Abstract
Nephrolithiasis is a complex disease of worldwide prevalence that is influenced by both genetic and environmental factors. About 75% of kidney stones are predominantly composed of calcium oxalate and urinary oxalate is considered a crucial risk factor. Microorganisms may have a role in the pathogenesis and prevention of kidney stones and the involvement of the intestinal microbiome in this renal disease has been a recent area of interest. Oxalobacter formigenes is a gram negative bacteria that degrades oxalate in the gut decreasing urinary oxalate excretion. In this review, we examine the data studying the role of Oxalobacter formigenes in kidney stone disease in humans and animals, the effect of antibiotics on its colonization, and the potential role of probiotics and whole microbial communities as therapeutic interventions.
Keywords: Microbiota, Nephrolithiasis, Urolithiasis
1. Introduction
Nephrolithiasis is a complex disease influenced by genetic and environmental factors. Twin studies have revealed a 56% heritability risk for stones while other implicated factors include diet, exercise, work environment and geography [1]. In recent years, the role of the intestinal microbiome in influencing the composition of the urine has been explored resulting in data suggesting that it affects kidney stone incidence. We will review here the evidence supporting this hypothesis. Not reviewed here is the well described role of infections of the urinary tract with Proteus species and other urease-producing organisms associated with struvite stone formation.
The enormous number of microorganisms that colonize the human body and form complex communities are referred to as the microbiome. Functionally, it communicates with host human cells and performs various biological processes. There is increasing concern that the ‘Western’ diet and lifestyle have altered the genetic composition and metabolic activity of the intestinal microbiome. The effects of these changes in the bacterial populations have been associated with the increasing incidence of diseases such as obesity, coronary vascular disease, allergies, and metabolic syndrome [2]. These effects make tenable the possibility that the gut microbiome also affects absorption and secretion of solutes relevant to kidney stone formation.
To date, relatively little is known about the general role of the gut microbiome in the pathophysiology of nephrolithiasis. A recent study has identified distinct differences in the gut microbiome of kidney stone patients compared to patients without stones [3]. Fecal and urine samples collected from both groups of patients revealed 178 genera, of which the five most abundant enterotypes, or distinct bacterial communities, within each group made up greater than 50% of the bacterial abundance identified. Prevotella genus was most abundant in the control group while the Bacteroides genus was most abundant in the kidney stone group. Eubacterium was inversely correlated with oxalate levels and Escherichia inversely correlated with citrate levels. Whether these differences in bacterial abundance seen in stone formers and controls are causative in the pathway of stone formation, or secondary to other variables such as antibiotic exposure or diet, is uncertain. Such broad characterizations of the microbiome will need more extensive investigations to link to specific solutes that compose kidney stones and specific agents affecting the crystallization process.
2. Oxalobacter formigenes
2.1. Genetic and microbiological characteristics
The discovery of an oxalate degrading bacteria, Oxalobacter formigenes (Oxf), by Allison and coworkers in 1985 has attracted considerable attention regarding its involvement in calcium oxalate stone disease [4]. Clinical findings have suggested that there is a direct correlation between the organism’s absence and hyperoxaluria and oxalate stone formation. Oxf is a Gram negative, obligate anaerobic bacterium, that is part of the normal bacterial flora in the large intestine of humans and other mammalian species. It is unique in that it requires oxalate both as a carbon source and for ATP generation, which it finds in the intestinal lumen [5]. It has been found in the gut of humans, rodents, dogs, pigs, and cattle. If present, it could degrade ingested oxalate and reduce intestinal absorption, and stimulate oxalate secretion from the colon, offering protection from hyperoxaluria.
