Abstract
We carried out geochemical analyses on a sediment core from Lake Harris, Florida (USA) to identify sources of organic matter to the sediment throughout the Holocene, and relate changes in those sources to shifts in past climate and environmental conditions. We hypothesized that the sources of organic matter changed in response to regional hydrologic shifts following de-glaciation, and to human population expansion in the state during the 20th century. Hydroclimate shifts in Florida were related to: 1) a steady rise in relative sea level and the fresh water table that began in the early Holocene, 2) wetland formation and expansion ca. 5,000 cal yrs BP, and 3) the onset of the modern El Niño (ENSO) cycle ~3,000 cal yrs BP. Stratigraphic changes in sediment variables from Lake Harris reflect each of these hydroclimate periods. Early in the Holocene, Lake Harris was a marsh-like system in a relatively dry, open-prairie environment. Organic sediments deposited at that time were derived largely from terrestrial sources, as inferred from high TOC/TN ratios, a dominance of longer-chain of n-alkanes (n-C29-31), relatively negative organic carbon isotope values (δ13CTOC), and low biogenic silica concentrations. In the middle Holocene, a positive shift in δ13CTOC coincided with the onset of wetter conditions in Florida. Submerged macrophyte biomarkers (n-C21-23) dominated, and during that period bulk organic carbon isotope values were most similar to δ13C values of mid-chain-length n-alkanes. In the late Holocene, δ13CTOC values declined, CaCO3 levels decreased to trace amounts, organic carbon concentrations increased and diatom biogenic silica concentrations increased from 10 to 120 mg g-1. Around 2,900 cal yrs BP, the effects of ENSO intensified and many Florida lakes deepened to their current limnetic state. Concentrations of algal and cyanobacterial biomarkers in the Lake Harris core increased by orders of magnitude after about AD 1940, in response to human-induced eutrophication, an inference supported by values of δ15N that fluctuate around zero.
Introduction
Lakes are critical components of the terrestrial carbon cycle. Their sediment contains an archived history of changes in organic carbon sources from the watershed (allochthonous) and water column (autochthonous). The sediments of lakes, reservoirs, and wetlands constitute a pool of organic carbon that is sequestered at a rate (0.07 Pg C yr-1) estimated to exceed the rate in the ocean by a factor of three [1, 2]. Analyses of organic carbon in a lacustrine sediment archive can be used to infer past environmental changes that occurred during the lake’s existence. In Florida, paleolimnological reconstructions based on organic biomarkers were used to document ecological succession [3] and hydrologic changes [4] during the transition from the last glacial period to the present.
Paleolimnological inferences regarding past climate and environment routinely employ total organic carbon/total nitrogen (TOC/TN) ratios and stable isotope values of total organic carbon (δ13CTOC) to identify sources of organic matter in the lake sediments [5, 6]. Low TOC/TN ratios in algae arise from their lack of carbohydrate-rich structural components [7]. Moreover, the extreme differences in the range of TOC/TN values in vascular (>20) versus non-vascular plants (4–10) have been used to estimate the relative contributions from these organic carbon sources to sediments.
Similarly, the δ13CTOC of bulk lake sediment can be interpreted as reflecting a mixture of values from various end-member groups. The δ13C values of higher plant and algal organic matter are governed primarily by the isotope value of the carbon substrate used for photosynthesis (atmospheric CO2 or dissolved HCO3-) and the enzymatic fractionations associated with fixing that carbon (i.e. ribulose 1,5 bisphosphate [RuBP]) [8]. Most terrestrial plants assimilate carbon through one of two photosynthetic pathways (C3 or C4) and the δ13C values of C3 plants (most trees, shrubs and herbs) differ from those of C4 plants (tropical grasses) [9]. Algae use the C3 pathway for photosynthesis, but in high-pH aquatic systems they typically display larger δ13C values because dissolved HCO3- is enriched in 13C relative to CO2(aq) by ~9‰ [8].
Recent studies, however, suggest that interpretation of organic carbon sources in lake sediments from TOC/TN ratios alone must be undertaken with caution. For instance, Cloern et al. [10] showed that TOC/TN ratios vary considerably at the species level. It has also been noted that TOC/TN can be greater in N-limited systems [11], and lower in systems where there is selective degradation of labile carbon [12]. Similarly, the interpretation of organic matter provenance from δ13CTOC can be equivocal. In lake sediments, stratigraphic shifts toward more positive δ13C values have been explained in multiple ways, such as reflecting greater input from algal material [13], increased autotrophic carbon fixation [14, 15], and a transition from a C3 to a C4 terrestrial plant community in the watershed [16].
An alternative to analysis of bulk organic matter in lake sediments is the application of compound-specific isotope measurements of molecular biomarkers to track the source and history of sediment organic matter. Biomarker studies have enabled reconstructions of past hydrological changes across millennial time scales. For example, carbon isotope values of lipid compounds in sediments from Mud Lake, Florida were used to document vegetation changes associated with Holocene climate shifts [17]. The authors found that shifts in alkane biomarkers corresponded to fluctuations in the regional water table that were caused by hydroclimate changes in the early Holocene. Sediment cores from other Florida lakes showed that changes recorded in the carbon isotopes of the n-alkanes were consistent with pollen-based environmental reconstructions [3].
A recent study of Lake Harris, Florida, USA, used multiple geochemical proxies from a sediment core dated to ~10,000 cal yrs BP, to identify prehistoric shifts in the primary producer community structure [18]. A shift from carbonate-dominated to organic-dominated sediment occurred at approximately 5,540 cal yrs BP [18]. The timing of that shift was probably related to the onset of modern hydrological conditions in Florida, as inferred from pollen data [3] and core studies of basal peat layers from the Mississippi Delta [19].
The primary objectives of this study were as follows: 1) assess changes in the major sources of organic matter to the sediments of Lake Harris from its nascent stages in the early Holocene to the present, and 2) determine the environmental and hydrological changes that drove the shifts in organic matter source. We employed a suite of geochemical variables in a complete Holocene sediment record from Lake Harris to evaluate these objectives quantitatively. The concentrations, ratios and stable isotope compositions (δ13C, δ15N) of sediment total organic carbon (TOC), total nitrogen (TN), and select biomarker hydrocarbons, were used as proxies for the sources of organic matter in the sediment. Geochemical analyses, including concentrations of carbonate (CaCO3), biogenic silica (BioSi), and total organic carbon (TOC), were used as proxies for hydrological changes, such as increases or decreases in groundwater input, water table elevation and lake stage. We hypothesized that the predominant sources of organic matter (i.e. allochthonous vs. autochthonous [macrophytes vs. algae]) to the lake’s sediments were controlled by regional hydrologic and environmental conditions that occurred as the Earth’s climate shifted through the Holocene, and human settlement expanded in the 20th century. Specifically, climate transitions included: 1) a steady rise in relative sea level in the Gulf of Mexico that began in the early Holocene and abruptly slowed ~7,000 cal yrs BP [2, 19], wetland formation and expansion in Florida ca. 5,000 cal yrs BP [3, 20] and the onset of the modern El Niño cycle ~3,000 cal yrs BP [21]. The sediment record in Lake Harris shows a response to each of these Holocene climate transitions, and the record from the last ~70–80 years reflects rapid cultural eutrophication.
Study site
Lake Harris is a shallow (mean depth = 3.5m), productive lake (mean annual chlorophyll a = 57 μg L-1, total phosphorus = 38 μg L-1, total nitrogen = 1707 μg L-1), with a surface area of ~75 km2 [22]. It is part of the Harris Chain of Lakes and is located in the Upper Ocklawaha River Basin, Central Florida, USA (Fig 1). Hydrologic flow through some water bodies in the Chain of Lakes begins at spring-fed, hypereutrophic Lake Apopka, which discharges through four other lakes and ultimately into the Ocklawaha River [23]. Three other lakes in the Chain, including Lake Harris, receive minimal or no flow from Lake Apopka and are considered mesotrophic to eutrophic. The hydraulic retention time for Lake Harris is relatively short (2.9 years), but can vary widely between periods of high versus low flow [22]. Most of the lakes in the Harris Chain receive surface and groundwater inputs that pass through organic-rich mineral soils, and thus are considered to be naturally productive and alkaline water bodies [22]. Geochemical analyses on the same 5.9-m sediment record from Lake Harris used in this study, however, indicated the lake was on a trajectory towards increasing oligotrophication throughout the Holocene [18]. This trajectory was interrupted abruptly after ca. AD 1940, when the human population in the region increased six-fold and the lake veered toward the highly productive end of the trophic state spectrum [18].
Fig 1. Location of Lake Harris within the Harris Chain of lakes; 28°46′4″N 81°48′57″W.
Reproduced from [23] with permission from Springer, license number 4111351386595.
