Abstract
Background
Primaquine (PQ) actively clears mature Plasmodium falciparum gametocytes but in glucose-6-phosphate dehydrogenase deficient (G6PDd) individuals can cause hemolysis. We assessed the safety of low-dose PQ in combination with artemether-lumefantrine (AL) or dihydroartemisinin-piperaquine (DP) in G6PDd African males with asymptomatic P. falciparum malaria.
Methods and findings
In Burkina Faso, G6PDd adult males were randomized to treatment with AL alone (n = 10) or with PQ at 0.25 (n = 20) or 0.40 mg/kg (n = 20) dosage; G6PD-normal males received AL plus 0.25 (n = 10) or 0.40 mg/kg (n = 10) PQ. In The Gambia, G6PDd adult males and boys received DP alone (n = 10) or with 0.25 mg/kg PQ (n = 20); G6PD-normal males received DP plus 0.25 (n = 10) or 0.40 mg/kg (n = 10) PQ. The primary study endpoint was change in hemoglobin concentration during the 28-day follow-up. Cytochrome P-450 isoenzyme 2D6 (CYP2D6) metabolizer status, gametocyte carriage, haptoglobin, lactate dehydrogenase levels and reticulocyte counts were also determined.
In Burkina Faso, the mean maximum absolute change in hemoglobin was -2.13 g/dL (95% confidence interval [CI], -2.78, -1.49) in G6PDd individuals randomized to 0.25 PQ mg/kg and -2.29 g/dL (95% CI, -2.79, -1.79) in those receiving 0.40 PQ mg/kg. In The Gambia, the mean maximum absolute change in hemoglobin concentration was -1.83 g/dL (95% CI, -2.19, -1.47) in G6PDd individuals receiving 0.25 PQ mg/kg. After adjustment for baseline concentrations, hemoglobin reductions in G6PDd individuals in Burkina Faso were more pronounced compared to those in G6PD-normal individuals receiving the same PQ doses (P = 0.062 and P = 0.022, respectively). Hemoglobin levels normalized during follow-up. Abnormal haptoglobin and lactate dehydrogenase levels provided additional evidence of mild transient hemolysis post-PQ.
Conclusions
Single low-dose PQ in combination with AL and DP was associated with mild and transient reductions in hemoglobin. None of the study participants developed moderate or severe anemia; there were no severe adverse events. This indicates that single low-dose PQ is safe in G6PDd African males when used with artemisinin-based combination therapy.
Trial registration
Clinicaltrials.gov NCT02174900
Clinicaltrials.gov NCT02654730
Background
Substantial reductions in malaria-related morbidity and mortality in the last decade [1] have encouraged Plasmodium falciparum elimination initiatives and increased interest in transmission-blocking interventions [2]. Although current first-line artemisinin-based combination therapies (ACTs) rapidly clear asexual parasites and early stage gametocytes, thereby limiting post-treatment transmission [3, 4], ACTs have incomplete activity against mature gametocytes [5, 6], the parasite stage responsible for transmission, and a proportion of patients may transmit malaria to mosquitoes after successful treatment [7, 8].
Primaquine (PQ) is the only available drug with established activity against mature P. falciparum gametocytes [9, 10] and was recommended at a single dose of 0.75 mg/kg until 2015. Recent studies in Uganda, Burkina Faso, Mali and The Gambia found that lower PQ doses of 0.20–0.40 mg/kg also clear gametocytes that persist after ACT [11–14] and prevent transmission to mosquitoes [12, 13]. These studies also demonstrated variation in transmission potential after different ACTs, with artemether-lumefantrine (AL) being associated with lower post-treatment gametocyte carriage and transmission potential compared to dihydroartemisinin-piperaquine (DP) [12, 13]. Current evidence on PQ efficacy supports the recent World Health Organization (WHO) guideline change to give a single 0.25 mg/kg PQ dose to all patients with confirmed P. falciparum (except pregnant women, infants aged < 6 months and breastfeeding women of infants aged < 6 months) on the first day of ACT in areas where there is a threat of artemisinin resistance or where P. falciparum elimination is considered [15].
However, the widespread use of PQ is limited by concerns about dose-dependent hemolysis in glucose-6-phosphate dehydrogenase deficient (G6PDd) individuals [9, 16–18]. Whilst formal safety assessments have been performed for the 14-day PQ regimen used for Plasmodium vivax radical cure [19–21], robust data on the safety of single low-dose PQ in G6PDd individuals is unavailable. Current evidence on the safety of a single low-dose PQ in G6PDd individuals is based on trials in which study participants were not or inefficiently screened for G6PD deficiency prior to enrolment. Three such studies in Africa reported mean absolute reductions in hemoglobin concentration 7 days post 0.75 mg/kg PQ of 1.1–2.5 g/dL, and mean relative reductions compared to baseline of 7–20% in G6PDd individuals [16, 22, 23]. Occassionally, moderate or severe transient anaemia was observed after a single PQ-dose of 0.75 mg/kg [16]. More recently, the safety of the 0.25 mg/kg PQ dose was reported as ancillary analysis after a malaria elimination campaign on the Thai-Myanmar border [24], in a gametocyte clearance efficacy study in Tanzania [25], and in a randomized controlled safety trial in Senegal [26]. The first two studies observed mean relative reductions in hemoglobin of 4–8% 5–8 days after 0.25 mg/kg PQ in a subpopulation of G6PDd participants [24, 25]. While all studies suggested the WHO-recommended PQ dose was safe, only the study from Tine et al. intensively monitored G6PDd individuals who have a higher risk of hemolysis [26]. Markers of hemolysis, including reticulocyte counts, lactate dehydrogenase (LDH), haptoglobin and bilirubin concentrations were not examined. Moreover, since the implementation of age-based dosing of PQ will inherently result in over- and under-dosing of individuals, it is important to assess PQ safety over a range of doses. Additional studies that intensively monitor changes in hemoglobin following different PQ doses in G6PDd individuals, alongside additional markers of hemolysis, may thus provide additional data on PQ safety that are needed to support policy recommendations [27].
Here, we report two linked randomized trials specifically designed to assess the tolerability and safety of two different doses of PQ in combination with artemether-lumefantrine (AL) or dihydroartemisinin-piperaquine (DP) in G6PDd African males with asymptomatic P. falciparum malaria.
Methods
Study design
Two open-label, randomized, dose-escalation trials were conducted from August 2014 to November 2015 in Banfora, in southwest Burkina Faso and from December 2015 to April 2016 in Basse Santa Su, Upper River Region, The Gambia. The studies received approval of the Interventions Research Ethics Committee of the London School of Hygiene and Tropical Medicine (#6523 for Burkina Faso; #8487 for The Gambia), Comité d’Ethique pour la Recherche en Santé, Ministère de la Santé du Burkina Faso (#2014-02-09) and Comité Technique d’Examen des Demandes d’Autorisation d’Essais Cliniques, Ministère de la Santé du Burkina Faso (#50002520146EC00000) and The Gambia Government/MRC Joint Ethics Committee (SCC 1391, 17 August 2015). Both studies were registered at ClinicalTrials.gov (NCT02174900 and NCT02654730). Written informed consent was obtained from all participants or from their parents or guardians prior to screening and enrolment.
We included G6PDd males to reduce the possibility of incorrect classification of G6PDd in heterozygous females. In Burkina Faso, a first phase of screening in the target population was performed in the community using the CareStart™ G6PD Rapid Diagnostic Test (G6PD RDT; Access Bio, Inc. Somerset, USA). To minimize the risk for trial participants in this first formal safety study on single low-dose PQ in G6PDd individuals, we excluded symptomatic patients and anemic individuals. Potentially eligible individuals were invited at the clinic to enroll males (age 18–45 years) with asymptomatic P. falciparum malaria, hemoglobin concentration ≥11 g/dL, and confirmed G6PD status by the “Beutler’s” fluorescent spot test [28]. Additional exclusion criteria were: fever (tympanic temperature >37.5°C) or history of fever in the last 24 hours; enrolled in another clinical trial; severe illness or danger signs; active infection other than malaria; history of severe chronic illness; known allergy to study medications; use of antimalarials in the previous 2 weeks; use of PQ in the previous 4 weeks; blood transfusion in the previous 90 days; non-falciparum malaria co-infection; current tuberculosis or anti-retroviral treatment; or current use of sulphonamides, dapsone, nitrofurantoin, nalidixic acid, ciprofloxacin, methylene blue, toluidine blue, phenazopyridine or co-trimoxazole. Enrolled participants were sequentially assigned to two cohorts. The first cohort consisted of one intervention group (G6PDd participants treated with AL and a single dose of 0.25 mg/kg PQ [n = 20]) and three control groups (G6PDd participants treated with AL alone [n = 10], and G6PD-normal participants treated with AL and a single dose of 0.25 mg/kg PQ [n = 10] or 0.40 mg/kg PQ [n = 10]). After the Data Safety Monitoring Board reviewed safety data, enrolment of the second cohort was initiated, consisting of one intervention group (G6PDd participants treated with AL and a single dose of 0.40 mg/kg PQ [n = 20]).
