Abstract
Objective
Transcranial magnetic stimulation (TMS) is a well-established clinical protocol with numerous potential therapeutic and diagnostic applications. Yet, much work remains in the elucidation of TMS mechanisms, optimization of protocols, and in development of novel therapeutic applications. As with many technologies, the key to these issues lies in the proper experimentation and translation of TMS methods to animal models, among which rat models have proven popular. A significant increase in the number of rat TMS publications has necessitated analysis of their relevance to human work. We therefore review the essential principles necessary for the approximation of human TMS protocols in rats as well as specific methods that addressed these issues in published studies.
Materials and Methods
We performed an English language literature search combined with our own experience and data. We address issues that we see as important in the translation of human TMS methods to rat models and provide a summary of key accomplishments in these areas.
Results
An extensive literature review illustrated the growth of rodent TMS studies in recent years. Current advances in the translation of single, paired-pulse, and repetitive stimulation paradigms to rodent models are presented. The importance of TMS in the generation of data for preclinical trials is also highlighted.
Conclusions
Rat TMS has several limitations when considering parallels between animal and human stimulation. However, it has proven to be a useful tool in the field of translational brain stimulation and will likely continue to aid in the design and implementation of stimulation protocols for therapeutic and diagnostic applications.
Keywords: repetitive transcranial magnetic stimulation, rTMS, rat, motor evoked potential, translation of human magnetic stimulation protocols
I. Introduction
Transcranial magnetic stimulation (TMS) is a well-established method for noninvasive focal cortical stimulation in humans that provides a means to investigate or modulate cortico-cortical and corticospinal circuits 1-5. In its most common embodiment, TMS is coupled with surface electromyography (EMG) and applied to stimulate the motor cortex of humans to elicit motor evoked potentials (MEPs) in targeted contralateral muscle groups. Quantitative attributes of these signals, such as stimulus threshold to activation, MEP amplitude and latency are then used as measures of cortical and corticospinal excitability. Such measures have been shown to change in correlation with a range of physiologic and disease states such as epilepsy, stroke and head trauma, which are characterized by pathologic alterations in cortical excitability 1, 3, 6, 7. The potential for future application in clinical diagnostics is quite clear.
TMS is also applied to cortical regions in repetitive trains; namely as repetitive transcranial magnetic stimulation (rTMS). rTMS enables use-dependent modulation of cortical excitability likely via mechanisms analogous to long-term potentiation (LTP) and long-term depression (LTD) 3, 8-13. The capacity of rTMS to induce changes in cortical excitability which outlast stimulation protocols has led to its experimental and clinical application in neuropsychiatric disease where the pathophysiology involves over- or under-activation of a cortical region 14, 15, 16.
Relevant to this discussion, TMS has recently been translated to use in rodent models 17-24 where more mechanistic insights into TMS-derived measures of cortical excitability and rTMS-mediated changes in cortical function can be obtained. The benefits of this translation are only now being realized as rodent, particularly rat, TMS methods are undergoing optimization, their underlying physiologic effect is being teased apart at the synaptic and molecular levels, and are ultimately applied in controlled disease models. Here we discuss the growing body of translational TMS research and address the hurdles which still need to be overcome in the translation of TMS techniques from human to rat.
II. Adapting TMS to Rats
II.A. TMS Coils and Stimulation Focality
Focalization of stimulation in TMS is of immense importance in experiments aimed to mimic human stimulation protocols. While steps towards focal rat TMS are not without their caveats, its introduction and evolution are vital to the field of non-invasive brain stimulation, especially in its capacity to measure cortical-specific excitability. The first and most formidable impediment is the discrepancy in the ratio of TMS coil size to head size. In human studies where focal stimulation is desired, TMS is often applied unilaterally, usually with a figure-of-eight coil to activate or alter the function of discrete brain regions. Most human TMS coils are capable of safe cortical stimulation at depths up to approximately 1.5 centimeters from the coil and provoke direct stimulation of volumes as focal as half a cubic centimeter 25. Yet this volume of stimulated brain would not produce a focal effect in a rat. Thus special adaptation to mimic focal human TMS in the rat is necessary.
A variety of different coil shapes have been used in rat TMS studies. Among them are round, Helmholtz, tear-drop, and figure-of-eight (supplemental Tables S1, S2, and S3). More focal stimulation results from the commonly used figure-of-eight coil 26. Interestingly, both documented 27 and empiric evidence from our laboratory demonstrates that the average adult rat brain size is on the order of 1.5-1.6 cubic centimeters, depending on age. Although TMS-induced electrical fields in rats are expected to be smaller than in humans due to the relatively poor electromagnetic coupling of the small rat head with the large magnetic field 28, nevertheless one can expect the relatively diffuse stimulation of the rat brain to impair translation of human focal TMS techniques to rodent models, at least when neuroanatomical considerations affect the outcome related of the research question.
To improve the focality of TMS, smaller TMS coils may, in principle, be used in rats. However, their development has been limited by a requirement for smaller electrical current loads to limit intracoil repulsive Lorentz forces 29, while also requiring a greater electrical intracoil current to produce an effective field due to greater cancellation induced in smaller coils 25, 30. In addition to smaller coil size, although untested in-vivo, is the development of an attachment or shield to use concomitantly with a human coil to more accurately direct the magnetic field 31.
Recent studies from our lab demonstrate that orienting the TMS coil eccentrically to targeted regions in the rat brain allows for at least uni-hemispheric stimulation 22. In this work, we obtained input-output (I/O) curves from 13 adult male rats anesthetized with intraperitoneal pentobarbital. The pentobarbital dose was optimized to allow for the comfortable placement of the rats in a stereotactic frame while preserving their withdrawal response to foot pinch. We utilized a relatively small figure-of-eight coil (20 mm outer lobe diameter) positioned lateral to the midline of the dorsal scalp. We then gradually increased the machine output (MO) while recording MEPs from the brachioradialis muscle bilaterally with electromyography (EMG) needle electrodes. The I/O curves demonstrated that motor threshold (MT), defined as the stimulus intensity required to achieve MEPs with a peak-to-peak voltage ≥ 15 μV in at least 5 of 10 consecutive trials, was reached in all rats for the target muscle contralateral to stimulation. As the stimulus intensity was increased, only 15% of the animals reached the MT of their ipsilateral limb. Figure 1 further illustrates the ipsilateral-contralateral MEP discrepancy as a function of TMS intensity.
Fig.1. I/O curves for rat TMS MEPs.
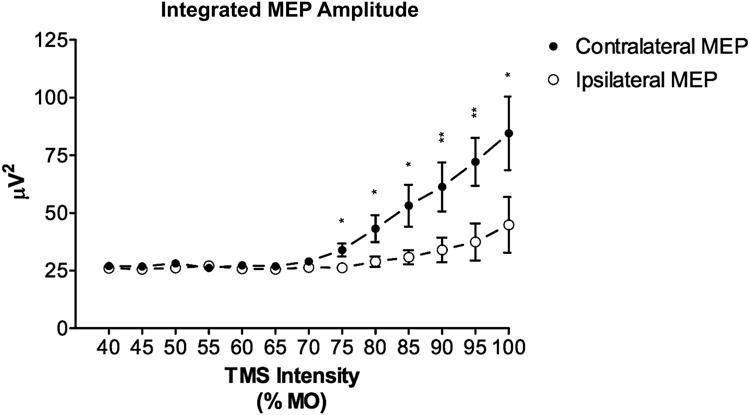
Data averaged across 13 male adult rats demonstrating MEP metric discrepancy between the contralateral and ipsilateral brachioradialis muscle as a function of TMS intensity. Demonstrates the lower MT of the contralateral target muscle group as well as the ability to activate the contralateral limb without ipsilateral activation. Error bars represent the standard error of the mean; * p < 0.05, ** p < 0.01. Adapted from Rotenberg et al 2010 22.
These data prove the feasibility of using conventional TMS equipment, with a relatively small coil, in rats to obtain focal brain stimulation at the resolution of a single hemisphere.
II.B. Anesthesia In Rat TMS Experiments
Another difficulty in translating TMS techniques is the requirement for anesthesia in order to provide humane, reproducible focal stimulation in rats. In contrast to human studies, experimenters are unable to control volitional motor activity in rats. Also, rather than the frameless stereotactic system used in humans, accuracy in rats is greatly increased by the use of a fixed stereotactic frame. Therefore, to suppress spontaneous motor activity and to eliminate pain and distress associated with placement in the stereotactic frame, anesthesia becomes a necessity. Early studies suggested a strict dependence of the success of TMS to elicit motor evoked potentials (MEPs) on the choice and dose of anesthesia 19. To date, a majority of rat studies use either urethane or propofol anesthesia, as both have been shown to preserve spinal reflexes 32, 33 (supplemental Tables S1, S2, and S3). More recent studies in our lab demonstrate the capacity to achieve TMS evoked MEPs under intraperitoneal pentobarbital, ketamine-atropine-xylazine (KAX) 22, 24, or inhaled isoflurane (Vahabzadeh-Hagh, AM. Lo, E. Pascual-Leone, A. and Rotenberg, A. unpublished) anesthesia with equal success. The broad range of anesthetics successfully used in our work, as well as current and previous studies, allows for experimental optimization and avoidance of anesthetic confounding of data relevant to testing contributions of specific neurotransmitter receptor families.
While anesthesia provides a vital tool to allow for focal stimulation, it is important to keep in mind that the effect of TMS is reliant upon the underlying activity of the brain at the time of stimulation; e.g the brain state 34. In human studies the effect of both sleep and anesthesia on state-dependent neural activity and the resulting changes in TMS wave propagation have been well documented 34, 35. In rats, a recent study from our group 36 compared the lasting neurochemical effects of repetitive TMS (rTMS) in awake and anesthetized animals. We found that the effects of stimulation on brain-derived neurotrophic factor (BDNF) and 2-amino-3-(5-methyl-3-oxo-1,2-oxazol-4-yl) propanoic acid (AMPA) receptor GluR1 subunit levels were opposite for the awake versus anesthetized states. These data emphasize the importance of anesthesia selection in translational rTMS experimental design.
In some experiments, such as those designed to simulate clinical rTMS applications, mechanical restraint, rather than anesthesia, has been used 37. Also, to facilitate translational studies in awake rats, our group has also been looking at unanesthetized TMS with a new alternative method to electromyography, mechanomyography (MMG) 38. However, what is lost in the conscious awake state is the ability to target a single hemisphere or more focal anatomical structure. In many cases, this loss of focality is not significant, as whole volume stimulation may indiscriminately activate all circuits thus allowing for the desired metric to possibly be isolated post-acquisition. However, where neuromodulatory stimulation paradigms are utilized, the need to target specific sites becomes more imperative to exclude the contribution of multiple areas to the observed effect. The present review highlights this continued hurdle to the application of focal TMS in rats. Thirty-seven of 100 studies analyzed in this review employed anesthesia. This comprises only 30% of the non-focal (holocranial) TMS studies, but is seen in 92% of focal TMS reports.