Methods
Sediment sampling
Lake Harris is a public resource, with free access, thus no permitting was required for core collection. Furthermore, no endangered species were involved in this study. We sampled sediment from the 5.9-m core retrieved by [18]. The upper portion of the core was measured for 210Pb activity, and the CRS model was used to calculate dates. A date of ~1911 was determined for sediments at 44 cm depth. The remaining portion of the core was AMS-14C dated using four charcoal and three bulk sediment samples. Radiocarbon dates were calibrated using INTCAL13. A date on charcoal near the core base had a range of 10500–10250 cal yrs BP, and linear regression of calibrated age versus depth indicated a near-constant sedimentation rate (R2 = 0.98). For details of the chronology, see [18].
The core was split in half lengthwise and one half was sampled at 4-cm intervals for geochemical analyses. Wet subsamples were frozen, freeze-dried, and then ground and homogenized for analyses. CaCO3, biogenic silica [BioSi] (both diatom- and sponge-spicule-derived), TN, and percent organic matter (%OM) analysis methods are presented in [18]. TOC was measured as the difference between total carbon, measured on a Carlo Erba NA1500 CNS elemental analyzer, and inorganic (carbonate) carbon, measured on a UIC-Coulometrics coulometer, coupled with an Auto-Mate automated carbonate preparation device (AutoMateFX.com). Dried sediment for δ15N and δ13CTOC analyses was pretreated with 1N HCl to remove inorganic carbon, and then combusted in a Carlo Erba NA1500 CNS elemental analyzer interfaced with a Thermo Scientific Delta V Advantage isotope ratio mass spectrometer. Carbon isotopic compositions were normalized to the VPDB scale and nitrogen isotope values are relative to air. All isotope results are reported in standard delta notation:
| (1) |
Lipid extraction and quantification
Lipids were extracted from 1-2g of freeze-dried sediment with an Accelerated Solvent Extractor ASE200 (Dionex), using 2:1 (v/v) dichloromethane (DCM):methanol through three extraction cycles at 10.3 MPa (1500 psi) and 100°C. Total lipid extracts (TLE) were concentrated under a gentle stream of nitrogen, and the neutral lipid fraction was obtained after base saponification of the TLE. Neutral lipids were further separated, based on polarity, into compound classes by column chromatography, using 5% deactivated silica gel, according to methods modified from [24]. Hydrocarbons were eluted from the silica gel column with 4.5 mL of 9:1 Hexane:DCM, and saturated hydrocarbons were separated from alkenes on 5% Ag-impregnated silica gel (w/w) with 4 mL of hexane and ethyl acetate, respectively. Branched and cyclic saturated hydrocarbons were isolated from n-alkanes with triple urea adduction.
Alkane concentrations were measured and identified on a Thermo Scientific Trace 1310 gas chromatograph with a Supelco Equity 5 column, interfaced to a Thermo Scientific TSQ 8000 triple quadrupole mass spectrometer with electron ionization. The inlet was operated in splitless mode at 280°C. The column flow rate was set to 2.0 mL min-1 and the oven was programmed to an initial temperature of 60°C and held for 1 minute, then ramped to 140°C at 15°C min-1, and to 320°C at 4°C min-1 and held for 25 minutes. Quantification was based on the calibration curves generated from the peak areas of external standards (C7-C40) with concentrations ranging from 5 to 250 μg mL-1. Concentration measurements were reproducible within ±10%.
Compound-specific isotope measurements on n-alkanes
Compound-specific carbon isotope values for n-alkanes were measured on an Agilent 6890 GC connected to a Thermo Scientific Delta V Plus IRMS interfaced with a GC-C-III combustion system. The GC flow rate was set to 2.0 mL min-1 and the oven was programmed as follows: 60°C for 1 minute, then increased at a rate of 6°C min-1 to 320°C, and held for 20 minutes. Compounds were combusted over a nickel/platinum/copper wire with O2 at 960°C. The isotope ratios of carbon in CO2 were measured and normalized to the VPDB scale using the Uncertainty Calculator [25] and are reported in standard delta notation as above. Standard errors of the mean (SE) were calculated using the Uncertainty Calculator, which yielded a 1σ SE value of ± 0.39‰ [25].
Results
Bulk geochemistry and isotopic composition
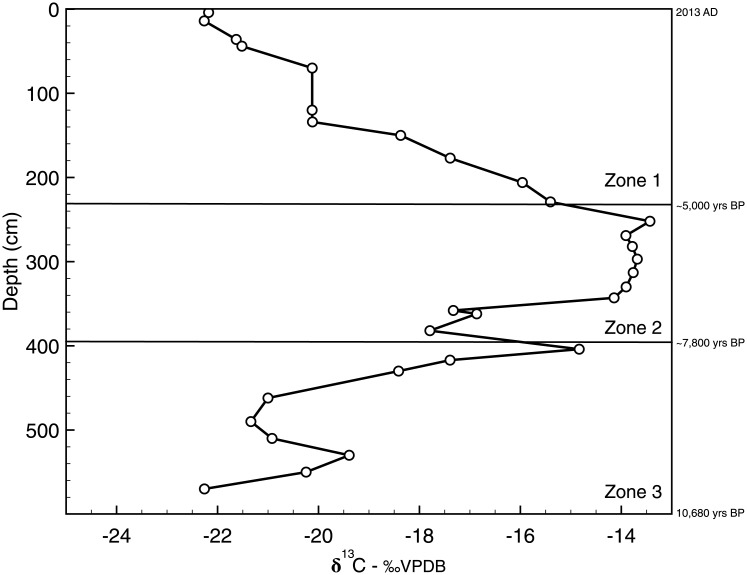
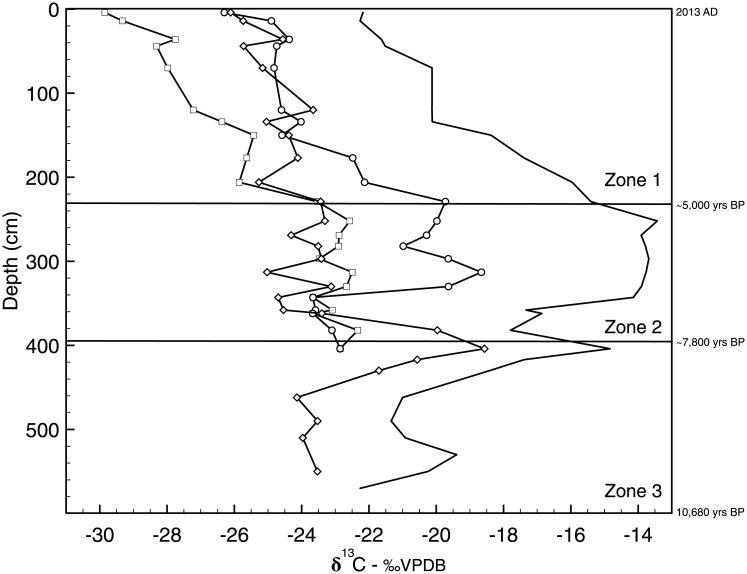
The core was divided into three zones based on shifts in δ13CTOC: zone 3 spanned from the bottom of the core to 403 cm (~10,000–7,800 cal yrs BP), zone 2 was from 402 to 241 cm (~7,800–5,000 cal yrs BP), and zone 1 encompassed from 240 cm depth to the surface (~5,000 cal yrs BP-AD 2013) (Fig 2). Zone 3 was marked by an increase in δ13CTOC values upcore, from a minimum of -24.29‰ to a maximum of -15.78‰. Bulk carbon isotope values remained relatively stable in zone 2 (mean -14.79‰), before decreasing sharply in zone 1 from -13.27‰ to a minimum value of -22.18‰ at the surface.
Fig 2. TOC isotope values (δ13CTOC) versus depth in the Lake Harris core.
The three zones in the core are delineated by major shifts in δ13CTOC (shown in the figure as solid horizontal lines). Zone 3 is from 590 to 403 cm, zone 2 is from 402 to 241 cm, and zone 1 extends from 240 cm to the core surface.
