Abstract
Background
Given the diversity of species from which adipose-derived stromal cells are derived and studied, the authors set out to delineate the differences in the basic cell biology that may exist across species. Briefly, the authors found that significant differences exist with regard to proliferation and osteogenic potentials of adipose-derived stromal cells across species.
Methods
Adipose-derived stromal cells were derived from human, mouse, and canine sources as previously described. Retinoic acid, insulin-like growth factor-1, and bone morphogenetic protein-2 were added to culture medium; proliferation and osteogenic differentiation were assessed by standardized assays. In vivo methods included seeding 150,000 adipose-derived stromal cells on a biomimetic scaffold and analyzing healing by micro–computed tomography and histology.
Results
Adipose-derived stromal cells from all species had the capability to undergo osteogenic differentiation. Canine adipose-derived stromal cells were the most proliferative, whereas human adipose-derived stromal cells were the most proliferative, whereas human adipose-derived stromal cells were the most osteogenic (p < 0.05). Human cells, however, had the most significant osteogenic response to osteogenic media. Retinoic acid stimulated osteogenesis in mouse and canine cells but not in human adipose-derived stromal cells. Insulin-like growth factor-1 enhanced osteogenesis across all species, most notably in human- and canine-derived cells.
Conclusions
Adipose-derived stromal cells derived from human, mouse, and canine all have the capacity to undergo osteogenic differentiation. Canine adipose-derived stromal cells appear to be the most proliferative, whereas human adipose-derived stromal cells appear to be the most osteogenic. Different cytokines and chemicals can be used to modulate this osteogenic response. These results are promising as attempts are made to optimize tissue-engineered bone using adipose-derived stromal cells.
As the field of skeletal tissue engineering expands, surgeons and scientists continue to study the differentiation of mesenchymal stem cells. Earlier studies of mesenchymal stem cells focused on bone marrow–derived cells.1–5 It has since been discovered that stromal cells obtained from adipose tissue (or adipose-derived stromal cells) share many of the same properties as mesenchymal stem cells.6–14 To translate basic science findings of adipose-derived stromal cells to the clinical realm, robust in vitro and in vivo data must be obtained to demonstrate the osteogenic capacity of adipose-derived stromal cells. To date, in vivo studies using mesenchymal stem cells have included large, immunocompetent animals such as sheep15 and dogs, 16,15 and smaller, immunocompromised animals such as rabbits,18 rats,6 and mice.19 Recent studies have also used human adipose-derived stromal cells, demonstrating their robust capacity to undergo osteogenesis in vitro and in vivo.20–24 Given the diversity of species from which adipose-derived stromal cells are derived and studied, we set out to delineate the differences in the basic cell biology of adipose-derived stromal cells that may exist across species. In this article, we compare the osteogenic and proliferative capacity of adipose-derived stromal cells from the following: mouse (the most common small animal used), canine (a common large animal used), and human adipose tissue.
The study of mouse adipose-derived stromal cells is attractive because of the ease of cell harvest and availability of laboratory mice. Moreover, the widespread use of genetic knockout mice makes for potential profitable avenues of investigation. However, important differences exist between human and mouse adipose-derived stromal cells, differences that have not yet been fully investigated. For example, human cells have been found to be more osteogenic in vitro than mouse stromal cells.25 To undergo significant in vitro osteogenesis, mouse cells require additional osteogenic stimuli, such as retinoic acid.26 Moreover, cytokines such as fibroblast growth factor (FGF)-2 inhibit osteogenic differentiation in mouse cells, whereas osteogenic differentiation of human adipose-derived stromal cells proceeds relatively uninhibited in the presence of FGF-2.25,27
As the use of adipose-derived stromal cells becomes more translational, scientists have begun using large-animal models such as canines. Multiple investigators have demonstrated that canine adipose-derived stromal cells can undergo adipogenic, osteogenic, chondrogenic, and myogenic differentiation in vitro.28 Scientists have also demonstrated the use of canine cells in vivo for chronic osteoarthritis and for skeletal tissue engineering when overexpressing bone morphogenetic protein (BMP)-2.29,30 Importantly, comparing the differentiation of canine adipose-derived stromal cells to that of other species has not been investigated.
In our laboratory, both mouse and human adipose-derived stromal cells contribute to osseous healing of mouse calvarial defects.19,24 A critical-sized mouse calvarial defect shows no healing without adipose-derived stromal cell engraftment up to 16 weeks after injury. On human adipose-derived stromal cell engraftment, significant healing is observed as early as 4 weeks after injuiy.24 In this study, we compared the in vitro and in vivo differences among adipose-derived stromal cells of human, mouse, and canine origin. Briefly, we found that significant differences exist across species in their potential to undergo osteogenic differentiation. Moreover, differences exist in adipose-derived stromal cell response to commonly studied cytokines based on species of derivation.
MATERIALS AND METHODS
Chemicals, Supplies, and Animals
Dulbecco’s Modified Eagle Medium, α-Minimal Essential Medium, fetal bovine serum, and penicillin/streptomycin were purchased from Gibco Life Technologies (Carlsbad, Calif.). Insulin-like growth factor (IGF-1) and BMP-2 were purchased from R&D Systems (Minneapolis, Minn.). Cell culture wares were purchased from Corning, Inc. (San Mateo, Calif.). Unless otherwise specified, all other chemicals were purchased from Sigma-Aldrich (St. Louis, Mo.). CD-1 nude mice (Crl:CD-1 Foxnlnu) were obtained from Charles River Laboratories (Wilmington, Mass.).
Cell Harvest
Adipose-derived stromal cells were harvested from human lipoaspirate from the thigh and flank regions of five patients younger than 60 years Without any comorbidities as described previously.22 [See Table, Supplemental Digital Content 1, which shows patient demographics (all female patients), http://links.lww.com/PRS/A351]. For these experiments, five different batches of human adipose-derived stromal cells were used. Because of different operation dates, each experiment was run independently and in triplicate. Numerical data from all five patients were pooled for analysis.
For all mouse adipose-derived stromal cell harvests, five female mice were euthanized at a given time. Inguinal fat pads were dissected and finely minced, and the cells were isolated, as for the human adipose-derived stromal cell harvest, to render a single and homogenous population of mouse adipose-derived stromal cells. This process was repeated such that multiple isolation dates were used for analysis. For three separate derivations of mouse adipose-derived stromal cells, a total of 15 mice were used. Thus, all experiments were performed on three separate batches of the mouse cells. Inguinal or ventral fat was used, as most subcutaneous white fat is located ventrally on mice and dorsal fat is mostly brown fat.31
To obtain canine adipose-derived stromal cells, fat harvests were performed on the dorsal surface of two canines. The adipose tissue was resected and separated from the skin. Next, the fat from each canine was minced and processed using technique identical to that used for human cell harvest, to derive two separate batches of canine adipose-derived stromal cells. A third batch was derived from a separate region of dorsal fat from both animals and combined.
Adipose-derived stromal cells were cultured in standard growth medium, containing Dulbecco’s Modified Eagle Medium, 10% fetal bovine serum, and 100 IU/ml penicillin/streptomycin, and passaged on confluence by trypsinization. The cells passaged once and twice only were used for the following assays.
Scanning Electron Microscopy
To examine cellular morphology, scanning electron microscopy was performed on cells after 7 days in culture in either standard growth medium or osteogenic differentiation medium as described previously.23 To quantify cell size, cells were normalized and plated at equal number. Forty cells per group were quantified at 600× magnification using Adobe Photoshop (Adobe Systems, Inc., San Jose, Calif.).