Also relevant to the issue of TMS focality in translational studies, the development of a very small, potentially implantable coil might forgo the use of anesthesia altogether. However, development of such a coil is not being actively pursued and presents a multitude of engineering problems 25, 30.
III. TMS Protocols In Rats
III.A. Paired-Pulse Tms In Rats
Among the advances in translation of human diagnostic TMS techniques to rodents is the adaptation of paired-pulse stimulation (ppTMS) to rats. ppTMS applied with variable interstimulus intervals (ISIs) is a tool capable of noninvasive interrogation of both excitatory and inhibitory cortico-cortical circuits 2, 39-41. In ppTMS protocols, a conditioning stimulus (CS) precedes each successive test stimulus (TS). The provocation of inhibitory or excitatory cortical circuits has been shown to largely depend on CS intensity and ISI. Short (1 – 6 ms) ISIs with subthreshold CS and suprathreshold TS leads to suppression of the MEP produced by the TS; i.e. short-interval intracortical inhibition (SICI). Longer (8 – 30 ms) ISIs with a subthreshold CS and suprathreshold TS results in facilitation of the MEP; i.e. intracortical facilitation (ICF) 2, 40-45. Still longer (50 – 300+ ms) ISIs with suprathreshold CS and TS results in MEP inhibition; i.e. long-interval intracortical inhibition (LICI) 39. Last, discrete ISIs (1.1 – 1.5 ms, 2.3 – 3.0 ms, and 4.1 – 5.0 ms) with a supra- or at motor threshold CS followed by a subthreshold TS, provokes MEP facilitation; short-interval intracortical facilitation (SICF) also known as facilitatory I-wave interaction 44-46. Thus, with a pair of stimuli applied to the same cortical location there are four main circuits one can activate in humans; namely SICI, ICF, LICI, and SICF.
In a recent study, we sought to translate human LICI protocol to anesthetized rats. LICI is a commonly used and robust ppTMS technique with previously demonstrated potential clinical applications. Notably, LICI is suppressed in diseases of excess cortical excitability such as epilepsy 42, 47-50. In this study we sought to demonstrate the ability to obtain LICI-like measures in rats, the dependence of these measures on two commonly used injectable anesthetics, and the capacity of these measures to detect acute changes in cortical inhibition as provoked by a chemoconvulsant, pentylenetetrazole (PTZ). Here we used a figure-of-eight double 40 mm coil (Magstim Company, Wales, UK). We applied stimulation using the same eccentric positioning described in Rotenberg et al 2010 22. One subset of rats was anesthetized with intraperitoneal (i.p.) pentobarbital while the other with i.p. KAX. We then provided both single and paired-pulse stimulation (ISIs of 50, 100, 200, 300, 400, and 500 ms) at 120% MT presented in a randomized order with an inter-trial interval (ITI) of 8 seconds. In a separate subset of rats, anesthetized with pentobarbital, we then assessed the capacity of these LICI-like measures to detect acute changes in cortical excitability as provoked by PTZ. Here we obtained baseline long-interval ppTMS (LI-ppTMS) measures, injected PTZ (70 mg/kg i.p.) or the equivalent volume of 0.9% saline (control) and then repeated LI-ppTMS measures at 5, 30, and 45 minutes post injection while recording the electroencephalogram (EEG) to confirm electrographic seizures.
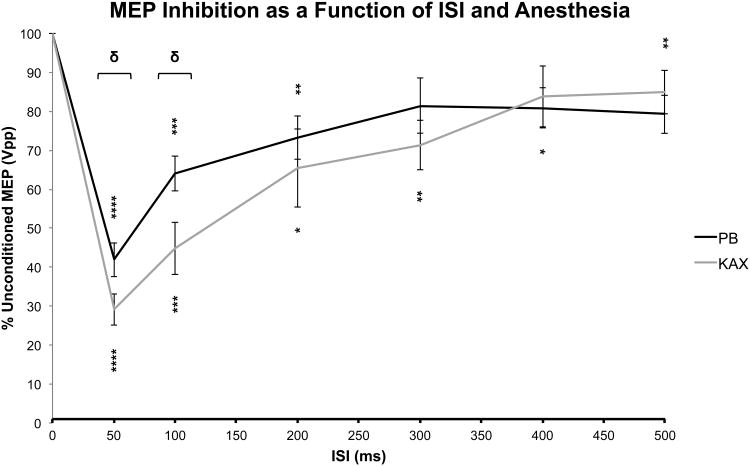
Figure 2 demonstrates the paired-pulse inhibition profile as a function of ISI for pentobarbital and KAX anesthesia. We found that both single-pulse TMS evoked MEPs and human-like measures of long-interval intracortical inhibition were preserved under either anesthetic. In addition, the rat LI-ppTMS inhibitory profile, as in Figure 2, strongly resembles the human LICI profile, suggesting the interrogation of perhaps similar intracortical processes. PTZ administration was shown to reduce LI-ppTMS inhibition by as much as 53% when compared to saline injected control subjects 24. This study was the first successful translation of a ppTMS protocol to rat models as well as the first to evaluate the sensitivity of LI-ppTMS measures to changes in cortical excitability during acute chemoconvulsant-induced seizures. This work also underscores the importance of animal TMS studies in helping us to better understand human TMS mechanisms.
Fig.2. Long interval paired-pulse transcranial magnetic stimulation (LI-ppTMS) inhibitory profile in rats.
Graph depicts the conditioned MEP size as a function of both anesthetic and ISI. Values were derived as the conditioned MEP peak-to-peak amplitude normalized to that of the unconditioned MEP. MEP inhibition is represented by values < 100%. Significant inhibition relative to baseline unconditioned MEP values is noted by asterisks (* p < 0.05, ** p < 0.02, *** p < 0.002, **** p ≤ 0.0002). ISIs demarcated with brackets indicate significant intergroup (e.g. interanesthetic) differences (δ < 0.05). Values indicate the mean ± se. Modified from Vahabzadeh-Hagh et al 2011 24.
LICI has generally been accepted to be mediated by the activation of gamma-aminobutyric acid B receptors (GABABR) based upon limited data for the enhancement of LICI via the administration of baclofen, a GABABR agonist. However, such dependence has proven difficult to replicate, suggesting that LICI is a more multifaceted process 51, 52. The data from our study, demonstrating the reduction of LI-ppTMS, a LICI-like measure, in the presence of a GABAAR antagonist (e.g. PTZ), corroborate the notion that LICI may not be an exclusive GABABR mediated process. Additionally, in more recent years, studies have demonstrated an intricate cross-talk that exists between GABAA and GABAB receptor subclasses. For example, the interaction of GABABR1 with GABAA receptor gamma2S has been shown to regulate surface expression and internalization of GABABR1 under different circumstances53, 54. As such, while GABABR activity may still be the final mediator of LICI, we cannot exclude that GABAAR activity may be at least indirectly involved. The establishment of this ppTMS protocol in rats opens the door to additional pharmacologic studies that will help us to understand these neurophysiologic processes even further.
III.B. Repetitive Transcranial Magnetic Stimulation (rTMS) In Rats
Translational rTMS has been applied in rodents largely for one of two objectives: (1) to study rTMS effects on biochemical or electrophysiologic markers of cell injury or synaptic plasticity, and (2) to test the capacity of rTMS to intervene in models of neuropsychiatric disease. Ex-vivo changes in molecular markers of altered neuronal excitability and alterations in hippocampal electrophysiology, suggest the modulation of neuronal activity following rTMS 13, 36, 55-57. Rat data also demonstrate the potential for rTMS-induced measures to serve as markers of neuronal plasticity. For example, Luft and colleagues demonstrated that simulated motor learning (by somato-sensory afferent stimulation) leads to an enhanced cortical motor excitability in anesthetized rats 18.
rTMS may be customized by frequency and the pattern (continuous or intermittent) of stimulation. When applied in a low frequency manner (i.e. 1 Hz) rTMS suppresses cortical excitability, while high frequency (i.e. 5-20 Hz) enhances cortical excitability. In the majority of rat rTMS publications, stimulation has been applied globally without confirmation of lateralized stimulation by limb EMG 17, 21, 37, 58, 59. More recently, we adapted lateralized TMS protocols in anesthetized rats to a rTMS experiment. We applied low frequency rTMS to induce an LTD-like suppression of the MEP and further utilized the rodent preparation to demonstrate the dependence of this process on the N-methyl-D-aspartate (NMDA)-type glutamate receptor 60. Demonstration of a reliable, focal increase or decrease in excitability by rTMS holds implications for studying disease states affecting discrete brain regions or overall cortical activity and helps to broaden the potential applications of this technique.
Utilization of animals to study the neurophysiologic effects of rTMS is beneficial even without the anatomical localization that can be achieved in humans. For example, experiments where rTMS was delivered non-focally showed how rTMS parameters can differentially affect neuronal excitability 55 and neurochemical markers of neuroplasticity such as immediate-early genes (IEGs), brain derived neurotrophic factor (BDNF) or AMPA 36 and NMDA 56 receptors, depending upon stimulation parameters and animal conditions. Some studies in animal models evaluated the acute effects of either high-frequency 61 or low-frequency 57 rTMS on plasticity markers or clinically-relevant neurochemical alterations such as the release of dopamine, glutamate or acetylcholine 62, 63. An early landmark study found that specific stimulation patterns of rTMS can trigger the expression of IEGs, surrogate measures of induced neuronal activity 17. IEGs are rapidly expressed following synaptic stimulation or behavioral experience and typically assume either a regulatory transcription factor (RTF) or an effector phenotype. C-fos and Zif268, two RTFs, have been implicated in the processes of synaptic plasticity and memory consolidation 64. From the work of Aydin-Abidin and colleagues it was shown that c-fos protein expression was enhanced by either low- or high-frequency rTMS, but only partially by intermittent theta-burst stimulation (iTBS). On the other hand, Zif268 expression was increased by iTBS, partially by high-, but not by low-frequency stimulation. Thus, c-fos appears to have a more non-specific pattern of activation induced by rTMS. By measuring the expression of these genes in different brain regions, Aydin-Abidin et. al. deciphered that different brain regions appear to exhibit different susceptibilities to entrainment by rTMS. Interestingly, they also found a reflexive increase in c-fos expression in the limbic or arousal system in response to sham rTMS, indicating a general level of stress on the animal associated with experimentation 17. This only reiterates a major hurdle we must overcome in rodent TMS; namely, minimization of both experimentally induced stress and of the utilization of anesthesia. Furthermore, recent animal studies have also helped to elucidate the target neuronal populations of theta burst stimulation; namely fast-spiking interneurons and GABAergic interneuronal synapses 65. As we learn more about the specific effectors of rTMS we may optimize the research and clinical applications of these techniques.
