Summary
White matter hyperintensities are frequently encountered in clinical practice. In adulthood, white matter hyperintensities are generally related to acquired disorders, such as vascular, inflammatory, or demyelinating diseases. Symmetrical and confluent white matter abnormalities on the first available MRI suggest a genetic disorder. In this article, we provide keys to recognize and classify the adult-onset forms of inherited leukoencephalopathies and to identify their causes by targeting specific biochemical or molecular biomarkers.
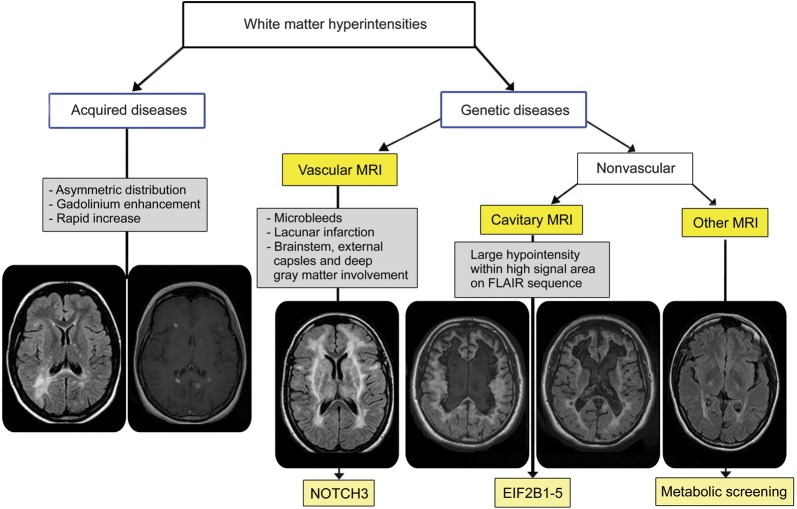
White matter hyperintensities in adulthood are frequently encountered by neurologists in clinical practice and are mainly related to acquired diseases, like cerebral microangiopathy or inflammatory disease such as multiple sclerosis (MS). In rare cases, inherited leukoencephalopathy may be diagnosed. Although rarely present, clinicians should inquire regarding a positive familial history, peripheral neuropathy, occurrence of sensorial involvement (visual loss, hypoacousia), and non-neurologic signs (xanthomas, endocrine dysfunction, syndactyly) (table e-1 at Neurology.org/cp). Neurologic symptoms are often nonspecific and not helpful in making a diagnosis. Patients are commonly referred for cognitive/mood changes, progressive paraparesis/ataxia, or both. Careful analysis of cerebral MRI helps to distinguish acquired from genetic disorders. Asymmetric distribution, gadolinium enhancement, and rapidly evolving lesions strongly argue for acquired etiologies, whereas persistent, symmetric, and confluent demyelination on serial MRI argues for a genetic cause (see diagnostic algorithm, figure 1).
Diagnostic algorithm of the white matter hyperintensities
Figure 1. FLAIR = fluid-attenuated inversion recovery.
Once a genetic cause of the white matter disease is suspected, MRI pattern analysis is a key to investigate for specific biochemical or molecular biomarkers. In adult-onset leukoencephalopathies, we propose considering 3 groups of MRI presentation: vascular, cavitary, and other types (figure 1).
Genetic vascular disorders of the white matter
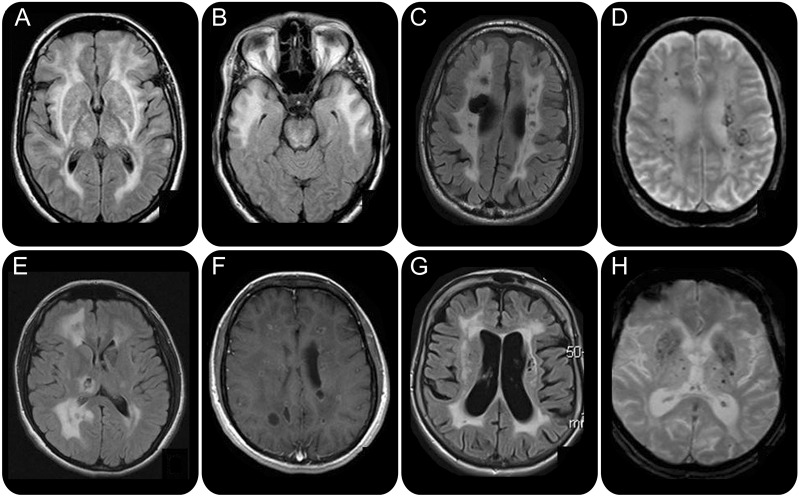
MRI findings suggestive of a vascular mechanism consist of T2/fluid-attenuated inversion recovery (FLAIR) sequences hyperintensities, involving the deep gray matter, the pons, the temporal lobe, and the external capsule. Associated subcortical infarcts, lacunes, microbleeds, calcifications, and dilated perivascular spaces reinforce this hypothesis (figure 2).