In Vitro Culture Assays
For osteogenic differentiation, cells were seeded onto six-well plates at a density of 100,000 cells/well and a 12-well plate at a density of 35,000 cells/well.23 Briefly, after attachment, adipose-derived stromal cells were treated with osteogenic differentiation medium containing Dulbecco’s Modified Eagle Medium, 10% fetal bovine serum, 100 μg/ml ascorbic acid, 10 mM β-glycerophosphate, and 100 IU/ml penicillin/streptomycin.32 For specific assays, all-trans retinoic acid (5 and 10 μM), recombinant IGF-1 (25 and 50 ng/ml), and recombinant BMP-2 (200 ng/ml) were supplemented to the medium. Medium was changed every 3 days. Alkaline phosphatase staining and quantification were performed at 3 days to assess early osteogenic differentiation as described previously.33 Alizarin red staining and photometric quantification were performed at 7 days.34
Proliferation of human adipose-derived stromal cells was assessed by cell counting and bromodeoxyuridine incorporation as described previously.33 Cell counting was performed on 1, 3, 5, and 7 days of growth. For bromodeoxyuridine incorporation assays, cells were grown in 96-well plates and seeded at a density of 1000 cells/well. After 1, 2, and 4 days in culture, bromodeoxyuridine labeling was performed for a period of 8 hours. Next, bromodeoxyuridine incorporation was quantified using photometric enzyme-linked immunosorbent assay (Roche Applied Science. Indianapolis, Ind.).
Scaffold Creation
Apatite-coated poly(lactic-co-glycolic acid) scaffolds were fabricated from 85/15 poly(lactic-co-glycolic acid) (Birmingham Polymers, Inc., Pelham, Ala.) by solvent casting and a particulate leaching process as described previously.19,24
Calvarial Defects
Critical-sized (4 mm) calvarial defects were created in the right parietal bones of 60-day-old CD-1 nude mice using a high-speed dental drill as described previously.24 In preparation for cell engraftment, scaffolds were seeded with human, mouse, or canine adipose-derived stromal cells, as described previously.24 Next, 150,000 human, mouse, or canine cells were resuspended in 25 μl of growth media and left for 12 hours’ incubation. Before engraftment, scaffolds were rinsed in sterile phosphate-buffered saline. Animals were split equally into five treatment groups: (1) empty defects in which a 4-mm defect was left empty; (2) scaffold only, in which a scaffold without cells was placed in the defect site; (3) 150,000 human adipose-derived stromal cells seeded onto a poly(lactic-co-glycolic acid) scaffold; (4) 150,000 mouse cells seeded onto a poly(lactic-co-glycolic acid) scaffold; and (5) 150,000 canine cells seeded onto a poly(lactic-co-glycolic acid) scaffold (n = 5 per group).
Micro–Computed Tomography
Micro–computed tomography was performed using a high-resolution MicroCAT II (ImTek, Inc., Knoxville, Tenn.) small-animal imaging system as described previously.24,25 Percentage healing was calculated relative to comptued tomographic images taken immediately postoperatively, and with the use of Adobe Photoshop.
Histology
Animals were euthanized by carbon dioxide asphyxiation and cervical dislocation to confirm radiographic findings. Briefly, tissues were formalin-fixed, decalcified with 19% ethylenediaminetetraacetic acid, paraffin embedded, and sectioned at 8-μm width according to standard protocols.36 To assess healing, aniline blue staining was performed on every tenth section throughout the sample to provide detailed histology of the regenerate. Next, select slides were stained with pentachrome and alkaline phosphatase.36,37 Aniline blue staining was quantified by using the magic wand tool in Adobe Photoshop (n = 5 slides per group).
Statistical Analysis
Means and standard deviations were calculated from numerical data, as presented in the text, figures, and figure legends. In figures, bar graphs represent means, whereas error bars represent 1 SD. Statistical analysis was performed using the appropriate analysis of variance test as described. For Figures 2, 3, 5, and 6, a one-factor analysis of variance was used to compare the differences between species. In Figure 4, a one-factor analysis of variance was used to compare the differences between different doses of cytokine treatment. Statistical analysis between groups was assessed by using a post hoc t test; a value of p ≤ 0.05 was considered to be significant.
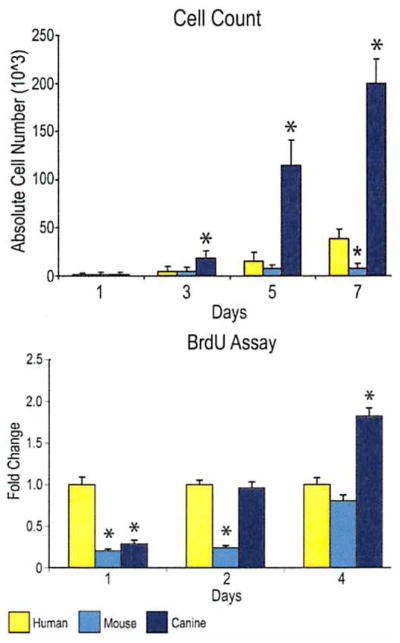
Fig. 2.
Cellular proliferation among human, mouse, and canine adipose-derived stromal cells. (Above) Cell counting assays were performed over 7 days in standard growth medium (Dulbecco’s Modified Eagle Medium, 10% fetal bovine serum) by trypsinization and hemocytometry. (Below) Bromodeoxyuridine (BrdU) incorporation assays were performed over 4 days in standard growth medium by enzyme-linked immunosorbent assay. Labeling reagent was applied for 8 hours in culture (n = 3 for cell counting, n = 6 for bromodeoxyuridine) (*p < 0.05).
Fig. 3.
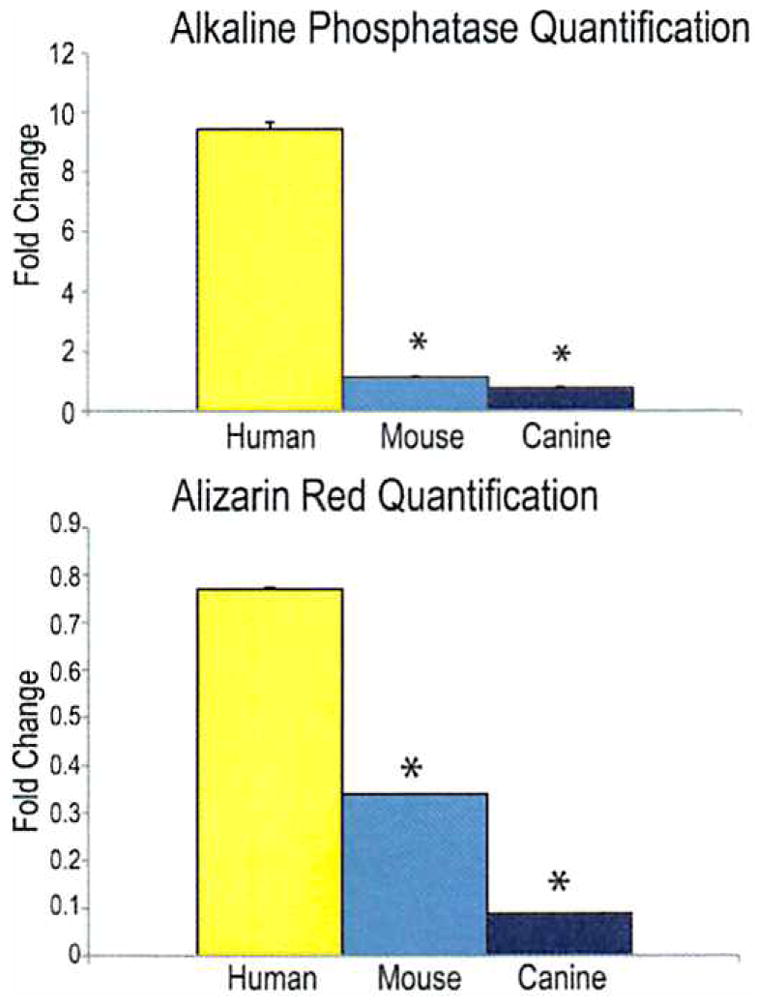
(Above) Alkaline phosphatase quantification normalized to total protein content of human, mouse, and canine adipose-derived stromal cells after 3 days of growth in osteogenic differentiation medium. Human cells had significantly higher levels of alkaline phosphatase activity than either mouse or canine cells. (Below) Alizarin red quantification of human, mouse, and canine adipose-derived stromal cells after 7 days of growth in osteogenic differentiation medium, demonstrating significantly more calcification in human cells than either mouse or canine cells (*p < 0.05).