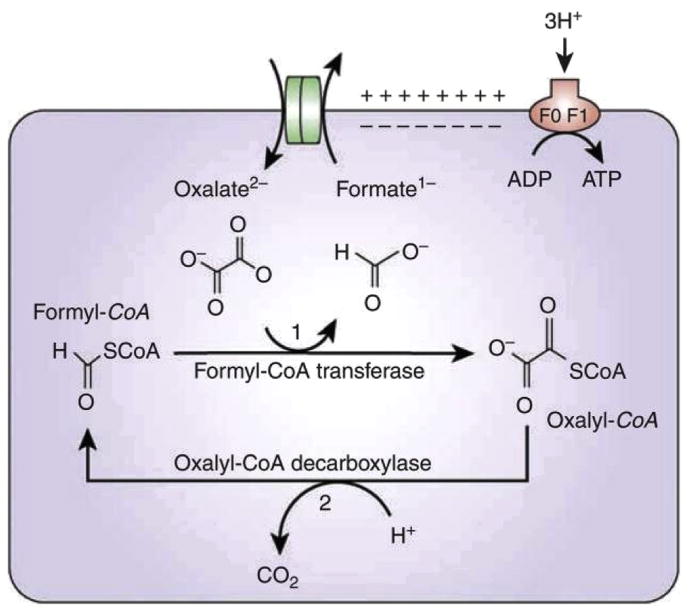
Oxalate metabolism by Oxf requires uptake of extracellular oxalate in exchange for formate by the membrane transporter called OxlT, encoded by the oxIT gene (see Fig. 1). The frc gene encodes formyl CoA transferase, Frc, which activates oxalate by adding a coenzyme A molecule to form oxalyl-CoA. Oxalyl-CoA is then decarboxylated to CO2 and formate, and the latter is then utilized by oxlT to take up more oxalate. The decarboxylation reaction is catalyzed by the enzyme oxalyl-CoA-decarboxylase, encoded by the gene oxc [6]. An inward gradient for protons results, driving ATP production.
Fig. 1.
Metabolism of oxalate by Oxf [6]. Reproduced with permission.
While, O. formigenes is thought to be the most effective oxalate-degrader, the role of other oxalate-degrading microbiota in the human intestine is not fully elucidated. Multiple bacterial species have both oxc and frc and demonstrate oxalate-degrading activity in vitro [7]. Recently, Hatch et al. demonstrated that Bifidobacterium lactis colonization decreases urinary oxalate by degrading dietary oxalate and reducing its intestinal absorption in a mouse model [8]. In a study of South African men, Lactobacillus species with high oxalate degrading capacity have been identified and associated with a lower prevalence of calcium oxalate kidney stones [9].
Comparison of the profiles of cellular fatty acids of 17 strains of Oxf has separated these strains into two main groups, currently designated as Group 1 (e.g. strain OXCC13) and Group 2 (e.g. strain HOxBLS). The sequencing of the genomes of these 2 strains as part of the Human Microbiome Project has provided an opportunity to increase our understanding of the important biological properties of the organism [10]. Additional proteomic analysis of Oxf in log and stationary growth phase cultures has allowed for the identification of specific proteins that are important for its growth and survival [11].
The development of a PCR-based detection assay specific for the oxc and/or frc genes in Oxf has allowed for the study of the role of this organism in oxalate metabolism. The rapid detection of Oxf in fecal cultures and fresh stool specimens is possible with a high degree of sensitivity and specificity [12]. Measurement of the oxalate-degrading capacity of the stool is another way to determine indirectly the presence or absence and activity of the organism [13].
Studies have reported an extensive variation in the degree to which Oxf colonizes the normal human gut. There may be undetectable levels of the bacterium or it may be present with as many as 107 per gram of feces. The levels of Oxf in fecal samples increased about 10 fold with a 10 fold increase of dietary oxalate. In contrast, abundance of the organism decreased with increasing calcium intake, which would bind oxalate and reduce its availability [14].
We recently described the prevalence, relative abundance and stability of Oxf in the human gut microbiome as revealed by Human Microbiome Project (HMP) data [15]. Fecal samples from 242 healthy young adults were analyzed using whole-genomic shotgun (WGS) sequencing and V13 or V35 16S rRNA sequencing. Analysis of the WGS dataset showed that 29 (31%) of 94 subjects were Oxf-positive while analysis of the V13 and V35 data showed Oxf prevalence at 15% (22/155) and 11% (23/210), respectively. Thus, detection of Oxf by the HMP investigators very much depended on methods used: WGS was more sensitive than 16S rRNA sequencing. We found that all 29 of the Oxf-positive subjects in the WGS analysis were colonized with strain OXCC13. However, of these 29, 59% were simultaneously colonized with strain HOxBLS. Thus, co-colonization with both strains was common. It has not been established whether the two strains have differing clinical significance.
