Abstract
Introduction
The number of diabetic patients has recently been increasing worldwide, and numerous anti-diabetic drugs have been developed to induce good glycemic control. In particular, metformin, which exhibits glucose-lowering effects by suppressing gluconeogenesis in the liver, is widely used as a first line oral anti-diabetic drug for type 2 diabetes mellitus.
Material and Methods
In this study, the pharmacological effects of metformin were investigated using female and male Spontaneously Diabetic Torii (SDT) fatty rats, a new obese type 2 diabetic model.
Results
Two experiments were performed: an assessment of repeated treatment with metformin in female SDT fatty rats 5 to 13 weeks of age (experiment 1), and an assessment of repeated treatment with metformin in male SDT fatty rats 6 to 10 weeks of age (experiment 2). In female SDT fatty rats, metformin treatment led to good glycemic control, increases in sensory nerve conduction velocity, and improvements in pancreatic abnormalities such as irregular boundaries and vacuole form of islets. In male SDT fatty rats, metformin decreased blood glucose levels 4 weeks after treatment.
Conclusion
Metformin treatment led to maintained good glycemic control and improved neuropathy and pancreatic lesions in female SDT fatty rats. The SDT fatty rat is useful for the development of novel anti-diabetic agents that show potential to improve glucose metabolic disorders in the liver.
Keywords: Anti-diabetic drugs, Metformin, SDT fatty rat, Type 2 diabetes
1. INTRODUCTION
Metabolic diseases, such as diabetes, obesity, and dyslipidemia, have become health problems worldwide, and the number of patients suffering from these diseases has rapidly increased with changes in lifestyle, including leading a sedentary life and consuming a high-calorie diet (1–3). In particular, type 2 diabetes is a serious challenge to healthcare services (4). The growing population of patients with type 2 diabetes has resulted in an increase in the number of patients who have micro-vascular complications, such as nephropathy, retinopathy, and neuropathy (5–7). In addition to the deterioration in quality of life of such patients, the growing number of patients contributes to an increase in medical costs (8). Preventing the development of type 2 diabetes is extremely important; however, the pathophysiology is complex, involving multiple mechanisms affecting multiple organs. Insulin resistance in peripheral tissues and the inability to secrete sufficient insulin to cover this reduction in insulin sensitivity are pivotal to the pathophysiology (9).
Since the critical causes for diabetes in patients vary, including genetic and environmental factors and a complex pathophysiology, various anti-diabetic drugs that demonstrate different mechanisms have been developed (10). Metformin is a first line oral anti-diabetic drug, and has been used to treat type 2 diabetic patients for over 60 years. Moreover, metformin is taken by over 150 million people each year because of its efficacy as a therapeutic agent and due to its affordable price (11). Guidelines for the treatment of type 2 diabetes, published by the American Diabetes Association and the European Association for the Study of Diabetes in 2012, recommend metformin as the initial drug for treatment (12). Metformin ameliorates hyperglycemia in type 2 diabetes, chiefly through the direct suppression of hepatic glucose production (11).
Diabetic animal models are indispensable for investigating the pathophysiology of diabetes and developing novel anti-diabetic drugs. The Spontaneously Diabetic Torii (SDT) fatty rat was established by introducing the fa allele of the Zucker fatty rat into the SDT rat genome, leading to the onset of hyperphagia, obesity, hyperglycemia and dyslipidemia in these rats at a young age compared with normal rats. Furthermore, with the early onset of diabetes mellitus, diabetes-associated complications, such as nephropathy, retinopathy, and neuropathy, in the SDT fatty rat are observed at a young age compared with lean rats (13). Female SDT fatty rats also develop diabetes and related complications at a young age, suggesting the potential to become an important diabetic animal model, especially for women, for which few models currently exist (14). In fact, the SDT fatty rat is considered to be a suitable model to understand the properties of obese type 2 diabetes. In addition to the pathophysiological analysis of diabetes in the model, the assessment of pharmacological effects of anti-diabetic drugs to elucidate the properties of the rats as a diabetic animal model is important. In this study, metformin was repeatedly administered to female and male SDT fatty rats, and the pharmacological effects of metformin were investigated.
