Summary
Neurologists often evaluate patients whose symptoms cannot be readily explained even after thorough clinical and diagnostic testing. Such medically unexplained symptoms are common, occurring at a rate of 10%–30% among several specialties. These patients are frequently diagnosed as having somatoform, functional, factitious, or conversion disorders. Features of these disorders may include symptom exaggeration and inadequate effort. Symptom validity tests (SVTs) used by psychologists when assessing the validity of symptoms and impairments are structured, validated, and objectively scored. They could detect poor effort, underperformance, and exaggeration. In settings with appropriate prior probabilities, detection rates for symptom exaggeration have diagnostic utility. SVTs may help in moderating expensive diagnostic testing and redirecting treatment plans. This article familiarizes practicing neurologists with their merits, shortcomings, utility, and applicability in practice.
Neurologists often encounter patients who exhibit symptoms that are not readily explained by traditional clinical and diagnostic evaluations. Commonly reported symptoms in this category are pain, cognitive changes, altered awareness, and weakness. Medically unexplained symptoms (MUS) are a broad category of related systemic phenomena that encompasses somatoform disorders, conversion disorders, functional disorders, symptom exaggeration, feigning, and malingering. Estimates of the prevalence of MUS in general neurologic practice range from 10% to 30%.1 This prevalence varies depending on the clinical setting: it is typically highest in compensation-seeking cases and lowest in nonlitigating general medical populations.2 The diagnosis of MUS is largely reliant on patients' self-report. Therefore, it may include exaggeration of symptoms beyond those likely or expected based on physical findings. While the related term “malingering” requires an intentional exaggeration or manufacture of symptoms for secondary gain, the terms “symptom exaggeration” or “inadequate effort” make no such presumption of intent.3 In practice, it is exceedingly difficult to assess intent; therefore, we prefer the terms MUS or symptom exaggeration in this article. Although use of these interchangeable terminologies varies among specialties, the core descriptive and uniting characteristics are similar, namely, a set of medically unlikely or inexplicable symptoms. Despite over a century of study, neurologic MUS remain complex and poorly understood, with multiple explanatory models and theories.4,5
When evaluating patients with hard-to-explain symptoms, it is difficult to be confident about the absence of an organic basis, even after diagnostic testing. Smith and Jozefowicz6 provide a helpful list of symptoms and bedside diagnostic findings that may identify somatoform disorders. However, a recent systematic narrative review suggests that many commonly accepted clinical signs of conversion disorder are of limited diagnostic utility.7 Thus, the practicing neurologist could benefit from any means that increases the confidence about the presence of a somatoform, or functional disorder. In such instances, neuropsychological testing often provides ancillary help. If these tests also indicate the probability of symptom exaggeration, then the symptoms may likely be due to a nonorganic etiology. Such an attribution could reduce the need for further expensive testing and follow-up.
For a clinician to accurately assess a patient's impairments, the patient must perform to the best of his or her ability, and respond accurately to questions regarding symptoms. Neuropsychologists and forensic psychologists frequently utilize a variety of standardized symptom validity tests (SVTs) in their assessments. SVTs are designed to detect if there may be an exaggeration of symptoms and impairments, including exaggerated cognitive, memory, motor, and psychiatric symptoms. Even patients with mild cognitive impairment (MCI) typically show little difficulty on these tasks. Patients with a need to exaggerate, however, are likely to perform worse than patients with documented injuries; some even perform at a below chance level. Their performance curves may also exhibit a pattern that indicates exaggeration. SVT results may affirm or negate the clinician's prior impression regarding the origins of the unexplained symptoms. Familiarity with these tests will assist neurologists with the interpretation of neuropsychological test reports and with subsequent management of patients with MUS.
