Summary
We examined the King-Devick (K-D) test, a vision-based test of rapid number naming, as a complement to components of the Sport Concussion Assessment Tool, 3rd edition (SCAT3) for diagnosis of concussion. Baseline and postconcussion data for the University of Florida men's football, women's soccer, and women's lacrosse teams were collected, including the K-D test, Standardized Assessment of Concussion (SAC), and Balance Error Scoring System (BESS). Among 30 athletes with first concussion during their athletic season (n = 217 total), differences from baseline to postinjury showed worsening of K-D time scores in 79%, while SAC showed a ≥2-point worsening in 52%. Combining K-D and SAC captured abnormalities in 89%; adding the BESS identified 100% of concussions. Adding a vision-based test may enhance the detection of athletes with concussion.
Recent studies of sports-related concussion have demonstrated that underreporting of symptoms is common in collegiate athletes.1,2 This indicates a critical need for accurate, easily applied, objective tests to aid diagnosis acutely on the sidelines or in locker room/athletic training facility settings. While previous clinical definitions of concussion required loss of consciousness or changes in mental status,3 more recent definitions include any impulse blow to the head or body accompanied by neurologic signs or symptoms. Early and accurate diagnosis is critical in order to remove the concussed athlete from play and decrease the risks of further injury.4,5 This applies particularly to younger athletes who may be susceptible to external pressures to return to play preemptively.4–6
Multiple assessment tools have been developed for the acute setting; among these, the Standardized Assessment of Concussion (SAC) for cognition7,8 and the Balance Error Scoring System (BESS) are most commonly used at the collegiate level.9 In addition to the SAC and BESS as performance measures, the Post-Concussion Scale (PCS) is used to assess reporting of symptoms.10 The Sport Concussion Assessment Tool, 3rd edition (SCAT3) and the prior SCAT2 capture cognition and balance.8,9,11 Vision is another frequently affected system in concussion,12 and adding a vision-based test may increase diagnostic power for the clinician. There have been several high-profile cases in which current sideline protocols have failed to confirm a diagnosis of concussion, emphasizing the need to examine other tools, such as those that capture the dimension of vision.12
The King-Devick (K-D) test is a <1-minute assessment of rapid number naming that requires vision and saccadic eye movements, features shown to reflect suboptimal brain function in concussion.12 We examined the potential for the K-D test to complement the SAC and BESS for the acute assessment of concussion in collegiate athletes.
METHODS
Study participants
Data from athletes from the University of Florida varsity men's football, women's soccer, and women's lacrosse teams were collected. K-D, PCS, SAC, BESS, and Immediate Post-Concussion Assessment and Cognitive Testing (ImPACT) tests were obtained for routine clinical purposes, with baseline assessments at the beginning of each athlete's collegiate career. This was a retrospective study of data from baseline and postinjury testing performed for clinical purposes; as such, there was no control group. Institutional review boards (IRBs) at the University of Florida approved all data collection protocols, with agreements for data analysis and publication with IRBs at the University of Pennsylvania and New York University School of Medicine.
King-Devick test
The K-D test is based on the time to perform rapid number naming and involves reading aloud a series of single digit numbers quickly from left to right on 3 laminated plastic test cards or screens on an iPad application (format used in this study).13–17 Standardized instructions are used. The sum of time for the 3 test cards or iPad screens (in seconds) constitutes the summary score for the entire test. A higher time compared to baseline indicates worsening of score. Test-retest reliability for the K-D test has been shown to be high, with intraclass correlations of 0.97 (95% confidence interval [CI] 0.90, 1.0) between measurements in the absence of concussion in one study14 and 0.96 (95% CI 0.93, 0.99) in another.17 Studies of boxers/mixed martial arts (MMA) fighters14 and collegiate athletes13 have also shown that the K-D test can distinguish athletes with reported concussion/overt head trauma from those without, and that worsening from preseason baseline is not generally observed solely on the basis of postcompetition fatigue (basketball scrimmage, for example).13 Average preseason baseline scores in published collegiate cohorts of athletes have been 36–40 seconds; these studies have also shown that scores are generally better than baseline following competition/exercise in the absence of concussion.11 Therefore, any worsening of K-D score from baseline may be suggestive of injury; this threshold for change was used in these analyses.
