Abstract
Underwater visual census (UVC) is the most common approach for estimating diversity, abundance and size of reef fishes in shallow and clear waters. Abundance estimation through UVC is particularly problematic in species occurring at low densities and/or highly aggregated because of their high variability at both spatial and temporal scales. The statistical power of experiments involving UVC techniques may be increased by augmenting the number of replicates or the area surveyed. In this work we present and test the efficiency of an UVC method based on diver towed GPS, the Tracked Roaming Transect (TRT), designed to maximize transect length (and thus the surveyed area) with respect to diving time invested in monitoring, as compared to Conventional Strip Transects (CST). Additionally, we analyze the effect of increasing transect width and length on the precision of density estimates by comparing TRT vs. CST methods using different fixed widths of 6 and 20 m (FW3 and FW10, respectively) and the Distance Sampling (DS) method, in which perpendicular distance of each fish or group of fishes to the transect line is estimated by divers up to 20 m from the transect line. The TRT was 74% more time and cost efficient than the CST (all transect widths considered together) and, for a given time, the use of TRT and/or increasing the transect width increased the precision of density estimates. In addition, since with the DS method distances of fishes to the transect line have to be estimated, and not measured directly as in terrestrial environments, errors in estimations of perpendicular distances can seriously affect DS density estimations. To assess the occurrence of distance estimation errors and their dependence on the observer’s experience, a field experiment using wooden fish models was performed. We tested the precision and accuracy of density estimators based on fixed widths and the DS method. The accuracy of the estimates was measured comparing the actual total abundance with those estimated by divers using FW3, FW10, and DS estimators. Density estimates differed by 13% (range 0.1–31%) from the actual values (average = 13.09%; median = 14.16%). Based on our results we encourage the use of the Tracked Roaming Transect with Distance Sampling (TRT+DS) method for improving density estimates of species occurring at low densities and/or highly aggregated, as well as for exploratory rapid-assessment surveys in which divers could gather spatial ecological and ecosystem information on large areas during UVC.
Introduction
A variety of methods has been used to estimate reef fish diversity, abundance and size; sampling methods include both capture techniques such as ichthyocides, nets and traps, and observational (non-destructive) methods such as video recording and underwater visual censuses (UVC) [1–5]. UVC techniques are the most commonly used method in shallow and clear waters worldwide [6–10]. Beyond being non-lethal, thus appropriate to be used in marine protected areas (MPAs) and for long-lived, rare and/or threatened species, UVC techniques are suitable for a wide range of fish sizes and behaviours as well as habitat types (especially in architecturally complex ones), and, importantly, they are easy to learn by divers, who may simultaneously and rapidly register information on environmental variables and fish behaviour [5,11]; for these reasons, UVC techniques are extremely flexible for any sampling design to be implemented in the field, so that a great variety of ecological questions can be dealt with [7,12]. Drawbacks of UVC techniques, however, also exist, such as physiological constraints of SCUBA diving (especially in deep, cold, strong current and/or turbid conditions), individual response of fishes (either attraction or escape), and bias in the estimates of abundance of cryptic, elusive, hidden or/and small-sized fishes [7, 12–16]. Consequently, a series of methodological studies dealing with these potential biases have been carried out in the last decades [5, 11, 15, 17–19].
Standard UVC techniques can be classified on the basis of the shape of the sampling unit, typically distinguishing among (1) strip transects [20], in which the diver records all fish detected along a path of fixed width and length; (2) roaming transects [21], in which the diver records all fish detected along a free path (or following a given isobath) of fixed width during a prefixed time; and (3) stationary point counts [22], in which the diver records all fish detected within a circular area of fixed radius. Strip transects are currently adopted in most ichthyofauna monitoring programs because they are better suited for a wide range of environments and types of reef fish assemblages [23,24].
Most UVC methods were conceived to efficiently gather information on fish assemblages that can be composed by a wide spectrum of species, sizes, behaviours and densities [12, 25, 26]. However, the selection of a proper UVC technique is particularly problematic in species occurring at low densities and/or highly aggregated (i.e., schooling species, such as damselfishes or bogues), or patchily distributed species (such as groupers) [27, 28] due to the high variability of their density at both spatial and temporal scales [11, 13, 29, 30, 31, 32]. Among these scarce, patchy and/or highly aggregated species, charismatic and commercially important reef fishes are often found, generally targeted by monitoring programs because of their relevance in fisheries management, conservation and MPA effectiveness analysis [33, 34]. Regarding the spatial perspective, the patchy distribution of some species increases the variability between replicates, while species occurring at low densities could not be detected [27]. On the other hand, as fishes are mobile animals, estimations vary at different temporal scales (minutes, days, weeks), and furthermore, the magnitude of this variability increases systematically as the mean of abundances decreases [11, 30]. Consequently, determining population changes on this species group might be difficult without having a large number of replicates.
The statistical power of UVC-based studies could be increased by augmenting either the number of replicates, the area surveyed [11, 30, 35] or using the distance sampling method, which considers intra-transect variance that is absent in other methods [35]. The number of replicates is usually constrained by sampling costs, logistics or/and dive time. In the case of strip transects, census area is determined by the length and width of the sampling units. Transect length is determined by a trade-off between time consumed for each replicate and the maximum possible length (which, in turn, is constrained by tape length, reef size, habitat heterogeneity, diving time, etc.). [7, 12, 19, 36]. Additionally, long transects could be constrained by habitat heterogeneity or by the size of the study site in the case of very long transects. Transect width, which could be limited by visibility constraints (particularly in temperate environments),is susceptible to optimization analysis [37]. Furthermore, it is noteworthy that both dimensions of the transect are subject to methodological considerations which may influence the results, as is the case of the reported impact of transect’s dimensions in diversity indices and the first-and-end transect section effect on fish abundance estimations [19, 36]. In some cases, the area surveyed can be maximized by performing roaming censuses [21], in which the diver swims in a free direction or following a predetermined direction. These surveys usually have a prefixed time, hence sighting frequency rather than fish density is quantified [21] since the method does not account for the area covered by the survey. This limitation is probably one of the main reasons of its limited use in studies involving UVC. From this perspective, the roaming transect could be refined by tracking the transect path and obtaining an accurate estimation of the area covered by the survey [27, 38].