Neurologic symptoms that come under the category of MUS
MUS are relatively common across all medical specialties: for instance, irritable bowel syndrome in gastroenterology and pelvic pain in urology.6,8,9 It is estimated that up to 40% of medical office visits are for symptoms without adequate medical foundation, and such patients require disproportionately greater physician time and a considerable share of health care resources.10 Their poor response to most interventions is a source of frustration both to them and their physicians.11
Memory and cognitive difficulties, chronic pain, altered consciousness, fatigue, and weakness are some of the leading neurologic complaints among patients with MUS. There is some evidence from the literature that diagnoses that are based primarily on symptom report may be more susceptible to distortion by the patient. Examples include postconcussion syndrome and complex regional pain syndrome.12–14 Stone et al15 reviewed the records of several thousand patients admitted to a consultation neurology service between 2002 and 2004. Presenting symptoms included a variety of complaints such as paralysis, weakness, and blurred vision. Approximately 12% of patients had symptoms that were either “not at all” or “somewhat” explained by disease. Patients whose symptoms were medically unexplained differed from other patients in important ways: they reported more pain and neurologic and psychiatric symptoms than patients whose symptoms were explained by disease. Similar studies1 have found rates of MUS to be approximately 30% in a general neurologic population. On follow-up for 18 months, fewer than 1% of 1,114 neurologic MUS patients were diagnosed with an organic brain disorder. Thus, MUS is a relatively common, and possibly stable, finding in a typical neurologic practice, with important health care cost and practice implications.
SVT in the psychological evaluation of MUS patients
Referrals to neuropsychologists for the assessment of neurologic MUS are common. Neuropsychologists and forensic psychologists frequently evaluate patients where the prevalence of symptom exaggeration is high, such as patients seeking a legal determination of injury or disability (e.g., Social Security disability, workers' compensation, personal injury). The term MUS is not generally used by neuropsychologists. Instead, they typically use terms such as inadequate effort and symptom exaggeration to describe unexpectedly poor neuropsychological test performance or symptoms disproportionate to the underlying injury. Psychological testing for MUS is broadly termed SVT or effort testing. The leading association of American Neuropsychologists (the American Academy of Clinical Neuropsychology) recommends routine use of SVTs throughout the neuropsychological assessment. Whereas the word “effort” denotes motor effort for neurologists, in SVT it refers to whether the patient is putting forth maximal cognitive or motoric effort to perform the required tests.
Failure in SVT denotes a performance score below a certain threshold. It has significant value as an ancillary diagnostic aid for MUS. SVT tests utilize several strategies that make them useful in detecting neurologic symptom exaggeration or MUS. These include the following:
Responding at a below chance level. This is a strong indicator of poor effort. For example, a purely chance score on a 100-item, true-false test is 50. A patient's score well below chance level (25%, for instance) suggests a deliberate selection of incorrect answers.
Abnormally poor performance on easy test items combined with a chance-only performance on extremely difficult items is worthy of note. This type of performance curve is an indicator of symptom exaggeration.
Performance on most SVTs is designed to be relatively unaffected by mild to moderate cognitive or neurologic deficits. In testing, this is termed a floor effect.16 When patients with MUS perform worse than patients with moderate/severe injuries, it is suggestive of exaggeration.
See the glossary for an explanation of terms.
Types of SVTs
Although clinicians may use many informal tests of symptom validity and consistency (e.g., Waddell signs, coin-in-hand test), such tests frequently have a limited empirical base and uncertain validity.17 In contrast, SVTs used in neuropsychology are objectively scored; they have empirically based norms and demonstrated validity with clinical populations.13,18 Recent texts by Rogers3 and Carone and Bush19 are comprehensive resources on this topic.
There are 2 general categories of SVTs: cognitive and psychological. Extensive research demonstrates that failure on cognitive SVTs is associated with impaired performance across nearly all cognitive and psychological domains, including learning, memory, executive functioning, and motor performance. Similarly, patients failing psychological SVTs report significantly worse pain and psychiatric symptoms (e.g., depression, anxiety). In both cases, patients who fail SVTs generally report greater impairment and more disabling symptoms than patients with documented neurologic injuries. Whereas our focus in this article is primarily on MUS involving cognitive symptoms (e.g., those found in mild traumatic brain injury [mTBI] or postconcussion syndrome), SVTs can be of use in sensory and motor functional disorders. For instance, Heintz et al20 found that patients with functional tremor performed worse than organic patients on SVTs.