3. Human studies
3.1. O. formigenes prevalence in humans
A large percentage of the population is colonized with Oxf. In US adults, the colonization rate of Oxf has been estimated to vary between 38 and 62%, but worldwide, the colonization rate is higher in populations with limited exposure to antibiotics. For instance, in India, the prevalence was reported at around 60%; in Korea, prevalence was at 77% [16,17]. Low Oxf colonization rates have been noted in several pathologic conditions, including inflammatory bowel disease, recurrent nephrolithiasis, morbid obesity, cystic fibrosis and idiopathic calcium nephrolithiasis, all of which are associated with calcium oxalate stones (Table 1).
Table 1.
Reported Oxf colonization rates in various adult populations.
| Reported Oxf colonization rates in various adult populations | |||
|---|---|---|---|
| Country | Population | Number of subjects | % colonization |
| India | Normal | 48 | 56 |
| Inflammatory Bowel Disease | 48 | 10 | |
| USA | Normal | 26 | 62 |
| Inflammatory Bowel Disease | 16 | 9 | |
| USA | Normal | 259 | 38 |
| Recurrent CaOx Stone formers | 247 | 17 | |
| Germany | Normal | 61 | 69 |
| CaOx Stone formers | 145 | 43 | |
| Korea | Normal | 233 | 77 |
| CaOx Stone formers | 103 | 46 | |
Colonization by Oxf has been investigated in a cross sectional study examining children from Ukraine [18]. This population was chosen due to the limited access to routine use of antibiotics during childhood. The organism could not be detected in infants less than 6–9 months of age and began appearing in the intestinal tracts of children around 1 year of age. By 3–4 years of age, all children showed colonization, with the number of children colonized declining between 8 and 12 years of age. Another group of patients of particular interest is those with cystic fibrosis (CF), who are known to have an increased prevalence of kidney stones. Patients with CF are subjected to multiple courses of antibiotics as a result of their increased susceptibility to pulmonary infections. In a study of urinary oxalate excretion in patients with CF, 71% of 21 non-CF control patients were colonized by Oxf compared with only 16% of 43 patients with CF [19]. All 7 patients with CF colonized by the bacterium had normal urinary oxalate excretion, whereas 53% of 36 patients not colonized had hyperoxaluria supporting the hypothesis that the presence of the organism protected against hyperoxaluria.
3.2. Association of O. formigenes and kidney stones
There are multiple epidemiological studies suggesting a protective role for Oxf. Human studies have also shown a strong inverse association between Oxf colonization and recurrent calcium oxalate renal stones. A case control study of 247 patients with recurrent episodes of calcium oxalate stones and 259 subjects without stone disease matched by age, gender and region found a strong inverse association between colonization with Oxf and recurrent calcium oxalate stones with a 70% risk reduction [20]. Among control subjects, an increase in the prevalence of Oxf was seen with increased oxalate consumption; the inverse was seen with antibiotic use. 24-h urine collections revealed a strong trend in the risk of stones with increasing urinary oxalate excretion. However there was no difference in median urinary oxalate excretion in patients who tested positive or negative for Oxf.
A key unanswered question is whether the absence of Oxf increases the risk of calcium oxalate stone formation by increasing urinary oxalate excretion. Under a controlled and standardized diet, urinary oxalate excretion has been shown to be lower in Oxf positive patients than in Oxf negative patients [21]. Results of a diet controlled study in 22 non-stone forming patients who were naturally colonized or noncolonized with Oxf suggests that differences in urinary oxalate excretion may be affected by differences in dietary calcium and oxalate intake [14].
Duncan et al. showed that the oral ingestion of a single dose of Oxf, followed by a dietary oxalate load, resulted in reduced urinary oxalate excretion, recovery of oxalate-degrading activity in feces, and prolonged colonization in 3 of 3 participants. A randomized, multicenter study of patients with primary hyperoxaluria failed to show a clear treatment effect of Oxf to reduce urinary oxalate excretion. The dose and viability of the administered bacterial treatment were questioned though the results did suggest a treatment effect when urine oxalate was normalized for creatinine [22].