Otherwise, transect-based samplings (usually terrestrial) take advantage of the Distance Sampling method (DS), a methodology that allows estimating the density of animals, plants or any event of interest taking into account large width census areas [39, 40]. Underwater, the DS method is a technique in which the observation/count distance could be increased up to the visibility limits since the distance of the observed individual from the transect (in transect-based sampling) or the observation centre (in stationary point count sampling) is used to estimate densities [39–41], thus avoiding the constraints of limiting the survey to a given width or radius. DS is based on the probability of detection of each observed individual, which can be estimated from the frequency distribution of the recorded sighting distances [39, 42, 43]. Detection probability generally decreases with increasing distance from the observer, and the shape of the sightings distribution is highly influenced by fish size, wariness and crypticity [42, 44]. Besides their usefulness for integrating information from large census areas, distances may provide further information on the behaviour of animals, which is extremely relevant in comparative studies among sites with different levels of human disturbance (i.e. MPA effectiveness evaluation studies) [16, 45, 46]. For example, in areas open to fishing, individuals of commercial species may keep themselves away from divers at distances longer than the transect width, while in areas with fishing restrictions they could show no diver avoidance [44, 45]. From this perspective, opposite to the case of terrestrial ecology, the use of DS to address species behavioural issues is concentrated in studies and monitoring programs carried out in the eastern Pacific Ocean [35, 42, 43, 45].
Here we describe and test the efficiency (estimated as its capacity to maximize the area surveyed) and precision (estimated as the coefficients of variations of density estimations) of the Tracked Roaming Transect (TRT), an UVC method designed to maximize transect length (and thus the surveyed area) with respect to diving time invested in monitoring. The method was specifically designed to survey commercial and charismatic, medium- and large-size fish species commonly found at low densities (such as groupers or sharks) and/or threatened species [28, 31, 32]. Furthermore, we analyzed the effect of increasing transect width on the precision of abundance estimations by comparing different fixed transect widths and the Distance Sampling (DS) which could maximize the transect width up to the visibility limits. Additionally, since with the DS method distances of fishes to the transect line have to be estimated, and not measured directly as in terrestrial environments [46], errors in estimations of perpendicular distances can seriously affect DS density estimations as well as estimations based on fixed belt transects [17, 19]. To assess the occurrence of these errors and their dependence on the experience of the observers performing fish UVC, a field experiment using wooden fish models was performed to test the precision and accuracy of density estimations with DS method as well as with fixed transect widths used.
Methods
Species and study sites
This study was carried out in July 2014 in Cabo de Palos—Islas Hormigas marine reserve (hereafter CP), and in Cabo Tiñoso (CT), a neighbouring unprotected area at the time of sampling (it is protected since July 2016), both locations situated along the coast of Murcia (SE Spain, Mediterranean Sea) (Fig 1). The marine reserve of CP was established in 1995 under fisheries legislation, and it occupies 1931 ha, from which 270 ha form the core no-take area (centred in 37°39'21"N, 0°38'57" W, around the Hormigas islands), where this study was undertaken. For its part, CT is the projection into the sea of a coastal cliff that extends for ~7 km (Fig 1).The two locations are characterized by clear water (visibility > 20 m most of the year in normal conditions) and long sections of steep reefs and large rocky boulders down to ~ 40 m depth.
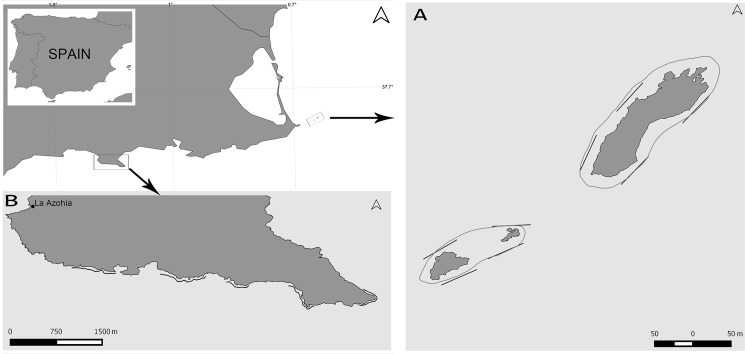
Fig 1. Study sites.
(A) Islas Hormigas and Hormigon in Cabo de Palos Marine Reserve (37.6550° N -0.6497° E). The distance between islands is not to scale to ease the representation. (B) Cabo Tiñoso (37.5370° N -1.1419° E). Straight lines: 50-m Conventional Strip Transects. Curved lines: Tracks of the Tracked Roaming Transects.
Six species were selected to be surveyed in this study, three grouper species (Epinephilidae) [dusky grouper Epinephelus marginatus, goldblotch grouper Epinephelus costae, and mottled grouper Mycteroperca rubra], common dentex (Dentex dentex, Sparidae), brown meagre (Sciaena umbra, Sciaenidae) and common eagle ray (Mylobatis aquila, Myliobatidae). All these species are medium-to-large sized, nekton-benthic fishes with similar habitat requirements and together compose part of the ichthyofauna of coastal rocky habitats [7, 12, 25]. These species are among the most important for commercial and recreational fisheries [47] and among the most charismatic for SCUBA divers in the Western Mediterranean Sea [48]. They are also among the most favoured by protection measures within MPAs in the Mediterranean Sea [49].
Comparison of underwater visual census methods
Two types of transects were performed: the Conventional Strip Transects (CST), in which the transect length is marked with a 50-m fiberglass tape measure [25] and the Tracked Roaming Transects (TRT) method, for which transects of variable length were performed (see below for further explanation). For both CST and TRT methods, visual counts were done in such a way that three census widths of 6, 20 and 40 m (3, 10 and 20 m each side of the transect, respectively) could be considered simultaneously in the same dive/sampling operation (see below). While the 6- and 20-m stretches were of fixed widths (called FW3 and FW10, respectively), density within the 40-m width was computed using a Distance Sampling (DS) methodology, so that six census methods (called CST-FW3, CST-FW10, CST-DS, TRT-FW3, TRT-FW10, and TRT-DS respectively) were compared. It is noteworthy that using a posteriori recorded distances it is likely that abundance estimates differ from yields resulting from fixed widths considered from the start. However, since this work is not focused on the comparisons of abundance estimations, this possible bias are not relevant.