Standardized Assessment of Concussion
The SAC score (maximum 30) includes Orientation (maximum 5), Immediate Memory (maximum 15), Concentration (maximum 5), and Delayed Recall (maximum 5). Reductions in scores from baseline indicate worsening. Recent studies of SCAT2 suggest a 2–4-point threshold for SAC total score as a minimum for detecting change from baseline; the lower limit of this range (2 points) was examined in our analyses.9
Balance Error Scoring System
The BESS score is based on the total number of errors made in 3 different stances (double leg, single leg, tandem) on 2 different surfaces (firm and foam—both were used for BESS testing in this cohort, although the SCAT3 uses a modified BESS with firm surface only).9 Athletes are required to undergo each trial for 20 seconds, with a point added for each error committed (lifting hands off hips, opening eyes, stepping, stumbling, falling, moving hip into more than 30° flexion or abduction, lifting forefoot or heel, or remaining out of test position for >5 seconds). The maximum score for any trial is 10, with a maximum (worst possible) total score of 60 (30 for modified BESS in SCAT3). A recently published minimum threshold for BESS score total change in one analysis was 3–6 points.9 The lower limit of this range (3 points) was examined in our primary analyses, while the upper bound of 6-point change was explored.
Post-Concussion Scale
The PCS is a symptom-evaluation scale rating 22 symptoms associated with concussion.10 It is not a performance measure but is used to assess reporting of symptoms. Athletes are asked to rate each symptom on a severity scale (0 = none to 6 = severe). Two scores are calculated: (1) Total Number of Symptoms (TNS), maximum score 22; and (2) Symptom Severity (SS), sum of severity scores for each symptom, maximum score 132 (maximum severity rating 6 × 22 symptoms). Increases in score from baseline indicate worsening. The PCS differs in several symptoms and subtle wording from the “Symptom Evaluation” scale contained in the SCAT3; however, both scales have 22 symptoms.
Immediate Post-Concussion Assessment and Cognitive Testing
ImPACT is a computerized neurocognitive testing tool that takes 25–30 minutes to administer.18 It includes of a number of indicators, each reported separately, including verbal memory, visual memory, visual motor speed, reaction time, impulse control, and cognitive efficiency index (simultaneous measure of speed and accuracy). Higher scores in verbal or visual memory and visual motor speed indicate better performance, whereas lower scores in reaction time and impulse control reflect better performance for those components.
Testing procedures
Athletic trainers or team physicians performed all testing during preseason sessions (baseline) and at the time of concussion (or reporting of concussion symptoms if there was a delay between injury and reporting). As such, testing was performed as soon as the athletic training or physician staff was made aware that the athlete had a potential concussion. Because not all concussion symptoms were immediately reported, the time between injury and postinjury testing was variable. BESS data were not available for all athletes in this retrospective cohort.
Concussion was defined as occurrence of a direct or indirect impulsive blow to the head, witnessed or reported, with any accompanying neurologic symptom, including headache. Judgments with regard to the occurrence of concussion were made as per standard practice by the athletic trainers or team physicians immediately following injury or reporting. Some of the concussion events were not witnessed; these athletes instead reported symptoms following the game or practice. Delays in reporting may reflect the current climate of underreporting among some collegiate athletes.1,2
Statistical analysis
Data were analyzed using Stata 13.0 (StataCorp, College Station, TX). Analyses examined changes in scores from baseline to postinjury using the Wilcoxon signed-rank test. The relationship of changes in scores for the K-D test vs the SAC and BESS was examined using linear regression. Proportions of athletes who met criteria for concussion and also worsened by published minimum change thresholds for the K-D test, SAC, and BESS were determined.