3.3. Antibiotic effect on O. formigenes in humans and mice
The hypothesis that antibiotic use could be responsible for the decrease in the prevalence of Oxf in adults has been investigated in recent studies. The effect of antibiotics on Oxf colonization was evaluated in patients receiving oral antibiotic treatment for Helicobacter pylori (HP) [23]. Oxf strains are susceptible to multiple antibiotics including quinolones, macrolides, tetracyclines and metronidazole. In a prospective study, the prevalence of Oxf colonization was compared between an HP-positive group who were treated with either clarithromycin or metronidazole and an HP-negative control group who did not receive antibiotics. 92% of the control group of 12 patients who were positive for Oxf on initial stool testing and were not administered antibiotics remained positive for Oxf on stool tests at 1 month and 6 months. In comparison, only 38% the 19 subjects who were positive for Oxf and who were administered antibiotic therapy for HP, remained positive for Oxf in the stool at both follow-up points. Only one of the participants whose colonization with Oxf was eliminated with antibiotics regained Oxf colonization at 6 months. These findings suggest that the lasting elimination of Oxf after antibiotic exposure may be a risk factor for kidney stone formation [23].
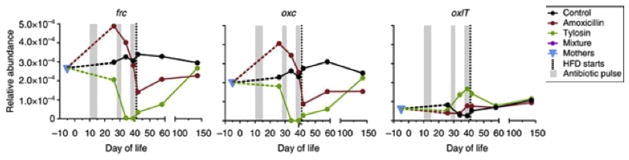
Another study examined the effect of antibiotic pulses in addition to dietary modifications in mice to understand the resulting physiologic perturbations [24]. In the pulsed antibiotic treatment (PAT) in early life mouse model, mice were divided into 3 groups. The control group did not receive antibiotics while the other two received 3 pulses of tylosin (a macrolide) or amoxicillin. To see the effect of the PAT model on oxalate degradation, the mean relative abundance of each of the three genes involved in oxalate metabolism was measured over time. The oxc, frc, and oxlT were not specific to Oxf but could be from other oxalate-degrading bacteria. Fig. 2 shows that antibiotic pulses and dietary modifications caused significant changes in the relative abundance of gene expression of oxc, frc, and oxlT during development; however the direction of the change was not uniform. This might indicate a differential effect of these variables on oxalate-degrading bacteria including Oxf.
Fig. 2.
Changes in the relative abundance of gene expression of oxc, frc, and oxlT during development with pulsed antibiotic treatment [24] (HFD: high fat diet reproduced with permission.
4. Animal studies
Multiple experiments have investigated the role of Oxf in reducing urinary oxalate excretion in animal models. Sidhu et al. showed that in rats, colonization with O. formigenes resulted in reduction of urinary oxalate excretion [25]. Likewise, in a mouse model of primary hyperoxaluria, a genetic disorder causing increased endogeneous oxalate production, O. formigenes induces enteric oxalate secretion, ultimately reducing net urinary oxalate excretion [26]. The urinary oxalate was similarly reduced when lysate of the bacterium was used in lieu of whole bacterium [27]. Chen et al. transfected mouse stem cells with oxc and frc genes, encoding the oxalate decarboxylase and the formyl Co-A transferase, and demonstrated reduction in oxalate levels in the media [28].
An alternative hypothesis is that Oxf possesses a unique characteristic that allows it to reduce urinary oxalate excretion not only by reducing intestinal absorption, but also by enhancing enteric oxalate secretion. Hatch et al. reported that Oxf interacts with colonic epithelium by inducing distal colonic secretion with a net secretive flux of oxalate from serosa to mucosa, leading to reduced urinary excretion [27]. This was shown by the studies on mice using two strains of Oxf, a human and rat strain. There was no change in colonic expression of the chloride/oxalate exchange protein, Slc26a6, in mice colonized by Oxf as compared with non-colonized wild type mice.