TRT method
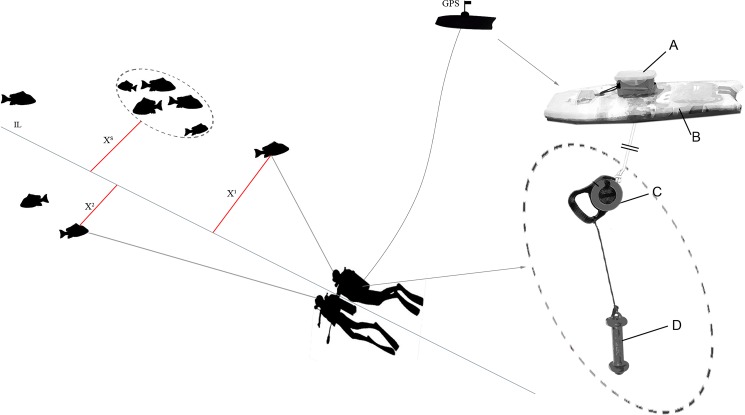
The TRT method proposed here is based on a GPS density survey designed to asses reef fish spawning aggregations [38] and other experiences of diver towed GPS techniques [50, 51, 28]. With this technique it is possible to measure the distance covered by geo-referencing start/end census points (along with the track recorded by the GPS) or to geo-reference any object, event or spatial feature of interest. The equipment, constructed ad hoc for this study, consists of a GPS mounted on a body-board, a diving reel with a monofilament of 1 mm diameter and a 3 kg weight (Fig 2). The reel is tied 0.8 m above the weight, thus the monofilament length can be regulated easily by the diver (Fig 2). The diver regulates the line length before starting the census so that the weight hangs from the board. Then the diver drags the equipment pulling on the line and thus the weight maintains the board vertically over the diver, minimizing the displacement of the board from the diver position. Photographs, which record the time or the day time registered during the dive, are used to geo-locate points or events (transect start/end point, fish counts, habitat features, etc.). The only requirement is the exact correspondence between camera or diving computer and GPS clocks or to take a picture of the GPS screen with the underwater camera before or after the dive to calculate the time difference between devices. Then, using a geographic information system (GIS) software, the time of the start/end point is associated with a waypoint of the track recorded by the synchronized GPS. The resulting track between start and end points is equal to the UVC transect length (for this study the GPS was set to construct the track, loading a waypoint every 3 seconds). Additionally, using free software for geo-referencing of photographs (in our case: http://jriguera.github.io/photoplace) it is possible to match the GPS data timestamps with the digital image timestamps, extracted from their embedded EXIF tags, and create a geospatial data set.
Fig 2. Scheme of the count method using the Distance Sampling method.
IL = imaginary line. X1-2 = perpendicular distances of solitary individuals to the transect line. X3 = perpendicular distance of a school of fishes to the perpendicular line. The equipment (Fig 2) is illustrated above divers, like at the surface. Inset: The Tracked Roaming Transect equipment. A = Waterproof case for GPS. B = Body Board Slate. C = Diving reel. D = Weight.
Procedure of visual census sampling operation
For both CST and TRT methods, divers conducted censuses in pairs as follows: once the observers defined the starting point of the census, they followed an imaginary line at a constant average depth of 16 m. Each left or right diver was responsible for counting fishes present only along his/her side of the sampling path, i.e. the left and right half of the transect, respectively. The two divers swam ahead simultaneously and continuously, while one of them unrolled the measuring tape (in the case of CST) or pulled GPS positioned at the surface above their vertical axis (in the case of TRT).
In all cases, divers recorded individuals of the selected fish species within 20 m from their side of the imaginary line. Divers estimated the first-sight perpendicular distance (to the nearest meter) between each observed individual fish encountered and the imaginary line (Fig 2). For groups of fish of the same species (schools), the distance from the closest fish of the group (Fig 2) was recorded and then the number of individuals was estimated. This information is necessary for the use of DS density estimations methods (see below). Distances were noted in 1-m intervals and fish observed at estimated distances beyond 20 m were not recorded. Fish size was recorded in 10-cm classes, and only fishes larger than 10 cm were included in the census in order to avoid possible biases associated with small size categories [13]. When fish moved from one side of the transect to the other, this was registered, so that, after the census, both divers immediately cross-checked this information in order to avoid a double count of individuals.
Sampling design and data analysis
In CP, 4 TRT following the 16-m isobath were conducted encircling each of the two islands of the Hormigas archipelago in the no-take zone (Fig 2), with one transect situated on the windward side and another one in the leeward side of each island. Eight CST 50-m long were randomly located on the leeward and windward side of the two islands also following the 16-m isobath (Fig 2). The number of CSTs was determined in order to spend a diving time comparable to the time invested for TRT. In CT, 5 TRT were conducted, but in this case, due to the extremely low abundances of the studied species in this location, CST was disregarded. Instead, CST-like transects were simulated taking advantage of the fact that observations of each individual or school during TRT were geo-located and their distance to the transect line was noted. With this recorded spatial distribution of fishes, a Monte Carlo simulation was conducted to reproduce 19 random 50-m CST laid over the TRT tracks, avoiding overlaps among them; this number is the time-equivalent number of CST in relation to the TRT sampling effort. For each simulation, fishes located within the 19 CST tracks were counted as "detected fish". Detection within 3 and 10 meters from transect were used to calculate the FW3 and FW10 estimators, respectively. Mean and standard deviation of the number of fishes detected in the CST were computed for each simulation (among the 19 CST) for each species. A total of 105 simulations were computed and global mean and the coefficient of variation among them were calculated for each species.
Comparisons of the six census methods were focused on the efficiency (calculated as the area covered per unit time) and precision (estimated as the coefficients of variation) of abundance estimates. In all cases, densities (D) were referred to as the number of fish per 300 m2 (which is equivalent to a CST-FW3 transect), and the coefficient of variation (CV) was considered for each location and combination of transect method as a standardized estimator of the variability. The CV was derived from the square root of the variance (i.e. standard error) divided by the density estimate. Densities and CV in DS method were calculated using the free software DISTANCE [39, 40]. Unlike the methods mentioned before in which variance is derived from differences in the number of individuals recorded per unit of area among transects, the sources of uncertainty in DS estimates involve three components that are combined additively to compute the CV: the empirical variability in the encounter rate of clusters; the variability in cluster size; and the uncertainty due to the maximum likelihood estimation of the detection-function parameters [39, 40]. Accuracy in this section was not analysed since true density and fish distribution among different census areas comprised in this study were unknown.
Accuracy of abundance estimations: A field experiment
In order to simulate a situation with fishes situated at known distances from the census line, 18 light brown wooden fish of 40 cm in length were fixed along a 50-m transect line placed at 10 m depth on a rocky reef within the CP marine reserve. The wooden fish were suspended between 0.3 and 0.7 m over the bottom and placed at random distances between 0 to 20 m on the right side of a transect line by a pair of divers who did not participate in the experiment.
