Summary
Cervical spondylotic myelopathy (CSM) is the leading cause of myelopathy in patients over age 50 years. Despite advances, CSM remains a clinical diagnosis and its natural history remains unclear. The treatment of CSM is controversial, especially in patients with mild or moderate clinical disease without rapid progression of symptoms. Herein, we begin with a clinical vignette followed by a brief description of the clinical problems. We discuss evaluation, treatment, and recommendations for the treatment of CSM. Emphasis is drawn to areas of uncertainty and present level of evidence for the treatment modalities of CSM.
Cervical spondylotic myelopathy (CSM) is a degenerative condition of the cervical spine and the leading cause of myelopathy in those over the age of 50.1–3 The exact incidence and prevalence of CSM is unknown.1 Initially, patients may present with any of the following symptoms: neck pain and decreased mobility of the cervical spine, numb or clumsy hands, unsteady gait, hyperreflexia, and spasticity. In time, upper extremity weakness, muscle atrophy, or sphincter dysfunction might develop.4–7
Clinical vignette
A 61-year-old teacher with a history of intermittent neck pain is seen after 5 months of continuing neck pain. MRI 2 months after the onset of pain showed degenerative changes with spinal stenosis in the cervical region. He is very anxious because the first physician he consulted recommended decompressive surgery and the second recommended conservative treatment and to avoid surgery. He read on the Internet that he could eventually become paralyzed and therefore seeks further opinions. How should this patient be evaluated and treated?
The pathophysiology of CSM involves 3 main components. The first component is the static factor, where structural changes cause spinal canal stenosis and cord compression. A strong correlation has been demonstrated between narrowing of the sagittal diameter of the cervical spine and the development of CSM.8–12 The second component is the dynamic mechanical factor, characterized by repetitive movement of the compressed spinal cord. In flexion, the spinal cord lengthens, which results in axial tension and, potentially, ischemia.13–15 In extension, the spinal canal shortens so that its cross-sectional area decreases. Furthermore, the ligamentum flavum buckles inward. Both changes result in a high risk of compression of the cervical spinal cord.2,11,15,16 The third component consists of histopathologic and vascular changes resulting in ischemia, infarction, apoptosis, and other toxic cell alterations.17–20
The natural history of CSM seems dependent on the severity of the condition. It is generally accepted that patients with severe symptoms will not improve or will undergo a steady progression.e1 However, the natural history of mild to moderate forms of CSM is unclear. Some have reported that approximately one-third of patients will deteriorate and require surgical treatment and others have reported that deterioration is rare.5,e2–e4 Due to this uncertainty, there is an ongoing debate regarding the best management strategy for these patients.
Evaluation
The most commonly used measures to evaluate CSM are the Nurick grade6 and the modified Japanese Orthopedic Association (mJOA) scoring system (tables 1 and 2). Patients with an mJOA score of ≥12 or 0–3 Nurick grade are considered to have mild to moderate forms of the disease. Otherwise the disease is considered severe.
Table 1 Nurick grades

Table 2 Modified Japanese Orthopedic Association scoring system

Imaging
MRI of the cervical spine (figure) is the modality of choice, not only because the etiology of the canal stenosis can be identified, but also because the magnitude of cord compression, number of levels affected, and intramedullary signal intensity (SI) change. MRI parameters have been used as predictors of functional outcome. However, there is conflicting evidence as to whether spinal cord atrophy or the degree of spinal canal stenosis may predict outcome.e5 Increased intramedullary SI on T2-weighted images and decreased SI on T1-weighted images (figure) are often seen with CSM and are related to unfavorable surgical outcome.e6 Plain radiographs and CT are also useful in identifying changes in the disc space, osteophytes, spinal alignment,e7 and instability of the cervical spine.e8
