Abstract
Collection of abdominal subcutaneous adipose tissue (SAT) for research testing is traditionally performed using punch biopsy or needle-aspiration techniques, yielding small amounts of very superficial SAT (100–500 milligrams). Although liposuction techniques can be used to obtain large amounts of SAT, these approaches can compromise the integrity of the adipose tissue. Therefore, we investigated a novel method using a 6-mm Bergström side-cutting biopsy needle to acquire suitable amounts of intact abdominal SAT for multiple complex studies such as flow cytometry cell sorting, RNA extraction, ex vivo expression of molecular and post-translational protein mediators, and histology. Fifty biopsies were obtained from 29 participants using a Bergström biopsy needle, applying transient manual suction, and shearing large pieces of fat within the inner cutting trochar. Eighteen of the biopsies were performed under ultrasound guidance, whereby we successfully sampled deep SAT (dSAT) from below Scarpa’s fascia. The average weight of SAT sampled was 1.5±0.4 grams. There was no clinically important bleeding or ecchymosis on the abdominal wall and no infection occurred with this procedure. The 6-mm Bergström biopsy needle yielded substantially more SAT than what has been obtained from superficial procedures and for the first time allowed sampling of dSAT by a percutaneous approach.
Keywords: Adipose tissue, biopsy, Bergström, needle, obesity, superficial subcutaneous adipose tissue, deep subcutaneous adipose tissue
Introduction
In obese persons, specific patters of adipose tissue distribution, particularly upper body fat, are closely linked to increased risk for cardiovascular disease (CVD) and insulin resistance (1–4). Upper body fat accumulation includes visceral adipose tissue (VAT), or intra-abdominal fat, and subcutaneous adipose tissue (SAT). Within the abdominal SAT depot, there are two distinct regions divided by the Scarpa’s fascia into superficial SAT (sSAT) and deep SAT (dSAT) (4,5). VAT shows the greatest linkage with risk for inflammation, CVD, and metabolic syndrome (6–8). However, compared to sSAT, VAT and dSAT share more similar inflammatory characteristics and associations with disease risk (4,5,9–11).
Traditionally, invasive surgical procedures are required to obtain VAT and sufficient amounts of fat for a portfolio of laboratory tests (9,12). Since sSAT is easily accessible and can reflect some biological aspects of VAT (9,13), non-invasive aspiration and punch biopsy procedures are used to sample this depot (2,13). However, these methods typically acquire only 100–500 milligrams of sSAT (14–16), far less than what is needed for research studies that may include fluorescent activated cell sorting, RNA extraction, ex vivo expression of molecular and post-translational protein mediators, and histology. Further, some of these techniques, including liposuction type approaches, require vigorous suctioning, which may disrupt the integrity of adipose tissue for multiple, and complex research studies. Although two studies reported using the Bergström biopsy needle to sample SAT, neither provided detailed information regarding their methodology, the quantity of adipose tissue obtained, or the feasibility of sampling dSAT (17,18). Therefore, the aims of this study were to determine: 1. whether a Bergström side-cutting biopsy needle could be used to safely obtain large amounts of intact SAT (≥1 gram) and 2. whether this method could be used in conjunction with ultrasound guidance to specifically sample dSAT because of its greater similarities to VAT.
Methods
SAT biopsies were obtained at the bedside from study participants who were enrolled in a study at the University of Southern California (USC). The USC Institutional Review Board approved the protocol and participants signed informed consent before testing. To be eligible, participants had to be 18–35 years-of-age, obese (BMI ≥30 kg/m2), non-diabetic, and free of any active medical problems. Eligible participants were scheduled for two biopsies and 3-Tesla whole abdominal MRI scans one month apart.