2. MATERIALS AND METHODS
Animals and drug treatment
Female and male SDT fatty rats were purchased from CLEA Japan Inc. (Tokyo, Japan). Age-matched female and male Sprague-Dawley (SD) rats (CLEA Japan Inc.) were used as normal control animals. Rats were housed in suspended bracket cages and given a standard laboratory diet (CRF-1, Oriental Yeast Co., Ltd. Tokyo, Japan) and water ad libitum in a controlled room for temperature, humidity and lightning. Female SDT fatty rats (Experiment 1) and male SDT fatty rats (Experiment 2) were prepared to investigate the gender difference in pharmacological responses. For the convenience of work, the experiment 1 was started from 5 weeks of age, and the experiment 2 was started from 6 weeks of age in the male rats.
Experiment 1: Metformin (Wako, Osaka, Japan) (100, 300 mg/kg) was administered to female SDT fatty rats that were 5 to 9 weeks of age (n=5) in a dietary mixture. After 9 weeks of age, the administration period in the metformin 300 mg/kg group was prolonged for 4 weeks.
Experiment 2: Metformin (300 mg/kg) was administered to male SDT fatty rats that were 6 to 10 weeks of age (n=5) in a dietary mixture.
Measurement of biological parameters
Food intake, body weight, and non-fasting serum biochemical parameters, such as glucose, insulin, triglyceride (TG) and total cholesterol (TC) levels, were evaluated every two weeks. The glucose, TG, and TC levels were measured using commercial kits (Roche Diagnostics, Basel, Switzerland) and an automatic analyzer (Hitachi, Tokyo, Japan). Serum insulin levels were measured using a rat-insulin enzyme-linked immunosorbent assay (ELISA) kit (Morinaga Institute of Biological Science, Yokohama, Japan).
Measurement of nerve conduction velocity
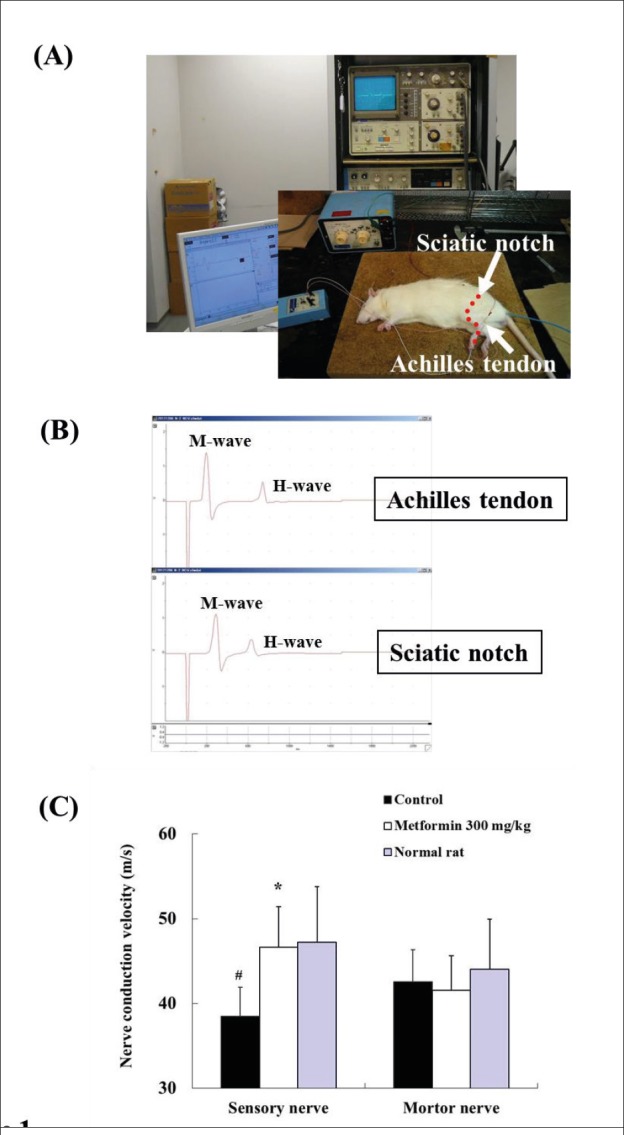
The evaluation of neuropathy, via nerve conduction velocity, was performed at 10 weeks of age in female SDT fatty rats. Nerve conduction velocity was measured in accordance with previously described methods (15). The sciatic nerve was stimulated at the sciatic notch and the Achilles tendon using adequate intensity under 37.5 mg/kg of sodium pentobarbital (Kanto chemical, Tokyo, Japan) and 3 mg/kg of diazepam anesthesia. The action potential in the muscle was recorded via PowerLab through a needle electrode (Figure 1A). Motor nerve conduction velocity (MNCV) was calculated from the delta latency between M-wave peaks divided by the distance of the nerve length measured. Sensory nerve conduction velocity (SNCV) was also calculated from H-wave peaks (Figure 1B).