RESULTS
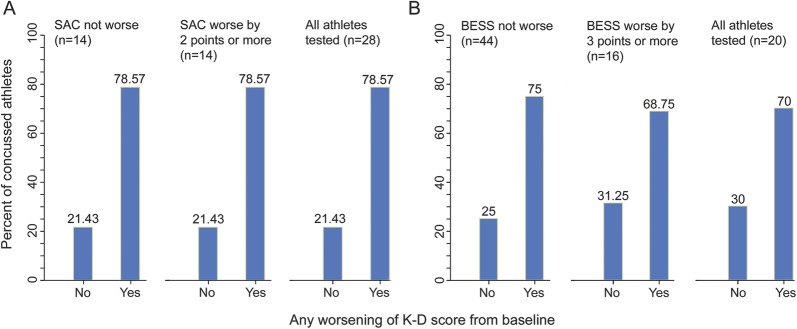
Baseline and postconcussion scores for 217 athletes are presented in table 1. Average baseline scores were similar across sports (150 men's football, 38 women's lacrosse, 33 women's soccer). Thirty athletes had a witnessed concussion or were diagnosed based on reported symptoms. Most were from football (n = 25) while others were from women's lacrosse (n = 1) and women's soccer (n = 4). Baseline K-D testing was available for 29 of 30 players with concussion; primary analyses were based on these 29 athletes. The SAC was also administered in 29 of the 30 concussed athletes; 28 of these 29 underwent both SAC and K-D testing at first postinjury evaluation (total n = 28 for figure 1 and figure 2A). BESS data were available for 20 athletes for first evaluation postinjury (total n = 20 for figure 2B). The median time from the concussive event to evaluation was 87 minutes. While most athletes (23/28) reported their symptoms on the day of injury, a few (5/28) first reported their symptoms days later.
Table 1 Test scores at baseline, postconcussion, and differences from baseline
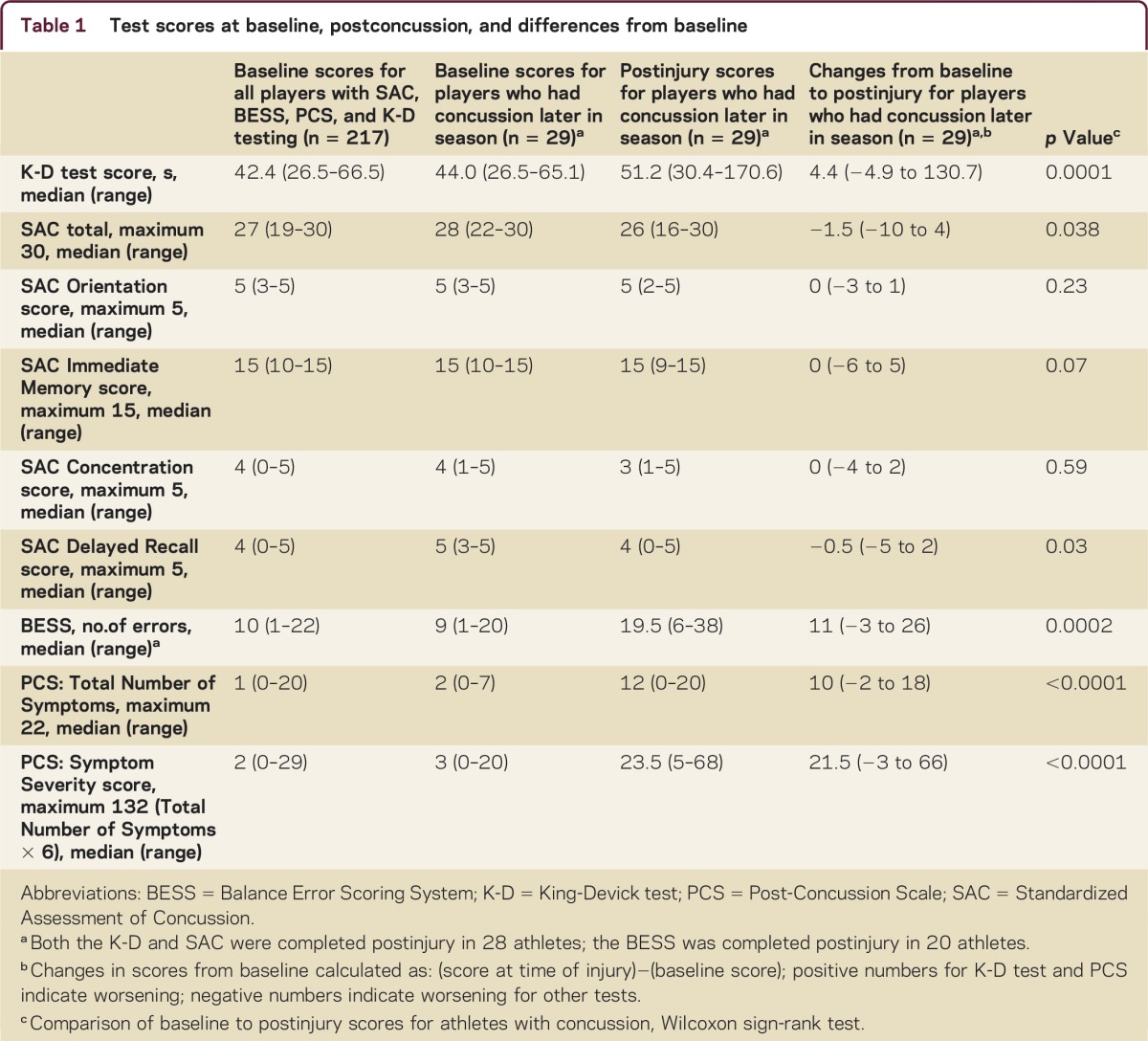
Percentages of athletes diagnosed with concussion who demonstrated worsening postinjury from baseline on the King-Devick (K-D) test (any worsening) and the Standardized Assessment of Concussion (SAC, 2 points based on recently published recommended levels of minimum change) among athletes who underwent both K-D and SAC postinjury (n = 28)9
Percentages of athletes diagnosed with concussion who demonstrated worsening postinjury from baseline on (A) SAC and (B) BESS total scores by recently published recommended levels of minimum change9