Other rat rTMS studies have focused on the longer lasting effects of several rTMS sessions, perhaps more relevant to the clinical approach in humans 36. As mentioned above, Gersner and colleagues looked at the long-term effects of rTMS using BDNF and the GluR1 AMPA receptor subunit expression as markers of neuronal plasticity. They found that 10 daily treatments of high frequency rTMS induced lasting change (e.g. 3 days) in these markers, the direction of which was dependent upon whether the animal was awake versus anesthetized 36. The sharp contrast in their findings between these states underscores the fact that these states are unequal and that caution must be taken when interpreting results obtained under anesthesia. This study also highlights a potential benefit of such animal studies; namely, the capacity to measure brain levels, as opposed to peripheral / serum levels, of plasticity markers. Such capacity may improve the sensitivity and validity of studies as well as the consistency of findings across reports to better inform clinical translation. Overall, these findings obtained in animal studies support the hypothesis that changes in cortical excitability induced by rTMS are accompanied by acute and lasting alterations in molecular markers for neuroplasticity.
IV. Translational TMS Literature
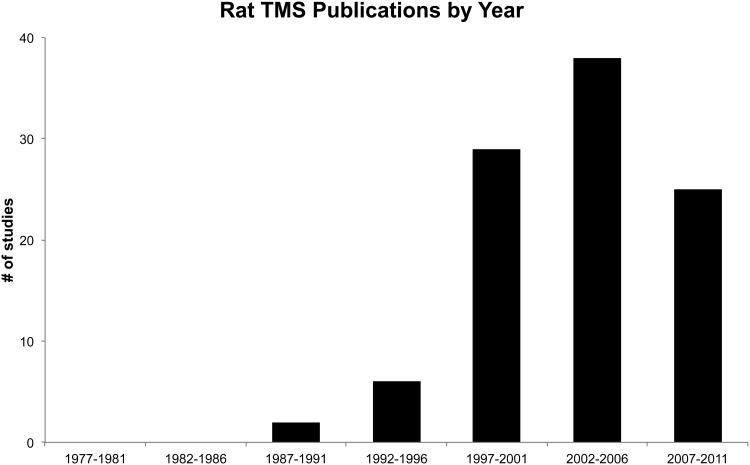
At the time of this writing, there are more than one hundred published reports that contain rat TMS data (supplemental Tables S1, S2, and S3) 17-19, 21, 22, 24, 31-33, 36, 37, 55-59, 61-63, 66-150. Our MEDLINE search strategy to gather these publications was performed for reports from 1966 through March of 2012 that contained keywords “rat(s)”, “tcMMEP” (transcranial magnetic motor evoked potential) “TMS”, “rTMS”, “repetitive transcranial magnetic stimulation” or “transcranial magnetic stimulation.” Notably, from the first rat TMS publication in 1990 there has been an exponential increase in published rat TMS studies (Figure 3), which underscores the TMS community's growing interest in the subject.
Fig.3. Recent growth in rodent TMS publications.
PubMed search was performed from 1966 through December 2010 for “rat(s)”, “tcMMEP”, “TMS”, “rTMS”, “repetitive transcranial magnetic stimulation” and “transcranial magnetic stimulation.” Publications from 2012 were not included as the year was not yet complete at the time of submission.
IV.A. TMS Safety
Since TMS in humans has an exceptionally favorable safety profile and is relatively easy to apply, the technique has become widespread in clinical literature 10, 151-155. Because of its wide safety margin, the development and application of TMS in humans has been less reliant upon foundational findings from animal models. In fact, TMS in rats, particularly if coupled with EMG or EEG, has required specialized technical adaptation, contributing to a lag in basic science data acquisition from rodent experiments. Nevertheless, rat studies have proliferated to meet investigators' need to explore TMS safety, basic TMS mechanisms and TMS efficacy in high-throughput rat disease models with neuroscience techniques that are not available in humans. Safety, beyond mechanism, is arguably highest among the concerns of TMS users. To date, a number of studies have used rat models to provide a deeper evaluation of the safety of rTMS. Chalet de Sauvage and colleagues implemented a rTMS protocol in rats similar in design to the protocol for treatment of depression in humans. They demonstrated that the brain current density profiles were similar between rat and human rTMS and that rTMS (2000 pulses at 100% MT) did not cause measurable genotoxicity when compared to sham treated rats 37. Additional studies have evaluated the safety of more chronic rTMS exposure and failed to demonstrate an alteration in the cerebral metabolite profile or any associated brain or brainstem lesions 58, 132, 156. These safety reports emphasize only a fraction of the beneficial knowledge gained from rat TMS work to date and highlight their potential for great contribution to the field of non-invasive brain stimulation.
IV.B. TMS Functional, Diagnostic, and Therapeutic Applications
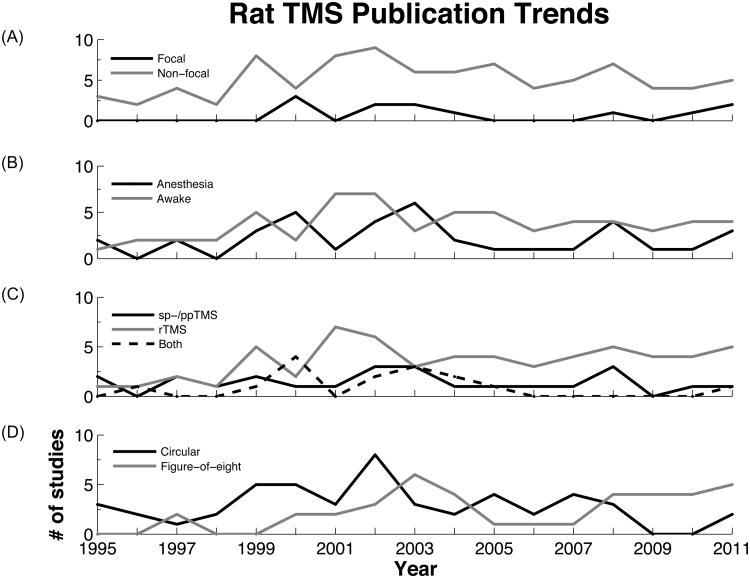
The articles reviewed were stratified on a number of key principles in rat TMS work; namely whether TMS was applied in a focal or non-focal manner, whether it was applied to awake or anesthetized animals, and whether single-/paired-pulse, repetitive TMS, or some combination of protocols was utilized. Only those articles detailing their stimulation protocol were used in this analysis. 100 were included dating back to 1995. Focal TMS was utilized in 12% of these studies while the remainder was unconcerned for its localized application. Among these publications, the first report with focal TMS occurred in 2000 with a steady interest in its application ever since, although of a less productive magnitude when compared to non-focal TMS studies (Figure 4A). As we are beginning to learn more about our capacity to achieve focal rat TMS, a growing trend for the future is anticipated. Figure 4C demonstrates a general increasing trend in the number of rTMS reports annually since 1995. With increasing clinical applications, we anticipate the maintenance of this trend for the foreseeable future. For specific details and citations of these reviewed articles organized by TMS protocol and year see supplemental Tables S1, S2, and S3.
Fig.4. Trends in stratified rat TMS publications.
The articles from the above-mentioned PubMed search were used with the exception of those that did not detail their TMS protocol. 100 articles were used in total. A: Number of publications regarding focal and non-focal TMS over time. B: Number of publications that used TMS on anesthetized versus awake rats. C: Number of publications using single- or paired-pulse TMS, repetitive TMS, or some combination thereof. D: Number of publications stratified by their usage of a circular versus figure-of-eight coil.
IV.C. Rat TMS Study Flaws
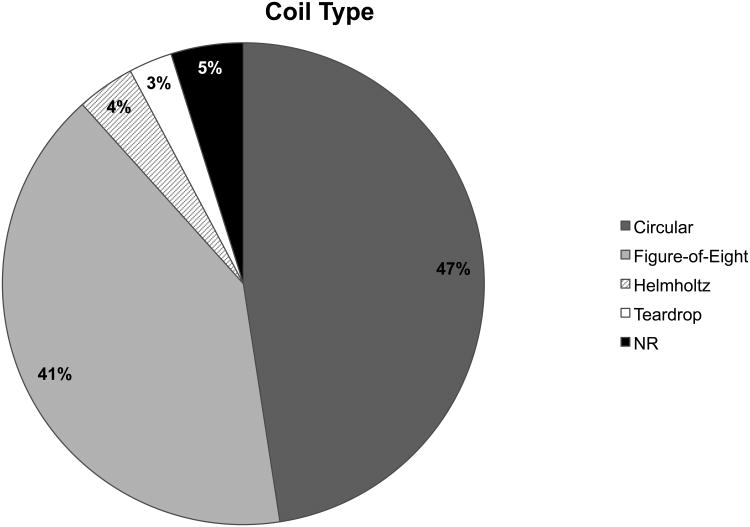
Over time coil shape usage has been almost evenly split between circular and figure-of-eight types, 51% versus 42% respectively (Figure 5). However, looking at the distribution of coil shapes utilized over time there has been a recent shift, from 2009-present, away from circular coils toward predominantly figure-of-eight coils (Figure 4D). The predilection to a more focal coil in recent years would suggest that studies are beginning to place a greater emphasis on stimulation specificity. Surprisingly, this does not appear to be the case as non-focal TMS studies continue to dominate the field. While circular coils and non-focal stimulation have and continue to provide valuable information on TMS mechanisms, their overall scientific utility is more limited. Stimulation of the majority of the rodent brain provides too much background precluding our ability to draw more spatially relevant and mechanistic conclusions. In order to truly uncover underlying biological processes in TMS, it is the opinion of our group that studies should dedicate more effort to refining and utilizing focal stimulation protocols whether via TMS or by focal electrical stimulation as an approximation of focal TMS in humans.
Fig.5. Distribution of coil type utilized in rat TMS reports.
This pie chart depicts the percentage of reports from among the 100 studies analyzed (supplemental Tables S1, S2, and S3), which utilized different types of TMS coils; namely, circular, figure-of-eight, Helmholtz, or teardrop coils. Since 1995, studies have mainly used the circular or figure-of-eight type coils at a near equal frequency.
The use and type of anesthesia in both therapeutic and diagnostic TMS applications is highly variable amongst publications. A wide range of anesthetics have been used including; ketamine, pentobarbital, isoflurane, propofol, and urethane. Any anesthesia will play a significant role in altering steady-state biochemistry and neuronal activity as demonstrated recently by our group 36. Different anesthetics have differing effects on excitability, as demonstrated in our recent study where ketamine significantly depressed MT compared to pentobarbital and enhanced LI-ppTMS inhibition 24. As previously stated, TMS effects are inherently state-dependent 34, 35, 157, and both the use and variability of anesthesia selection warrant caution in the interpretation of data and comparison across studies. Studies dedicated to the effect of each anesthetic on TMS outcome measures is needed and standardization of anesthetic use would benefit the field. Overall, the use of anesthesia remains a considerable difference between human and rat TMS studies, which merits further attention hopefully leading to novel and creative solutions.
