Summary
Management of seizure emergencies substantially changed after the introduction of rectal diazepam in Europe in the 1970s and in the United States in the 1990s. Although safe and effective, social objections and legal concerns have limited use of rectal diazepam products in out-of-hospital treatment of seizure emergencies. Shortly after the introduction of Diastat (diazepam rectal gel), commercial development of innovative formulations began involving several benzodiazepines and routes of administration, including buccal, IM, nasal, and subcutaneous. All benzodiazepines have the same mechanism of action; however, there are major differences in physicochemical properties and pharmacokinetic characteristics, which affect the choice of drug and route. This article highlights some of those differences and their effect on selection of therapies for treating seizure emergencies. We also present results from key clinical studies of these drugs and provide an update on current status of new products under development.
Rectal diazepam gel administered after the onset of acute repetitive and prolonged seizures has been shown to significantly reduce seizure recurrence and emergency department admissions.1 Nonetheless, social objections to and legal concerns2 about rectal administration have limited its use. A need for better, more patient-friendly therapies for seizure emergencies such as prolonged seizures, acute repetitive seizures, or status epilepticus has resulted in development of innovative benzodiazepine formulations using nonrectal routes. Each of these routes and results from key clinical studies of 3 benzodiazepines (diazepam, lorazepam, and midazolam) undergoing development as rescue therapies are reviewed in this article.
Current guidelines to treating seizure emergencies
Rescue therapy, for which benzodiazepines are considered the drugs of choice, is administered to abort a seizure or interrupt progression to a more prolonged episode or prevent recurrence.3 The Neurocritical Care Society recommends use of IV lorazepam for treatment of status epilepticus when skilled health care personnel are available. When IV therapy is not feasible, Society guidelines state that benzodiazepines can be administered by IM, rectal, intranasal (IN), or buccal routes.4 However, the only product available in the United States for rescue therapy is rectal diazepam gel. Until such time as Food and Drug Administration (FDA)–approved IM and IN formulations are available, clinicians outside of Europe, where buccal midazolam is approved in patients up to the age of 17 years, can consider the off-label use of diazepam or midazolam oral solutions given buccally or injectable formulation administered intranasally, when patients decline rectal therapy. However, these therapies have not been evaluated in controlled trials, resulting in uncertainty about their efficacy or optimal dosage regimens.
Ideal drug characteristics for seizure emergencies
Drugs used in the management of seizure emergencies must be sufficiently potent to permit small dose volumes and have wide therapeutic index. They should exhibit a rapid onset of action (minutes) with an intermediate duration of action (hours). Drug administration should be quick, easy, and safe. When treating seizure emergencies by an extravascular route, the formulation should exhibit high, consistent, and rapid (minutes) absorption.
Lipid solubility and pharmacokinetics
Lipid solubility affects the rate at which a drug diffuses across biological barriers, i.e., the greater a drug's lipid solubility, the faster its absorption across tissue membranes. Diazepam and midazolam are 4 and 6 times more lipid soluble than lorazepam (see lipid solubility, table 1). When given IV, diazepam, lorazepam, and midazolam enter the CNS within a few minutes with a corresponding rapid onset of effect. However, lorazepam more slowly redistributes out of the CNS into muscle and fat tissue, resulting in a longer duration of effect. This property makes lorazepam the drug of choice when given IV to treat SE.
