Abstract
In previous studies, variability was high among replicate acute cadmium (Cd) Daphnia magna lethality tests (e.g., >10-fold range of median effect concentrations [EC50s]), less among zinc (Zn) tests, and relatively low for copper (Cu) and nickel (Ni) tests. Although the US Environmental Protection Agency’s (USEPA’s) protocol includes starting toxicity tests with neonates less than 24 h old, the authors hypothesized that age-related differences in sensitivity to metals might occur even within that relatively narrow age range. Daphnia magna neonates were collected during 3 age windows (0–4 h, 10–14 h, and 20–24 h old) and immediately exposed to each of the 4 metals for 48 h using the standard USEPA protocol. In repeated sets of tests during different weeks, the Cd EC50 of the youngest neonates was approximately 10-fold greater than the EC50 of the oldest neonates (i.e., Cd was less toxic to the youngest neonates) and the EC50 of neonates aged 10 h to 14 h was intermediate. Age-related differences were negligible in Cu, Ni, and Zn tests. Therefore, variability in toxicity of Cd may partly be caused by temporal variability in neonate age at the start of toxicity tests. Decreasing the age range of D. magna used in toxicity tests could help to improve the accuracy and precision of toxicity models, particularly for metal mixtures.
Keywords: Developmental toxicity, Aquatic toxicology, Invertebrate toxicology, Median effect concentration (EC50), Sensitivity, Test protocol, Metal toxicity
INTRODUCTION
The cladoceran Daphnia magna is one of the most commonly used invertebrate species for evaluating the toxicity of chemicals to freshwater organisms [1,2]. Although toxicity tests have been conducted with D. magna for more than half a century [3], methodology for evaluating acute toxicity with these organisms was not standardized until the mid-1980s [4,5]. To control for age-related differences in sensitivity to toxicants, the US Environmental Protection Agency (USEPA) and the Organisation for Economic Co-operation and Development (OECD) stipulate that the age of D. magna should be ≤24 h at the start of acute toxicity tests [6,7]. However, based on reported variability in the toxicity of some metals to D. magna neonates [8,9], we hypothesized that even the relatively narrow 24-h age window might introduce considerable variability in acute lethality tests with some toxicants.
The typical life span of D. magna is approximately 2 mo, with the organisms reaching reproductive maturity in the first 5 d to 10 d [10]. Daphnia magna neonates grow 0.126 mm d−1 to 0.388 mm d−1 [11] and shed their first carapace [10] within 24 h after being released from their mother’s brood chamber. As a result of this rapid life cycle, the variability in toxicity to contaminants may be more apparent if the organisms’ sensitivity is highly age-dependent. Previous authors have reported that changes in age-dependent sensitivity of <1-d-old to 7-d-old D. magna were often not statistically significant [12] and that adult daphnids tend to be less sensitive than younger organisms [2]. However, those studies were limited in their temporal resolution, which prevented their ability to evaluate changes in neonate sensitivity during the first 24 h after release from the brood chamber.
Daphnia magna neonates were previously exposed to binary and ternary mixtures of cadmium (Cd), copper (Cu), nickel (Ni), and zinc (Zn) in acute lethality tests in the authors’ laboratory [8,9]. When individual metals were tested concurrently with the mixtures at concentrations ranging from nonlethal to lethal, variability of mortality was very high in the Cd-only tests (e.g., >10-fold range of median effect concentrations [EC50 values]), less in Zn-only tests, and relatively low in Cu-only and Ni-only tests. This same pattern of variability was evident in the binary and ternary mixtures, which makes predicting mixture toxicity from variable individual-metal results even more challenging than if one only had to account for physical-chemical metal–metal interactions [13]. In the present study, we demonstrate that the among-test variability was caused at least in part by large age-related differences in sensitivity of D. magna neonates to some metals during their first 24 h postbirth. Therefore, in addition to the influence of test design [14], early postnatal age of daphnids at the start of single-metal and metal-mixture toxicity tests can influence their results and interpretation.
MATERIALS AND METHODS
Test organisms
Daphnia magna were purchased from Aquatic BioSystems. Gravid D. magna females were shipped to the Colorado School of Mines in USEPA moderately hard reconstituted water [6] with the green alga Pseudokirchneriella subcapitata as food (approximately 3 × 107 cells/L or 3.8 × 105 cells/D. magna adult). On arrival, the organisms were transferred to a skimmer tank, which allowed for the removal of neonates that had emerged during shipping. Gravid D. magna were maintained in skimmer tanks containing freshly prepared moderately hard reconstituted water and P. subcapitata. All neonates that emerged during the next 4 h were collected and either immediately placed into exposure water to begin a toxicity test or held in a separate tank of moderately hard reconstituted water for either 10 h or 20 h before being placed into exposure water. Neonates that were held in the separate tank for an additional amount of time were fed P. subcapitata (approximately 3.6 × 107 cells/L or 4.5 × 105 cells/neonate) 4 h before being placed in the exposure waters and were not fed during the exposure.