Animal restraint is another area in which rat TMS publications offer inconsistent methods. Restraint techniques are in some cases elegant, utilizing fixation screws attached to the occipital bone followed by subsequent fixing to a stereotactic frame 56, 80 or the use of a stereotactic frame with a TMS coil attached to a manipulator 22, 24. However, in the majority of studies animals and TMS coils are simply held and approximated by hand. Use of a proper restraint in order to facilitate reproducible stimulation is imperative to TMS experimental design. While global changes induced by TMS can be analyzed with less intricate methods, one cannot assume that stimulation is identical between animals. Thus, an effort should be made to immobilize the head in a maximally humane and efficient manner and provide a method for accurately placing the stimulation coil to reduce stimulation and outcome variability.
While EMG use is not a required readout, its use in determining stimulation strength should be considered indispensible. Among the publications analyzed in this review, EMG is used in 47% of reports without a discernable chronological trend. Approximation to human protocols alone would require that stimulation strength be standardized to a percentage of MT. Crude observation of limb twitching, while an improvement, cannot replace the sensitivity provided by EMG recording. Whether MT is unreliably determined, or a set MO is provided, each will contribute to variable stimulation among animals and likely introduce error.
Stimulation protocol is one final area of inconsistency amongst rat TMS studies. It is clear that rTMS is the more popular form of TMS used in these studies to date (Figure 4C). However, the use of spTMS and ppTMS, in this groups' opinion, should be used in combination with rTMS as both a readout and method of ensuring that the desired excitation or depression of targeted cortical regions has been achieved. Additionally, there is a lack of rationale concerning the choice of rTMS frequency, as the field is lacking a comprehensive overview of low and high frequency rTMS effects. Although great strides have been made in the study of TMS in rats, a more concerted effort is needed to refine our tools and techniques.
V. Conclusions and Practical Significance
The growing body of translational TMS research in rodents has realistic potential to complement the already established field of clinical TMS. Although limited by stimulation focality, specialized technical requirements, and by essential differences in neuronal circuitry between rat and human, rat data has provided important insights into basic TMS mechanisms and its prospects in the treatment of neuropsychiatric disease. For instance, a demonstration of seizure suppression by rTMS in the rat 21 has influenced the design of an ongoing clinical trial for rTMS in patients with epilepsy. In the authors' view, as with other translational fields, basic science exploration of TMS, particularly rTMS and ppTMS protocols, will continue to direct clinical trial development. The steady increase in rodent TMS research over the recent years seems to uphold this prospect. As rodent TMS models continue to be refined, we can expect to learn more about TMS and the physiologic underpinnings of neurologic and neuropsychiatric disease, while expanding upon our diagnostic and therapeutic armamentarium.
Supplementary Material
Table.S1 organizes retrieved articles that utilized a rTMS protocol.
Table.S2 organizes retrieved articles that utilized a single- or paired-pulse TMS protocol.
Table.S3 organizes retrieved articles that utilized a single- or paired-pulse TMS protocol as well as a rTMS protocol.
Acknowledgments
The authors would like to thank the financial support that helped fund the salaries of some of the contributors to this review article. Andrew Vahabzadeh-Hagh received support from the Howard Hughes Medical Institute medical research training fellowship program and the Center for Integration of Medicine and Innovative Technology (CIMIT). Dr. Rotenberg received support from CIMIT, the Department of Defense, and the NIH (K08NS055895).
Financial Support: Andrew M. Vahabzadeh-Hagh received support from the Howard Hughes Medical Institute research training fellowship program.
Footnotes
Authorship Statement: Mr. Vahabzadeh-Hagh and Mr. Muller prepared the manuscript draft with important intellectual input and feedback from Drs. Rolenberg, Zangen and Gersner. Mr. Vahabzadeh-Hagh and Mr. Muller coordinated subsequent revisions among the authors and prepared the manuscript in its final form. All authors approved the final manuscript.
Financial Disclosure and Conflict of Interest: Andrew M. Vahabzadeh-Hagh reports no biomedical financial interest or potential conflicts of interest. Paul A. Muller reports no biomedical financial interest or potential conflicts of interest. Roman Gersner reports no biomedical financial interest or potential conflicts of interest. Abraham Zangen is a consultant for and has financial interest in Brainsway Inc., a company that develops TMS coils designed for stimulation of deeper brain areas. Alexander Rotenberg does not currently serve on any advisory board, but does hold intellectual property for TMS technology and the combination of TMS with EEG.
References
- 1.Kobayashi M, Pascual-Leone A. Transcranial magnetic stimulation in neurology. The Lancet Neurology. 2003;2:145–156. doi: 10.1016/s1474-4422(03)00321-1. [DOI] [PubMed] [Google Scholar]
- 2.Kujirai T, Caramia MD, Rothwell JC, Day BL, Thompson PD, Ferbert A, et al. Corticocortical inhibition in human motor cortex. J Physiol. 1993;471:501–519. doi: 10.1113/jphysiol.1993.sp019912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pascual-Leone A, Tormos JM, Keenan J, Tarazona F, Canete C, Catala MD. Study and modulation of human cortical excitability with transcranial magnetic stimulation. J Clin Neurophysiol. 1998;15:333–343. doi: 10.1097/00004691-199807000-00005. [DOI] [PubMed] [Google Scholar]
- 4.Pascual-Leone A, Walsh V, Rothwell J. Transcranial magnetic stimulation in cognitive neuroscience--virtual lesion, chronometry, and functional connectivity. Curr Opin Neurobiol. 2000;10:232–237. doi: 10.1016/s0959-4388(00)00081-7. [DOI] [PubMed] [Google Scholar]
- 5.Tormos JM, Catala MD, Pascual-Leone A. transcranial magnetic stimulation. Rev Neurol. 1999;29:165–171. [PubMed] [Google Scholar]
- 6.Demirtas-Tatlidede A, Vahabzadeh-Hagh AM, Bernabeu M, Tormos JM, Pascual-Leone A. Noninvasive brain stimulation in traumatic brain injury. J Head Trauma Rehabil. 2011:17. doi: 10.1097/HTR.0b013e318217df55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rotenberg A. Prospects for clinical applications of transcranial magnetic stimulation and real-time eeg in epilepsy. Brain Topogr. 2010;22:257–266. doi: 10.1007/s10548-009-0116-3. [DOI] [PubMed] [Google Scholar]
- 8.Khedr EM, Rothwell JC, Ahmed MA, Shawky OA, Farouk M. Modulation of motor cortical excitability following rapid-rate transcranial magnetic stimulation. Clin Neurophysiol. 2007;118:140–145. doi: 10.1016/j.clinph.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 9.Maeda F, Keenan JP, Tormos JM, Topka H, Pascual-Leone A. Modulation of corticospinal excitability by repetitive transcranial magnetic stimulation. Clin Neurophysiol. 2000;111:800–805. doi: 10.1016/s1388-2457(99)00323-5. [DOI] [PubMed] [Google Scholar]
- 10.Wassermann EM. Risk and safety of repetitive transcranial magnetic stimulation: Report and suggested guidelines from the international workshop on the safety of repetitive transcranial magnetic stimulation, june 5-7, 1996. Electroencephalogr Clin Neurophysiol. 1998;108:1–16. doi: 10.1016/s0168-5597(97)00096-8. [DOI] [PubMed] [Google Scholar]
- 11.Fitzgerald PB, Fountain S, Daskalakis ZJ. A comprehensive review of the effects of rtms on motor cortical excitability and inhibition. Clin Neurophysiol. 2006;117:2584–2596. doi: 10.1016/j.clinph.2006.06.712. [DOI] [PubMed] [Google Scholar]
- 12.Hoogendam JM, Ramakers GM, Di Lazzaro V. Physiology of repetitive transcranial magnetic stimulation of the human brain. Brain Stimul. 2009;3:95–118. doi: 10.1016/j.brs.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 13.Pell GS, Roth Y, Zangen A. Modulation of cortical excitability induced by repetitive transcranial magnetic stimulation: Influence of timing and geometrical parameters and underlying mechanisms. Prog Neurobiol. 2011;93:59–98. doi: 10.1016/j.pneurobio.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 14.Muller PA, Pascual-Leone A, Rotenberg A. Safety and tolerability of repetitive transcranial magnetic stimulation in patients with pathologic positive sensory phenomena: A review of literature. Brain Stimul. 2011;14:14. doi: 10.1016/j.brs.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fregni F, Otachi PT, Do Valle A, Boggio PS, Thut G, Rigonatti SP, et al. A randomized clinical trial of repetitive transcranial magnetic stimulation in patients with refractory epilepsy. Ann Neurol. 2006;60:447–455. doi: 10.1002/ana.20950. [DOI] [PubMed] [Google Scholar]
- 16.Fitzgerald PB, Daskalakis ZJ. The effects of repetitive transcranial magnetic stimulation in the treatment of depression. Expert Rev Med Devices. 2011;8:85–95. doi: 10.1586/erd.10.57. [DOI] [PubMed] [Google Scholar]
- 17.Aydin-Abidin S, Trippe J, Funke K, Eysel UT, Benali A. High- and low-frequency repetitive transcranial magnetic stimulation differentially activates c-fos and zif268 protein expression in the rat brain. Exp Brain Res. 2008;188:249–261. doi: 10.1007/s00221-008-1356-2. [DOI] [PubMed] [Google Scholar]