The biopsy site was prepared with three betadine scrubs and covered with a fenestrated sterile drape. The dermis at the biopsy site (at the right anterior axillary line at the level of the umbilicus) was infiltrated with 0.5 cc of 1% lidocaine followed by injection of 4 cc in the very superficial layers of adipose tissue immediately below the skin (no more than ½-inch deep). As shown in Figure 1, a 6–7 mm incision was made in the skin and a 6-mm Bergström side-cutting needle (Micrins Surgical, Inc; Lake Forest, IL) was introduced approximately 1–1.5 inches through the incision into the deeper SAT. After the needle was angled obliquely, an assistant applied brief suction from a 60 cc irrigation syringe attached to the Bergström needle with gastrointestinal irrigation tubing (Kendall; no. 1; 16 Fr/Ch × 48 inches; Mansfield, MA).
Figure 1. Biopsy Method With the Bergström Biopsy Needle.
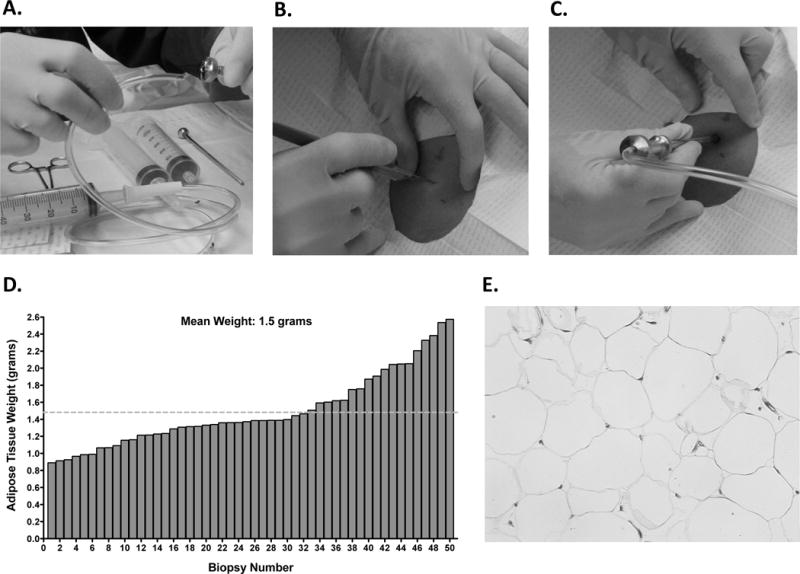
A. Tubing was cut at a 45-degree angle in order to fit more securely into the top of the Bergström biopsy needle for generating suction. Tubing inserted into the cutting trochar of the Bergström biopsy needle. B. A 6–7 mm incision was made through the skin up to the hub of a number 11 Bard Parker blade. C. When US guidance was not used, the Bergström biopsy needle was inserted approximately 1.5 inches in the incision site. D. The average SAT biopsy weight taken was 1.5±0.4 grams. E. Representative hematoxylin and eosin staining showing integrity of adipose tissue is maintained when using the Bergström biopsy needle.
Four cuts were made with the cutting trochar as the needle was further advanced and rotated 90 degrees. The procedure was repeated with a second pass to generate eight total cuts. In 18 of the biopsies performed, we utilized ultrasound (US) guidance (Toshiba Aplio 500 with iStyle) to ensure sampling from dSAT below the Scarpa’s fascia, which is often 2–3 inches below the skin in obese participants. We also utilized color Doppler imaging to verify that there were no large blood vessels present in our sampling region in dSAT. Some participants experienced minor discomfort when the needle was advanced through Scarpa’s fascia. To rectify the discomfort we now routinely anesthetize the superficial layer of Scarpa’s fascia with 1% lidocaine administered through a spinal needle guided by US.
After the biopsy procedure, manual compression was applied for 10 minutes to prevent bleeding. Following compression, a single 2.0 Ethicon nylon suture was used to close the wound. Bacitracin ointment was applied to the wound, which was then covered with Tegaderm (3M Health Care, St. Paul, MN) dressing. After this, the participant rolled onto their right side with a cold pack (T-pak instant cold pack) over the wound for an additional 15–20 minutes. Participants were given instructions on how to care for their wound including no water immersion (swimming, bath, spa) and avoidance of heavy lifting (more than 5 pounds) for 72 hours. The suture was removed 5–7 days following the biopsy.