Figure 2. Total numbers of athletes are those tested postinjury using both the K-D and SAC (A, n = 28) or the K-D and BESS (B, n = 20). In panel A, 11/14 (79%) had worsening on the K-D test without change in the SAC; 11/14 (79%) also had K-D worsening and SAC worsening by 2 points or more. Overall, 22/28 (79%) had K-D worsening. BESS = Balance Error Scoring System; K-D = King-Devick test; SAC = Standardized Assessment of Concussion.
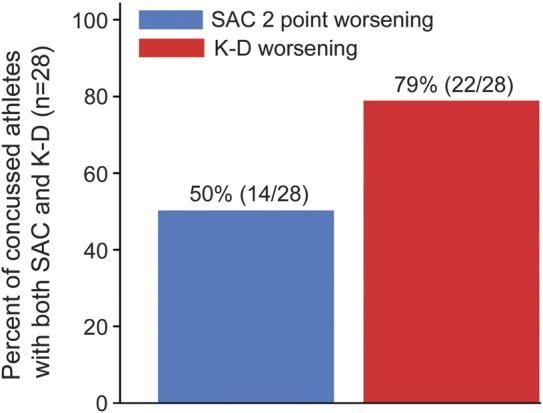
In terms of K-D testing, 79% (23/29) of concussed athletes had worsening from baseline to postinjury (figure 1). The SAC worsened by 2 points or more (recently established threshold)9 in 52% (15/29). Combining the K-D test and the SAC captured 89% (25/28) of concussed athletes tested with both measures. Sixteen of 20 athletes (80%) who underwent BESS had increases (worsening) in their scores by ≥3 points. When BESS was added in a smaller cohort of athletes, 100% of concussions were identified. Using an upper bound of 6 points' change in BESS as a criterion reduced the sensitivity somewhat, resulting in the test identifying 70% (14/20) of concussed athletes. Seventy-nine percent (11/14) of those who worsened by ≥2 points on the SAC also had worsening on the K-D test (figure 2A). However, 77% (10/13) of those with concussion had worsening on the K-D test without any (even a 1-point) change in the SAC. Similar patterns were noted when comparing the K-D test and the BESS (figure 2B). Two of 20 athletes examined with all 3 tests had worsening of the K-D test in the absence of change in the SAC or BESS, while 90% (18/20) worsened on either the SAC or BESS. In terms of symptoms, average baseline PCS TNS score was 1–2 symptoms. Consistent with diagnosis of concussion, this average increased to 12 at the postinjury assessment, with corresponding increase in SS score (table 1).
