Abstract
Background/purpose
Patients treated with chemoradiotherapy for locally advanced non-small-cell lung carcinoma (LA-NSCLC) were analyzed for local-regional failure (LRF) and overall survival (OS) with respect to radiotherapy dose intensity (BED).
Materials/Methods
This study combined data from seven Radiation Therapy Oncology Group (RTOG) trials in which chemoradiotherapy was used for LA NSCLC: RTOG 88-08 (chemoradiation arm only), 90-15, 91-06, 92-04, 93-09 (nonoperative arm only), 94-10 and 98-01. The radiotherapeutic biologically effective dose (BED) received by each individual patient was calculated, as was the overall treatment time-adjusted BED (tBED) using standard formulae. Heterogeneity testing was done with Chi-squared statistics and weighted pooled hazard ratio estimates were used. Cox and Fine and Gray's proportional hazard models were used for OS and LRF, respectively, to test the associations between BED/tBED adjusted for other covariates.
Results
A total of 1,356 patients were analyzed for BED (1,348 for tBED). The 2-year and 5-year OS rates were 38% and 15%. The 2-year and 5-year LRF rates were 46% and 52%. BED (and tBED) were highly significantly associated with both OS and LRF, with or without adjustment for other covariates on multivariate analysis (p<0.0001). A 1 Gy BED increase in radiotherapy dose intensity was statistically significantly associated with approximately 4% relative improvement in survival – this is another way of expressing the finding that the pool adjusted HR for survival as a function of BED was 0.96. Similarly, a 1 Gy tBED increase in radiotherapy dose intensity was statistically significantly associated with approximately 3% relative improvement in local-regional control – this is another way of expressing the finding that the pool adjusted HR as a function of tBED was 0.97.
Conclusions
Higher radiotherapy dose intensity is associated with improved local-regional control and survival in the setting of chemoradiotherapy.
Keywords: Radiation Oncology, advanced non-small-cell lung carcinoma, chemoradiotherapy, local-regional failure, survival with respect to radiotherapy dose intensity
Introduction
Radiotherapy is an important part of treatment for locally advanced (LA) but non-metastatic non-small cell lung carcinoma (NSCLC). Prior to the 1990's, single modality radiotherapy was considered the standard of care; it offered palliation to many patients and prolonged survival for a small minority of patients(1). More recently, it has become clear that combination chemoradiotherapy is better than radiotherapy alone(2). While radiotherapy alone offers approximately 9 month median survival, induction chemotherapy followed by radiotherapy offers approximately 13 month median survival(3), and concurrent chemoradiotherapy offers approximately 17 month median survival(4). Perhaps more importantly, a finite number of patients will be long term survivors with aggressive multimodality therapy.
Most studies of radiotherapy or chemoradiotherapy have used a prescription dose of radiotherapy of approximately 60 Gy, over approximately 6 weeks. This is a modest dose compared to ‘curative’ radiotherapy dose/schedules for other types of cancer, particularly head and neck cancer or uterine cervix cancer. Furthermore, not all NSCLC patients receive their planned dose/schedule of radiotherapy, due to acute toxicity and/or logistical reasons. We previously reported the negative impact of radiation treatment interruptions on outcomes in chemoradiotherapy for NSCLC(5). This current paper examines the association of radiotherapy dose intensity with overall survival and local-regional control/failure in the setting of chemoradiotherapy.
Methods
Patients and Trials
This is a retrospective analysis of patients treated with chemoradiotherapy in prospective Radiation Therapy Oncology Group (RTOG) trials from 1988 through 2002. Patients who received a BED < 40 Gy were excluded from this analysis, since it is likely that these particular patients were noncompliant with protocol therapy and thus not assessable for this exploratory clinical-biological study.
The trials analyzed were as follows (See Table 1):
RTOG 88-08 (chemo-RT arm)(6): This consisted of two cycles of cisplatin/vinblastine chemotherapy followed by definitive radiotherapy (63 Gy).
RTOG 90-15(7): Phase I/II trial of concurrent cisplatin/vinblastine with definitive bid radiotherapy (69.6 Gy)
RTOG 91-06(8): Phase I/II trial of concurrent cisplatin/etoposide with definitive bid radiotherapy (69.6 Gy)
RTOG 92-04(9): Phase IIR trial; one arm was the same treatment as in RTOG 91-06, while the second arm was induction chemotherapy (vinblastine/cisplatin) followed by concurrent chemoradiotherapy (63 Gy with vinblastine/cisplatin).
RTOG 93-09(10): Phase III study of immediate concurrent chemoradiotherapy (cisplatin/etoposide/RT) with or without surgical resection (potentially operable IIIA only) – for this analysis only the patients randomized to no surgery were included.
RTOG 94-10(11): Phase III trial comparing chemo-RT similar to that given in RTOG 88-08 versus immediate concurrent chemo-RT (cisplatin/vinblastine/RT) versus the RTOG 91-06 regimen.
RTOG 98-01(12): Phase III trial of induction chemotherapy (carboplatin/paclitaxel) followed by concurrent chemoradiotherapy (carboplatin/paclitaxel/bid RT to a dose of 69.6 Gy), with or without amifostine.
Table 1.
Description of “Arms” analyzed in this study.
| “Arm” | Description | (Prescribed) RT dose/fractionation | Prescribed BED | Chemo | Actual BED delivered [median (min-max) 25th 75th] |
|---|---|---|---|---|---|
| 1 | 88-08 Arm A | 60 Gy (2 Gy qd) | 72 | Induction Cisplatin/vinblastine | 72.00 (48.00-86.40) 72.00, 72.00 |
| 2 | 90-15 | 69.6 Gy (1.2 Gy bid) | 77.95 | Concurrent Cisplatin/vinblastine | 77.95 (40.07, 79.81) 77.46, 77.95 |
| 3 | 91-06 | 69.6 Gy (1.2 Gy bid) | 77.95 | Concurrent Cisplatin/etoposide | 77.95 (45.77-77.95) 77.95, 77.95 |
| 4 | 92-04 Arm A | 69.6 Gy (1.2 Gy bid) | 77.95 | Concurrent Cisplatin/etoposide | 74.67 (47.00-81.73) 74.34, 74.67 |
| 5 | 92-04 Arm B | 63 Gy (1.8/2 Gy qd) | 74.9 | Induction and concurrent Cisplatin/vinblastine | 77.95 (57.79-81.71) 77.95, 77.95 |
| 6 | 93-09 Arm B | 61 Gy (1.8/2 Gy qd) | 72.3 | Concurrent cisplatin/etoposide | 72.28 (53.10-76.46) 72.28, 72.28 |
| 7 | 94-10 Arm A | 63 Gy (1.8/2 Gy qd) | 74.9 | Induction Cisplatin/vinblastine | 74.67 (42.48-77.80) 74.67, 74.67 |
| 8 | 94-10 Arm B | 63 Gy (1.8/2 Gy qd) | 74.9 | Concurrent Cisplatin/vinblastine | 74.67 (42.48-77.80) 74.67, 74.67 |
| 9 | 94-10 Arm C | 69.6 Gy (1.2 Gy bid) | 77.95 | Concurrent Cisplatin/etoposide | 77.95 (40.32-82.35) 77.95, 77.95 |
| 10 | 98-01 Arm A | 69.6 Gy (1.2 Gy bid) | 77.95 | Induction and Concurrent Carboplatin/paclitaxel | 77.95 (56.45-85.63) 77.95, 77.95 |
| 11 | 98-01 Arm B | 69.6 Gy (1.2 Gy bid) | 77.95 | Induction and Concurrent Carboplatin/paclitaxel | 77.95 (54.98-82.35) 77.95, 77.95 |
From these 7 prospective trials, there were a total of 11 “arms” that were analyzed for this study. In some cases e.g. 88-08 and Arm 1 of 94-10), the prescribed treatment for two or more arms was exactly the same. However, for this analysis, these were still considered as different arms since they came from different prospective studies.