Cerebral MRI of genetic vascular disorders of the white matter
Figure 2. (A, B) Cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy. Fluid-attenuated inversion recovery (FLAIR) sequence: symmetrical hyperintensities of external capsules, thalami (A), and anterior part of the temporal lobe (B). (C, D) COL4A1 mutation. (C) FLAIR sequence. Right porencephalic cavity. (D) Gradient echo sequence. Numerous microbleeds. (E, F) Leukoencephalopathy with calcifications and cysts. (E) FLAIR sequence. Cystic aspect in the demyelinating area. (F) T1 weighted with gadolinium injection. Enhancement of the cysts by the contrast product. (G, H) Fabry disease. (G) FLAIR sequence. Lacunes and hyperintensities of the white matter involving the external capsules. (H) Gradient echo sequences. Numerous deep microbleeds.
Three diseases representing the main causes of inherited cerebral microangiopathy must be considered: cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL), the most frequent; COL4A1 mutations–related disease; and cerebroretinal microangiopathy with calcifications and cysts (CRMCC).
CADASIL
CADASIL is by far the most frequent cause of inherited adult-onset cerebral microangiopathy related to heterozygous mutations of the NOTCH3 gene (19p13). Clinical symptoms are classically related to the age of the patients: migraine with aura before the fourth decade, lacunar infarcts and mood disturbances during the fifth decade, and cognitive decline in the sixth. Late onset and incomplete phenotypes are increasingly recognized.1
Familial history suggesting an autosomal dominant transmission could be absent, since incomplete penetrance, variable expressivity, and de novo mutations have been described.
MRI features suggestive of a vascular origin are observed: T2/FLAIR hyperintensities of external capsules, thalami, and pons (figure 2, A and B), associated with deep microbleeds on gradient echo sequences. In this context of vascular MRI, marked hyperintensities of the temporal lobe strongly suggest CADASIL diagnosis (figure 2B), since they are observed in 90% of NOTCH 3 mutated patients.2 Milder MRI phenotypes are increasingly recognized. Age of the patient is important when considering a diagnosis of CADASIL. NOTCH3 gene analysis must be investigated in (1) patients under age 50, with a vascular MRI pattern, even in the absence of the characteristic temporal lobe involvement; and (2) patients above age 50 without any cardioembolic disease or atherosclerosis, and with a typical MRI pattern.
A rare and recessive form mimicking CADASIL, cerebral autosomal recessive arteriopathy with subcortical infarcts and leukoencephalopathy (CARASIL), has been reported. CARASIL is mainly described in Asia, with peculiar extraneurologic symptoms such as alopecia and spondylosis. The mutated gene, HTRA1, should not be routinely screened, except for patients with recessive inheritance and extraneurologic signs.3
COL4A1 mutations–related diseases
Mutations in the COL4A1 gene were initially described in children as familial porencephaly. Common features were initially characterized by dominant inheritance, cerebral palsy with porencephalic cavities, and recurrent brain hemorrhages.4 Porencephaly is defined by a fluid-filled cavity, communicating with the lateral ventricle.
Phenotype is now widening, including adult-onset cases, sporadic cases, mild phenotype, ophthalmologic involvement (retinal arteriolar tortuosities, cataracts, retinal hemorrhages, Axenfeld-Rieger anomaly), and intracranial aneurysms. Association of nephropathy, muscle cramps, and aneurysms has been described as hereditary angiopathy with nephropathy, aneurysms, and cramps (HANAC) syndrome.5
MRI features consist of a classical vascular pattern. In addition, occurrence of deep hemorrhages (deep microbleeds, parenchymal hematomas), calcifications, intracranial aneurysms, and porencephalic cavities (figure 2, C and D) and absence of temporal lobe involvement strongly suggest to search for a COL4A1 gene mutation.