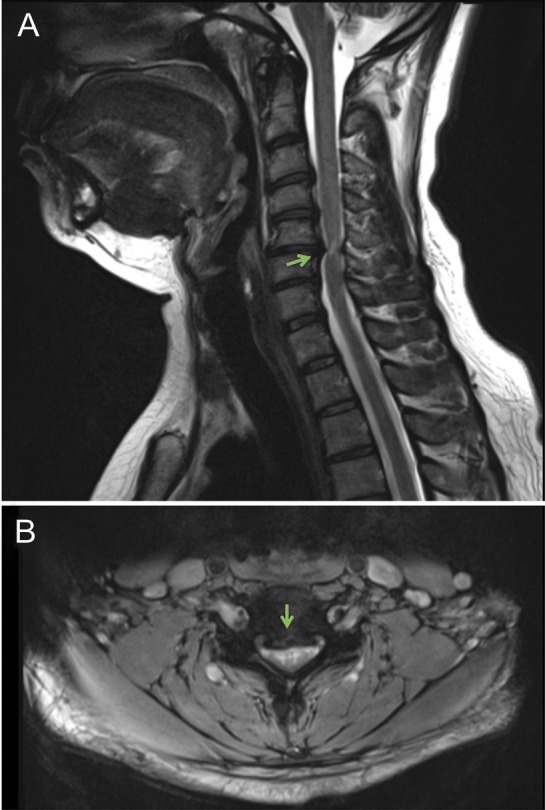
MRI of the cervical spine
Figure. T2-weighted MRI of the cervical spine in a sagittal view (A) illustrates herniated disks at C3-C4, C4-C5, and C5-C6 as well as an osteophytic spur at C5-C6 with spinal cord compression. T2-weighted imaging in an axial view (B) at C5-C6 shows the level of maximum compression. At the level of maximum compression, C5-C6, a hyperintensity signal (arrow), is illustrated.
Evoked potentials
Somatosensory evoked potentials (SEP) and motor evoked potentials (MEP) are an objective and sensitive method for detecting spinal cord compromise in patients with CSM.e9 In asymptomatic patients with spinal cord compression, with or without intramedullary SI changes on MRI, the presence of abnormal SEP and MEP could predict progression into clinically evident CSM in most cases.e10 However, the use of SEP and MEP as predictors of surgical outcome is unclear. Limited data suggest that SEP could have a role in predicting surgical success. Favorable surgical outcome is associated with normal preoperative or early postoperative improvement of median SEP.e11,e12 Although MEP are sensitive in detecting CSM, data reflecting their predictive value are lacking.e13
Treatment
Treatment of CSM is primarily based on the severity and dynamics of the condition. Operative treatment is favorable for patients with rapid progression or severe forms of the disease (mJOA < 12). However, there is no consensus in practice about how to manage patients with mild to moderate forms of CSM (mJOA ≥ 12). Because the natural history in the long term of mild to moderate forms of CSM remains uncertain,e1 it is not clear if surgical decompression is necessary.e2,e3,e14–e18
Surgical treatment
Various surgical approaches have been proposed for patients with CSM. These include laminectomy with or without fusion, laminoplasty, corpectomy with grafting, anterior discectomy and fusion, or combined anterior–posterior procedures. Many studies have attempted to clarify which surgical approach is best suited to patients with CSM.7,e19–e24 Some advocate the strategy of “treat anterior pathology anteriorly and posterior pathology posteriorly.” However, the surgical outcome in CSM is dependent on more than just the approach. The patient's age, the radiographic transverse area of the level of maximal compression of the spinal cord, and duration of symptoms are some of the factors affecting the surgical outcome in patients with CSM.e19,e20
A systematic review comparing the efficacy of different surgical techniques for the treatment of CSM showed that several well-accepted surgical techniques using the anterior or posterior approach produce similar results.e25 The study group concluded that because of the high relative effectiveness and similarity of costs and complications of these different surgical approaches, it may not be necessary to devote substantial resources to clinical trails designed to determine small advantages of one technique over the other.
In a recent multicenter study of 302 patients,e26 the authors assessed the rates of and risk factors related to perioperative and delayed complications associated with the surgical treatment of CSM. There was no standardization of surgical treatment and the patients were treated with anterior-only (n = 176), posterior-only (n = 107), or combined anterior–posterior (n = 19) approaches. Perioperative complications were encountered in 47 (15.6%) patients. The most common perioperative complications were minor cardiopulmonary events (3.0%), dysphagia (3.0%), and superficial wound infection (2.3%). The extent of myelopathy worsened in 4 patients (1.3%) and 1 patient died postoperatively (0.33%). At 2-year follow-up, 275 patients (follow-up rate of 91%) were available and presented an overall delayed complications rate of 4.4%. Significant difference between the surgical approaches with regards to complication rates was seen for infection rates following anterior-only or posterior-only approaches (0.6% vs 4.7%) and dysphagia was more often encountered following the combined anterior–posterior approach (21.1%) compared with the anterior-only approach (2.3%) or posterior-only approach (0.9%). The authors were able to identify greater age, increased operative time, and use of combined anterior–posterior procedures as risk factors for complications.
