Abstract
Background
Since 2010, there has been an increase in serogroup W Neisseria meningitidis (MenW) disease in many countries due to an emerging sequence type-11 clonal complex (ST-11 CC). In 2016, a small increase in MenW disease due to the ST-11 CC was documented in Ontario, Canada.
Objective
To examine the trends in MenW disease in Canada and to assess whether there have been changes in the type of ST clonal complex causing MenW disease between 2009 to 2016.
Methods
Invasive N. meningitidis isolates routinely submitted from across the country to the National Microbiology Laboratory were analyzed. The proportional distribution of MenW compared with other serogroups was calculated. The MenW isolates were then further characterized by serotype, serosubtype and ST clonal complex. The geographic distribution of the emerging ST-11 CC was documented and the age of patients with ST-11 CC was compared with the traditional ST-22 CC.
Results
Of the 888 invasive isolates examined, 63 were MenW giving an average annual rate of 7.1%. However, the percentage of MenW varied from 2.7% in 2012 to 18.8% in 2016. From 2009 to 2013, 91% of the MenW were typed as the traditional ST-22 CC while from 2014 to 2016, 75% were typed to be the emerging ST-11 CC. ST-11 MenW CC was documented in five provinces across Canada (British Columbia, Alberta, Manitoba, Ontario and Quebec). The median age of patients infected with the emerging ST-11 MenW CC was 53.5 years, while for patients with the traditional ST-22 CC it was 23.5 years.
Conclusion
MenW meningococcal disease is growing in prevalence in Canada and is associated with an increase in the emerging ST-11 CC. This emerging clonal complex has now been identified in five provinces in Canada. It appears to be more common in older patients than the traditional ST-22 CC, which occurs more often in younger patients.
Introduction
Invasive meningococcal disease (IMD) has been a notifiable disease in Canada since 1924 (1). It is caused by Neisseria meningitidis, which normally resides in the upper respiratory tract of healthy carriers. For reasons not completely understood, N. meningitidis may invade the blood stream and cause serious systemic infection leading to meningitis, septicemia, septic arthritis, bacteremic pneumonia and pericarditis (2). Initial clinical presentation of IMD can be nonspecific but it may progress rapidly, leading to septic shock. The disease has an average case-fatality rate of 10% (3).
N. meningitidis is classified into 12 serogroups based on antigenic specificities of their polysaccharide capsules. Most invasive diseases are caused by six serogroups: A (MenA), B (MenB), C (MenC), W (MenW), X (MenX) and Y (MenY). These invasive strains belong to a handful of genetic lineages known as hypervirulent clones, such as sequence type (ST)-32[electrophoretic type (ET)-5], ST-41/44 (lineage 3), ST-11 (ET-37), ST-8 (cluster A4), ST-5 (subgroup III) and ST-269 clonal complexes (CCs) (4), (5).
The strains causing IMD have been described as “shifting sands”, with unique strains emerging with the potential to spread regionally and internationally. For example, MenA of subgroup III caused epidemics in China in the 1960s and subsequently spread to Russia and then globally (6). ET-5 MenB caused an intercontinental outbreak with a wide geographic spread that lasted for over a decade (7). The ET-15 MenC clone first emerged in Canada in the mid-1980s and led to worldwide dissemination, which ultimately led to the introduction of MenC conjugate vaccine programs in many countries. Other notable MenB clones that have caused epidemics include cluster A4 and lineage 3 (5).
The first report of MenW causing a major outbreak or epidemic occurred in 2000; this outbreak started in Saudi Arabia during the Hajj and involved more than 400 cases. The strain was characterized as ST-11 CC (8). With pilgrims returning to their countries in Africa, Asia, Europe, North America and South America, this strain was disseminated globally. The gradual increase in MenW disease in recent years was first reported in sub-Saharan Africa (9) and South America (10), (11). Since 2010, other countries have reported an increase in IMD caused by ST-11 MenW (12), (13), (14), (15).
In December 2016, Tsang et al. (16) reported an increase in invasive MenW strains in Ontario, Canada. This increase started in 2014 and was associated with a replacement of the traditional ST-22 CC with the ST-11 CC (16). There was also a small increase in the number of MenW IMD cases in that province. To determine if clonal replacement had occurred in MenW disease nationally, this study examines the trends in MenW disease and changes in clonal complex in Canada between 2009 and 2016.