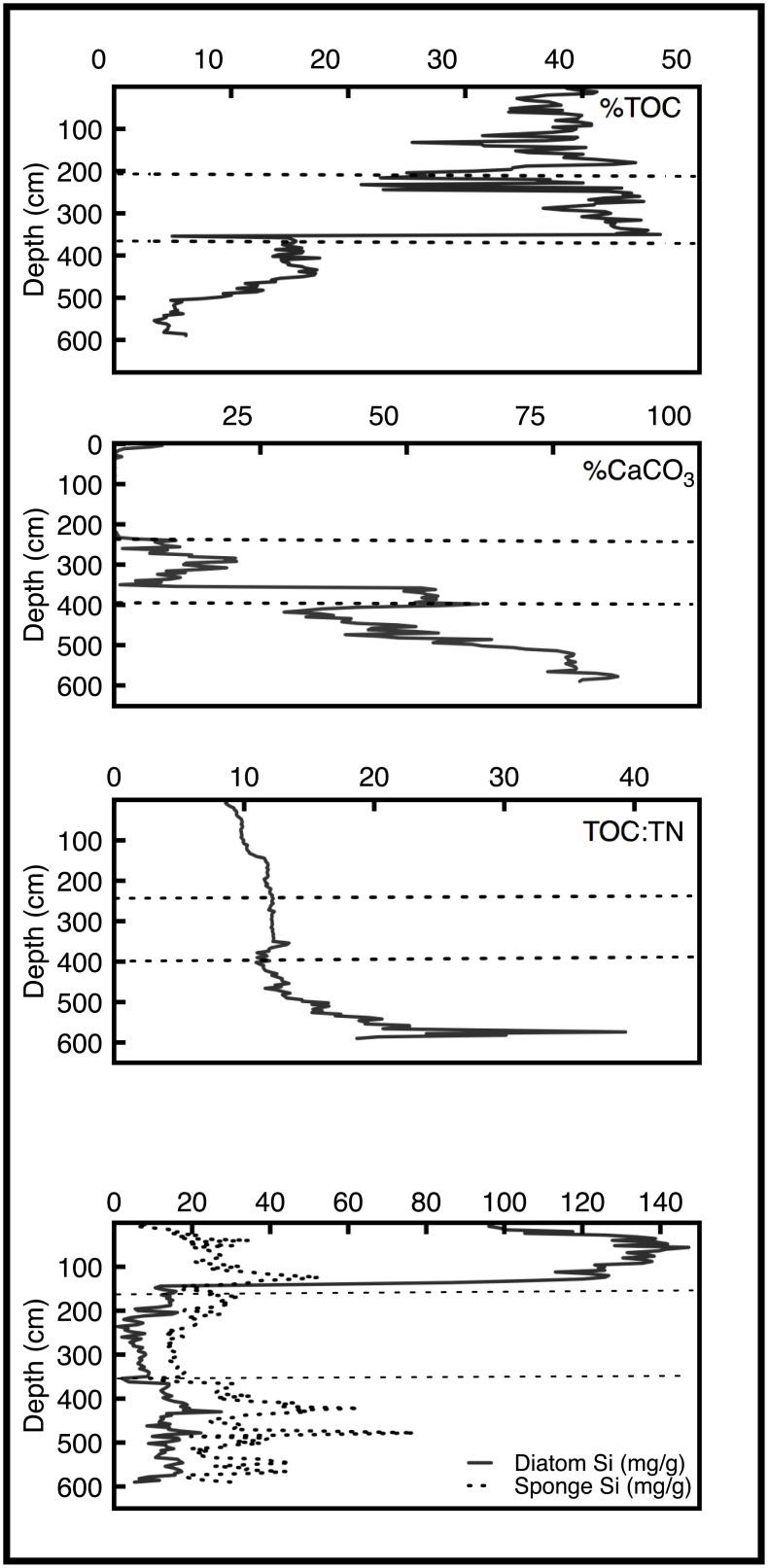
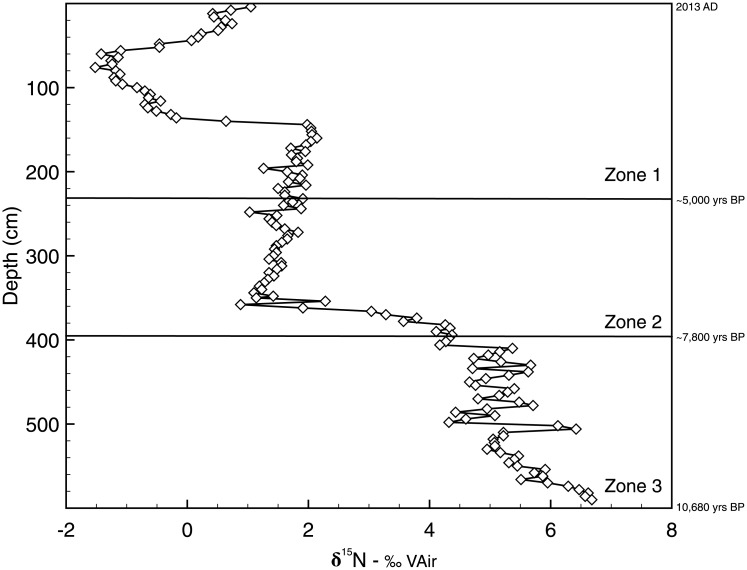
Organic carbon percentages remained below 15% throughout zone 3, while %CaCO3 decreased from a maximum value of 86% at 579 cm depth (~10,000 cal yrs BP), to just under 30% at 418 cm (~8,000 cal yrs BP), with an average value of ~60% throughout zone 3 (Fig 3). Values of %TOC and %CaCO3 changed markedly at ~350 cm depth (~6,500 cal yrs BP) in zone 2: organic carbon increased from 2.7% to 38.1% and carbonate dropped to 1% and never increased above 25% in zone 2. In zone 1, %TOC remained at elevated concentrations and varied about its mean of 21.3%. Carbonate values in zone 1 never exceeded 11% from 240 cm (~5,000 cal yrs BP) to the surface of the core and displayed an average value of 0.6%. δ15N displayed a maximum value of 6.70‰ at the base of the core, in zone 3, but values declined upward, to about 4.00‰ at the top of the zone (Fig 4). Thereafter, values declined rapidly in zone 2, reaching a low of 1.10‰ at ~340 cm. This was followed by a slow increase in δ15N values to 2.14 at 160 cm (zone 1), followed by an abrupt decline to a minimum of -1.52‰ at 75 cm core depth. Values then increased to 1.05‰ at the top of the core.
Fig 3. Bulk geochemical variability in the Lake Harris core.
From the top panel to the bottom: percent total organic carbon, percent calcium carbonate, molecular total organic carbon to total nitrogen, and diatom and sponge derived silica concentrations.
Fig 4. Nitrogen isotope values (δ15N) versus depth in the Lake Harris core.
Shifts in δ15N in the Lake Harris core. Core zones are delineated with solid horizontal lines, and labeled accordingly.
In zone 3, TOC:TN values reached a maximum of 39.3 at 574 cm (~10,000 cal yrs BP), before decreasing rapidly in zone 2 to a minimum value of 10.9. TOC:TN values continued to decrease slowly from zone 2 to zone 1 and reached a minimum value of 8.6 at 8 cm depth (ca. AD 2009) (Fig 3).
Biogenic silica, both diatom- and sponge-derived, displayed considerable variability throughout all zones. Of note was an exponential increase in diatom silica at 144 cm (~2,600 cal yrs BP), where the concentration increased from 12.0 to 52.0 mg g-1. These values continued to rise in zone 1, reaching a maximum value of 147.3 mg g-1 at 52 cm (~490 cal yrs BP) (Fig 3).
Concentrations and isotopic compositions of hydrocarbons
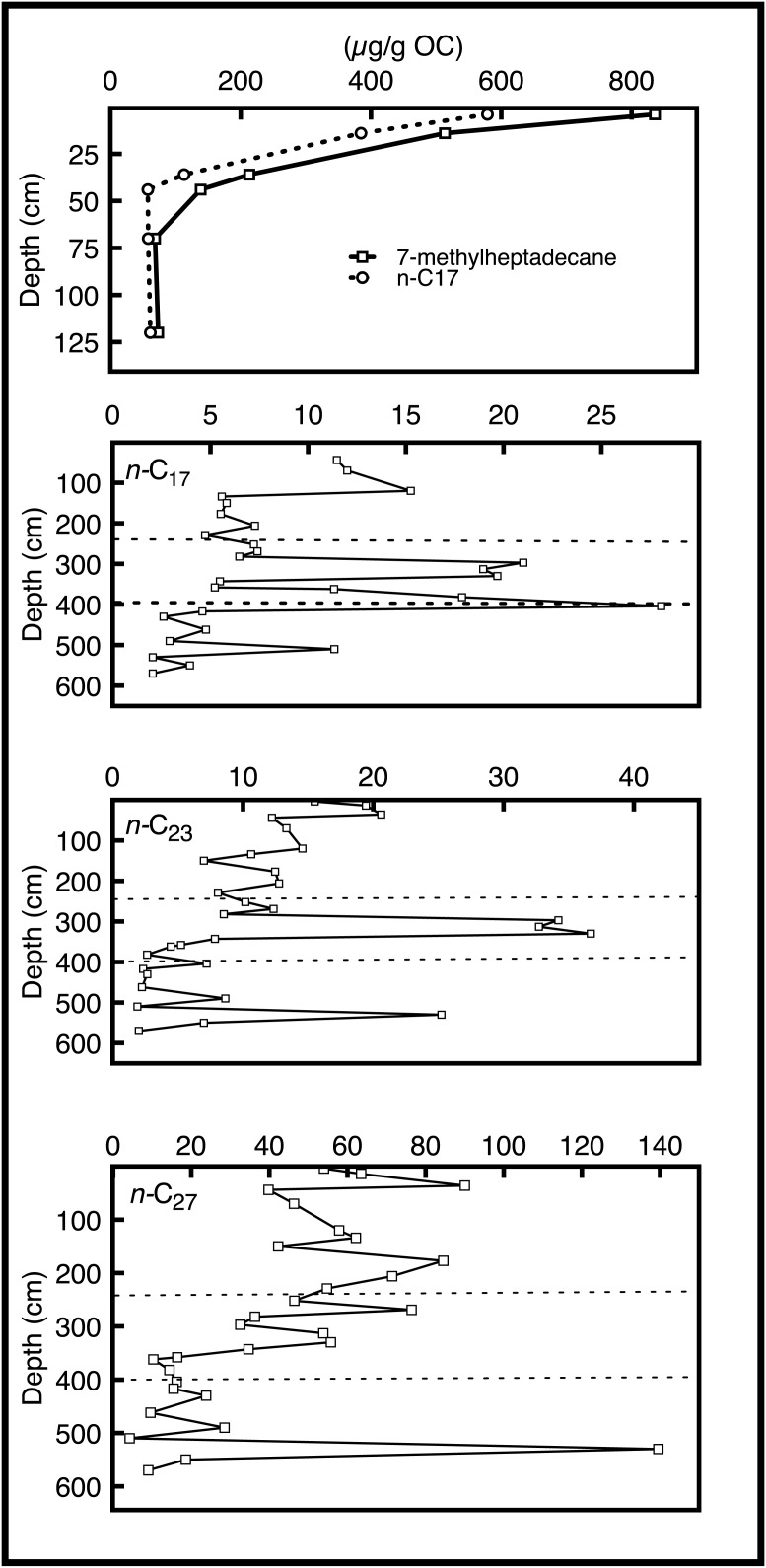
Concentrations for select n-alkane chain lengths are displayed in Fig 5. Mean n-alkane concentrations for these chain lengths are displayed in Table 1. The n-alkane concentrations were dominated by long-chain alkanes (i.e. >n-C25). The most abundant n-alkane in our record was n-C27, with an average concentration of 36.0 μg g-1 OC throughout the entire core. We calculated the average chain length (ACL) for all samples using the Eglinton and Hamilton [26] equation:
| (2) |
Fig 5. n-alkanes.