In The Gambia, males (age ≥10 years) with asymptomatic P. falciparum malaria were screened using identical procedures to Burkina Faso and randomized to one intervention group (G6PDd participants treated with DP and a single dose of 0.25 mg/kg PQ [n = 20]) or one of three control groups (G6PDd participants treated with DP alone [n = 10], or G6PD-normal participants treated with DP and a single dose of 0.25 mg/kg PQ [n = 10] or 0.40 mg/kg PQ [n = 10]). Whilst the Data Safety Monitoring Board review was favorable to escalating the treatment to a single dose of 0.40 mg/kg PQ, this could not be completed for logistical and financial reasons.
Procedures
All participants in Burkina Faso received 6 doses of 4 tablets of AL twice daily over three days (Coartem [20 mg artemether and 120 mg lumefantrine], Novartis Pharma AG, Basel, Switzerland). In The Gambia, participants received a daily dose of DP (Eurartesim [40mg piperaquine and 320mg dihydroartemisinin], Sigma-Tau IFR S.p.A, Italy) over three days; the dose was calculated based on body weight, on an empty stomach. For groups receiving PQ, doses were prepared as previously described [11]: PQ tablets containing 26.3 mg PQ phosphate USP, equivalent to 15 mg of PQ base (Sanofi, New York, USA) were crushed and dissolved in 15 mL of drinking water to produce a stable 1 mg/mL PQ base solution. We drew up the assigned dose to the nearest 0.5 mL. In Burkina Faso, PQ was administered under supervision together with the first AL dose with biscuits and juice. In The Gambia, PQ was administered under supervision with a glass of orange juice 30 minutes after the first dose of DP.
Participants returned to the study clinic for follow-up on the evening of day 0, twice daily on days 1, 2, and 3 (morning and evening), and once daily on days 4, 5, 7, 10, 14 and 28, and on additional days if they developed symptoms. All adverse events (AEs) were scored based on the Division of Microbiology and Infectious Diseases (DMID) toxicity table and graded as mild/grade 1 (awareness of symptoms that were easily tolerated and did not interfere with usual daily activity), moderate/grade 2 (discomfort that interfered with or limited usual daily activity), or severe/grade 3 (disabling, with inability to perform usual daily activity). Tympanic temperature was measured and recorded as fever grade 1 (37.6–38.0°C), grade 2 (> 38.0–39.0°C), or grade 3 (> 39.0°C). Causality of AEs was classified as unrelated, unlikely, possibly, probably or definitely related to the trial drugs.
Hemoglobin concentration was assessed at each visit by self-calibrating HemoCue 201+ photometers (Hemocue; Ängelholm, Sweden). Blood samples were taken for microscopy on days 0, 1, 2, 3, 7, 10, 14 and 28, and stained with 10% Giemsa for 10 minutes before screening for asexual parasites and gametocytes in 100 microscopic fields and quantifying against 200 and 500 leukocytes, respectively, and translating to parasite counts/μl assuming 8000 leukocytes per μl. Standard hematological and biochemical tests were done twice daily on days 0 and 1, and once daily on days 3, 7, 14 and 28 using for hematology the Cell-Dyn Ruby [Abbott Diagnostics, Wiesbaden, Germany] in Burkina Faso and the Medonic M-Series analyzer [Boule Medical AB, Spånga, Sweden] in The Gambia and for biochemistry the BS-200 analyzer [Shenzhen Mindray Bio-Medical Electronics Co., Ltd, Guangdong Sheng, China] in Burkina Faso and the Vitros 350 analyzer [Ortho Clinical Diagnostics, Raritan, NJ, US] in The Gambia. Assessments include total bilirubin and reticulocyte counts. Gametocyte detection by Pfs25 mRNA quantitative nucleic acid sequence-based amplification (QT-NASBA, Burkina Faso) or Pfs25 mRNA quantitative reverse-transcriptase PCR (qRT-PCR, The Gambia) [29] was done on days 0, 3 and 7. Urine dipstick to detect blood/hemoglobin and urobilinogen was done twice daily on days 0, 1, 2 and 3 and once daily on days 4, 5, 7 and 28. Remaining plasma samples from biochemical tests from the study in Burkina Faso were used for haptoglobin and LDH measurements.
Whole blood samples collected on day 0 were used for genotyping the gene for human cytochrome P-450 isoenzyme 2D6 (CYP2D6) to investigate any influence on PQ pharmacokinetics [30–32], and G6PD sequence variants 202A and 376G, which define the major African G6PD deficient variant A-. CYP2D6 and G6PD genotyping was performed as described previously [13]. For G6PD the two sequence variants (202A and 376G) were genotyped in OpenArray or single assay format (Thermo Fisher). For CYP2D6 21 sequence variants were genotyped for detection of major alleles *2, *3, *4, *6, *7, *8, *9, *10, *11, *15, *17, *18, *19, *20, *29, *40, and *41 with Quantstudio 12K Flex OpenArray with TaqMan assays (Thermo Fisher). If no CYP2D6 mutations were detected, participants were genotyped as *1. Also three copy number variation (CNV) assays targeting introns 2 and 6 as well as exon 9 of the gene were used for copy number analysis, allowing for partial detection of potential CYP2D6 hybrid alleles with the pseudogene CYP2D7 [33]. From genotype, Activity Score (AS) for each participant was calculated and metabolizer phenotype was inferred accordingly: an AS of 0.0 = poor metabolizer (PM) phenotype, AS of 0.5 or 1.0 = intermediate metabolizer (IM) phenotype, AS of 1.5 or 2.0 = extensive metabolizer phenotype, and an AS of 2.5 or more = ultra-rapid metabolizer (UM) phenotype [34–36].
Primary and secondary endpoints
The study objective was to evaluate the tolerability and safety of single doses of PQ at two different dosages when administered with AL or DP to G6PDd males with asymptomatic P. falciparum infections. The primary study outcome was the hemoglobin concentration during the 28-day follow-up relative to baseline value (safety). Secondary outcomes included frequency and severity of AEs, and changes in hemoglobin concentration > -2.5 g/dL (safety outcomes), and post-treatment gametocyte prevalence (efficacy outcome). Secondary outcomes that were only assessed for Burkina Faso included other hematological (e.g. haptoglobin) and biochemical (e.g. LDH) parameters during follow-up.
Statistical analysis
Statistical analysis was performed using Stata (v13, StataCorp). Student t-test was used to compare hemoglobin concentration (g/dL) at baseline between study groups; other continuous variables were compared by non-parametric Wilcoxon-rank sum. Proportions were compared by chi-square test. Repeated measures mixed models (xtmixed with independent covariance structure) were used for pairwise comparisons of hemoglobin concentrations. Hemoglobin concentration during follow-up relative to baseline value was expressed as absolute and relative changes. To limit multiple comparisons, we restricted comparisons of changes in hemoglobin concentration to days 3, 7, 14 and 28 [16, 22, 23]. None of the P-values were adjusted for multiple comparisons. We adjusted all comparisons for baseline hemoglobin concentration using multivariate linear regression; models only contained treatment group and baseline hemoglobin. A drop of >2.5 g/dL at any time-point during follow-up was analyzed as dichotomous variable. Moderate anemia was defined as a hemoglobin concentration <8 g/dL; severe anemia as a hemoglobin concentration <5 g/dL [37]. The difference in AEs among groups was calculated as i) the proportion of individuals developing AEs in each group and ii) the frequency of AEs in each group (absolute number of AEs per participant). This study was not powered to assess PQ gametocytocidal efficacy [11, 12] and analyses of gametocyte prevalence by molecular methods at baseline and during follow-up are descriptive.
We explored whether genetically inferred CYP2D6 metabolizer status was a relevant factor in determining hemolysis after PQ by adding inferred CYP2D6 metabolizer status (poor/intermediate versus extensive/ultra rapid) to multivariate linear regression models with absolute or relative reductions in hemoglobin concentration on day 7 compared to baseline as dependent variable and baseline hemoglobin and phenotypically determined G6PD status as independent variables. This analysis was restricted to PQ-treated study participants.
Sample size
We did not perform a formal sample size calculation for this safety study. We assumed the probability of the occurrence of at least one serious adverse event (SAE) was 10% for G6PDd individuals. Twenty individuals in each of the 2 intervention groups (G6PDd participants receiving PQ) provide an 88% probability of detecting at least one individual with an SAE and 61% probability of detecting 2 or more individuals with SAEs. The control groups were included to support the interpretation of hemoglobin concentrations following treatment; the size of these control groups was based on expert opinion (Walter Reed Army Institute of Research, Tafenoquine group).