Results
This study included 17 male and 12 female obese (BMI 40.5±6.9 kg/m2) Hispanic adults (22.4±4.8 years) who underwent 50 abdominal SAT biopsies. The time period from prepping the biopsy site to tissue processing averaged about 20 minutes. Other than minor discomfort from the intradermal lidocaine injection, there was no evidence of substantial participant pain during the procedure and the mild discomfort penetrating the Scarpa’s fascia was alleviated by first anesthetizing the fascia.
We obtained an average of 1.5 grams (0.9–2.6 grams) of intact SAT, which ranged from 5–10 times greater than we previously obtained using a punch biopsy method (Figure 1 D) (14). As illustrated in the MRI scan (Figure 2 A), SAT is divided into sSAT and dSAT by the Scarpa’s fascia. For 18 biopsies we used US guidance to sample intact dSAT (Figure 2 B–C). We performed histology on SAT samples, which confirmed that this procedure did not damage the adipose tissue (Figure 1 E). Twenty-one participants had two biopsies and the weight of the second biopsy did not differ from the first (1.6±0.46 vs. 1.5±0.46 grams, P=0.35). There was no clinically important bleeding (<1cc at the site of incision), abdominal wall ecchymosis, or infections. Occasionally, biopsies would result in several mm of bruising at the biopsy site.
Figure 2. Location of Subcutaneous Adipose Tissue Biopsies with MRI and Ultrasound Guidance.
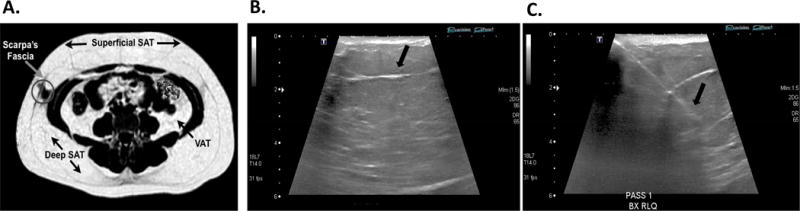
A. Single slice from 3-Tesla MRI scan shows VAT as well as sSAT and dSAT separated by the Scarpa’s fascia. Adipose tissue was obtained from dSAT (denoted by circle) below the Scarpa’s fascia (arrowhead). B-C. Representative US images are from 1 of the 18 biopsies performed under US guidance. B. Bergström biopsy needle above the Scarpa’s fascia (arrowhead). C. Bergström biopsy needle below the Scarpa’s fascia, approximately 2–3 inches below the skin (arrowhead), where we sampled dSAT.
Discussion
This novel abdominal fat biopsy procedure used a Bergström side-cutting biopsy needle and yielded an average of 1.5 grams of intact SAT, which was several fold greater than what has been previously reported using punch biopsy and needle aspiration techniques (14–16). Additionally, to our knowledge, we are the first to report the ability to safely and consistently sample adipose tissue from the dSAT compartment by a percutaneous bedside approach. This is particularly important since dSAT better reflects VAT than sSAT in regards to inflammation, macrophage accumulation, cardiometabolic risk factors and insulin resistance (4,5,9–11).
Although the current study was limited in that we did not directly compare our biopsy method to aspiration, punch, liposuction techniques, the Bergström biopsy needle required only mild and brief suction to aspirate adipose tissue into the side window of the large bore Bergström needle. This did not damage the integrity of the tissue for histology because of the size of the pieces cut. This is in contrast to other procedures whereby SAT samples are obtained by forceful extraction with tissue forceps, continued manual suction using smaller gauge cutting needles, or wall suction as utilized during liposuction. For example, although a tumescent mini liposuction method may yield 3–15 grams of adipose tissue (19), it utilizes forceful continued suction, increasing the potential for disruption of adipose tissue and extensive abdominal wall ecchymosis as commonly occurs with liposuction. In contrast, we found that using the Bergström method did not damage our SAT and only occasionally resulted in minor bruising. Although two prior studies used the Bergström biopsy needle to sample SAT (17,18), this is the first to report a detailed description of this approach, including the quantity of adipose tissue obtained and the ability to sample dSAT.