Characterization of the apparent secretagogue made by Oxf strengthening the plausibility of Oxf-stimulated oxalate secretion has recently proceeded. While the responsible molecule has not yet been identified, culture media exposed to Oxf, administered rectally to knockout mice with primary hyperoxaluria type 1, reduced urinary oxalate excretion (>32.5%) and stimulated distal colonic oxalate secretion (>42%) in Ussing chamber-mounted bowel cross-sections [29]. The mechanism of action included activation of the SLC26A6 transporter by protein kinase A without any increased expression of the protein. These effects have not yet been recapitulated in human colon but described in human intestinal Caco-2-BBE cells. Since maintaining sustained Oxf colonization in the absence of high exogenous oxalate remains difficult, the identification of Oxf-derived bioactive factors that induce colonic oxalate secretion, thereby reducing urinary oxalate excretion, may be of important therapeutic potential.
The recent successful mono-colonization of germ free mice with Oxf suggests that Oxf does not require other organisms for its survival. Experiments showed that these mono-colonized mice had significantly reduced urinary oxalate excretion compared to germ free mice. This finding may play an important role in the development of Oxf as probiotic [30].
4.1. Potential role of probiotics and whole microbial communities
The recent microbial transplants of oxalate-degrading bacteria from the mammalian herbivore Neotoma albigula into a laboratory rat resulted in a significant increase and persistent colonization of oxalate-degrading bacteria. This result may represent a new target for therapeutic intervention to confer persistent oxalate degradation across species [31].
Attempts to introduce oxalate-degrading microbes though oral probiotic formulations into the human or rat gut have temporarily resulted in a decrease in urinary oxalate excretion. These oral probiotic preparations include Oxf alone, or different combinations of Lactobacillus, Bifidobacterium, Enterococcus, and other oxalate degraders. With all formulations, the probiotics tested in both humans and rodents initially lead to a reduction in urinary oxalate excretion; however the bacteria and their oxalate-degrading function were usually undetectable as early as 5 days after oxalate is removed from the diet [7,32].
5. Conclusion
Although research establishing a direct causal relationship between alterations in the gut microbiome and the incidence of kidney stones is lacking, the reviewed literature is highly suggestive. While research in this field is still in its early stages, the advancement of sequencing technologies and analytical tools offer a unique opportunity to explore previously unanswered questions on the role of gut and urine bacteria in stone pathophysiology. (To date, there are no studies linking the presence of non-pathogenic bacteria in the urine, as opposed to the intestinal lumen, with kidney stones).
A few limitations in previous studies can be identified and considered in developing further studies. All the animal studies manipulated rodents’ microbiome with the addition of Oxf and changes in diet. We know that the human microbiome and diet are significantly different than the rodent’s, so finding a more representative model might be necessary in order to translate this work to humans. In addition, the understanding of the gut microbiome as a network of bacterial species performing a function, e.g. oxalate degradation, instead of as a single species, will likely be of important therapeutic implications.
HIGHLIGHTS.
Oxalobacter formigenes.
Genetic and microbiological characteristics.
O. formigenes prevalence in humans.
Association of O. formigenes and kidney stones.
Antibiotic effect on O. formigenes in humans and mice.
Potential role of probiotics and whole microbial communities.
Acknowledgments
Sources of funding
None.
This work was also supported by the Rare Kidney Stone Consortium (U54KD083908), which is a part of the NIH Rare Diseases Clinical Research Network, supported through collaboration between the NIH Office of Rare Diseases Research at the National Center for Advancing Translational Sciences and National Institute of Diabetes and Digestive and Kidney Disease.
Footnotes
Ethical approval
None.
Author contribution
Mansi Mehta.
David S. Goldfarb MD.
Lama Nazzal.
All authors participated in writing and completing the paper.
Conflict of interest
None.
Guarantor
David Goldfarb.
Lama Nazzal.
Mansi Mehta.
Disclosures
Mehta: none; Goldfarb: consultant: Allena, AstraZeneca, Cymabay, Ironwood, Revive; owner: Ravine Group; funding from NIDDK, NCATS; Nazzal: none.