Exposure water
The exposure water in the toxicity tests was moderately hard reconstituted water to which 3 mg dissolved organic carbon (DOC)/L was added as Suwannee River fulvic acid (International Humic Substances Society). Metal salts (Ni[II] nitrate, Cd nitrate, Cu[II] nitrate, Zn nitrate; reagent grade, Mallinckrodt Chemical [Ni] and Baker Chemical [Cd, Cu, Zn]) were added to that exposure matrix. Exposure solutions were prepared 24 h to 36 h before the start of a toxicity test, to allow equilibration of the metals with the DOC as recommended by Ma et al. [15].
Toxicity tests
The toxicity of individual metals was determined in 48-h static, nonrenewal lethality tests, following USEPA-recommended procedures [6]. The toxicity tests comprised a series of 6 metal concentrations in a gradient designed to produce mortalities ranging from 0% to 100%. In all tests, each metal concentration was tested in 4 replicate chambers (50-mL polycarbonate cups), each containing 25 mL of exposure water and 5 organisms (i.e., a total of 20 organisms were exposed to each concentration in the metal gradient). The number of dead organisms was recorded at 24 h and 48 h, with immobilization as the indicator of mortality [6].
Individual-metal toxicity tests that had not been controlled for age were conducted from September 2012 through November 2013 in a previous study [8]. Age-controlled individual-metal tests were conducted from September through December 2014. All tests were conducted in incubators (VWR International) at 20 ± 2 °C, with a 16:8-h light:dark cycle.
Chemical analyses
At 0 h and 48 h, temperature and dissolved oxygen were measured using a YSI 55 probe, and pH was measured using an Orion ROSS electrode and Orion 2 STAR meter (Thermo Fisher Scientific) calibrated with pH 4, 7, and 10 buffers. Alkalinity was analyzed in the moderately hard reconstituted water that was used to prepare all the exposure waters by titration with sulfuric acid to the bromo-cresol green/methyl red end point [16]. Unfiltered water samples for analysis of total organic carbon (TOC) concentration were collected from the control and the highest metal concentration at the beginning of each test and preserved by addition of phosphoric acid (reagent grade, EM Science) to pH <2. Concentrations of TOC were analyzed by ultraviolet light–catalyzed persulfate oxidation using a Sievers 900 TOC Analyzer (GE Analytical Instruments).
At the beginning of each test, unfiltered water from each treatment in the metal-concentration series was acidified to pH < 2 with concentrated Optima nitric acid (HNO3; Mallinckrodt Chemical) and then submitted for elemental analysis. Exposure waters were not filtered before analysis because preliminary tests demonstrated that commercial filters can either sorb metals from or leach metals into initial volumes of water that are passed through the membranes [9]. Consequently, the small volumes (<100 mL) of exposure waters used in these D. magna toxicity tests were not sufficient to adequately rinse the filters or exceed their sorption capacity. Because additional tests demonstrated that the metals added to moderately hard reconstituted water were >90% dissolved [9], the total-metal concentrations were assumed to closely approximate the dissolved-metal concentrations.
All controls and exposure concentrations were analyzed for total concentrations of metals (including Cd, Cu, Ni, and Zn), major inorganic cations (Ca2+, Mg2+, Na+, K+), and sulfur using an Optima 5300 inductively coupled plasma optical emission spectrometer (ICP-OES; PerkinElmer). Sulfate concentrations in all controls and metal exposures were calculated by assuming all the sulfur measured by ICP-OES was present as SO42−. Chloride (Cl) concentrations were calculated by assuming the molar Cl– concentration equaled the measured molar potassium (K) concentration (because the only Cl– in the moderately hard reconstituted water recipe was added as KCl [6]).