Fig. 5.
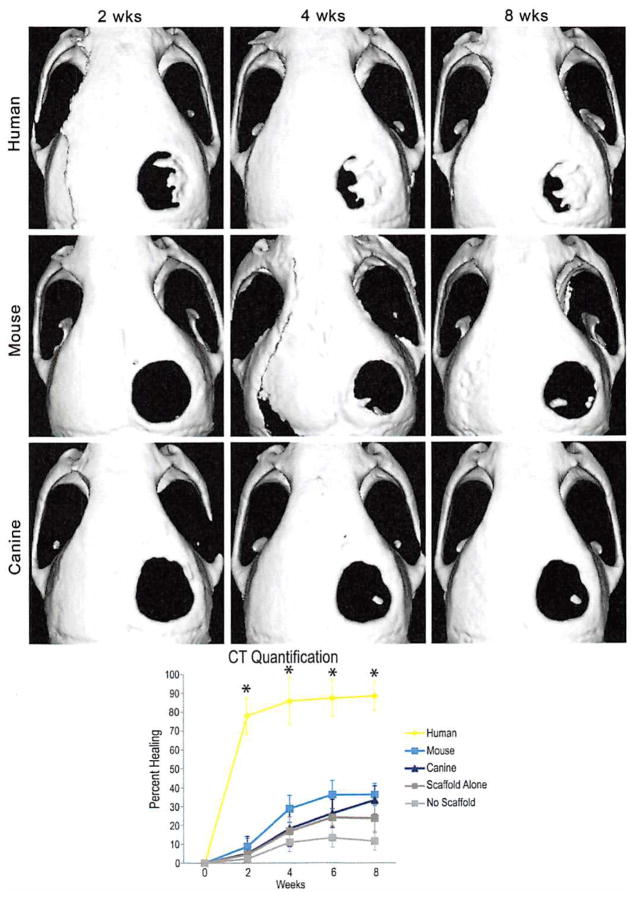
(Above) Micro–computed tomographic scans of live mouse calvariae after a 4-mm parietal defect and placement of a 4-mm hydroxyapatite coated poly(lactic-co-glycolic acid) scaffold with 150,000 human (first row), mouse (second row), or canine adipose-derived stromal ceils (last row). Human cells demonstrate a significant amount of healing as early as 2 weeks and nearly complete healing by 8 weeks; mouse cells demonstrate some osseous healing by 4 weeks but never completely heal. Canine cells demonstrate minimal healing around the edges of the defect. (Below) Quantification of mean calvarial healing calculated by dividing the size of the defect at each time point by the size of the defect at day 0 of injury. Human adipose-derived stromal cells have significantly greater healing at all time points (n = 4 mice per group; *p < 0.05). CT, computed tomography.
Fig. 6.
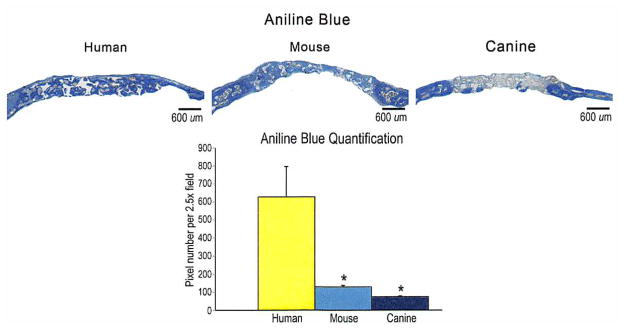
(Above) Histology of calvarial defects. Four-millimeter calvarial defects were allowed to heal for 8 weeks before histologic analysis by aniline blue stain. Pictures were taken of the midpoint of the defect site. In aniline blue stains, bone appears dark blue. (Below) At 8 weeks, aniline blue–positive bone per 2.5 × field was quantified (n = 50 slides per group, n = 5 animals per group). A one-factor analysis of variance was used, followed by a post hoc t test to assess significance (*p < 0.05).
Fig. 4.
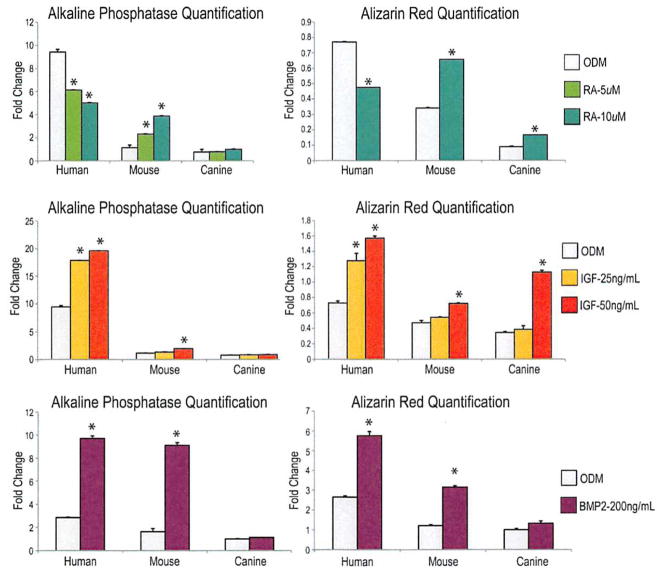
(Above, left) Alkaline phosphatase quantification normalized to total protein content of human, mouse, and canine adipose-derived stromal cells after 3 days of growth with treatment of osteogenic differentiation medium (ODM) and osteogenic differentiation medium supplemented with 5 and 10 μM of retinoic acid (RA). With retinoic acid supplementation, mouse cells show a significant increase in alkaline phosphatase activity, whereas human cells show a significant decrease. Alkaline phosphatase activity in canine cells is relatively unaffected by the presence of retinoic acid. (Above, right) Alizarin red quantification of human, mouse, and canineadipose-derived stromal cells after 7 days of growth. Mouse cells and canine cells demonstrate a significant increase in calcification when treated with retinoic acid, whereas human cells demonstrate a significant decrease. (Center, left) Alkaline phosphatase quantification of human, mouse, and canine adipose-derived stromal cells after 3 days of growth in osteogenic differentiation medium and osteogenic differentiation medium supplemented with 25 and 50 ng/ml of IGF-1. Human and mouse cells demonstrate increased alkaline phosphatase activity with IGF-1 supplementation. Canine cells demonstrate only a paucity of alkaline phosphatase activity even with IGF-1 supplementation. (Center, right) Alizarin red quantification of human, mouse, and canine adipose-derived stromal cells after 7 days of growth in either osteogenic differentiation medium or osteogenic differentiation medium with IGF-1 supplementation. Similar to alkaline phosphatase, human and mouse cells demonstrate increased mineralization with IGF-1 supplementation. However, canine cells also demonstrate enhanced mineralization (a late marker for osteogenic differentiation) with IGF-1 supplementation, which is not appreciated in the earlier stages of osteogenesis (*p <0.05).(Below, left) Alkaline phosphatase quantification of human, mouse, and canine adipose-derived stromal cells after 3 days of growth in osteogenic differentiation medium and osteogenic differentiation medium supplemented with 200 ng/ml of BMP-2. Human and mouse cells demonstrate increased alkaline phosphatase activity with BMP-2 supplementation. Canine cells demonstrate only minimal alkaline phosphatase activity even with BMP-2 supplementation. (Below, right) Alizarin red quantification of human, mouse, and canine adipose-derived stromal cells after 7 days of growth in either osteogenic differentiation medium or osteogenic differentiation medium with BMP-2 supplementation. Similar to alkaline phosphatase, human and mouse cells demonstrate increased mineralization with BMP-2 supplementation. Canine cells demonstrate only minimally enhanced mineralization (a late marker for osteogenic differentiation) with BMP-2 (*p < 0.05).