Results
Overview of trials
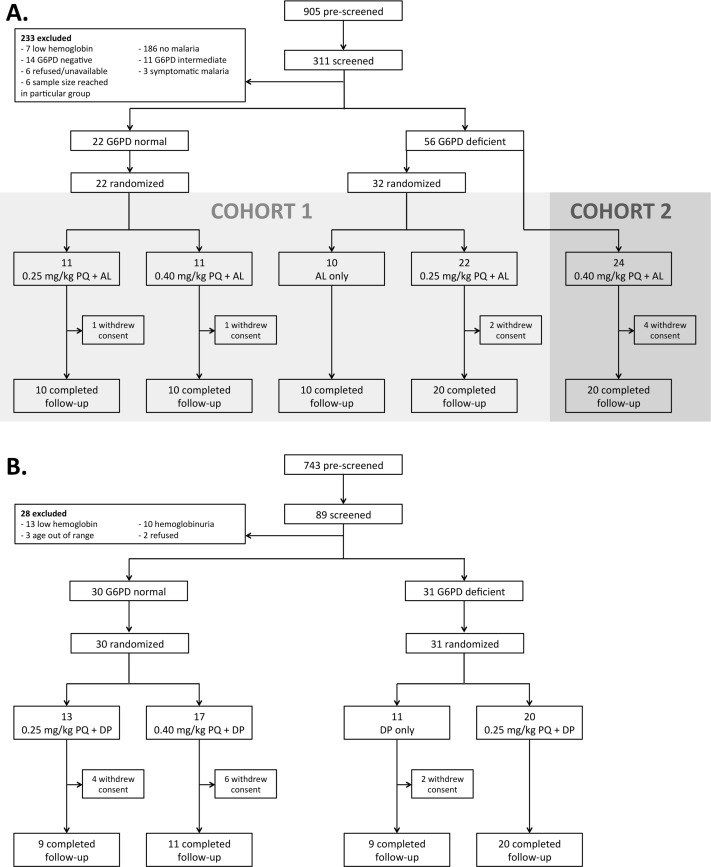
Initial G6PD-status screening by G6PD RDT in Burkina Faso involved 905 males; 311 were further screened in the clinic and 78 were enrolled (Fig 1A). Seventy participants completed follow-up, 8 were lost to follow-up prior to day 3 (Fig 1A). In The Gambia 743 males were pre-screened and 61 were enrolled of whom 49 completed follow-up and 12 withdrew within the first week (Fig 1B). Mean hemoglobin concentration at baseline was higher in G6PD-normal compared to G6PDd individuals in Burkina Faso (mean difference 0.84 g/dL; 95% CI, 0.16–1.53; P = 0.016) but not in The Gambia (mean difference -0.33 g/dL; 95% CI, -1.09–0.43; P = 0.38). Other baseline characteristics were not significantly different between G6PDd and G6PD-normal individuals or between study groups (Table 1). Median asexual parasite density at enrolment was 85.5 (interquartile range [IQR], 43.0–269.0 parasites/μL) in Burkina Faso; gametocyte prevalence was 3.8% (3/78) by microscopy and 89.9% (62/69) by QT-NASBA (Table 1). In The Gambia, only 9.8% (6/61) of study participants had asexual parasites detected by microscopy at enrolment with a median parasite density of 768.0 (IQR, 320.0–1520.0 parasites/μL) in parasite positive individuals. Gametocyte prevalence was 1.6% (1/61) by microscopy and 18.0% (11/61) by qRT-PCR (Table 1).
Fig 1.
Clinical trial profile for Burkina Faso (A) and The Gambia (B). Abbreviations: AL, artemether-lumefantrine; G6PD, glucose-6-phosphate dehydrogenase; PQ, primaquine; DP, dihydroartemisinin-piperaquine.
Table 1. Baseline characteristics of enrolled subjects.
| Burkina Faso | ||||||
|---|---|---|---|---|---|---|
| G6PD Normal | G6PD Deficient | |||||
| Characteristic | 0.25 mg/kg PQ + AL (n = 11) |
0.40 mg/kg PQ + AL (n = 11) |
AL only (n = 10) |
0.25 mg/kg PQ + AL (n = 22) |
0.40 mg/kg PQ + AL (n = 24) | |
| Age, y, median (IQR) | 26.0 (22.0–27.0) | 31.0 (21.0–40.0) | 33.0 (23.0–38.0) | 24.5 (21.0–32.0) | 30.0 (25.5–36.0) | |
| Hemoglobin, g/dL, mean (SD) | 14.5 (1.4) | 15.0 (1.6) | 13.6 (0.9) | 14.0 (1.6) | 14.0 (1.2) | |
| Asexual parasite density by microscopy, parasites/μL, median (IQR) | 117 (44.0–537.0) | 43.0 (32.0–107.0) | 140.5 (28.0–276.0) | 67.0 (40.0–175.0) | 100.5 (54.5–821.5) | |
| Gametocyte prevalence by microscopy, % (n/N) | 0.0 (0/11) | 0.0 (0/11) | 10.0 (1/10) | 0.0 (0/22) | 8.3 (2/24) | |
| Gametocyte prevalence by QT-NASBA, % (n/N) | 100.0 (10/10) | 100.0 (10/10) | 80.0 (8/10) | 85.0 (17/20) | 89.5 (17/19) | |
| CYP2D6 inferred poor metabolizer phenotype, % (n/N) | 0.0 (0/11) | 0.0 (0/11) | 0.0 (0/10) | 0.0 (0/20) | 4.2 (1/24) | |
| CYP2D6 inferred intermediate metabolizer phenotype, % (n/N) | 27.3 (3/11) | 36.4 (4/11) | 30.0 (3/10) | 55.0 (11/20)a | 62.5 (15/24) | |
| The Gambia | ||||||
| G6PD Normal | G6PD Deficient | |||||
| Characteristic | 0.25 mg/kg PQ + DP (n = 13) |
0.40 mg/kg PQ + DP (n = 17) |
DP only (n = 11) |
0.25 mg/kg PQ + DP (n = 20) |
||
| Age, y, median (IQR) | 13.0 (12.0–16.0) | 15.0 (11.0–20.0) | 13.0 (11.0–29.0) | 15.5 (12.5–20.0) | ||
| Hemoglobin, g/dL, mean (SD) | 13.1 (1.5) | 12.8 (1.4) | 12.9 (1.8) | 13.5 (1.4) | ||
| Asexual parasite prevalence by microscopy, % (n/N) | 15.4 (2/13) | 11.8 (2/17) | 18.2 (2/11) | 0.0 (0/20) | ||
| Asexual parasite density by microscopy, parasites/μLb | 1520; 2384 | 288; 320 | 400; 1136 | - | ||
| Gametocyte prevalence by microscopy, % (n/N) | 0.0 (0/13) | 0.0 (0/17) | 9.1 (1/11) | 0.0 (0/20) | ||
| Gametocyte prevalence by qRT-PCR, % (n/N) | 7.7 (1/13) | 29.4 (5/17) | 27.3 (3/11) | 10.0 (2/20) | ||
| CYP2D6 inferred poor metabolizer phenotype, % (n/N) | 0.0 (0/13) | 0.0 (0/15) | 0.0 (0/11) | 5.3 (1/19) | ||
| CYP2D6 inferred intermediate metabolizer phenotype, % (n/N) | 30.8 (4/13) | 40.0 (6/15) | 54.5 (6/11) | 26.3 (5/19) | ||
Abbreviations: G6PD, glucose-6-phosphate dehydrogenase; PQ, primaquine; AL, artemether-lumefantrine; IQR, interquartile range; QT-NASBA, quantitative nucleic acid sequence-based amplification; SD, standard deviation; CYP2D6, cytochrome P-450 isoenzyme 2D6; DP, dihydroartemisinin-piperaquine; qRT-PCR, quantitative reverse transcriptase PCR.
CYP2D6 inferred poor metabolizer phenotype = activity score 0.0; CYP2D6 inferred intermediate metabolizer phenotype = activity score 0.5–1.0.
a Potential CYP2D7/CYP2D6 hybrid allele in 2 subjects.
b Individual asexual parasite densities for parasite positive individuals only.
Genotyping of the 202A and 376G mutations in the G6PD gene broadly confirmed the phenotypic status of study participants (S1 Table). In Burkina Faso all individuals with the 202A mutation were G6PDd by fluorescent spot test; two lacked the 376G mutation. In The Gambia the 202A mutation was detected in 13/30 individuals who were phenotypically G6PDd whilst 14 had the 376G mutation only and 3 were wild-type for the 202A and 376G mutation (S1 Table). CYP2D6 metabolizer phenotypes were inferred from genotypes for all participants enrolled except for 2 who potentially had a CYP2D7/2D6 hybrid allele with unknown metabolizer phenotype in Burkina Faso and 3 individuals with missing samples in The Gambia (Table 1, S2 Table). Two participants in the Gambia were also potential hybrid CYP2D7/2D6 allele carriers, but one was homozygous for the *4-allele, which strongly suggests this person to be a poor metabolizer and was therefore classified as such, the other of the two could not have a CYP2D6 metabolizer phenotype inferred.
All participants from both study sites cleared their asexual parasites by day 2, based on microscopy results. The current trials with limited study population sizes were not designed to determine gametocyte clearance; larger efficacy trials have addressed this recently [11–14]. In Burkina Faso, gametocyte prevalence by QT-NASBA in the AL only group was 80% (8/10) on day 3 and 33.3% (2/6) on day 7, which was numerically higher than in individuals receiving 0.25 mg/kg PQ (69.0% [20/29; P = 0.50] on day 3 and 7.7% [2/26; P = 0.09] on day 7) or 0.40 mg/kg PQ (68.2% [15/22; P = 0.68] on day 3 and 7.1% [1/14; P = 0.20] on day 7). In The Gambia, gametocyte prevalence by QT-NASBA in the DP only group was 30.0% (3/10) on day 3 and 22.2% (2/9) on day 7, which was statistically significantly higher than in individuals receiving 0.25 mg/kg PQ (0% [0/20, P = 0.03] on day 3 and 0.0% [0/29; P = 0.05] on day 7) and non-significantly higher than in individuals receiving 0.40 mg/kg PQ (16.7% [2/12; P = 0.21] on day 3 and 0.0% [0/11; P = 0.19] on day 7).