Radiotherapy technology and techniques were similar for all of these trials. These trials included elective nodal irradiation to the entire mediastinum and in some cases the supraclavicular and/or contralateral hilar nodes to 45 Gy. These comprehensive radiotherapy treatment fields were then followed by a boost to gross disease to at least 60 Gy (maximum 69.6 Gy in 1.2 Gy bid fractionation). “High technology” forms of modern radiotherapy such as intensity modulated radiation therapy (IMRT), image guided radiation therapy (IGRT), adaptive radiotherapy, respiratory gated radiotherapy, or air/tissue inhomogeneity-corrected radiotherapy dosimetry were not used.
As noted above, all of the studies used a platinum based chemoradiotherapy regimen (all used cisplatin except for RTOG 98-01). Concurrent chemoradiotherapy was more commonly used than sequential chemotherapy followed by radiotherapy.
Assessment for tumor control was consistent among these trials. Specifically, all patients were required to undergo a post-radiotherapy CT scan of the chest (including liver/adrenals) 3-6 months after completing radiotherapy and then every 6 months for two years, and then annually. Bone scan and/or head CT/MRI scanning was only performed if metastatic disease was suggested by clinical evaluation. PET scans were not used for pre-treatment staging or post-treatment assessment in this study.
Definitions of BED and Outcomes
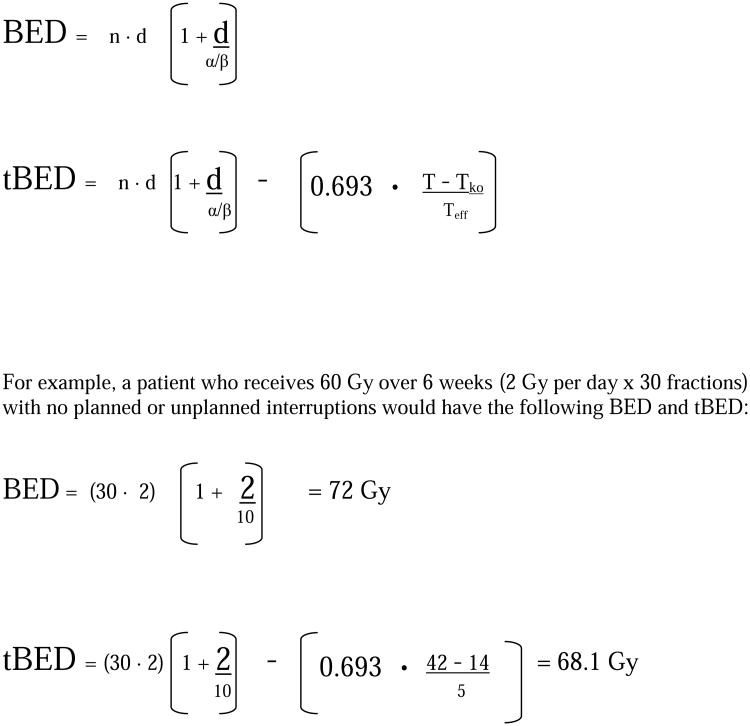
Radiotherapy dose intensity was assessed for this analysis using the biologically effective dose (BED) modeling, adapted from Fowler et al. (13) (See Figure 1) BED is a function of the nominal radiotherapy dose, number of radiotherapy fractions, radiotherapy fraction size, and several biological factors (alpha and beta, based upon preclinical models of rapidly proliferating epithelial tumor cell lines studied using the linear quadratic model for radiation survival. For this study, ‘alpha’ was presumed to = 0.35 for all patients, and the alpha/beta ratio was presumed to = 10. This BED formula does not take into account altered fractionation. For twice daily (bid) fractionation, with an interfraction time of at least 6 hours (as has been standard in RTOG protocols), it is assumed that there is complete repair between fractions. Thus, for the typical RTOG hyperfractionation schedule of 69.6 Gy delivered 1.2 Gy bid, BED = 77.95 (69.6 Gy × (1 + 1.2/10).
Figure 1.
BED and tBED formulae
Time-adjusted BED (tBED) is BED (as calculated above) corrected for overall treatment time via subtraction of a correction factor for treatment time. This time correction factor is a function of the patient's overall treatment time (T) and three putative biological factors: alpha (as above), Tko (the window time period before accelerated repopulation of tumor cells begins) and Teff (the tumor effective doubling time). For this study, Tko was presumed to = 14 days and Teff = 5 days, reflecting the aggressive and highly proliferative nature of typical NSCLC.(14) It should be noted that as values for Tko and/or Teff are increased, the tBED formula more closely approaches and eventually becomes identical to the BED formula. We did not perform a “sensitivity analysis” for varying values of Tko and/or Teff.
The outcomes for this study are overall survival (OS) and local regional control. Local-regional control (LRC) and local-regional failure (LRF) was assessed using traditional RTOG methodology, corresponding to Freedom from Local-regional Progression (FFLP): With this definition, all patients are considered to have local-regional control at Day 0 (Date of registration/randomization). Subsequently, the development of progressive lung cancer within or adjacent to the radiotherapy field is considered to be a local-regional failure (LRF) event. Central review of local-regional failures was not performed; the decision of whether to consider progressive lung cancer “in field” versus “marginal” was at the discretion of the local investigator/treating physician, based on his/her review of the data. Given the potential for variable criteria among investigators for defining in-field versus marginal failure, for this study, we have included both types of failure within our definition of LRF.
Patients were “censored” if/when they died from distant metastases and/or died without documented progressive cancer. A failure event for OS is defined as death due to any cause. Time to LRF or OS was measured from the date of randomization to the date of a failure event.
Statistical Methods
Heterogeneity test was performed is to see if one estimate can be used to represent the combined data from different trials or not. The Chi-square test to assess heterogeneity among the 11 treatment arms was applied to this data at the significance level of 0.1. The pooled hazard ratio (HR)(15)(16) estimator with the weight of the inverse of variance of estimator was used if they were homogeneous among the treatment arms. The missing at random (MAR) assumption was assumed and a multiple imputation method (20 imputed datasets) with Markov chain Monte Carlo (MCMC) estimation was applied to impute missing BED/tBED values. The following covariates were considered in all outcomes as default in the multiple regression models:
Age -- Continuous variable.