Since milder phenotypes have been recognized, COL4A1 gene analysis is also recommended in case of unexplained isolated profound hemorrhage in patients under age 50.
More recently, COL4A2 gene mutations have been identified in adult patients with porencephalic cavities and brain hemorrhage.6
CRMCC
The association of white matter changes with calcifications and intracranial cysts should evoke CRMCC, a rare recessive multisystem disorder initially described in infancy or early childhood. Extraneurologic symptoms are frequent, including retinal telangiectasias, osteopenia, sparse hair, and dystrophic nails. Intracranial cysts could be very large, evolving with increasing size or appearance of new cysts on serial MRI (figure 2, E and F). CRMCC includes 2 entities: leukoencephalopathy with calcifications and cysts (LCC), restricted to the CNS, and Coats plus disease, which associates retinal telangiectasia with retinal exudation, intracranial calcifications, white matter changes, and extraneurologic symptoms (digestive hemorrhage, skeletal abnormalities, hepatopathy, disorder of skin appendages). Coats plus disease and LCC are likely the same clinical entity, with a common primary pathogenesis based on a small-vessel obliterative microangiopathy. Mutations in the conserved telomere maintenance component 1 gene (CTC1) were recently identified in patients with CRMCC.7 Further molecular analysis is needed in order to confirm the genetic heterogeneity of CRMCC.
Familial cerebral amyloid angiopathy
Cerebral amyloid angiopathy (CAA) is characterized by leukoaraiosis, intracerebral hemorrhage, and lobar microbleeds. Compared with the sporadic forms, familial CAA (fCAA) is characterized by an earlier age at onset and a more severe clinical course. fCAA is excessively rare. In the absence of relatives with dementia or brain hemorrhage, genes involved in fCAA (at first APP gene) should not be routinely investigated.
Rare but treatable diseases: Fabry disease and cerebrotendinous xanthomatosis
Inherited metabolic disorders affecting small cerebral vessels such as Fabry disease and cerebrotendinous xanthomatosis (CTX) must be considered in case of vascular white matter MRI pattern since specific treatment can be prescribed. Extraneurologic features (painful neuropathy, angiokeratomas or cutaneous xanthomas, renal insufficiency, cataracts) must be meticulously searched. Symmetrical involvement of the dentate nucleus argues for a diagnosis of CTX. T1 hyperintensity of the thalami (the pulvinar sign) and basilar artery dilation are classically described in Fabry disease.8 As a rule, cholestanol and α-galactosidase A must be measured in patients with a vascular white matter MRI pattern without any explanation (figure 2, G and H).
New and unknown vascular leukoencephalopathies
New phenotypes of dominant vascular leukoencephalopathies are increasingly recognized. Recently, an autosomal dominant inherited vascular leukoencephalopathy sharing some MRI features with CADASIL was published. The hallmark of this new disease was a constant involvement of the pons sparing the medulla and cerebellum, and absence of ischemic or hemorrhagic strokes. The gene was mapped to 20q13.9
In patients without any diagnosis, careful definition of MRI patterns can help to classify them in order to identify new biochemical or molecular markers.
Cavitary leukoencephalopathies
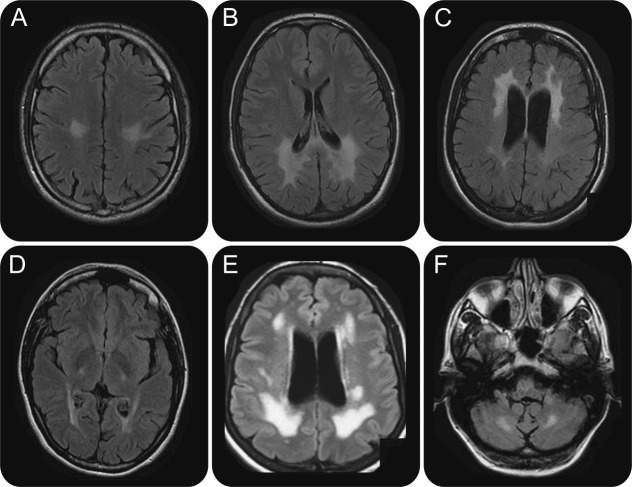
A cavitary leukoencephalopathy is radiologically defined by the association of hypointensities within large areas of demyelination on FLAIR sequences (figure 1). In contrast to porencephaly, they are not connected to the ventricles and they are within the demyelination.10 They are not enhanced by gadolinium injection, unlike cysts. This rare aspect should suggest an adult-onset form of childhood ataxia with CNS hypomyelination (CACH) diagnosis.