Hematological changes after treatment
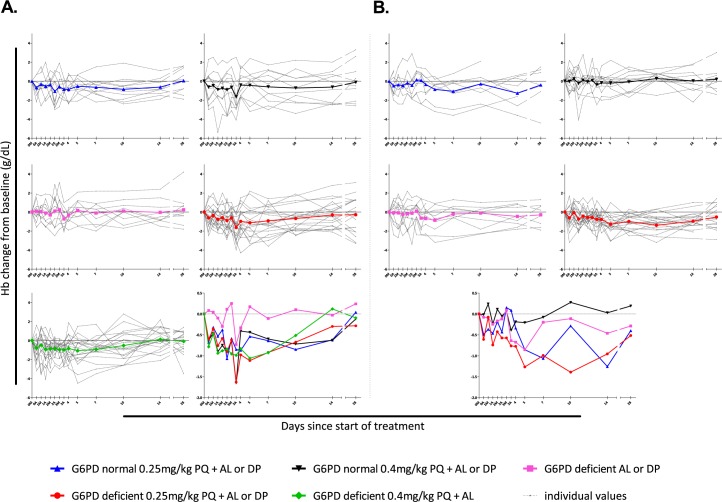
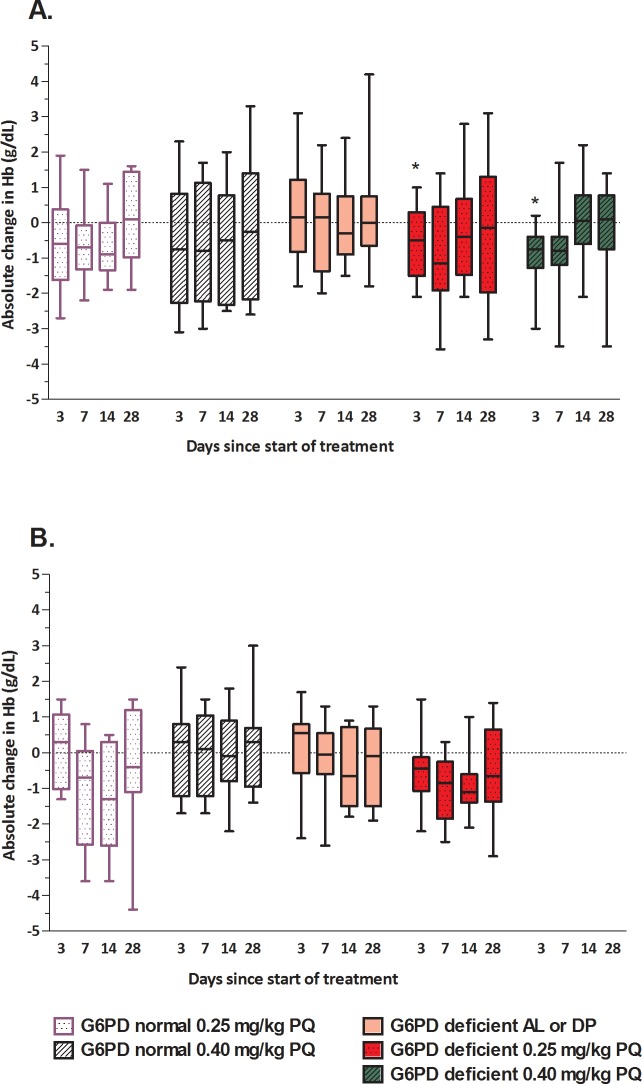
We first describe changes in hemoglobin relative to baseline values (Fig 2) and absolute changes in hemoglobin concentration (Fig 3) for each of the study arms and study settings separately. In all groups receiving PQ, mean hemoglobin concentrations, presented as relative or absolute change, declined in the first week after treatment and returned to baseline values during follow-up (Figs 2 and 3). Similarly, paired analysis of hemoglobin concentrations relative to baseline indicated reductions in G6PDd individuals in the first week after receiving 0.25mg/kg or 0.4mg/kg PQ (Fig 3). These reductions were statistically significant on day 3 after 0.25 mg/kg PQ (coefficient, -0.92; 95% CI, -1.81, -0.024; P = 0.044) and 0.40 mg/kg PQ (coefficient, -1.17; 95% CI, -2.06, -0.28; P = 0.010) in Burkina Faso. None of the control groups showed meaningful reductions in hemoglobin concentrations during follow-up (Figs 2 and 3). Analyses of hemoglobin as absolute value reflected the same patterns with an absolute decline in hemoglobin levels for G6PDd study participants one week after treatment with PQ, followed by a return to baseline values (Fig 3 and S3 Table).
Fig 2.
Hemoglobin levels during 28-day follow-up Burkina Faso (A) and The Gambia (B). Hemoglobin concentrations (g/dL) during follow-up are expressed relative to that at enrolment for each individual (grey dotted lines) and for each treatment group (colored lines). Abbreviations: 0M, day 0 morning; 0A, day 0 afternoon, etc.; Hb, hemoglobin; G6PD, glucose-6-phosphate dehydrogenase; PQ, primaquine; AL, artemether-lumefantrine; DP, dihydroartemisinin-piperaquine.
Fig 3.
Absolute changes in hemoglobin levels during 28-day follow-up for Burkina Faso (A) and The Gambia (B). Hemoglobin concentrations (g/dL) on days 3, 7, 14 and 28 during follow-up are expressed relative to that at enrolment for each treatment group. Asterisks indicate a statistically significant reduction compared to baseline by repeated measures mixed models. Abbreviations: Hb, hemoglobin; G6PD, glucose-6-phosphate dehydrogenase; PQ, primaquine.
Whilst mean changes in hemoglobin concentration allow a general comparison between groups, maximum reductions may better reflect severe hemolysis. We thus calculated the maximum reductions at absolute and relative hemoglobin values that were experienced by each trial participant and the proportion of participants experiencing a reduction >2.5 g/dL. The maximum reductions in hemoglobin concentrations, both as absolute and relative change, were strongly influenced by baseline hemoglobin concentrations with larger reductions for individuals with higher baseline concentrations (S1 Fig). Subsequent comparisons, even those on relative hemoglobin concentration, were therefore adjusted for baseline hemoglobin concentration [23]. In Burkina Faso, maximum drops in hemoglobin concentration (the largest reduction in hemoglobin compared to baseline at any time point during follow-up) after 0.25 mg/kg PQ were larger in G6PDd than G6PD-normal participants, both at absolute (mean difference, -0.64 g/dL; 95% CI, -1.31, 0.04; P = 0.062) and relative (mean difference, -4.23%; 95% CI, -9.17, 0.70; P = 0.09) scales (Fig 2 and S3A Table). Similarly, maximum drops in hemoglobin concentration in Burkina Faso were significantly larger in G6PDd than G6PD-normal participants after 0.40 mg/kg PQ, both at an absolute scale (mean difference, -0.82 g/dL; 95% CI, -1.51, -0.13; P = 0.022) and expressed as proportion of baseline hemoglobin concentration (mean difference, -6.23%; 95% CI, -11.22, -1.25; P = 0.016) (Fig 2 and S3A Table). In The Gambia, we observed no statistically significant difference in maximum drops in hemoglobin concentration between G6PDd and G6PD-normal participants treated with 0.25 mg/kg PQ at the absolute (P = 0.93) or relative scale (P = 0.95) (S3B Table). In Burkina Faso, the proportion of participants with a decrease in hemoglobin of >2.5 g/dL was 45.0% (9/20) in the G6PDd group receiving 0.25 mg/kg PQ and 40.0% (4/10) in the G6PD-normal group receiving the same PQ dose (P = 0.167). The proportion of participants with a decrease in hemoglobin of >2.5 g/dL was 35.0% (7/20) in the G6PDd group receiving 0.40 mg/kg PQ and 50.0% (5/10) in the G6PD-normal group receiving the same dose (P = 0.687). In The Gambia, the proportion of participants with a decrease in hemoglobin of >2.5 g/dL was 20.0% (4/20) in the G6PDd group receiving 0.25 mg/kg PQ and 33.3% (3/9) in the G6PD-normal group receiving the same dose (P = 0.34, S3A Table).