Methods
Provincial public health laboratories receive case isolates from hospitals and clinical diagnostic laboratories for identification and serogroup typing. As part of the enhanced surveillance program on IMD, all provinces and territories in Canada routinely submit all their invasive N. meningitidis isolates from culture-confirmed cases to the National Microbiology Laboratory (NML) for serogroup confirmation and additional strain characterization (17). This study included all N. meningitidis isolates obtained from culture-confirmed IMD cases submitted to the NML between 2009 and 2016.
Typing of meningococci
At the NML, serogrouping is done by slide agglutination using rabbit anti-grouping antisera produced in-house and/or polymerase chain reaction (PCR) (18). Serotyping and serosubtyping is done by whole-cell enzyme-linked immunosorbent assay (ELISA) using monoclonal antibodies (19). PorA genotyping and multilocus sequence typing were conducted according to previously described standard methods (20), (21).
Geographic distribution, source and patient characteristics
Based on the requisition information provided by the provincial public health laboratories, the NML collects and analyzes information on the geographic origin of the specimens, the source (e.g. blood, cerebral spinal, pericardial or intra-articular fluid) and the age and sex of patients from whom the specimens were drawn.
Results
Trends in MenW meningococcal disease
In Canada, between 2009 and 2016, a total of 888 N. meningitidis isolates were recovered from individual IMD cases and sent to the NML. Of these, 63 were grouped as MenW. The percentage of MenW isolates varied by year from a low of 2.7% in 2012 to a high of 18.8% in 2016 (Table 1), for an average percentage of 7.1%.
Table 1. Contribution of Neisseria meningitidis serogroup W (MenW) in culture-positive invasive meningococcal disease cases, Canada 2009–2016.
| Year | Number of MenW case isolates | Total number of IMD case isolates | MenW case isolates as a percentage of IMD case isolates |
|---|---|---|---|
| 2009 | 12 | 168 | 7.1% |
| 2010 | 6 | 118 | 5.1% |
| 2011 | 10 | 133 | 7.5% |
| 2012 | 3 | 112 | 2.7% |
| 2013 | 5 | 103 | 4.9% |
| 2014 | 6 | 86 | 7.0% |
| 2015 | 6 | 88 | 6.8% |
| 2016 | 15 | 80 | 18.8% |
| All years | 63 | 888 | 7.1% |
Abbreviations: IMD, invasive meningococcal disease; MenW, Neisseria meningitidis serogroup W
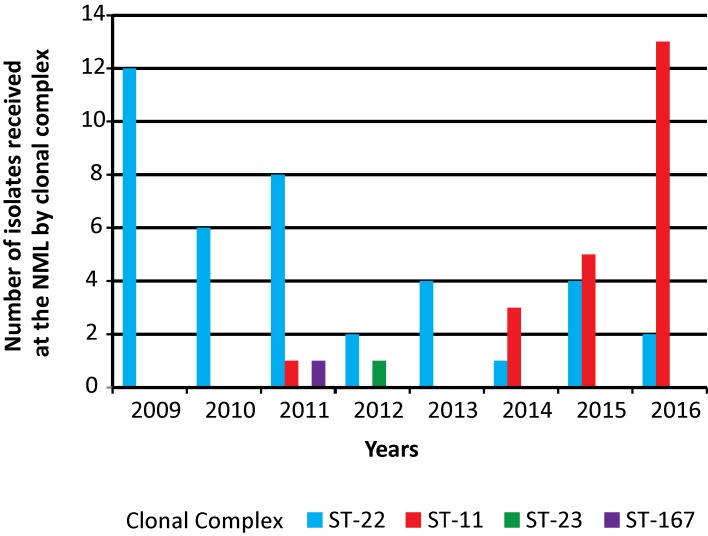
Changes in clonal complex
The increase in the number of MenW isolates from IMD cases coincided with the identification of the ST-11 (ET-37) CC (Figure 1). From 2009 to 2013, 91% (32 out of 35) MenW isolates were typed as the traditional ST-22 CCs whereas from 2014 to 2016, only 25% (7 out of 28) MenW isolates belonged to this clonal complex. Over this same period, the emerging ST-11 CC increased from 3% (1 out of 35 isolates) to 75% (21 out of 28 isolates).