Quality assurance/quality control procedures for the toxicity tests that were controlled for age (September–December 2014) were the same as in the individual-metal toxicity tests that had not been controlled for age (September 2012–November 2013) [8]. The pH probe was calibrated daily using certified buffers at pH 4, 7, and 10. In all ICP-OES analytical runs, a scandium (Sc) internal calibration standard was continuously introduced into the plasma along with each sample, and samples were analyzed in triplicate. Quality assurance/quality control samples included deionized water blanks (Barnstead Nanopure system; Thermo Fisher Scientific) that contained trace-metal–grade HNO3 (Thermo Fisher Scientific) and certified continuing concentration verification standards. The quality assurance/ quality control samples were analyzed immediately after instrument calibration, after every 10 samples, and at the end of each set of samples. Additionally, National Institute of Standards and Technology–certified standard reference materials 1640a and 1643e [17] were analyzed before and at the end of each set of samples. All samples were reanalyzed in any analytical run in which acceptable quality assurance/quality control results were not obtained. Unacceptable quality assurance/quality control results could include deviations of the internal Sc standard >20% from the known concentration, deviations of the continuing concentration verification samples >10% from the known concentrations, or relative standard deviations of triplicate analyses of a sample >10%. The ranges of instrument detection limits for the metals, major cations, and sulfur during the age-controlled toxicity tests were as follows: 4.2 μg Ca/L to 7.0 μg Ca/L, 0.1 μg Cd/L to 0.3 μg Cd/L, 0.3 μg Cu/L to 0.4 μg Cu/L, 18 μg K/L to 40 μg K/L (equivalent to 16–36 mg Cl/L calculated), 0.1 μg magnesium/L to 0.4 μg magnesium/L, 6.0 μg sodium/L to 7.0 μg sodium/L, 1.8 μg sulfur/L to 6.5 μg sulfur/L (equivalent to 5.4–19.5 μg sulfate/L calculated), 0.1 μg Ni/L to 0.4 μg Ni/L, and 0.3 μg Zn/L to 0.6 μg Zn/L.
Data analyses
Nonoverlap of 84% confidence intervals (CIs) was used to infer significant differences between 2 means at the 95% confidence level, as recommended by statisticians Julious [18] and Payton et al. [19]. In the present study, the EC50 values and the slopes of the associated concentration–response curves were calculated using the logit-regression method in OriginPro 9.1 (OriginLab). The 84% CIs on the averages of several EC50 values or slopes were calculated at n – 1 degrees of freedom using Microsoft Excel.
RESULTS AND DISCUSSION
General variability
In the individual-metal toxicity tests started with 0-h-old to 24-h-old neonates (i.e., using the standard USEPA protocol for neonate age [6]), Cu EC50 values were the least variable (i.e., lowest coefficient of variation [CV]) of the 4 metals tested, followed by Zn, Ni, and Cd in sequence of increasing variability (Table 1). Similar results have been reported elsewhere for Cd, Cu, and Zn toxicity [9], supporting this observation that the acute toxicity of Cd to 0-h-old to 24-h-old D. magna neonates is highly variable. The intermediate variability of Zn toxicity to D. magna was similar to the ranking of toxicity variability among Cd, Cu, and Zn reported by Meyer et al. [9] in the same exposure-water recipe.
Table 1.
Average 48-h median effect concentration and relevant parameters for immobilization of Daphnia magna neonates exposed to Cd, Cu, Ni, or Zn in repeated individual-metal static, nonrenewal lethality tests conducted with 0-h-old to 24-h-old neonates from September 2012 through November 2013a
| Metal | Average EC50 (mg/L)b (84% CI) | Range (mg/L) | SD (mg/L) | CV | Slopeb,c (84% CI) | nd |
|---|---|---|---|---|---|---|
| Cd | 0.054 A (0.039–0.069) | 0.002–0.125 | 0.036 | 0.661 | 0.683 A (0.642–0.724) | 13 |
| Cu | 0.100 B (0.093–0.108) | 0.074–0.128 | 0.019 | 0.192 | 6.654 B (6.372–6.936) | 15 |
| Ni | 1.633 C (1.510–1.756) | 0.606–2.45 | 0.475 | 0.291 | 2.933 C (2.846–3.020) | 31 |
| Zn | 0.928 D (0.773–1.083) | 0.604–1.28 | 0.256 | 0.276 | 1.428 D (1.336–1.521) | 7 |
Modified from Traudt et al. [8].
Different capital letters within a column indicate statistically significant differences among the metals.
Slope of logit(mortality) versus log(concentration) curve.
Number of individual-metal toxicity tests conducted.
EC50 = median effect concentration; CI = confidence interval; SD = standard deviation; CV = coefficient of variation (= SD/average).
The logit-regression slopes differed significantly among all 4 metals (Table 1). In this analysis, Cd had the shallowest slope and Cu had the steepest slope, with the slopes of Ni and Zn falling in the intermediate range (Table 1). The steepness of its concentration–response curves was inversely related to the CV of the EC50s of the metals (average slope = 0.3024 × CV−1.686, R2 = 0.815); however, this might have been a statistical artifact whereby more disperse data generally have a shallower least-squares regression slope than less disperse data.