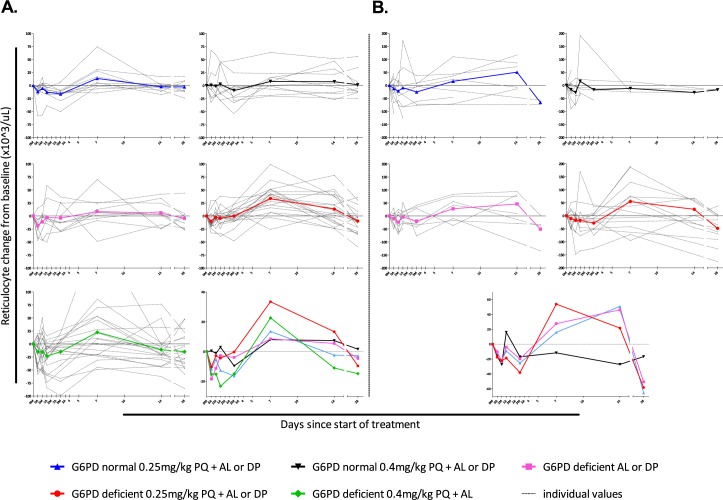
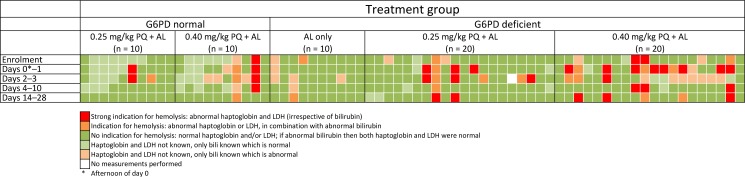
None of the study participants developed moderate or severe anemia. There was a general trend towards an increase in reticulocyte count at day 7 in all treatment arms in both Burkina Faso and The Gambia, particularly in G6PDd trial participants receiving PQ (Fig 4). Haptoglobin and LDH concentrations were determined in all available plasma samples for Burkina Faso only. Haptoglobin concentrations <0.3 g/L in combination with LDH levels >250 U/L were considered indicative of hemolysis [38, 39]. In addition, total bilirubin concentration (abnormal defined as ≥20.53 μmol/L) was used as indicator of hemolysis. Abnormal values for either haptoglobin or LDH, and total bilirubin were strongly correlated (P ≤ 0.008). The proportion of individuals with haptoglobin, LDH and/or total bilirubin concentrations indicative of hemolysis was higher in PQ-treated individuals (Fig 5). Genetically inferred CYP2D6 metabolizer status was not associated with absolute and relative reductions in hemoglobin concentration on day 7 (S4 Table).
Fig 4.
Absolute reticulocyte counts during 28-day follow-up for Burkina Faso (A) and The Gambia (B). Absolute reticulocyte counts (x103/μL) during follow-up are expressed relative to that at enrolment for each individual (grey dotted lines) and for each treatment group (colored lines). The value not displayed in the G6PD-deficient 0.40 mg/kg PQ group was 216 x103/μL. Abbreviations: 0M, day 0 morning; 0A, day 0 afternoon, etc.; G6PD, glucose-6-phosphate dehydrogenase; PQ, primaquine; AL, artemether-lumefantrine; DP, dihydroartemisinin-piperaquine.
Fig 5. Haptoglobin, lactate dehydrogenase (LDH) and total bilirubin levels as parameters of hemolysis during follow-up in Burkina Faso.
Each individual represents one vertical column. Abnormal levels were defined as: haptoglobin <0.3 g/L; LDH ≥250 U/L; total bilirubin ≥20.53 μmol/L. Abbreviations: G6PD, glucose-6-phosphate dehydrogenase; PQ, primaquine; AL, artemether-lumefantrine.
Adverse events and clinical assessment of hemolysis
There were 82 AEs recorded for the 70 participants from Burkina Faso who completed follow-up. All reported AEs were graded as mild or moderate (Table 2). In all 10 participants from Burkina Faso with hemoglobinuria, the diagnosis was made between the evening visit of day 0 (8 hours after enrolment and first treatment dose) and the morning visit of day 3 (72 hours after enrolment), with no difference between treatment groups (Table 2). In The Gambia, a total of 26 AEs were recorded in 20 participants and all were classified as mild. Sixteen participants had hemoglobinuria at enrollment (before the first dose of DP) that did not worsen during follow-up. There was no association between hemoglobinuria and randomization group. Microscopic urinalysis confirmed a diagnosis of schistosomiasis and participants were subsequently treated with praziquantel (Table 3). We recorded no SAEs, no cases of blackwater fever, and no red, black, or tea-colored urine. None of the participants required a blood transfusion.
Table 2. Adverse events of any severity in the different treatment groups in Burkina Faso.
| Treatment Group | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| G6PD Normal | G6PD Deficient | ||||||||||||||
| 0.25 mg/kg PQ + AL (n = 10) |
0.4 mg/kg PQ + AL (n = 10) |
AL only (n = 10) |
0.25mg/kg PQ + AL (n = 20) |
0.4mg/kg PQ + AL (n = 20) |
|||||||||||
| Adverse Event | No. subjectsa | No. AEs | Days after PQ | No. subjects | No. AEs | Days after PQ | No. subjects | No. AEs | Days after ALb | No. subjects | No. AEs | Days after PQ | No. subjects | No. AEs | Days after PQ |
| Abdominal pain | 2 | 2 | 0, 0 | ||||||||||||
| Abscess | 1 | 1 | 1 | ||||||||||||
| Anorexia | 1 | 1 | 0 | ||||||||||||
| Arthralgia | 2 | 2 | 1, 2 | 3 | 4 | 2, 2, 4, 13 | |||||||||
| Bronchitis | 1 | 1 | 1 | 1 | 2 | 4, 26 | 1 | 1 | 12 | 1 | 1 | 7 | |||
| Cough | 1 | 1 | 26 | ||||||||||||
| Constipation | 1 | 1 | 13 | ||||||||||||
| Dizziness | 1 | 1 | 13 | 4 | 4 | 0, 3, 8, 9 | |||||||||
| Dolor at blood draw site | 1 | 1 | 14 | ||||||||||||
| Drowsiness | 1 | 1 | 1 | ||||||||||||
| Dysuria | 2 | 2 | 8, 13 | ||||||||||||
| Enteritis | 1 | 1 | 3 | ||||||||||||
| Fatigue | 1 | 1 | 13 | 2 | 2 | 1, 1 | 1 | 1 | 27 | 1 | 1 | 3 | |||
| Fever | 1 | 1 | 1 | 1 | 1 | 0 | |||||||||
| Gastralgia | 1 | 1 | 12 | ||||||||||||
| Hemoglobinuriac | 1 | 1 | 2 | 2 | 2 | 0, 2 | 1 | 1 | 1 | 4 | 4 | 0, 2, 3, 3 | 2 | 4 | 0, 0, 28, 28 |
| Headache | 1 | 2 | 2, 13 | 2 | 2 | 2, 20 | 2 | 2 | 1, 13 | 6 | 6 | 1, 2, 2, 4, 5, 13 | |||
| Hepatitis B | 1 | 1 | 28 | ||||||||||||
| Low back pain | 1 | 1 | 5 | ||||||||||||
| Myalgia | 1 | 1 | 2 | 2 | 2 | 2, 4 | |||||||||
| Parasitosis | 1 | 1 | 9 | 2 | 2 | 1, 13 | 1 | 1 | 6 | ||||||
| Pharyngitis | 1 | 1 | 14 | ||||||||||||
| Pruritus | 2 | 2 | 0, 13 | ||||||||||||
| Rash | 1 | 1 | 10 | ||||||||||||
| Rhinitis | 2 | 2 | 3, 5 | 2 | 2 | 3, 8 | 3 | 3 | 6, 13, 13 | ||||||
| Superficial mycosis | 1 | 1 | 1 | ||||||||||||
| Tooth pain | 1 | 1 | 4 | ||||||||||||
| Wound on thigh | 1 | 1 | 21 | ||||||||||||
| Total no. subjects experiencing any AE, % | 3 (30%) | 7 | 9 (90%) | 18 | 4 (40%) | 6 | 10 (50%) | 16 | 13 (65%) | 35 | |||||
| Total no. subjects experiencing possible/probable AEs, % | 1 (10%) | 1 | 4 (40%) | 5 | 2 (20%) | 3 | 5 (25%) | 6 | 9 (45%) | 18 | |||||
Abbreviations: G6PD, glucose-6-phosphate dehydrogenase; PQ, primaquine; AL, artemether-lumefantrine; No., number; AE, adverse event.
a Subjects could have more than one adverse event.
b First dose of artemether-lumefantrine.
c Hemoglobinuria was reported as adverse event in combination with a positive urine dipstick. All cases of hemoglobinuria were probably or possibly related to the trial, except for two cases occurring on day 28 in the G6PD-deficient group receiving 0.40 mg/kg PQ.
Table 3. Adverse events of any severity in the different treatment groups in The Gambia.
| Treatment Group | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| G6PD Normal | G6PD Deficient | |||||||||||
| 0.25 mg/kg PQ + DP (n = 9)a |
0.4 mg/kg PQ + DP (n = 11)a |
DP only (n = 9)a |
0.25mg/kg PQ + DP (n = 20)a |
|||||||||
| Adverse Event | No. subjectsb | No. AEs | Days after PQ | No. subjects | No. AEs | Days after PQ | No. subjects | No. AEs | Days after DPc | No. subjects | No. AEs | Days after PQ |
| Abdominal pain | 1 | 1 | 9 | 1 | 1 | 5 | ||||||
| Chest pain | 1 | 1 | 9 | 1 | 1 | 28 | ||||||
| Hemoglobinuriad | 3 | 3 | 0 | 3 | 3 | 0 | 1 | 1 | 0 | 7 | 7 | 0 |
| Headache | 2 | 2 | 2,9 | 2 | 2 | 13,14 | 2 | 2 | 5,8 | |||
| Trauma | 1 | 1 | 14 | |||||||||
| Vomiting | 1 | 1 | 9 | |||||||||
| Total no. subjects experiencing any AE, % | 4 (44%) | 8 | 3 (27%) | 4 | 4 (44%) | 4 | 9 (45%) | 10 | ||||
| Total no. subjects experiencing possible/probable AEs, % | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | ||||||||
Abbreviations: G6PD, glucose-6-phosphate dehydrogenase; PQ, primaquine; DP, dihydroartemisinin-piperaquine; No., number; AE, adverse event.
a Individuals who completed follow-up
b Subjects could have more than one adverse event.
c First dose of dihydroartemisinin-piperaquine.
d Hemoglobinuria was observed at enrollment (before PQ administration) and microscopic urinalysis confirmed a diagnosis of schistosomiasis. All participants were treated with praziquantel.