Figure 1. Clonal analysis of invasive Neisseria meningitidis serogroup W (MenW) in Canada, 2009–2016.
Abbreviations: CC, Clonal complex; ST, sequence type
Antigenic and genetic characterization
Almost 70% (27 out of 39) of the traditional ST-22 CC MenW isolates were typed as either W:NT (non-serotypeable):P1.6 (n=19) or W:NT:P1.– (non-serosubtypeable; n=8) with the PorA genotype of P1.18-1,3,38. Fiveother ST-22 MenW were typed as W:NT:P1.– with a deletion in their PorA genes. The other seven ST-22 CC MenW were non-serotypeable with serosubtype antigens of either P1.5 (n=1), P1.5,2 (n=1), P1.14 (n=2), P1.16 (n=2) or P1.– (n=1). Twelve different sequence types were identified among these 39 ST-22 CC MenW, with 15 isolates belonging to ST-184, 11 to ST-22, three to ST-8974 and two to ST-1617. The remaining eight isolates each belonged to a different sequence type (ST-1221, ST-1224, ST-1476, ST-2625, ST-3137, ST-3849, ST-8230 and ST-10188).
In contrast, 100% (all 22) of the ST-11 CC MenW isolates were typed as C:2a:P1.5,2 (n=21) or C:2a:P1.2 (n=1), with the PorA genotype of P1.5,2,36-2. Twenty-one belonged to ST-11 and one to ST-10826. There was also one MenW isolate that typed as ST-23 (ST-23 CC) and one isolate typed as ST-3705 (ST-167 CC). The former had the antigenic formula of W:19:P1.– with PorA genotype of P1.5-2,10-1,36-2 while the later was W:19:P1.5 with PorA genotype of P1.5-1,10-4,36-2.
Geographic, demographic and source data
MenW isolates of the ST-11 CC were found in British Columbia (n=5), Alberta (n=3), Manitoba (n=2), Ontario (n=8) and Quebec (n=4). The sex and age distribution of cases due to ST-22 and ST-11 CCs are shown in Table 2. For the traditional ST-22 CC MenW cases, the mean and median ages were 31.7 years and 23.5 years, respectively. The mean and median ages of patients with emerging ST-11 CC MenW were 47.9 years and 53.5 years, respectively. Eight patients under 2 years old were identified with ST-22 CC MenW; no one in this age group was diagnosed with ST-11 CC MenW. Of the ST-22 CC MenW isolates, 33 were from blood cultures, three from cerebral spinal fluid cultures, two from articular fluids and one from the pericardial fluid. Of the 22 isolates of ST-11 CC MenW from across Canada, 21 were from blood cultures and only one from cerebral spinal fluid culture.
Table 2. Demographic characteristics and specimen source for invasive Neisseria meningitidis serogroup W (MenW) cases according to clonal complex in Canada, 2009–2016.
| Demographics and specimen source | ST-22 CC isolates n (%) |
ST-11 CC isolates n (%) |
|---|---|---|
| Sex | ||
| Male | 20 (51%) | 13 (59%) |
| Female | 19 (49%) | 9 (41%) |
| Age | ||
| < 12 months | 4 (10%) | 0 |
| 12–23 months | 4 (10%) | 0 |
| 2–5 years | 6 (15%) | 2 (10%) |
| 6–10 years | 1 (3%) | 0 |
| 11–20 years | 5 (13%) | 4 (18%) |
| 21–40 years | 5 (13%) | 2 (10%) |
| 41–60 years | 5 (13%) | 7 (31%) |
| > 60 years | 8 (21%) | 7 (31%) |
| Unknown | 1 (3%) | 0 |
| Specimen source | ||
| Blood | 33 (84.6%) | 21(95%) |
| Cerebral spinal fluid | 3 (7.7%) | 1 (5%) |
| Others1 | 3 (7.7%) | 0 |
| Total number of samples | 39 (100%) | 22 (100%) |
Abbreviations: CC, clonal complex; n, number; ST, sequence type 1 Shoulder, pericardial or articular fluid
Discussion
From 2009 to 2016, while the overall number of IMD cases in Canada decreased, the percentage of cases due to MenW increased from 2.7% in 2012 to 18.8% in 2016. This increase in MenW disease was associated with a clonal shift in the MenW strain from ST-22 CC to ST-11 CC. This emerging ST-11 CC MenW clone has now been documented in five provinces. It tends to occur in middle-aged and older adults.