CACH is an autosomal recessive leukodystrophy, initially described as having childhood onset (2–5 years), leading to death in a few years. It was also named vanishing white matter disease (VWM) due to its peculiar MRI pattern with progressive cavitation of diffuse edematous white matter. Mutations in 1 of the 5 EIF2B1–5 genes, encoding the 5 subunits of the eukaryotic initiation factor eIF2B, were further identified in classical CACH/VWM as well as in congenital and juvenile-/adult-onset forms. Adult-onset phenotypes have been more recently described. In our series of 16 consecutive patients, a sex imbalance (male/female = 3/13) and a mean age at onset of 46 years (range 16–62) were observed.10 Two findings are characteristic: influence of stress (that worsened and revealed the symptoms in 38% of the cases) and premature ovarian failure. Occurrence of ovarian failure, termed as ovarioleukodystrophy, was effectively present in the majority of the female patients of our adult series.10
MRI shows a typical abnormal extensive signal of the cerebral white matter with a diffuse T1-weighted sequence hypointensity and decreased FLAIR signal within the T2-weighted hyperintensity observed in the majority (15/16 patients of our series) of the cases. MRI features also include a constant cerebral atrophy, sparing the U-fibers, and frequent involvement of the corpus callosum and the cerebellum.10
In adult-onset forms of EIF2B-related disorders, mutations are mainly found in the EIF2B5 gene, with a recurrent R113H mutation observed in 71% of the cases.10
Rarely, cavitary leukoencephalopathies have been reported in mitochondrial diseases, megalencephalic leukoencephalopathy, and childhood-onset form of Alexander disease. In contrast to CACH/VWM, these cystic lesions are less extensive and well-delineated.
In fact, the main differential diagnoses of cavitary leukodystrophies in adult patients are acquired disease, mostly MS. In our series of 9 patients with MS with large cavitary lesions,11 initially referred to as a cavitary leukodystrophy, the MS diagnosis was based on 2 arguments: (1) absence of mutation of the 5 EIF2B genes and (2) a careful analysis of the clinical data, including previous relapses, presence of oligoclonal bands on CSF study, and partial transverse myelitis on spinal cord MRI.
MRI without any vascular or cavitary pattern
This heterogenous group includes patients with white matter hyperintensities, without any argument, to search for a vascular mechanism or a cavitary leukoencephalopathy.
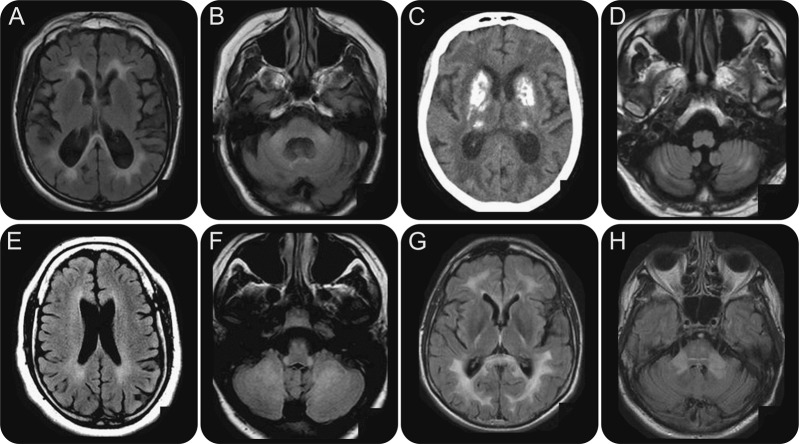
Most of the patients present as sporadic cases, because of a frequent recessive inheritance. An inborn error of metabolism has to be searched first in these cases. Since an inborn error of metabolism is found in almost 25% of childhood leukodystrophies,12 we propose to carry out a systematic biochemical workup in adult patients without vascular or cavitary demyelinating MRI pattern (table e-2). The most frequent metabolic leukodystrophies in adult-onset cases are X-linked adrenoleukodystrophy (figure 3, A and B), metachromatic leukodystrophy (figure 3C), Krabbe disease (figure 3D), and CTX (figure 3, E and F). Interestingly, patients with CTX can have either a vascular or a nonspecific leukoencephalopathy. The diagnosis is based on very long-chain fatty acid level (increased), arylsulfatase or galactocerebrosidase activity (decreased), or cholestanol (increased). However, as the clinical symptoms and MRI features of most inherited leukoencephalopathies are not specific, sometimes with isolated corticospinal tract involvement, a standardized biochemical investigation is proposed to search for rarer diseases (table e-2). The enzyme deficiency has to be systematically confirmed by the genetic analysis.