Age-related differences in sensitivity of neonates
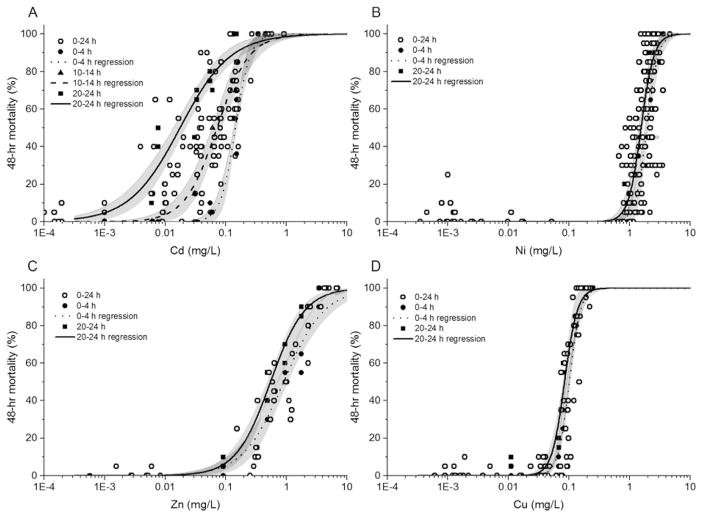
Within the first 24 h after a daphnid was released from the brood chamber, its sensitivity to some metals was highly age-dependent. Similar to the results observed for the general variability of Cd, in which the EC50 values spanned over an order of magnitude for neonates that started the tests at 0 h to 24 h old, the average EC50 values for neonates that started the age-controlled Cd toxicity tests at 0 h to 4 h, 10 h to 14 h, and 20 h to 24 h old ranged from 0.020 mg Cd/L to 0.141 mg Cd/L (Figure 1 and Table 2). The youngest neonates were the most tolerant of Cd (average EC50 [84% CI] = 0.141 mg Cd/L [0.114–0.168 mg Cd/L], n = 3 tests), whereas the oldest neonates experienced statistically significantly greater toxicity from the same exposures (average EC50 [84% CI] = 0.020 mg Cd/L [0.004–0.035 mg Cd/L], n = 3 tests). Consistent with these results, the EC50 in the single toxicity test conducted with the intermediate age group (0.071 mg Cd/L) was intermediate between the other EC50 values.
Figure 1.
Concentration–response curves for Daphnia magna neonates exposed to (A) Cd, (B) Ni, (C) Zn, or (D) Cu as a function of age at the start of 48-h static, nonrenewal lethality tests. “0–24 h” refers to individual-metal toxicity tests conducted from September 2012 through November 2013, in which the age range of the neonates at the start of the test was 0 h to 24 h. In age-controlled trials with all 4 metals, 2 age windows of neonates (0–4 h and 20–24 h postbirth) were used to start the toxicity tests. A third 4-h age window (10–14 h postbirth) was used in Cd toxicity tests. The regression curves were fitted to the composite data set of each age-controlled group, and the shaded region represents the 84% confidence interval [18].
Table 2.
Average 48-h median effect concentration and relevant parameters for immobilization of Daphnia magna neonates exposed to Cd, Cu, Ni, or Zn in repeated individual-metal static, nonrenewal lethality tests designed to evaluate the sensitivity of the organisms within several different 4-h age windows during the first 24 h postbirtha
| Metal | Organism age at start of toxicity test (h) | Average EC50 (mg/L) (84% CI) | Range (mg/L) | SD (mg/L) | CV | Slopec(84% CI) | nd |
|---|---|---|---|---|---|---|---|
| Cd | 0–4 | 0.141 (0.114–0.168)b | 0.122–0.164 | 0.021 | 0.150 | 4.613 (1.94–7.29)b | 3 |
| 10–14 | 0.071 (N/A) | N/A | N/A | N/A | 1.640 (N/A) | 1 | |
| 20–24 | 0.020 (0.004–0.035)b | 0.010–0.034 | 0.012 | 0.619 | 1.222 (0.65–1.80)b | 3 | |
| Cu | 0–4 | 0.101 (0.101–0.102)b | 0.101–0.101 | 0.00017 | 0.0020 | 4.715 (3.49–5.94) | 2 |
| 20–24 | 0.082 (0.071–0.093)b | 0.079–0.085 | 0.0040 | 0.049 | 6.820 (0–20.6) | 2 | |
| Ni | 0–4 | 2.065 (2.043–2.087)b | 2.059–2.071 | 0.0080 | 0.0039 | 6.520 (0–14.5) | 2 |
| 20–24 | 1.560 (1.251–1.868)b | 1.481–1.639 | 0.112 | 0.072 | 4.470 (3.07–5.87) | 2 | |
| Zn | 0–4 | 0.904 (0.767–1.040) | 0.869–0.939 | 0.050 | 0.055 | 1.244 (0.48–2.01) | 2 |
| 20–24 | 0.553 (0.132–0.975) | 0.445–0.662 | 0.153 | 0.277 | 1.637 (1.48–1.79) | 2 |
Tests were conducted from September through December 2014.