ST-11 CC is a long-established hypervirulent clonal complex that was first identified in 1917 in a serogroup B strain (5). In the 1960s and 1970s, ST-11 CC was associated with MenB in North America and Europe (5). In the mid-1980s, a genetic variant of ST-11 CC that caused IMD in teenagers in Ontario appeared in a MenC clone designated as ET-15 (22). Like others in the ST-11 CC, this ET-15 clone spread rapidly in North America and eventually globally (23). The MenC conjugate vaccine was first included in the publically funded childhood immunization program in the United Kingdom, in 1999 (24). In Canada, the MenC conjugate vaccine was licensed in April 2001. Beginning in 2002, some provinces started routine MenC conjugate vaccine immunisation programs and by 2007 all provinces and territories have implemented such programs (25).
The first major ST-11 CC MenW outbreak occurred during the Hajj pilgrimage in 2000. The pilgrims returning to their countries initiated the global dissemination of this clone (26). In England and Wales, the increase in the ST-11 MenW clone first became apparent in 2009/2010 (27) and its prevalence increased yearly until 2015 when a targeted vaccination program was introduced (28). A similar increase in MenW disease as a result of the same clone has been seen in Australia since 2013 (29), also leading to the introduction of a targeted vaccination program (30). The ST-11 CC MenW has now been documented in a number of other countries around the world (9), (10), (11), (12), (13), (14), (15).
The Canadian ST-11 CC MenW isolates have serotype antigen 2a and serosubtype antigen P1.5,2, typical of isolates of this clonal complex (31). They also differ antigenically from meningococci of the ST-22 CC. Currently there is no evidence to suggest that these ST-11 MenW arose by capsule switching from MenC ST-11 strain. Investigations into the MenB ST-11 that arose from MenC ST-11 by capsule switching suggest that these capsule-switched strains may not be stable for endemic spread (32). Rather, the increase in MenW ST-11 isolates in Canada and elsewhere is likely due to clonal expansion of an endemic strain (25), (26).
This study has two limitations. First, it included only bacteriologic culture-confirmed cases and not those confirmed using PCR. However, only about 10% of the IMD cases confirmed in Canada between 2006 and 2011 were diagnosed by PCR (17) and there is no evidence to suggest that PCR-diagnosed cases differ from culture-confirmed cases. Second, this study does not include data on the meningococcal vaccination history of patients with MenW. The quadrivalent meningococcal A, C, W and Y conjugate vaccine has protective immunity against MenW, but determining if any of the patients had been vaccinated prior to their illness was not possible.
Some provinces in Canada have quadrivalent meningococcal A, C, W and Y conjugate vaccine programs targeting primary or high school students (33). The protective immunity offered by this quadrivalent vaccine in the student population may have moderated the effect of the expansion of the ST-11 MenW clone in Canada. Of note, fewer MenW cases have been identified in Canada than in the United Kingdom or Australia, where a vaccine program was more recently introduced (28), (30).
It is important to note that IMD due to MenW ST-11 CC may have an atypical clinical presentation. In England, for example, a review of MenW cases in teenagers (aged 15 to 19 years) found that 7 out of 15 patients initially presented with acute gastrointestinal symptoms of nausea, vomiting and diarrhea; four were sent home from hospital, delaying the diagnosis (34). In another study of 129 MenW cases in England and Wales from 2010 to 2013, half of which were diagnosed in patients aged 45 years or above, 23% were with atypical clinical presentations of pneumonia (12%), septic arthritis (7%) and epiglottitis or supraglottitis (4%) (26). The unusual initial clinical symptoms may have implications in the early diagnosis of the disease. Timely diagnosis of IMD is important for patient treatment, contact tracing and public health control of the disease. Ongoing surveillance of these trends is indicated.
