, total fat, sat fat, oleic acid, linoleic acid, protein, fiber, cholesterol & sodium intakes | Multivariable Logistic Regression | No significant associations with DR (Data not reported) | |
| Alcubierre, 2016 | NS | Case-Control | 10 | PUFA | Case: 146 Ctrl: 148 |
Prevalence | Age, gender, diabetes duration, energy intake, educational level, physical activity, waist circumference, systolic BP, HDL cholesterol & diabetes treatment | Multivariable Logistic Regression | High PUFA consumption vs Low MUFA consumption, OR: 0.99 (0.69–1.41) | |
| Roy, 1989 | NS | Cross-Sectional | 5 | PUFA | 34 | Prevalence | Energy Intake | t-test | No significant associations with DR (Data not reported) | |
| Oleic Acid | ||||||||||
| Alcubierre, 2016 | Protective | Case-Control | 10 | Oleic Acid | Case: 146 Ctrl: 148 |
Prevalence | Age, gender, diabetes duration, energy intake, educational level, physical activity, waist circumference, systolic BP, HDL cholesterol & diabetes treatment | Multivariable Logistic Regression | High Intake Tertile (T3) vs Lowest Intake Tertile (T1), OR: 0.37 (0.16–0.85) | |
| Roy, 2010 | NS | Prospective | 9 | Oleic Acid | 469 | Progression & Incidence | Total caloric intake, total fat, sat fat, oleic acid, linoleic acid, protein, fiber, cholesterol & sodium intakes | Multivariable Logistic Regression | No Significant associations with DR (Data not reported) | |
| Interventional Studies | ||||||||||
| Houtsmuller, 1979 | Protective | Interventional | High Bias | Unsaturated Fats | 96 | Progression | Matched for gender | Saturated Fat Diet Vs Unsaturated Fat Diet Males (n = 52, 26 on each diet) P<0.001 Females (n = 44, 22 on each diet) P<0.025 |
||
| Howard-williams, 1985 | NS | Interventional | High Bias | PUFA | 149 | Incidence | Matched for age, sex & BMI | Persons on modified fat diet (PUFA: saturated fat ratio, 0.3) vs persons on low carb diet (PUFA: Saturated fat ratio, 0.9) All patients (n = 149) No difference between two groups (chi-squared, p = 0.69) Dietary compliers (n = 58) No difference between two groups (chi-squared, p = 0.13) |
||
| Carbohydrates | ||||||||||
| Cundiff, 2005 | Protective | Prospective | 8 | Carbohydrates | 1412 | Progression | Energy Intake | Spearman Correlation | Carbohydrates in %/kcal against DR progression rate, r = -0.11 (p<0.001) | |
| Roy, 1989 | Protective | Cross-Sectional | 5 | Carbohydrates | 34 | Prevalence | Energy Intake | t-test | Persons without retinopathy vs Persons with retinopathy (p<0.05) | |
| Horikawa, 2017 | NS | Prospective | 10 | Carbohydrates | 978 | Incidence and Progression | Age, sex, BMI, HbA1C, Diabetes Duration, systolic BP, LDL-cholesterol, HDL-cholesterol, triglycerides, treatment by insulin, treatment by antihypertensive agents, treatment by lipid-lowering agents, current smoker, alcohol intake, energy intake & physical activity | Multivariable Cox Regression Models | Highest Intake Tertile (T3) vs lowest Intake Tertile (T1), HR: 1.00 (0.72–1.38) | |
| Roy, 2010 | NS | Prospective | 9 | Carbohydrates | 469 | Progression & Incidence | Total caloric intake, total fat, sat fat, oleic acid, linoleic acid, protein, fiber, cholesterol & sodium intakes | Multivariable Logistic Regression | No significant associations with DR (Data not reported) | |
| Alcubierre, 2016 | NS | Case-Control | 10 | Carbohydrates | Case: 146 Ctrl: 148 |
Prevalence | Age, gender, diabetes duration, energy intake, educational level, physical activity, waist circumference, systolic BP, HDL cholesterol & diabetes treatment | Multivariable Logistic Regression | High Intake Tertile (T3) vs lowest intake tertile (T1), OR: 1.18 (0.45–3.09) | |
| Sasaki, 2015 | NS | Cross Sectional | 8 | Carbohydrates | 379 | Prevalence | Energy Intake | Chi-Squared | No significant associations with DR (data not reported) | |
| Protein | ||||||||||
| Cundiff, 2005 | Protective | Prospective | 8 | Protein | 1412 | Progression | Energy Intake | Spearman Correlation | Protein in %/kcal against DR progression rate, r = -0.6 (p = 0.0188) | |
| Roy, 1989 | Risk | Cross-Sectional | 5 | Protein | 34 | Prevalence | Energy Intake | t-test | Persons without retinopathy vs Persons with retinopathy (p<0.02) | |
| Roy, 2010 | NS | Prospective | 9 | Protein | 469 | Progression & Incidence | Total caloric intake, total fat, sat fat, oleic acid, linoleic acid, protein, fiber, cholesterol & sodium intakes | Multivariable Logistic Regression | No Significant associations with DR (Data not reported) | |
| Alcubierre, 2016 | NS | Case-Control | 10 | Protein | Case: 146 Ctrl: 148 |
Prevalence | Age, gender, diabetes duration, energy intake, educational level, physical activity, waist circumference, systolic BP, HDL cholesterol & diabetes treatment | Multivariable Logistic Regression | Highest protein intake tertile (T3) vs lowest protein intake tertile (T1), OR: 1.24 (0.49–3.16) | |
| Sasaki, 2015 | NS | Cross Sectional | 8 | Protein | 379 | Prevalence | Energy Intake | Chi-Squared | No Significant associations with DR (Data not reported) | |
Poly-Unsaturated Fatty Acids (PUFA)
A prospective study by Sala-Vila and associates[45] found those adhering to the dietary long-chain omega-3 PUFA (LCω3PUFA) recommendation of at least 500mg/day to be at lower risk of incident DR than those who did not adhere (HR, 95% CI: 0.52, 0.31–0.99). Similarly, though Sasaki and colleagues[12] found no overall association between PUFA intake and DR, they reported a protective asociation within patients with well-controlled diabetes (OR, 95% CI: 0.18, 0.06–0.59). In contrast to these two studies, Cundiff and colleagues[13] reported a risk association between a larger percentage of caloric intake as PUFA’s and DR progression, though again this result was not adjusted for DR confounders. The remaining three other studies[10, 14, 39] reported no significant relationships between PUFA intake and DR.
Interventional studies have been equally equivocal; a 1979 study by Houtsmuller and associates[57] reported a significant reduction in DR progression among participants on an unsaturated fat diet rich in linoleic acid, compared to those on a saturated fat diet. In contrast, a later study by Howard-Williams and colleagues[58] reported no significant differences in incident DR between compliers of a modified fat diet (high PUFA to saturated fat ratio) and those on a low carbohydrate diet (low PUFA to saturated fat ratio).