One example of a common cognitive SVT test is the Test of Memory Malingering (TOMM).21 The TOMM takes advantage of the fact that human visual recognition memory is extraordinarily robust, and is relatively unimpaired in most neurologic patients.22 In this test, the patient is briefly presented with a series of 50 line drawings. Next, in a recognition trial, they are asked to choose the drawings they have previously seen from a group of 3 distractors. Even patients with documented mild traumatic brain injury (mTBI) or MCI generally score at or near 100%.23 Subjects who recognize 45 or fewer drawings are considered to have failed this SVT. The utility of the TOMM is not that it is a test of visual memory; it is not, since even impaired neurologic patients do well on it. Rather, it has face validity, and appears relevant to the unsophisticated patient. This superficial validity presumably leads the patient with MUS to distort their true ability. Its empirical usefulness lies in the fact that patients who fail SVT tests like the TOMM perform differently than valid neurologic patients in a variety of ways. These include (1) worse performance on most cognitive measures of visual and verbal memory, concentration, IQ, and executive functioning24; (2) more severe symptoms on measures associated with neurologic injury and concussion (e.g., concentration, headache, memory problems, pain); and (3) greater self-report of current functional impairment, combined with idealized reports of premorbid functioning.25
The TOMM is a somewhat older SVT test. Newer, computer-based tests often use multiple strategies to detect symptom exaggeration and inadequate effort. Such tests include the Word Memory Test (WMT)26 and the Validity Indicator Profile (VIP),27 which utilize more sophisticated strategies such as below chance responding and the performance curve. The figure illustrates the essential concept behind an early visual memory test that is seldom used now.
The Rey 15-item test
Figure. An early test for memory malingering (Rey, 1958). After a 10-second presentation of this card, the patient is asked to draw as many items as possible from memory. Although seemingly difficult because it contains 15 items, the task is actually simple because the logical progression of stimuli makes them very easy to remember. The total number of items recalled, with a cutoff of ⩽7, shows high specificity but low sensitivity for symptom exaggeration. Although more sophisticated symptom validity tools have replaced this test, we show this figure as a prototype of visual memory testing.
Limitations of SVTs
SVTs are not infallible. Because of the danger of potentially misidentifying a patient with valid symptoms, the cutoff values for SVTs are deliberately designed to have high specificity; that is, only a small portion of true patients making valid effort should fail. Research suggests most SVTs are roughly 90% specific, which leaves open the possibility of a 10% rate of false-positives. The tradeoff for good specificity is only moderate sensitivity: SVT tests detect approximately 60%–80% of patients demonstrating inadequate effort.3 Thus, SVTs are not a panacea for the detection of MUS; they are subject to the same limitations as other medical or psychological diagnostic tests.
The classification utility of SVTs varies (as do other tests) with the disease prevalence in the population tested (table 1). If the prevalence of MUS in a clinical population is very low (e.g., 10%), then SVT testing may not be helpful. On the other hand, if the prevalence is 40% (not unusual in compensation-seeking populations), SVT tests can be used individually or combined to provide strong clinical confidence in their results. Neurologists can maximize the utility of SVT testing by either relying on empirically established prevalence rates1,2 or by employing their own locally based experiential a priori assumptions in calculating results.