Conclusion
In summary, the traditional endemic MenW ST-22 CC has been replaced by an abrupt emergence of MenW ST-11 CC in five provinces in Canada. Although the overall number of MenW cases in Canada remains small, MenW is responsible for 19% of all IMD cases. Of note for clinicians and public health professionals, this ST-11 MenW clone has the potential to cause outbreaks, has occurred in an older age group in Canada and may have an atypical clinical presentation. The NML will continue its surveillance program on this disease including laboratory characterization of strains.
Authors’ statement
All authors (RSWT, LH, GJT, GH, PVC, FJ, BL, DH, RRG, GJG, and GZ) are involved in the surveillance of invasive meningococcal disease in Canada. RSWT prepared the first draft and all authors contributed to the final version with comments and suggestions.
Acknowledgements
We thank the staff at the provincial public health laboratories for identifying and sending N. meningitidis isolates to the NML. We also thank Dennis Law, Jianwei Zhou, and Saul Deng for providing laboratory assistance in the analysis of strains, and the NML’s DNA Core Service for providing assistance in nucleotide sequencing. The authors made use of the Neisseria Multi Locus Sequence Typing website developed by Keith Jolley and sited at the University of Oxford (BMC Bioinformatics). The development of this site was funded by the Wellcome Trust and the European Union.
Conflict of interest: None.
Funding: Laboratory surveillance of invasive meningococcal disease is funded by the Public Health Agency of Canada.
References
- 1.Varughese PV, Carter AO. Meningococcal disease in Canada. Surveillance summary to 1987. Can Dis Wkly Rep 1989. Apr;15(17):89–96. [PubMed] [Google Scholar]
- 2.Apicella MA. Neisseria meningitidis. In: Mandell G, Bennett JE, Dolin R, editors. Mandell, Douglas, and Bennett’s principles and practice of infectious diseases, 7th edition, Philadelphia (PA): Churchill Livingstone Elsevier; 2009. p. 2737-52. [Google Scholar]
- 3.Rosenstein NE, Perkins BA, Stephens DS, Popovic T, Hughes JM. Meningococcal disease. N Engl J Med 2001. May;344(18):1378–88. . 10.1056/NEJM200105033441807 [DOI] [PubMed] [Google Scholar]
- 4.Harrison LH, Trotter CL, Ramsay ME. Global epidemiology of meningococcal disease. Vaccine 2009. Jun;27 Suppl 2:B51–63. . 10.1016/j.vaccine.2009.04.063 [DOI] [PubMed] [Google Scholar]
- 5.Caugant DA. Population genetics and molecular epidemiology of Neisseria meningitidis. APMIS 1998. May;106(5):505–25. . 10.1111/j.1699-0463.1998.tb01379.x [DOI] [PubMed] [Google Scholar]
- 6.Olyhoek T, Crowe BA, Achtman M. Clonal population structure of Neisseria meningitidis serogroup A isolated from epidemics and pandemics between 1915 and 1983. Rev Infect Dis 1987. Jul-Aug;9(4):665–92. . 10.1093/clinids/9.4.665 [DOI] [PubMed] [Google Scholar]
- 7.Caugant DA, Frøholm LO, Bøvre K, Holten E, Frasch CE, Mocca LF et al. Intercontinental spread of a genetically distinctive complex of clones of Neisseria meningitidis causing epidemic disease. Proc Natl Acad Sci USA 1986. Jul;83(13):4927–31. . 10.1073/pnas.83.13.4927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Taha MK, Achtman M, Alonso JM, Greenwood B, Ramsay M, Fox A et al. Serogroup W135 meningococcal disease in Hajj pilgrims. Lancet 2000. Dec;356(9248):2159. . 10.1016/S0140-6736(00)03502-9 [DOI] [PubMed] [Google Scholar]
- 9.Parent du Châtelet I, Traore Y, Gessner BD, Antignac A, Naccro B, Njanpop-Lafourcade BM et al. Bacterial meningitis in Burkina Faso: surveillance using field-based polymerase chain reaction testing. Clin Infect Dis 2005. Jan;40(1):17–25. . 10.1086/426436 [DOI] [PubMed] [Google Scholar]