Cerebral MRI of leukodystrophies with biochemical markers (axial fluid-attenuated inversion recovery sequences)
Figure 3. (A, B) X-linked adrenoleukodystrophy. Symmetrical hyperintensities of the white matter with parieto-occipital predominance. (C) Metachromatic leukodystrophy. Symmetrical hyperintensities of the white matter with frontal predominance. (D) Krabbe disease. Hyperintensities of the corticospinal tracts. (E, F) Cerebrotendinous xanthomatosis. Diffuse leukoencephalopathy (E) and hyperintensities of the dentate nuclei (F).
In cases of negative metabolic investigation, a careful analysis of the MRI features is important to guide the genetic analysis (table e-3).
Cerebellar involvement is suggestive of CTX (figure 3, E and F). Association with middle cerebellar peduncle involvement suggests fragile X–associated tremor/ataxia syndrome13 (notably in the presence of late-onset tremor, ataxia, and cognitive impairment) (figure 4, A and B).14
Calcifications (mainly cerebellar or deep basal ganglia nuclei) and cerebellar hyperintensities/atrophy suggest first a mitochondrial disorder (figure 4, C and D). This diagnosis is reinforced by occurrence of sensorial symptoms (hypoacousia, optic atrophy) and diabetes. Intracranial calcifications are also found in the Nasu-Hakola disease,15 including rheumatologic symptoms (osteopenia, bone cysts) and frontal cognitive involvement.
Subtle periventricular white matter hyperintensities with corticospinal tract involvement can be found in hereditary spastic paraplegia mainly related to spastic paraplegia (SPG)–10 and SPG-11 (corpus callosum atrophy is an additional indication of the latter) (figure 4E). The diagnosis is reinforced by occurrence of chronic spastic paraparesis.
Mesencephalic trigeminal tract involvement with cerebellar involvement and hyperintensities of the posterior part of the spinal cord is suggestive of leukoencephalopathy with brainstem and spinal cord involvement and lactate elevation syndrome (DARS2 gene mutation) (figure 4F).14
Temporal cysts strongly suggest a megalencephalic leukoencephalopathy with subcortical cysts diagnosis.16
Hyperintensities predominant in the internal and external capsules, pons, and medulla with spinal cord atrophy suggest a diagnosis of adult polyglucosan body disease. This suspicion is reinforced by occurrence of progressive spastic paraplegia with neurogenic bladder and peripheral axonal neuropathy. Diagnosis is supported by the glycogen branching enzyme deficiency and GBE1 gene mutation.17
A hypomyelinated MRI pattern, defined by T1 isointensity or hyperintensity of the white matter, is rarely observed in adult-onset forms of leukodystrophies. Two diseases can be suspected in adult patients: PLP1 (SPG2) or GJC2 (SPG44) mutations with a predominant spastic paraplegia and hypomyelination with hypodontia and hypogonadotropic hypogonadism (4H) syndrome with a predominant cerebellar ataxia.
Neuroradiologic examinations of leukoencephalopathies with molecular markers
Figure 4. (A, B) Fragile X–associated tremor/ataxia syndrome. Axial fluid-attenuated inversion recovery (FLAIR): hyperintensities involve the splenium of the corpus callosum (A) and the middle cerebellar peduncles (B). (C, D) Mitochondrial disorders. (C) CT scan. Bilateral and profound calcifications. (D) Axial FLAIR MRI: hyperintensities of the cerebellar white matter. (E) Spastic paraplegia–11. Axial FLAIR sequence: subtle hyperintensities of the periventricular white matter. (F) Leukoencephalopathy with brainstem and spinal cord involvement and lactate elevation syndrome. Axial FLAIR sequence: hyperintense signal of the inferior cerebellar peduncles, pyramidal tracts, and cerebellar white matter. (G, H) Adult-onset autosomal dominant leukodystrophy. Axial FLAIR sequences: hyperintensities of the splenium of the corpus callosum, corticospinal tracts (G), and middle cerebellar peduncles (H).