Discussion
We present the results from two linked randomized controlled trials that evaluated the tolerability and safety of a single low-dose PQ in combination with AL or DP in G6PDd African males with asymptomatic P. falciparum malaria. Following PQ treatment, we observed transient reductions in hemoglobin concentrations in both G6PDd and G6PD-normal individuals. None of the study participants experienced SAEs or developed severe anemia.
Primaquine-associated hemolysis is dose-dependent and related to the degree of G6PD deficiency [23, 40–43]. In the current trials we specifically assessed the safety of single low-dose PQ in G6PDd individuals, with G6PD normal individuals receiving the same PQ dose as controls. In both Burkina Faso and The Gambia, G6PDd individuals had a statistically significant reduction in hemoglobin concentration after PQ treatment [16, 40–42]. Absolute and relative reductions in hemoglobin concentrations were most pronounced in the first week after treatment, and normalized during follow-up. None of the study participants developed moderate or severe anemia, experienced SAEs or required blood transfusion or hospitalization. Contrary to a study in Tanzania that observed no difference in hemoglobin concentrations between G6PDd and G6PD-normal individuals [25], we report a larger reduction in hemoglobin concentration in G6PDd individuals in Burkina Faso following treatment with 0.25 mg/kg and 0.40 mg/kg PQ. Whilst this association between G6PDd and hemoglobin reductions is in line with the majority of studies that measured hemoglobin after single dose PQ [16, 22–24], we did not observe a statistically significant difference between G6PDd and G6PD-normal individuals in The Gambia. In general, we observed considerable individual variation in hemoglobin dynamics during follow-up and a marked reduction in hemoglobin concentration in a proportion of G6PD-normal individuals who received PQ [16, 22–24]. In our study populations the fluctuations in hemoglobin concentrations during follow-up are probably influenced by malaria-associated hemolysis [44]. We excluded symptomatic malaria patients and imposed a conservative minimum hemoglobin concentration prior to treatment. Since symptomatic malaria patients and anemic individuals are likely to display different patterns in hemoglobin concentrations following treatment, our findings cannot be extrapolated to this population. We observed a strong association between baseline hemoglobin concentration and absolute or relative reductions in hemoglobin during follow up [23], probably reflecting the influence of the age distribution of the red blood cell population on the severity of drug-induced hemolysis [45].
Large inter- and intra-individual variation was also observed in reticulocyte counts without consistent differences between PQ-treated and non-treated individuals or between G6PDd and G6PD-normal individuals. Reticulocytosis is potentially an important marker for acute hemolysis and reticulocyte counts were elevated following various doses of PQ in an in vivo drug screening model using G6PDd mice [46]. We aimed to obtain further insights in hemolysis after PQ by measuring LDH, released from hemolysed red blood cells, and haptoglobin, which binds to hemoglobin released during intravascular or extravascular hemolysis. Whilst we performed these analyses post-hoc and only for individuals with remaining plasma samples from Burkina Faso, we noticed a trend towards more abnormal values on days 1–3 in G6PDd individuals, particularly in those receiving 0.40 mg/kg PQ. Although an elevated LDH is a non-specific marker of tissue damage and can be found in many conditions other than hemolysis, the combination of an increased serum LDH and a reduced haptoglobin is highly specific for diagnosing hemolysis [38, 39]. Our sparse observations on reticulocytes, LDH and haptoglobin support indications of transient hemolysis in the first week following PQ. Our findings suggest minimal safety concerns related to PQ at 0.25 mg/kg in G6PDd individuals, which is in line with other recent studies that used this PQ-dose [24, 25]. However, it is unclear how these findings can be extrapolated to individuals with lower starting hemoglobin concentrations, particularly children. Moreover, individuals with more severe forms of G6PD deficiency than the African A- variant may also experience more severe hemolysis following PQ [41, 47] and warrant safety studies in these populations. Severe hemolytic events may be rare [16, 43] and unlikely to be detected in small safety or efficacy studies. Incorporation of pharmacovigilance and G6PD genotyping into larger community based studies with PQ will help establish a solid base for broader implementation of single low-dose PQ.
Our study enrolled G6PDd individuals based on the phenotypic “Beutler’s” fluorescent spot test. The correlation between G6PD genotyping and phenotyping is typically mixed [48]. Compared to our study population in Burkina Faso, a large fraction of G6PDd study participants in The Gambia lacked the 202A mutation. This may be explained by the fact that G6PD deficiency alleles other than the 202A/376G G6PD A- allele are relatively common in The Gambia; alternative alleles include the 542T/376G Santamaria and the 968C/376G G6PD Betica-Selma allele [49]. There is increasing evidence for a role of CYP2D6 metabolizer status in determining the efficacy of PQ in preventing P. vivax relapses [30, 36]. CYP2D6 activity is hypothesized to be a rate-limiting step in the formation of active metabolite(s) of PQ for P. vivax. It is currently unclear whether CYP2D6 activity is also associated with PQ efficacy for P. falciparum gametocyte clearance or PQ-associated hemolysis. Our study was underpowered to detect such effects; our exploratory analysis showed no effect of genetically inferred CYP2D6 metabolizer status on PQ-associated reductions in hemoglobin concentrations.
Our findings on PQ safety fill an important gap in considerations on PQ implementation [27] and need to be considered in combination with evidence on PQ efficacy. The rationale for PQ treatment in P. falciparum is that it results in a community benefit in terms of reducing malaria transmission that outweighs potential individual-level risks associated with PQ use. Efficacy trials indeed demonstrate that the addition of PQ to ACTs results in a substantially reduced duration of gametocyte carriage [11–14]. The actual implications of PQ-associated gametocyte clearance in terms of reductions in the number of infected mosquitoes strongly depend on the study population and potentially on the type of ACT that is used in combination with PQ. In P. falciparum gametocyte carriers with high gametocyte densities and high transmission potential prior to treatment, 0.25 mg/kg PQ in combination with DP reduced transmission to mosquitoes by >90% within 48 hours after administration whilst a considerable proportion of DP-treated individuals remained infectious to mosquitoes for at least one week after treatment [13]. In asymptomatic parasite carriers with lower gametocyte densities prior to treatment, residual transmission after treatment with DP [14] and AL [12] may be considerably smaller and the added benefit of PQ may therefore be more modest [12, 14]. Decisions on the benefit of PQ for P. falciparum malaria control and elimination have to consider target populations and take into account that the proportion of infected individuals that can be covered with efficacious antimalarials is a major determinant of impact [50, 51]. Our findings on PQ safety form a relevant addition to these considerations and indicate that single low-dose PQ is associated with hemolysis post treatment but that this hemolysis is not severe and is self-limiting. Evidence is accumulating that single low-dose PQ can be used in Africa without prior G6PD screening.
Supporting information
(DOCX)
(DOCX)
(DOCX)
(DOCX)
The figure shows the association between baseline hemoglobin concentration and changes in the maximum absolute change in hemoglobin concentration during follow-up (left panel: Pearson r = -0.69; P < 0.0001); and changes in the maximum relative change in hemoglobin concentration during follow-up expressed as % of baseline values (Pearson r = -0.59; P < .0001).
(TIF)
(PDF)
(PDF)
(DOC)
Acknowledgments
We thank the study participants, members of the field teams, and staff of the Banfora Hospital in Burkina Faso and the Basse Health Centre in The Gambia for their cooperation throughout the study. We appreciate the advice of prof. Dennis Shanks, dr. Bernard Ogutu, dr. Emily Webb, and dr. Issaka Ouédraogo as members of the Data Safety Monitoring Board in Burkina Faso and dr. Roly Gosling, dr. Mahamadou Sissoko, dr. Jozefien Buyze and dr. Jaap ten Oever as members of the Data Safety Monitoring Board in The Gambia. We also thank dr. Rob ter Heine for arranging the shipment of Coartem, and Wouter Graumans and Marion Ybema-Antoine for laboratory analysis. We thank Sanofi for their kind donation of primaquine.