Table 1 Physicochemical properties and pharmacokinetic parameters of diazepam, midazolam, and lorazepam14,15
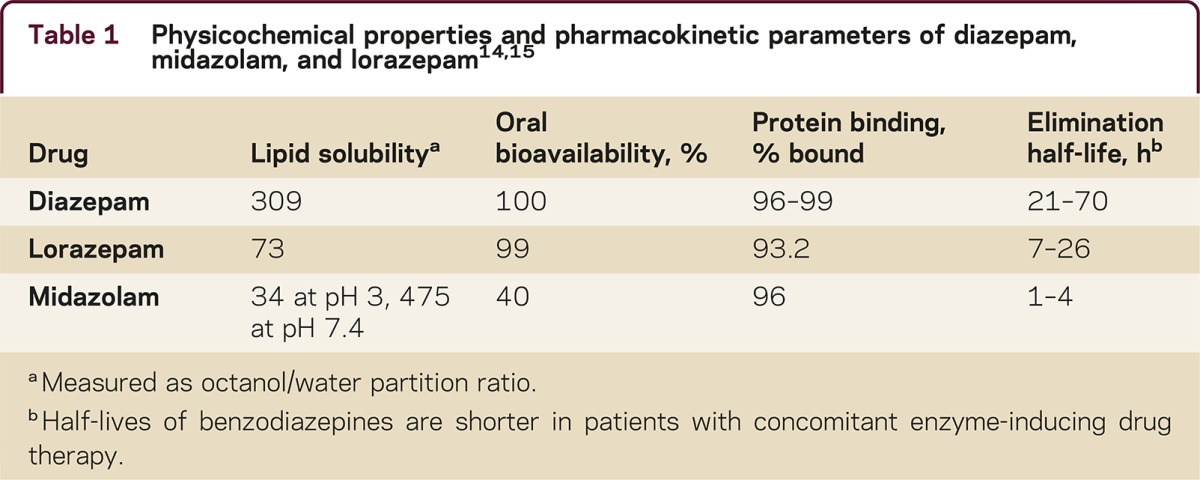
When treating a seizure emergency by an extravascular route, the differences in lipid solubility and pharmacokinetics result in a different choice of benzodiazepines. Diazepam and midazolam when given extravascularly display significantly faster rates of absorption than lorazepam due to their high lipid solubility and redistribution is not as pronounced. Consequently, diazepam and midazolam are preferred when non-IV routes are indicated, but their performance will vary by route and type of seizure emergency.
Status of extravascular formulation development
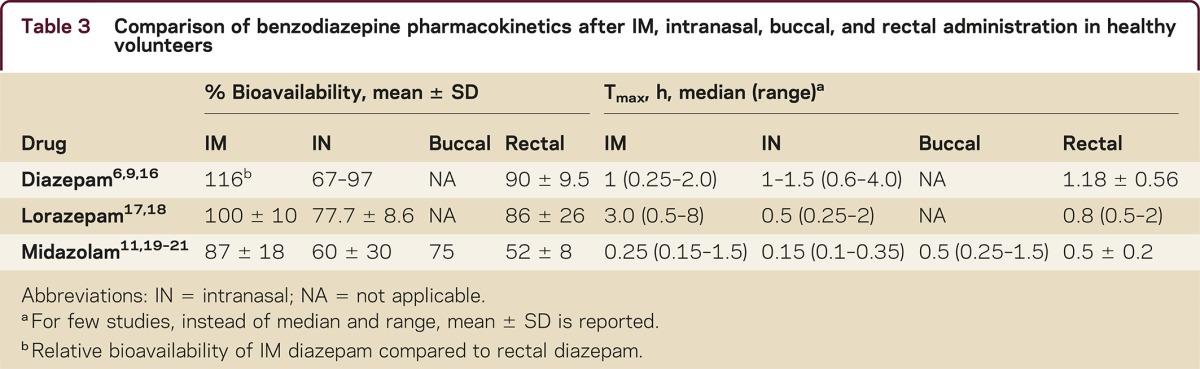
Table 2 is an overview of benzodiazepine rescue therapies that are approved, used off-label, or under development. Currently rectal diazepam and buccal midazolam (Europe only) are approved. Investigational products under development include IN, IM, and subcutaneous formulations. Table 3 provides a comparison of bioavailability and time to maximum concentration (Tmax) of 3 benzodiazepines via different routes of administration.