Conservative treatment
Conservative treatment is feasible and safe for most patients with mild to moderate CSM.e4,e18,e27 In prospective cohort study of 60 patients with mild CSM, all patients were treated by in-bed Good Samaritan cervical traction for 8 hours a day for 2 weeks and were encouraged to avoid risky activities. For final evaluation, 55 patients were assessed. There was no significant difference in mean JOA score in all cases between first visit and endpoint. Forty-one of the 55 (74.5) patients remained stable after more than 5 years. However, progression of the condition was seen in 14 (25.5%) patients.e27 In a prospective study of 70 patients with mild CSM,e4 the authors assessed outcome following conservative treatment. All patients were treated by in-bed Good Samaritan cervical traction for 8 hours a day for approximately 2 weeks with or without anti-inflammatory drugs. The patients were then advised to avoid risky activities. For final analyses (mean follow-up of 35.6 ± 25.2 months), 56 patients were examined. There was also no significant difference in mean JOA score in all cases between first visit and endpoint. However, 11 of 56 (19.6%) deteriorated and therefore were referred to surgery. Others have implemented more applicable regimens of conservative treatment. Others used intermittent cervical immobilization with a soft collar, anti-inflammatory drugs, and intermittent bed rest for patients with mild to moderate CSM (mJOA ≥ 12).e2,e3 The patients were also advised to avoid high-risk activities and to avoid risky environments involving physical overloading, manipulation therapy, or rigorous or prolonged flexion of the head. The authors concluded that the symptoms of mild CSM in patients younger than 75 years do not worsen in the majority of patients (80%). A retrospective evaluation of 45 patients with mild CSM following conservative treatment revealed that the majority of patients did not require surgery 5 years (82%) or 10 years (56%) following initial treatment.e18 Total cervical range of motion, segmental kyphosis in the maximum compressed segment, and local slip were significant prognostic factors for worse outcome.e18
Because chronic compression of the spinal cord is believed to cause ischemia and a range of inflammatory and cytotoxic cell changes,19,20 anti-inflammatory drugs have been advocated. However, none of the commonly recommended drugs has been tested in randomized, placebo-controlled trials. Currently, a multicenter randomized controlled trial (http://clinicaltrials.gov/ct2/show/NCT01257828) is examining the adjuvant effect of the neuroprotective drug riluzole following decompressive surgery for CSM. Riluzole, Food and Drug Administration–approved for the treatment of amyotrophic lateral sclerosis, blocks glutamate receptors and increases glutamate receptor activity and is thereby thought to decrease glutamate excitotoxicity.e28
Areas of uncertainty
The main uncertainty regarding CSM remains its natural history. Although sufficient evidence is lacking, there is a general understanding that patients with severe symptoms (mJOA < 12) and a long duration of impairment do not improve following conservative treatment.e1 Therefore, it would be unethical to conduct a randomized trial in order to provide high-level evidence for those patients. For patients with mild to moderate CSM, progression of the disease can be slow, with stepwise decline, or with long periods of quiescence.e1 Due to this uncertainty, there is an ongoing debate regarding the best management strategy for CSM. The only 2 prospective comparative studies on this topic provide conflicting results.e2,e3,e17
Kadanka et al.e2 performed a single-center randomized study with 68 patients presenting with mild to moderate forms of CSM, in which 33 patients underwent surgical decompression and 35 patients received conservative treatment. At 3-year follow-up, there were on average no differences in clinical outcome between the groups. At 10-year follow-up, 25 patients in the conservatively treated group and 22 in the surgically treated group were evaluated. There were no statistical differences between the groups in mJOA score or subjective evaluation by the patients themselves.e3 Although this was a randomized study, the evidence provided is considered to be low, because the study seems to have had an inappropriately small sample size, as evidenced by the large SD in the demographic factors of each group.e29
Sampath et al.e17 examined 43 patients with CSM in a multicenter study, in which 23 patients received conservative treatment and the rest underwent surgical decompression. According to their findings, surgically treated patients showed a substantial improvement in functional status and in neurologic symptoms compared with patients treated conservatively. Therefore, the authors concluded that surgical treatment is superior to nonsurgical management. However, this study is considered to provide low-level evidence because the results may have been biased by a short term of follow-up (mean 11.2 months), a low follow-up rate (68%), and the fact that the patients were not randomized, and were selected without regard to the degree of their myelopathy.