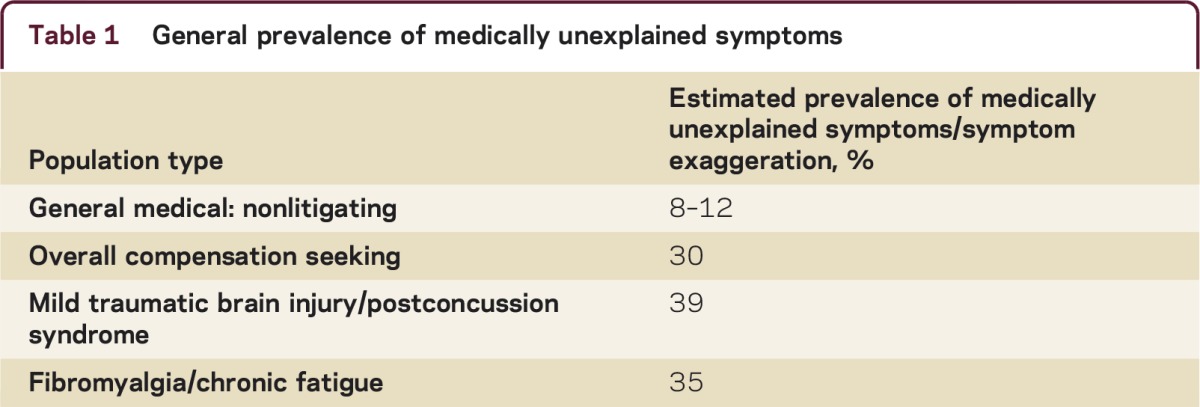
Table 1 General prevalence of medically unexplained symptoms
Utilizing SVT results when evaluating MUS
SVTs can be used in multiple ways: most are stand-alone, and can be given either individually or as part of a broader neurologic or neuropsychological evaluation. Many of the newer SVTs (e.g., the WMT and VIP) can be administered by computer in an office location.
Familiarity with the neuropsychologist's vocabulary will aid the neurologist when reviewing a neuropsychological report, especially regarding concerns about MUS, symptom exaggeration, or inadequate effort. Neuropsychologists are often guarded in their description of SVT failure, due to the difficulty in ascertaining the patient's motivation for failing an SVT, and concern about labeling a patient as a potential malingerer. They frequently use alternative, equivalent terms such as inadequate effort, underperformance, negative response distortion, symptom exaggeration, and, of course, SVT failure. All of these should be red flags for the neurologist to consider dialogue with the neuropsychologist to discuss implications of the SVT failure when formulating diagnosis and treatment.
There are few established guidance documents on how the neurologist should react to a patient who significantly fails SVTs. If used early during an anticipated extensive neurologic evaluation, an SVT failure will reduce the reliability of a patient's reported history, current symptoms, and neuropsychological testing results. At the very least, following SVT failure the neurologist should primarily rely on more objective tests that are less likely to be affected by alterations in effort or self-report. Within an appropriate patient population, SVTs can provide useful information as an integral part of a thorough neurologic evaluation.
Even if the patient with MUS fails SVTs, the neurologist must remain sensitive to the patient's perceived distress. Along with a thorough neurologic assessment, the physician should be sure to inquire about psychosocial stressors, including family issues, physical and sexual abuse, anxiety, and depressive symptoms. It is also important to obtain a current history regarding potential disability, compensation, and litigation issues.
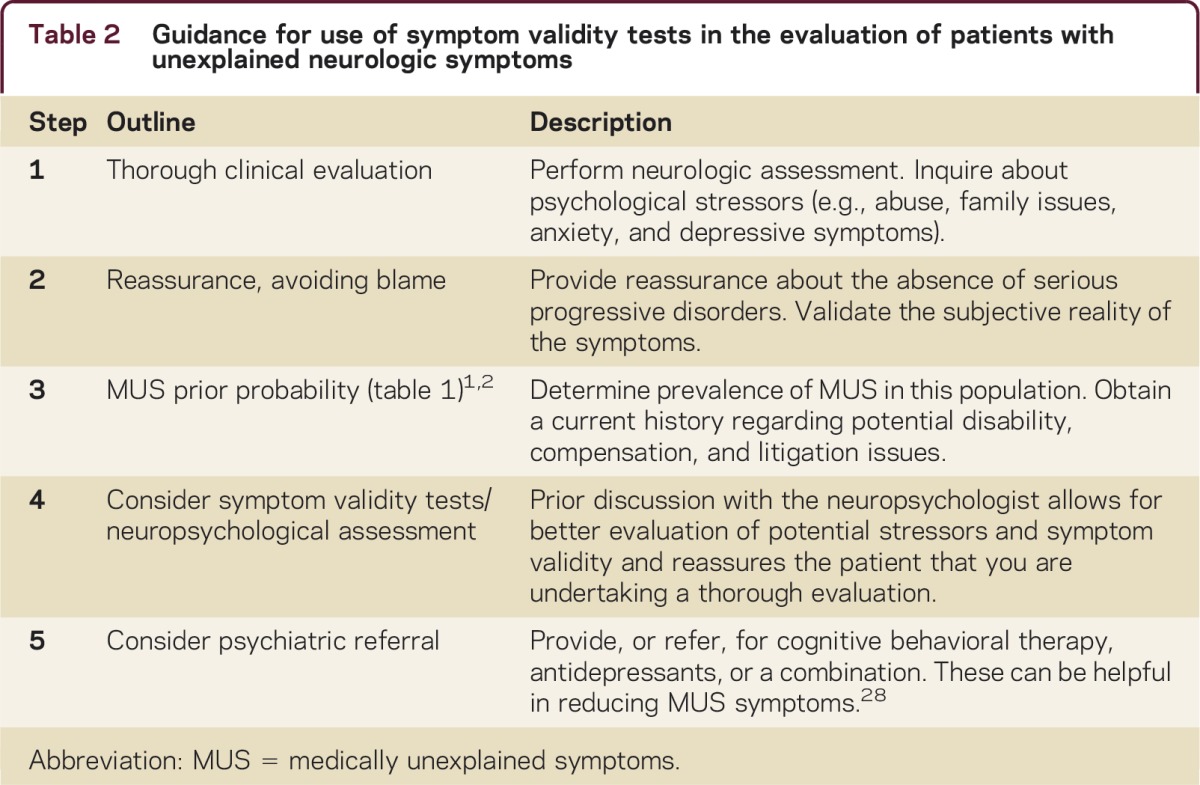