Statistically different EC50 values or slopes (as determined by nonoverlapping confidence intervals) between 0–4 h and 20–24 h tests for each metal.
Slope of logit(mortality) versus log(concentration) curve.
Number of toxicity tests conducted.
EC50 = median effect concentration; CI = confidence interval; SD = standard deviation; CV = coefficient of variation (= SD/average).
Along with the gradient in Cd EC50 values, the steepness of and variability around the Cd concentration–response curves were also age-dependent (Table 2). The youngest neonates, which had the highest average Cd EC50, also had the steepest toxicity curves and the lowest variability. In contrast, the oldest neonates had a 3.8-fold shallower concentration–response curve (as indicated by the slope) and a 4-fold greater variability around the average Cd EC50 (as indicated by the CV) within a 4-h age window. These results may suggest that the daphnids were released from the parent daphnid with a physiological defense mechanism [20]; however, such a mechanism might have waned as they aged and the neonates became susceptible to a variety of external factors that may have led to increased variability in the susceptibility to some metals. Alternatively, Cd (and to some extent Zn) might have interfered with important age-dependent developmental/ physiological processes that occur between 48 h and 72 h postbirth (i.e., after a test started with daphnids 0–4 h old had ended, when the daphnids were 48–52 h old, but before a test started with daphnids 20–24 h old had ended, when the daphnids were 68–72 h old). However, as discussed above for the general differences among the 4 metals, the shallower slope for the 20-h-old to 24-h-old organisms could have been a statistical artifact resulting from increased variability of toxicity responses in that age class.
In tests started with 0-h-old to 24-h-old neonates, the Zn EC50 values ranged from 0.3 mg Zn/L to 2.0 mg Zn/L. The among-age variability with Zn was considerably smaller than that with Cd (i.e., the average Zn EC50 for neonates 0–4 h old was only 1.6 times the average Zn EC50 for neonates 20–24 h old), and the average Zn EC50 values did not differ significantly between the youngest and oldest neonates (Figure 1 and Table 2). Once again, the older neonates exhibited greater variability around the EC50 than the younger organisms; however, in contrast to Cd, the concentration–response curves for the older neonates were slightly steeper (but not significantly different) than for the younger neonates. This difference between Cd and Zn could indicate different modes of toxicity of the 2 metals at that early life stage in D. magna.
Sensitivity to Cu and Ni also differed significantly as a function of age (Figure 1 and Table 2). However, the average EC50 of the 0-h-old to 4-h-old neonates was only 1.2 to 1.3 times the average EC50 of the 20-h-old to 24-h-old neonates for Cu and Ni compared with 7-fold and 1.6-fold differences for Cd and Zn, respectively. Therefore, the age-related differences in EC50 values were small for Cu and Ni. Additionally, the average slopes of the concentration–response curves for either age group were similar for Cu and Ni and did not differ significantly between the 2 age groups for either of those 2 metals.
Age-related differences in 24-h mortality
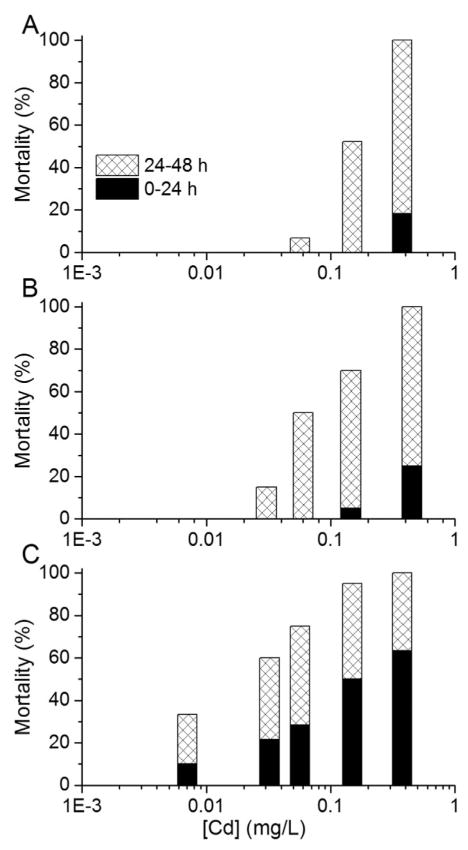
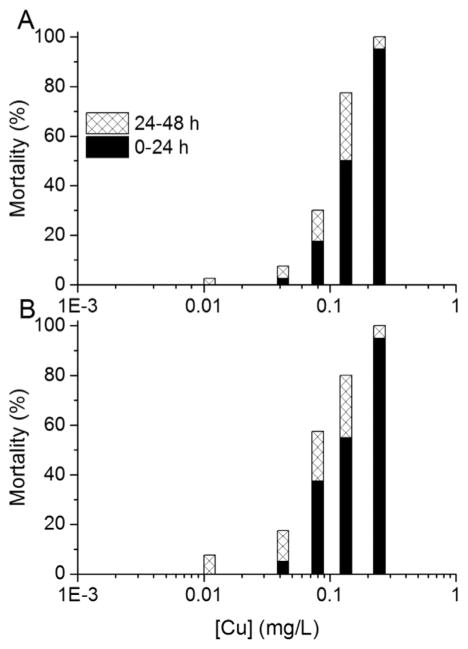
Following the trend in total mortality at 48 h of exposure, the older organisms were more likely than the younger organisms to become immobilized during the initial 24 h of exposure to some of the metals. Again, Cd exhibited the greatest difference in sensitivity among age groups, with only approximately 20% mortality after 24 h in the highest exposure concentration (~400 μg Cd/L) in tests started with 0-h-old to 4-h-old neonates, whereas tests started with 20-h-old to 24-h-old neonates had approximately the same mortality percentage in the lowest exposure concentration (~8 μg Cd/L; Figure 2).