Amid potential cases considered for surgery, there is no consensus on the best surgical approach or timing of surgery. All applied surgical approaches seem to yield similar results without significant differences in radiologic or clinical outcome.e25
Recommendation
It is recommended that for patients younger than 75 years with mild to moderate CSM (mJOA ≥ 12), both operative and nonoperative management options should be offered, as objectively measured deterioration in function is rarely seen acutely.e30 It is also recommended that operative therapy be offered to patients with severe symptoms and longer duration of symptoms, because the likelihood of improvement with nonoperative measures is low.e1 For patients with symptomatic spondylotic cervical stenosis without clinically evident myelopathy (mJOA = 18), in whom either abnormal electrophysiology findings or clinical radiculopathy are observed, decompressive surgery may be considered as both factors are associated with an approximately 5% annual risk of CSM development in this patient population.e1 For conservative treatment, prolonged immobilization in a cervical collar, “low-risk” activity modification or bed rest, and anti-inflammatory drugs, depending on the patient's preference, are recommended.e30
As the authors of these guidelines stated, guidelines can be only as strong as the underlying evidence. Because there is no high-level evidence for patients with CSM, these recommendations are also a matter of debate.
DISCUSSION
For the patient described in the vignette, we would recommend conservative treatment and yearly follow-up examinations if electrophysiologic examinations were normal. In case abnormalities are depicted in electrophysiologic examinations, in particular median SEP, then surgery could be considered. It is important to convey to the patient the uncertainties regarding this condition and the lack of evidence supporting a best treatment option. Also, the patient needs to know that this particular treatment decision is based on our opinion and other surgeons might recommend surgery. For conservative treatment, we would prescribe an anti-inflammatory drug and physiotherapy. Cervical immobilization in a cervical collar and low-risk activity modifications are discussed with the patient but are not recommended due to the lacking evidence. Surgery should be preserved for patients with severe forms of cervical myelopathy (mJOA < 12) and could be considered for patients presenting with mild to moderate forms (mJOA ≥ 12) with abnormal electrophysiologic examinations or who show progression while being treated conservatively.
As the population ages, the incidence of CSM will continue to rise. The overwhelming majority of patients have mild to moderate forms of CSM. To date, there are no high-evidence studies providing recommendations for the best treatment modality. It is surprising that such a common and interdisciplinary encountered condition, with a potentially devastating outcome, has no well-founded recommendations. It is time for a well-designed, randomized, and multicenter trial.
STUDY FUNDING
No targeted funding reported.
DISCLOSURES
The authors report no disclosures. Full disclosure form information provided by the authors is available with the full text of this article at Neurology.org/cp.
Correspondence to: Ehab.Shiban@lrz.tum.de
Funding information and disclosures are provided at the end of the article. Full disclosure form information provided by the authors is available with the full text of this article at Neurology.org/cp.