- 18.Luft AR, Kaelin-Lang A, Hauser TK, Buitrago MM, Thakor NV, Hanley DF, et al. Modulation of rodent cortical motor excitability by somatosensory input. Exp Brain Res. 2002;142:562–569. doi: 10.1007/s00221-001-0952-1. [DOI] [PubMed] [Google Scholar]
- 19.Luft AR, Kaelin-Lang A, Hauser TK, Cohen LG, Thakor NV, Hanley DF. Transcranial magnetic stimulation in the rat. Exp Brain Res. 2001;140:112–121. doi: 10.1007/s002210100805. [DOI] [PubMed] [Google Scholar]
- 20.Nielsen JB, Perez MA, Oudega M, Enriquez-Denton M, Aimonetti JM. Evaluation of transcranial magnetic stimulation for investigating transmission in descending motor tracts in the rat. Eur J Neurosci. 2007;25:805–814. doi: 10.1111/j.1460-9568.2007.05326.x. [DOI] [PubMed] [Google Scholar]
- 21.Rotenberg A, Muller P, Birnbaum D, Harrington M, Riviello JJ, Pascual-Leone A, et al. Seizure suppression by eeg-guided repetitive transcranial magnetic stimulation in the rat. Clin Neurophysiol. 2008;119:2697–2702. doi: 10.1016/j.clinph.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rotenberg A, Muller PA, Vahabzadeh-Hagh AM, Navarro X, Lopez-Vales R, Pascual-Leone A, et al. Lateralization of forelimb motor evoked potentials by transcranial magnetic stimulation in rats. Clin Neurophysiol. 2010;121:104–108. doi: 10.1016/j.clinph.2009.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang YP, Shields LB, Zhang Y, Pei J, Xu XM, Hoskins R, et al. Use of magnetic stimulation to elicit motor evoked potentials, somatosensory evoked potentials, and h-reflexes in non-sedated rodents. J Neurosci Methods. 2007;165:9–17. doi: 10.1016/j.jneumeth.2007.05.021. [DOI] [PubMed] [Google Scholar]
- 24.Vahabzadeh-Hagh AM, Muller PA, Pascual-Leone A, Jensen FE, Rotenberg A. Measures of cortical inhibition by paired-pulse transcranial magnetic stimulation in anesthetized rats. J Neurophysiol. 2011;105:615–624. doi: 10.1152/jn.00660.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roth Y, Amir A, Levkovitz Y, Zangen A. Three-dimensional distribution of the electric field induced in the brain by transcranial magnetic stimulation using figure-8 and deep h-coils. J Clin Neurophysiol. 2007;24:31–38. doi: 10.1097/WNP.0b013e31802fa393. [DOI] [PubMed] [Google Scholar]
- 26.Cohen LG, Roth BJ, Nilsson J, Dang N, Panizza M, Bandinelli S, et al. Effects of coil design on delivery of focal magnetic stimulation. Technical considerations. Electroencephalogr Clin Neurophysiol. 1990;75:350–357. doi: 10.1016/0013-4694(90)90113-x. [DOI] [PubMed] [Google Scholar]
- 27.Sahin B, Aslan H, Unal B, Canan S, Bilgic S, Kaplan S, et al. Brain volumes of the lamb, rat and bird do not show hemispheric asymmetry: A stereological study. Image Analysis & Stereology. 2001;20:9–13. [Google Scholar]
- 28.Zheng J, Li L, Huo X. Analysis of electric field in real rat head model during transcranial magnetic stimulation. Conf Proc IEEE Eng Med Biol Soc. 2005;2:1529–1532. doi: 10.1109/IEMBS.2005.1616724. [DOI] [PubMed] [Google Scholar]
- 29.Wagner T, Valero-Cabre A, Pascual-Leone A. Noninvasive human brain stimulation. Annu Rev Biomed Eng. 2007;9:527–565. doi: 10.1146/annurev.bioeng.9.061206.133100. [DOI] [PubMed] [Google Scholar]
- 30.Cohen D, Cuffin BN. Developing a more focal magnetic stimulator. Part i: Some basic principles. J Clin Neurophysiol. 1991;8:102–111. doi: 10.1097/00004691-199101000-00013. [DOI] [PubMed] [Google Scholar]
- 31.Gasca F, Richter L, Schweikard A. Simulation of a conductive shield plate for the focalization of transcranial magnetic stimulation in the rat. Conf Proc IEEE Eng Med Biol Soc. 2010;2010:1593–1596. doi: 10.1109/IEMBS.2010.5626674. [DOI] [PubMed] [Google Scholar]
- 32.Fishback AS, Shields CB, Linden RD, Zhang YP, Burke D. The effects of propofol on rat transcranial magnetic motor evoked potentials. Neurosurgery. 1995;37:969–974. doi: 10.1227/00006123-199511000-00017. [DOI] [PubMed] [Google Scholar]
- 33.Kohn DF. Anesthesia and analgesia in laboratory animals. San Diego: Academic Press; 1997. [Google Scholar]
- 34.Ferrarelli F, Massimini M, Sarasso S, Casali A, Riedner BA, Angelini G, et al. Breakdown in cortical effective connectivity during midazolam-induced loss of consciousness. Proc Natl Acad Sci U S A. 2010;107:2681–2686. doi: 10.1073/pnas.0913008107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Massimini M, Ferrarelli F, Murphy M, Huber R, Riedner B, Casarotto S, et al. Cortical reactivity and effective connectivity during rem sleep in humans. Cogn Neurosci. 2010;1:176–183. doi: 10.1080/17588921003731578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gersner R, Kravetz E, Feil J, Pell G, Zangen A. Long-term effects of repetitive transcranial magnetic stimulation on markers for neuroplasticity: Differential outcomes in anesthetized and awake animals. J Neurosci. 2011;31:7521–7526. doi: 10.1523/JNEUROSCI.6751-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.de Sauvage RC, Lagroye I, Billaudel B, Veyret B. Evaluation of the potential genotoxic effects of rtms on the rat brain and current density mapping. Clin Neurophysiol. 2008;119:482–491. doi: 10.1016/j.clinph.2007.09.137. [DOI] [PubMed] [Google Scholar]
- 38.Hsieh TH, Dhamne SC, Chen JJ, Pascual-Leone A, Jensen FE, Rotenberg A. A new measure of cortical inhibition by mechanomyography and paired-pulse transcranial magnetic stimulation in unanesthetized rats. J Neurophysiol. 2012;107:966–972. doi: 10.1152/jn.00690.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Valls-Sole J, Pascual-Leone A, Wassermann EM, Hallett M. Human motor evoked responses to paired transcranial magnetic stimuli. Electroencephalogr Clin Neurophysiol. 1992;85:355–364. doi: 10.1016/0168-5597(92)90048-g. [DOI] [PubMed] [Google Scholar]
- 40.Ziemann U, Lonnecker S, Steinhoff BJ, Paulus W. The effect of lorazepam on the motor cortical excitability in man. Exp Brain Res. 1996;109:127–135. doi: 10.1007/BF00228633. [DOI] [PubMed] [Google Scholar]
- 41.Ziemann U, Rothwell JC, Ridding MC. Interaction between intracortical inhibition and facilitation in human motor cortex. J Physiol. 1996;496(Pt 3):873–881. doi: 10.1113/jphysiol.1996.sp021734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen R. Interactions between inhibitory and excitatory circuits in the human motor cortex. Exp Brain Res. 2004;154:1–10. doi: 10.1007/s00221-003-1684-1. [DOI] [PubMed] [Google Scholar]
- 43.Chen R, Tam A, Butefisch C, Corwell B, Ziemann U, Rothwell JC, et al. Intracortical inhibition and facilitation in different representations of the human motor cortex. J Neurophysiol. 1998;80:2870–2881. doi: 10.1152/jn.1998.80.6.2870. [DOI] [PubMed] [Google Scholar]
- 44.Ilic TV, Meintzschel F, Cleff U, Ruge D, Kessler KR, Ziemann U. Short-interval paired-pulse inhibition and facilitation of human motor cortex: The dimension of stimulus intensity. J Physiol. 2002;545:153–167. doi: 10.1113/jphysiol.2002.030122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Peurala SH, Muller-Dahlhaus JF, Arai N, Ziemann U. Interference of short-interval intracortical inhibition (sici) and short-interval intracortical facilitation (sicf) Clin Neurophysiol. 2008;119:2291–2297. doi: 10.1016/j.clinph.2008.05.031. [DOI] [PubMed] [Google Scholar]
- 46.Ziemann U, Chen R, Cohen LG, Hallett M. Dextromethorphan decreases the excitability of the human motor cortex. Neurology. 1998;51:1320–1324. doi: 10.1212/wnl.51.5.1320. [DOI] [PubMed] [Google Scholar]
- 47.Badawy RA, Macdonell RA, Jackson GD, Berkovic SF. Why do seizures in generalized epilepsy often occur in the morning? Neurology. 2009;73:218–222. doi: 10.1212/WNL.0b013e3181ae7ca6. [DOI] [PubMed] [Google Scholar]
- 48.Brodtmann A, Macdonell RA, Gilligan AK, Curatolo J, Berkovic SF. Cortical excitability and recovery curve analysis in generalized epilepsy. Neurology. 1999;53:1347–1349. doi: 10.1212/wnl.53.6.1347. [DOI] [PubMed] [Google Scholar]
- 49.Molnar GF, Sailer A, Gunraj CA, Cunic DI, Wennberg RA, Lozano AM, et al. Changes in motor cortex excitability with stimulation of anterior thalamus in epilepsy. Neurology. 2006;66:566–571. doi: 10.1212/01.wnl.0000198254.08581.6b. [DOI] [PubMed] [Google Scholar]
- 50.Valzania F, Strafella AP, Tropeani A, Rubboli G, Nassetti SA, Tassinari CA. Facilitation of rhythmic events in progressive myoclonus epilepsy: A transcranial magnetic stimulation study. Clin Neurophysiol. 1999;110:152–157. doi: 10.1016/s0013-4694(98)00115-1. [DOI] [PubMed] [Google Scholar]
- 51.Florian J, Muller-Dahlhaus M, Liu Y, Ziemann U. Inhibitory circuits and the nature of their interactions in the human motor cortex a pharmacological tms study. J Physiol. 2008;586:495–514. doi: 10.1113/jphysiol.2007.142059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McDonnell MN, Orekhov Y, Ziemann U. The role of gaba(b) receptors in intracortical inhibition in the human motor cortex. Exp Brain Res. 2006;173:86–93. doi: 10.1007/s00221-006-0365-2. [DOI] [PubMed] [Google Scholar]
- 53.Balasubramanian S, Teissere JA, Raju DV, Hall RA. Hetero-oligomerization between gabaa and gabab receptors regulates gabab receptor trafficking. J Biol Chem. 2004;279:18840–18850. doi: 10.1074/jbc.M313470200. [DOI] [PubMed] [Google Scholar]
- 54.Kuczewski N, Fuchs C, Ferrand N, Jovanovic JN, Gaiarsa JL, Porcher C. Mechanism of gabab receptor-induced bdnf secretion and promotion of gabaa receptor membrane expression. J Neurochem. 2011;118:533–545. doi: 10.1111/j.1471-4159.2011.07192.x. [DOI] [PubMed] [Google Scholar]