Figure 1. Effects of metformin on assessments of nerve conduction velocity at 10 weeks of age in female SDT fatty rats. (A) The appearance of the measurement device. (B) Representative data of M- and H- waves. (C) Changes in sensory and motor nerve conduction velocities after metformin 300 mg/kg treatment. Data represent mean ± standard deviation (n=5). * p<0.05; significantly different from the control. # p<0.05; significantly different from normal rats.
The formulas used to calculate MNCV and SNCV were as follows.
MNCV (m/sec) = distance between the sciatic notch and Achilles tendon (mm)/peak latency of an M-wave in the sciatic notch – peak latency of an M-wave in the Achilles tendon (msec).
SNCV (m/sec) = distance between the sciatic notch and Achilles tendon (mm)/peak latency of an H-wave in the Achilles tendon – peak latency of an H-wave in the sciatic notch (msec).
Histopathological analysis
Necropsy was performed at 13 weeks of age in female SDT fatty rats. The pancreas was fixed in 10% neutral buffered formalin. After resection, the tissue was paraffin-embedded using standard techniques and thin-sectioned (3 to 5 μm). The sections were stained with hematoxylin and eosin (HE).
Statistical analysis
Results of biological parameters and assessments of nerve conduction velocity were expressed as means ± standard deviation. A statistical analysis of differences between mean values was performed using a One-way analysis of variance (ANOVA) followed by Dunnett’s two-tailed test.
3. RESULTS
Biological parameters
Changes in food intake, body weight, and blood chemical parameters in female SDT fatty rats are shown in Table 1. The female SDT fatty rat developed metabolic disorders, such as hyperphagia, obesity, hyperglycemia, hyperinsulinemia, and hyperlipidemia as previously reported. Blood glucose levels in the metformin 300 mg/kg group significantly decreased from 7 to 9 weeks of age compared with those in the control group. Effects on metabolic disorders were not observed in the metformin 100 mg/kg group.
Table 1. Quantitative analysis in metformin treatment in female SDT fatty rats. Data represent mean ± standard deviation (n = 5). *p<0.05: significantly different from Control group. #p<0.05, ##p<0.01: significantly different from Normal rat. N.D.: not determined.