Carbohydrates
Two studies (one cross-sectional, one prospective) found carbohydrate intake to be protective for DR[13, 14] (Table 3), but neither study adjusted for relevant confounders. In contrast, the remaining four studies[10, 12, 39, 43]—three of which used fully adjusted multivariable models—reported non-significant relationships between carbohydrate intake and DR.
Protein
A prospective study by Cundiff and colleagues reported those with a larger percentage of caloric intake as proteins to be at lower risk of DR progression. In contrast, a cross-sectional study by Roy and associates reported a risk association between protein intake and prevalent DR[13, 14]. However, both studies did not adjust for relevant confounders. The remaining three studies[10, 12, 39] that adjusted for confounders reported non-significant relationships between dietary protein intake and DR (Table 3).
Associations between food and beverage intake and DR
Fruits, vegetables and dietary fibre
Two studies (one prospective, one cross-sectional) reported a protective association between the intake of fruits and vegetables and DR, in contrast to one cross-sectional study that reported non-significant associations. Similarly, for dietary fibre, the majority of studies(two prospective, two cross-sectional) reported a protective effect of increased dietary fibre intake on DR[13–16, 41] (Table 4), in contrast to two other studies that reported non-significant associations.
Table 4. Dietary intake of foods, beverages, dietary patterns and DR.
| Author, year | Association | Study Design | Quality | Dietary Factor | Sample size | DR outcome type | Confounders adjusted for | Statistical methods | Main Findings |
|---|---|---|---|---|---|---|---|---|---|
| Dietary Fibre | |||||||||
| Tanaka, 2013 | Protective | Prospective | 10 | Fruits, Vegetables, & Dietary Fibre | 978 | Incidence | Age, sex, BMI, HBA1C, Duration of Diabetes, Treatment by insulin, treatment by oral hypoglycemic agents without insulin, systolic blood Pressure, LDL Cholesterol, HDL cholesterol, Triglycerides, smoking, alcohol, physical activity, total energy intake, proportions of dietary protein, fat, carbohydrate, saturated fatty acids, n-6 PUGA and n-3 PUFA, cholesterol & Sodium | Multivariate Cox Regression | Veg & Fruit intake Q4 vs Q1, HR: 0.59 (0.37–0.92) Fruit intake Q4 Vs Q1, HR: 0.48(0.32–0.71) Dietary Fibre intake Q4 Vs Q1, HR: 0.63 (0.38–1.03) |
| Cundiff, 2005 | Protective | Prospective | 8 | Dietary Fibre | 1412 | Progression | Energy Intake | Spearman Correlation | Dietary fibre in g/1000kcal against DR progression rate, r = -0.10 (p = 0.002) |
| Ganesan, 2012 | Protective | Cross Sectional | 10 | Dietary Fibre | 1261 | Prevalence | Age, Gender, duration of diabetes, BP, BMI, glycosylated hemoglobin, serum lipids, smoking status & SES. | Multivariable Logistic Regression | Low-fibre diet Vs High fibre diet for any DR, OR: 1.41 (1.02–1.94) Low fibre diet Vs High fibre diet for VTDR, OR: 2.24 (1.01–5.02) |
| Roy, 1989 | Protective | Cross-Sectional | 5 | Dietary Fibre | 34 | Prevalence | Duration of diabetes | t-test | Persons without retinopathy vs Persons with retinopathy, (p<0.01) |
| Roy, 2010 | NS | Prospective | 9 | Dietary Fibre | 469 | Progression & Incidence | Total caloric intake, total fat, sat fat, oleic acid, linoleic acid, protein, fiber, cholesterol & sodium intakes | Multivariable Logistic Regression | No significant associations with DR (Data not reported) |
| Alcubierre, 2016 | NS | Case-Control | 10 | Dietary Fibre | Case: 146 Ctrl: 148 |
Prevalence | Age, gender, diabetes duration, energy intake, educational level, physical activity, waist circumference, systolic BP, HDL Cholesterol & Diabetes treatment | Multivariable Logistic Regression | Highest Fibre intake tertile (T3) vs lowest Fibre intake tertile (T1), OR: 0.76 (0.33–1.76) |
| Fruits & vegetables | |||||||||
| Mahoney, 2014 | Protective | Cross Sectional | 8 | Fruit & Vegetables | 155 | Prevalence | Age, Gender, Ethnicity, BMI, HbA1C, Physical activity, diabetic medications, CVD, cancer, stroke, & homocysteine | Multivariable Logistic Regression | Per 10 Unit increase in HFVC (High-flavonoid Fruit and Vegetable consumption) Index, OR:0.67 (0.45–0.99) With adjustment for duration of diabetes (n = 115) per 10 unit increase in HFVC index, OR:0.59 (P = 0.03) |
| Lugo-Radillo, 2013 | NS | Cross-Sectional | 5 | Fruit & Vegetables | 88 | Prevalence | NIL | Binary Logistic Regression | High fruit & vegetable diet vs low fruit & vegetable diet, OR: (OR = 1.2, 0.3–6.2) High fruit consumption vs Low fruit consumption, OR: 1.8 (0.4–8.9) High vegetable consumption vs Low vegetable consumption, OR: 0.9 (0.3–2.9) |
| Fish | |||||||||
| Sala-Vila, 2016 | Protective | Prospective | 9 | "Oily Fish" | 3482 | Incidence | Age, gender, BMI, intervention group, year after diagnosis of diabetes, use of insulin, use of oral hypoglycemic agents, smoking, systolic BP, hypertension, physical activity & adherence to med diet. | Cox Proportional Hazard Model | >2 servings a week vs <2 servings a week, HR: 0.41 (0.23–0.72) |
| Millen, 2016 | Protective | Cross-Sectional | 9 | Fish | 1305 | Prevalence | Race, duration of diabetes, HBA1C & Hypertension | Multivariate Logistic Regression | Dark fish >1 a week vs never, OR: 0.32 (0.14–0.78) Other fish >1 a week vs never, OR: 1.16 (0.70–1.92) |
| Green Tea | |||||||||
| Ma, 2014 | Protective | Case-Control | 8 | Green Tea | Case:100 Ctrl: 100 |
Prevalence | Education, BMI, systolic BP, smoking, alcohol, duration of diabetes, insulin therapy, family history of diabetes, physical activity & fasting blood glucose | Multivariable logistic regression | Regular chinese green tea drinker vs non-regular chinese green tea drinker, OR: 0.48 (0.24–0.97) |
| Coffee | |||||||||