Abbreviations
- ACT
artemisinin-based combination therapy
- AE
adverse event
- AL
artemether-lumefantrine
- AS
Activity Score
- CI
confidence interval
- CNV
copy number variation
- CYP2D6
Cytochrome P-450 isoenzyme 2D6
- DMID
Division of Microbiology and Infectious Diseases
- DP
dihydroartemisinin-piperaquine
- G6PD(d)
glucose-6-phosphate dehydrogenase (deficient)
- IM
intermediate metabolizer
- LDH
lactate dehydrogenase
- PM
poor metabolizer
- PQ
primaquine
- qRT-PCR
quantitative reverse-transcriptase polymerase chain reaction
- QT-NASBA
quantitative nucleic acid sequence-based amplification
- RDT
rapid diagnostic test
- SAE
serious adverse event
- SD
standard deviation
- UM
ultra-rapid metabolizer
- WHO
World Health Organization
Data Availability
The datasets generated and/or analyzed during the current study are available in the Dryad repository: https://doi.org/10.5061/dryad.230ps.
Funding Statement
This work was supported by: i) the Bill & Melinda Gates Foundation [AFIRM grant OPP1034789]; ii) the Medical Research Council (MRC), UK, the Department for International Development, UK Government, under the MRC/DFID Concordat Agreement, and the Wellcome Trust through the joint Global Health Trials Scheme (Grant number: MR/K007203/1); and iii) TB is further supported by the European Research Council [ERC-2014-StG 639776 to TB]. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.World Health Organization. World Malaria Report 2016. Geneva: WHO: 2016. [Google Scholar]
- 2.malERA Consultative Group on Diagnoses and Diagnostics. A research agenda for malaria eradication: diagnoses and diagnostics. PLoS Med. 2011;8(1):e1000396 doi: 10.1371/journal.pmed.1000396 ; PubMed Central PMCID: PMC3026696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sinclair D, Zani B, Donegan S, Olliaro P, Garner P. Artemisinin-based combination therapy for treating uncomplicated malaria. Cochrane Database Syst Rev. 2009;(3):CD007483 doi: 10.1002/14651858.CD007483.pub2 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Okell LC, Drakeley CJ, Ghani AC, Bousema T, Sutherland CJ. Reduction of transmission from malaria patients by artemisinin combination therapies: a pooled analysis of six randomized trials. Malar J. 2008;7:125 doi: 10.1186/1475-2875-7-125 ; PubMed Central PMCID: PMCPMC2491628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Adjalley SH, Johnston GL, Li T, Eastman RT, Ekland EH, Eappen AG, et al. Quantitative assessment of Plasmodium falciparum sexual development reveals potent transmission-blocking activity by methylene blue. Proc Natl Acad Sci U S A. 2011;108(47):E1214–23. doi: 10.1073/pnas.1112037108 ; PubMed Central PMCID: PMCPMC3223476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bousema T, Drakeley C. Epidemiology and infectivity of Plasmodium falciparum and Plasmodium vivax gametocytes in relation to malaria control and elimination. Clinical microbiology reviews. 2011;24(2):377–410. Epub 2011/04/13. doi: 10.1128/CMR.00051-10 ; PubMed Central PMCID: PMC3122489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Targett G, Drakeley C, Jawara M, von Seidlein L, Coleman R, Deen J, et al. Artesunate reduces but does not prevent posttreatment transmission of Plasmodium falciparum to Anopheles gambiae. J Infect Dis. 2001;183(8):1254–9. doi: 10.1086/319689 . [DOI] [PubMed] [Google Scholar]
- 8.Sawa P, Shekalaghe SA, Drakeley CJ, Sutherland CJ, Mweresa CK, Baidjoe AY, et al. Malaria transmission after artemether-lumefantrine and dihydroartemisinin-piperaquine: a randomized trial. J Infect Dis. 2013;207(11):1637–45. doi: 10.1093/infdis/jit077 . [DOI] [PubMed] [Google Scholar]
- 9.White NJ. Primaquine to prevent transmission of falciparum malaria. Lancet Infect Dis. 2013;13(2):175–81. Epub 2012/11/28. doi: 10.1016/S1473-3099(12)70198-6 . [DOI] [PubMed] [Google Scholar]
- 10.Baird JK, Hoffman SL. Primaquine therapy for malaria. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2004;39(9):1336–45. Epub 2004/10/21. doi: 10.1086/424663 . [DOI] [PubMed] [Google Scholar]
- 11.Eziefula AC, Bousema T, Yeung S, Kamya M, Owaraganise A, Gabagaya G, et al. Single dose primaquine for clearance of Plasmodium falciparum gametocytes in children with uncomplicated malaria in Uganda: a randomised, controlled, double-blind, dose-ranging trial. Lancet Infect Dis. 2014;14(2):130–9. doi: 10.1016/S1473-3099(13)70268-8 . [DOI] [PubMed] [Google Scholar]
- 12.Goncalves BP, Tiono AB, Ouedraogo A, Guelbeogo WM, Bradley J, Nebie I, et al. Single low dose primaquine to reduce gametocyte carriage and Plasmodium falciparum transmission after artemether-lumefantrine in children with asymptomatic infection: a randomised, double-blind, placebo-controlled trial. BMC Med. 2016;14:40 doi: 10.1186/s12916-016-0581-y ; PubMed Central PMCID: PMCPMC4782330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dicko A, Brown JM, Diawara H, Baber I, Mahamar A, Soumare HM, et al. Primaquine to reduce transmission of Plasmodium falciparum malaria in Mali: a single-blind, dose-ranging, adaptive randomised phase 2 trial. Lancet Infect Dis. 2016. doi: 10.1016/S1473-3099(15)00479-X . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Okebe J, Bousema T, Affara M, DiTanna G, Eziefula AC, Jawara M, et al. The gametocytocidal efficacy of primaquine in malaria asymptomatic carriers treated with dihydroartemisinin-piperaquine in The Gambia (PRINOGAM): study protocol for a randomised controlled trial. Trials. 2015;16:70 doi: 10.1186/s13063-015-0597-1 ; PubMed Central PMCID: PMC4349754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.World Health Organization. Guidelines for the treatment of malaria - 3rd edition Geneva: WHO: 2015. [PubMed] [Google Scholar]
- 16.Shekalaghe SA, ter Braak R, Daou M, Kavishe R, van den Bijllaardt W, van den Bosch S, et al. In Tanzania, hemolysis after a single dose of primaquine coadministered with an artemisinin is not restricted to glucose-6-phosphate dehydrogenase-deficient (G6PD A-) individuals. Antimicrob Agents Chemother. 2010;54(5):1762–8. Epub 2010/03/03. doi: 10.1128/AAC.01135-09 ; PubMed Central PMCID: PMC2863610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alving AS, Johnson CF, Tarlov AR, Brewer GJ, Kellermeyer RW, Carson PE. Mitigation of the haemolytic effect of primaquine and enhancement of its action against exoerythrocytic forms of the Chesson strain of Piasmodium vivax by intermittent regimens of drug administration: a preliminary report. Bulletin of the World Health Organization. 1960;22:621–31. Epub 1960/01/01. PubMed ; PubMed Central PMCID: PMC2555355. [PMC free article] [PubMed] [Google Scholar]
- 18.Reeve PA, Toaliu H, Kaneko A, Hall JJ, Ganczakowski M. Acute intravascular haemolysis in Vanuatu following a single dose of primaquine in individuals with glucose-6-phosphate dehydrogenase deficiency. The Journal of tropical medicine and hygiene. 1992;95(5):349–51. Epub 1992/10/01. PubMed . [PubMed] [Google Scholar]
- 19.Betuela I, Bassat Q, Kiniboro B, Robinson LJ, Rosanas-Urgell A, Stanisic D, et al. Tolerability and safety of primaquine in Papua New Guinean children 1 to 10 years of age. Antimicrobial agents and chemotherapy. 2012;56(4):2146–9. doi: 10.1128/AAC.05566-11 ; PubMed Central PMCID: PMC3318393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sutanto I, Tjahjono B, Basri H, Taylor WR, Putri FA, Meilia RA, et al. Randomized, open-label trial of primaquine against vivax malaria relapse in Indonesia. Antimicrobial agents and chemotherapy. 2013;57(3):1128–35. doi: 10.1128/AAC.01879-12 ; PubMed Central PMCID: PMC3591862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nelwan EJ, Ekawati LL, Tjahjono B, Setiabudy R, Sutanto I, Chand K, et al. Randomized trial of primaquine hypnozoitocidal efficacy when administered with artemisinin-combined blood schizontocides for radical cure of Plasmodium vivax in Indonesia. BMC Med. 2015;13:294 doi: 10.1186/s12916-015-0535-9 ; PubMed Central PMCID: PMC4676167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shekalaghe S, Drakeley C, Gosling R, Ndaro A, van Meegeren M, Enevold A, et al. Primaquine clears submicroscopic Plasmodium falciparum gametocytes that persist after treatment with sulphadoxine-pyrimethamine and artesunate. PLoS One. 2007;2(10):e1023 Epub 2007/10/11. doi: 10.1371/journal.pone.0001023 ; PubMed Central PMCID: PMC1995753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eziefula AC, Pett H, Grignard L, Opus S, Kiggundu M, Kamya MR, et al. Glucose-6-phosphate dehydrogenase status and risk of hemolysis in Plasmodium falciparum-infected African children receiving single-dose primaquine. Antimicrob Agents Chemother. 