Select n-alkane chain length abundances versus depth in the Lake Harris core. Dashed lines delineate core zones 1–3.
Table 1. n-alkane homologues.
| n-alkane | C17 μg g-1 OC | C23 μg g-1 OC | C27 μg g-1 OC | C29 μg g-1 OC | C35 μg g-1 OC |
|---|---|---|---|---|---|
| Average | 34.1 | 10.0 | 36.1 | 30.3 | 7.0 |
| Max | 482.6 | 32.5 | 120.0 | 79.7 | 50.5 |
| Min | 0.0 | 0.8 | 1.7 | 1.7 | 0.0 |
| Range | 482.6 | 31.7 | 118.4 | 78.0 | 50.5 |
| Zone 1 Average | 81.4 | 11.2 | 51.0 | 40.2 | 8.1 |
| Zone 2 Average | 10.6 | 12.5 | 29.2 | 26.3 | 4.6 |
| Zone 3 Average | 1.2 | 5.0 | 25.2 | 22.3 | 8.8 |
Concentrations (average, maxima, minima, and ranges) for the five n-alkane homologues used for organic matter source interpretation. Zones are delineated by δ13CTOC values.
There was low variability in ACL throughout δ13CTOC zones 1 and 3 (ranges = 1.2 and 1.1, respectively), however ACL values in zone 2 vary by 2.8, with a high of 29.7 and a low of 26.9 (Table 2).
Table 2. Proxies for organic matter source.
| Depth (cm) | ACL | TARHC | Paq | Age | Dating Method |
|---|---|---|---|---|---|
| 4 | 28.72 | 0.15 | 0.47 | 2012 | 210Pb-AD |
| 14 | 29.67 | 0.44 | 0.29 | 1998 | 210Pb-AD |
| 36 | 29.27 | 2.56 | 0.30 | 1943 | 210Pb-AD |
| 44 | 28.99 | 6.62 | 0.38 | 1912 | 210Pb-AD |
| 70 | 29.17 | 6.93 | 0.30 | 900 | C14-yrs BP |
| 120 | 29.05 | 7.02 | 0.34 | 1920 | C14-yrs BP |
| 134 | 28.44 | 28.86 | 0.31 | 2400 | C14-yrs BP |
| 150 | 29.38 | 19.48 | 0.25 | 2780 | C14-yrs BP |
| 177 | 29.31 | 44.10 | 0.24 | 3570 | C14-yrs BP |
| 206 | 29.12 | 19.48 | 0.29 | 4770 | C14-yrs BP |
| 229 | 29.25 | 32.83 | 0.23 | 4840 | C14-yrs BP |
| 252 | 29.27 | 15.01 | 0.27 | 5200 | C14-yrs BP |
| 269 | 29.29 | 26.94 | 0.21 | 5450 | C14-yrs BP |
| 282 | 29.48 | 13.61 | 0.24 | 5620 | C14-yrs BP |
| 297 | 27.50 | 3.32 | 0.61 | 5840 | C14-yrs BP |
| 313 | 29.06 | 7.61 | 0.42 | 6050 | C14-yrs BP |
| 330 | 26.92 | 3.12 | 0.81 | 6250 | C14-yrs BP |
| 343 | 29.50 | 13.76 | 0.24 | 6430 | C14-yrs BP |
| 358 | 29.53 | 14.86 | 0.22 | 6740 | C14-yrs BP |
| 362 | 29.73 | 1.57 | 0.21 | 6830 | C14-yrs BP |
| 382 | 28.71 | 2.08 | 0.22 | 7770 | C14-yrs BP |
| 404 | 29.64 | 1.72 | 0.30 | 7850 | C14-yrs BP |
| 417 | 30.15 | 13.94 | 0.12 | 8020 | C14-yrs BP |
| 430 | 29.85 | 74.75 | 0.13 | 8170 | C14-yrs BP |
| 462 | 29.43 | 4.80 | 0.20 | 8480 | C14-yrs BP |
| 490 | 29.29 | 45.66 | 0.29 | 8830 | C14-yrs BP |
| 510 | 29.45 | 1.46 | 0.34 | 9300 | C14-yrs BP |
| 530 | 29.97 | 51.22 | 0.32 | 9470 | C14-yrs BP |
| 550 | 29.03 | 11.58 | 0.37 | 9830 | C14-yrs BP |
| 570 | 29.77 | n/a | 0.30 | 9900 | C14-yrs BP |
Average chain length (ACL) and the ratio of submerged to emergent vegetation (Paq) in the Lake Harris core. Dates determined by the 210Pb method are in Anno Domini (AD) and carbon-14 dated layers are in calibrated radiocarbon years before present (cal yrs BP).
Alkane concentrations showed differences across zones in the Lake Harris core (Fig 5). We subdivided alkanes into groups based upon their most probable source(s): cyanobacterial (7-methylheptadecane and diploptene), algal (n-C17), emergent/submerged macrophytes (n-C23), woody terrestrial vegetation (n-C27 and n-C29), and mixed woody terrestrial/C4 grasses (n-C35). We then related changes in these organic matter sources to regional environmental changes. In broadest terms, the following three zones were discernible based on relative alkane concentrations: zone 3) dominance of terrestrial biomarkers throughout the lower section of the core (below 390 cm), with a concentration maximum at 530 cm (~9,500 cal yrs BP), zone 2) a rapid increase in submerged macrophyte biomarker concentrations at 390 cm (~7,800 cal yrs BP), which persisted until 330 cm (~6,250 cal yrs BP), and zone 1) proliferation of algal and cyanobacterial biomarkers in the top 40 cm of the core (AD 1940 to present).
Specifically, zone 3 was dominated by terrestrial alkane inputs, with n-C27 and n-C29 concentrations ~5x that of n-C23 and ~20x that of n-C17. Paq values also indicate primarily terrestrial inputs in this zone. A sudden increase in concentrations of n-C23, n-C27, n-C29, and n-C35 occurs at 530 cm (~9,500 cal yrs BP). Both terrestrial hydrocarbon biomarkers, i.e. n-C27 and n-C29, attain maximum values at this depth.
In zone 2, the average concentration of n-C17 decreases to about 12% of its previous mean value and the terrestrial alkane concentrations are approximately halved, although they remain the most abundant chain lengths in this zone. Of note is an increase in n-C23 concentrations from 6.2 μg g-1 OC at 340 cm (~6,400 cal yrs BP) to 32.5 μg g-1 OC at 330 cm, which then dropped back down to 6.9 μg g-1 OC at 280 cm (~5,600 cal yrs BP). A similar, albeit smaller increase in n-C17 concentrations also occurred during this interval (Fig 5).
We evaluated the relative contributions of submerged and floating aquatic macrophytes to emergent terrestrial vegetation using the Paq proxy [27], described by the formula (C23 + C25)/(C23 + C25 + C29 + C31). Paq values from 330 cm (~6,250 cal yrs BP) to 290 cm (~5,700 cal yrs BP) ranged from 0.42 to 0.81, indicative of a freshwater, submerged vegetation source. This is the only section of the Lake Harris core that has Paq values that fall in the range of submerged vegetation.