Treatment of patients with MUS is based primarily on enabling the patient to feel understood, broadening the agenda beyond physical symptoms, and making the link with psychosocial issues. Once serious and rapidly progressive disorders have been ruled out, the neurologist can communicate this reassurance to the patient, while not challenging the subjective reality of his or her symptoms. Some patients respond to cognitive-behavioral therapy, antidepressant treatment, or a combination of both.28 In the case of psychogenic gait disorder, a combination of physical therapy with positive behavioral reinforcement has been shown to be effective.29 We have provided a 5-step outline (table 2) that could serve as guidance for the evaluation of patients with MUS.
Table 2 Guidance for use of symptom validity tests in the evaluation of patients with unexplained neurologic symptoms
STUDY FUNDING
No targeted funding reported.
DISCLOSURE
J.J. Lockhart has received funding for travel from SAMHSA/NIDA for training and developing Veterans' Treatment Courts and serves on the editorial advisory board for Open Access Journal of Forensic Psychology. S. Satya-Murti has participated in telephone consultations and/or in-person Medical Advisory Board meetings for Evidera (formerly a division of United BioSource Corporation [UBC]), Medical Market Strategists, Pfizer, Simon-Kucher Consultants, Stratas Consultants, AstraZeneca, Avalere LLC, Covidien, Michael J. Fox Foundation, Foley Hoag and Baxter, and 1798 Consultants; and has received funding for travel from Evidera (formerly a division of UBC), Pfizer, Medical Market Strategists, AstraZeneca, Covidien, Avalere LLC, Baxter, and Michael J. Fox Foundation; serves on the editorial board of Neurology® Clinical Practice; and served as panelist and later (2010–2011) Vice-Chair of CMS-Medcac (Medicare Evidence Development and Coverage Advisory Committee). For the duration of the Medcac meeting, usually 1–2 days, he was considered a Special Government Employee (SGE). Full disclosure form information provided by the authors is available with the full text of this article at Neurology.org/cp.
Correspondence to: josephjlockhart@gmail.com
* These authors contributed equally to this work.
Funding information and disclosures are provided at the end of the article. Full disclosure form information provided by the authors is available with the full text of this article at Neurology.org/cp.
Footnotes
Correspondence to: josephjlockhart@gmail.com
Funding information and disclosures are provided at the end of the article. Full disclosure form information provided by the authors is available with the full text of this article at Neurology.org/cp.