- 55.Benali A, Trippe J, Weiler E, Mix A, Petrasch-Parwez E, Girzalsky W, et al. Theta-burst transcranial magnetic stimulation alters cortical inhibition. J Neurosci. 2011;31:1193–1203. doi: 10.1523/JNEUROSCI.1379-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kole MH, Fuchs E, Ziemann U, Paulus W, Ebert U. Changes in 5-ht1a and nmda binding sites by a single rapid transcranial magnetic stimulation procedure in rats. Brain Res. 1999;826:309–312. doi: 10.1016/s0006-8993(99)01257-3. [DOI] [PubMed] [Google Scholar]
- 57.Zhang X, Mei Y, Liu C, Yu S. Effect of transcranial magnetic stimulation on the expression of c-fos and brain-derived neurotrophic factor of the cerebral cortex in rats with cerebral infarct. J Huazhong Univ Sci Technolog Med Sci. 2007;27:415–418. doi: 10.1007/s11596-007-0416-3. [DOI] [PubMed] [Google Scholar]
- 58.Liebetanz D, Fauser S, Michaelis T, Czeh B, Watanabe T, Paulus W, et al. Safety aspects of chronic low-frequency transcranial magnetic stimulation based on localized proton magnetic resonance spectroscopy and histology of the rat brain. J Psychiatr Res. 2003;37:277–286. doi: 10.1016/s0022-3956(03)00017-7. [DOI] [PubMed] [Google Scholar]
- 59.Wang F, Geng X, Tao HY, Cheng Y. The restoration after repetitive transcranial magnetic stimulation treatment on cognitive ability of vascular dementia rats and its impacts on synaptic plasticity in hippocampal ca1 area. J Mol Neurosci. 2010;41:145–155. doi: 10.1007/s12031-009-9311-7. [DOI] [PubMed] [Google Scholar]
- 60.Muller P, Dhanme S, Vahabzadeh-Hagh A, Pascual-Leone A, Jensen F, Rotenberg A. Demonstration of long-term depression in rat motor cortex with low frequency rtms. Society for Neuroscience 40th Annual Meeting. 2010 OOO28. [Google Scholar]
- 61.Muller MB, Toschi N, Kresse AE, Post A, Keck ME. Long-term repetitive transcranial magnetic stimulation increases the expression of brain-derived neurotrophic factor and cholecystokinin mrna, but not neuropeptide tyrosine mrna in specific areas of rat brain. Neuropsychopharmacology. 2000;23:205–215. doi: 10.1016/S0893-133X(00)00099-3. [DOI] [PubMed] [Google Scholar]
- 62.Keck ME, Welt T, Muller MB, Erhardt A, Ohl F, Toschi N, et al. Repetitive transcranial magnetic stimulation increases the release of dopamine in the mesolimbic and mesostriatal system. Neuropharmacology. 2002;43:101–109. doi: 10.1016/s0028-3908(02)00069-2. [DOI] [PubMed] [Google Scholar]
- 63.Zangen A, Hyodo K. Transcranial magnetic stimulation induces increases in extracellular levels of dopamine and glutamate in the nucleus accumbens. Neuroreport. 2002;13:2401–2405. doi: 10.1097/00001756-200212200-00005. [DOI] [PubMed] [Google Scholar]
- 64.Kubik S, Miyashita T, Guzowski JF. Using immediate-early genes to map hippocampal subregional functions. Learn Mem. 2007;14:758–770. doi: 10.1101/lm.698107. [DOI] [PubMed] [Google Scholar]
- 65.Funke K, Benali A. Modulation of cortical inhibition by rtms - findings obtained from animal models. J Physiol. 2011;589:4423–4435. doi: 10.1113/jphysiol.2011.206573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Akamatsu N, Fueta Y, Endo Y, Matsunaga K, Uozumi T, Tsuji S. Decreased susceptibility to pentylenetetrazol-induced seizures after low-frequency transcranial magnetic stimulation in rats. Neurosci Lett. 2001;310:153–156. doi: 10.1016/s0304-3940(01)02116-4. [DOI] [PubMed] [Google Scholar]
- 67.Arias-Carrion O, Verdugo-Diaz L, Feria-Velasco A, Millan-Aldaco D, Gutierrez AA, Hernandez-Cruz A, et al. Neurogenesis in the subventricular zone following transcranial magnetic field stimulation and nigrostriatal lesions. J Neurosci Res. 2004;78:16–28. doi: 10.1002/jnr.20235. [DOI] [PubMed] [Google Scholar]
- 68.Belmaker RH, Grisaru N. Magnetic stimulation of the brain in animal depression models responsive to ecs. J ECT. 1998;14:194–205. [PubMed] [Google Scholar]
- 69.Ben-Shachar D, Belmaker RH, Grisaru N, Klein E. Transcranial magnetic stimulation induces alterations in brain monoamines. J Neural Transm. 1997;104:191–197. doi: 10.1007/BF01273180. [DOI] [PubMed] [Google Scholar]
- 70.Ben-Shachar D, Gazawi H, Riboyad-Levin J, Klein E. Chronic repetitive transcranial magnetic stimulation alters beta-adrenergic and 5-ht2 receptor characteristics in rat brain. Brain Res. 1999;816:78–83. doi: 10.1016/s0006-8993(98)01119-6. [DOI] [PubMed] [Google Scholar]
- 71.Cao Q, Zhang YP, Iannotti C, DeVries WH, Xu XM, Shields CB, et al. Functional and electrophysiological changes after graded traumatic spinal cord injury in adult rat. Exp Neurol. 2005;191(Suppl 1):S3–S16. doi: 10.1016/j.expneurol.2004.08.026. [DOI] [PubMed] [Google Scholar]
- 72.Chiba A, Ohta Y, Oshio K, Inase M. Motor evoked potentials in rats with congenital hydrocephalus. Neurol Res. 2003;25:305–308. doi: 10.1179/016164103101201391. [DOI] [PubMed] [Google Scholar]
- 73.Chiba A, Oshio K, Inase M. Magnetically evoked emgs in rats. Neurol Res. 2003;25:87–91. doi: 10.1179/016164103101200987. [DOI] [PubMed] [Google Scholar]
- 74.Cruz-Orengo L, Figueroa JD, Torrado A, Puig A, Whittemore SR, Miranda JD. Reduction of epha4 receptor expression after spinal cord injury does not induce axonal regeneration or return of tcmmep response. Neurosci Lett. 2007;418:49–54. doi: 10.1016/j.neulet.2007.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Czeh B, Welt T, Fischer AK, Erhardt A, Schmitt W, Muller MB, et al. Chronic psychosocial stress and concomitant repetitive transcranial magnetic stimulation: Effects on stress hormone levels and adult hippocampal neurogenesis. Biol Psychiatry. 2002;52:1057–1065. doi: 10.1016/s0006-3223(02)01457-9. [DOI] [PubMed] [Google Scholar]
- 76.Deng YB, Liu Y, Zhu WB, Bi XB, Wang YZ, Ye MH, et al. The co-transplantation of human bone marrow stromal cells and embryo olfactory ensheathing cells as a new approach to treat spinal cord injury in a rat model. Cytotherapy. 2008;10:551–564. doi: 10.1080/14653240802165673. [DOI] [PubMed] [Google Scholar]
- 77.Dimar JR, 2nd, Glassman SD, Raque GH, Zhang YP, Shields CB. The influence of spinal canal narrowing and timing of decompression on neurologic recovery after spinal cord contusion in a rat model. Spine (Phila Pa 1976) 1999;24:1623–1633. doi: 10.1097/00007632-199908150-00002. [DOI] [PubMed] [Google Scholar]
- 78.Dimar JR, 2nd, Shields CB, Zhang YP, Burke DA, Raque GH, Glassman SD. The role of directly applied hypothermia in spinal cord injury. Spine (Phila Pa 1976) 2000;25:2294–2302. doi: 10.1097/00007632-200009150-00006. [DOI] [PubMed] [Google Scholar]
- 79.Doi W, Sato D, Fukuzako H, Takigawa M. C-fos expression in rat brain after repetitive transcranial magnetic stimulation. Neuroreport. 2001;12:1307–1310. doi: 10.1097/00001756-200105080-00050. [DOI] [PubMed] [Google Scholar]
- 80.Ebert U, Ziemann U. Altered seizure susceptibility after high-frequency transcranial magnetic stimulation in rats. Neurosci Lett. 1999;273:155–158. doi: 10.1016/s0304-3940(99)00636-9. [DOI] [PubMed] [Google Scholar]
- 81.Erhardt A, Sillaber I, Welt T, Muller MB, Singewald N, Keck ME. Repetitive transcranial magnetic stimulation increases the release of dopamine in the nucleus accumbens shell of morphine-sensitized rats during abstinence. Neuropsychopharmacology. 2004;29:2074–2080. doi: 10.1038/sj.npp.1300493. [DOI] [PubMed] [Google Scholar]
- 82.Fa Z, Zhang P, Wu W, Wang Z, Huang F, Yang L, et al. Functional mapping of rat brain activation following rtms using activity-induced manganese-dependent contrast. Neurol Res. 2011;33:563–571. doi: 10.1179/1743132810Y.0000000009. [DOI] [PubMed] [Google Scholar]
- 83.Feng HL, Yan L, Cui LY. Effects of repetitive transcranial magnetic stimulation on adenosine triphosphate content and microtubule associated protein-2 expression after cerebral ischemia-reperfusion injury in rat brain. Chin Med J (Engl) 2008;121:1307–1312. [PubMed] [Google Scholar]
- 84.Feng HL, Yan L, Guan YZ, Cui LY. Effects of transcranial magnetic stimulation on motor cortical excitability and neurofunction after cerebral ischemia-reperfusion injury in rats. Chin Med Sci J. 2005;20:226–230. [PubMed] [Google Scholar]
- 85.Fleischmann A, Hirschmann S, Dolberg OT, Dannon PN, Grunhaus L. Chronic treatment with repetitive transcranial magnetic stimulation inhibits seizure induction by electroconvulsive shock in rats. Biol Psychiatry. 1999;45:759–763. doi: 10.1016/s0006-3223(98)00211-x. [DOI] [PubMed] [Google Scholar]
- 86.Fleischmann A, Prolov K, Abarbanel J, Belmaker RH. The effect of transcranial magnetic stimulation of rat brain on behavioral models of depression. Brain Res. 1995;699:130–132. doi: 10.1016/0006-8993(95)01018-q. [DOI] [PubMed] [Google Scholar]
- 87.Fleischmann A, Sternheim A, Etgen AM, Li C, Grisaru N, Belmaker RH. Transcranial magnetic stimulation downregulates beta-adrenoreceptors in rat cortex. J Neural Transm. 1996;103:1361–1366. doi: 10.1007/BF01271196. [DOI] [PubMed] [Google Scholar]
- 88.Funamizu H, Ogiue-Ikeda M, Mukai H, Kawato S, Ueno S. Acute repetitive transcranial magnetic stimulation reactivates dopaminergic system in lesion rats. Neurosci Lett. 2005;383:77–81. doi: 10.1016/j.neulet.2005.04.018. [DOI] [PubMed] [Google Scholar]
- 89.Gao F, Wang S, Guo Y, Wang J, Lou M, Wu J, et al. Protective effects of repetitive transcranial magnetic stimulation in a rat model of transient cerebral ischaemia: A micropet study. Eur J Nucl Med Mol Imaging. 2010;37:954–961. doi: 10.1007/s00259-009-1342-3. [DOI] [PubMed] [Google Scholar]