Below 120 cm (~1,900 cal yrs BP) in zone 1, concentrations of n-C17 and 7-methylheptadecane were < 5 μg g-1 OC. In this lower section of zone 1, terrestrial biomarkers n-C27 and n-C29 were the most abundant hydrocarbons, with average concentrations of 52.3 and 43.3 μg g-1 OC, respectively. In the upper 40 cm of zone 1 (AD 1940 to present), however, there was a ~50-fold increase in the concentration of n-C17 and a ~40-fold increase in 7-methylheptadecane concentration.
Meyers [13] developed a hydrocarbon-based proxy, known as the terrestrial to aquatic ratio (TARHC), to distinguish between autochthonous and allochthonous sources of organic matter in sediments. TARHC results matched our comparative analyses of single-chain-length alkane concentrations (Table 2). Values <1 reflect dominance of aquatic hydrocarbons, and are only observed in the top 15 cm of our core, i.e. after AD 1998. TARHC are highly variable, but average 16.4, which indicates that contributions of carbon from terrestrial plants dominated the record. The maximum TARHC (74.8) was found at 530 cm (~9,500 cal yrs BP), whereas values <4 occur at 510 (~9,300 cal yrs BP), 400–360 (~7,800–6,750 cal yrs BP), 330–290 (~6,250–5,700 cal yrs BP), and 35 cm (AD 1945).
Isotopic compositions of selected n-alkane chain lengths are shown in Fig 6. Based upon n-alkane concentrations and organic matter source assignments, we interpreted isotope values for the following hydrocarbons in this study: 7-methylheptadecane, n-C17, n-C23, n-C27, and n-C29. Reliable carbon isotope ratios could not be measured on n-C17 and n-C23 below 415 cm depth.
Fig 6. n-alkane isotopic variability.
Carbon isotope variability in select n-alkane chain lengths in the Lake Harris core.
The carbon isotopes of 7-methylheptadecane had the highest average value for any biomarker in our core. From 45 cm to the sediment surface, the δ13C values of this branched alkane averaged -21.30‰, and varied by only 0.7‰ across those depths. The δ13C signature of n-C17 decreased progressively across all zones from a maximum value of -22.3‰ at 380 cm depth, to a minimum value of -29.9‰ at the top of the core. From 400 to 313 cm (~7,800–6,050 cal yrs BP) (zone 2), δ13C of n-C23 increased from -22.8‰ to -18.7‰, then stabilized at a mean value of -19.9‰ throughout the rest of zone 2. In zone 1, carbon isotope values of n-C23 decreased from a maximum of -19.73‰ to a minimum value of -26.30‰ at 4 cm depth (AD 2011). From the bottom of the core to 400 cm (the base of zone 2, ~7,800 cal yrs BP), δ13C values of vascular plant alkanes n-C27 and n-C29 both increase in a pattern that follows the increase in δ13CTOC. Carbon isotope values then decreased rapidly at 362 cm (~6,820 cal yrs BP) for n-C27, and at 400 cm for n-C29. These chain lengths returned relatively constant δ13C values in core zone 1, with average values of 24.8‰ and 28.0‰ for n-C27 and n-C29, respectively (Fig 6).
Discussion
Shifts in values of geochemical variables in the sediments of Lake Harris coincide with major environmental changes that occurred in the region during the Holocene. Hydrocarbon biomarker data indicate a series of hydrological shifts that resulted following Northern Hemisphere deglaciation. These shifts in Lake Harris are temporally linked to records from other lakes in north and central Florida [3, 27, 28]. Broadly speaking, Lake Harris evolved from a marsh-like system in the early Holocene, to a shallow lake in the middle Holocene, and then deepened to its modern state after ~2,800 years BP. Carbon isotope values of the autochthonous and allochthonous organic matter pools are proxies for these environmental changes and reflect multiple sources of inorganic carbon utilization by the primary producer communities.
Geochemical biomarkers
Biological sources of alkanes are described in numerous publications as reviewed in Bianchi and Canuel [11]. The n-C27/29 homologues of n-alkanes represent organic matter sourced from woody terrigenous plants [29]. C4 graminoids contain n-C35 alkanes in their leaves at concentrations an order of magnitude greater than those in woody C3 angiosperms [30, 31]. Submerged and emergent macrophytes typically produce alkanes with chain lengths of n-C21/23 [27], and algae synthesize alkanes with a predominant chain length of n-C17 [29]. Additionally, branched alkanes 7- and 8-methylheptadecane are produced exclusively by cyanobacteria and account for 90% of their total branched alkanes [32]. Although hydrocarbon biomarkers are source-specific, they represent only a small fraction of the total carbon content of bacteria and plants. Biomarker data gleaned from this study complement the bulk sediment carbon analyses that characterize much larger fractions of the organic matter.
The earliest portion of the core (zone 3) is dominated by n-alkanes derived from terrestrial and, to a lesser extent, macrophyte sources, as well as abundant sponge-spicule-derived biogenic silica. At a depth of 550 cm in the core, approximately 800 years after the lake began to fill with water in the early Holocene (10,600 cal yrs BP), there was a pulse of n-C23, n-C27, and n-C29 to the sediments (Fig 5). This occurred shortly after TOC:TN values increased from 24 to 39 at 574 cm (~10,000 cal yrs BP). TOC:TN values continued to increase toward maximum values, in agreement with the high abundances of terrestrial biomarkers. During this earliest stage in the lake’s evolution, it was a shallow, low-productivity, marsh-like system that received abundant vascular plant input from littoral communities (e.g. Taxodium spp., Salix spp. and Cephalanthus occidentalis), but also supported macrophyte growth. The temporal correlation between the TOC:TN, sponge spicules, and terrestrial hydrocarbon biomarkers is in strong agreement with palynological [33] and diatom-based paleolimnological reconstructions [34], all of which describe central Florida lakes as low-nutrient, shallow-water systems during the early Holocene.
Zone 2 is highlighted by localized maxima of algae and macrophyte biomarkers (Fig 5). The increase in n-C23 begins at 358 cm (~6,700 cal yrs BP), which corresponds to a shift from predominantly CaCO3 sediments to organic-rich sediments without measurable carbonate by 232 cm (~5,000 cal yrs BP) (Fig 3). The decrease in carbonate content in lake sediments coincided with decreases in proxy variables indicative of extensive macrophyte coverage in Lake Harris, e.g. sponge-derived biogenic silica, ACL values < 27, and average Paq values of 0.61 (Fig 3). Kenney et al. (2016) correlated these geochemical changes with the proliferation of Pinus pollen in Florida and the onset of wetter conditions in upland areas during the middle Holocene [3]. We infer that during this interval, hydrologic input to Lake Harris shifted from a predominantly groundwater source, early in zone 2, to primarily direct rainfall and runoff, later in zone 2.
Lake Panasoffkee, in central Florida, is a modern analogue for Lake Harris during the early to middle Holocene. Currently, Lake Panasoffkee receives substantial groundwater inputs and is a hard-water, macrophyte-dominated system. TOC:TN values (14–22) of submerged aquatic vegetation (SAV) in Lake Panasoffkee [35] are only slightly greater than the average TOC:TN value in zone 2 of the Lake Harris core (12.1). From this, we infer that sediment organic carbon in Lake Harris from ~8,000–5,000 cal yrs BP was sourced from a mixture of macrophytes and low-TOC:TN algae and periphyton. This inference is further supported by vegetation studies from shallow, low-nutrient lakes that have primary producer communities dominated by SAV [36]. The n-alkane biomarker concentrations, with maxima of n-C17 (at ~400 and 330 cm; ~7,800 and 6,250 cal yrs BP, respectively) and n-C23 (at ~330 cm; ~6,250 cal yrs BP), the sponge-derived silica concentrations, and the TOC:TN data from modern SAV, periphyton, and algae, coincide with one another in zone 2 of our core. Together they indicate a primarily groundwater-fed system with abundant submerged and emergent macrophyte populations.