In few cases, the pedigree analysis argues for a dominant transmission, a mode of inheritance rarely observed in leukodystrophies with metabolic markers. Some genes are identified in dominant leukodystrophies:
Involvement of the brainstem, cerebellum, middle cerebellar peduncles, and corticospinal tracts (figure 4, G and H) in a context of a severe ataxia, dysautonomia, and tremor is suggestive of adult-onset autosomal dominant leukodystrophy diagnosis (related to a lamin B1 gene duplication).18
Predominantly frontal hyperintensities are in favor of the pigmentary orthochromatic leukodystrophy/hereditary diffuse leukoencephalopathy with spheroids diagnosis (CSF1R gene mutation).19 Association with brainstem atrophy and palatal tremor suggests Alexander disease (GFAP gene mutation).20
Dominant inherited hypomyelination, reinforced by occurrence of syndactylia, suggests a diagnosis of the oculodentodigital syndrome (connexin 43/GJA1 gene mutation).
Patients without any diagnosis
Finally, some patients can remain without any diagnosis. Acquired diseases or new phenotypes could explain negative results. Repeat clinical, sensorial, neurophysiological, and MRI evaluation must be performed according to the disease progression. In these latter cases, technological advances including next-generation sequencing will probably allow the identification of new pathogenic genes.
DISCUSSION
Neurologists should be aware of the genetic form of white matter diseases, even in the absence of family history. Symmetrical involvement on the first available MRI is the essential distinctive finding since it strongly suggests an inherited disorder and allows a classification into 3 groups: vascular, cavitary, and nonspecific. In the vascular group, CADASIL should be evoked first. Cavitary aspects strongly suggest EIF2B-related disorders. In the third group, a standardized approach by a metabolic screening remains mandatory. Confirmation of the inborn metabolism error requires the presence of a pathogenic mutation. In patients without any enzyme deficiency, a careful analysis of the MRI and the clinical data are important to search for a mutation in specific pathogenic genes.
STUDY FUNDING
No targeted funding reported.
DISCLOSURES
P. Labauge reports no disclosures. C. Dalliere has received funding for travel from Merck Serono. N. Menjot de Champfleur and X. Ayrignac report no disclosures. O. Boespflug-Tanguy receives research support from the European Leukodystrophies Foundation. Full disclosure form information provided by the authors is available with the full text of this article at Neurology.org/cp.
Correspondence to: labauge@yahoo.fr
Funding information and disclosures are provided at the end of the article. Full disclosure form information provided by the authors is available with the full text of this article at Neurology.org/cp.
Footnotes
Supplemental data: Neurology.org/cp
Correspondence to: labauge@yahoo.fr
REFERENCES
- 1.Chabriat H, Joutel A, Dichgans M, Tournier-Lasserve E, Bousser MG. CADASIL. Lancet Neurol. 2009;7:643–653. doi: 10.1016/S1474-4422(09)70127-9. [DOI] [PubMed] [Google Scholar]
- 2.Pantoni L, Pescini F, Nannucci S. Comparison of clinical, familial, and MRI features of CADASIL and NOTCH3-negative patients. Neurology. 2010;74:57–63. doi: 10.1212/WNL.0b013e3181c7da7c. [DOI] [PubMed] [Google Scholar]
- 3.Hara K, Shiga A, Fukutake T, Nozaki H. Association of HTRA1 mutations and familial ischemic cerebral small-vessel disease. N Engl J Med. 2009;360:1729–1739. doi: 10.1056/NEJMoa0801560. [DOI] [PubMed] [Google Scholar]
- 4.Vahedi K, Alamowitch S. Clinical spectrum of type IV collagen (COL4A1) mutations: a novel genetic multisystem disease. Curr Opin Neurol. 2011;1:63–68. doi: 10.1097/WCO.0b013e32834232c6. [DOI] [PubMed] [Google Scholar]