- 90.Glassman SD, Zhang YP, Shields CB, Linden RD, Johnson JR. An evaluation of motor-evoked potentials for detection of neurologic injury with correction of an experimental scoliosis. Spine (Phila Pa 1976) 1995;20:1765–1775. doi: 10.1097/00007632-199508150-00004. [DOI] [PubMed] [Google Scholar]
- 91.Godlevskii LS, Kobolev EV. The effects of l-dopa and transcranial magnetic stimulation on behavioral reactions in kindled rats. Neurosci Behav Physiol. 2005;35:313–317. doi: 10.1007/s11055-005-0065-6. [DOI] [PubMed] [Google Scholar]
- 92.Godlevsky LS, Kobolev EV, van Luijtelaar EL, Coenen AM, Stepanenko KI, Smirnov IV. Influence of transcranial magnetic stimulation on spike-wave discharges in a genetic model of absence epilepsy. Indian J Exp Biol. 2006;44:949–954. [PubMed] [Google Scholar]
- 93.Gur E, Lerer B, Dremencov E, Newman ME. Chronic repetitive transcranial magnetic stimulation induces subsensitivity of presynaptic serotonergic autoreceptor activity in rat brain. Neuroreport. 2000;11:2925–2929. doi: 10.1097/00001756-200009110-00019. [DOI] [PubMed] [Google Scholar]
- 94.Gur E, Lerer B, van de Kar LD, Newman ME. Chronic rtms induces subsensitivity of post-synaptic 5-ht1a receptors in rat hypothalamus. Int J Neuropsychopharmacol. 2004;7:335–340. doi: 10.1017/S1461145703003985. [DOI] [PubMed] [Google Scholar]
- 95.Hausmann A, Weis C, Marksteiner J, Hinterhuber H, Humpel C. Chronic repetitive transcranial magnetic stimulation enhances c-fos in the parietal cortex and hippocampus. Brain Res Mol Brain Res. 2000;76:355–362. doi: 10.1016/s0169-328x(00)00024-3. [DOI] [PubMed] [Google Scholar]
- 96.Hedges DW, Higginbotham BJ, Salyer DL, Lund TD. Transcranial magnetic stimulation effects on one-trial learning and response to anxiogenic stimuli in adult male rats. J ECT. 2005;21:25–30. doi: 10.1097/01.yct.0000154051.73269.62. [DOI] [PubMed] [Google Scholar]
- 97.Hedges DW, Massari C, Salyer DL, Lund TD, Hellewell JL, Johnson AC, et al. Duration of transcranial magnetic stimulation effects on the neuroendocrine stress response and coping behavior of adult male rats. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:633–638. doi: 10.1016/S0278-5846(03)00052-6. [DOI] [PubMed] [Google Scholar]
- 98.Hedges DW, Salyer DL, Higginbotham BJ, Lund TD, Hellewell JL, Ferguson D, et al. Transcranial magnetic stimulation (tms) effects on testosterone, prolactin, and corticosterone in adult male rats. Biol Psychiatry. 2002;51:417–421. doi: 10.1016/s0006-3223(01)01266-5. [DOI] [PubMed] [Google Scholar]
- 99.Hong B, Kuwaki T, Ju K, Kumada M, Akai M, Ueno S. Changes in blood pressure and heart rate by repetitive transcranial magnetic stimulation in rats. Neurosci Lett. 2002;329:57–60. doi: 10.1016/s0304-3940(02)00592-x. [DOI] [PubMed] [Google Scholar]
- 100.Iannotti C, Ping Zhang Y, Shields CB, Han Y, Burke DA, Xu XM. A neuroprotective role of glial cell line-derived neurotrophic factor following moderate spinal cord contusion injury. Exp Neurol. 2004;189:317–332. doi: 10.1016/j.expneurol.2004.05.033. [DOI] [PubMed] [Google Scholar]
- 101.Isogawa K, Fujiki M, Akiyoshi J, Tsutsumi T, Horinouchi Y, Kodama K, et al. Anxiety induced by repetitive transcranial magnetic stimulation is suppressed by chronic treatment of paroxetine in rats. Pharmacopsychiatry. 2003;36:7–11. doi: 10.1055/s-2003-38085. [DOI] [PubMed] [Google Scholar]
- 102.Isogawa K, Fujiki M, Akiyoshi J, Tsutsumi T, Kodama K, Matsushita H, et al. Anxiolytic suppression of repetitive transcranial magnetic stimulation-induced anxiety in the rats. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:664–668. doi: 10.1016/j.pnpbp.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 103.Jennum P, Klitgaard H. Repetitive transcranial magnetic stimulations of the rat. Effect of acute and chronic stimulations on pentylenetetrazole-induced clonic seizures. Epilepsy Res. 1996;23:115–122. doi: 10.1016/0920-1211(95)00088-7. [DOI] [PubMed] [Google Scholar]
- 104.Kaga A, Fujiki M, Hori S, Nakano T, Isono M. Motor evoked potentials following transcranial magnetic stimulation after middle cerebral artery and/or basilar artery occlusions in rats. J Clin Neurosci. 2003;10:470–475. doi: 10.1016/s0967-5868(03)00082-1. [DOI] [PubMed] [Google Scholar]
- 105.Kamida T, Fujiki M, Hori S, Isono M. Conduction pathways of motor evoked potentials following transcranial magnetic stimulation: A rodent study using a “figure-8” coil. Muscle Nerve. 1998;21:722–731. doi: 10.1002/(sici)1097-4598(199806)21:6<722::aid-mus3>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 106.Kanno M, Matsumoto M, Togashi H, Yoshioka M, Mano Y. Effects of repetitive transcranial magnetic stimulation on behavioral and neurochemical changes in rats during an elevated plus-maze test. J Neurol Sci. 2003;211:5–14. doi: 10.1016/s0022-510x(03)00030-3. [DOI] [PubMed] [Google Scholar]
- 107.Kanno M, Matsumoto M, Togashi H, Yoshioka M, Mano Y. Effects of acute repetitive transcranial magnetic stimulation on extracellular serotonin concentration in the rat prefrontal cortex. J Pharmacol Sci. 2003;93:451–457. doi: 10.1254/jphs.93.451. [DOI] [PubMed] [Google Scholar]
- 108.Kanno M, Matsumoto M, Togashi H, Yoshioka M, Mano Y. Effects of acute repetitive transcranial magnetic stimulation on dopamine release in the rat dorsolateral striatum. J Neurol Sci. 2004;217:73–81. doi: 10.1016/j.jns.2003.08.013. [DOI] [PubMed] [Google Scholar]
- 109.Keck ME, Engelmann M, Muller MB, Henniger MS, Hermann B, Rupprecht R, et al. Repetitive transcranial magnetic stimulation induces active coping strategies and attenuates the neuroendocrine stress response in rats. J Psychiatr Res. 2000;34:265–276. doi: 10.1016/s0022-3956(00)00028-5. [DOI] [PubMed] [Google Scholar]
- 110.Keck ME, Sillaber I, Ebner K, Welt T, Toschi N, Kaehler ST, et al. Acute transcranial magnetic stimulation of frontal brain regions selectively modulates the release of vasopressin, biogenic amines and amino acids in the rat brain. Eur J Neurosci. 2000;12:3713–3720. doi: 10.1046/j.1460-9568.2000.00243.x. [DOI] [PubMed] [Google Scholar]
- 111.Keck ME, Welt T, Post A, Muller MB, Toschi N, Wigger A, et al. Neuroendocrine and behavioral effects of repetitive transcranial magnetic stimulation in a psychopathological animal model are suggestive of antidepressant-like effects. Neuropsychopharmacology. 2001;24:337–349. doi: 10.1016/S0893-133X(00)00191-3. [DOI] [PubMed] [Google Scholar]
- 112.Kim EJ, Kim WR, Chi SE, Lee KH, Park EH, Chae JH, et al. Repetitive transcranial magnetic stimulation protects hippocampal plasticity in an animal model of depression. Neurosci Lett. 2006;405:79–83. doi: 10.1016/j.neulet.2006.06.023. [DOI] [PubMed] [Google Scholar]
- 113.Kling JW, Yarita M, Yamamoto T, Matsumiya Y. Memory for conditioned taste aversions is diminished by transcranial magnetic stimulation. Physiol Behav. 1990;48:713–717. doi: 10.1016/0031-9384(90)90216-q. [DOI] [PubMed] [Google Scholar]
- 114.Kraus KH, Welch JA, Levy WJ, Gugino VD, Wells MR. Partial regeneration of the sciatic nerve in rats enhances motor excitability to magnetic stimulation. Exp Neurol. 1997;143:18–24. doi: 10.1006/exnr.1996.6343. [DOI] [PubMed] [Google Scholar]
- 115.Kudo K, Yamada M, Takahashi K, Nishioka G, Tanaka S, Hashiguchi T, et al. Repetitive transcranial magnetic stimulation induces kf-1 expression in the rat brain. Life Sci. 2005;76:2421–2429. doi: 10.1016/j.lfs.2004.10.046. [DOI] [PubMed] [Google Scholar]
- 116.Levkovitz Y, Grisaru N, Segal M. Transcranial magnetic stimulation and antidepressive drugs share similar cellular effects in rat hippocampus. Neuropsychopharmacology. 2001;24:608–616. doi: 10.1016/S0893-133X(00)00244-X. [DOI] [PubMed] [Google Scholar]
- 117.Levkovitz Y, Marx J, Grisaru N, Segal M. Long-term effects of transcranial magnetic stimulation on hippocampal reactivity to afferent stimulation. J Neurosci. 1999;19:3198–3203. doi: 10.1523/JNEUROSCI.19-08-03198.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Levkovitz Y, Segal M. Aging affects transcranial magnetic modulation of hippocampal evoked potentials. Neurobiol Aging. 2001;22:255–263. doi: 10.1016/s0197-4580(00)00195-0. [DOI] [PubMed] [Google Scholar]
- 119.Li L, Yin Z, Huo X. The influence of low-frequency rtms on eeg of rats. Neurosci Lett. 2007;412:143–147. doi: 10.1016/j.neulet.2006.10.054. [DOI] [PubMed] [Google Scholar]
- 120.Li W, Yang Y, Ye Q, Yang B, Wang Z. Effect of chronic and acute low-frequency repetitive transcranial magnetic stimulation on spatial memory in rats. Brain Res Bull. 2007;71:493–500. doi: 10.1016/j.brainresbull.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 121.Linden RD, Zhang YP, Burke DA, Hunt MA, Harpring JE, Shields CB. Magnetic motor evoked potential monitoring in the rat. J Neurosurg. 1999;91:205–210. doi: 10.3171/spi.1999.91.2.0205. [DOI] [PubMed] [Google Scholar]
- 122.Loy DN, Magnuson DS, Zhang YP, Onifer SM, Mills MD, Cao QL, et al. Functional redundancy of ventral spinal locomotor pathways. J Neurosci. 2002;22:315–323. doi: 10.1523/JNEUROSCI.22-01-00315.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Loy DN, Talbott JF, Onifer SM, Mills MD, Burke DA, Dennison JB, et al. Both dorsal and ventral spinal cord pathways contribute to overground locomotion in the adult rat. Exp Neurol. 2002;177:575–580. doi: 10.1006/exnr.2002.7959. [DOI] [PubMed] [Google Scholar]
- 124.Magnuson DS, Trinder TC, Zhang YP, Burke D, Morassutti DJ, Shields CB. Comparing deficits following excitotoxic and contusion injuries in the thoracic and lumbar spinal cord of the adult rat. Exp Neurol. 1999;156:191–204. doi: 10.1006/exnr.1999.7016. [DOI] [PubMed] [Google Scholar]