RESULTS
Characterization of Surface Morphology and Proliferation of Human, Mouse, and Canine Adipose-Derived Stromal Cells
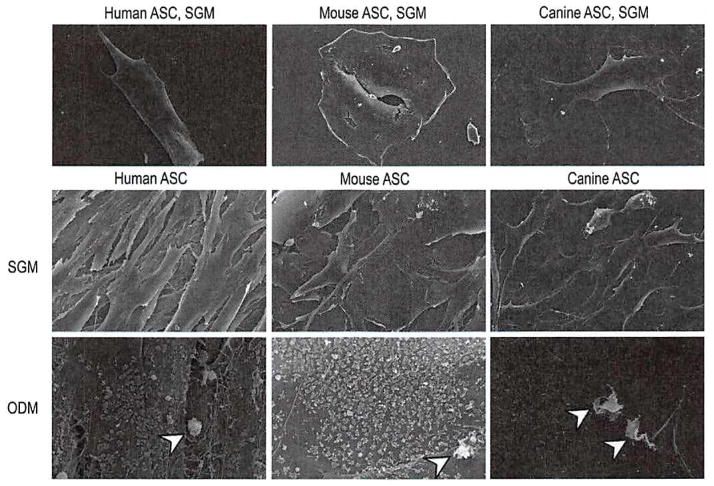
To gain insight into the differences among human, mouse, and canine adipose-derived stromal cells, the appearance of human, mouse, and canine adipose-derived stromal cells was observed by scanning electron microscopy (Fig. 1). [See Figure, Supplemental Digital Content 2, above, which shows scanning electron microscopy comparing human, mouse, and canine adipose-derived stromal cells, http://links.lww.com/PRS/A353. Cells were allowed to grow for 7 days in standard growth medium and imaged by scanning electron microscopy technology at 100× magnification. Mouse adipose-derived stromal cells were observed to have a spread and rounded appearance. The human cells were tightly aligned in a thin and elongated pattern. The canine cells were somewhere in between that of mouse and human adipose-derived stromal cells in morphology, with a more fibroblastic appearance than mouse cells but less spindle-shaped than human cells. The canine cells were also noted to be smaller than either human or mouse adipose-derived stromal cells.] Cells were seeded at equal confluency and treated in culture for 1 week in standard growth medium (Fig. 1, above and center rows, and Supplemental Digital Content 2, above). After 7 days in standard growth medium, mouse adipose-derived stromal cells were observed to adopt a flattened and more rounded phenotype (Fig. 1, above, center, and center, center, and Supplemental Digital Content 2, above, center). In contrast, human adipose-derived stromal cells exhibited a more spindle-shaped appearance, with thin, elongated cells Running in parallel (Fig. 1, left, and Supplemental Digital Content 2, above, left). Canine adipose-derived stromal cells were somewhere in between human and mouse cells in morphology), with a more fibroblastic appearance than mouse cells but less spindle-shaped than human cells (Fig. 1, above, right, and center, right, and Supplemental Digital Content 2, above, right). When grown in standard growth medium (Fig. 1, above and center rows), the cell surface of human, mouse, and canine adipose-derived stromal cells was smooth and without significant contour irregularities.
Fig. 1.
Scanning electron microscopy comparing human, mouse, and canine adipose-derived stromal cells. (Above and center rows) Cells were allowed to grow for 7 days in standard growth medium and imaged by scanning electron microscopy technology at 1000× (above) and 600× (center row) magnification. Mouse cells were observed to have a spread and rounded appearance. Human cells were tightly aligned in a thin and elongated pattern. Canine cells were somewhere in between that of mouse and human cells in morphology, with a more fibroblastic appearance than mouse but less spindle-shaped than human cells. (Below) Cells were allowed to grow in osteogenic differentiation medium (ODM) and were imaged by scanning electron microscopy technology at 600× magnification. Under osteogenic conditions, human, mouse, and canine adipose-derived stromal cells adopted intricate cell surface features, including a studding of the cell surface with numerous, small, rounded projections representing bone nodules (arrowheads).
Next, cell surface features were observed after 7 days in osteogenic differentiation medium (Fig. 1, below). (See Figure, Supplemental Digital Content 2, center, which shows cells allowed to grow in osteogenic differentiation medium and imaged by scanning election microscopy technology at 100× magnification, http://links.lww.com/PRS/A353. At this low magnification, few changes were observed under osteogenic conditions of human, mouse, and canine adipose-derived stromal cells, with the exception of a subtle increase in three-dimensional topography.) At low magnification, few changes were observed in either cell type, with the exception of a subtle Increase in the three-dimensional topography of human, mouse, and canine adipose-derived stromal cells (See Supplemental Digital Content 2, center). At high magnification, however, the addition of osteogenic differentiation medium had dramatic results: human, mouse, and canine adipose-derived stromal cells demonstrated studding of the majority of cell surfaces with spheroid and umbilicated cell surface projections. These were accompanied by the appearance of calcified bone nodules, appearing lighter in contrast (Fig. 1, below). Thus, although human, mouse, and canine adipose-derived stromal cells differ in shape and cell arrangement, they share similar cell surface features, depending on their in vitro environment.
To quantify cell size, cells were normalized and plated at equal number. Forty cells per group were quantified at 600× magnification using Adobe Photoshop. Canine adipose-derived stromal cells were slightly smaller than either human or mouse cells, but this difference was not statistically significant. (See Supplemental Digital Content 2, below, which shows cells normalized and plated at equal number, http://links.lww.com/PRS/A353. Forty cells per group were quantified at 600× magnification using Adobe Photoshop. Canine adipose-derived stromal cells were slightly smaller than either human or mouse cells, but the difference was not statistically significant.)
Proliferation of Human, Mouse, and Canine Adipose-Derived Stromal Cells
Cellular proliferation was then assessed by cell counting and bromodeoxyuridine incorporation assays (Fig. 2). After equal seeding, cell counting was performed over a period of 7 days (Fig. 2, above) (n = 3). Results showed that canine adipose-derived stromal cells proliferated in standard culture conditions to a significantly greater degree than human and mouse cells (p < 0.05 at 3, 5, and 7 days). Bromodeoxyuridine incorporation assays performed at 1, 2, and 4 days of growth (Fig. 2, below) (n = 6) resulted in a significant increase in bromodeoxyuridine incorporation among human adipose-derived stromal cells (p < 0.05) and canine cells (p < 0.05) at day 2, and among canine cells (p < 0.05) at day 4.