| Kumari, 2014 | NS | Cross Sectional | 9 | Coffee | 353 | Prevalence | Age, gender, smoking, BMI, HbA1c, creatinine, education level, duration of diabetes, family history of diabetes, history of hypertension, ischemic heart disease, stroke, dyslipidemia & cancer | Multivariable logistic regression | Coffee drinker vs never/rarely, OR: 1.36 (0.69–2.69) |
| Milk | |||||||||
| Millen, 2016 | NS | Cross-Sectional | 9 | Milk | 1305 | Prevalence | Race, duration of diabetes, HBA1C & Hypertension | Multivariate Logistic Regression | Skim Milk, OR: 1.13 (0.67–1.91) Whole Milk, OR: 0.88 (0.35–2.23) |
| Alcohol | |||||||||
| Beulens, 2008 | Protective | Cross-Sectional | 10 | Alcohol | 1857 | Prevalence | Age, gender, centre, smoking, physical activity, duration of diabetes, systolic BP, BMI, presence of CVD and HbA1C | Multivariable Logistic Regression | Mod Vs Abstain, OR: 0.60 (0.37–0.99) |
| Fenwick, 2015 | Protective | Cross-Sectional Study | 10 | Alcohol | 395 | Prevalence | Age, Gender, Poor Diabetes Control, Diabetes Duration, Smoking BMI, SBP, insulin use and presence of at least one other diabetic Complication | Multivariable Logistic Regression | Mod Vs Abstain, OR: 0.47 (0.26–0.95) Mod White Wine Vs Abstain, OR:0.48 (0.25–0.91) Mod Fortified Wine Vs Abstain, OR: 0.15 (0.04–0.62) |
| Moss, 1992 | Protective | Cross Sectional | 9 | Alcohol | Younger: 891 Older:987 |
Prevalence | Duration of diabetes, age, glycosylated hemoglobin, diastolic BP, use of insulin | Multivariable Logistic Regression |
Younger onset diabetics Per 1oz/day increase in alcohol consumption for PDR, OR: 0.49, (0.27–0.92) Older onset: no significant associations |
| Harjutsalo, 2013 | Protective | Cross-Sectional | 8 | Alcohol | 3608 | Prevalence | Age at onset of diabetes, sex, duration of diabetes, triglycerides, HDL cholesterol, HbA1C, social class, BMI, smoking status, hypertension and lipid-lowering medication | Multivariable Logistic Regression | Abstain Vs Light, OR: 1.42 (1.11–1.82) Former Use Vs Light, OR: 1.73 (1.07–2.79) |
| Young, 1984 | Risk | Prospective | 8 | Alcohol | 296 | Incidence | Duration of diabetes, glycemic control & impotence | Multivariable Logistic Regression | Heavy consumption Vs None-Mod consumption, RR: 2.25 (1.15–4.42) |
| Cundiff, 2005 | NS | Prospective | 8 | Alcohol | 1412 | Progression | Energy Intake | Spearman Correlation | No Significant association with DR (p = 0.26) |
| Lee, 2010 | NS | Prospective | 9 | Alcohol | 1239 | Progression | Age, Gender, Smoking, BMI, HbA1C, Systolic BP, duration of diabetes and ethnicity | Multivariable Logistic Regression | Mod Vs None, OR: 1.08 (0.70–1.67) Heavy Vs None, OR: 1.07 (0.54–2.13) |
| Moss, 1993 | NS | Prospective | 9 | Alcohol | Younger: 439 Older:478 |
Incidence & progression | Glycosylated Hemoglobin, Age, Sex | Multivariable Logistic Regression |
Younger onset diabetics Per 1oz/day increase in alcohol consumption on DR incidence, OR: 2.09 (0.04–1.07) Per 1oz/day increase in alcohol consumption on DR progression, OR: 1.25 (0.75–2.08) Older onset diabetics Per 1oz/day increase in alcohol consumption on DR incidence, OR: 0.75 (0.4–1.42) Per 1oz/day increase in alcohol consumption on DR progression, OR: 0.73 (0.4–1.20) |
| Giuffre, 2004 | NS | Case-Control | 7 | Alcohol | Case: 45 Ctrl: 87 |
Prevalence | Duration of Diabetes, Duration of Treatment with oral drugs, Duration of insulin treatment | Multivariable Logistic Regression | No Significant Association with DR (Data not reported) |
| Mediterranean Diet | |||||||||
| Diaz-Lopez, 2015 | Protective | Interventional | Moderate Bias | Med Diet | 3614 | Incidence of DR | Age, sex, BMI, Waist circumference, Smoking, physical activity, educational level, hypertension, dyslipidemia, family history of premature coronary heart disease, and baseline adherence. | Multivariate Cox Regression | Med Diet vs Control Diet, HR: 0.60 (0.37–0.96) |
| Caloric Intake | |||||||||
| Roy, 2010 | Risk | Prospective | 10 | Caloric Intake | 469 | Progression & Incidence | Total caloric intake, age, sex, physical exercise, glycated hemoglobin, oleic acid intake, protein intake, carbohydrate intake & hypertension | Multivariable Logistic Regression | Higher Caloric Intake, OR: 1.48 (1.15–1.92) |
| Cundiff, 2005 | Risk | Prospective | 8 | Caloric Intake | 1412 | Progression | NIL | Spearman Correlation | Calories in kcal against DR progression rate, r = 0.07 (p = 0.007) |
| Alcubierre, 2016 | NS | Case-Control | 10 | Caloric Intake | Case: 146 Ctrl: 148 |
Prevalence | Age, gender, diabetes duration, energy intake, educational level, physical activity, waist circumference, systolic BP, HDL Cholesterol and Diabetes treatment | Multivariable Logistic Regression | Highest energy intake tertile (T3) vs lowest energy intake tertile (T1), OR: 0.73 (0.37–1.46) |
Fish
Two prospective studies reported a protective association between oily fish intake and DR (Table 4). Sala-Vila and associates[45] reported a decrease in risk of incident DR between those who consumed two or more weekly servings of oily fish and those who did not (HR: 0.41, 0.23–0.72). Similarly, Millen and colleagues[9] found a protective effect on DR in dark (oily) fish (consume dark fish >1 times a week vs. <1 times, OR, 95% CI: 0.32, 0.14–0.78), but not in white fish (OR, 95% CI: 1.16, 0.70–1.92).
Alcohol
All cross-sectional studies[40, 46, 48, 52] have reported an independent protective association between light to moderate alcohol intake and prevalence of DR, even in multivariable logistic regression models (Table 4). However, a prospective study by Young and associates[54] found a risk association between alcohol and DR, but only in those with heavy alcohol intake (heavy vs. none-moderate, relative risk, 95% CI: 2.24, 1.15–4.42). In contrast, three prospective studies and a case-control study all reported no significant associations between alcohol and DR[13, 47, 50, 53].