- 125.Matsumiya Y, Yamamoto T, Yarita M, Miyauchi S, Kling JW. Physical and physiological specification of magnetic pulse stimuli that produce cortical damage in rats. J Clin Neurophysiol. 1992;9:278–287. doi: 10.1097/00004691-199204010-00008. [DOI] [PubMed] [Google Scholar]
- 126.Michaluk J, Antkiewicz-Michaluk L, Vetulani J. Conditions of application of repeated transcranial magnetic stimulation to rats may mask the effects of the treatment. Pol J Pharmacol. 2001;53:685–687. [PubMed] [Google Scholar]
- 127.Mix A, Benali A, Eysel UT, Funke K. Continuous and intermittent transcranial magnetic theta burst stimulation modify tactile learning performance and cortical protein expression in the rat differently. Eur J Neurosci. 2010;32:1575–1586. doi: 10.1111/j.1460-9568.2010.07425.x. [DOI] [PubMed] [Google Scholar]
- 128.Muller PA, Pascual-Leone A, Rotenberg A. Safety and tolerability of repetitive transcranial magnetic stimulation in patients with pathologic positive sensory phenomena: A review of literature. 2011 doi: 10.1016/j.brs.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ogiue-Ikeda M, Kawato S, Ueno S. The effect of repetitive transcranial magnetic stimulation on long-term potentiation in rat hippocampus depends on stimulus intensity. Brain Res. 2003;993:222–226. doi: 10.1016/j.brainres.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 130.Ogiue-Ikeda M, Kawato S, Ueno S. Acquisition of ischemic tolerance by repetitive transcranial magnetic stimulation in the rat hippocampus. Brain Res. 2005;1037:7–11. doi: 10.1016/j.brainres.2004.10.063. [DOI] [PubMed] [Google Scholar]
- 131.Okada K, Matsunaga K, Yuhi T, Kuroda E, Yamashita U, Tsuji S. The long-term high-frequency repetitive transcranial magnetic stimulation does not induce mrna expression of inflammatory mediators in the rat central nervous system. Brain Res. 2002;957:37–41. doi: 10.1016/s0006-8993(02)03582-5. [DOI] [PubMed] [Google Scholar]
- 132.Post A, Muller MB, Engelmann M, Keck ME. Repetitive transcranial magnetic stimulation in rats: Evidence for a neuroprotective effect in vitro and in vivo. Eur J Neurosci. 1999;11:3247–3254. doi: 10.1046/j.1460-9568.1999.00747.x. [DOI] [PubMed] [Google Scholar]
- 133.Ravnborg M, Knudsen GM, Blinkenberg M. No effect of pulsed magnetic stimulation on the blood-brain barrier in rats. Neuroscience. 1990;38:277–280. doi: 10.1016/0306-4522(90)90392-h. [DOI] [PubMed] [Google Scholar]
- 134.Roman A, Vetulani J, Nalepa I. Effect of combined treatment with paroxetine and transcranial magnetic stimulation (tms) on the mitogen-induced proliferative response of rat lymphocytes. Pol J Pharmacol. 2002;54:633–639. [PubMed] [Google Scholar]
- 135.Sachdev PS, McBride R, Loo C, Mitchell PM, Malhi GS, Croker V. Effects of different frequencies of transcranial magnetic stimulation (tms) on the forced swim test model of depression in rats. Biol Psychiatry. 2002;51:474–479. doi: 10.1016/s0006-3223(01)01298-7. [DOI] [PubMed] [Google Scholar]
- 136.Shaldivin A, Kaptsan A, Belmaker RH, Einat H, Grisaru N. Transcranial magnetic stimulation in an amphetamine hyperactivity model of mania. Bipolar Disord. 2001;3:30–34. doi: 10.1034/j.1399-5618.2001.030104.x. [DOI] [PubMed] [Google Scholar]
- 137.Shin HI, Han TR, Paik NJ. Effect of consecutive application of paired associative stimulation on motor recovery in a rat stroke model: A preliminary study. Int J Neurosci. 2008;118:807–820. doi: 10.1080/00207450601123480. [DOI] [PubMed] [Google Scholar]
- 138.Tasset I, Drucker-Colin R, Pena J, Jimena I, Montilla P, Medina FJ, et al. Antioxidant-like effects and protective action of transcranial magnetic stimulation in depression caused by olfactory bulbectomy. Neurochem Res. 2010;35:1182–1187. doi: 10.1007/s11064-010-0172-9. [DOI] [PubMed] [Google Scholar]
- 139.Trippe J, Mix A, Aydin-Abidin S, Funke K, Benali A. Theta burst and conventional low-frequency rtms differentially affect gabaergic neurotransmission in the rat cortex. Exp Brain Res. 2009;199:411–421. doi: 10.1007/s00221-009-1961-8. [DOI] [PubMed] [Google Scholar]
- 140.Tsutsumi T, Fujiki M, Akiyoshi J, Horinouchi Y, Isogawa K, Hori S, et al. Effect of repetitive transcranial magnetic stimulation on forced swimming test. Prog Neuropsychopharmacol Biol Psychiatry. 2002;26:107–111. doi: 10.1016/s0278-5846(01)00227-5. [DOI] [PubMed] [Google Scholar]
- 141.Tunez I, Drucker-Colin R, Jimena I, Medina FJ, Munoz Mdel C, Pena J, et al. Transcranial magnetic stimulation attenuates cell loss and oxidative damage in the striatum induced in the 3-nitropropionic model of huntington's disease. J Neurochem. 2006;97:619–630. doi: 10.1111/j.1471-4159.2006.03724.x. [DOI] [PubMed] [Google Scholar]
- 142.Ueyama E, Ukai S, Ogawa A, Yamamoto M, Kawaguchi S, Ishii R, et al. Chronic repetitive transcranial magnetic stimulation increases hippocampal neurogenesis in rats. Psychiatry Clin Neurosci. 2011;65:77–81. doi: 10.1111/j.1440-1819.2010.02170.x. [DOI] [PubMed] [Google Scholar]
- 143.Vetulani J, Roman A, Kowalska M, Nalepa I. Paroxetine pretreatment does not change the effects induced in the rat cortical beta-adrenergic receptor system by repetitive transcranial magnetic stimulation and electroconvulsive shock. Int J Neuropsychopharmacol. 2010;13:737–746. doi: 10.1017/S1461145709990459. [DOI] [PubMed] [Google Scholar]
- 144.Vieyra-Reyes P, Mineur YS, Picciotto MR, Tunez I, Vidaltamayo R, Drucker-Colin R. Antidepressant-like effects of nicotine and transcranial magnetic stimulation in the olfactory bulbectomy rat model of depression. Brain Res Bull. 2008;77:13–18. doi: 10.1016/j.brainresbull.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Wang HY, Crupi D, Liu J, Stucky A, Cruciata G, Di Rocco A, et al. Repetitive transcranial magnetic stimulation enhances bdnf-trkb signaling in both brain and lymphocyte. J Neurosci. 2011;31:11044–11054. doi: 10.1523/JNEUROSCI.2125-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Yang X, Song L, Liu Z. The effect of repetitive transcranial magnetic stimulation on a model rat of parkinson's disease. Neuroreport. 2010;21:268–272. doi: 10.1097/WNR.0b013e328335b411. [DOI] [PubMed] [Google Scholar]
- 147.Yang Y, Li W, Zhu B, Liu Y, Yang B, Wang H, et al. Sex differences in antidepressant-like effect of chronic repetitive transcranial magnetic stimulation in rats. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:735–740. doi: 10.1016/j.pnpbp.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 148.Yue L, Xiao-Lin H, Tao S. The effects of chronic repetitive transcranial magnetic stimulation on glutamate and gamma-aminobutyric acid in rat brain. Brain Res. 2009 doi: 10.1016/j.brainres.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 149.Zhang YP, Burke DA, Shields LB, Chekmenev SY, Dincman T, Zhang Y, et al. Spinal cord contusion based on precise vertebral stabilization and tissue displacement measured by combined assessment to discriminate small functional differences. J Neurotrauma. 2008;25:1227–1240. doi: 10.1089/neu.2007.0388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Zyss T, Gorka Z, Kowalska M, Vetulani J. Preliminary comparison of behavioral and biochemical effects of chronic transcranial magnetic stimulation and electroconvulsive shock in the rat. Biol Psychiatry. 1997;42:920–924. doi: 10.1016/S0006-3223(96)00518-5. [DOI] [PubMed] [Google Scholar]
- 151.Machii K, Cohen D, Ramos-Estebanez C, Pascual-Leone A. Safety of rtms to non-motor cortical areas in healthy participants and patients. Clin Neurophysiol. 2006;117:455–471. doi: 10.1016/j.clinph.2005.10.014. [DOI] [PubMed] [Google Scholar]
- 152.Rossi S, Hallett M, Rossini PM, Pascual-Leone A. Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin Neurophysiol. 2009;120:2008–2039. doi: 10.1016/j.clinph.2009.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.George MS, Lisanby SH, Avery D, McDonald WM, Durkalski V, Pavlicova M, et al. Daily left prefrontal transcranial magnetic stimulation therapy for major depressive disorder: A sham-controlled randomized trial. Arch Gen Psychiatry. 2010;67:507–516. doi: 10.1001/archgenpsychiatry.2010.46. [DOI] [PubMed] [Google Scholar]
- 154.Janicak PG, O'Reardon JP, Sampson SM, Husain MM, Lisanby SH, Rado JT, et al. Transcranial magnetic stimulation in the treatment of major depressive disorder: A comprehensive summary of safety experience from acute exposure, extended exposure, and during reintroduction treatment. J Clin Psychiatry. 2008;69:222–232. doi: 10.4088/jcp.v69n0208. [DOI] [PubMed] [Google Scholar]
- 155.O'Reardon JP, Solvason HB, Janicak PG, Sampson S, Isenberg KE, Nahas Z, et al. Efficacy and safety of transcranial magnetic stimulation in the acute treatment of major depression: A multisite randomized controlled trial. Biol Psychiatry. 2007;62:1208–1216. doi: 10.1016/j.biopsych.2007.01.018. [DOI] [PubMed] [Google Scholar]
- 156.Nishikiori O. studies on transcranial magnetic stimulation--part 2. Pathological findings of rabbit brain after long-term stimulation based on characteristics of intracerebral induced voltage. Nihon Ganka Gakkai Zasshi. 1996;100:489–495. [PubMed] [Google Scholar]
- 157.Silvanto J, Pascual-Leone A. State-dependency of transcranial magnetic stimulation. Brain Topogr. 2008;21:1–10. doi: 10.1007/s10548-008-0067-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table.S1 organizes retrieved articles that utilized a rTMS protocol.
Table.S2 organizes retrieved articles that utilized a single- or paired-pulse TMS protocol.
Table.S3 organizes retrieved articles that utilized a single- or paired-pulse TMS protocol as well as a rTMS protocol.