References
- 1.Goldfarb DS, Fischer ME, Keich Y, et al. A twin study of genetic and dietary influences on nephrolithiasis: a report from the Vietnam Era Twin (VET) Registry. Kidney Int. 2005;67:1053. doi: 10.1111/j.1523-1755.2005.00170.x. [DOI] [PubMed] [Google Scholar]
- 2.Blaser MJ, Falkow S. What are the consequences of the disappearing human microbiota? Nat Rev Microbiol. 2009;7:887. doi: 10.1038/nrmicro2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stern JM, Moazami S, Qiu Y, et al. Evidence for a distinct gut microbiome in kidney stone formers compared to non-stone formers. Urolithiasis. 2016;44:399. doi: 10.1007/s00240-016-0882-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Allison MJ, Dawson KA, Mayberry WR, et al. Oxalobacter formigenes gen. nov., sp. nov.: oxalate-degrading anaerobes that inhabit the gastrointestinal tract. Arch Microbiol. 1985;141:1. doi: 10.1007/BF00446731. [DOI] [PubMed] [Google Scholar]
- 5.Allison MJ, Cook HM, Milne DB, et al. Oxalate degradation by gastrointestinal bacteria from humans. J Nutr. 1986;116:455. doi: 10.1093/jn/116.3.455. [DOI] [PubMed] [Google Scholar]
- 6.Anantharam V, Allison MJ, Maloney PC. Oxalate:formate exchange. The basis for energy coupling in Oxalobacter. J Biol Chem. 1989;264:7244. [PubMed] [Google Scholar]
- 7.Abratt VR, Reid SJ. Oxalate-degrading bacteria of the human gut as probiotics in the management of kidney stone disease. Adv Appl Microbiol. 2010;72:63. doi: 10.1016/S0065-2164(10)72003-7. [DOI] [PubMed] [Google Scholar]
- 8.Klimesova K, Whittamore JM, Hatch M. Bifidobacterium animalis subsp. lactis decreases urinary oxalate excretion in a mouse model of primary hyperoxaluria. Urolithiasis. 2015;43:107. doi: 10.1007/s00240-014-0728-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Magwira CA, Kullin B, Lewandowski S, et al. Diversity of faecal oxalate-degrading bacteria in black and white South African study groups: insights into understanding the rarity of urolithiasis in the black group. J Appl Microbiol. 2012;113:418. doi: 10.1111/j.1365-2672.2012.05346.x. [DOI] [PubMed] [Google Scholar]
- 10.Knight J, Deora R, Assimos DG, et al. The genetic composition of Oxalobacter formigenes and its relationship to colonization and calcium oxalate stone disease. Urolithiasis. 2013;41:187. doi: 10.1007/s00240-013-0566-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ellis ME, Mobley JA, Holmes RP, et al. Proteome dynamics of the specialist oxalate degrader Oxalobacter formigenes. J Proteom Bioinform. 2016;9:19. doi: 10.4172/jpb.1000384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sidhu H, Holmes RP, Allison MJ, et al. Direct quantification of the enteric bacterium Oxalobacter formigenes in human fecal samples by quantitative competitive-template PCR. J Clin Microbiol. 1999;37:1503. doi: 10.1128/jcm.37.5.1503-1509.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Duncan SH, Richardson AJ, Kaul P, et al. Oxalobacter formigenes and its potential role in human health. Appl Environ Microbiol. 2002;68:3841. doi: 10.1128/AEM.68.8.3841-3847.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jiang J, Knight J, Easter LH, et al. Impact of dietary calcium and oxalate, and Oxalobacter formigenes colonization on urinary oxalate excretion. J Urol. 2011;186:135. doi: 10.1016/j.juro.2011.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barnett C, Nazzal L, Goldfarb DS, et al. The presence of Oxalobacter formigenes in the microbiome of healthy young adults. J Urol. 2015:499. doi: 10.1016/j.juro.2015.08.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mittal RD, Kumar R, Mittal B, et al. Stone composition, metabolic profile and the presence of the gut-inhabiting bacterium Oxalobacter formigenes as risk factors for renal stone formation. Med Princ Pract. 2003;12:208. doi: 10.1159/000072285. [DOI] [PubMed] [Google Scholar]