Other beverages
Limited studies–only one study per beverage—have been conducted on the associations of other beverages with DR (Table 4). A case-control study by Ma and associates[17] reported a protective effect of green tea consumption on prevalent DR (consumers vs. non-consumers, OR, 95% CI: 0.48, 0.24–0.97). Respective studies by Kumari and associates, and Millen and colleagues found no significant associations between coffee and milk with DR[9, 49].
Associations between broader dietary patterns / characteristics and DR
Mediterranean (Med) diet
Evidence from an interventional study by Diaz-Lopez and associates[19] suggests a protective association of a Med diet on incident DR (Table 4). 3614 patients with type 2 diabetes from the PREDIMED trial were split between a control (low-fat) diet, and two types of Med diets. Using a multivariable cox regression model, a protective effect of the Med diet on incident DR was found (any Med diet vs. control diet, HR, 95% CI: 0.60, 0.37–0.96).
Total caloric intake
While the case-control study by Alcubierre and associates[39] reported no significant relationship between high caloric intake as a whole and DR (Table 4), two prospective studies by Cundiff and colleagues, and Roy and associates (OR, 95% CI: 1.49, 1.15–1.92) both reported risk associations between a high total caloric intake and DR[10, 13].
Discussion
In our broad-based systematic review of the relationship between dietary intake and DR, a majority of studies found that intake of dietary fibre, oily fish, and a Med diet were protective of DR. In contrast, sodium and carbohydrates were not associated with DR, while high total caloric intake may be associated with greater DR risk. Importantly, the relationship between DR and several common dietary components including antioxidants, fatty acids, proteins, alcohol, and beverages, such as tea and coffee remained unclear, suggesting that more research, including longitudinal studies, are required to better understand these relationships. Our study may contribute to DR-specific dietary recommendation and complement existing DR management guidelines.
Our review provides evidence of a protective effect of dietary fibre, fruits and vegetables, and oily-fish on DR, consistent with the current literature[9, 12–16, 41]. Most fruits and vegetables are low-glycemic index foods rich in antioxidants and dietary fibre.[59] The ingestion of dietary fibres tends to modulate the postprandial glucose response[60], and is thus proposed to reduce glucose-induced damage to the retina[15]. Likewise, antioxidants are proposed to decrease oxidative stress in the retina[61], though till date there exists no clear association between antioxidants and DR (discussed later). Oily-fish is a rich source of Vitamin D and LCω3PUFAs, and it is proposed that the immune-modulatory and anti-angiogenic properties of these nutrients may play a role in the inhibition of DR[62–64]. However, it should be noted that Millen and associates found the protective effects of oily fish on DR to be independent of LCω3PUFA intake[9], suggesting these protective effects may stem from more than just the presence of LCω3PUFA’s in oily fish alone, with further research needed to confirm the exact underpinning mechanisms.
Our finding that a Med diet is protective for DR is similarly unsurprising as it is recognized as one of the healthiest dietary patterns[65–68], with several components of the Med diet, including olive oil, red wine, fibre and cereals proposed to alleviate pathogenic factors of diabetic microvascular complications such as inflammation, oxidative stress, and insulin resistance[69]. However, given that only one study has focused on the Med dietary pattern and DR, with relatively low number of incident DR cases (n = 74), our results should be interpreted with caution[19].
Alternatively, our finding that a high total caloric intake may increase the risk of DR incidence and progression[10, 13] concurs with experimental and clinical evidence suggesting that higher caloric intake increases the metabolic burden and oxidative stress in persons with diabetes, and may increase the risk of developing DR in the oxidative stress-susceptible retina[70–73]. Interestingly, evidence unequivocally suggests that there is no significant association of increased carbohydrate intake, one of the key contributors to total caloric intake, with DR[10, 43]. In fact, two studies reported protective associations between carbohydrate intake and DR;[13, 14] however, these results should be viewed with caution, as they did not adjust for important confounders such as duration of diabetes, insulin use, the quality of carbohydrates (e.g. high vs low glycemic index). In spite of a lack of significant association with DR, the monitoring of carbohydrate intake is still important for improving postprandial glucose control in patients with diabetes[74]. However, greater focus on the quality of carbohydrates (consumption of low-glycemic index foods), and on reducing total caloric intake, may be more beneficial in preventing the development and progression of the disease[75, 76].
While prior experimental studies have suggested a protective association between antioxidants and DR[77, 78], we found a lack of consensus over the effects of Vitamin C, E and carotenoid intake on DR in humans, similar to a review by Lee and associates[22]. Additionally, while experimental studies have also suggested PUFA intake to be protective against DR[79–81] through anti-inflammatory and anti-angiogenic properties[82], current evidence remains inconclusive[10, 12, 39, 45]. Our findings also support those of a recent meta-analysis by Zhu and associates that could not confirm a protective effect of alcohol consumption on DR[83]. While moderate alcohol consumption has been postulated to be protective of DR through multiple proposed mechanisms, including improving increasing insulin sensitivity[84] and decreasing platelet aggregability[21], such protective associations have only been reported in cross-sectional studies, with longitudinal studies reporting no clear associations. Lastly, research on the effect of popular beverages such as coffee, tea, milk[9, 17, 49] on DR remains limited, with only one study on each beverage, and no data available on the effects of soft drinks on DR. Given that the above dietary factors constitute a large component of diet, future large-scale prospective studies are warranted to elucidate their impact on DR incidence and progression.
Our findings generally support existing American Diabetic Association (ADA) guidelines for overall diabetes management that acknowledge the beneficial effects of a Med diet[7], encourage people with diabetes to consume a diet rich in fruits and vegetables[7], and recommend lower caloric intakes[7, 8]. The ADA likewise states insufficient evidence for the benefits of antioxidant supplementation[74], or to recommend an ideal amount of protein intake[85] for diabetic individuals. For other dietary components however, while we found no conclusive evidence to suggest that increased sodium and carbohydrate intake have a detrimental effect on DR risk, the ADA still recommends patients with diabetes to monitor their sodium and carbohydrate intake[85]. Likewise, while the evidence for the effect of MUFA / PUFA intake, and moderate alcohol intake on DR remains inconclusive, the ADA does recommend PUFAs and MUFAs as substitutes for saturated or trans fat[74], and also recommends moderate alcohol consumption[7] (should persons wish to drink) for patients with diabetes. The findings of our review are meant to complement and should be viewed in conjunction with the existing dietary guidelines for overall diabetes management.