- 5.Plaisier E, Gribouval O, Alamowitch S. COL4A1 mutations and hereditary angiopathy, nephropathy, aneurysms, and muscle cramps. N Engl J Med. 2007;357:2687–2695. doi: 10.1056/NEJMoa071906. [DOI] [PubMed] [Google Scholar]
- 6.Verbeek E, Meuwissen ME, Verheijen FW. COL4A2 mutation associated with familial porencephaly and small-vessel disease. Eur J Hum Genet. 2012;8:844–851. doi: 10.1038/ejhg.2012.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Polvi A, Linnankivi T, Kivelä T. Mutations in CTC1, encoding the CTS telomere maintenance complex component 1, cause cerebroretinal microangiopathy with calcifications and cysts. Am J Hum Genet. 2012;3:540–549. doi: 10.1016/j.ajhg.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zarate YA, Hopkin RJ. Lysosomal storage disease 3: Fabry's disease. Lancet. 2008;372:1427–1435. doi: 10.1016/S0140-6736(08)61589-5. [DOI] [PubMed] [Google Scholar]
- 9.Herve D, Chabriat H, Rigal M. A novel hereditary extensive vascular leukoencephalopathy mapping to chromosome 20q13. Neurology. 2012;79:2283–2287. doi: 10.1212/WNL.0b013e3182768954. [DOI] [PubMed] [Google Scholar]
- 10.Labauge P, Horzinski L, Ayrignac X. Natural history of 16 patients with adult onset vanishing white matter disease. Brain. 2009;132:2161–2169. doi: 10.1093/brain/awp171. [DOI] [PubMed] [Google Scholar]
- 11.Renard D, Brochet B, Vukusic S. Clinical and radiological characteristics in multiple sclerosis patients with large cavitary lesions. Eur Neurol. 2012;68:156–161. doi: 10.1159/000338476. [DOI] [PubMed] [Google Scholar]
- 12.Bonkowsky JL, Nelson C, Kingston JL, Filloux FM, Mundorff MB, Srivastava R. The burden of inherited leukodystrophies in children. Neurology. 2010;75:718–725. doi: 10.1212/WNL.0b013e3181eee46b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Apartis E, Blancher A, Meissner WG. FXTAS: new insights and the need for revised diagnostic criteria. Neurology. 2012;79:1898–1907. doi: 10.1212/WNL.0b013e318271f7ff. [DOI] [PubMed] [Google Scholar]
- 14.Labauge P, Dorboz I, Eymard-Pierre E, Dereeper O, Boespflug-Tanguy O. Clinically asymptomatic adult patient with extensive LBSL MRI pattern and DARS2 mutations. J Neurol. 2011;258:335–337. doi: 10.1007/s00415-010-5755-5. [DOI] [PubMed] [Google Scholar]
- 15.Klünemann HH, Ridha BH, Magy L. The genetic causes of basal ganglia calcification, dementia, and bone cysts: DAP12 and TREM2. Neurology. 2005;64:1502–1507. doi: 10.1212/01.WNL.0000160304.00003.CA. [DOI] [PubMed] [Google Scholar]
- 16.Leegwater PA, Boor PK, Yuan BQ. Identification of novel mutations in MLC1 responsible for megalencephalic leukoencephalopathy with subcortical cysts. Hum Genet. 2002;110:279–283. doi: 10.1007/s00439-002-0682-x. [DOI] [PubMed] [Google Scholar]
- 17.Mochel F, Schiffmann R, Steenweg ME. Adult polyglucosan body disease: natural history and key magnetic resonance imaging findings. Ann Neurol. 2012;72:433–441. doi: 10.1002/ana.23598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Padiath QS, Saigoh K, Schiffmann R. Lamin B1 duplications cause autosomal dominant leukodystrophy. Nat Genet. 2006;38:1114–1123. doi: 10.1038/ng1872. [DOI] [PubMed] [Google Scholar]
- 19.Nicholson AM, Baker MC, Finch NA. CSF1R mutations link POLD and HDLS as a single disease entity. Neurology. 2013;80:1033–1040. doi: 10.1212/WNL.0b013e31828726a7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li R, Johnson AB, Salomons G. Glial fibrillary acidic protein mutations in Infantile, juvenile, and adult forms of Alexander disease. Ann Neurol. 2005;57:310–326. doi: 10.1002/ana.20406. [DOI] [PubMed] [Google Scholar]