- 10.Weidlich L, Baethgen LF, Mayer LW, Moraes C, Klein CC, Nunes LS et al. High prevalence of Neisseria meningitidis hypervirulent lineages and emergence of W135:P1.5,2:ST-11 clone in Southern Brazil. J Infect 2008. Oct;57(4):324–31. . 10.1016/j.jinf.2008.07.014 [DOI] [PubMed] [Google Scholar]
- 11.Efron AM, Sorhouet C, Salcedo C, Abad R, Regueira M, Vázquez JA. W135 invasive meningococcal strains spreading in South America: significant increase in incidence rate in Argentina. J Clin Microbiol 2009. Jun;47(6):1979–80. . 10.1128/JCM.02390-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Collard JM, Maman Z, Yacouba H, Djibo S, Nicolas P, Jusot JF et al. Increase in Neisseria meningitidis serogroup W135, Niger, 2010. Emerg Infect Dis 2010. Sep;16(9):1496–8. . 10.3201/eid1609.100510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hossain MJ, Roca A, Mackenzie GA, Jasseh M, Hossain MI, Muhammad S et al. Serogroup W135 meningococcal disease, The Gambia, 2012. Emerg Infect Dis 2013;19(9):1507–10. . 10.3201/eid1909.130077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou H, Liu W, Xu L, Deng L, Deng Q, Zhuo J et al. Spread of Neisseria meningitidis serogroup W clone, China. Emerg Infect Dis 2013;19(9):1496–9. . 10.3201/eid1909.130160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hu S, Zhang W, Li F, Hu Z, Ma E, Zheng T et al. Neisseria meningitidis serogroup W135 sequence type 11, Anhui Province, China, 2011-2013. Emerg Infect Dis 2014. Jul;20(7):1236–8. . 10.3201/eid2007.131138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tsang RS, Deeks SL, Wong K, Marchand-Austin A, Jamieson FB. Invasive serogroup W Neisseria meningitides (MenW) in Ontario, Canada shows potential clonal replacement during the period January 1, 2009 to June 30, 2016. Can Commun Dis Rep 2016;42(12):263–6. Available from: http://www.phac-aspc.gc.ca/publicat/ccdr-rmtc/16vol42/dr-rm42-12/ar-06-eng.php [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li YA, Tsang R, Desai S, Deehan H. Enhanced surveillance of invasive meningococcal disease in Canada, 2006-2011. Can Commun Dis Rep 2014;40(9):160–9. Available from: http://www.phac-aspc.gc.ca/publicat/ccdr-rmtc/14vol40/dr-rm40-09/dr-rm40-09-surv-eng.php [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tsang R, Taha MK. Diagnosis of meningococcal disease. In: Feavers I, Pollard AJ, Sadaranghi M, editors. Handbook of meningococcal disease management. Cham (CH) Springer International Publishing Switzerland; 2016. p. 45-55. [Google Scholar]
- 19.Abdillahi H, Poolman JT. Whole cell ELISA for typing Neisseria meningitidis with monoclonal antibodies. FEMS Microbiol Lett 1987;48(3):367–71. . 10.1111/j.1574-6968.1987.tb02626.x [DOI] [PubMed] [Google Scholar]
- 20.Jamieson FB, Rawte P, Deeks SL, Zhou J, Law DK, Deng S et al. Genetic and antigenic characterization of invasive endemic serogroup B Neisseria meningitidis from Ontario, Canada, in 2001-2010 [Internet]. J Med Microbiol 2013. Jan;62(Pt 1):46–55. . 10.1099/jmm.0.050369-0 [DOI] [PubMed] [Google Scholar]
- 21.Zhou J, Lefebvre B, Deng S, Gilca R, Deceuninck G, Law DK et al. Invasive serogroup B Neisseria meningitidis in Quebec, Canada, 2003 to 2010: persistence of the ST-269 clone since it first emerged in 2003. J Clin Microbiol 2012. May;50(5):1545–51. . 10.1128/JCM.06835-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ashton FE, Ryan JA, Borczyk A, Caugant DA, Mancino L, Huang D. Emergence of a virulent clone of Neisseria meningitidis serotype 2a that is associated with meningococcal group C disease in Canada [Internet]. J Clin Microbiol 1991. Nov;29(11):2489–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jelfs J, Munro R, Ashto FE, Caugant DA. Genetic characterization of a new variant within the ET-37 complex of Neisseria meningitidis associated with outbreaks in various parts of the world [Internet]. Epidemiol Infect 2000. Oct;125(2):285–98. . 