Other studies have shown that open surgical procedures are able to sample large quantities of intact adipose tissue from sSAT, dSAT, and VAT (20,21); however, these methods are invasive and are not always feasible for research testing. Additionally, ascertainment of surgical adipose tissue during bariatric or lap band procedures is restricted to persons with morbid obesity who may have metabolic dysregulation different than patients with lesser amounts of upper body obesity. Surgical biopsies are also limited by an inability to sample SAT over time, for example, during research treatment interventions.
In summary, the 6-mm Bergström biopsy procedure with brief manual suction allowed us to obtain an average of 1.5 grams of SAT before and after treatment interventions in obese healthy adults. The procedure was associated with minimal discomfort and also afforded us the ability to obtain substantial amounts of intact adipose tissue from the dSAT compartment that has been shown to have metabolic and histologic properties more similar to VAT (4,9).
Acknowledgments
This work was supported by the SC CTSI (NIH/NCRR/NCATS) Grant # UL1TR000130 (T.L.A.) and the Robert C. and Veronica Atkins Foundation (M.I.G). T.L.A. (principal investigator) supervised all aspects of the study, assisted with biopsies, and was responsible for data analysis and manuscript preparation. M.I.G. (co-investigator) acted as study sponsor and mentor. F.R.S. (co-investigator) was the study physician and performed biopsies. E.G.G. helped develop ultrasound-imaging procedures while S.D.M., X.S., and J.T. developed the adipose tissue processing protocols. All co-authors assisted with manuscript preparation.
Footnotes
Disclosure:
The authors declared no conflict of interest.
References
- 1.Fox CS, Massaro JM, Hoffmann U, Pou KM, Maurovich-Horvat P, Liu C-Y, et al. Abdominal visceral and subcutaneous adipose tissue compartments: association with metabolic risk factors in the Framingham Heart Study. Circulation. 2007;116:39–48. doi: 10.1161/CIRCULATIONAHA.106.675355. [DOI] [PubMed] [Google Scholar]
- 2.Goodpaster B, Thaete F, Simoneau J, Kelley D. Subcutaneous abdominal fat and thigh muscle composition predict insulin sensitivity independently of visceral fat. Diabetes. Am Diabetes Assoc Am Diabetes Assoc. 1997;46:1579–1585. doi: 10.2337/diacare.46.10.1579. [DOI] [PubMed] [Google Scholar]
- 3.Patel P, Abate N. Body fat distribution and insulin resistance. Nutrients. 2013;5:2019–2027. doi: 10.3390/nu5062019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kelley DE, Thaete FL, Troost F, Huwe T, Goodpaster BH. Subdivisions of subcutaneous abdominal adipose tissue and insulin resistance. Am J Physiol Endocrinol Metab. 2011;278:E941–948. doi: 10.1152/ajpendo.2000.278.5.E941. [DOI] [PubMed] [Google Scholar]
- 5.Walker GE, Verti B, Marzullo P, Savia G, Mencarelli M, Zurleni F, et al. Deep subcutaneous adipose tissue: a distinct abdominal adipose depot. Obesity (Silver Spring) 2007;15:1933–1943. doi: 10.1038/oby.2007.231. [DOI] [PubMed] [Google Scholar]
- 6.Item F, Konrad D. Visceral fat and metabolic inflammation: the portal theory revisited. Obes Rev. 2012;13(Suppl 2):30–39. doi: 10.1111/j.1467-789X.2012.01035.x. [DOI] [PubMed] [Google Scholar]
- 7.Tchernof A, Després JP. Pathophysiology of Human Visceral Obesity: An Update. Physiological Reviews. 2013;93:359–404. doi: 10.1152/physrev.00033.2011. [DOI] [PubMed] [Google Scholar]
- 8.Alvehus M, Burén J, Sjöström M, Goedecke J, Olsson T. The human visceral fat depot has a unique inflammatory profile. Obesity. 2010;18:879–883. doi: 10.1038/oby.2010.22. [DOI] [PubMed] [Google Scholar]