Characterization of Osteogenic Differentiation of Human, Mouse, and Canine Adipose-Derived Stromal Cells and the Effects of Retinoic Acid
Alkaline phosphatase and alizarin red staining at 3 and 7 days in osteogenic differentiation medium was performed to assess early and late osteogenic differentiation, respectively. With regard to early osteogenic differentiation, human adipose-derived stromal cells demonstrated significantly more alkaline phosphatase activity, assessed by alkaline phosphatase stain and quantification (Fig. 3, above), than either mouse or canine adipose-derived stromal cells. [See Figure, Supplemental Digital Content 3, above, left, which shows alkaline phosphatase stain of human, mouse, and canine adipose-derived stromal cells after 3 days of growth in osteogenic differentiation medium (ODM) and osteogenic differentiation medium supplemented with 5 and 10 μM of retinoic acid (RA), http://links.lww.com/PRS/A354. Top row shows human adipose-derived stromal cells, which demonstrate the most robust alkaline phosphatase activity with osteogenic differentiation medium alone and high but decreased expression with retinoic acid supplementation. Mouse adipose-derived stromal cells (middle row) demonstrate a significant increase in alkaline phosphatase activity with retinoic acid supplementation. The bottom row shows canine adipose-derived stromal cells, which demonstrate a paucity of alkaline phosphatase staining even with addition of retinoic acid. See also Supplemental Digital Content 3, center, left, which shows alkaline phosphatase stain of human, mouse, and canine adipose-derived stromal cells after 3 days of growth in osteogenic differentiation medium and osteogenic differentiation medium supplemented with 25 and 50 ng/ml of IGF-1. Human and mouse adipose-derived stromal cells demonstrate increased alkaline phosphatase activity with IGF-1 supplementation. Canine adipose-derived stromal cells demonstrate only a paucity of alkaline phosphatase activity even with IGF-1 supplementation. However, canine adipose-derived stromal cells fail to demonstrate significant mineralization with BMP-2 supplementation.] Similarly, bone nodule formation, as assessed by alizarin red stain and quantification (Fig. 3, below), demonstrated significantly more calcification in human than either mouse or canine adipose-derived stromal cells. [See Supplemental Digital Content 3, above, right, which shows alizarin red stain of human, mouse, and canine adipose-derived stromal cells after 7 days of growth with osteogenic differentiation medium versus osteogenic differentiation medium with retinoic acid supplementation. Similar to the alkaline phosphatase stain, human adipose-derived stromal cells (top row) exhibit significantly higher levels of mineralization in osteogenic differentiation medium alone than either mouse adipose-derived stromal cells or canine adipose-derived stromal cells, as assessed by alizarin red staining. However, on retinoic acid supplementation, mineralization was significantly decreased in human adipose-derived stromal cells. Mouse adipose-derived stromal cells demonstrate the most robust response, demonstrating significantly increased calcification following retinoic acid supplementation (center row). Canine adipose-derived stromal cells show minimal but improved calcification with retinoic acid supplementation (bottom row). See also Supplemental Digital Content 3, center, right, which shows alizarin red stain of human, mouse, and canine adipose-derived stromal cells after 7 days of growth in either osteogenic differentiation medium or osteogenic differentiation medium with IGF-1 supplementation. Similar to the alkaline phosphatase stain, human and mouse adipose-derived stromal cells demonstrate increased mineralization with IGF-1 supplementation. However, canine adipose-derived stromal cells also demonstrate increased calcification (a late marker of osteogenic differentiation) with IGF-1 supplementation, which is not appreciated in the earlier stages of osteogenesis.]
Results from studies have demonstrated that retinoic acid enhances osteogenic differentiation of mouse adipose-derived stromal cells.26,38 Furthermore, we have demonstrated that IGF-1 stimulates osteogenesis of human adipose-derived stromal cells.22 In this study, we chose to investigate the effects of these two supplements in the osteogenic differentiation of adipose-derived stromal cells of human, mouse, and canine origin. To this end, all-trans-retinoic acid and recombinant IGF-1 were added to osteogenic differentiation medium; standard osteogenic assays were again performed.
As previously shown, retinoic acid demonstrated a significant pro-osteogenic effect in mouse adipose-derived stromal cells, observed at both early (alkaline phosphatase staining and quantification) and late (alizarin red staining and quatification) time points (Fig. 4, above). (See Supplemental Digital Content 3, above, http://links.tww.com/PRS/A354). Interestingly, retinoic acid demonstrated the reverse in human adipose-derived stromal cells, significantly inhibiting osteogenesis among human adipose-derived stromal cells. Retinoic acid also failed to stimulate early osteogenesis in canine adipose-derived stromal cells, assessed by alkaline phosphatase stain (see Supplemental Digital Content 3, above, left) and quantification (Fig. 4, above, left). Bone nodule formation, however, as defined by alizarin red staining (see Supplemental Digital Content 3, above, right) and quantification (Fig. 4, above, right), resulted in a small but significant increase among canine adipose-derived stromal cells with retinoic acid addition. Collectively, these data suggest that retinoic acid has a pro-osteogenic effect on mouse and canine but not human adipose-derived stromal cells.
Effects of IGF-1 on Osteogenic Differentiation of Human, Mouse, and Canine Adipose-Derived Stromal Cells
Previous studies have shown IGF-1 to enhance osteogenesis in human adipose-derived stromal cells; we next set out to investigate whether it had the same effect on mouse and canine adipose-derived stromal cells. Interestingly, IGF-1 had a substantial and significant effect on early osteogenesis in human adipose-derived stromal cells, demonstrated by alkaline phosphatase stain (see Supplemental Digital Content 3, center left, http://links.lww.com/PRS/A354) and quantification (Fig. 4, center, left). IGF-1 had a small, significant effect on early osteogenesis in mouse adipose-derived stromal cells and no effect on canine cells. With regard to late osteogenesis, recombinant IGF-1 led to a significant increase in bone nodule formation after 7 days in culture by both gross visualization (see Supplemental Digital Content 3, center, right) and photometric quantification (Fig. 4, center, right) across all species, demonstrating the greatest relative augmentation in canine adipose-derived stromal cells.
Effects of BMP-2 on Osteogenic Differentiation of Human, Mouse, and Canine Adipose-Derived Stromal Cells
We have previously demonstrated the osteogenic effect of BMP-2 on human adipose-derived stromal cells in vitro and in vivo.24,39 Furthermore, BMP-2 has been successfully used in a clinical setting for spinal fusion.40 These promising studies make BMP-2 a likely candidate for future use as a cytokine for tissue engineering purposes. We thus treated human, mouse, and canine adipose-derived stromal cells with BMP-2. When assessing osteogenesis in vitro, human and mouse adipose-derived stromal cells showed a significant increase in alkaline phosphatase and alizarin red quantification (Fig. 4, below) and alkaline phosphatase and alizarin red stain. (See Supplemental Digital Content 3, below, left, which shows alkaline phosphatase stain of human, mouse, and canine adipose-derived stromal cells after 3 days of growth in osteogenic differentiation medium and osteogenic differentiation medium supplemented with 200 ng/ml of BMP-2, http://links.lww.com/PRS/A354. Human and mouse cells demonstrate increased alkaline phosphatase activity with BMP-2 supplementation. Canine cells demonstrate only a paucity of alkaline phosphatase activity even with BMP-2 supplementation. See also Supplemental Digital Content 3, below, right, which shows alizarin red stain of human, mouse, and canine adipose-derived stromal cells after 7 days of growth in either osteogenic differentiation medium or osteogenic differentiation medium with BMP-2 supplementation. Similar to the alkaline phosphatase stain, human and mouse cells demonstrate increased mineralization with BMP-2 supplementation. However, canine cells fail to demonstrate significant mineralization with BMP-2 supplementation.) Canine cells, however, showed only a slight increase in late osteogenesis, as shown by alizarin red, which did not reach statistical significance (Fig. 4, below).
Effects of Human, Mouse, and Canine Adipose-Derived Stromal Cells on In Vivo Healing of Calvarial Defects
Having demonstrated increased osteogenic differentiation of human adipose-derived stromal cells in vitro, we next set out to compare the ability of human, mouse, and canine adipose-derived stromal cells to heal a critical-sized mouse calvarial defect (Fig. 5). By 2 weeks, human cells showed patchy bone formation (Fig. 5, above). By 4 weeks, the majority of human adipose-derived stromal cell-engrafted defects and some mouse adipose-derived stromal cell-treated defects showed significant healing (Fig. 5, (above, first and second rows). Finally, canine adipose-derived stromal cell-laden scaffolds demonstrated the least amount of healing across all time points (Fig. 5, above, third row, and below). These findings were in comparison with defects left empty (no scaffold) or treated with a scaffold alone, which showed significantly less healing (Fig. 5, below, and data not shown). Results were quantified and presented as average fraction healing of the original defect size (Fig. 5, below).
After 8-week serial computed tomographic scans, animals were euthanized and histologic analysis was performed. With aniline blue staining and quantification, in which osteoid appears dark blue, human adipose-derived stromal cell–engrafted scaffolds demonstrated significantly more bone formation within the defect site than other groups (Fig. 6). Adjacent slides were stained with pentachrome and alkaline phosphatase, which appear yellow and purple, respectively. [See Figure, Supplemental Digital Content 4, which shows histology of calvarial defects, http://links.lww.com/PRS/A355. Four-millimeter calvarial defects were allowed to heal for 8 weeks before histologic analysis by pentachrome and alkaline phosphatase. Photographs were taken of the midpoint of the defect site. In pentachrome stains, bone appears yellow (above), and in alkaline phosphatase stains, alkaline phosphatase activity appears purple (below).] These stains confirmed the increased osteogenesis observed among human adipose-derived stromal cell–treated defects. In fact, complete bony bridging of the human adipose-derived stromal cell–treated defects was found by 8 weeks after injury. Collectively, these in vivo data replicated our in vitro findings on the differences in osteogenic potential of adipose-derived stromal cells based on species of derivation. Human adipose-derived stromal cells lead to significant osseous healing when placed in a critical-sized calvarial defect with an appropriate osteoinductive scaffold. In contrast, neither mouse nor canine adipose-derived stromal cells led to comparable healing under identical experimental conditions.