Table 2 Benzodiazepine seizure rescue therapies
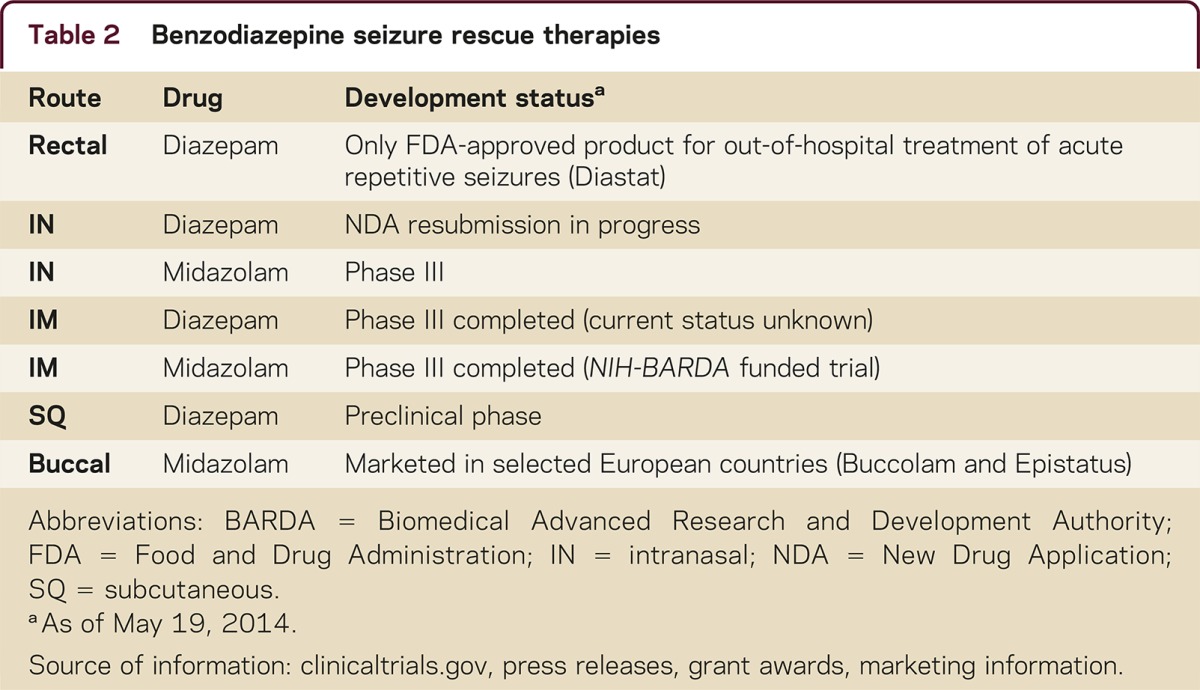
Table 3 Comparison of benzodiazepine pharmacokinetics after IM, intranasal, buccal, and rectal administration in healthy volunteers
Intramuscular administration
IM autoinjector devices can be used to easily administer medications by nonmedical personnel and patients. Studies of IM administration of diazepam, midazolam, and lorazepam show that the former 2 are absorbed more quickly than lorazepam (table 3). In a controlled clinical trial funded by the National Institutes of Health and Biomedical Advanced Research and Development Authority, the efficacy and safety of IM midazolam (5–10 mg) was compared with IV lorazepam (2–4 mg) in children and adults with status epilepticus who were treated by paramedics. Results of this study showed that IM midazolam was superior to IV lorazepam in terminating seizures.5 Both treatment groups were similar with respect to need for endotracheal intubation and recurrence of seizures. As the IM route does not require establishing an IV line, the median time to initiate treatment was shorter (1.2 minutes) for midazolam than IV lorazepam (4.8 minutes) and the corresponding median time from initiating treatment to cessation of convulsions for midazolam was only slightly slower than lorazepam: 3.3 minutes and 1.6 minutes, respectively. In contrast, autoinjector IM diazepam exhibits somewhat slower absorption, approximately the same as rectal diazepam.6 Recently, Abou-Khalil et al.7 reported the results of a randomized, placebo-controlled study of IM diazepam autoinjector for treatment of acute repetitive seizures in which the drug was significantly better than placebo at preventing seizure recurrence.
Intranasal administration
Intranasal administration is simple, convenient, does not require patient cooperation, and generally offers rapid absorption of selected drugs, resulting in a rapid onset of effect. Challenges in developing nasal formulations include overcoming small dose volumes (<200 μL per nostril) limitations and variable absorption. These challenges appeared to have been largely overcome by the investigational diazepam and midazolam products under development. Additional caveats include determining if seizures affect nasal absorption and minimizing the irritation of nasal tissues by organic solvents used in the formulations. An open-label trial conducted in children with prolonged (>10 minutes) febrile seizures, comparing the safety and efficacy of midazolam injectable solution (0.2 mg/kg) given intranasally with IV diazepam (0.3 mg/kg), demonstrated the potential of IN therapy. The results of the study showed that IN midazolam was similar in effectiveness to IV diazepam, but resulted in earlier cessation of seizures due to rapid initiation of IN therapy.8
Pharmacokinetics in healthy volunteers show that absorption following IN administration may be somewhat faster than rectal diazepam, but bioavailability is widely variable. Diazepam and midazolam formulations are being commercially developed in the United States, which can be administered in small dose volumes with no evidence of second peaks in plasma concentration–time profiles due to delayed absorption from swallowing a portion of a dose.9–11 Recent studies evaluating the pharmacokinetics of a cyclodextrin (CD)-based IN midazolam formulation containing the absorption enhancer chitosan in healthy volunteers showed average Tmax and bioavailability to be <10 minutes (range 5–30 minutes) and ∼80% (range 41%–102%), respectively.12,13 Therefore, absorption from CD-based IN midazolam appears to be relatively rapid compared to IN diazepam, but variability in bioavailability was wide with both diazepam and midazolam formulations. Nasal irritation following drug administration was noted in most studies, with one exception (Agarwal et al9). Taken together, these studies demonstrate that IN benzodiazepine formulations under development, despite some limitations, have pharmacokinetic and safety profiles that support their use for management of seizure emergencies.