The location, position above the bottom and colour of wooden fish were chosen in order to simulate an equivalent detection probability of real fishes, including those situated behind rocks in the census area. Each diver had to estimate independently the distance of each wooden fish perpendicular to the transect line while swimming along the transect at a constant speed, this procedure being repeated 3 times by the set of divers, with the wooden fish placed at different positions each time (thus, N = 54, i.e. 18 per transect, for each diver). During data analysis, the three passes were considered as a unique transect of 150 m in length. Divers had no previous information on the number of wooden fish present in the transect nor the distance range of the fish from the transect line. This procedure was replicated in two different occasions. On a first occasion, five divers performed the experiment: three experienced divers (with > 10 years of experience in visual census) and two non-experienced divers (with no previous experience in UVC). One of the non-experienced divers had a vast experience as scuba diver and the other had much less (< 20 dives). On a second occasion, three divers, one of them an experienced diver and the other two non-experienced, performed the experiment. In this second experiment, wooden fish were numbered and divers annotated the number of each fish, so that real versus estimated distance could be compared. The accuracy of the estimates of each occasion was measured comparing actual total abundance with those estimated from each diver’s observations using FW3, FW10, and DS estimators.
Results
Comparison among census methods
Nine TRTs were performed in both studied locations (CP and CT) covering a distance of 4829 m for a total of 255 minutes of diving time per pair of divers (Fig 1): 4 TRTs were performed in CP (covering 1221 m in 69 min) and 5 TRTs in CT (3608 m in 186 min). In CP, 8 CSTs were performed in four dives, requiring a total of 90 min of diving time per pair of divers. In CP, the ratio between the distance covered in an CST compared with a TRT (distance in meters covered on CST per minute of diving time / distance covered on TRT per minute of diving time) was 0.26 (i.e.: the TRT method was 4 times more efficient than CST). A total of 1266 individuals of the 6 selected fish species were counted in CP, 827 by the 9 TRTs and the remaining 439 fishes by the 8 CSTs. Overall, 93.8% of the fish were counted in CP, despite the sampling effort (in terms of distance surveyed) in this site being 52% lower than in CT. Groupers (E. marginatus, E. costae and M. rubra) and M. aquila were observed mostly solitary or in isolated pairs. On the other hand, S. umbra was mostly recorded in schools (75% of detections–up to 80 individuals per detection) and D. dentex was recorded in schools up to 30 individuals in 25% of cases.
In order to make valid estimations for distance distribution, at least ~30 observations are necessary [39, 40]. For this reason, density estimates following the DS theory were calculated only for E. marginatus and D. dentex, and the total number of groupers (i.e. considering the three species jointly).
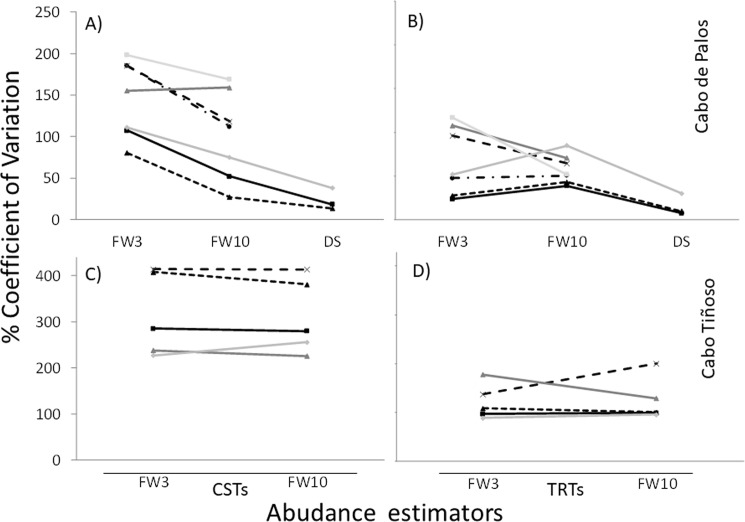
The coefficients of variation (CVs) of estimations based on TRT counts were smaller than those based on CST (Table 1; Fig 3). When CVs are compared between transects methods, CVs attain on average 57.3, 42.4 and 47.7% for the FW3, FW10 and DS estimators, respectively, considering both sampling locations (Table 1; Fig 3).
Table 1. Density estimations (D) (fish/300m2) and coefficients of variations (CV) for studied species and the total of groupers for different combinations of transects types and census widths in Cabo de Palos and Cabo Tiñoso.
Note that CST values in Cabo Tiñoso were derived from simulations. CST = Conventional Strip Transect. TRT = Tracked Roaming Transect. FW3 and FW10 = Fixed Width of 3 and 10 m for each side of the transects, respectively. DS = Distance Sampling Method. The census area of each survey is detailed in m2 considering total transect lengths and 6 and 20 m width for FW´s estimators and 40 m width for the DS estimator.
| Site | Transect type/ Census method/ Area (m2) | Total groupers | E. marginatus | M. rubra | E.costae | S. umbra | D. dentex | M. aguila | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D | %cv | D | %cv | %cv | D | %cv | D | %cv | D | %cv | D | %cv | |||
| Cabo Palos | CST FW3 2400 m2 | 3.8 | 107.4 | 2.0 | 80.2 | 1.3 | 185.2 | 0.5 | 185.2 | 0.8 | 155.3 | 1.3 | 111.1 | 0.4 | 198.4 |
| TRT FW3 7400 m2 | 4.4 | 23.6 | 3.0 | 27.3 | 1.0 | 47.3 | 0.3 | 96.0 | 6.8 | 107.8 | 0.6 | 51.5 | 0.1 | 116.7 | |
| CST FW10 8000 m2 | 3.3 | 52.0 | 2.1 | 27.0 | 1.0 | 112.0 | 0.2 | 118.0 | 12.2 | 159.0 | 0.6 | 75.0 | 0.2 | 169.0 | |
| TRT FW10 24666 m2 | 1.9 | 38.7 | 1.5 | 42.8 | 0.3 | 50.3 | 0.1 | 64.2 | 3.6 | 70.5 | 1.4 | 84.7 | 0.1 | 51.6 | |
| CST DS 16000 m2 | 4.2 | 18.1 | 3.6 | 13.5 | - | - | - | - | - | - | 1.5 | 38.0 | - | - | |
| TRT DS 49000 m2 | 2.9 | 7.4 | 2.6 | 9.6 | - | - | - | - | - | - | 1.2 | 30.0 | - | - | |
| Cabo Tiñoso | CST FW3 5700 m2 | 0.1 | 285.0 | 0.1 | 408.0 | - | - | 0.1 | 414.0 | 0.2 | 238.0 | 0.3 | 227.0 | - | - |
| TRT FW3 21600 m2 | 0.1 | 96.9 | 0.1 | 108.3 | - | - | 0.0 | 137.3 | 0.2 | 177.3 | 0.2 | 88.7 | - | - | |
| CST FW10 19000 m2 | 0.1 | 279.6 | 0.1 | 381.0 | - | - | 0.1 | 413.6 | 0.4 | 225.6 | 0.5 | 255.6 | - | - | |
| TRT FW10 72000 m2 | 0.1 | 99.0 | 0.0 | 100.2 | - | - | 0.0 | 200.0 | 0.1 | 128.6 | 0.1 | 95.9 | - | - | |
Fig 3. Coefficients of variation in percentage of density estimations of the different methods combinations in Cabo de Palos and Cabo Tiñoso.