While the majority of the studies included in our review had sound methodological and study qualities (with high NOS scores), there remain several restrictions in the current literature on the relationship between dietary intake and DR that limits our ability to derive more conclusive outcomes. First, most studies used FFQs to assess dietary intakes, and these questionnaires were administered only once at study baseline. While FFQs are widely used as the primary tool of dietary assessment in epidemiological studies, they also have limitations due to recall bias, and subjectivity across individuals and time-frames[86]. Future studies using more objective measures of diet such as food consumption records or which collect data across multiple time-points will provide more robust measurements of dietary intake. Second, most studies are cross-sectional, which limits their ability to establish a causal relationship between dietary factors and DR. As such, more longitudinal studies are hence warranted. Third, most studies only assessed a single dietary component or nutrient, and did not consider a broader concept of dietary intake, which is often a combination of many meals, foods and nutrients. Rather than continued focus on single nutrients, studies should also place emphasis on foods, beverages or even dietary patterns, to better reflect real world consumption habits which can be translated into clearer dietary guidelines[87, 88]. Forth, research regarding the impact of dietary intake on DME remains sparse, and future research is needed to better understand the mechanisms of diet in those with DME which may differ from DR. Lastly, it should be noted that many studies did not differentiate between type 1 and type 2 diabetes. This is important because there are pathophysiological, etiological, epidemiological and disease management differences between diabetes types, all of which may influence on the effect of dietary intake on DR and DME. Future studies should clearly identify diabetes types and provide specific data for each.
There are several strengths in our systematic review. Firstly, we sought to specifically evaluate dietary intake exposures and DR within human subjects, rather than including experimental, bio-mechanism or bio-marker studies, which allows for a more direct translation of results into dietary recommendations for patients. Secondly, as included studies were conducted on a wide variety of populations (over more than 10 different countries), this increases the generalizability of our results. However, there are also certain limitations to our study. For example, the methodological diversity across studies in assessing dietary intake exposures, and DR outcomes may affect their comparability. For example, studies using only fundus examinations, two-field or non-mydriatic fundus photography may have underestimated the number of DR cases, compared to studies using stereoscopic 7-field fundus photographs (the reference standard to detect DR as defined by the ETDRS)[29]. We were also unable to conduct a formal meta-analysis to synthesize overall findings, as within each dietary component there were few studies sufficiently similar in design, outcome, and exposure measurements to be suitable for meta-analysis.
In conclusion, our systematic review demonstrates that diet can form a crucial aspect of DR prevention and management, with evidence suggesting that dietary fibre, oily fish, and a Med diet are protective of DR, while a higher caloric intake was associated with greater DR risk. These findings may enable clinicians to make evidence-based dietary recommendations when counseling patients with diabetes who are at risk of DR. However, further prospective studies and experimental models to untangle the effects of other key dietary components on DR, such as antioxidants, fatty acids, proteins, alcohol and popular beverages, are needed in order to better inform clinical guidelines.
Supporting information
(XLSX)
(DOC)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Mohamed Q, Gillies MC, Wong TY. Management of diabetic retinopathy: A systematic review. Jama. 2007;298(8):902–16. doi: 10.1001/jama.298.8.902 [DOI] [PubMed] [Google Scholar]
- 2.Fong DS, Aiello L, Gardner TW, King GL, Blankenship G, Cavallerano JD, et al. Retinopathy in diabetes. Diabetes care. 2004;27 Suppl 1:S84–7. [DOI] [PubMed] [Google Scholar]
- 3.Wong TY, Cheung CMG, Larsen M, Sharma S, Simó R. Diabetic retinopathy. 2016;2:16012. [DOI] [PubMed] [Google Scholar]
- 4.Cheung N, Mitchell P, Wong TY. Diabetic retinopathy. The Lancet.376(9735):124–36. [DOI] [PubMed] [Google Scholar]
- 5.Funnell MM, Anderson RM. Empowerment and Self-Management of Diabetes. Clinical Diabetes. 2004;22(3):123–7. [Google Scholar]
- 6.Lopez JMS, Katic BJ, Fitz-Randolph M, Jackson RA, Chow W, Mullins CD. Understanding preferences for type 2 diabetes mellitus self-management support through a patient-centered approach: a 2-phase mixed-methods study. BMC Endocrine Disorders. 2016;16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.American Diabetes A. Standards of Medical Care in Diabetes—2013. Diabetes care. 2013;36(Suppl 1):S11–S66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Franz MJ, Wylie-Rosett J. The 2006 American Diabetes Association nutrition recommendations and interventions for the prevention and treatment of diabetes. Diabetes Spectrum. 2007. 2007 Winter:49+. [Google Scholar]
- 9.Millen AE, Sahli MW, Nie J, LaMonte MJ, Lutsey PL, Klein BE, et al. Adequate vitamin D status is associated with the reduced odds of prevalent diabetic retinopathy in African Americans and Caucasians. Cardiovascular diabetology. 2016;15(1):128 doi: 10.1186/s12933-016-0434-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roy MS, Janal MN. High caloric and sodium intakes as risk factors for progression of retinopathy in type 1 diabetes mellitus. Archives of Ophthalmology. 2010;128(1):33–9. doi: 10.1001/archophthalmol.2009.358 [DOI] [PubMed] [Google Scholar]
- 11.Millen AE, Klein R, Folsom AR, Stevens J, Palta M, Mares JA. Relation between intake of vitamins C and E and risk of diabetic retinopathy in the Atherosclerosis Risk in Communities Study. American Journal of Clinical Nutrition. 2004;79(5):865–73. [DOI] [PubMed] [Google Scholar]
- 12.Sasaki M, Kawasaki R, Rogers S, Man RE, Itakura K, Xie J, et al. The Associations of Dietary Intake of Polyunsaturated Fatty Acids With Diabetic Retinopathy in Well-Controlled Diabetes. Investigative ophthalmology & visual science. 2015;56(12):7473–9. [DOI] [PubMed] [Google Scholar]