DISCUSSION
Neurologists should include in their therapeutic armamentarium rescue therapy options for patients who experience seizure emergencies. Available therapies include an FDA-approved rectal diazepam as well as off-label use of buccal and nasal diazepam, lorazepam, and midazolam. However, the safety and efficacy of off-label drugs and routes remains to be established as level I evidence is lacking. IN and IM diazepam and midazolam formulations are under development that will offer clinicians and patients effective, safe, and more acceptable alternatives to treating seizure emergencies outside the hospital.
STUDY FUNDING
No targeted funding reported.
DISCLOSURES
S.K. Agarwal reports no disclosures. J.C. Cloyd serves on scientific advisory boards for CurX Pharmaceuticals and Tansna and a DSMB for University of California at San Diego; serves as a consultant for CurX Pharmaceuticals, Upsher Smith Laboratories, and Zogenix Inc.; and has patents pending re: IV carbamazepine, IV topiramate, and benzodiazepine prodrug/enzyme systems for rescue therapy. Full disclosure form information provided by the authors is available with the full text of this article at Neurology.org/cp.
Supplementary Material
Correspondence to: agar0152@umn.edu
Funding information and disclosures are provided at the end of the article. Full disclosure form information provided by the authors is available with the full text of this article at Neurology.org/cp.
Footnotes
Supplemental data at Neurology.org/cp
Correspondence to: agar0152@umn.edu
Funding information and disclosures are provided at the end of the article. Full disclosure form information provided by the authors is available with the full text of this article at Neurology.org/cp.
REFERENCES
- 1.Kriel RL, Cloyd JC, Hadsall RS, Carlson AM, Floren KL, Jones-Saete CM. Home use of rectal diazepam for cluster and prolonged seizures: efficacy, adverse reactions, quality of life, and cost analysis. Pediatr Neurol. 1991;7:13–17. doi: 10.1016/0887-8994(91)90099-7. [DOI] [PubMed] [Google Scholar]
- 2.Terry D, Paolicchi J, Karn M. Acceptance of the use of diazepam rectal gel in school and day care settings. J Child Neurol. 2007;22:1135–1138. doi: 10.1177/0883073807306254. [DOI] [PubMed] [Google Scholar]
- 3.Poukas VS, Pollard JR, Anderson CT. Rescue therapies for seizures. Curr Neurol Neurosci Rep. 2011;11:418–422. doi: 10.1007/s11910-011-0207-x. [DOI] [PubMed] [Google Scholar]
- 4.Brophy GM, Bell R, Claassen J. Guidelines for the evaluation and management of status epilepticus. Neurocrit Care. 2012;17:3–23. doi: 10.1007/s12028-012-9695-z. [DOI] [PubMed] [Google Scholar]
- 5.Silbergleit R, Durkalski V, Lowenstein D. Intramuscular versus intravenous therapy for prehospital status epilepticus. N Engl J Med. 2012;366:591–600. doi: 10.1056/NEJMoa1107494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lamson MJ, Sitki-Green D, Wannarka GL, Mesa M, Andrews P, Pellock J. Pharmacokinetics of diazepam administered intramuscularly by autoinjector versus rectal gel in healthy subjects: a phase I, randomized, open-label, single-dose, crossover, single-centre study. Clin Drug Investig. 2011;31:585–597. doi: 10.2165/11590250-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 7.Abou-Khalil B, Wheless J, Rogin J. A double-blind, randomized, placebo-controlled trial of a diazepam auto-injector administered by caregivers to patients with epilepsy who require intermittent intervention for acute repetitive seizures. Epilepsia. 2013;54:1968–1976. doi: 10.1111/epi.12373. [DOI] [PubMed] [Google Scholar]