Increases in the K-D test were significantly associated with worsening of the SAC and, to a lesser degree, the BESS. On average, for every 1-point worsening of overall SAC score, there was a corresponding 4.7-second increase in K-D score from baseline to postinjury (p = 0.001, linear regression models); for the BESS, the association of change was less strong, but a 1-point worsening corresponded to a 1.6-point increase in K-D time score (p = 0.043). Increases in the SS score of the PCS from baseline to postinjury were also associated with worsening of K-D times (p < 0.001) and SAC total scores (p = 0.003) but not BESS scores (p = 0.66, linear regression). Among specific symptoms, light and noise sensitivities correlated well with K-D worsening.
The relationship of ImPACT subscores to K-D, SAC, and BESS scores was examined at baseline only (table 2) because ImPACT was used as a management tool for return to play and not for acute diagnosis of concussion. Longer K-D times were associated with reduced ImPACT visual motor speed and visual memory. SAC total score had the strongest correlations at baseline with ImPACT components, emphasizing the cognitive basis for ImPACT testing.
Table 2 Immediate Post-Concussion Assessment and Cognitive Testing (ImPACT) baseline scores
DISCUSSION
Results of this study provide evidence that a composite of rapid performance tests, including the SAC, BESS, and K-D test, may be both feasible and effective for augmenting the clinical diagnosis of concussion in the acute setting for collegiate athletes.
Our findings highlight the value of adding a visual test that measures neurologic dysfunction not captured by sideline cognitive or balance tests. While no single tool or group of tests should replace the judgments of athletic trainers, physicians, or others who know players well, our data suggest that a combination of cognitive, balance, and visual measures may be best to augment subjective symptom assessments and help diagnose concussion shortly after injury. Furthermore, correlations in this study between K-D scores and ImPACT visual motor scores at baseline, now seen in 2 independent studies,18 support the validity of the K-D test as a test that requires visual function.
Adding a test that captures saccadic (fast) eye movements as well as other aspects of visual function adds a critical dimension to postinjury assessment in concussion for several reasons. First, studies with formal eye movement recordings in patients postconcussion have shown that impairments of saccades, anti-saccades, and smooth pursuit are greater in those with worse outcomes with regard to symptom severity and problems of activities of daily living.12 That study demonstrated a potential role for tests that capture vision and eye movements not only in the acute setting but also as a measure of recovery. Second, the brain pathways for saccades, or fast eye movements, are widely distributed and involve the frontal eye fields, supplementary eye field, dorsolateral prefrontal cortex (DLPFC), parietal lobes, and deeper structures including the brainstem (figure e-1 at Neurology.org/cp). All of these areas are susceptible to injury in concussion, and such injury may result in combinations of visual, cognitive, and balance disturbances. In fact, a recent study of the K-D test in professional hockey players showed that worse baseline scores were associated with reductions in immediate memory as captured by the SAC.15 Known as the highest cortical area responsible for motor planning and working memory, the DLPFC has also been implicated in the control of saccadic eye movements (particularly anticipatory saccades, anti-saccades, object scanning, and memory-guided saccades).12 Since the DLPFC and other areas of the visual pathway are vulnerable to injury based on their location (in the case of the DLPFC) and widespread extent throughout the brain, assessment of saccades and other aspects of vision by rapid tests is a complementary addition to postinjury tests of cognition and balance.