| 5 weeks | 7 weeks | 9 weeks | ||
|---|---|---|---|---|
| Food intake (g/day) | Control | N.D. | 33.7 ± 2.0 ## | 33.3 ± 1.9 ## |
| Metformin 100 mg/kg | N.D. | 30.5 ± 3.0 | 33.3 ± 5.4 | |
| Metformin 300 mg/kg | N.D. | 32.0 ± 2.8 | 32.2 ± 2.6 | |
| Normal rat | N.D. | 18.8 ± 4.0 | 19.6 ± 5.0 | |
| Body weight (g) | Control | 210.3 ± 11.7 ## | 333.6 ± 14.2 ## | 422.0 ± 30.4 ## |
| Metformin 100 mg/kg | 211.8 ± 8.6 | 335.0 ± 8.1 | 424.2 ± 8.4 | |
| Metformin 300 mg/kg | 209.0 ± 5.6 | 331.6 ± 5.6 | 430.0 ± 8.1 | |
| Normal rat | 179.4 ± 5.7 | 239.9 ± 10.0 | 290.5 ± 10.0 | |
| Blood glucose level (mg/dl) | Control | 159.4 ± 16.3 ## | 464.4 ± 170.0 ## | 466.6 ± 172.9 ## |
| Metformin 100 mg/kg | 157.0 ± 13.9 | 404.0 ± 98.8 | 464.8 ± 199.4 | |
| Metformin 300 mg/kg | 155.6 ± 12.1 | 279.8 ± 37.8 * | 193.2 ± 37.5 * | |
| Normal rat | 133.4 ± 6.3 | 146.4 ± 15.7 | 127.2 ± 13.4 | |
| Blood insulin level (ng/ml) | Control | 13.7 ± 2.7 ## | 21.5 ± 5.0 ## | 21.4 ± 15.6 # |
| Metformin 100 mg/kg | 11.2 ± 2.2 | 22.9 ± 1.9 | 21.1 ± 7.6 | |
| Metformin 300 mg/kg | 21.1 ± 7.6 | 25.1 ± 6.4 | 28.4 ± 4.0 | |
| Normal rat | 1.6 ± 0.9 | 2.4 ± 0.6 | 1.4 ± 0.4 | |
| Blood triglyceride level (mg/dl) | Control | 340.8 ± 43.5 ## | 668.0 ± 191.4 ## | 377.1 ± 57.6 ## |
| Metformin 100 mg/kg | 320.9 ± 41.9 | 659.2 ± 244.1 | 303.3 ± 124.8 | |
| Metformin 300 mg/kg | 300.2 ± 59.1 | 641.3 ± 163.8 | 291.2 ± 69.8 | |
| Normal rat | 64.4 ± 31.6 | 96.2 ± 20.5 | 81.4 ± 33.0 | |
| Blood total chlesterol level (mg/dl) | Control | 128.1 ± 3.5 ## | 111.7 ± 5.2 ## | 128.0 ± 20.7 ## |
| Metformin 100 mg/kg | 121.6 ± 9.0 | 114.4 ± 9.4 | 143.1 ± 31.1 | |
| Metformin 300 mg/kg | 131.4 ± 3.5 | 112.5 ± 6.9 | 132.3 ± 10.8 | |
| Normal rat | 78.6 ± 7.3 | 77.1 ± 3.2 | 82.2 ± 7.9 |
Changes in food intake, body weight, and blood chemical parameters in male SDT fatty rats are shown in Table 2. The male SDT fatty rat also developed metabolic disorders, such as hyperphagia, obesity, hyperglycemia, hyperinsulinemia, and hyperlipidemia. Blood insulin levels at 10 weeks of age in the SDT fatty rat were comparable to that in normal rats. Food intake and blood glucose levels in the metformin 300 mg/kg group significantly decreased at 10 weeks of age compared with those in the control group. The blood triglyceride levels in the metformin 300 mg/kg group significantly increased at 10 weeks of age compared with that in the control group, and the total cholesterol levels in blood in the metformin 300 mg/kg group significantly increased from 8 to 10 weeks of age.
Table 2. Quantitative analysis in metformin treatment in male SDT fatty rats. Data represent mean ± standard deviation (n = 5). *p<0.05, **p<0.01: significantly different from Control group. ##p<0.01: significantly different from Normal rat group. N.D.: not determined.