DISCUSSION
As adipose-derived stromal cells become more widely studied, scientists will need to appropriately defend their choice of species from which to derive these cells, and provide a thorough explanation as to how their choice may affect or confound their results. The ultimate translational goal of skeletal tissue engineering is, within the course of a single operation, to harvest human adipose-derived stromal cells from a patient and place them onto an osteoinductive scaffold that is then placed in the defect site. Although human adipose-derived stromal cells have been used in large clinical trials for inflammatory disorders, only case reports exist for their use for skeletal tissue repair. To receive approval of federal regulatory agencies, surgeons must perform more animal studies of adipose-derived stromal cells. Mouse and canine represent suitable species for small- and large-animal testing, respectively. However, scientists must also understand the basic differences in the biology of these adipose-derived stromal cells from different species. One could, perhaps, foresee stimulating mouse adipose-derived stromal cells with retinoic acid, canine cells with IGF-1, and human cells with BMP-2 to augment osteogenesis.
Previous studies have demonstrated differences between individuals with regard to the adipose tissue harvested.41,42 Fat distribution between men and women and within each sex demonstrate significant differences. Adipose tissue from subcutaneous locations, or depots, have different blood supplies, cytokine signaling, and gene expression profiles, leading to differences in osteogenic capacity. Stimulated by the rising incidence of obesity and the need for a more thorough understanding of adipose biology, a growing number of studies have investigated the differences between subcutaneous and visceral fat depots, with visceral fat depots having a greater osteogenic potential.43
Although the anatomical distribution of white adipose tissue differs between species, we attempted to control for these differences by using only subcutaneous white adipose tissue. For our canine and human samples, we used fat from only the thigh, flank, or dorsal surface. For our mouse adipose tissue, however, we used inguinal fat, as dorsal mouse fat has been shown to be mostly brown adipose tissue, which is biologically different from white fat.31 Furthermore, none of the species was obese, as young CD-1 mice, human patients younger than 50 years, and dogs between 1 and 2 years old were used. With regard to sex, all human lipoaspirate patients were women during the study period. For canine adipose-derived stromal cell derivation, we had only the male sex available. Previous studies have shown that male human adipose-derived stromal cells are more osteogenic than female human adipose-derived stromal cells and thus we would expect the male canine adipose-derived stromal cells to have a greater osteogenic potential than female canine adipose-derived stromal cells. Thus, the fact that our male canine adipose-derived stromal cells were much less osteogenic than the female human and mouse cells further underscores their impaired osteogenesis.44
The fact that human adipose-derived stromal cells appear to be the most osteogenic is very encouraging. Furthermore, they do not require pretreatment to heal a critical-sized mouse defect within 4 weeks. In this study, we demonstrate that differences exist in the osteogenic potential of adipose-derived stromal cells across species. We have previously demonstrated that untreated human and mouse adipose-derived stromal cells can heal a critical-sized calvarial defect when placed on an osteoinductive scaffold.19,24 In this study, we demonstrate close to 50 percent healing of a 4-mm defect at 8 weeks. Previous studies from our laboratory demonstrated similar healing using adult mouse adipose-derived stromal cells, although more robust healing was noted with juvenile adipose-derived stromal cells.19 With regard to canine adipose-derived stromal cells, however, untreated cells fail to undergo osteogenic differentiation in vivo, within the time frame observed. In previous studies, BMP-2 gene expression was up-regulated to heal a critical-sized defect with canine adipose-derived stromal cells.30
Standard supplements for osteogenic differentiation medium include beta-glycerol phosphate and ascorbic acid.32 Other potential additives include vitamin D, retinoic acid, and dexamethasone.32 Retinoic acid clearly has pleiotropic effects on bone-forming cells, in some instances inhibiting45 and in some instances promoting osteogenic differentiation.46 These effects are most likely both cell type and dosage dependent. Previously, we have observed that pharmacologic dosages of retinoic acid enhance the osteogenic differentiation of calvaria-derived osteoblasts47 and calvaria-derived mesenchymal cells.48 Interestingly, this may be through a hedge-hog-dependent mechanism.48 Moreover, in certain instances, retinoic acid has been shown to increase bone resorption because of osteoclast activation.38 Overall, in the current study, we chose a specific pharmacologic dosage of retinoic acid that we anticipated would increase the osteogenic differentiation of adipose-derived stromal cells.
IGF-1 is a known mediator of skeletal growth and bone formation.49–52 Specifically, it has been shown to promote the differentiation of bone cells in an autocrine and paracrine fashion.53,54 Prior investigators have targeted IGF-1 to stimulate osteogenesis in vitro in the treatment of bone marrow–derived osteoblastic cells,55 and in vivo in an aged rat model.52 We have demonstrated that IGF-1 has an osteogenic effect on human, mouse, and canine adipose-derived stromal cells similar to its effect on bone marrow–derived osteoblasts. When differentiating adipose-derived stromal cells from human, mouse, or canine species down an osteogenic lineage, IGF-1 is a potential protein that could be added in vitro to cell culture or in vivo onto a cell scaffold for bone tissue engineering purposes.
Interestingly, canine adipose-derived stromal cells were the least osteogenic in vitro and in vivo. In vitro, they also appeared to be the most proliferative. Although the mechanism behind this enhanced proliferation and weak osteogenic potential is unknown, future studies will look at FGF-2 signaling across species. FGF-2 is a known mitogen across several cell types, including osteoblasts.56 Furthermore, our laboratory has demonstrated that in mouse adipose-derived stromal cells, FGF-2 functions to inhibit osteogenic differentiation and promote continued proliferation, maintaining the mouse cells in a more undifferentiated state.27 Future studies aim to delineate whether there is in fact enhanced FGF-2 signaling in canine adipose-derived stromal cells, compared with human and mouse adipose-derived stromal cells.
CONCLUSIONS
Adipose-derived stromal cells from human, mouse, and canine sources have the capacity to undergo osteogenesis. Canine adipose-derived stromal cells are the most proliferative, whereas human adipose-derived stromal cells are the most osteogenic. These results are promising as we move from the bench to the bedside and attempt to optimize tissue-engineered bone using adipose-derived stromal cells.
Supplementary Material
Acknowledgments
This study was supported by National Institutes of Health, National Institute of Dental and Craniofacial Research grants 1 R21 DEO19274-02 and 1 RC2 DE020771-01, the National Endowment of Plastic Surgery, the Oak Foundation and the Hagey Laboratory for Pediatric Regenerative Medicine (to M.T.L.); National Institutes of Health, National Institute of Arthritis and Musculoskeletal and Skin Diseases grant 1F32AR057302-01 (to B.L.); and grants R01EB009689 and RC1HL099117 (to J. C. W.).
Footnotes
The first three authors share responsibility for the work presented in this article.
Disclosure: The authors have no financial interest to declare in relation to the content of this article.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text; simply type the URL address into any Web browser to access this content. Click-able links to the material are provided in the HTML text of this article on the Journal’s Web site (WWW.PRSJournal.com).