Figure 2.
Mortality percentages during 0 h to 24 h and 24 h to 48 h intervals in 48-h static, nonrenewal lethality tests with Daphnia magna neonates exposed to Cd. Three 4-h age windows of neonates were used to start the toxicity tests: (A) 0 h to 4 h, (B) 10 h to 14 h, and (C) 20 h to 24 h postbirth. For the 0 h to 4 h and the 10 h to 14 h age groups, the averages of 3 tests are shown; only 1 test was conducted for the 20 h to 24 h age group.
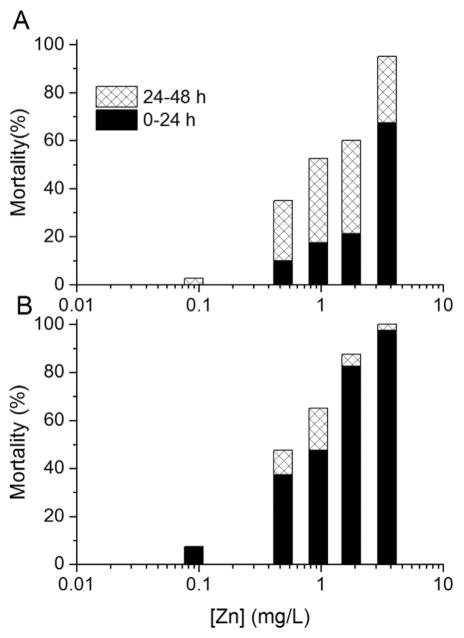
After Cd, Zn exhibited the next greatest difference in immobilization between 0-h-old to 4-h-old and 20-h-old to 24-h-old neonates after 24 h of exposure (Figure 3). In the tests started with 20-h-old to 24-h-old neonates, at least 70% of the total number of deaths in each exposure concentration occurred in the first 24 h of exposure, whereas much lower percentages of total mortality occurred in the first 24 h of exposure in the tests started with 0-h-old to 4-h-old neonates.
Figure 3.
Mortality percentages during 0 h to 24 h and 24 h to 48 h intervals in 48-h static, nonrenewal lethality tests with Daphnia magna neonates exposed to Zn. Two 4-h age windows of neonates were used to start the toxicity tests: (A) 0 h to 4 h and (B) 20 h to 24 h postbirth. The averages of 2 tests are shown for each age group.
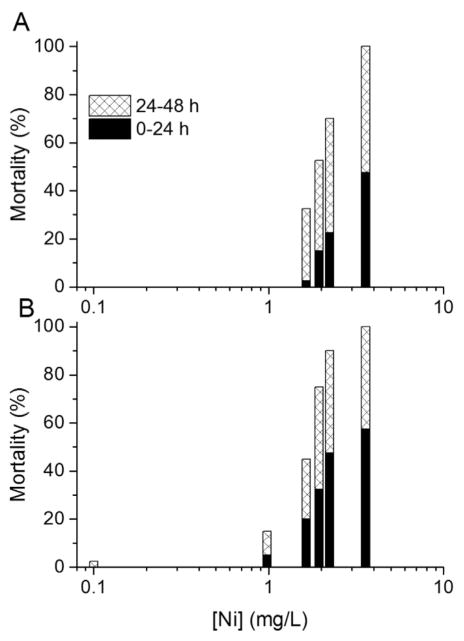
With Ni, the tests started with 0-h-old to 4-h-old neonates had only slightly less mortality in the first 24 h of exposure than the tests started with 20-h-old to 24-h-old neonates (Figure 4). Even smaller differences between tests started with the 2 different age groups occurred with Cu (Figure 5).