| 6 weeks | 8 weeks | 10 weeks | ||
|---|---|---|---|---|
| Food intake (g/day) | Control | N.D. | 44.0 ± 2.3 ## | 43.9 ± 1.8 ## |
| Metformin 300 mg/kg | N.D. | 41.4 ± 1.5 | 38.4 ± 2.7 ** | |
| Normal rat | N.D. | 25.2 ± 1.5 | 24.0 ± 4.5 | |
| Body weight (g) | Control | 283.6 ± 26.7 ## | 377.3 ± 8.8 ## | 460.9 ± 11.0 ## |
| Metformin 300 mg/kg | 270.4 ± 25.5 | 380.2 ± 20.9 | 470.7 ± 27.2 | |
| Normal rat | 215.5 ± 15.7 | 312.4 ± 37.0 | 393.2 ± 31.7 | |
| Blood glucose level (mg/dl) | Control | 589.0 ± 211.2 ## | 690.8 ± 75.8 ## | 739.2 ± 82.5 ## |
| Metformin 300 mg/kg | 582.2 ± 195.6 | 703.6 ± 130.4 | 627.6 ± 53.2 * | |
| Normal rat | 159.8 ± 6.8 | 148.0 ± 7.4 | 165.6 ± 9.5 | |
| Blood insulin level (ng/ml) | Control | 18.9 ± 4.5 ## | 6.8 ± 0.7 ## | 5.6 ± 1.5 |
| Metformin 300 mg/kg | 16.8 ± 3.9 | 7.1 ± 2.3 | 5.8 ± 1.9 | |
| Normal rat | 2.0 ± 0.6 | 3.9 ± 0.8 | 5.7 ± 1.8 | |
| Blood triglyceride level (mg/dl) | Control | 516.9 ± 77.0 ## | 431.4 ± 93.1 ## | 281.7 ± 74.1 ## |
| Metformin 300 mg/kg | 471.8 ± 42.5 | 540.5 ± 100.4 | 613.9 ± 152.5 ** | |
| Normal rat | 108.0 ± 11.2 | 145.8 ± 30.1 | 131.0 ± 24.9 | |
| Blood total chlesterol level (mg/dl) | Control | 103.3 ± 5.4 ## | 103.4 ± 3.6 ## | 104.0 ± 8.9 ## |
| Metformin 300 mg/kg | 106.4 ± 1.9 | 118.3 ± 10.2 * | 119.0 ± 9.9 * | |
| Normal rat | 74.6 ± 2.8 | 75.2 ± 3.3 | 79.5 ± 8.3 |
Nerve conduction velocity
The device used to measure nerve conduction velocity and the results are shown in Figure 1. The SNCV in the control group significantly decreased compared with that in the normal rat group, and the SNCV in the metformin 300 mg/kg group recovered to levels similar to those observed in the normal rat group (control group, 38.5 ± 3.4 m/s; metformin 300 mg/kg group, 46.6 ± 4.8 m/s; normal rat group, 47.2 ± 6.6 m/s). The MNCV in the control group was comparable to that in the normal rat group, and the effect of metformin on MNCV was not shown.
Histopathology
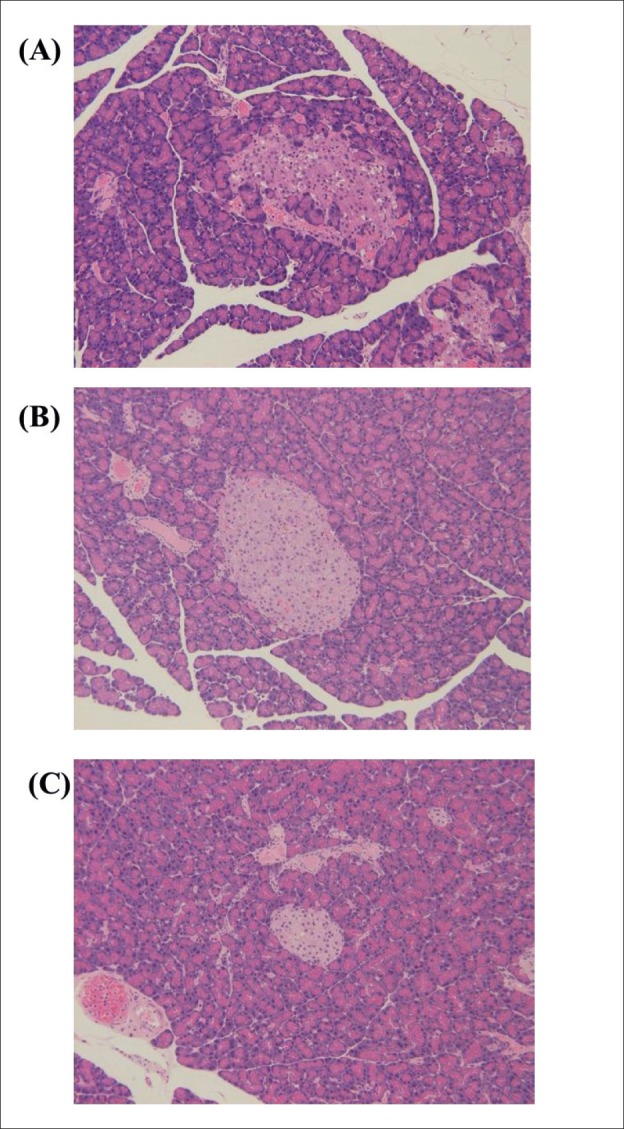
Histopathological changes in the pancreas are shown in Figure 2. Histopathological abnormalities, such as irregular boundaries and vacuole form in islets, were observed in the pancreas of female SDT fatty rats. These changes were not observed in the metformin 300 mg/kg group.