REFERENCES
- 1.Stone J, Carson A, Duncan R. Symptoms “unexplained by organic disease” in 1144 new neurology out-patients: how often does the diagnosis change at follow-up? Brain. 2009;132:2878–2888. doi: 10.1093/brain/awp220. [DOI] [PubMed] [Google Scholar]
- 2.Mittenberg W, Patton C, Canyock EM, Condit DC. Base rates of malingering and symptom exaggeration. J Clin Exp Neuropsychol. 2002;24:1094–1102. doi: 10.1076/jcen.24.8.1094.8379. [DOI] [PubMed] [Google Scholar]
- 3.Rogers R. Clinical Assessment of Malingering and Deception. New York: The Guilford Press; 2012.
- 4.Parees I, Saifee TA, Kassavetis P. Believing is perceiving: mismatch between self-report and actigraphy in psychogenic tremor. Brain. 2011;135:117–123. doi: 10.1093/brain/awr292. [DOI] [PubMed] [Google Scholar]
- 5.Pareés I, Kassavetis P, Saifee TA. Failure of explicit movement control in patients with functional motor symptoms: explicit control and functional symptoms. Mov Disord. 2013;28:517–523. doi: 10.1002/mds.25287. [DOI] [PubMed] [Google Scholar]
- 6.Smith JK, Jozefowicz RF. Diagnosis and treatment of somatoform disorders. Neurol Clin Pract. 2012;2:94–102. doi: 10.1212/CPJ.0b013e31825a6183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Daum C, Hubschmid M, Aybek S. The value of “positive” clinical signs for weakness, sensory and gait disorders in conversion disorder: a systematic and narrative review. J Neurol Neurosurg Psychiatry. 2014;85:180–190. doi: 10.1136/jnnp-2012-304607. [DOI] [PubMed] [Google Scholar]
- 8.Nimnuan C, Hotopf M, Wessely S. Medically unexplained symptoms: an epidemiological study in seven specialties. J Psychosom Res. 2001;51:361–367. doi: 10.1016/s0022-3999(01)00223-9. [DOI] [PubMed] [Google Scholar]
- 9.Kemp S, Spilling C, Hughes C, Pauw K. Medically unexplained symptoms (MUS): what do current trainee psychologists, neurologists, psychiatrists believe? Open J Med Psychol. 2013;2:12–20. [Google Scholar]
- 10.Konnopka A, Schaefert R, Heinrich S. Economics of medically unexplained symptoms: a systematic review of the literature. Psychother Psychosom. 2012;81:265–275. doi: 10.1159/000337349. [DOI] [PubMed] [Google Scholar]
- 11.Smith RC, Gardiner JC, Luo Z, Schooley S, Lamerato L, Rost K. Primary care physicians treat somatization. J Gen Intern Med. 2009;24:829–832. doi: 10.1007/s11606-009-0992-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hou R, Moss-Morris R, Peveler R, Mogg K, Bradley BP, Belli A. When a minor head injury results in enduring symptoms: a prospective investigation of risk factors for postconcussional syndrome after mild traumatic brain injury. J Neurol Neurosurg Psychiatry. 2012;83:217–223. doi: 10.1136/jnnp-2011-300767. [DOI] [PubMed] [Google Scholar]
- 13.Martelli MF, Nicholson K, Zasler ND, Bender MC. Assessing, addressing response bias. Brain Inj Med [Internet], 2nd ed. New York: Demos Medical Publishing; 2013. Available at: http://www.researchgate.net/publication/230788479_Assessment_of_Response_Bias_in_Clinical_and_Forensic_Evaluations_of_Impairment_Following_Brain_Injury/file/d912f50b5ac3d0e6db.pdf. Accessed December 4, 2014.