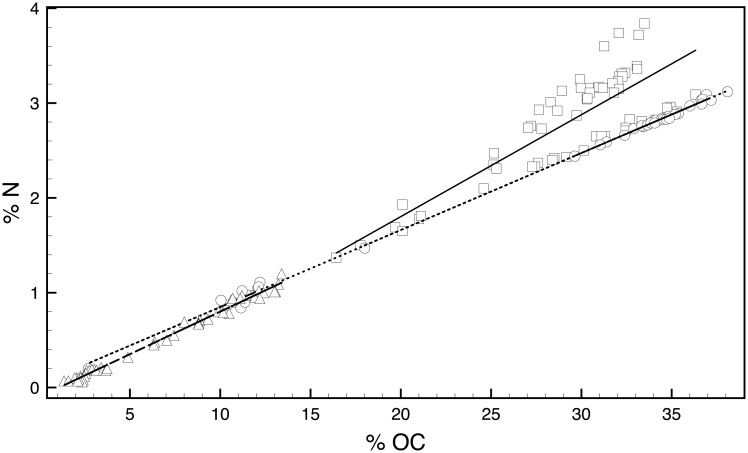
The largest shift in any of the hydrocarbon concentrations occurred in zone 1. Between about 240 cm (~5,000 cal yrs BP) and 40 cm (AD 1940), concentrations of biomarkers for the three major organic matter sources are fairly evenly distributed. Indeed, within that sediment depth range, combined algal and macrophyte n-alkane concentrations equate to approximately half of the terrestrial n-alkane concentrations. Terrestrial (n-C27) and macrophyte (n-C23) biomarker concentrations fluctuate between high and low values, but display no discernible trend across zone 1. In the top ~40 cm of the core, ca. AD 1940 to present, however, n-C17 and 7-methylheptadecane increased by about 50-fold and 40-fold, respectively. This rapid increase in algal and cyanobacterial biomarkers corresponds with the timing of an increase in trophic status of many other Florida lakes and a six-fold population increase in the counties surrounding the Harris Chain of Lakes after about AD 1940 [22]. The 7-methylheptadecane concentrations, in particular, are indicative of a highly eutrophic, possibly N-limited lake that supports cyanobacteria proliferation [37]. The decreasing trends in δ15N values (Fig 4), that approach 0‰, further support the relative increase in N-fixing cyanobacteria during the top of zone 1 [38]. In some ecosystems, the presence of inorganic nitrogen (e.g. ammonium) sorbed to clays can alter the δ15N signal. This is not applicable in Lake because its sediments lack clay and have high organic matter content. Furthermore, a plot of TOC vs TN is strongly coupled (r2 = 0.95), and the intercept of the regression line is nearly 0 (Fig 7). If inorganic nitrogen contributed to the sediment N pool, the intercept of Fig 7 would be >0. It is possible that the isotopic signature of organic matter is altered, in situ, but this alteration would have been minor and remained consistent throughout the record.
Fig 7. Plot of total organic carbon percent versus total nitrogen.
The plot is divided according to core zone: zone 1, squares with solid trend line (R2 = 0.72, b = -0.72), zone 2, circles with dashed trend line (R2 = 0.99, b = 0.03), and zone 3, triangles with dotted trend line (R2 = 0.99, b = -0.09). For all samples R2 = 0.95 and b = -0.09).
Trends in the biomarker data, however, are not fully supported by the bulk geochemical data. Whereas concentrations of algal and bacterial biomarkers are an order of magnitude greater than concentrations of any terrestrial biomarker throughout the top 40 cm of the core, the TOC:TN values do not display a decrease, as would be expected with an increase in non-vascular plant sources. Instead, TOC:TN values remain relatively stable throughout zone 1, with an average value of 10.2, nevertheless indicative of a primarily algal source [39]. Post-depositional alteration of TOC:TN values occurs in sediment pore waters [11], and decomposition can remove as much as 20% of the organic matter that is buried on the lake bottom [39, 40]. Kenney et al. [41] compared cores collected in 1999 and 2013 at the same location in Lake Harris and detected loss of organic matter from sediments < 50 years old during the ~13-year interval between core collection dates. Because Lake Harris is shallow and polymictic, organic matter can degrade in the water column year-round, which likely results in significant pre-depositional decay. In fact, in Lake Michigan, it was shown that only 6% of the autochthonously produced organic matter reached the sediment surface [42]. Although loss of sinking organic matter to heterotrophic oxidation in a shallow water body such as Lake Harris (3.5 m mean depth) is expected to be considerably less than in a deep lake like Lake Michigan (85 m mean depth), we can assume that some fraction is lost before burial in the Harris sediment. Overall, TOC:TN data are limited in their ability to reflect source information because of selective degradation and organic matter processing in the water column. Additionally, TOC:TN values in zone 1 have the weakest correlation, suggesting multiple N contributions to the lake during this period (Fig 7) [43]. Because the measured TOC:TN value of a sample reflects multiple organic matter sources and post-depositional processes, its utility for source inference is compromised relative to the hydrocarbon biomarker data.
δ13CTOC and hydrocarbon biomarkers
Measures of δ13CTOC fluctuate from low values in the earliest part of the core (~10,000–7,800 cal yrs BP) to the greatest values in the middle section (~7,800–5,000 cal yrs BP), and then return to lower values in the most recent section (~5,000 cal yrs BP to present) (Fig 2). This variability reflects, in part, the changing sources of carbon utilized by the primary producer communities. In the early Holocene, Lake Harris began to fill as the water table in the region started to rise in response to deglaciation and sea level rise [44]. Most lakes in Florida began to accumulate sediment at that time, but remained shallow marsh systems for millennia, as precipitation and water table elevations both stayed relatively low during the early Holocene [28, 45, 46]. The carbon isotope values of n-C27 show a stratigraphic pattern similar to that of δ13CTOC throughout zone 3. The δ13C values of n-C27, adjusted for the 4.4‰ depletion during alkane synthesis, plot within the isotopic range δ13CTOC values. The environmental reconstructions and carbon isotope data indicate that the major source of organic carbon to the lake sediments in zone 3 was from terrestrial plants that utilized atmospheric CO2.
As Florida gradually became wetter during the middle Holocene (between 7,000 and 5,500 cal yrs BP, zone 2 in our record) the sclerophyllous oak and open prairie plant communities were replaced by modern vegetation communities, largely dominated by pine forests, at least in upland sites [3, 47]. The first significant change in δ13CTOC values occurred at ~6,500 cal yrs BP (350 cm core depth) and corresponds to this environmental change. The increase in δ13CTOC by ~10‰ from zones 3 to 2 implies that a new source of inorganic carbon was being utilized by the primary producers. The average δ13CTOC value (14.8‰) is within the expected range of C4 vegetation, meaning a shift from C3 to C4 communities could also be a cause of the δ13CTOC increase. This, however, is improbable for two reasons. First, C4 plants are adapted to low pCO2 and arid environments [48], and the middle Holocene was a period of increasing precipitation and relatively high pCO2 [3, 49]. Second, our n-C35 record, which is a proxy for graminoid abundance [31], and the record of C3/C4 changes from nearby Lake Tulane [50], do not indicate an increase in the relative abundance of C4 plants during this time interval. It is also possible that the 13C enrichment was a consequence of decreased carbon isotope discrimination among phytoplankton under highly eutrophic conditions. This has been observed in several high-productivity lakes in Florida [15, 51], and has been attributed to recent cultural eutrophication in others [14]. If this were the cause of the higher δ13CTOC values, then Lake Harris would have been eutrophic during the middle Holocene, but Kenney et al. [18] demonstrated that nutrient accumulation was much lower at that time compared to modern nutrient accumulation rates.
The δ13CTOC record in zone 2 reflects a shift to a more 13C-enriched carbon source for the in-lake primary producer community. The δ13C value of n-C23 is the probable source for this enrichment, as: 1) this chain length exhibits the highest δ13C values across the depth interval 350–250 cm (~6,500–5,150 cal yrs BP), 2) these values, once corrected for the 3.2‰ offset during alkane synthesis, nearly overlap with the TOC isotope data, and 3) the n-C23 carbon isotope profile tracks the total organic carbon isotope data throughout zone 2. Lake Harris deepened as the modern hydrosphere developed. Algae initially filled this newly created niche. From 430 to 400 cm (~8,200–7,800 cal yrs BP) there was a ~15-fold increase in n-C17 concentrations. In Lake Griffin, which is part of the Harris Chain of Lakes, a similar mid-Holocene peak in algal abundance was recorded in the form of sedimented cyanotoxins [52]. We speculate that a relatively rapid rise in water levels initially supported phytoplankton populations, which later gave way to the proliferation of slower-growing macrophytes, the latter indicated by a ~30-fold increase in n-C23 contributions to the sediment at 380 to 330 cm (~7,700–6,250 cal yrs BP).
We propose two possible explanations for the 13C enrichment of the TOC. First, high CaCO3 concentrations in the sediment from the beginning of zone 3 to 350 cm (~6,500 cal yrs BP) indicate a well-buffered system with high pH (>9). Bicarbonate (HCO3-) is the major form of DIC at pH >9, with dissolved CO2 absent above pH ~8.3, and active uptake of this HCO3- would have enriched the phytoplankton and SAV biomass by 8‰ relative to CO2(aq) [53]. This could explain the high δ13C values measured in n-C23. Secondly, Brenner et al. [35] measured modern δ13C values in two SAV taxa (Vallisneria sp. and Potamogeton sp.) from nearby Lake Panasoffkee, and their δ13C range (-13.2‰ to -15.5‰) is enough to explain the elevated δ13C of the bulk organic matter in zone 2 of Lake Harris. The relatively high δ13C values in zone 2 suggest proliferation of Vallisneria sp. and Potamogeton sp. in Lake Harris during this time period.