References
- 1.Deans RJ, Moseley AB. Mesenchymal stem cells: Biology and potential clinical uses. Exp Hematol. 2000;28:875–884. doi: 10.1016/s0301-472x(00)00482-3. [DOI] [PubMed] [Google Scholar]
- 2.Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 3.Gronthos S, Graves SE, Ohta S, Simmons PJ. The STRO-1 + fraction of adult human bone marrow contains the osteogenic precursors. Blood. 1994;84:4164–4173. [PubMed] [Google Scholar]
- 4.Ashton BA, Allen TD, Howlett CR, Eaglesom CC, Hattori A, Owen M. Formation of bone and cartilage by marrow stromal cells in diffusion chambers in vivo. Clin Orthop Relat Res. 1980;151:294–307. [PubMed] [Google Scholar]
- 5.Till JE, McCulloch EA. A direct measurement of the radiation sensitivity of normal mouse bone marrow cells. Radiat Res. 1961;14:213–222. [PubMed] [Google Scholar]
- 6.Yoon E, Dhar S, Chun DE, Gharibjanian NA, Evans GR. In vivo osteogenic potential of human adipose-derived stem cells/poly lactide-co-glycolic acid constructs for bone regeneration in a rat critical-sized calvarial defect model. Tissue Eng. 2007;13:619–627. doi: 10.1089/ten.2006.0102. [DOI] [PubMed] [Google Scholar]
- 7.Yoshimura K, Shigeura T, Matsumoto D, et al. Characterization of freshly isolated and cultured cells derived from the fatty and fluid portions of liposuction aspirates. J Cell Physiol. 2006;208:64–76. doi: 10.1002/jcp.20636. [DOI] [PubMed] [Google Scholar]
- 8.Izadpanah R, Trygg C, Patel B, et al. Biologic properties of mesenchymal stem cells derived from bone marrow and adipose tissue. J Cell Biochem. 2006;99:1285–1297. doi: 10.1002/jcb.20904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dicker A, Le Blanc K, Astrōm G, et al. Functional studies of mesenchymal stem cells derived from adult human adipose tissue. Exp Cell Res. 2005;308:283–290. doi: 10.1016/j.yexcr.2005.04.029. [DOI] [PubMed] [Google Scholar]
- 10.Boquest AC, Shahdadfar A, Frønsdal K, et al. Isolation and transcription profiling of purified uncultured human stromal stem cells: Alteration of gene expression after in vitro cell culture. Mol Biol Cell. 2005;16:1131–1141. doi: 10.1091/mbc.E04-10-0949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Ugarte DA, Morizono K, Elbarbary A, et al. Comparison of multi-lineage cells from human adipose tissue and bone marrow. Cells Tissues Organs. 2003;174:101–109. doi: 10.1159/000071150. [DOI] [PubMed] [Google Scholar]
- 12.De Ugarte DA, Alfonso Z, Zuk PA, et al. Differential expression of stem cell mobilization-associated molecules on multi-lineage cells from adipose tissue and bone marrow. Immunol Lett. 2003;89:267–270. doi: 10.1016/s0165-2478(03)00108-1. [DOI] [PubMed] [Google Scholar]
- 13.Zuk PA, Zhu M, Ashjian P, et al. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13:4279–4295. doi: 10.1091/mbc.E02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zuk PA, Zhu M, Mizuno H, et al. Multilineage cells from human adipose tissue: Implications for cell-based therapies. Tissue Eng. 2001;7:211–228. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
- 15.Kon E, Muraglia A, Corsi A, et al. Autologous bone marrow stromal cells loaded onto porous hydroxyapatite ceramic accelerate bone repair in critical-size defects of sheep long bones. J Biomed Mater Res. 2000;49:328–337. doi: 10.1002/(sici)1097-4636(20000305)49:3<328::aid-jbm5>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 16.Bruder SP, Kraus KH, Goldberg VM, Kadiyala S. The effect of implants loaded with autologous mesenchymal stem cells on the healing of canine segmental bone defects. J Bone Joint Surg Am. 1998;80:985–996. doi: 10.2106/00004623-199807000-00007. [DOI] [PubMed] [Google Scholar]
- 17.Arinzeh TL, Peter SJ, Archambault MP, et al. Allogeneic mesenchymal stem cells regenerate bone in a critical-sized canine segmental defect. J Bone Joint Surg Am. 2003;85:1927–1935. doi: 10.2106/00004623-200310000-00010. [DOI] [PubMed] [Google Scholar]
- 18.Dudas JR, Marra KG, Cooper GM, et al. The osteogenic potential of adipose-derived stem cells for the repair of rabbit calvarial defects. Ann Plast Surg. 2006;56:543–548. doi: 10.1097/01.sap.0000210629.17727.bd. [DOI] [PubMed] [Google Scholar]
- 19.Cowan CM, Shi YY, Aalami OO, et al. Adipose-derived adult stromal cells heal critical-size mouse calvarial defects. Nat Biotechnol. 2004;22:560–567. doi: 10.1038/nbt958. [DOI] [PubMed] [Google Scholar]
- 20.Levi B, James AW, Glotzbach JP, Wan DC, Commons GW, Longaker MT. Depot specific variation in the osteogenic and adipogenic potential of human adipose-derived stromal cells. Plast Reconstr Surg. 2010;126:822–834. doi: 10.1097/PRS.0b013e3181e5f892. [DOI] [PubMed] [Google Scholar]
- 21.Levi B, James AW, Xu Y, Commons GW, Longaker MT. Divergent modulation of adipose-derived stromal cell differentiation by TGF-beta1 based on species of derivation. Plast Reconstr Surg. 2010;126:412–425. doi: 10.1097/PRS.0b013e3181df64dc. [DOI] [PubMed] [Google Scholar]
- 22.Levi B, James AW, Wan D, Glotzbach JP, Commons GW, Longaker MT. Regulation of human adipose-derived stromal cell osteogenic differentiation by insulin-like growth factor-1 and platelet-derived growth factor-alpha. Plast Reconstr Surg. 2010;126:41–52. doi: 10.1097/PRS.0b013e3181da8858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Levi B, James AW, Nelson ER, et al. Human adipose-derived stromal cells stimulate autogenous skeletal repair via paracrine hedgehog signaling with calvarial osteoblasts. Stem Cells Dev. 2011;20:243–257. doi: 10.1089/scd.2010.0250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Levi B, James AW, Nelson ER, et al. Human adipose derived stromal cells heal critical size mouse calvarial defects. PLoS One. 2010;5:e11177. doi: 10.1371/journal.pone.0011177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Quarto N, Longaker MT. Differential expression of specific FGF ligands and receptor isoforms during osteogenic differentiation of mouse Adipose-derived Stem Cells (mASCs) recapitulates the in vivo osteogenic pattern. Gene. 2008;424:130–140. doi: 10.1016/j.gene.2008.07.029. [DOI] [PubMed] [Google Scholar]
- 26.Wan DC, Shi YY, Nacamuli RP, et al. Osteogenic differentiation of mouse adipose-derived adult stromal cells requires retinoic acid and bone morphogenetic protein receptor type IB signaling. Proc Natl Acad Sci USA. 2006;103:12335–12340. doi: 10.1073/pnas.0604849103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Quarto N, Longaker MT. FGF-2 inhibits osteogenesis in mouse adipose tissue-derived stromal cells and sustains their proliferative and osteogenic potential state. Tissue Eng. 2006;12:1405–1418. doi: 10.1089/ten.2006.12.1405. [DOI] [PubMed] [Google Scholar]
- 28.Vieira NM, Brandalise V, Zucconi E, Secco M, Strauss BE, Zatz M. Isolation, characterization, and differentiation potential of canine adipose-derived stem cells. Cell Transplant. 2010;19:279–289. doi: 10.3727/096368909X481764. [DOI] [PubMed] [Google Scholar]