Data from the present study demonstrate the additive effect of using multiple sideline tools that examine a wide range of neurologic dysfunction; while cognitive and balance tests were normal in certain subjects, a visual test, the K-D test, showed abnormalities. Likewise, the K-D test was not able to detect all concussions, and the SAC and BESS identified concussions in these athletes. Similar observations in the field of multiple sclerosis (MS) have demonstrated that adding a vision test to existing composites of performance measures enhanced physicians' abilities to distinguish MS patients from controls. Like previous studies of MMA fighters, boxers, rugby league athletes, professional ice hockey players, and collegiate cohorts, the present investigation showed that worsening of the K-D test occurs in most athletes following concussion.13–16,18 It is notable that our athletes' K-D scores correlated with certain common symptoms, most notably light and noise sensitivities. Although these findings may be nonlocalizing, they may also reflect functional injury to the brainstem. Correlation of test scores with specific symptoms may help determine which sideline tests are most appropriate to detect concussion in individual athletes.
Diagnosis of concussion on the sidelines requires a battery of high-sensitivity tests, particularly since reliance on symptom reporting can lead to false-negatives. There are several lines of evidence for underreporting: (1) anonymous online surveys demonstrating that 40%–70% may have previously hidden a concussion to stay in a game, while 23% were likely to hide a concussion in the future1,2; (2) a New Zealand Rugby League study in which athletes commonly had undetected concussions16; and (3) investigations in which 26% of athletes reporting no concussion symptoms had deficits on formal neurocognitive testing.6,19
Sideline testing that captures key neurologic dimensions of concussion, in addition to assessing symptoms, is essential. A recent study in the New Zealand Rugby League demonstrated that unrecognized concussion is far more common than witnessed events.16 While 22 concussions were detected during a single season, only 5 of these were diagnosed at the time of the event. Since the authors of that study routinely examined all athletes postmatch, an additional 17 were diagnosed during the season. This additional group was interviewed when their K-D tests showed worsening from baseline to postmatch; they admitted to signs and symptoms consistent with concussion. These findings emphasize the need to have not only a symptom checklist but also objective measures that can diagnose concussion. Athletes who want to continue to play or who do not understand the seriousness of concussion may fail to report symptoms of concussion at the time of the event. Still others do not want to let their teammates down.19 When responding to anonymous athlete surveys, athletes may report concussive events up to 10 times more frequently than when athletic trainers are surveyed.1,2
In this cohort, the BESS was administered to fewer athletes than the K-D test or the SAC, particularly when the diagnosis of concussion was made by these other measures. BESS testing may have been targeted based on athlete symptomatology at the time of the concussion assessment. Ongoing prospective studies will address this potential source of ascertainment bias that is inherent in retrospective analyses. The BESS is also known to have a wide range of interrater reliability, with intraclass correlation coefficients of 0.57 and interrater minimum detectable change scores of 9.4.9,20 These changes are much higher than the change threshold of ≥3 used in our primary analyses, and greater than the upper bound of recently recommended ranges (3–6 points).9 Varying experience with the BESS in our cohort (graduate assistants vs athletic trainers) may have led to lower (better) baseline scores, thus making worsening more likely.
Since our analyses were based on data acquired for clinical purposes, there were no nonconcussed controls in this cohort. While this aspect of design is a part of ongoing studies and will be helpful to estimate sensitivity, it presents challenges at the collegiate or professional level since testing a control athlete requires removing an uninjured athlete from play. In terms of clinically meaningful change in this cohort, our analyses used any worsening in K-D score as a criterion. This was the case since prior studies of collegiate athletes showed improvements in scores following athletic competition and exercise in the absence of concussion.13 Specifically, collegiate athletes undergoing K-D testing immediately following a 2-hour basketball scrimmage had improvement of their scores from preseason baseline, providing evidence that fatigue and exercise alone may not be the cause of worsening on this measure.