KPS -- 70-80 was considered the reference level (reference level; RL), versus 90-100.
Gender -- Female (RL), versus Male.
Histology -- Non-squamous (RL), versus Squamous.
Stage -- II/IIIA (RL), versus. IIIB.
RT Delivery Method – Hyperfractionated (RL), versus Standard fractionation.
Chemotherapy sequence -- Concurrent (RL), vs. Sequential.
BED or tBED – Continuous variable
We compared the pretreatment characteristics and outcomes of patients with and without missing data to determine if there was a bias due to missing data by Chi-square test statistics.
The Kaplan-Meier method(17)was used to estimate the survival rate and the cumulative incidence method(18) was used to estimate the local-regional failure (LRF) rate. To analyze whether each covariate was independently associated with outcomes while adjusting for other covariates, Cox proportional hazards regression models(19) were used for OS. To consider competing risk events for LRF, Fine and Gray's proportional hazards regression models(20) were used. The competing risk for a failure event of LRF is death without local-regional failure. Unadjusted and adjusted hazard ratios were calculated for all covariates using either the Cox or Fine and Gray's proportional hazards model with associated 95% confidence intervals (C.I.s) and p-values. If the 95% C.I. contains 1, the corresponding HR is not considered statistically significant. All statistical tests were two-sided, and a p-value <0.05 was considered statistically significant. Statistical Analysis System® (SAS Institute, Cary, NC) were used for all statistical analyses, except Fine and Gray's modeling which was analyzed using R software.
Results
The patients were accrued and treated from 1988 through 2002. There were 1,356 (1348 for tBED) analyzable patients whose BED (or tBED) are not less than 40 Gy among 1390 eligible patients. Among those analyzable patients, 133 (10%) patients have missing data for BED (158 patients (12%) were for tBED) (lack of detailed information on total radiotherapy dose, fractionation and/or treatment time). Those missing data were imputed using MCMC as described in the statistical method section.
Patient characteristics used for BED analysis are shown in Table 2. The median age was 61 and most patients (87%) were relatively young (≤70 years old) and had excellent KPS (77% had KPS 90-100). A total of 63% of patients had non-squamous histology and 65% were male; 50% of the patients had stage II/IIIAN2 disease and 50% had stage IIIB disease. The median followup for all patients is 17.5 months (72 months for surviving patients).
Table 2. Characteristics of Patients with and without Missing Data For BED Analysis.
| With Complete Data (n=1223) | With Missing Data (n=133) | Total (n=1356) | |||||
|---|---|---|---|---|---|---|---|
| Age | |||||||
| Median | 61 | 60.5 | 61 | ||||
| Range | 31-84 | 32-78 | 31-84 | ||||
| n | % | n | % | p-value* | n | % | |
|
|
|||||||
| Age | |||||||
| ≤ 70 | 1066 | 87 | 111 | 83 | 0.23 | 1177 | 87 |
| > 70 | 157 | 13 | 22 | 17 | 179 | 13 | |
| Gender | |||||||
| Female | 434 | 35 | 39 | 29 | 0.16 | 473 | 35 |
| Male | 789 | 65 | 94 | 71 | 883 | 65 | |
| KPS | |||||||
| 90-100 | 943 | 77 | 106 | 80 | 0.50 | 1049 | 77 |
| 70-80 | 280 | 23 | 27 | 20 | 307 | 23 | |
| Histology | |||||||
| Non-squamous | 771 | 63 | 77 | 58 | 0.24 | 848 | 63 |
| Squamous | 452 | 37 | 56 | 42 | 508 | 37 | |
| AJCC Stage | |||||||
| II/IIIA | 606 | 50 | 95 | 71 | <0.0001 | 701 | 52 |
| IIIB | 617 | 50 | 38 | 29 | 655 | 48 | |
| Chemotherapy Sequence | |||||||
| Concurrent | 917 | 75 | 98 | 74 | 0.74 | 1015 | 75 |
| Sequential | 306 | 25 | 35 | 26 | 341 | 25 | |
| Radiation Method | |||||||
| Hyperfractionated (HFX) | 699 | 57 | 27 | 20 | <0.0001 | 726 | 54 |
| Single daily fraction (SFX) | 524 | 43 | 106 | 80 | 630 | 46 | |
Chi-square test
The median BED for 1,223 patients who have BED data was 74.7 Gy (range 40-86.4 Gy) and the median tBED for 1,190 patients who have tBED was 63.8 Gy (range 40-82.6 Gy). The 2year and 5-year overall survival rates for these patients who do not have missing data were 38% and 15%. Local-regional failure rates at 2 and 5 years were 46% and 52% respectively.
Local-regional failure/control and BED/t-BED
The 11 treatment arms are homogeneous with respect to LRF for BED and tBED (Table 3, p- values > 0.1). LRF rates were associated with BED (or tBED), i.e. a higher BED or tBED was associated with a lower risk of LRF. Patients who have a 1 Gy increase in BED were statistically less likely to have a LRF (pooled adjusted HR =0.97 (95% CI = 0.96, 0.98). Similarly, patients who have a 1 Gy increase in tBED were statistically less likely to have a LRF (pooled adjusted HR =0.96 (95% CI = 0.95, 0.98). This (HR's of 0.97 for BED or 0.96 for tBED) means that as BED or tBED increases by 1 Gy, the relative decrease in the risk of LRF was approximately 3% for BED and 4% for tBED.
Table 3. Heterogeneity Testing by Treatment Arm/Study.