Footnotes
Supplemental data: Neurology.org/cp
Correspondence to: Ehab.Shiban@lrz.tum.de
REFERENCES
- 1.Kalsi-Ryan S, Karadismas SK, Fehlings MG. Cervical spondylotic myelopathy: the clinical phenomenon and the current pathobiology of an increasingly prevalent and devastating disorder. Neuroscientist. 2013;19:409–421. doi: 10.1177/1073858412467377. [DOI] [PubMed] [Google Scholar]
- 2.Wilkinson M. The morbid anatomy of cervical spondylotic and myelopathy. Brain. 1960;83:589–616. doi: 10.1093/brain/83.4.589. [DOI] [PubMed] [Google Scholar]
- 3.Young WF. Cervical spondylotic myelopathy: a common cause of spinal cord dysfunction in older persons. Am Fam Physician. 2000;62:1064–1070. [PubMed] [Google Scholar]
- 4.Clark E, Robinson PK. Cervical myelopathy: complication of cervical spondylosis. Brain. 1956;79:483–510. doi: 10.1093/brain/79.3.483. [DOI] [PubMed] [Google Scholar]
- 5.Lees F, Turner JW. Natural history and prognosis of cervical spondylosis. Br Med J. 1963;2:1607–1610. doi: 10.1136/bmj.2.5373.1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nurick S. The natural history and the results of surgical treatment of the spinal cord disorder associated with cervical spondylosis. Brain. 1972;95:101–108. doi: 10.1093/brain/95.1.101. [DOI] [PubMed] [Google Scholar]
- 7.Houten JK, Cooper PR. Laminectomy and posterior cervical plating for multilevel cervical spondylotic myelopathy and ossification of the posterior longitudinal ligament: effects on cervical alignment, spinal cord compression, and neurological outcome. Neurosurgery. 2003;52:1081–1087. [PubMed] [Google Scholar]
- 8.Arnold JGJ. The clinical manifestations of spondylochondrosis (spondylosis) of the cervical spine. Ann Surg. 1955;141:872–889. doi: 10.1097/00000658-195514160-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Adams CB, Logue V. Studies in cervical spondylotic myelopathy: II: the movement and contour of the spine in relation to the neural complications of cervical spondylosis. Brain. 1971;94:568–586. [PubMed] [Google Scholar]
- 10.Ogino H, Tada K, Okada K. Canal diameter, anteroposterior compression ratio, and spondylotic myelopathy of the cervical spine. Spine. 1983;8:1–15. doi: 10.1097/00007632-198301000-00001. [DOI] [PubMed] [Google Scholar]
- 11.Parke WW. Correlative anatomy of cervical spondylotic myelopathy. Spine. 1988;13:831–837. doi: 10.1097/00007632-198807000-00023. [DOI] [PubMed] [Google Scholar]
- 12.Penning L, Wilmink JT, van Woerden HH, Knol E. CT myelographic findings in degenerative disorders of the cervical spine: clinical significance. AJR Am J Roentgenol. 1986;146:793–801. doi: 10.2214/ajr.146.4.793. [DOI] [PubMed] [Google Scholar]
- 13.Breig A, Turnbull I, Hassler O. Effects of mechanical stresses on the spinal cord in cervical spondylosis: a study on fresh cadaver material. J Neurosurg. 1966;25:45–56. doi: 10.3171/jns.1966.25.1.0045. [DOI] [PubMed] [Google Scholar]
- 14.Panjabi M, White AD. Biomechanics of nonacute cervical spinal cord trauma. Spine. 1988;13:838–842. doi: 10.1097/00007632-198807000-00024. [DOI] [PubMed] [Google Scholar]
- 15.White AA, Panjabi MM. Biomechanical considerations in the surgical management of cervical spondylotic myelopathy. Spine. 1988;13:856–860. doi: 10.1097/00007632-198807000-00029. [DOI] [PubMed] [Google Scholar]
- 16.Penning L, Zwaag PVD. Biomechanical aspects of spondylotic myelopathy. Acta Radiol Diagn. 1966;5:1090–1103. doi: 10.1177/02841851660050p253. [DOI] [PubMed] [Google Scholar]
- 17.Fehling MG, Skaf G. A review of the pathophysiology of cervical spondylotic myelopathy with insights for potential novel mechanism drawn from traumatic spinal cord injury. Spine. 1988;23:2730–2737. doi: 10.1097/00007632-199812150-00012. [DOI] [PubMed] [Google Scholar]
- 18.Bohlmann HH, Emory SE. The pathophysiology of cervical spondylosis and myelopathy. Spine. 1988;13:843–846. doi: 10.1097/00007632-198807000-00025. [DOI] [PubMed] [Google Scholar]
- 19.Beattie MS, Manley GT. Tight squeeze, slow burn: inflammation and the aetiology of cervical myelopathy. Brain. 2011;134:1259–1261. doi: 10.1093/brain/awr088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yu WR, Liu T, Kiehl TR, Fehlings MG. Human neuropathological and animal model evidence supporting a role for Fas-mediated apoptosis and inflammation in cervical spondylotic myelopathy. Brain. 2011;134:1277–1292. doi: 10.1093/brain/awr054. [DOI] [PubMed] [Google Scholar]