2014;58(8):4971–3. doi: 10.1128/AAC.02889-14 ; PubMed Central PMCID: PMC4136063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bancone G, Chowwiwat N, Somsakchaicharoen R, Poodpanya L, Moo PK, Gornsawun G, et al. Single low dose primaquine (0.25mg/kg) does not cause clinically significant haemolysis in G6PD deficient subjects. PLoS One. 2016;11(3):e0151898 doi: 10.1371/journal.pone.0151898 ; PubMed Central PMCID: PMCPMC4807095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mwaiswelo R, Ngasala BE, Jovel I, Gosling R, Premji Z, Poirot E, et al. Safety of a single low-dose of primaquine in addition to standard artemether-lumefantrine regimen for treatment of acute uncomplicated Plasmodium falciparum malaria in Tanzania. Malar J. 2016;15:316 doi: 10.1186/s12936-016-1341-3 ; PubMed Central PMCID: PMCPMC4901409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tine RC, Sylla K, Faye BT, Poirot E, Fall FB, Sow D, et al. Safety and efficacy of adding a single low dose of primaquine to the treatment of adult patients with Plasmodium falciparum malaria in Senegal, to reduce gametocyte carriage: a randomized controlled trial. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2017. doi: 10.1093/cid/cix355 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eziefula AC, Gosling R, Hwang J, Hsiang MS, Bousema T, von Seidlein L, et al. Rationale for short course primaquine in Africa to interrupt malaria transmission. Malar J. 2012;11:360 doi: 10.1186/1475-2875-11-360 ; PubMed Central PMCID: PMC3502539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Beutler E, Blume KG, Kaplan JC, Lohr GW, Ramot B, Valentine WN. International Committee for Standardization in Haematology: recommended screening test for glucose-6-phosphate dehydrogenase (G-6-PD) deficiency. Br J Haematol. 1979;43(3):465–7. PubMed . [DOI] [PubMed] [Google Scholar]
- 29.Pett H, Goncalves BP, Dicko A, Nebie I, Tiono AB, Lanke K, et al. Comparison of molecular quantification of Plasmodium falciparum gametocytes by Pfs25 qRT-PCR and QT-NASBA in relation to mosquito infectivity. Malar J. 2016;15(1):539 doi: 10.1186/s12936-016-1584-z ; PubMed Central PMCID: PMC5100312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bennett JW, Pybus BS, Yadava A, Tosh D, Sousa JC, McCarthy WF, et al. Primaquine failure and cytochrome P-450 2D6 in Plasmodium vivax malaria. N Engl J Med. 2013;369(14):1381–2. doi: 10.1056/NEJMc1301936 . [DOI] [PubMed] [Google Scholar]
- 31.Pybus BS, Marcsisin SR, Jin X, Deye G, Sousa JC, Li Q, et al. The metabolism of primaquine to its active metabolite is dependent on CYP 2D6. Malar J. 2013;12:212 doi: 10.1186/1475-2875-12-212 ; PubMed Central PMCID: PMCPMC3689079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Potter BM, Xie LH, Vuong C, Zhang J, Zhang P, Duan D, et al. Differential CYP 2D6 metabolism alters primaquine pharmacokinetics. Antimicrob Agents Chemother. 2015;59(4):2380–7. doi: 10.1128/AAC.00015-15 ; PubMed Central PMCID: PMCPMC4356838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sim SC, Daly AK, Gaedigk A. CYP2D6 update: revised nomenclature for CYP2D7/2D6 hybrid genes. Pharmacogenet Genomics. 2012;22(9):692–4. doi: 10.1097/FPC.0b013e3283546d3c . [DOI] [PubMed] [Google Scholar]
- 34.Gaedigk A, Simon SD, Pearce RE, Bradford LD, Kennedy MJ, Leeder JS. The CYP2D6 activity score: translating genotype information into a qualitative measure of phenotype. Clin Pharmacol Ther. 2008;83(2):234–42. doi: 10.1038/sj.clpt.6100406 . [DOI] [PubMed] [Google Scholar]
- 35.Hicks JK, Swen JJ, Gaedigk A. Challenges in CYP2D6 phenotype assignment from genotype data: a critical assessment and call for standardization. Curr Drug Metab. 2014;15(2):218–32. PubMed . [DOI] [PubMed] [Google Scholar]
- 36.St Jean PL, Xue Z, Carter N, Koh GC, Duparc S, Taylor M, et al. Tafenoquine treatment of Plasmodium vivax malaria: suggestive evidence that CYP2D6 reduced metabolism is not associated with relapse in the Phase 2b DETECTIVE trial. Malar J. 2016;15:97 doi: 10.1186/s12936-016-1145-5 ; PubMed Central PMCID: PMCPMC4757974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sumbele IU, Kimbi HK, Ndamukong-Nyanga JL, Nweboh M, Anchang-Kimbi JK, Lum E, et al. Malarial anaemia and anaemia severity in apparently healthy primary school children in urban and rural settings in the Mount Cameroon area: cross sectional survey. PloS one. 2015;10(4):e0123549 doi: 10.1371/journal.pone.0123549 ; PubMed Central PMCID: PMC4403990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marchand A, Galen RS, Van Lente F. The predictive value of serum haptoglobin in hemolytic disease. JAMA. 1980;243(19):1909–11. PubMed . [PubMed] [Google Scholar]
- 39.Galen RS. Application of the predictive value model in the analysis of test effectiveness. Clin Lab Med. 1982;2(4):685–99. PubMed . [PubMed] [Google Scholar]
- 40.Beutler E. G6PD deficiency. Blood. 1994;84(11):3613–36. Epub 1994/12/01. PubMed . [PubMed] [Google Scholar]
- 41.Cappellini MD, Fiorelli G. Glucose-6-phosphate dehydrogenase deficiency. Lancet. 2008;371(9606):64–74. Epub 2008/01/08. doi: 10.1016/S0140-6736(08)60073-2 . [DOI] [PubMed] [Google Scholar]
- 42.Dern RJ, Beutler E, Alving AS. The hemolytic effect of primaquine. V. Primaquine sensitivity as a manifestation of a multiple drug sensitivity. The Journal of laboratory and clinical medicine. 1955;45(1):30–9. Epub 1955/01/01. PubMed . [PubMed] [Google Scholar]
- 43.Ashley EA, Recht J, White NJ. Primaquine: the risks and the benefits. Malar J. 2014;13:418 doi: 10.1186/1475-2875-13-418 ; PubMed Central PMCID: PMCPMC4230503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Price RN, Simpson JA, Nosten F, Luxemburger C, Hkirjaroen L, ter Kuile F, et al. Factors contributing to anemia after uncomplicated falciparum malaria. The American journal of tropical medicine and hygiene. 2001;65(5):614–22. PubMed ; PubMed Central PMCID: PMC4337986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Beutler E, Dern RJ, Alving AS. The hemolytic effect of primaquine. IV. The relationship of cell age to hemolysis. The Journal of laboratory and clinical medicine. 1954;44(3):439–42. PubMed . [PubMed] [Google Scholar]
- 46.Zhang P, Gao X, Ishida H, Amnuaysirikul J, Weina PJ, Grogl M, et al. An in vivo drug screening model using glucose-6-phosphate dehydrogenase deficient mice to predict the hemolytic toxicity of 8-aminoquinolines. Am J Trop Med Hyg. 2013;88(6):1138–45. doi: 10.4269/ajtmh.12-0682 ; PubMed Central PMCID: PMCPMC3752814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Beutler E, Duparc S, Group GPDW. Glucose-6-phosphate dehydrogenase deficiency and antimalarial drug development. Am J Trop Med Hyg. 2007;77(4):779–89. PubMed . [PubMed] [Google Scholar]
- 48.Domingo GJ, Satyagraha AW, Anvikar A, Baird K, Bancone G, Bansil P, et al. G6PD testing in support of treatment and elimination of malaria: recommendations for evaluation of G6PD tests. Malar J. 2013;12:391 doi: 10.1186/1475-2875-12-391 ; PubMed Central PMCID: PMC3830439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Clark TG, Fry AE, Auburn S, Campino S, Diakite M, Green A, et al. Allelic heterogeneity of G6PD deficiency in West Africa and severe malaria susceptibility. European journal of human genetics: EJHG. 2009;17(8):1080–5. doi: 10.1038/ejhg.2009.8 ; PubMed Central PMCID: PMC2986558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Slater HC, Okell LC, Ghani AC. Mathematical modelling to guide drug development for malaria elimination. Trends Parasitol. 2017;33(3):175–84. doi: 10.1016/j.pt.2016.09.004 ; PubMed Central PMCID: PMCPMC5347022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Johnston GL, Gething PW, Hay SI, Smith DL, Fidock DA. Modeling within-host effects of drugs on Plasmodium falciparum transmission and prospects for malaria elimination. PLoS Comput Biol. 2014;10(1):e1003434 doi: 10.1371/journal.pcbi.1003434 ; PubMed Central PMCID: PMCPMC3900379. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
(DOCX)
(DOCX)
The figure shows the association between baseline hemoglobin concentration and changes in the maximum absolute change in hemoglobin concentration during follow-up (left panel: Pearson r = -0.69; P < 0.0001); and changes in the maximum relative change in hemoglobin concentration during follow-up expressed as % of baseline values (Pearson r = -0.59; P < .0001).
(TIF)
(PDF)
(PDF)
(DOC)
Data Availability Statement
The datasets generated and/or analyzed during the current study are available in the Dryad repository: https://doi.org/10.5061/dryad.230ps.