| Local-Regional Failure | ||||
|---|---|---|---|---|
|
| ||||
| Adjusted Hazard Ratio** for BED | Adjusted Hazard Ratio** for tBED | |||
|
| ||||
| Treatment arm/Study | Hazard Ratio (95% CI*) | p-value | Hazard Ratio (95% CI*) | p-value |
| 8808 | 0.95 (0.91, 0.98) | 0.005 | 0.93 (0.88, 0.97) | 0.001 |
| 9015 | 0.97 (0.95, 0.99) | 0.002 | 0.96 (0.94, 0.99) | 0.006 |
| 9106 | 1.00 (0.97, 1.02) | 0.73 | 0.99 (0.95, 1.03) | 0.59 |
| 9204: Arm 1 | 1.00 (0.93, 1.07) | 0.94 | 1.01 (0.97, 1.05) | 0.69 |
| 9204: Arm 2 | 0.95 (0.92, 0.98) | 0.001 | 0.96 (0.92, 0.99) | 0.009 |
| 9309** | 1.01 (0.94, 1.07) | 0.87 | 1.00 (0.93, 1.08) | 0.94 |
| 9410: Arm 1 | 0.93 (0.89, 0.98) | 0.005 | 0.93 (0.87, 0.99) | 0.03 |
| 9410: Arm 2 | 0.93 (0.90, 0.97) | <0.0001 | 0.93 (0.89, 0.97) | 0.0008 |
| 9410: Arm 3 | 0.97 (0.96, 0.99) | 0.0004 | 0.96 (0.95, 0.98) | 0.0002 |
| 9801: Arm 1 | 0.99 (0.95, 1.04) | 0.81 | 0.99 (0.94, 1.04) | 0.62 |
| 9801: Arm 2 | 0.98 (0.91, 1.05) | 0.50 | 0.96 (0.90, 1.02) | 0.21 |
|
| ||||
| Chi-Square T.S. = 17.2 | p-value = 0.93 | Chi-Square T.S. = 15.6 | p-value = 0.89 | |
|
| ||||
| Pooled HR | 0.97 (0.96, 0.98) | 0.96 (0.95, 0.98) | ||
|
| ||||
| Overall Survival | ||||
|
| ||||
| Adjusted Hazard Ratio†† for BED | Adjusted Hazard Ratio†† for tBED | |||
|
| ||||
| Treatment arm/Study | Hazard Ratio (95% CI*) | p-value | Hazard Ratio (95% CI*) | p-value |
|
| ||||
| 8808 | 0.95 (0.90, 0.996) | 0.04 | 0.95 (0.91, 0.99) | 0.03 |
| 9015 | 0.98 (0.94, 1.03) | 0.40 | 0.92 (0.86, 0.98) | 0.01 |
| 9106 | 0.97 (0.93, 1.02) | 0.24 | 1.01 (0.94, 1.07) | 0.83 |
| 9204: Arm 1 | 0.92 (0.85, 1.003) | 0.06 | 0.99 (0.95, 1.03) | 0.59 |
| 9204: Arm 2 | 0.97 (0.91, 1.03) | 0.30 | 1.00 (0.96, 1.03) | 0.82 |
| 9309† | 0.99 (0.93, 1.05) | 0.70 | 1.00 (0.94, 1.06) | 1.00 |
| 9410: Arm 1 | 0.95 (0.92, 0.98) | 0.005 | 0.95 (0.89, 0.99) | 0.047 |
| 9410: Arm 2 | 0.94 (0.91, 0.97) | 0.0001 | 0.93 (0.90, 0.97) | 0.0009 |
| 9410: Arm 3 | 0.97 (0.94, 1.003) | 0.07 | 0.95 (0.92, 0.98) | 0.002 |
| 9801: Arm 1 | 0.94 (0.89, 0.998) | 0.04 | 0.96 (0.90, 1.01) | 0.13 |
| 9801: Arm 2 | 0.95 (0.91, 1.004) | 0.07 | 0.87 (0.82, 0.98) | 0.02 |
|
| ||||
| Chi-Square T.S. =6.7 | p-value = 0.24 | Chi-Square T.S. =15.7 | p-value = 0.89 | |
|
| ||||
| Pooled HR | 0.96 (0.95, 0.97) | 0.96 (0.95, 0.98) | ||
CI = Confidence Interval; RL = Reference Level; T.S.=test statistics
It's from Fine and Gray's regression and is adjusted for: Age (continuous), Gender (female vs. male), KPS (90,100 vs. 70-80), Histology (non-squamous vs. squamous) and Stage group (II/IIIA vs. IIIB) Chemotherapy sequence(Concurrent (RL) vs. Sequential) RT Delivery Method (only for BED; HFX (RL) vs. SFX)
It's from Cox regression and is adjusted for the same as above
Not adjusted for Stage group; all patients are II/IIIA.
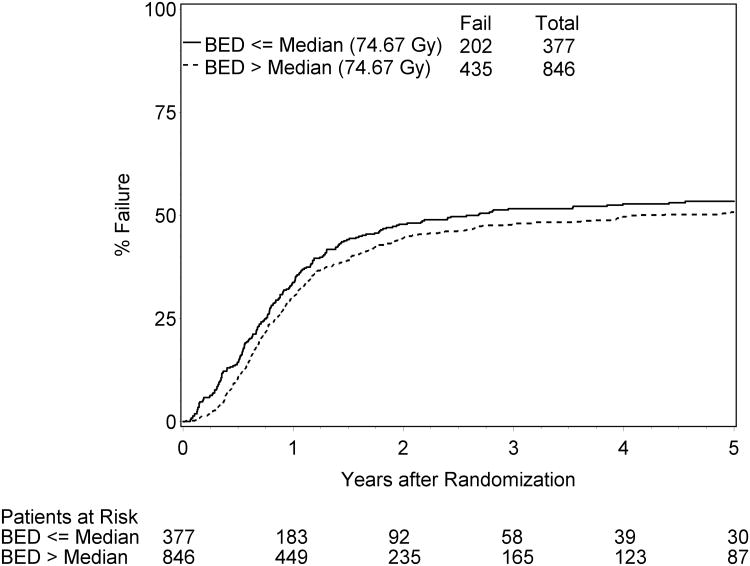
The results remain the same when Fine and Gray's proportional hazard models were applied (Table 4. p<0.0001). Figure 2 displays LRF rates from cumulative incidence estimation for the subgroups of patients with BED above versus below the median value (74.67 Gy).Other factors associated with lower risk of LRF on this multivariate analysis were Age (HR=0.77, p=0.04); Gender (HR=1.32; p=0.0008); Stage (HR =1.17, p=0.04), and chemotherapy sequencing (HR=1.28; p=0.003). These factors were also statistically significant factors when tBED is used in place of BED for this LRF model.
Table 4. Multiple Proportional Hazards Regression Models* for Local-Regional Failure and Overall Survival.
| Local-Regional Failure* | |||
|---|---|---|---|
|
| |||
| Parameter | Comparison | Hazard Ratio (95% CI**) | p-value |
| BED | Continuous | 0.98 (0.97, 0.99) | <0.0001 |
| Age | Continuous | 0.77 (0.61, 0.99) | 0.04 |
| Gender | Female vs. Male | RL 1.32 (1.12, 1.55) | 0.0008 |
| KPS | 90-100 vs. 70-80 | RL 1.06 (0.89, 1.27) | 0.52 |
| Histology | Non-Squamous vs. Squamous | RL 1.04 (0.89, 1.21) | 0.61 |
| Stage Group | II/IIIA vs. IIIB | RL 1.17 (1.00, 1.36) | 0.04 |
| RT Delivery Method | HFX vs. SFX | RL 1.08 (0.93, 1.25) | 0.33 |
| Chemotherapy | Sequential vs. Induction | RL 1.28 (1.09, 1.51) | 0.003 |
|
| |||
| Parameter | Comparison | Hazard Ratio (95% CI**) | p-value |
|
| |||
| tBED | Continuous | 0.97 (0.96, 0.98) | <0.0001 |
| Age | Continuous | 0.76 (0.60, 0.97) | 0.03 |
| Gender | Female vs. Male | RL 1.34 (1.13, 1.57) | 0.0005 |
| KPS | 90-100 vs. 70-80 | RL 1.06 (0.89, 1.27) | 0.5 |
| Histology | Non-squamous vs. Squamous | RL 1.05 (0.90, 1.22) | 0.54 |
| Stage Group | II/IIIA vs. IIIB | RL 1.16 (1.003, 1.35) | 0.045 |
| Chemotherapy sequence | Sequential vs. Induction | RL 1.35 (1.15, 1.60) | 0.0003 |
|
| |||
| Overall Survival | |||
|
| |||
| Parameter | Comparison | Hazard Ratio (95% CI**) | p-value |
|
| |||
| BED | Continuous | 0.97 (0.96, 0.98) | <0.0001 |
| Age | Continuous | 1.19 (1.02, 1.41) | 0.03 |
| Gender | Female vs. Male | RL 1.14 (1.01, 1.29) | 0.03 |
| KPS | 90-100 vs. | RL 1.22 (1.07, 1.40) | 0.003 |
| Histology | Non-squamous vs. squamous | RL 1.15 (1.02, 1.29) | 0.02 |
| Stage Group | II/IIIA vs. IIIB | RL 1.13 (1.01, 1.26) | 0.04 |
| RT Delivery Method | HFX vs. SFX | RL 0.94 (0.84, 1.06) | 0.31 |
| Chemotherapy sequence | Sequential vs. Induction | RL 1.24 (1.09, 1.41) | 0.0009 |
|
| |||
| Parameter | Comparison | Hazard Ratio (95% CI**) | p-value |
|
| |||
| tBED | Continuous | 0.97 (0.96, 0.98) | <0.0001 |
| Age | Continuous | 1.16 (0.99, 1.37) | 0.07 |
| Gender | Female vs. Male | RL 1.16 (1.03, 1.31) | 0.02 |
| KPS | 90-100 vs. 70-80 | RL 1.25 (1.10, 1.43) | 0.0009 |
| Histology | Non-squamous vs. Squamous | RL 1.15 (1.02, 1.29) | 0.02 |
| Stage Group | II/IIIA vs. IIIB | RL 1.16 (1.04, 1.30) | 0.009 |
| Chemotherapy sequence | Sequential vs. Induction | RL 1.29 (1.14, 1.47) | <0.0001 |
Fine and Gray's Proportional Hazards Regression Model was used for LRF and Cox Proportional Hazards Regression was for OS.