- 9.Tordjman J, Divoux A, Prifti E, Poitou C, Pelloux V, Hugol D, et al. Structural and inflammatory heterogeneity in subcutaneous adipose tissue: relation with liver histopathology in morbid obesity. J Hepatol. 2012;56:1152–1158. doi: 10.1016/j.jhep.2011.12.015. [DOI] [PubMed] [Google Scholar]
- 10.Marinou K, Hodson L, Vasan SK, Fielding BA, Banerjee R, Brismar K, et al. Structural and functional properties of deep abdominal subcutaneous adipose tissue explain its association with insulin resistance and cardiovascular risk in men. Diabetes Care. 2014;37:821–829. doi: 10.2337/dc13-1353. [DOI] [PubMed] [Google Scholar]
- 11.Smith SR, Lovejoy JC, Greenway F, Ryan D, deJonge L, la Bretonne de J, et al. Contributions of total body fat, abdominal subcutaneous adipose tissue compartments, and visceral adipose tissue to the metabolic complications of obesity. Metab Clin Exp. 2001;50:425–435. doi: 10.1053/meta.2001.21693. [DOI] [PubMed] [Google Scholar]
- 12.Kolak M, Westerbacka J, Velagapudi VR, Wågsäter D, Yetukuri L, Makkonen J, et al. Adipose tissue inflammation and increased ceramide content characterize subjects with high liver fat content independent of obesity. Diabetes. 2007;56:1960–1968. doi: 10.2337/db07-0111. [DOI] [PubMed] [Google Scholar]
- 13.Bigornia SJ, Farb MG, Mott MM, Hess DT, Carmine B, Fiscale A, et al. Relation of depot-specific adipose inflammation to insulin resistance in human obesity. Nutr Diabetes. 2012;2:e30. doi: 10.1038/nutd.2012.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lê K-A, Mahurkar S, Alderete TL, Hasson RE, Adam TC, Kim JS, et al. Subcutaneous adipose tissue macrophage infiltration is associated with hepatic and visceral fat deposition, hyperinsulinemia, and stimulation of NF-κB stress pathway. Diabetes. 2011;60:2802–2809. doi: 10.2337/db10-1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Campbell KL, Makar KW, Kratz M, Foster-Schubert KE, McTiernan A, Ulrich CM. A pilot study of sampling subcutaneous adipose tissue to examine biomarkers of cancer risk. Cancer Prev Res (Phila) 2009;2:37–42. doi: 10.1158/1940-6207.CAPR-08-0073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Daum SM, Knittle J, Roseman K, Rom WN, Holstein EC. A simple technique for fat biopsy of PBB-exposed individuals. Environ Health Perspect. 1978;23:183–185. doi: 10.1289/ehp.7823183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pasarica M, Gowronska-Kozak B, Burk D, Remedios I, Hymel D, Gimble J, et al. Adipose tissue collagen VI in obesity. J Clin Endocrinol Metab. 2009;94:5155–5162. doi: 10.1210/jc.2009-0947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tam CS, Covington JD, Bajpeyi S, Tchoukalova Y, Burk D, Johannsen DL, et al. Weight gain reveals dramatic increases in skeletal muscle extracellular matrix remodeling. J Clin Endocrinol Metab. doi: 10.1210/jc.2013-4381. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bastard JP, Cuevas J, Cohen S, Jardel C, Hainque B. Percutaneous adipose tissue biopsy by mini-liposuction for metabolic studies. JPEN J Parenter Enteral Nutr. 1994;18:466–468. doi: 10.1177/0148607194018005466. [DOI] [PubMed] [Google Scholar]
- 20.van Beek L, Lips MA, Visser A, Pijl H, Ioan-Facsinay A, Toes R, et al. Increased systemic and adipose tissue inflammation differentiates obese women with T2DM from obese women with normal glucose tolerance. Metab Clin Exp. 2014;63:492–501. doi: 10.1016/j.metabol.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 21.Hagman DK, Kuzma JN, Larson I, Foster-Schubert KE, Kuan L-Y, Cignarella A, et al. Characterizing and quantifying leukocyte populations in human adipose tissue: impact of enzymatic tissue processing. J Immunol Methods. 2012;386:50–9. doi: 10.1016/j.jim.2012.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]