(A) Conventional Strip Transects in Cabo de Palos. (B) Tracked Roaming Transects in Cabo de Palos. (C) Conventional Strip Transects in Cabo Tiñoso (data obtained from simulations). (D) Tracked Roaming Transects in Cabo Tiñoso. Black solid lines with square markers: total groupers. Short dashed line with triangle markers: Ephinepelus marginatus. Long dashed line with x marker: Ephinepelus costae. Dotted and dashed line with circle markers: Mycteroperca rubra. Grey solid lines with triangle marker: Sciena umbra. Soft grey solid lines with diamond marker: Dentex dentex. Ligth grey solid line with square marker: Myliobatis aquila. DS: Distance Sampling. FW3: Fixed width of 3 m for each side of the transect. FW10: Fixed width of 10 m for each side of the transect.
On the other hand, within counts on CSTs and TRTs, values of CV tended to diminish as the abundance estimators incorporated wider census areas in Cabo de Palos (with the exception of three species using TRT-FW10 method) and remained of similar magnitude for counts performed in Cabo Tiñoso (Table 1; Fig 3).
Accuracy of abundance estimations—Field experiment
Non-experienced divers systematically underestimated perpendicular distances (mean distance estimated = 5.2 m; actual mean distance = 8.7 m), and consequently overestimated abundance values (average = 64%) for the three estimators calculated [39] (Tables 2 and 3). Without considering the estimations made by non-experienced divers, abundance estimations of wooden fish using the FW3, FW10 and DS estimators, merging data from the two experimental occasions, ranged between 0.1 to 31% of difference compared to actual abundances (average = 13.1%; median = 14.2%). On the second occasion, in which the actual distance from each wooden fish was known, the average distance estimation error was 19.2% ± 19.86 standard error. Regarding the DS technique, the abundance estimations of the four experienced and three of the non-experienced divers were similar to the actual abundance of wooden fish (number of wooden fish = 54 and density = 0.0180 individuals m2) (Table 2), thus the actual abundance was within the confidence intervals estimated with count data from these seven divers. The number of wooden fish estimated ranged between 53 and 70, and the coefficients of variation (CV) of the estimations ranged between 13.1 and 17%. The number of wooden fish observed by each diver ranged between 39 and 46 out of the 54 wooden fish, with no trend observed between the number of fish detections per diver and the accuracy of abundance estimation. The actual mean of perpendicular distances of wooden fish to the transect line was 8.7 m in both experiments, and the mean distances estimated by the divers ranged between 7.7 and 9.6 m (Table 2).
Table 2. Number (N) of wooden fishes estimated with the Distance Sampling (DS) method by the experienced and non-experienced divers for the total census area (3000 m2) and the confidence interval (N CI 95%) at p < 0.05.
DS estimator: the best fit from the nine estimators tested with the DISTANCE software. D = Density per 300 m2. %CV = Coefficient of variation of the estimation in percentage. N detected (%) = Number of wooden fishes detected by divers and the percentage of the total wooden fish. MDD = Mean distance detection derived from visual estimations and standard deviation (SD) by divers. "*" Actual number of wooden fishes. "**" Actual mean distance.
| Diver | DS estimator | N* | N CI 95% | D (300 m2) | %CV | N detected (%) | MDD**(SD) | |
|---|---|---|---|---|---|---|---|---|
| Experienced | 1 | Half-normal/Cosine | 53 | 39–73 | 5.3 | 15.9 | 46 (85) | 9.6 (5.5) |
| 2 | Half-normal/Cosine | 60 | 45–80 | 6.0 | 14.1 | 40 (74) | 8.1 (5.1) | |
| 3 | Uniform/Cosine | 53 | 39–77 | 5.3 | 15.9 | 39 (72) | 9.2 (6.2) | |
| 4 | Half-normal/Cosine | 56 | 39–79 | 5.6 | 17.1 | 42 (78) | 9.1 (5.7) | |
| Non Experienced | 1 | Half-normal/Cosine | 55 | 38–79 | 5.5 | 17.0 | 41 (75) | 9.4 (5.7) |
| 2 | Half-normal/Hermite | 99 | 57–173 | 10.0 | 28.0 | 44 (81) | 5.2 (3.1) | |
| 3 | Uniform/Cosine | 70 | 54–91 | 7.0 | 13.1 | 44 (81) | 7.7 (5.6) | |
| 4 | Half-normal/Cosine | 65 | 48–87 | 6.5 | 14.7 | 44 (81) | 7.9 (5.3) | |
* Actual N = 54
** Actual mean distance of experiments 1 and 2 = 8,7
Table 3. Density of wooden fishes/300 m2 and percentage CV estimated by the experienced and non-experienced divers using the three abundance estimators in this study (DS, FW3 and FW10).
% Average estimation error: The average estimation error in percentage without considering data from one of the inexperienced divers who systematically underestimated distances.
| Diver | DS | %DS CV | FW10 | %FW10 CV | FW3 | %FW3 CV | |
|---|---|---|---|---|---|---|---|
| Experiment 1 | Experienced | 5.3 | 15.9 | 5.5 | 12.0 | 4.6 | 50.0 |
| 6.0 | 14.1 | 5.5 | 12.0 | 4.0 | 50.0 | ||
| 5.3 | 15.9 | 5.1 | 26.0 | 2.3 | 65.0 | ||
| Non Experienced | 5.5 | 17.0 | 5.1 | 17.0 | 4.0 | 60.0 | |
| 10.0 | 28.0 | 7.8 | 15.0 | 9.2 | 32.0 | ||
| Actual abundances | 5.4 | 6.7 | 4.6 | ||||
| Experiment 2 | Experienced | 5.6 | 17.1 | 4.8 | 21.7 | 6.0 | 33.3 |
| Non Experienced | 7.0 | 13.1 | 6.8 | 33.4 | 6.7 | 17.3 | |
| 6.5 | 14.7 | 6.4 | 37.9 | 7.3 | 31.5 | ||
| Actual abundances | 5.4 | 7.0 | 6.7 | ||||
| % Average estimations error | 9.9 | 18.1 | 9.1 |
Discussion
The TRT method proposed and tested in this study increased the efficiency of UVC in comparison with CST. It thus provides new information to ecologists for selecting an UVC method to study commercial and charismatic, medium- and large-size, nektobenthic reef fish species that are commonly found at low densities. We also showed that, for a comparable time effort, variability of abundance estimations can be reduced by enlarging the transect, either by using the TRT method (i.e. increasing transect length) and/or using abundance estimators that incorporate information from larger census widths (DS or FW10) (Table 1; Fig 3). These results are in concordance with a previous DS test of UVC [19, 35, 36] and studies which suggest that increasing the census area for species occurring at low densities (provided they have high detectability) could be used as a strategy to diminish the variability of abundance estimations. However, it is important to note that it is not the case for cryptic or camouflaged species as encounter does not necessarily lead to detection [11, 13, 27, 30].