CI=Confidence Interval; RL=Reference Level
Figure 2.
Median Local Failure
Overall survival and BED/t-BED
The 11 treatment arms are homogeneous with respect to OS for BED and tBED (Table 3, p- values > 0.1).
The median and 3-year overall survival data were 17.5 months and 26%. The 5-year survival rate was 15%. Patients who have a 1 Gy BED or tBED increase in radiotherapy dose were statistically less likely to die, with a pooled adjusted HR = 0.96 (95 C.I.=0.95, 0.97) and 0.96 (95% CI=0.95, 0.98), respectively. This (HR's of 0.96 for survival as a function of BED or tBED) means that as BED or tBED increase by 1 Gy, the relative decrease in the risk of death was approximately 4% for both BED and tBED.
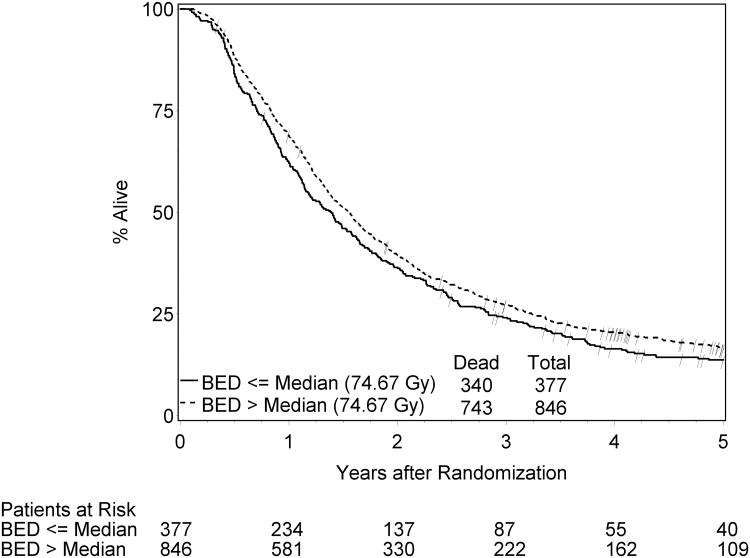
The results remain same when Cox proportional hazard models were applied (Table 4, p-values < 0.0001). Figure 3 displays OS rates from Kaplan-Meier estimation for the subgroups of patients with BED above versus below the median value (74.67 Gy).
Figure 3.
Median Overall Survival
With BED in the model for overall survival, other factors statistically significantly associated with OS were age (HR=1.19, p=0.03), gender (HR=1.14, p=0.03), KPS (HR=1.22, p-value=0.003), histology (HR=1.15, p-value=0.02), stage group (HR= 1.13, p=0.04), and chemotherapy sequence (HR=1.24, p<0.001). These factors were also statistically significant factors except age (HR=1.16, p-value =0.07) when tBED is used in place of BED in the model for OS.
Discussion
We found a strong association between outcomes (local-regional control/failure and survival) with radiotherapy dose intensity as measured by the BED/tBED models, using a large database of patients treated in RTOG chemoradiotherapy trials. This indicates that radiotherapy dose intensity remains important despite the establishment of chemotherapy in stage III NSCLC.
Our study has some limitations and potential biases related to its retrospective nature. First, this study does not prove a causal relationship between radiotherapy dose intensity and outcome. Some patients may have received lower dose due to rapid disease progression (and discontinuation of radiotherapy in favor of palliative care) or rapid decline in performance status. Either of these events limits survival independently of his/her radiotherapy dose received. Unfortunately, we do not have detailed data within the RTOG database to analyze and correct for these potential biases; however, the relatively young age and high performance status nature of this patient population would suggest that these events are relatively uncommon. Additionally, we attempted to control for this confounding factor by excluding patients who did not receive at least a ‘minimal’ therapeutic dose of radiotherapy (at least 40 Gy).
A second potential limitation is that the model used for calculating BED may be imperfect. It is based upon presumptions for several values, such as the alpha/beta ratio, Tko and/or Teff that are relatively well validated in preclinical models of lung cancer but suboptimally studied in the clinical setting. Our results (relationship between BED and tumor control probability) partially validate this BED model for lung cancer, but it is quite possible that a better model could be developed in the future.
A third limitation is that we did not perform central review of all cases for local-regional failure versus control. The definition of LRF was based on local RTOG investigators. Although the criteria for analyzing local-regional failure/control were specified in the protocol, there are still some subjectivities that probably differ across the broad group of investigators. One way in which we address this limitation is to include in our definition of LRF both “in field” and “marginal” tumor progression. This eliminates the potential bias of different investigators' using different criteria for determining in-field versus marginal progression.
Ultimately, a prospective randomized chemoradiotherapy trial comparing ‘standard’ radiotherapy dose versus intensified radiotherapy dose is needed. Prior studies addressing this have not been optimal. For example, RTOG 94-10 included a pre-planned statistical comparison between Arm 2 (once daily radiotherapy to 63 Gy) versus Arm 3 (twice daily radiotherapy to 69.6 Gy). There was no statistically significant difference, although Arm 2 was numerically better. However, RTOG 94-10 Arm 3 utilized 1.2 Gy bid (smaller radiation fraction size than standard once daily radiotherapy) and a different chemotherapy added to cisplatin (oral etoposide as opposed to intravenous vinblastine)(21). The increased acute toxicity (mainly esophagitis) of RTOG 94-10 Arm 3 may have also affected compliance to this nominally more dose intense regimen. A more recent randomized trial by Yuan et al. compared standard, extended field (EF) radiotherapy versus involved field (IF)(22). Patients in the IF arm received a higher total radiotherapy dose (68-74 Gy) than patients in the EF arm (60-64 Gy). Results showed a trend toward improved survival in the IF (higher dose) arm (2-year survival 39% versus 26%, p=0.048). There was less radiation pneumonitis in the IF arm as well, supporting the hypothesis that volume irradiated may have more impact on lung toxicity than the nominal tumor dose.