Figure 2. Histopathological analysis of the pancreas. (A) female SDT fatty rat at 13 weeks of age. (B) A metformin 300 mg/kg-treated female SDT fatty rat. (C) A normal SD rat at 13 weeks of age. Original magnification x 200. HE staining.
4. DISCUSSION
The pathogenesis of type 2 diabetes is heterogeneous, and genetic and environmental factors are complexly related with the development of the disease. Metabolic abnormalities including hyperglycemia and dyslipidemia in diabetic patients are caused by defects in insulin secretion from the pancreas and insulin sensitivity in peripheral tissues (16, 17). Improvements in insulin deficiency in pancreatic β cells and the enhancement of insulin sensitivity are necessary to achieve tight blood glucose control, and numerous drugs have been developed to treat type 2 diabetic patients. Sulfonylureas and dipeptidyl peptidase (DPP)-4 inhibitors have been developed to improve insulin deficiency, and biguanides and thiazolidinedione-based agents have been developed to enhance insulin sensitivity (16, 18).
In 1929, dimethylbiguanides, including phenformin and metformin, were developed, with phenformin being more potent than metformin in terms of glucose-lowering effect; however, phenformin was withdrawn from the market due to the high occurrence of the serious side effect lactic acidosis in 1970s (11). As a treatment for patients with type 2 diabetes, metformin decreased hepatic glucose output by suppressing gluconeogenesis (19, 20). In animal studies, metformin also reduced hepatic glucose production (21, 22). In this study, the pharmacological effects of metformin were investigated to elucidate the properties of SDT fatty rats, a new obese type 2 diabetic model.
A significant glucose-lowering effect in female SDT fatty rats was observed with metformin 300 mg/kg treatment, and the sustained glycemic control ameliorated neuropathy in sensory neurons. In this study, female SDT fatty rats showed dysfunctions in sensory neurons, but not in motor neurons. Since the thinner nerve is easily damaged, leading to hyperglycemia, only dysfunctions in sensory neurons may be represented in female SDT fatty rats at 10 weeks of age. In female SDT fatty rats at 29 weeks of age, sodium glucose co-transporter (SGLT) inhibitors reportedly improve decreases in SNCV and MNCV (23). Moreover, good glycemic control reportedly leads to improvements in SNCV in other diabetic models, including in streptozotocin (STZ) – induced diabetic rats and Zucker diabetic fatty (ZDF) rats (24). The female SDT fatty rat is considered to be useful for the evaluation of the glucose-lowering effects and the potential effects of anti-diabetic drugs on diabetic complications.
The irregular boundaries and the vacuole form in pancreatic islets are considered to be caused by sustained hyperglycemia in SDT fatty rats, and these changes improved with glycemic control observed after metformin treatment. In ZDF rats, pancreatic abnormalities, such as irregular boundaries and vacuole form in islets, as well as fibrosis, were also reportedly improved by chronic glycemic control (25).
In male SDT fatty rats, metformin 300 mg/kg treatment transiently decreased blood glucose levels; however, food intake decreased and, furthermore, lipid levels increased with metformin treatment. Since blood glucose levels in male SDT fatty rats are significantly higher than those in female SDT fatty rats, an increase in the administration dosage may be necessary to observe a glucose-lowering effect with metformin in male SDT fatty rats. In our preliminary study, a single administration of metformin 1000 mg/kg in male SDT fatty rats led to significant glucose-lowering effects (blood glucose levels at 3 h after oral administration, Control group: 537 ± 109 mg/dl, Metformin treatment group: 310 ± 98 mg/dl). The metformin treatment group in male SDT fatty rats showed increases in blood lipid levels; however, the mechanism is unknown.