Figure 4.
Mortality percentages during 0 h to 24 h and 24 h to 48 h intervals in 48-h static, nonrenewal lethality tests with Daphnia magna neonates exposed to Ni. Two 4-h age windows of neonates were used to start the toxicity tests: (A) 0 h to 4 h and (B) 20 h to 24 h postbirth. The averages of 2 tests are shown for each age group.
Figure 5.
Mortality percentages during 0 h to 24 h and 24 h to 48 h intervals in 48-h static, nonrenewal lethality tests with Daphnia magna neonates exposed to Cu. Two 4-h age windows of neonates were used to start the toxicity tests: (A) 0 h to 4 h and (B) 20 h to 24 h postbirth. The averages of 2 tests are shown for each age group.
Synthesis
The present results are consistent with those of Nebeker et al. [12], who exposed various age groups of D. magna to Cd and Cu in sediments to determine if the sensitivities change as a function of the organism’s age. Those age groups ranged from less than 4 h old up to 6 d old, with intervals relevant to the present study at <4 h, <24 h, 1 d, and 2 d. Although their younger daphnids qualitatively appeared to be less sensitive to Cd than the older daphnids, Nebeker et al. [12] concluded that, at the 95% confidence level, the EC50 values for Cd and Cu did not often differ significantly among the age groups. However, those authors did not report the standard deviations or 95% CIs on the average EC50 values, thus precluding the more appropriate comparison of 84% CIs to infer statistically significant differences at the 95% confidence level [18]. In another study, 1-d-old D. magna were significantly more sensitive to dichloroaniline than were 7-d-old D. magna, but the 2 age groups had the same sensitivity to trichloroethane, dieldrin, and pentachlorophenol regardless of the organism’s age [2]. That author did not test for age-related differences within the 0-h-old to 24-h-old age group.
De Laender et al. [14] concluded that nonsimultaneous testing of individual chemicals and chemical mixtures can greatly reduce the confidence of accurately classifying a mixture as additive or nonadditive. The age-dependent sensitivity to some metals within the first 24 h postbirth that we have demonstrated in D. magna contributes to the variability introduced by nonsimultaneous testing and thus should be considered when interpreting results of metal-mixture toxicity tests.
Several mechanisms might explain age-related differences in sensitivity to some metals during the first 24 h postbirth. For example, 0-h-old to 4-h-old neonates might retain a yolk that provides nutrition [20] that is mostly consumed or no longer available in 20-h-old to 24-h-old neonates, thus increasing the susceptibility of the 20-h-old to 24-h-old neonates to Cd and Zn. This difference would not be related to general nutrition, because the 20-h-old to 24-h-old neonates in the present study were fed immediately before their toxicity tests were begun and were treated identically to the 20-h-old to 24-h-old neonates in the Cu and Ni tests, in which minimal age-related toxicity differences occurred. Instead, this explanation depends on a yet-to-be-identified attribute of the egg yolk that would preferentially protect against specific metals such as Cd. Another possible explanation is that Cd might interfere with important age-dependent developmental or physiological processes, such as the molting of the carapace, that occur between 24 h and 72 h postbirth (i.e., after the first 24 h of exposure ends in toxicity tests begun with 0-h-old to 4-h-old daphnids). In these 48-h toxicity tests, the oldest organisms (i.e., those started at age 20–24 h) usually molted before the end of the tests, but the youngest organisms (i.e., those started at age 0–4 h) only rarely molted before the end of the tests.
CONCLUSIONS
The sensitivity of D. magna neonates to Cd (and to some extent Zn, Ni, and Cu) is highly variable even within their first 24 h postbirth. Although the physiological cause of the age-related differences in sensitivity to metals is not known, the consequences to the precision of toxicity tests involving these metals and the implications for predictive models of toxicity that use those results could be important. For some routine purposes, less precise EC50 values might suffice; however, the imprecision inherent in testing neonates born during the 0-h to 24-h age window recommended by the USEPA and the OECD might introduce greater uncertainty to the determination and modeling of the toxicity of metal mixtures that contain Cd and Zn. Furthermore, in testing mixtures for additivity, uncertainties in each individual metal test may result in very large windows of uncertainty around the computed additive toxicity. Thus, small but real nonadditive effects may be missed. Additionally, some other metals that have not yet been tested for age-related differences in sensitivity might be of concern.
Supplementary Material
Acknowledgments
The present research was funded by the Copper Alliance, the Nickel Producers Environmental Research Association, the International Zinc Association, Rio Tinto, and the National Institute of Environmental Health Sciences (1R01ES020917-01). E.M. Traudt was partially supported by a teaching assistantship from the Colorado School of Mines. K. Lucas (Colorado School of Mines) assisted with the chemical analyses, and M. Ramiro Pastorinho collaborated on the experimental design.