The RTOG and other groups have been studying intensified chemoradiotherapy in phase I and II studies (See Table 5). RTOG 0117 demonstrated the feasibility of 74 Gy (2 Gy once daily fractionation) with concurrent and adjuvant carboplatin/paclitaxel. Preliminary results show a median survival of approximately 26 months, which is significantly better than the results seen in previous RTOG studies(24). The CALGB demonstrated similar results with a very similar regimen(25). The University of North Carolina (Carolina Consortium) experience also reported approximately 2-year median survival with 74 Gy plus chemotherapy(26). These data formed the basis for a recently opened Phase III randomized trial comparing conventional dose radiotherapy (60-63 Gy) against intensified radiotherapy (74 Gy), with chemotherapy in both arms (RTOG 0617). This study is also testing the utility of adding the anti-EGFR antibody drug cetuximab, in a 2×2 factorial statistical design(27). Given the limitations of a retrospective analysis such as our current paper, we believe it is critical to support RTOG 0617, and we caution against the routine use of ultra-high dose (74 Gy) thoracic radiotherapy with concurrent chemotherapy outside of a clinical trial.
Table 5. Chemoradiotherapy Dose Intensity and Outcome – Review of Selected Literature.
| Study | # of Pts | Nominal RT Dose/fraction size | Approx. RT Dose Intensity | Type of Chemo | Survival |
|---|---|---|---|---|---|
| Conventional RT Dose Intensity | |||||
| West Japan (4) | 148 | 56 Gy/2 | 67 | MVdP | 16.5 mo. |
| RTOG 9410 Arm #2 (11) | 201 | 60 Gy/2 | 72 | Cis/Vlb | 17.0 mo. |
| LAMP(37) | 92 | 63 Gy/1.8 | 74 | Cbo/Tax | 16.1 mo. |
| SWOG 9504(33) | 83 | 61.2 Gy/1.8 | 72 | Cis/Etop/Doc | 26.0 mo. |
| Intermediate Dose Intensity RT | |||||
| Japanese Jeremic trial(38) | 65 | 69.6 Gy/1.2 bid | 78 | Cbo/Etop | 22.0 mo. |
| RTOG 9410 Arm #3(11) | 193 | 69.6 Gy/1.2 bid | 78 | Cis/Etop | 15.2 mo. |
| CALGB 39801 (31) | 366 | 66 Gy/2 | 79 | Cbo/Tax | 14.0 mo. |
| ‘Dose Intense’ RT | |||||
| RTOG 0117(24) | 63 | 74 Gy/2 | 89 | Cbo/Tax | 26 mo. |
| CALGB 30105 (25) | 43 | 74 Gy/2 | 89 | Cbo/Tax | 25 mo. |
| NCCTG N0028(39) | 20 | 70-78Gy/2 | 89 | Cbo/Tax | 42 mo |
| Univ. North Carolina Consortium(26) | 62 | 74 Gy/2 | 89 | Cbo/Tax | 25 mo. |
Cis = Cisplatin; Vlb = Vinblastine; Tax = Paclitaxel; Cbo = Carboplatin; Etop =Etoposide; Doc = Docetaxel.
MVdP = Mitomycin/Vindesine/cisplatin
As radiotherapy technology improves, the question arises regarding the safety and efficacy of even further dose intensification of radiotherapy for NSCLC. Doses above 74 Gy are probably necessary to achieve more reliable local-regional control. In medically inoperable stage I NSCLC, stereotactic irradiation using three very large radiation fractions (20 Gy × 3), which has a BED > 100 Gy, provides superb 3-year local control (>95%) without chemotherapy(28). Onishi et al. showed that stereotactic radiotherapy with a BED < 100 Gy had significantly lower local control and survival(29). However, the maximum tolerated dose of radiotherapy with concurrent chemotherapy for locally advanced (Stage III) NSCLC is considerably lower than the feasible dose for Stage I NSCLC. In the early, Phase I portion of RTOG 0117, an attempt was made to escalate radiotherapy dose-intensity (with concurrent chemotherapy) to 75.25 Gy in 2.15 Gy daily fractionation. This was unsuccessful, due to toxicity(30). It appears that dose escalation beyond 74 Gy (with concurrent chemotherapy) for NSCLC will require significant technological advances beyond that traditionally available with 3-D conformal radiotherapy. Some examples of technology improvements may include IMRT, respiratory gating, image guided/adaptive radiotherapy, and/or particle beam radiotherapy.
Of note, our study did not show any major differences in the results whether BED or tBED was used as the variable of interest. This was somewhat surprising, considering our previous paper (5), in which we showed that overall treatment time was an important factor in outcomes after chemoradiotherapy. We do not interpret our current analysis to mean that the time factor is unimportant. It should be noted that the vast majority of the patients in this study had rather similar overall treatment times (approximately 6 weeks), irrespective of their treatment arm. This would make it difficult to identify a modest time factor effect.
Another controversy exists regarding the ‘optimal’ chemotherapy regimen with radiotherapy. In the U.S. weekly carboplatin/paclitaxel has become an accepted community standard despite the lack of a direct randomized trial comparing this against older regimens such as cisplatin/etoposide. A CALGB Phase III study showed disappointing results with radiotherapy/carboplatin/paclitaxel(31), contrasting with other prospective studies (12, 32). One example of a study with exceptionally high survival despite modest radiotherapy dose is SWOG 9504(33), an ‘outlier’ among the standard radiotherapy dose trials. SWOG 9504 had a 26-month median survival despite a radiotherapy dose of 61.2 Gy; the authors hypothesize that intensification of chemotherapy (addition of post-RT docetaxel) may have led to the improved outcomes(33). However, phase III studies have not confirmed the efficacy of this approach(34-35) The use of a variety of different chemotherapy regimens and schedules in our study (cisplatin/vinblastine; cisplatin/etoposide; carboplatin/paclitaxel) is certainly a limitation. We have in fact shown once again that concurrent chemoradiotherapy appears to be better than sequential/induction chemotherapy followed by radiotherapy. Our multivariable model does account for induction versus concurrent chemotherapy. In contrast to the sequencing question, there is no clear evidence that any one platinum-based doublet chemotherapy combination during XRT is significantly superior (or inferior) to another.