- 14.Greiffenstein M, Gervais R, Baker WJ, Artiola L, Smith H. Symptom validity testing in medically unexplained pain: a chronic regional pain syndrome type 1 case series. Clin Neuropsychol. 2013;27:138–147. doi: 10.1080/13854046.2012.722686. [DOI] [PubMed] [Google Scholar]
- 15.Stone J, Carson A, Duncan R. Which neurological diseases are most likely be associated with “symptoms unexplained by organic disease”. J Neurol. 2012;259:33–38. doi: 10.1007/s00415-011-6111-0. [DOI] [PubMed] [Google Scholar]
- 16.Green P, Rohling ML, Lees-Haley PR, Allen LM. Effort has a greater effect on test scores than severe brain injury in compensation claimants. Brain Inj. 2001;15:1045–1060. doi: 10.1080/02699050110088254. [DOI] [PubMed] [Google Scholar]
- 17.Fishbain DA, Cutler RB, Rosomoff HL, Rosomoff RS. Is there a relationship between nonorganic physical findings (Waddell signs) and secondary gain/malingering? Clin J Pain. 2004;20:399–408. doi: 10.1097/00002508-200411000-00004. [DOI] [PubMed] [Google Scholar]
- 18.Sollman MJ, Berry DTR. Detection of inadequate effort on neuropsychological testing: a meta-analytic update and extension. Arch Clin Neuropsychol. 2011;26:774–789. doi: 10.1093/arclin/acr066. [DOI] [PubMed] [Google Scholar]
- 19.Carone D, Bush S. Mild Traumatic Brain Injury: Symptom Validity Assessment and Malingering. New York: Springer Publishing Company; 2012.
- 20.Heintz CEJ, van Tricht MJ, van der Salm SM. Neuropsychological profile of psychogenic jerky movement disorders: importance of evaluating non-credible cognitive performance and psychopathology. J Neurol Neurosurg Psychiatry. 2013;84:862–867. doi: 10.1136/jnnp-2012-304397. [DOI] [PubMed] [Google Scholar]
- 21.Tombaugh TN, Tombaugh PW. Test of Memory Malingering: TOMM [Internet]. North Tonawanda, NY: Multi-Health Systems; 1996. Available at: http://www.v-psyche.com/doc/Clinical%20Test/Test%20of%20Memory%20of%20Malingering.doc. Accessed December 4, 2014.
- 22.Brady TF, Konkle T, Alvarez GA, Oliva A. Visual long-term memory has a massive storage capacity for object details. Proc Natl Acad Sci USA. 2008;105:14325–14329. doi: 10.1073/pnas.0803390105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Merten T, Bossink L, Schmand B. On the limits of effort testing: symptom validity tests and severity of neurocognitive symptoms in nonlitigant patients. J Clin Exp Neuropsychol. 2007;29:308–318. doi: 10.1080/13803390600693607. [DOI] [PubMed] [Google Scholar]
- 24.Demakis GJ, Gervais RO, Rohling ML. The effect of failure on cognitive and psychological symptom validity tests in litigants with symptoms of post-traumatic stress disorder. Clin Neuropsychol. 2008;22:879–895. doi: 10.1080/13854040701564482. [DOI] [PubMed] [Google Scholar]
- 25.Iverson GL, Lange RT, Brooks BL, Rennison VLA. “Good old days” bias following mild traumatic brain injury. Clin Neuropsychol. 2010;24:17–37. doi: 10.1080/13854040903190797. [DOI] [PubMed] [Google Scholar]
- 26.Green P, Allen L, Astner K. The Word Memory Test: A User's Guide to the Oral and Computer-Administered Forms, US Version 1.1. Durham, NC: CogniSyst; 1996.
- 27.Frederick R. VIP: Validity Indicator Profile. Minneapolis, MN: National Computer Systems; 1997.
- 28.Edwards TM, Stern A, Clarke DD, Ivbijaro G, Kasney LM. The treatment of patients with medically unexplained symptoms in primary care: a review of the literature. Ment Health Fam Med. 2010;7:209–221. [PMC free article] [PubMed] [Google Scholar]
- 29.Jordbru AA, Smedstad LM, Klungsoyr O, Martinsen EW. Psychogenic gait disorder: a randomized controlled trial of physical rehabilitation with one-year follow-up. J Rehabil Med. 2014;46:181–187. doi: 10.2340/16501977-1246. [DOI] [PubMed] [Google Scholar]