10.1017/S0950268899004471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miller E, Salisbury D, Ramsay M. Planning, registration, and implementation of an immunisation campaign against meningococcal serogroup C disease in the UK: a success story. Vaccine 2001. Oct;20 Suppl 1:S58–67. . 10.1016/S0264-410X(01)00299-7 [DOI] [PubMed] [Google Scholar]
- 25.National Advisory Committee on Immunization (NACI). An update on the invasive meningococcal disease and meningococcal vaccine conjugate recommendations. An Advisory Committee Statement (ACS). Can Commun Dis Rep 2009. Apr;35 ACS-3:1–40. [PubMed] [Google Scholar]
- 26.Mayer LW, Reeves MW, Al-Hamdan N, Sacchi CT, Taha MK, Ajello GW et al. Outbreak of W135 meningococcal disease in 2000: not emergence of a new W135 strain but clonal expansion within the electophoretic type-37 complex. J Infect Dis 2002. Jun;185(11):1596–605. . 10.1086/340414 [DOI] [PubMed] [Google Scholar]
- 27.Ladhani SN, Beebeejaun K, Lucidarme J, Campbell H, Gray S, Kaczmarski E et al. Increase in endemic Neisseria meningitidis capsular group W sequence type 11 complex associated with severe invasive disease in England and Wales. Clin Infect Dis 2015. Feb;60(4):578–85. . 10.1093/cid/ciu881 [DOI] [PubMed] [Google Scholar]
- 28.Campbell H, Saliba V, Borrow R, Ramsay M, Ladhani SN. Targeted vaccination of teenagers following continued rapid endemic expansion of a single meningococcal group W clone (sequence type 11 clonal complex), United Kingdom 2015. Euro Surveill 2015. Jul;20(28):21188. . 10.2807/1560-7917.ES2015.20.28.21188 [DOI] [PubMed] [Google Scholar]
- 29.Martin NV, Ong KS, Howden BP, Lahra MM, Lambert SB, Beard FH et al. ; Communicable Diseases Network Australia MenW Working Group. Rise in invasive serogroup W meningococcal disease in Australia 2013-2015. Commun Dis Intell Q Rep 2016. Dec;40(4):E454–9. [PubMed] [Google Scholar]
- 30.Government of Western Australia. Media Statement. Targeted campaign against meningococcal W [Internet]. 2016 Dec 8 [cited 2017 May 1]. Available from: https://www.mediastatements.wa.gov.au/Pages/Barnett/2016/12/Targeted-campaign-against-meningococcal-W.aspx
- 31.Wang JF, Caugant DA, Morelli G, Koumaré B, Achtman M. Antigenic and epidemiologic properties of the ET-37 complex of Neisseria meningitidis. J Infect Dis 1993. Jun;167(6):1320–9. . 10.1093/infdis/167.6.1320 [DOI] [PubMed] [Google Scholar]
- 32.Tyler S, Tsang R. Genetic analysis of Canadian isolates of C:2a:P1.2,5 and B:2a:P1.2,5 Neisseria meningitidis strains belonging to the hypervirulent clone of ET-15. Can J Microbiol 2004. Jun;50(6):433–43. . 10.1139/w04-024 [DOI] [PubMed] [Google Scholar]
- 33.Public Health Agency of Canada. Canada's provincial and territorial routine (and catch-up) vaccination programs for infants and children [Internet]. Ottawa (ON): Government of Canada; [modified: 2017 Apr 3; cited 2017 May 1]. Available from: https://www.canada.ca/en/public-health/services/provincial-territorial-immunization-information/provincial-territorial-routine-vaccination-programs-infants-children.html
- 34.Campbell H, Parikh SR, Borrow R, Kaczmarski E, Ramsay ME, Ladhani SN. Presentation with gastrointestinal symptoms and high case fatality associated with group W meningococcal disease (MenW) in teenagers, England, July 2015 to January 2016 [Internet]. Euro Surveill 2016;21(12):30175. . 10.2807/1560-7917.ES.2016.21.12.30175 [DOI] [PubMed] [Google Scholar]