- 13.Cundiff DK, Nigg CR. Diet and diabetic retinopathy: Insights from the Diabetes Control and Complications Trial (DCCT). MedGenMed Medscape General Medicine. 2005;7(1). [PMC free article] [PubMed] [Google Scholar]
- 14.Roy MS, Stables G, Collier B, Roy A, Bou E. Nutritional factors in diabetics with and without retinopathy. The American journal of clinical nutrition. 1989;50(4):728–30. [DOI] [PubMed] [Google Scholar]
- 15.Tanaka S, Yoshimura Y, Kawasaki R, Kamada C, Tanaka S, Horikawa C, et al. Fruit intake and incident retinopathy in japanese patients with type 2 diabetes-nutritional analysis in the Japan Diabetes Complications Study (JDCS). Diabetes. 2012;61:A77. [Google Scholar]
- 16.Mahoney SE, Loprinzi PD. Influence of flavonoid-rich fruit and vegetable intake on diabetic retinopathy and diabetes-related biomarkers. Journal of diabetes and its complications. 2014;28(6):767–71. doi: 10.1016/j.jdiacomp.2014.06.011 [DOI] [PubMed] [Google Scholar]
- 17.Ma Q, Chen D, Sun HP, Yan N, Xu Y, Pan CW. Regular Chinese Green Tea Consumption Is Protective for Diabetic Retinopathy: A Clinic-Based Case-Control Study. Journal of diabetes research. 2015;2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alcubierre N, Valls J, Rubinat E, Cao G, Esquerda A, Traveset A, et al. Vitamin D Deficiency Is Associated with the Presence and Severity of Diabetic Retinopathy in Type 2 Diabetes Mellitus. Journal of diabetes research. 2015;2015:374178 doi: 10.1155/2015/374178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Diaz-Ĺopez A, Babio N, Martinez-González MA, Corella D, Amor AJ, Fitó M, et al. Mediterranean diet, retinopathy, nephropathy, and microvascular diabetes Complications: A post Hoc analysis of a randomized trial. Diabetes care. 2015;38(11):2134–41. doi: 10.2337/dc15-1117 [DOI] [PubMed] [Google Scholar]
- 20.Williams M, Hogg RE, Chakravarthy U. Antioxidants and diabetic retinopathy. Current diabetes reports. 2013;13(4):481–7. doi: 10.1007/s11892-013-0384-x [DOI] [PubMed] [Google Scholar]
- 21.Wang S, Wang JJ, Wong TY. Alcohol and Eye Diseases. Survey of Ophthalmology. 2008;53(5):512–25. doi: 10.1016/j.survophthal.2008.06.003 [DOI] [PubMed] [Google Scholar]
- 22.Lee CTC, Gayton EL, Beulens JWJ, Flanagan DW, Adler AI. Micronutrients and Diabetic Retinopathy. A Systematic Review. Ophthalmology. 2010;117(1):71–8. doi: 10.1016/j.ophtha.2009.06.040 [DOI] [PubMed] [Google Scholar]
- 23.Sharma Y, Saxena S, Mishra A, Saxena A, Natu SM. Nutrition for diabetic retinopathy: plummeting the inevitable threat of diabetic vision loss. European journal of nutrition. 2017. [DOI] [PubMed] [Google Scholar]
- 24.Sanhueza C, Ryan L, Foxcroft DR. Diet and the risk of unipolar depression in adults: systematic review of cohort studies. Journal of human nutrition and dietetics: the official journal of the British Dietetic Association. 2013;26(1):56–70. [DOI] [PubMed] [Google Scholar]
- 25.Murakami K, Sasaki S. Dietary intake and depressive symptoms: a systematic review of observational studies. Molecular nutrition & food research. 2010;54(4):471–88. [DOI] [PubMed] [Google Scholar]
- 26.Burrows T, Goldman S, Pursey K, Lim R. Is there an association between dietary intake and academic achievement: a systematic review. Journal of human nutrition and dietetics: the official journal of the British Dietetic Association. 2017;30(2):117–40. [DOI] [PubMed] [Google Scholar]
- 27.O'Neil A, Quirk SE, Housden S, Brennan SL, Williams LJ, Pasco JA, et al. Relationship between diet and mental health in children and adolescents: a systematic review. American journal of public health. 2014;104(10):e31–42. doi: 10.2105/AJPH.2014.302110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, et al. The PRISMA Statement for Reporting Systematic Reviews and Meta-Analyses of Studies That Evaluate Health Care Interventions: Explanation and Elaboration. PLOS Medicine. 2009;6(7):e1000100 doi: 10.1371/journal.pmed.1000100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grading diabetic retinopathy from stereoscopic color fundus photographs—an extension of the modified Airlie House classification. ETDRS report number 10. Early Treatment Diabetic Retinopathy Study Research Group. Ophthalmology. 1991;98(5 Suppl):786–806. [PubMed] [Google Scholar]
- 30.Wilkinson CP, Ferris FL 3rd, Klein RE, Lee PP, Agardh CD, Davis M, et al. Proposed international clinical diabetic retinopathy and diabetic macular edema disease severity scales. Ophthalmology. 2003;110(9):1677–82. doi: 10.1016/S0161-6420(03)00475-5 [DOI] [PubMed] [Google Scholar]
- 31.von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: guidelines for reporting observational studies. International journal of surgery (London, England). 2014;12(12):1495–9. [DOI] [PubMed] [Google Scholar]
- 32.Deeks JJ, Dinnes J, D'Amico R, Sowden AJ, Sakarovitch C, Song F, et al. Evaluating non-randomised intervention studies. Health technology assessment (Winchester, England). 2003;7(27):iii–x, 1–173. [DOI] [PubMed] [Google Scholar]
- 33.Herzog R, Alvarez-Pasquin MJ, Diaz C, Del Barrio JL, Estrada JM, Gil A. Are healthcare workers' intentions to vaccinate related to their knowledge, beliefs and attitudes? A systematic review. BMC public health. 2013;13:154 doi: 10.1186/1471-2458-13-154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wells G SB, O'Connell J, Robertson J, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analysis 2011 [http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
- 35.McPheeters ML, Kripalani S, Peterson NB, Idowu RT, Jerome RN, Potter SA, et al. Closing the quality gap: revisiting the state of the science (vol. 3: quality improvement interventions to address health disparities). Evidence Report/Technology Assessment. 2012(2083):1–475. [PMC free article] [PubMed] [Google Scholar]
- 36.Chmielowska M, Fuhr DC. Intimate partner violence and mental ill health among global populations of Indigenous women: a systematic review. Social Psychiatry and Psychiatric Epidemiology. 2017:1–16. [DOI] [PubMed] [Google Scholar]