Investigations with prospective athlete enrollment/data ascertainment and control group design are under way to address these and other important aspects of visual testing in contact sport athletes. Our study shows that adding a visual dimension to current testing components may increase our capacity to identify concussed athletes in the acute setting, and that a continued stepwise approach to research will advance the field of sports-related concussion.
STUDY FUNDING
Supported by NIH K24 EY 018136 (LJB).
DISCLOSURES
Z. Marinides, K. Galetta, C. Andrews, and J. Wilson report no disclosures. D. Herman's spouse owns/has owned stock/stock options in Xhale, Inc. C. Robinson, M. Smith, and B. Bentley report no disclosures. S. Galetta serves on the editorial boards of Neurology and the Journal of Neuro-ophthalmology; serves as a consultant for Vaccinex and Biogen Idec; and has received speaker honoraria from Biogen Idec, Questcor, Vaccinex, and Teva. L. Balcer has served as a consultant for Biogen Idec, Novartis, Questcor, Vaccinex, and Acorda; has served on scientific advisory boards for Biogen Idec; and has received speaker honoraria from Biogen Idec, Bayer, and Novartis. J. Clugston has received research support from Banyan Biomarkers. Full disclosure form information provided by the authors is available with the full text of this article at Neurology.org/cp.
Supplementary Material
Correspondence to: laura.balcer@nyumc.org
Funding information and disclosures are provided at the end of the article. Full disclosure form information provided by the authors is available with the full text of this article at Neurology.org/cp.
Footnotes
Supplemental data at Neurology.org/cp
Correspondence to: laura.balcer@nyumc.org
Funding information and disclosures are provided at the end of the article. Full disclosure form information provided by the authors is available with the full text of this article at Neurology.org/cp.
REFERENCES
- 1.Torres DM, Galetta KM, Phillips HW. Sports-related concussion: anonymous survey of a collegiate cohort. Neurol Clin Pract. 2013;3:279–287. doi: 10.1212/CPJ.0b013e3182a1ba22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kroshus E, Daneshvar DH, Baugh CM, Nowinski CJ, Cantu RC. NCAA concussion education in ice hockey: an ineffective mandate. Br J Sports Med. 2014;48:135–140. doi: 10.1136/bjsports-2013-092498. [DOI] [PubMed] [Google Scholar]
- 3.McCrea M, Barr WB, Guskiewicz K. Standard regression-based methods for measuring recovery after sport-related concussion. J Int Neuropsychol Soc. 2005;11:58–69. doi: 10.1017/S1355617705050083. [DOI] [PubMed] [Google Scholar]
- 4.Covassin T, Moran R, Wilhelm K. Concussion symptoms and neurocognitive performance of high school and college athletes who incur multiple concussions. Am J Sports Med. 2013;41:2885–2889. doi: 10.1177/0363546513499230. [DOI] [PubMed] [Google Scholar]
- 5.Schneider KJ, Iverson GL, Emery CA, McCrory P, Herring SA, Meeuwisse WH. The effects of rest and treatment following sport-related concussion: a systematic review of the literature. Br J Sports Med. 2013;47:304–307. doi: 10.1136/bjsports-2013-092190. [DOI] [PubMed] [Google Scholar]
- 6.Dziemianowicz MS, Kirschen MP, Pukenas BA, Laudano E, Balcer LJ, Galetta SL. Sports- related concussion testing. Curr Neurol Neurosci Rep. 2012;12:547–559. doi: 10.1007/s11910-012-0299-y. [DOI] [PubMed] [Google Scholar]