- 17.Kwak C, Jeong BC, Kim HK, et al. Molecular epidemiology of fecal Oxalobacter formigenes in healthy adults living in Seoul, Korea. J Endourol. 2003;17:239. doi: 10.1089/089277903765444384. [DOI] [PubMed] [Google Scholar]
- 18.Sidhu H, Enatska L, Ogden S, et al. Evaluating children in the Ukraine for colonization with the intestinal bacterium Oxalobacter formigenes, using a polymerase chain reaction-based detection system. Mol Diagn. 1997;2:89. doi: 10.1054/MODI00200089. [DOI] [PubMed] [Google Scholar]
- 19.Sidhu H, Hoppe B, Hesse A, et al. Absence of Oxalobacter formigenes in cystic fibrosis patients: a risk factor for hyperoxaluria. Lancet. 1998;352:1026. doi: 10.1016/S0140-6736(98)03038-4. [DOI] [PubMed] [Google Scholar]
- 20.Kelly JP, Curhan GC, Cave DR, et al. Factors related to colonization with Oxalobacter formigenes in U.S. adults. J Endourol. 2011;25:673. doi: 10.1089/end.2010.0462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Siener R, Bangen U, Sidhu H, et al. The role of Oxalobacter formigenes colonization in calcium oxalate stone disease. Kidney Int. 2013;83:1144. doi: 10.1038/ki.2013.104. [DOI] [PubMed] [Google Scholar]
- 22.Hoppe B, Groothoff JW, Hulton SA, et al. Efficacy and safety of Oxalobacter formigenes to reduce urinary oxalate in primary hyperoxaluria. Nephrol Dial Transpl. 2011:3609. doi: 10.1093/ndt/gfr107. [DOI] [PubMed] [Google Scholar]
- 23.Kharlamb V, Schelker J, Francois F, et al. Oral antibiotic treatment of Helicobacter pylori leads to persistently reduced intestinal colonization rates with Oxalobacter formigenes. J Endourol. 2011;25:1781. doi: 10.1089/end.2011.0243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nobel YR, Cox LM, Kirigin FF, et al. Metabolic and metagenomic outcomes from early-life pulsed antibiotic treatment. Nat Commun. 2015;6:7486. doi: 10.1038/ncomms8486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sidhu H, Allison MJ, Chow JM, et al. Rapid reversal of hyperoxaluria in a rat model after probiotic administration of Oxalobacter formigenes. J Urol. 2001;166:1487. [PubMed] [Google Scholar]
- 26.Hatch M, Gjymishka A, Salido EC, et al. Enteric oxalate elimination is induced and oxalate is normalized in a mouse model of Primary Hyperoxaluria following intestinal colonization with Oxalobacter. Am J Physiol Gastrointest Liver Physiol. 2010;300:G461. doi: 10.1152/ajpgi.00434.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hatch M, Cornelius J, Allison M, et al. Oxalobacter sp. reduces urinary oxalate excretion by promoting enteric oxalate secretion. Kidney Int. 2006;69:691. doi: 10.1038/sj.ki.5000162. [DOI] [PubMed] [Google Scholar]
- 28.Chen Z, Liu G, Ye Z, et al. The construction of an oxalate-degrading intestinal stem cell population in mice: a potential new treatment option for patients with calcium oxalate calculus. Urol Res. 2012;40:131. doi: 10.1007/s00240-011-0420-8. [DOI] [PubMed] [Google Scholar]
- 29.Arvans D, Jung YC, Antonopoulos D, et al. Oxalobacter formigenes-derived bioactive factors stimulate oxalate transport by intestinal epithelial cells. J Am Soc Nephrol. 2016 doi: 10.1681/ASN.2016020132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li X, Ellis ML, Dowell AE, et al. Response of germ-free mice to colonization with O. formigenes and altered Schaedler flora. Appl Environ Microbiol. 2016 doi: 10.1128/AEM.02381-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miller AW, Oakeson KF, Dale C, et al. Microbial community transplant results in increased and long-term oxalate degradation. Microb Ecol. 2016;72:470. doi: 10.1007/s00248-016-0800-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lieske JC, Tremaine WJ, De Simone C, et al. Diet, but not oral probiotics, effectively reduces urinary oxalate excretion and calcium oxalate supersaturation. Kidney Int. 2010;78:1178. doi: 10.1038/ki.2010.310. [DOI] [PMC free article] [PubMed] [Google Scholar]