- 37.Lo CK-L, Mertz D, Loeb M. Newcastle-Ottawa Scale: comparing reviewers’ to authors’ assessments. BMC Medical Research Methodology. 2014;14:45-. doi: 10.1186/1471-2288-14-45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Higgins JPT, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ (Clinical research ed). 2011;343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Alcubierre N, Navarrete-Muñoz EM, Rubinat E, Falguera M, Valls J, Traveset A, et al. Association of low oleic acid intake with diabetic retinopathy in type 2 diabetic patients: a case-control study. Nutrition & metabolism. 2016;13:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fenwick EK, Xie J, Man RE, Lim LL, Flood VM, Finger RP, et al. Moderate consumption of white and fortified wine is associated with reduced odds of diabetic retinopathy. Journal of diabetes and its complications. 2015;29(8):1009–14. doi: 10.1016/j.jdiacomp.2015.09.001 [DOI] [PubMed] [Google Scholar]
- 41.Ganesan S, Raman R, Kulothungan V, Sharma T. Influence of dietary-fibre intake on diabetes and diabetic retinopathy: Sankara Nethralaya-Diabetic Retinopathy Epidemiology and Molecular Genetic Study (report 26). Clinical and Experimental Ophthalmology. 2012;40(3):288–94. doi: 10.1111/j.1442-9071.2011.02594.x [DOI] [PubMed] [Google Scholar]
- 42.Horikawa C, Yoshimura Y, Kamada C, Tanaka S, Tanaka S, Hanyu O, et al. Dietary sodium intake and incidence of diabetes complications in Japanese patients with type 2 diabetes: Analysis of the Japan diabetes complications study (JDCS). Journal of Clinical Endocrinology and Metabolism. 2014;99(10):3635–43. doi: 10.1210/jc.2013-4315 [DOI] [PubMed] [Google Scholar]
- 43.Horikawa C, Yoshimura Y, Kamada C, Tanaka S, Tanaka S, Matsunaga S, et al. Is the Proportion of Carbohydrate Intake Associated with the Incidence of Diabetes Complications?-An Analysis of the Japan Diabetes Complications Study. Nutrients. 2017;9(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sahli MW, Mares JA, Meyers KJ, Klein R, Brady WE, Klein BEK, et al. Dietary Intake of Lutein and Diabetic Retinopathy in the Atherosclerosis Risk in Communities Study (ARIC). Ophthalmic epidemiology. 2016;23(2):99–108. doi: 10.3109/09286586.2015.1129426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sala-Vila A, Díaz-López A, Valls-Pedret C, Cofán M, García-Layana A, Lamuela-Raventós RM, et al. Dietary marine ω-3 fatty acids and incident sight-threatening retinopathy in middle-aged and older individuals with type 2 diabetes: Prospective investigation from the PREDIMED trial. JAMA ophthalmology. 2016;134(10):1142–9. doi: 10.1001/jamaophthalmol.2016.2906 [DOI] [PubMed] [Google Scholar]
- 46.Beulens JW, Kruidhof JS, Grobbee DE, Chaturvedi N, Fuller JH, Soedamah-Muthu SS. Alcohol consumption and risk of microvascular complications in type 1 diabetes patients: the EURODIAB Prospective Complications Study. Diabetologia. 2008;51(9):1631–8. doi: 10.1007/s00125-008-1091-z [DOI] [PubMed] [Google Scholar]
- 47.Giuffrè G, Lodato G, Dardanoni G. Prevalence and risk factors of diabetic retinopathy in adult and elderly subjects: The Casteldaccia Eye Study. Graefe's archive for clinical and experimental ophthalmology = Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie. 2004;242(7):535–40. doi: 10.1007/s00417-004-0880-4 [DOI] [PubMed] [Google Scholar]
- 48.Harjutsalo V, Feodoroff M, Forsblom C, Groop PH. Patients with Type 1 diabetes consuming alcoholic spirits have an increased risk of microvascular complications. Diabetic medicine: a journal of the British Diabetic Association. 2014;31(2):156–64. [DOI] [PubMed] [Google Scholar]
- 49.Kumari N. Is Coffee Consumption associated with Age-related Macular Degeneration and Diabetic Retinopathy? All Res J Biol. 2014;5(2). [Google Scholar]
- 50.Lee C, Stolk RP, Adler AI, Beulens J. Association between alcohol consumption and diabetic retinopathy and visual acuity—The AdRem study. Diabetes. 2009;58. [DOI] [PubMed] [Google Scholar]
- 51.Lugo-Radillo A, Amezcua-Portillo A, Valdovinos-Ruiz DE, Perez-Chavira CJ. The consumption of a diet abundant in vegetables and fruits or the performance of non-exhaustive exercise do not protect against diabetic retinopathy: a study in a Mexican population. International Journal of Diabetes in Developing Countries. 2015;35(3):375–9. [Google Scholar]
- 52.Moss SE, Klein R, Klein BE. Alcohol consumption and the prevalence of diabetic retinopathy. Ophthalmology. 1992;99(6):926–32. [DOI] [PubMed] [Google Scholar]
- 53.Moss SE, Klein R, Klein BE. The association of alcohol consumption with the incidence and progression of diabetic retinopathy. Ophthalmology. 1994;101(12):1962–8. [DOI] [PubMed] [Google Scholar]
- 54.Young RJ, McCulloch DK, Prescott RJ, Clarke BF. Alcohol: Another risk factor for diabetic retinopathy? British Medical Journal. 1984;288(6423):1035–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mayer-Davis EJ, Bell RA, Reboussin BA, Rushing J, Marshall JA, Hamman RF. Antioxidant nutrient intake and diabetic retinopathy: The San Luis Valley Diabetes Study. Ophthalmology. 1998;105(12):2264–70. doi: 10.1016/S0161-6420(98)91227-1 [DOI] [PubMed] [Google Scholar]
- 56.Engelen L, Soedamah-Muthu SS, Geleijnse JM, Toeller M, Chaturvedi N, Fuller JH, et al. Higher dietary salt intake is associated with microalbuminuria, but not with retinopathy in individuals with type 1 diabetes: the EURODIAB Prospective Complications Study. Diabetologia. 2014;57(11):2315–23. doi: 10.1007/s00125-014-3367-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Houtsmuller AJ, Zahn KJ, Henkes HE. Unsaturated fats and progression of diabetic retinopathy. Documenta Ophthalmologica. 1980;48(2):363–71. [DOI] [PubMed] [Google Scholar]
- 58.Howard-Williams J, Patel P, Jelfs R. Polyunsaturated fatty acids and diabetic retinopathy. British Journal of Ophthalmology. 1985;69(1):15–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Foster-Powell K, Holt SH, Brand-Miller JC. International table of glycemic index and glycemic load values: 2002. The American journal of clinical nutrition. 2002;76(1):5–56. [DOI] [PubMed] [Google Scholar]
- 60.Blackwood AD, Salter J, Dettmar PW, Chaplin MF. Dietary fibre, physicochemical properties and their relationship to health. The journal of the Royal Society for the Promotion of Health. 2000;120(4):242–7. [DOI] [PubMed] [Google Scholar]
- 61.Madsen-Bouterse SA, Kowluru RA. Oxidative stress and diabetic retinopathy: pathophysiological mechanisms and treatment perspectives. Reviews in endocrine & metabolic disorders. 2008;9(4):315–27. [DOI] [PubMed] [Google Scholar]
- 62.Ma Q, Shen JH, Shen SR, Das UN. Bioactive lipids in pathological retinopathy. Critical reviews in food science and nutrition. 2014;54(1):1–16. doi: 10.1080/10408398.2011.565523 [DOI] [PubMed] [Google Scholar]