Radiotherapy dose intensification can be hypothesized to improve survival via improved local-regional control but would not be expected to improve survival significantly in patients who harbor occult or overt distant metastatic disease. Thus, it is important to select patients carefully for radiotherapy dose intensification. In recent years, the routine use of FDG-PET scanning prior to therapy has offered “enrichment” of the stage III population via detection of small volume Stage IV disease and/or such extensive bulky intrathoracic disease that radical radiotherapy is not feasible(36). However, FDG-PET is imperfect, as indicated by the persistently high rate of long-term distant progression among patients originally thought to have Stage III disease by PET. Other diagnostic studies, such as newer forms of functional imaging and/or blood or tissue biomarkers to predict outcomes and better select patients for aggressive local-regional therapy are needed. The benefit of intensified radiotherapy is expected to be most significant in patients whose tumors are local-regionally confined.
Conclusions
In summary, this analysis reveals that local-regional control and survival are associated with a higher radiotherapy dose intensity (BED) received among patients treated with chemoradiotherapy. These data strongly support the rationale for RTOG 0617/CALGB 30609/ECOG R0617, the recently activated Intergroup phase III trial comparing standard (60 Gy) versus intensified (74 Gy) radiotherapy for stage III NSCLC.
Acknowledgments
This paper is supported by RTOG U10 CA21661 and CCOP U10 CA37422 grants from the NCI. This paper's contents are the sole responsibility of the authors and do not necessarily represent the official views of the NCI
Footnotes
There is no conflict of interest for any of the authors.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Perez CA, Pajak TF, Rubin P, et al. Long-term observations of the patterns of failure in patients with unresectable non-oat cell carcinoma of the lung treated with definitive radiotherapy. Report by the RTOG Cancer. 1987;59:1874. doi: 10.1002/1097-0142(19870601)59:11<1874::aid-cncr2820591106>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 2.Marino P, Preatoni A, Cantoni A. Randomized trials of radiotherapy alone versus combined chemotherapy and radiotherapy in stages IIIa and IIIb nonsmall cell lung cancer: A meta-analysis. Cancer. 1995;76:593–601. doi: 10.1002/1097-0142(19950815)76:4<593::aid-cncr2820760409>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 3.Dillman RO, Herndon J, Seagren SL, Eaton WLJ, Green MR. Improved survival in stage III non-small cell lung cancer: seven-year followup of cancer and leukemia group B (CALGB) 8433 trial. J Natl Cancer Inst. 1996;88:1210–5. doi: 10.1093/jnci/88.17.1210. [DOI] [PubMed] [Google Scholar]
- 4.Furuse K, Fukuoka M, Kawahara M. Phase III study of concurrent versus sequential thoracic radiotherapy in combination with mitomycin, vindesine, and cisplatin in unresectable stage III non-small cell lung cancer. J Clin Oncol. 1999;17:2692–9. doi: 10.1200/JCO.1999.17.9.2692. [DOI] [PubMed] [Google Scholar]
- 5.Machtay M, Hsu C, Komaki R, et al. Effect of overall treatment time on outcome after concurrent chemoradiation for locaclly advnced non-small cell lung carcinoma: analysis of the Radiation Therapy Oncology Group (RTOG) experience. Int J Radiat Oncol Biol Phys. 2005;63(3):667–71. doi: 10.1016/j.ijrobp.2005.03.037. [DOI] [PubMed] [Google Scholar]
- 6.Sause W, Kolesar P, Taylor SI, Johnson D, et al. Final results of phase III trial in regionally advanced unresectable non-small cell lung cancer: radiation therapy oncology group, eastern cooperative oncology group and southwest oncology group. Chest. 2000;117:358–64. doi: 10.1378/chest.117.2.358. [DOI] [PubMed] [Google Scholar]
- 7.Byhardt R, Scott CB, Ettinger D, et al. Concurrent hyperfractionated irradiation and chemotheray for unresectable NSCLC: Results of RTOG 90-15. Cancer. 1995;75(9):2237–44. doi: 10.1002/1097-0142(19950501)75:9<2337::aid-cncr2820750924>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 8.Lee JS, Scott C, Komaki R, Fossella FV, Dundas GS, McDonald S, Byhardt RW, Curran WJ. Concurrent Chemoradiation Therapy with Oral Etoposide and Cisplatin for Locally Advanced Inoperable Non-Small Cell Lung Cancer: RTOG Protocol 91-06. J Clin Oncol. 1996;14:1055–64. doi: 10.1200/JCO.1996.14.4.1055. [DOI] [PubMed] [Google Scholar]
- 9.Komaki R, Seiferheld W, Ettinger D, Lee JS, Movsas B, Sause W. Randomized phase II chemotherapy and radiotherapy trial for patients with locally advanced inoperable NSCLC: long term followup of RTOG 92-04. Int J Radiat Oncol Biol Phys. 2002;53:548–57. doi: 10.1016/s0360-3016(02)02793-1. [DOI] [PubMed] [Google Scholar]
- 10.Albain KS, Swann RS, Rusch VW, et al. Radiotherapy plus chemotherapy with or without surgical resection for stage III non-small cell lung cancer: a phase IIi randomised controlled trial. Lancet. 2009;374:379–86. doi: 10.1016/S0140-6736(09)60737-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Curran WJ, Scott CB, Langer CJ, et al. Proc Am Soc Clin Oncol (ASCO) Chicago: 2003. Long-term benefit is observed in a phase III comparison of sequential vs concurrent chemo-radiation for patients with urnsected stage III NSCLC: RTOG 9410 (abstr. #2499) p. 621. 2003. [Google Scholar]
- 12.Movsas B, Scott C, Langer C, et al. Randomized trial of amifostine in locally advanced non-small cell lung cancer patients receiving chemotherapy and hyperfractionated radiation: RTOG 98-01. J Clin Oncol. 2005;23(10):2145–54. doi: 10.1200/JCO.2005.07.167. [DOI] [PubMed] [Google Scholar]
- 13.Fowler J. Brief summary of radiobiological principles in fractionated radiotherapy. Semin Radiat Oncol. 1992;2:16–21. [Google Scholar]
- 14.Fowler JF, Chappell R. Non-small cell lung tumors repopulate rapidly during radiation therapy. Int J Radiat Oncol Biol Phys. 2000;46:516–7. doi: 10.1016/s0360-3016(99)00364-8. [DOI] [PubMed] [Google Scholar]
- 15.Smith CT, Williamson PR, Marson AG. Investigating heterogeneity in an individual patient data meta-analysis of time to even outcomes. Stat Med. 2005;24:1307–1. doi: 10.1002/sim.2050. [DOI] [PubMed] [Google Scholar]
- 16.Collett D, editor. Modelling survival data in medical research. New York: Chapman & Hall; 1994. [Google Scholar]
- 17.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–81. [Google Scholar]