5. CONCLUSION
In female SDT fatty rats, metformin treatment led to a glycemic control and improved neuropathy and pancreatic abnormalities. The SDT fatty rat is useful for the development of novel anti-diabetic agents that show potential to improve glucose metabolic disorders in the liver.
Conflict of interest
none declared.
REFERENCES
- 1.King H, Aubert RE, Herman WH. Global burden of diabetes, 1995-2025: prevalence, numerical estimates, and projections. Diabetes Care. 1998;21(9):1414–31. doi: 10.2337/diacare.21.9.1414. [DOI] [PubMed] [Google Scholar]
- 2.Yoon KH, Lee JH, Kim JW, Cho JH, Choi YH, Ko SH, et al. Epidemic obesity and type 2 diabetes in Asia. Lancet. 2006;368:1681–8. doi: 10.1016/S0140-6736(06)69703-1. [DOI] [PubMed] [Google Scholar]
- 3.Szoke E, Shrayyef MZ, Messing S, Woerle HJ, van Haeften TW, Meyer C, et al. Effect of aging on glucose homeostasis: accelerated deterioration of beta-cell function in individuals with impaired glucose tolerance. Diabetes Care. 2008;31(3):539–43. doi: 10.2337/dc07-1443. [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization. Global report on diabetes; 2016:1-88. [November 4, 2016]; [Google Scholar]
- 5.Cortez M, Singleton JR, Smith AG. Glucose intolerance, metabolic syndrome, and neuropathy. Handb Clin Neurol. 2014;126:109–22. doi: 10.1016/B978-0-444-53480-4.00009-6. [DOI] [PubMed] [Google Scholar]
- 6.Hall JE, Kuo JJ, da Silva AA, de Paula RB, Liu J, Tallam L, et al. Obesity-associated hypertension and kidney disease. Curr Opin Nephrol Hypertens. 2003;12(2):195–200. doi: 10.1097/00041552-200303000-00011. [DOI] [PubMed] [Google Scholar]
- 7.Ritz E, Stefanski A. Diabetic nephropathy in type II diabetes. Am J Kidney Dis. 1996;27(2):167–94. doi: 10.1016/s0272-6386(96)90538-7. [DOI] [PubMed] [Google Scholar]
- 8.Calcutt NA, Cooper ME, Kern TS, Schmidt AM. Therapies for hyperglycaemia-induced diabetic complications: from animal models to clinical trials. Nat Rev Drug Discov. 2009;8(5):417–29. doi: 10.1038/nrd2476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brown A, Guess N, Dornhorst A, Taheri S, Frost G. Insulin-associated weight gain in obese type 2 diabetes mellitus patients: What can be done? Diabetes Obes Metab. 2017 May 16; doi: 10.1111/dom.13009. [DOI] [PubMed] [Google Scholar]
- 10.Bohannon NJ. Treating dual defects in diabetes: insulin resistance and insulin secretion. Am J Health Syst Pharm. 2002;59(Suppl9):S9–13. doi: 10.1093/ajhp/59.suppl_9.S9. [DOI] [PubMed] [Google Scholar]
- 11.An H, He L. Current understanding of metformin effect on the control of hyperglycemia in diabetes. J Endocrinol. 2016;228(3):R97–106. doi: 10.1530/JOE-15-0447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Inzucchi SE, Bergenstal RM, Buse JB, Diamant M, Ferrannini E, Nauck M, et al. Management of hyperglycaemia in type 2 diabetes: a patient-centered approach. Position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) Diabetologia. 2012;55(6):1577–96. doi: 10.1007/s00125-012-2534-0. [DOI] [PubMed] [Google Scholar]
- 13.Matsui K, Ohta T, Oda T, Sasase T, Ueda N, Miyajima K, et al. Diabetes-associated complications in Spontaneously Diabetic Torii fatty rats. Exp Anim. 2008;57(2):111–21. doi: 10.1538/expanim.57.111. [DOI] [PubMed] [Google Scholar]