- 7.McCrory P, Meeuwisse WH, Aubry M. Consensus statement on concussion in sport: the 4th International Conference on Concussion in Sport held in Zurich, November 2012. Br J Sports Med. 2013;47:250–258. doi: 10.1136/bjsports-2013-092313. [DOI] [PubMed] [Google Scholar]
- 8.McCrea M, Kelly JP, Kluge J, Ackley B, Randolph C. Standardized assessment of concussion in football players. Neurology. 1997;48:586–588. doi: 10.1212/wnl.48.3.586. [DOI] [PubMed] [Google Scholar]
- 9.Guskiewicz KM, Register-Mihalik J, McCrory P. Evidence-based approach to revising the SCAT2: introducing the SCAT3. Br J Sports Med. 2013;47:289–293. doi: 10.1136/bjsports-2013-092225. [DOI] [PubMed] [Google Scholar]
- 10.Lovell MR, Iverson GL, Collins MW. Measurement of symptoms following sports-related concussion: reliability and normative data for the post-concussion scale. Appl Neuropsychol. 2006;13:166–174. doi: 10.1207/s15324826an1303_4. [DOI] [PubMed] [Google Scholar]
- 11.Valovich McLeod TC, Bay C, Lam KC, Chhabra A. Representative baseline values on the Sport Concussion Assessment Tool 2 (SCAT2) in adolescent athletes vary by gender, grade, and concussion history. Am Sports J. 2012;40:927–933. doi: 10.1177/0363546511431573. [DOI] [PubMed] [Google Scholar]
- 12.Heitger MH, Jones RD, Macleod AD, Snell DL, Frampton CM, Anderson TJ. Impaired eye movements in post-concussion syndrome indicate suboptimal brain function beyond the influence of depression, malingering or intellectual ability. Brain. 2009;132:2850–2870. doi: 10.1093/brain/awp181. [DOI] [PubMed] [Google Scholar]
- 13.Galetta KM, Brandes LE, Maki K. The King-Devick test and sports-related concussion: study of a rapid visual screening tool in a collegiate cohort. J Neurol Sci. 2011;309:34–39. doi: 10.1016/j.jns.2011.07.039. [DOI] [PubMed] [Google Scholar]
- 14.Galetta KM, Barrett J, Allen M. The King-Devick test as a determinant of head trauma and concussion in boxers and MMA fighters. Neurology. 2011;76:1456–1462. doi: 10.1212/WNL.0b013e31821184c9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Galetta MS, Galetta KM, McCrossin J. Saccades and memory: baseline associations of the King-Devick and SCAT2 SAC tests in professional ice hockey players. J Neurol Sci. 2013;32:28–31. doi: 10.1016/j.jns.2013.02.008. [DOI] [PubMed] [Google Scholar]
- 16.King D, Clark T, Gissane C. Use of rapid visual screening tool for the assessment of concussion in amateur rugby league: a pilot study. J Neurol Sci. 2012;320:16–21. doi: 10.1016/j.jns.2012.05.049. [DOI] [PubMed] [Google Scholar]
- 17.Leong DF, Balcer LJ, Galetta SL, Liu Z, Master CL. The King-Devick test as a concussion screening tool administered by sports parents. J Sports Med Phys Fitness. 2014;54:70–77. [PubMed] [Google Scholar]
- 18.Tjarks BJ, Dorman JC, Valentine VD. Comparison and utility of King-Devick and ImPACT composite scores in adolescent concussion patients. J Neurol Sci. 2013;334:148–153. doi: 10.1016/j.jns.2013.08.015. [DOI] [PubMed] [Google Scholar]
- 19.McCrea M, Hammeke T, Olsen G, Leo P, Guskiewicz K. Unreported concussion in high school football players: implications for prevention. Clin J Sports Med. 2004;14:13–17. doi: 10.1097/00042752-200401000-00003. [DOI] [PubMed] [Google Scholar]
- 20.Finnoff JT, Peterson VJ, Hollman JH, Smith J. Intrarater and interrater reliability of the Balance Error Scoring System (BESS) PM&R. 2009;1:50–54. doi: 10.1016/j.pmrj.2008.06.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.