- 18.Gray RJ. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Annual Statistics. 1988;16:1141–3. [Google Scholar]
- 19.Cox DR. Regression models and life tables. J Royal Stat Soc. 1972;34:187–202. [Google Scholar]
- 20.Fine J, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 21.Curran WJ, Scott C, Langer C, Komaki R, Lee J, et al. Proc Am Soc Clin Oncol (ASCO) New Orleans: 2000. Phase III comparison of sequential vs. concurrent chemoradiation for pts with unrsected stage III NSCLC: Initial report of RTOG 9410 (abstr. #1891) p. 484a. 2000. [Google Scholar]
- 22.Yuan S, Sun X, Li M, et al. A randomized study of involved field irradiation versus elective nodal irradiation in combination with concurrent chemotherapy for inoperable stage III nonsmall cell lung cancer. Am J Clin Oncol. 2007;30(3):239–44. doi: 10.1097/01.coc.0000256691.27796.24. [DOI] [PubMed] [Google Scholar]
- 23.Bradley JD, Graham M, Swann RS, et al. Proc Am Soc Clin Oncol (ASCO) Orlando: 2005. Phase I results of RTOG L-0117; a phase I/II dose intensification study using 3DCRT and concurrent chemotherapy for patients with inoperable NSCLC; p. 7063. J Clin Oncol (PASCOsuppl); 2005. [Google Scholar]
- 24.Bradley J, Bae K, Graham M, et al. World Lung Congress of the International Association for the study of Lung Cancer (IASLC) San Fracisco: 2009. Initial primary analysis of phase II for RTOG 0117: A phase I/II dose intensification study using 3D conformal radiaiton therapy and concurrent chemotherapy for patients with inoperable, non-small cell lung cancer (NSCLC) (abstr. B.5.2) J Thor Oncol suppl., 2009. [Google Scholar]
- 25.Socinski MA, Blackstock AW, Bogart JA, et al. Randomized phase II trial of induction chemotherapy followed by concurrent chemotherapy and dose-escalated thoracic conformal radiotherapy (74 Gy) in sage III non-small cell lung cancer: CALGB 30105. J Clin Oncol. 2008;26(15):2457–63. doi: 10.1200/JCO.2007.14.7371. [DOI] [PubMed] [Google Scholar]
- 26.Stinchcombe TE, Morris DE, Lee CB, et al. Induction chemotherapy with carboplatin, irinotecan, and paclitaxel followed by high dose three-dimension conformal thoracic radiotherapy (74 Gy) with concurrent carboplatin, paclitaxel, and gefitinib in unresectable stage IIIA and stage IIIB non-small cell lung cancer. J Thorac Oncol. 2008;3(3):250–7. doi: 10.1097/JTO.0b013e3181653cf4. [DOI] [PubMed] [Google Scholar]
- 27.RTOG 0617. American College of Radiology. [Accessed 12/1/2009, 2009];2009 at http://www.rtog.org/members/protocols/0617/0617.pdf.
- 28.Timmerman RD, Paulus R, Galvin J, et al. Proc Am Soc Radiat Oncol (ASTRO) Chicago: 2009. RTOG 0236: Stereotactic body radiation therapy (SBRT) to treat medically inoperable early stage lung cancer patients (abstr. #5) p. 3. Int. J. Radiat. Oncol. BIol. Phys.; 2009. [Google Scholar]
- 29.Onishi H, Shirato H, Nagata Y, et al. Hypofractionated stereotactic radiotherapy (HypoFXSRT) for stage I non-small cell lung cancer: updated results of 257 patients in a Japanese multi-institutional study. J Thorac Oncol. 2007;2(7 Suppl 3):S94–100. doi: 10.1097/JTO.0b013e318074de34. [DOI] [PubMed] [Google Scholar]
- 30.Bradley JD, Graham MV, Moughan J, et al. IASLC. Seould: 2007. Phase I/II results of RTOG L-0117; a phase I/II dose intensification study using 3DCRT ahnd concurrent chemotherapy for patients with inoperable NSCLC: PD5-2-4; p. S476. J Thor Oncol; 2007. [Google Scholar]
- 31.Vokes EE, Herndon JEn, Kelley MJ, et al. Induction chemotherapy followed by chemoradiotherapy compared with chemoradiotherapy alone for regionally advanced unresectable stage III Non-small-cell lung cancer: Cancer and Leukemia Group B. J Clin Oncol. 2007;25(13):1698–704. doi: 10.1200/JCO.2006.07.3569. [DOI] [PubMed] [Google Scholar]
- 32.Choy H, DeVore RF, Hande KR, et al. A Phase II study of paclitaxel, carboplatin, and hyperfractionated radiation therapy for locally advanced inoperarble non-small cell lung cancer (A Vanderbilt cancer center affiliate network study) Int J Radiat Oncol Biol Phys. 2000;47:931–7. doi: 10.1016/s0360-3016(00)00420-x. [DOI] [PubMed] [Google Scholar]
- 33.Gandara DR, Chansky K, Albain KS, et al. Long-term survival with concurrent chemoradiation therapy followed by consolidation docetaxel in stage IIIB non-small-cell lung cancer: a phase II Southwest Oncology Group Study (S9504) Clin Lung Cancer. 2006;8(2):116–21. doi: 10.3816/CLC.2006.n.039. [DOI] [PubMed] [Google Scholar]
- 34.Kelly K, Chansky K, Gaspar LE, et al. Phase III trial of maintenance gefitinib or placebo after concurrent chemoradiotherapy and docetaxel consolidation in inoperable stage III non-small-cell lung cancer: SWOG S0023. J Clin Oncol. 2008;26(15):2450–6. doi: 10.1200/JCO.2007.14.4824. [DOI] [PubMed] [Google Scholar]
- 35.Hanna N, Neubauer M, Yiannoutsos C, et al. Phase III study of cisplatin, etoposide, and concurrent chest radiation with or without consolidation docetaxel in patients with inoperable stage III non-small cell lung cancer: the Hoosier Oncology Group and U.S. Oncology J Clin Oncol. 2008;26(35):5755–60. doi: 10.1200/JCO.2008.17.7840. [DOI] [PubMed] [Google Scholar]
- 36.MacManus MP, Hicks RJ, Ball DL, et al. F-18 fluorodeoxyglucose positron emission tomography staging in radical radiotherapy candidates with nonsmall cell lung carcinoma: powerful correlation with survival and high impact on treatment. Cancer. 2001;92:886–95. doi: 10.1002/1097-0142(20010815)92:4<886::aid-cncr1397>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 37.Belani CP, Choy H, Bonomi P, et al. Combined chemoradiotherapy regimens of paclitaxel and carboplatin for locally advanced non-small cell lung cancer: a randomized phase II locally advanced multi-modality protocol. J Clin Oncol. 2005;23(25):5883–91. doi: 10.1200/JCO.2005.55.405. [DOI] [PubMed] [Google Scholar]
- 38.Jeremic B, Shibamoto Y, Acimovic L, Milisavljevic S. Hyperfractionated radiation therapy with or without concurrent low-dose daily carboplatin/etoposide for stage III non-small cell lung cancer: a randomized study. J Clin Oncol. 1996;14:1065–70. doi: 10.1200/JCO.1996.14.4.1065. [DOI] [PubMed] [Google Scholar]
- 39.Schild S, Graham D, Hillman S, et al. Am Soc Clin Oncol (ASCO) Orlando: 2009. Survival of patients treated with high dose radiotherapy (RT) and concurrent chemotherapy for unresectable non-small cell lung cancer (NSCLC) (abstr. #7544) p. 7544. J. Clin. Oncol. ASCO proceedings; 2009. [Google Scholar]