A few studies used towed GPS devices to track UVC census measuring fish densities in roaming surveys [27, 38, 52]. According to Beck et al. [27], tracking the diver’s path optimizes the sampling effort, making surveys more efficient. In this work, the high efficiency found (74% higher in TRT than CSTs and 25% higher than found by [27]) could be due to the water currents prevailing in the study area. All censuses were conducted following the direction of the water current and hence the effort and time-air consumed against the current to roll the transect tapes in CST were high with respect to diver displacements during TRT surveys. Moreover, the additional sampling area increases the chances of encountering a greater abundance of rare species and hence improves the estimates of fish diversity [27, 28]. Furthermore, the potential of the TRT method to add spatial information to species detections, such as habitat features (e.g. caves, nests), geo-localized events (reproductive or feeding aggregations), etc., gives a powerful tool to interpret patterns and processes when analyzing data [28]. In cases such as part of the study area (Cabo de Palos—Islas Hormigas marine reserve, including the Hormigas archipelago) where the sampling area (at a certain depth range) comprises all or most of the home range of fishes, serious bias related to fish movement in response to environmental (e. g. currents), or behavioural (e. g. reproductive or feeding aggregations) variables could be avoided and/or studied [53].
The density estimates obtained by using the Distance Sampling method and fixed belt transects estimators were accurate and precise for most of the divers involved in the experiments with wooden fishes. The error levels for the three estimators found in this work were of similar or lower magnitude than the lowest values of instantaneous variability reported for reef fish visual census data [11, 30]. Although several authors [15, 22, 54, 55] studied the precision of distance estimates by divers with disparate results, to our knowledge our work is the first to directly test the accuracy of density estimations following the DS theory in real field conditions. Our results encourage the use of the DS method for surveying medium- and large-sized species. However, the transect width (i.e. maximum sighting distance) probably needs to be reduced when studying small-sized species (not visible at long distances) and/or for species with fast declining detectability curves [42]. The main virtues of the DS method are the relatively low variability of estimations (which increases the power of statistical analyses), and the potential integration of information of large width census areas; the latter feature is essential when comparing the abundance of target fish species in areas with different disturbance levels (e.g. MPAs and areas open to fishing) due to the different approach distances to divers (or wariness) of commercial species associated with human disturbance [42, 44, 45]. The drawbacks of the DS method in comparison with fixed width standard UVC were: (1) the higher post-processing requirements to calculate parameters in comparison with CST, which might not be justified when other methods (e.g. FW3 or FW10) are accurate [45], (2) the need for at least 30–50 observations to apply the DS density calculations, which is a serious constraint at the species level for most commercial species outside marine reserves, and (3) the need to test or train the observers’ ability to accurately and precisely estimate distances. Regarding the second shortcoming, it can be overcome by using the average distance of each species to the transect as a way to calculate densities, which has been shown to be a robust estimator [35, 56]. However, it is important to note that it is difficult to produce accurate density estimates for rare and/or difficult to detect species with any UVC method [27, 35, 42]. For its part, density estimates may be seriously biased if distance estimates are wrong, so as with other UVC methods, initial training and an accuracy test among observers are recommended both to apply the DS method as well as for counts in narrow fixed width transects (FW3 and FW10). Considerable non-systematic variation in the ability of different observers to estimate distance underwater has been found in previous studies [15]. Biases that are not systematic either through space, time, method or observer can lead to misleading conclusions [14].
Conclusions
Results of this study are relevant for the design of monitoring programs where the level of sampling effort would depend on a trade-off between the desired precision and logistic and material constraints. It is noteworthy that this method can be used for surveying other groups of marine animals (such as giant clams and mussels, sea turtles, lobsters, sharks, and rays) or objectives (e.g. locating fish nests, surveying caves used as refuges for fish and other groups, assessing spawning aggregations, etc.) [38, 52] or furthermore used to geo-locate survey sites (e.g. point counts). In addition, the spatial scale (width and length covered with TRT+DS) should be adjusted depending on environmental, ecological and logistic issues specific to each study. Specifically, we encourage the use of the TRT+DS as a rapid assessment method [57] for fish surveys in new sites not previously explored, in which divers could gather spatial ecological and ecosystem information on large areas while counting fishes.
Acknowledgments
We thank José Manuel Pereñíguez, Adrián Aguilar Rodríguez, Ramón Hernández and and Captain José Antonio Rodríguez Navarro ('Rodri') for field support. Katie Hogg helped with the English grammar and Andrea Marino for her guidance with the distance sampling method. We also warmly thank Michel Kulbicki and Neville Barret for his invaluable comments onearliest version of the manuscript. The authors wish to thank to the editor for their useful comments which have helped us to improve the manuscript.
Data Availability
All relevant data are within the paper.
Funding Statement
Field activities and data acquisition were accomplished in the course and funded by the following projects from Argentina and Spain: IRSES-RECOMPRA (FP7-People-Marie Curie Actions-IRSES-Contract nº 295213), Monitoring of the Cabo de Palos - Islas Hormigas marine reserve (Regional Fisheries and Aquaculture Service, Autonomous Community of the Region of Murcia), REDEMED (MINECO CGL2013-49039-R), ABHACO2DE (Fundación Séneca 19516/PI/14) and Agencia Nacional de Promocion de Ciencia y Tecnica (PICT 2012-0297).
References
- 1.Willis TJ, Anderson MJ. Structure of cryptic reef fish assemblages: relationships with habitat characteristics and predator density. Mar Ecol Prog Ser. 2003; 257: 209–221. [Google Scholar]
- 2.Cappo MC, Harvey ES, Shortis M. Counting and measuring fish with baited video techniques—an overview. In: Furlani D, Beumer JP, editors. Proceedings of the Australian Society for Fish Biology Workshop, Hobart, Australia. 2007 pp. 101–114.
- 3.Polunin NVC, Bloomfield HJ, Sweeting CJ, McCandless DT. Developing indicators of MPA effectiveness: finfish abundance and diversity in a Yorkshire prohibited trawl area, Report to MFA, London. 2009.