- 63.Das UN. Lipoxins, resolvins, and protectins in the prevention and treatment of diabetic macular edema and retinopathy. Nutrition (Burbank, Los Angeles County, Calif). 2013;29(1):1–7. [DOI] [PubMed] [Google Scholar]
- 64.Mora JR, Iwata M, von Andrian UH. Vitamin effects on the immune system: vitamins A and D take centre stage. Nature reviews Immunology. 2008;8(9):685–98. doi: 10.1038/nri2378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Martinez-Gonzalez MA, Salas-Salvado J, Estruch R, Corella D, Fito M, Ros E. Benefits of the Mediterranean Diet: Insights From the PREDIMED Study. Progress in cardiovascular diseases. 2015;58(1):50–60. doi: 10.1016/j.pcad.2015.04.003 [DOI] [PubMed] [Google Scholar]
- 66.Ros E, Martinez-Gonzalez MA, Estruch R, Salas-Salvado J, Fito M, Martinez JA, et al. Mediterranean diet and cardiovascular health: Teachings of the PREDIMED study. Advances in nutrition (Bethesda, Md). 2014;5(3):330s–6s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Salas-Salvado J, Bullo M, Estruch R, Ros E, Covas MI, Ibarrola-Jurado N, et al. Prevention of diabetes with Mediterranean diets: a subgroup analysis of a randomized trial. Annals of internal medicine. 2014;160(1):1–10. doi: 10.7326/M13-1725 [DOI] [PubMed] [Google Scholar]
- 68.Toledo E, Hu FB, Estruch R, Buil-Cosiales P, Corella D, Salas-Salvado J, et al. Effect of the Mediterranean diet on blood pressure in the PREDIMED trial: results from a randomized controlled trial. BMC medicine. 2013;11:207 doi: 10.1186/1741-7015-11-207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Safi SZ, Qvist R, Kumar S, Batumalaie K, Ismail IS. Molecular mechanisms of diabetic retinopathy, general preventive strategies, and novel therapeutic targets. Biomed Res Int. 2014;2014:801269 doi: 10.1155/2014/801269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Archer DB. Bowman Lecture 1998. Diabetic retinopathy: some cellular, molecular and therapeutic considerations. Eye (London, England). 1999;13 (Pt 4):497–523. [DOI] [PubMed] [Google Scholar]
- 71.Skrha J, Kunesova M, Hilgertova J, Weiserova H, Krizova J, Kotrlikova E. Short-term very low calorie diet reduces oxidative stress in obese type 2 diabetic patients. Physiological research. 2005;54(1):33–9. [DOI] [PubMed] [Google Scholar]
- 72.Masoro EJ. Overview of caloric restriction and ageing. Mechanisms of ageing and development. 2005;126(9):913–22. doi: 10.1016/j.mad.2005.03.012 [DOI] [PubMed] [Google Scholar]
- 73.Weindruch R, Keenan KP, Carney JM, Fernandes G, Feuers RJ, Floyd RA, et al. Caloric restriction mimetics: metabolic interventions. The journals of gerontology Series A, Biological sciences and medical sciences. 2001;56 Spec No 1:20–33. [DOI] [PubMed] [Google Scholar]
- 74.3. Foundations of Care and Comprehensive Medical Evaluation. Diabetes care. 2016;39 Suppl 1:S23–35. [DOI] [PubMed] [Google Scholar]
- 75.Chiu CJ, Taylor A. Dietary hyperglycemia, glycemic index and metabolic retinal diseases. Progress in retinal and eye research. 2011;30(1):18–53. doi: 10.1016/j.preteyeres.2010.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Thomas D, Elliott EJ. Low glycaemic index, or low glycaemic load, diets for diabetes mellitus. The Cochrane database of systematic reviews. 2009(1):Cd006296 doi: 10.1002/14651858.CD006296.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kowluru RA. Diabetic retinopathy, oxidative stress and antioxidants. Current Topics in Nutraceutical Research. 2005;3(4):209–18. [Google Scholar]
- 78.Kowluru RA, Menon B, Gierhart DL. Beneficial effect of zeaxanthin on retinal metabolic abnormalities in diabetic rats. Investigative ophthalmology & visual science. 2008;49(4):1645–51. [DOI] [PubMed] [Google Scholar]
- 79.Connor KM, SanGiovanni JP, Lofqvist C, Aderman CM, Chen J, Higuchi A, et al. Increased dietary intake of omega-3-polyunsaturated fatty acids reduces pathological retinal angiogenesis. Nature medicine. 2007;13(7):868–73. doi: 10.1038/nm1591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sapieha P, Chen J, Stahl A, Seaward MR, Favazza TL, Juan AM, et al. Omega-3 polyunsaturated fatty acids preserve retinal function in type 2 diabetic mice. Nutr Diabetes. 2012;2:e36 doi: 10.1038/nutd.2012.10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tikhonenko M, Lydic TA, Opreanu M, Li Calzi S, Bozack S, McSorley KM, et al. N-3 polyunsaturated Fatty acids prevent diabetic retinopathy by inhibition of retinal vascular damage and enhanced endothelial progenitor cell reparative function. PloS one. 2013;8(1):e55177 doi: 10.1371/journal.pone.0055177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lottenberg AM, Afonso Mda S, Lavrador MS, Machado RM, Nakandakare ER. The role of dietary fatty acids in the pathology of metabolic syndrome. The Journal of nutritional biochemistry. 2012;23(9):1027–40. doi: 10.1016/j.jnutbio.2012.03.004 [DOI] [PubMed] [Google Scholar]
- 83.Zhu W, Meng YF, Wu Y, Xu M, Lu J. Association of alcohol intake with risk of diabetic retinopathy: a meta-analysis of observational studies. Scientific reports. 2017;7(1):4 doi: 10.1038/s41598-017-00034-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Greenfield JR, Samaras K, Jenkins AB, Kelly PJ, Spector TD, Campbell LV. Moderate alcohol consumption, estrogen replacement therapy, and physical activity are associated with increased insulin sensitivity: is abdominal adiposity the mediator? Diabetes care. 2003;26(10):2734–40. [DOI] [PubMed] [Google Scholar]
- 85.Evert AB, Boucher JL, Cypress M, Dunbar SA, Franz MJ, Mayer-Davis EJ, et al. Nutrition therapy recommendations for the management of adults with diabetes. Diabetes care. 2014;37 Suppl 1:S120–43. [DOI] [PubMed] [Google Scholar]
- 86.Shim JS, Oh K, Kim HC. Dietary assessment methods in epidemiologic studies. Epidemiology and Health. 2014;36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Moeller SM, Reedy J, Millen AE, Dixon LB, Newby PK, Tucker KL, et al. Dietary patterns: challenges and opportunities in dietary patterns research an Experimental Biology workshop, April 1, 2006. Journal of the American Dietetic Association. 2007;107(7):1233–9. doi: 10.1016/j.jada.2007.03.014 [DOI] [PubMed] [Google Scholar]
- 88.Hu FB. Dietary pattern analysis: a new direction in nutritional epidemiology. Current opinion in lipidology. 2002;13(1):3–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
(DOC)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.