From 250 cm core depth (~5,000 cal yrs BP) to the present (AD 2013) (zone 1), the organic matter becomes progressively depleted in 13C. This depletion coincides with a second stage of increased precipitation in Florida that occurred between 5,000 and 3,000 cal yrs BP [47]. Pollen-based reconstructions of summer precipitation show persistently wetter conditions after 3,000 cal yrs BP, which were related to the intensification of ENSO [46]. During that period, south Florida transitioned from a wet prairie to swamp forest environment [54]. Farther north, Quillen et al. [46] linked changes in benthic diatom assemblages to an increase in water depth in Lake Annie around the same time that Donders et al. [54] noted ENSO intensification. In nearby Lake Apopka, a shift from dominance of benthic diatoms (>50%) to dominance of planktonic diatoms (>50%) ~2,800 cal yrs BP indicated that the system had increased in depth sufficiently to approximate its modern limnetic state [28]. Geochemical proxies in the Lake Harris core indicate it also achieved its modern water depth around that time. Organic matter abundance stabilized at levels >50% and CaCO3 dropped to trace levels after ~4,800 cal yrs BP (Fig 3). Diatom biogenic silica concentrations exceeded sponge-derived silica concentrations at ~2,800 cal yrs BP. Together these sediment variables reflect a transition from a primarily shallow, groundwater-fed lake, to a relatively deep lake, whose depth was controlled by precipitation and/or surface water inputs.
Our n-alkane data also support this interpretation. The more negative δ13CTOC values in zone 1 results from increased algal contributions to the sediment. The algal proxy n-C17 has the most depleted δ13C values among all n-alkanes analyzed, and the transition to more negative values in the bulk isotope data signifies the rising contribution of algae to the organic matter pool. Interestingly, this is the opposite trend observed by Filley et al. [17] in Mud Lake, Marion County, Florida. In that study, the greatest δ13C values were recorded in n-C17. We argue that the photic zone to water column ratio is larger in Mud Lake (mean depth = 1 m) than in Lake Harris (mean depth = 3.5 m), and therefore isotopic discrimination by the primary producers has a greater impact on increasing the δ13CTOC value. The dissolved inorganic carbon (DIC) pool in Lake Harris is larger, because it is deeper, thus primary productivity has a smaller impact on the isotopic signature of the DIC pool.
The cyanobacteria biomarker 7-methylheptadecane is the biomarker in highest abundance in the top 35 cm (AD ~1940-present) of the core. It also exhibits the highest average δ13C values among all hydrocarbons in zone 1. Despite this, the δ13CTOC values do not become more positive up-core, even though greater δ13CTOC values would be expected as the lake became more eutrophic and cyanobacterial blooms thrived [14]. The average δ13C values of 7-methylheptadecane (-21.3‰) are nearly identical to values measured in the top 30 cm of Mud Lake (-21.9‰) by Filley et al. [17]. Diagenetic alteration of the cyanobacteria signal is unlikely, as these compounds are present in abundant concentrations in our record, and are major components in the surface sediments of other Florida lakes [52, 55]. Our data show that diatom silica accounts for more than 10% of sediment mass in zone 1, so the discrepancy between δ13CTOC and the δ13C of 7-methylheptadecane is probably a result of high productivity of diatoms and other algae in Lake Harris.
Conclusions
The evolution of Lake Harris is recorded in the geochemical variables preserved within its sediments. The lake began to fill with water ~10,000 cal yrs BP, when many lakes in Florida began to accumulate sediment in response to wetter conditions caused by deglaciation and eustatic sea level rise. From the bottom of the core to 403 cm depth (~7,800 cal yrs BP), terrestrial carbon inputs dominated the record, with limited input from macrophytes and algae, and the primary carbon source was atmospheric CO2. Throughout the early Holocene, Lake Harris was a marsh-like system in a relatively dry, open-prairie environment. A rapid, positive shift in δ13CTOC values at 402 cm (~7,800 cal yrs BP), and stabilization of these values at 350 cm (~6,500 cal yrs BP), represents the onset of wetter conditions in Florida. High CaCO3 concentrations in the deepest sediments indicate that Lake Harris was fed primarily by groundwater, and isotopic values of n-alkanes and bulk organic matter suggest that organic matter was derived primarily from macrophytes that utilize 13C-enriched HCO3- as their carbon source. Alternatively, higher δ13CTOC values after ~7,000 cal yrs BP may have resulted from input of Vallisneria sp., with its higher δ13C signature. It was during that time, between 7,000 and 5,000 cal yrs BP, that Florida uplands transitioned from dry oak scrub vegetation to pine forests. Above 240 cm core depth (~5,000 cal yrs BP), δ13CTOC values begin to decline, CaCO3 concentrations in the sediments decrease to below trace levels, and organic carbon concentrations increase. Around 3,000 cal yrs BP the effects of ENSO intensified and many Florida lakes deepened to their current limnetic state. This is observed in Lake Harris ~2,900 cal yrs BP, when diatom biogenic silica concentrations increased from 10 to 120 mg g-1. Concentrations of algal and cyanobacteria biomarkers increased by orders of magnitude after about AD 1940 in response to human-induced eutrophication.
Supporting information
(XLSX)
(XLSX)
Acknowledgments
We thank the Water Institute and the Land Use and Environmental Change Institute at the University of Florida for funding this project, and Gianna Browne for sample preparations. We thank Dr. Andrea Dutton for valuable comments on the manuscript.
Data Availability
All relevant data are within the paper and its supporting files.
Funding Statement
We received funding from the Water Institute and the Land Use and Environmental Change Institute at the University of Florida.
References
- 1.Dean WE, Gorham E (1998) Magnitude and significance of carbon burial in lakes, reservoirs, and peatlands. Geology 26:535–538. [Google Scholar]
- 2.Tranvik LJ, Downing JA, Cotner JB, Loiselle SA, Striegl RG, Ballatore TJ, et al. (2009). Lakes and reservoirs as regulators of carbon cycling and climate. Limnology and Oceanography 54: 2298–2314. [Google Scholar]
- 3.Watts WA, Hansen BCS (1994) Pre-Holocene and Holocene pollen records of vegetation history from the Florida peninsula and their climatic implications. Palaeogeogr Palaeoclimatol Palaeoecol 109:163–176. [Google Scholar]
- 4.Castañeda IS, Schouten S (2011) A review of molecular organic proxies for examining modern and ancient lacustrine environments. Quat Sci Rev, 30:2851–2891. [Google Scholar]
- 5.Meyers PA (1994) Preservation of source identification of sedimentary organic matter during and after deposition. Chem Geol 144:289–302. [Google Scholar]
- 6.Silliman JE, Meyers PA, Bourbonniere RA (1996) Record of postglacial organic matter delivery and burial in sediments of Lake Ontario. Org Geochem 24:463–472. [Google Scholar]
- 7.Meyers PA, Ishiwatari R (1993) Lacustrine organic geochemistry—an overview of indicators of organic matter sources and diagenesis in lake sediments. Org Geochem 20:867–900. [Google Scholar]
- 8.O’Leary MH (1988) Carbon isotopes in photosynthesis. Bioscience, 38:328–336. [Google Scholar]
- 9.Farquhar GD, Ehleringer JR, Hubick KT (1989) Carbon isotope discrimination and photosynthesis. Annu Rev Plant Biol 40:503–537. [Google Scholar]
- 10.Cloern JE, Canuel EA, Harris D (2002) Stable carbon and nitrogen isotope composition of aquatic and terrestrial plants of the San Francisco Bay estuarine system. Limnol Oceanogr 47:713–729. [Google Scholar]
- 11.Bianchi TS, Canuel EA (2011) Chemical biomarkers in aquatic ecosystems. Princeton University Press. [Google Scholar]
- 12.Aller RC (1994) Bioturbation and remineralization of sedimentary organic matter: effects of redox oscillation. Chem Geol 11:331–345. [Google Scholar]
- 13.Meyers PA (1997) Organic geochemical proxies of paleoceanographic, paleolimnologic, and paleoclimatic processes. Org Geochem 27:213–250. [Google Scholar]
- 14.Brenner M, Whitmore TJ, Curtis JH, Hodell DA, Schelske CL (1999) Stable isotope (δ13C and δ15N) signatures of sedimented organic matter as indicators of historic lake trophic state. J Paleolimnol 22:205–221. [Google Scholar]