- 4.Colton MA, Swearer SE. A comparison of two survey methods: differences between underwater visual census and baited remote underwater video. Mar Ecol Prog Ser. 2010; 400: 19–36. [Google Scholar]
- 5.Katsanevakis S, Weber A, Pipitone C, Leopold M, Cronin M, Scheidat M, et al. Monitoring marine populations and communities: methods dealing with imperfect detectability. Aquat Biol. 2012; 16: 31–52. [Google Scholar]
- 6.Bortone SA, Martin T, Bundrick CM. Visual census of reef fish assemblages: a comparison of slate, audio, and video recording devices. Northeast Gulf Sci. 1991; 12(1): 17–23. [Google Scholar]
- 7.García-Charton JA, Pérez-Ruzafa A. Spatial pattern and the habitat structure of a Mediterranean rocky reef fish local assemblage. Mar Biol. 2001; 138: 917–934. [Google Scholar]
- 8.Stobart B, García-Charton JA, Espejo C, Rochel E, Goñi R, Reñones O, et al. A baited underwater video technique to assess shallow-water Mediterranean fish assemblages: Methodological evaluation. J Exp Mar Biol Ecol. 2007; 345(2): 158–174. [Google Scholar]
- 9.MacNeil MA1, Tyler EHM, Fonnesbeck CJ, Rushton SP, Polunin NVC, Conroy MJ. Accounting for detectability in reef-fish biodiversity estimates. Mar Ecol Prog Ser. 2008; 367: 249–260. [Google Scholar]
- 10.Ward-Paige C, Mills Flemming J, Lotze HK. Overestimating fish counts by non-instantaneous visual censuses: consequences for population and community descriptions. PLoS ONE 2010; 5(7): e11722 doi: 10.1371/journal.pone.0011722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McClanahan TR, Graham NAJ, Maina J, Chabanet P, Bruggemann JH, Polunin NVC. Influence of instantaneous variation on estimates of coral reef fish populations and communities. Mar Ecol Prog Ser. 2007; 340: 221–234. [Google Scholar]
- 12.García-Charton JA, Pérez-Ruzafa A. Correlation between habitat structure and a rocky reef fish assemblage in the Southwest Mediterranean. Mar Ecol. 1998; 19: 111–128. [Google Scholar]
- 13.Willis TJ. Visual census methods underestimate density and diversity of cryptic reef fishes. J Fish Biol. 2001; 59: 1408–1411. [Google Scholar]
- 14.Edgar GJ, Barrett NS, Morton AJ. Biases associated with the use of underwater visual census techniques to quantify the density and size-structure of fish populations. J Exp Mar Biol Ecol. 2004; 308: 269–290. [Google Scholar]
- 15.Bernard ATF, Gotz A, Kerwarth SE, Wilke CG. Observer bias and detection probability in underwater visual census of fish assemblages measured with independent double-observers. J Exp Mar Biol Ecol. 2013; 443: 75–84. [Google Scholar]
- 16.Usseglio P. Quantifying reef fishes: bias in observational approaches In: Mora C, editors. Ecology of Fishes on Coral Reefs, Cambridge University Press; 2015. pp. 270–273. [Google Scholar]
- 17.Samoilys MA, Carlos G. Determining methods of underwater visual census for estimating the abundances of coral reef fishes. Env Biol Fish. 2000; 57: 289–304. [Google Scholar]
- 18.Williams ID, Walsh WJ, Tissot BN, Hallacher LE. Impact of observers’ experience level on counts of fishes in underwater visual surveys. Mar Ecol Prog Ser. 2006; 310: 185–191. [Google Scholar]
- 19.Kulbicki M, Cornuet N, Vigliola L, Wantiez L, MouTham G, Chabanet P. Counting coral reef fishes: Interaction between fish life-history traits and transect design. J Exp Mar Biol Ecol. 2010; 387:15–23. [Google Scholar]
- 20.Brock VE. A preliminary report on a method of estimating reef fish populations. J Wild Manage. 1954; 18: 297–308. [Google Scholar]
- 21.Schmitt EF, Sluka RD, Sullivan-Sealey KM. Evaluating the use of roving diver and transect surveys to assess the coral reef fish assemblage off southeastern Hispaniola. Coral Reefs. 2002; 21: 216–223. [Google Scholar]
- 22.Bohnsack JA, Bannerot SP. A stationary visual census technique for quantitatively assessing community structure of coral reef fishes. NOAA Technical Report NMFS 1986; 41: 1–15.
- 23.Hodgson G, Kiene W, Mihaly J, Liebeler J, Shuman C, Maun L. Reef Check instruction manual: a guide to reef check coral reef monitoring. Reef Check, Institute of the Environment, University of California; at Los Angeles: 2004. [Google Scholar]
- 24.Hill J, Wilkinson C. Methods for ecological monitoring of coral reefs. Australian Institute of Marine Science, Townsville: 2004. [Google Scholar]
- 25.García-Charton JA, Pérez-Ruzafa A, Sánchez-Jerez P, Bayle-Sempere JT, Reñones O, Moreno D. Multiscale spatial heterogeneity, habitat structure, and the effect of marine reserves on Western Mediterranean rocky reef fish assemblages. Mar Biol. 2004; 144: 161–182. [Google Scholar]
- 26.Stuart-Smith RD, Bates AE, Lefcheck JS, Duffy JE, Baker SC, Thomson RJ, et al. Integrating abundance and functional traits reveals new global hotspots of fish diversity. Nature. 2013; 501(7468): 539–542. doi: 10.1038/nature12529 [DOI] [PubMed] [Google Scholar]
- 27.Beck HJ, Feary DA, Figueira WF, Booth DJ. Assessing range shifts of tropical reef fishes: a comparison of belt transect and roaming underwater visual census methods. Bull Mar Sci. 2014; 90(2): 705–721. [Google Scholar]
- 28.Lynch T, Green M, Davies C. Diver towed GPS to estimate densities of a critically endangered fish. Biol Conserv. 2015; 191: 700–706. [Google Scholar]
- 29.Thompson AA, Mapstone BD. Intra- versus inter-annual variations in counts of reef fish and interpretations of long-term monitoring studies. Mar Ecol Prog Ser. 2002; 232: 247–257. [Google Scholar]
- 30.Irigoyen AJ, Galván DE, Venerus LA, Parma AM. Variability in abundance of temperate reef fishes estimated by visual census. PLoS ONE. 2013; 8(4): e61072 doi: 10.1371/journal.pone.0061072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sadovy Y, Kulbicki M, Labrosse P, Letourneur Y, Lokani P, Donaldson TJ. The humphead wrasse, Cheilinus undulatus: synopsis of a threatened and poorly known giant coral reef fish. Reviews in fish Biology and Fisheries. 2003; 13(3): 327–364. [Google Scholar]
- 32.Colin PL, de Mitcheson YS, Donaldson TJ. Grouper spawning aggregations: be careful what you measure and how you measure it: a rebuttal of Golbuu and Friedlander (2011). Estuarine, Coastal and Shelf Science. 2013; 123: 1–6. [Google Scholar]
- 33.Anderson AB, Bonaldo RM, Barneche DR, Hackradt CW, Félix-Hackradt FC, García-Charton JA, et al. Recovery of grouper assemblages indicates effectiveness of a marine protected area in Southern Brazil. Mar Ecol Prog Ser. 2014; 514: 207–215. [Google Scholar]
- 34.Edgar GJ, Stuart-Smith RD, Willis TJ, Kininmonth S, Baker SC, Banks S, et al. Global conservation outcomes depend on marine protected areas with five key features. Nature 2014; 506: 216–220. doi: 10.1038/nature13022 [DOI] [PubMed] [Google Scholar]
- 35.Kulbicki M, Sarramégna S. Comparison of density estimates derived from strip transect and distance sampling for underwater visual censuses: a case study of Chaetodontidae and Pomacanthidae. Aquat Living Resour. 1999; 12: 315–325. [Google Scholar]
- 36.Loiseau N, Gaertner JC, Kulbicki M, Mérigot B, Legras G, Taquet M, Gaertner-Mazouni N. Assessing the multicomponent aspect of coral fish diversity: The impact of sampling unit dimensions. Ecological Indicators. 2016; 60: 815–823. [Google Scholar]
- 37.Cheal AJ, Thompson AA. Comparing visual counts of coral reef fish: implications of transect width and species selection. Mar Ecol Prog Ser. 1997; 158: 241–248. [Google Scholar]
- 38.Colin PL, Donaldson TJ, Martin LE. GPS Density Surveys: A New Method for Quantitatively Assessing Reef Fish Spawning Aggregations (and other populations of reef fishes).(Abs.) Seventh Indo-Pacific Fish Conference. In 7th Indo Pacific Reef Fish Conference. 2005 pp. 16–21.