- 8.Lahat E, Goldman M, Barr J, Bistritzer T, Berkovitch M. Comparison of intranasal midazolam with intravenous diazepam for treating febrile seizures in children: prospective randomised study. BMJ. 2000;321:83–86. doi: 10.1136/bmj.321.7253.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Agarwal SK, Kriel RL, Brundage RC, Ivaturi VD, Cloyd JC. A pilot study assessing the bioavailability and pharmacokinetics of diazepam after intranasal and intravenous administration in healthy volunteers. Epilepsy Res. 2013;105:362–367. doi: 10.1016/j.eplepsyres.2013.02.018. [DOI] [PubMed] [Google Scholar]
- 10.Ivaturi V, Kriel R, Brundage R, Loewen G, Mansbach H, Cloyd J. Bioavailability of intranasal vs. rectal diazepam. Epilepsy Res. 2013;103:254–261. doi: 10.1016/j.eplepsyres.2012.07.018. [DOI] [PubMed] [Google Scholar]
- 11.Wermeling DP, Record KA, Archer SM, Rudy AC. A pharmacokinetic and pharmacodynamic study, in healthy volunteers, of a rapidly absorbed intranasal midazolam formulation. Epilepsy Res. 2009;83:124–132. doi: 10.1016/j.eplepsyres.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 12.Hardmeier M, Zimmermann R, Ruegg S. Intranasal midazolam: pharmacokinetics and pharmacodynamics assessed by quantitative EEG in healthy volunteers. Clin Pharmacol Ther. 2012;91:856–862. doi: 10.1038/clpt.2011.316. [DOI] [PubMed] [Google Scholar]
- 13.Haschke M, Suter K, Hofmann S. Pharmacokinetics and pharmacodynamics of nasally delivered midazolam. Br J Clin Pharmacol. 2010;69:607–616. doi: 10.1111/j.1365-2125.2010.03611.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rey E, Treluyer JM, Pons G. Pharmacokinetic optimization of benzodiazepine therapy for acute seizures. Focus on delivery routes. Clin Pharmacokinet. 1999;36:409–424. doi: 10.2165/00003088-199936060-00003. [DOI] [PubMed] [Google Scholar]
- 15.Anderson M. Benzodiazepines for prolonged seizures. Arch Dis Child Educ Pract Ed. 2010;95:183–189. doi: 10.1136/adc.2009.176321. [DOI] [PubMed] [Google Scholar]
- 16.Cloyd JC, Lalonde RL, Beniak TE, Novack GD. A single-blind, crossover comparison of the pharmacokinetics and cognitive effects of a new diazepam rectal gel with intravenous diazepam. Epilepsia. 1998;39:520–526. doi: 10.1111/j.1528-1157.1998.tb01415.x. [DOI] [PubMed] [Google Scholar]
- 17.Graves NM, Kriel RL, Jones-Saete C. Bioavailability of rectally administered lorazepam. Clin Neuropharmacol. 1987;10:555–559. doi: 10.1097/00002826-198712000-00007. [DOI] [PubMed] [Google Scholar]
- 18.Wermeling DP, Miller JL, Archer SM, Manaligod JM, Rudy AC. Bioavailability and pharmacokinetics of lorazepam after intranasal, intravenous, and intramuscular administration. J Clin Pharmacol. 2001;41:1225–1231. doi: 10.1177/00912700122012779. [DOI] [PubMed] [Google Scholar]
- 19.Bell DM, Richards G, Dhillon S. A comparative pharmacokinetic study of intravenous and intramuscular midazolam in patients with epilepsy. Epilepsy Res. 1991;10:183–190. doi: 10.1016/0920-1211(91)90011-4. [DOI] [PubMed] [Google Scholar]
- 20.Clausen TG, Wolff J, Hansen PB. Pharmacokinetics of midazolam and alpha-hydroxy-midazolam following rectal and intravenous administration. Br J Clin Pharmacol. 1988;25:457–463. doi: 10.1111/j.1365-2125.1988.tb03330.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schwagmeier R, Alincic S, Striebel HW. Midazolam pharmacokinetics following intravenous and buccal administration. Br J Clin Pharmacol. 1998;46:203–206. doi: 10.1046/j.1365-2125.1998.00781.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