- 15.Torres IC, Inglett PW, Brenner M, Kenney WF, Reddy KR (2012) Stable isotope (δ13C and δ15N) values of sediment organic matter in subtropical lakes of different trophic status. J Paleolimnol 47:693–706. [Google Scholar]
- 16.Bianchi TS, Mitra S, McKee BA (2002) Sources of terrestrially-derived organic carbon in lower Mississippi River and Louisiana shelf sediments: implications for differential sedimentation and transport at the coastal margin. Mar Chem 77:211–223. [Google Scholar]
- 17.Filley TR, Freeman KH, Bianchi TS, Baskaran M, Colarusso L, Hatcher PG (2001) An isotopic biogeochemical assessment of shifts in organic matter input to Holocene sediments from Mud Lake, Florida. Org Geochem 32:1153–1167. [Google Scholar]
- 18.Kenney WF, Brenner M, Curtis JH, Arnold TE, Schelske CL (2016) A Holocene Sediment Record of Phosphorus Accumulation in Shallow Lake Harris, Florida (USA) Offers New Perspectives on Recent Cultural Eutrophication. PLOS One 11:e0147331 doi: 10.1371/journal.pone.0147331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Törnqvist TE, González JL, Newsom LA, van der Borg K, de Jong AF, Kurnik CW (2004) Deciphering Holocene sea-level history on the US Gulf Coast: A high-resolution record from the Mississippi Delta. Geol Soc Am Bull 116: 1026–1039. [Google Scholar]
- 20.Willard DA, Bernhardt CE (2011) Impacts of past climate and sea level change on Everglades wetlands: placing a century of anthropogenic change into a late-Holocene context. Climatic Change 107:59–80. [Google Scholar]
- 21.Donders TH, Wagner-Cremer F, Visscher H (2008) Integration of proxy data and model scenarios for the mid-Holocene onset of modern ENSO variability. Quat Sci Rev 27:571–579. [Google Scholar]
- 22.Fulton RS III, Smith D (2008) Development of phosphorus load reduction goals for seven lakes in the upper Ocklawaha river basin, Florida. Lake and Res Manag 24:139–154 [Google Scholar]
- 23.Kenney WF, Brenner M, Curtis JH, Schelske CL (2010). Identifying sources of organic matter in sediments of shallow lakes using multiple geochemical variables. J Paleolimnol 44:1039–1052. [Google Scholar]
- 24.Nichols JE (2011) Procedures for extraction and purification of leaf wax biomarkers from peats. Mires Peat 7:1–7. [Google Scholar]
- 25.Polissar PJ, D’Andrea WJ (2014) Uncertainty in paleohydrologic reconstructions from molecular δD values. Geochim Cosmochim Ac 129:146–156. [Google Scholar]
- 26.Eglinton G, Hamilton RJ (1967) Leaf epicuticular waxes. Science 156:1322–1335. [DOI] [PubMed] [Google Scholar]
- 27.Ficken KJ, Li B, Swain DL, Eglinton G (2000) An n-alkane proxy for the sedimentary input of submerged/floating freshwater aquatic macrophytes. Org Geochem geochemistry 31: 745–749. [Google Scholar]
- 28.Donar C, Stoermer EF, Brenner M (2009) The Holocene paleolimnology of Lake Apopka, Florida. Nova Hedwigia 135:57–70. [Google Scholar]
- 29.Cranwell PA (1982) Lipids of aquatic sediments and sedimenting particulates. Prog Lipid Res 21:271–308. [DOI] [PubMed] [Google Scholar]
- 30.Garcin Y, Schefuß E, Schwab VF, Garreta V, Gleixner G, Vincens A et al. (2014) Reconstructing C 3 and C 4 vegetation cover using n-alkane carbon isotope ratios in recent lake sediments from Cameroon, Western Central Africa. Geochim Cosmochim Ac 142:482–500. [Google Scholar]
- 31.Diefendorf AF, Freimuth EJ (2017) Extracting the most from terrestrial plant-derived n-alkyl lipids and their carbon isotopes from the sedimentary record: A review. Org Geochem 103:1–21. [Google Scholar]
- 32.Han J, Calvin M (1969) Hydrocarbon distribution of algae and bacteria, and microbiological activity in sediments. P Natl Acad Sci USA 64:436–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Watts WA (1975) A late Quaternary record of vegetation from Lake Annie, south-central Florida. Geology 3:344–346. [Google Scholar]
- 34.Quillen, A. K. (2009). Diatom-based paleolimnological reconstruction of quaternary environments in a Florida Sinkhole Lake. Florida International University, Electronic Theses and Dissertations. Miami, Florida, USA.
- 35.Brenner M, Hodell DA, Leyden BW, Curtis JH, Kenney WF, Gu B et al. (2006). Mechanisms for organic matter and phosphorus burial in sedimentsof a shallow, subtropical, macrophyte-dominated lake. J Paleolimnol 35:129–148. [Google Scholar]
- 36.Schelsk CL, Lowe EF, Battoe LE, Brenner M, Coveney MF, Kenney WF (2005) Abrupt biological response to hydrologic and land-use changes in Lake Apopka, Florida, USA. AMBIO 34:192–198. [PubMed] [Google Scholar]
- 37.Dolman AM, Rücker J, Pick FR, Fastner J, Rohrlack T, Mischke U, et al. (2012) Cyanobacteria and cyanotoxins: the influence of nitrogen versus phosphorus. PlOS one 7:e38757 doi: 10.1371/journal.pone.0038757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Flett R J, Schindler DW, Hamilton RD, Campbell NE (1980). Nitrogen fixation in Canadian Precambrian shield lakes. Can J Fish Aquat Sci 37:494–505. [Google Scholar]
- 39.Meyers PA (2003) Applications of organic geochemistry to paleolimnological reconstructions: a summary of examples from the Laurentian Great Lakes. Org Geochem 34:261–289. [Google Scholar]
- 40.Gälman V, Rydberg J, de-Luna SS, Bindler R, Renberg I (2008) Carbon and nitrogen loss rates during aging of lake sediment: Changes over 27 years studied in varved lake sediment. Limnol Oceanogr 53:1076–1082. [Google Scholar]
- 41.Kenney WF, Brenner M, Arnold TE, Curtis JH, Schelske CL (2016) Sediment cores from shallow lakes preserve reliable informative paleoenvironmental archives despite hurricane-force winds. Ecol Indic 60:963–969. [Google Scholar]
- 42.Eadie BJ, Chambers RL, Gardner WS, Bell GL (1984) Sediment trap studies in Lake Michigan: Resuspension and chemical fluxes in the southern basin. J Great Lake Res 10:307–321. [Google Scholar]
- 43.Fulton JM, Arthur MA, Freeman KH (2012). Black Sea nitrogen cycling and the preservation of phytoplankton δ15N signals during the Holocene. Global Biogeochem Cycles 26:1–15. [Google Scholar]
- 44.Watts WA, Stuiver M (1980) Late Wisconsin climate of northern Florida and the origin of species-rich deciduous forest. Science 210:325–327. doi: 10.1126/science.210.4467.325 [DOI] [PubMed] [Google Scholar]
- 45.Watts W. A. (1971). Postglacial and interglacial vegetation history of southern Georgia and central Florida. Ecology 52:676–690. doi: 10.2307/1934159 [DOI] [PubMed] [Google Scholar]
- 46.Quillen AK, Gaiser EE, Grimm EC (2013) Diatom-based paleolimnological reconstruction of regional climate and local land-use change from a protected sinkhole lake in southern Florida, USA. J Paleolimnol 49: 15–30. [Google Scholar]
- 47.Donders TH (2014) Middle Holocene humidity increase in Florida: Climate or sea-level? Quat Sci Rev 103:170–174. [Google Scholar]
- 48.Ehleringer JR, Cerling TE, Helliker BR (1997) C4 photosynthesis, atmospheric CO2, and climate. Oecologia, 112:285–299. doi: 10.1007/s004420050311 [DOI] [PubMed] [Google Scholar]
- 49.Broecker W S, Stocker T F (2006). The Holocene CO2 rise: anthropogenic or natural? Eos, 87: 27–29. [Google Scholar]
- 50.Huang Y, Shuman B, Wang Y, Webb T, Grimm EC, Jacobson GL (2006) Climatic and environmental controls on the variation of C 3 and C 4 plant abundances in central Florida for the past 62,000 years. Palaeogeogr Palaeoclimatol Palaeoecol 237:428–435. [Google Scholar]
- 51.Gu B, Chapman AD, Schelske CL (2006) Factors controlling seasonal variations in stable isotope composition of particulate organic matter in a soft water eutrophic lake. Limnol Oceanogr 51:2837–2848. [Google Scholar]
- 52.Waters MN (2016) A 4700-year history of cyanobacteria toxin production in a shallow subtropical lake. Ecosystems 19:426–436. [Google Scholar]
- 53.Mook WG, Bommerson JC, Staverman WH (1974) Carbon isotope fractionation between dissolved bicarbonate and gaseous carbon dioxide. Earth Planet Sc Lett, 22:169–176. [Google Scholar]
- 54.Donders TH, Wagner F, Dilcher DL, Visscher H (2005) Mid-to late-Holocene El Nino-Southern Oscillation dynamics reflected in the subtropical terrestrial realm. P Natl Acad Sci USA 102:10904–10908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Riedinger-Whitmore MA, Whitmore TJ., Smoak JM, Brenner M, Moore A, Curtis J, Schelske CL (2005). Cyanobacterial proliferation is a recent response to eutrophication in many Florida lakes: a paleolimnological assessment. Lake Reservoir Manag, 21:423–435. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
(XLSX)
Data Availability Statement
All relevant data are within the paper and its supporting files.