- 14.Ishii Y, Ohta T, Sasase T, Morinaga H, Ueda N, Hata T, et al. Pathophysiological analysis of female Spontaneously Diabetic Torii fatty rats. Exp Anim. 2010;59(1):73–84. doi: 10.1538/expanim.59.73. [DOI] [PubMed] [Google Scholar]
- 15.Katsuda Y, Sasase T, Tadaki H, Mera Y, Motohashi Y, Kemmochi Y, et al. Contribution of hyperglycemia on diabetic complications in obese type 2 diabetic SDT fatty rats: effects of SGLT inhibitor phlorizin. Exp Anim. 2015;64(2):161–9. doi: 10.1538/expanim.14-0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meece J. Pancreatic islet dysfunction in type 2 diabetes: a rational target for incretin-based therapies. Curr Med Res Opin. 2007;23(4):933–44. doi: 10.1185/030079906x167336. [DOI] [PubMed] [Google Scholar]
- 17.Turner NC, Clapham JC. Insulin resistance, impaired glucose tolerance and non-insulin-dependent diabetes, pathologic mechanisms and treatment: current status and therapeutic possibilities. Prog Drug Res. 1998;51:33–94. doi: 10.1007/978-3-0348-8845-5_2. [DOI] [PubMed] [Google Scholar]
- 18.Wilding JP. PPAR agonists for the treatment of cardiovascular disease in patients with diabetes. Diabetes Obes Metab. 2012;14(11):973–82. doi: 10.1111/j.1463-1326.2012.01601.x. [DOI] [PubMed] [Google Scholar]
- 19.Stumvoll M1, Nurjhan N, Perriello G, Dailey G, Gerich JE. Metabolic effects of metformin in non-insulin-dependent diabetes mellitus. N Engl J Med. 1995;333(9):550–4. doi: 10.1056/NEJM199508313330903. [DOI] [PubMed] [Google Scholar]
- 20.Hundal RS, Krssak M, Dufour S, Laurent D, Lebon V, Chandramouli V, et al. Mechanism by which metformin reduces glucose production in type 2 diabetes. Diabetes. 2000;49(12):2063–9. doi: 10.2337/diabetes.49.12.2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Takashima M, Ogawa W, Hayashi K, Inoue H, Kinoshita S, Okamoto Y, et al. Role of KLF15 in regulation of hepatic gluconeogenesis and metformin action. Diabetes. 2010;59(7):1608–15. doi: 10.2337/db09-1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Song S, Andrikopoulos S, Filippis C, Thorburn AW, Khan D, Proietto J. Mechanism of fat-induced hepatic gluconeogenesis: effect of metformin. Am J Physiol Endocrinol Metab. 2001;281(2):E275–82. doi: 10.1152/ajpendo.2001.281.2.E275. [DOI] [PubMed] [Google Scholar]
- 23.Katsuda Y, Sasase T, Tadaki H, Mera Y, Motohashi Y, Kemmochi Y, et al. Contribution of hyperglycemia on diabetic complications in obese type 2 diabetic SDT fatty rats: effects of SGLT inhibitor phlorizin. Exp Anim. 2015;64(2):161–9. doi: 10.1538/expanim.14-0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kürthy M, Mogyorósi T, Nagy K, Kukorelli T, Jednákovits A, Tálosi L, Bíró K. Effect of BRX-220 against peripheral neuropathy and insulin resistance in diabetic rat models. Ann N Y Acad Sci. 2002;967:482–9. doi: 10.1111/j.1749-6632.2002.tb04306.x. [DOI] [PubMed] [Google Scholar]
- 25.Hata T, Mera Y, Kawai T, Ishii Y, Kuroki Y, Kakimoto K, et al. JTT-130, a novel intestine-specific inhibitor of microsomal triglyceride transfer protein, ameliorates impaired glucose and lipid metabolism in Zucker diabetic fatty rats. Diabetes Obes. Metab. 2011;13(7):629–38. doi: 10.1111/j.1463-1326.2011.01387.x. [DOI] [PubMed] [Google Scholar]