- 29.Black LL, Gaynor J, Gahring D, et al. Effect of adipose-derived mesenchymal stem and regenerative cells on lameness in dogs with chronic osteoarthritis of the coxofemoral joints: A randomized, double-blinded, multicenter, controlled trial. Vet Ther. 2007;8:272–284. [PubMed] [Google Scholar]
- 30.Li H, Dai K, Tang T, Zhang X, Yan M, Lou J. Bone regeneration by implantation of adipose-derived stromal cells expressing BMP-2. Biochem Biophys Res Commun. 2007;356:836–842. doi: 10.1016/j.bbrc.2007.02.165. [DOI] [PubMed] [Google Scholar]
- 31.Murano I, Barbatelli G, Giordano A, Cinti S. Noradrenergic parenchymal nerve fiber branching after cold acclimatisation correlates with brown adipocyte density in mouse adipose organ. J Anat. 2009;214:171–178. doi: 10.1111/j.1469-7580.2008.01001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Malladi P, Xu Y, Yang GP, Longaker MT. Functions of vitamin D, retinoic acid, and dexamethasone in mouse adipose-derived mesenchymal cells. Tissue Eng. 2006;12:2031–2040. doi: 10.1089/ten.2006.12.2031. [DOI] [PubMed] [Google Scholar]
- 33.James AW, Xu Y, Wang R, Longaker MT. Proliferation, osteogenic differentiation, and fgf-2 modulation of postero-frontal/sagittal suture-derived mesenchymal cells in vitro. Plast Reconstr Surg. 2008;122:53–63. doi: 10.1097/PRS.0b013e31817747b5. [DOI] [PubMed] [Google Scholar]
- 34.Xu Y, James AW, Longaker MT. Transforming growth factor-beta1 stimulates chondrogenic differentiation of postero-frontal suture-derived mesenchymal cells in vitro. Plast Reconstr Surg. 2008;122:1649–1659. doi: 10.1097/PRS.0b013e31818cbf44. [DOI] [PubMed] [Google Scholar]
- 35.Lee J, Gupta D, Panetta NJ, et al. Elucidating mechanisms of osteogenesis in human adipose-derived stromal cells via microarray analysis. J Craniofac Surg. 2010;21:1136–1141. doi: 10.1097/SCS.0b013e3181e488d6. [DOI] [PubMed] [Google Scholar]
- 36.James AW, Theologis AA, Brugmann SA, et al. Estrogen/estrogen receptor alpha signaling in mouse posterofrontal cranial suture fusion. PLoS One. 2009;4:e7120. doi: 10.1371/journal.pone.0007120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xu Y, Hammerick KE, James AW, et al. Inhibition of histone deacetylase activity in reduced oxygen environment enhances the osteogenesis of mouse adipose-derived stromal cells. Tissue Eng Part A. 2009;15:3697–3707. doi: 10.1089/ten.tea.2009.0213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wan DC, Siedhoff MT, Kwan MD, Nacamuli RP, Wu BM, Longaker MT. Refining retinoic acid stimulation for osteogenic differentiation of murine adipose-derived adult stromal cells. Tissue Eng. 2007;13:1623–1631. doi: 10.1089/ten.2006.0283. [DOI] [PubMed] [Google Scholar]
- 39.Panetta NJ, Gupta DM, Lee JK, Wan DC, Commons GW, Longaker MT. Human adipose-derived stromal cells respond to and elaborate bone morphogenetic protein-2 during in vitro osteogenic differentiation. Plast Reconstr Surg. 2010;125:483–493. doi: 10.1097/PRS.0b013e3181c82d75. [DOI] [PubMed] [Google Scholar]
- 40.Glassman SD, Carreon LY, Djurasovic M, et al. RhBMP-2 versus iliac crest bone graft for lumbar spine fusion: A randomized, controlled trial in patients over sixty years of age. Spine (Phila Pa 1976) 2008;33:2843–2849. doi: 10.1097/BRS.0b013e318190705d. [DOI] [PubMed] [Google Scholar]
- 41.van Beek EA, Bakker AH, Kruyt PM, Hofker MH, Saris WH, Keijer J. Intra- and interindividual variation in gene expression in human adipose tissue. Pflugers Arch. 2007;453:851–861. doi: 10.1007/s00424-006-0164-4. [DOI] [PubMed] [Google Scholar]
- 42.Schipper BM, Marra KG, Zhang W, Donnenberg AD, Rubin JP. Regional anatomic and age effects on cell function of human adipose-derived stem cells. Ann Plast Surg. 2008;60:538–544. doi: 10.1097/SAP.0b013e3181723bbe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Peptan LA, Hong L, Mao JJ. Comparison of osteogenic potentials of visceral and subcutaneous adipose-derived cells of rabbits. Plast Reconstr Surg. 2006;117:1462–1470. doi: 10.1097/01.prs.0000206319.80719.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Aksu AE, Rubin JP, Dudas JR, Marra KG. Role of gender and anatomical region on induction of osteogenic differentiation of human adipose-derived stem cells. Ann Plast Surg. 2008;60:306–322. doi: 10.1097/SAP.0b013e3180621ff0. [DOI] [PubMed] [Google Scholar]
- 45.Chen M, Huang HZ, Wang M, Wang AX. Retinoic acid inhibits osteogenic differentiation of mouse embryonic palate mesenchymal cells. Birth Defects Res A Clin Mol Teratol. 2010;88:965–970. doi: 10.1002/bdra.20723. [DOI] [PubMed] [Google Scholar]
- 46.Zhang W, Deng ZL, Chen L, et al. Retinoic acids potentiate BMP9-induced osteogenic differentiation of mesenchymal progenitor cells. PLoS One. 2010;5:e11917. doi: 10.1371/journal.pone.0011917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Song HM, Nacamuli RP, Xia W, et al. High-dose retinoic acid modulates rat calvarial osteoblast biology. J Cell Physiol. 2005;202:255–262. doi: 10.1002/jcp.20115. [DOI] [PubMed] [Google Scholar]
- 48.James AW, Levi B, Xu Y, Carre AL, Longaker MT. Retinoic acid enhances osteogenesis in cranial suture-derived mesenchymal cells: Potential mechanisms of retinoid-induced craniosynostosis. Plast Reconstr Surg. 2010;125:1352–1361. doi: 10.1097/PRS.0b013e3181d62980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Canalis E. Effect of insulinlike growth factor I on DNA and protein synthesis in cultured rat calvaria. J Clin Invest. 1980;66:709–719. doi: 10.1172/JCI109908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schlechter NL, Russell SM, Spencer EM, Nicoll CS. Evidence suggesting that the direct growth-promoting effect of growth hormone on cartilage in vivo is mediated by local production of somatomedin. Proc Natl Acad Sci USA. 1986;83:7932–7934. doi: 10.1073/pnas.83.20.7932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schoenle E, Zapf J, Humbel RE, Froesch ER. Insulin-like growth factor I stimulates growth in hypophysectomized rats. Nature. 1982;296:252–253. doi: 10.1038/296252a0. [DOI] [PubMed] [Google Scholar]
- 52.Tanaka H, Quarto R, Williams S, Barnes J, Liang CT. In vivo and in vitro effects of insulin-like growth factor-I (IGF-I) on femoral mRNA expression in old rats. Bone. 1994;15:647–653. doi: 10.1016/8756-3282(94)90313-1. [DOI] [PubMed] [Google Scholar]
- 53.Canalis E, McCarthy TL, Centrella M. Growth factors and cytokines in bone cell metabolism. Annu Rev Med. 1991;42:17–24. doi: 10.1146/annurev.me.42.020191.000313. [DOI] [PubMed] [Google Scholar]
- 54.Rodan GA. Introduction to bone biology. Bone. 1992;13(Suppl 1):S3–S6. doi: 10.1016/s8756-3282(09)80003-3. [DOI] [PubMed] [Google Scholar]
- 55.Machwate M, Zerath E, Holy X, Pastoureau P, Marie PJ. Insulin-like growth factor-I increases trabecular bone formation and osteoblastic cell proliferation in unloaded rats. Endocrinology. 1994;134:1031–1038. doi: 10.1210/endo.134.3.8119139. [DOI] [PubMed] [Google Scholar]
- 56.Li S, Quarto N, Longaker MT. Dura mater-derived FGF-2 mediates mitogenic signaling in calvarial osteoblasts. Am J Physiol Cell Physiol. 2007;293:C1834–C1842. doi: 10.1152/ajpcell.00135.2007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.