- 39.Buckland ST, Anderson DR, Burnham KP, Laake JL, Borchers DL, Thomas L. Introduction to distance sampling: estimating abundance of biological populations. Oxford University Press, Oxford: 2001. [Google Scholar]
- 40.Buckland ST, Anderson DR, Burnham KP, Laake JL, Borchers DL, Thomas L. Advanced distance sampling: estimating the abundance of biological populations. Oxford University Press; 2004. [Google Scholar]
- 41.Burnham KP, Anderson DR, Laake JL. Estimation of density from line transect sampling of biological populations. Wildl. Monogr. 1980; 72: 1–202. [Google Scholar]
- 42.Bozec YM, Kulbicki M, Laloe F, Mou-Tham G, Gascuel D. Factors affecting the detection distances of reef fish: implications for visual counts. Mar Biol. 2011; 158: 969–981. [Google Scholar]
- 43.Labrosse P, Kulbicki M, Ferraris J. Underwater visual fish census surveys. Proper use and implementation. Reef resource assessment tools. Noumea, New Caledonia: Secretariat of the Pacific Community; 2002. [Google Scholar]
- 44.Goetze JS, Januchowski-Hartley FA, Claudet J, Langlois TJ, Wilson SK, Jupiter SD. Fish wariness is a more sensitive indicator to changes in fishing pressure than abundance, length or biomass. Ecol Appl. 2017; 27: 1178–1189. doi: 10.1002/eap.1511 [DOI] [PubMed] [Google Scholar]
- 45.Kulbicki M. How the acquired behaviour of commercial reef fishes may influence the results obtained from visual censuses. J Exp Mar Biol Ecol. 1998; 222: 11–30. [Google Scholar]
- 46.Marino AI, Johnson A. 2012 Behavioural response of free-ranging guanacos (Lama guanicoe) to land use change: habituation to motorised-vehicles in a recently created reserve. Wildlife Res. 2012; 39: 503–511. [Google Scholar]
- 47.Lloret J, Zaragoza N, Caballero D, Font T, Casadevall M, Riera V. Spearfishing pressure on fish communities in rocky coastal habitats in a Mediterranean marine protected area. Fish Res. 2008; 94: 84–91. [Google Scholar]
- 48.Louisy P. Peces Marinos de Europa y el Mediterráneo. Ediciones Omega, Barcelona, España: 2006. [Google Scholar]
- 49.García-Charton JA, Hackradt CW, Félix-Hackradt FC, Segovia-Viadero M, Martínez-Garrido J, et al. Estudios de seguimiento de la reserva marina de Cabo de Palos–Islas Hormigas 2010. Universidad de Murcia, Comunidad Autónoma de la Región de Murcia; 2010. [Google Scholar]
- 50.Siwiec T, Sheldrake S, Hess A, Thompson D, Macchio L, Duncan PB. Survey technique for underwater digital photography with integrated GPS location data In: Brueggeman P.S., Pollock N.W. editors. Diving for science; Dauphin Island, 2008; 159–166. [Google Scholar]
- 51.Schories D, Niedzwiedz G. Precision, accuracy, and application of diver-towed underwater GPS receivers. Environ Monit Assess. 2012; 184: 2359–2372. doi: 10.1007/s10661-011-2122-7 [DOI] [PubMed] [Google Scholar]
- 52.Rizzari JR, Frish A, Magnenat KA. Diversity, abundance, and distribution of reef sharks on outer-shelf reefs of the Great Barrier Reef, Australia. Mar Biol. 2014; 161: 2847–2855. [Google Scholar]
- 53.Zeller DC. Tidal current orientation of Plectropomus leopardus (Serranidae). Coral Reefs 2002; 21: 183–187. [Google Scholar]
- 54.Nolan RS, Taylor LR. An evaluation of transect methods for fish census on Hawaiian reefs. Ms Hawaiian Cooperative Fishery Unit University of Hawaii; 1980. [Google Scholar]
- 55.Thresher RE, Gunn JS. Comparative analysis of visual census techniques for highly mobile, reef associated piscivores (Carangidae). Environ Biol Fish. 1986; 17: 93–116. [Google Scholar]
- 56.Kulbicki M, Sarramégna S, Letourneur Y, Wantiez L, Galzin R, Mou-Tham G, et al. Opening of an MPA to fishing: Natural variations in the structure of a coral reef fish assemblage obscure changes due to fishing. J Exp Mar Biol Ecol. 2007; 353: 145–163. [Google Scholar]
- 57.Álvarez-Berastegui D, Amengual J, Coll J, Reñones O, Moreno-Navas J, Agardy T. Multidisciplinary rapid assessment of coastal areas as a tool for the design and management of marine protected areas. J Nat Conserv. 2014; 22: 1–14. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.