Footnotes
Supplemental Data—The Supplemental Data are available on the Wiley Online Library at DOI: 10.1002/etc.3507.
Data Availability—Data are available from the corresponding author at etraudt@mines.edu. Chemistry and toxicity data from the age-controlled tests are provided in the Supplemental Data.
This article includes online-only Supplemental Data.
References
- 1.Koivisto S. Is Daphnia magna an ecologically representative zooplankton species in toxicity tests? Environ Pollut. 1995;90:263–267. doi: 10.1016/0269-7491(95)00029-q. [DOI] [PubMed] [Google Scholar]
- 2.Adema D. Daphnia magna as a test animal in acute and chronic toxicity tests. Hydrobiologia. 1978;59:125–134. [Google Scholar]
- 3.Naumann E. Über die Anwendung von Daphnia magna Straus als Versuchstier zur experimentellen Klarlegung der Lebensverhältnisse im Wasser. Internationale Revue der gesamten Hydrobiologie und Hydrographie. 1934;31:421–431. [Google Scholar]
- 4.Peltier WH, Weber CI. Methods for Measuring the Acute Toxicity of Effluents to Freshwater and Marine Organisms. 3. US Environmental Protection Agency; Cincinnati, OH: 1985. EPA600/4-85/013. [Google Scholar]
- 5.Organisation for Economic Co-operation and Development. OECD Guidelines for the Testing of Chemicals. Paris, France: 1984. Test No. 202: Daphnia sp. acute immobilisation test. [Google Scholar]
- 6.US Environmental Protection Agency. Methods for Measuring the Acute Toxicity of Effluents and Receiving Waters to Freshwater and Marine Organisms. 5. Washington, DC: 2002. EPA821/R-02/012. [Google Scholar]
- 7.Organisation for Economic Co-operation and Development. OECD Guidelines for the Testing of Chemicals. Paris, France: 2004. Test No. 202: Daphnia sp. acute immobilisation test. [Google Scholar]
- 8.Traudt EM, Ranville JF, Smith SA, Meyer JS. A test of the additivity of acute toxicity of binary-metal mixtures of Ni with Cd, Cu, and Zn to Daphnia magna, using the inflection point of the concentration–response curves. Environ Toxicol Chem. 2015;35:1843–1851. doi: 10.1002/etc.3342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meyer JS, Ranville JF, Pontash M, Gorsuch JW, Adams WJ. Acute toxicity of binary and ternary mixtures of Cd, Cu, and Zn to Daphnia magna. Environ Toxicol Chem. 2015;34:799–808. doi: 10.1002/etc.2787. [DOI] [PubMed] [Google Scholar]
- 10.Ebert D. Ecology, Epidemiology, and Evolution of Parasitism in Daphnia. National Center for Biotechnology Information; Bethesda, MD, USA: 2005. [Accessed: 15 December 2015]. Introduction to Daphnia biology; pp. 5–18. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?db=Books. [Google Scholar]
- 11.McKee D, Ebert D. The effect of temperature on maturation threshold body-length in Daphnia magna. Oecologia. 1996;108:627–630. doi: 10.1007/BF00329035. [DOI] [PubMed] [Google Scholar]
- 12.Nebeker AV, Cairns MA, Onjukka ST, Titus RH. Effect of age on sensitivity of Daphnia magna to cadmium, copper and cyanazine. Environ Toxicol Chem. 1986;5:527–530. [Google Scholar]
- 13.Meyer JS, Farley KJ, Garman ER. Metal mixtures modeling evaluation project: 1. Background. Environ Toxicol Chem. 2015;34:726–740. doi: 10.1002/etc.2792. [DOI] [PubMed] [Google Scholar]
- 14.De Laender F, Janssen CR, De Schamphelaere KAC. Non-simultaneous ecotoxicity testing of single chemicals and their mixture results in erroneous conclusions about the joint action of the mixture. Chemosphere. 2009;76:428–432. doi: 10.1016/j.chemosphere.2009.03.027. [DOI] [PubMed] [Google Scholar]
- 15.Ma H, Kim SD, Cha DK, Allen HE. Effect of kinetics of complexation by humic acid on toxicity of copper to Ceriodaphnia dubia. Environ Toxicol Chem. 1999;18:828–837. [Google Scholar]
- 16.American Public Health Association, American Water Works Association, Water Environment Federation. Standard Methods for the Examination of Water and Wastewater. 22. Denver, CO: 2012. [Google Scholar]
- 17.Zeisler R, Murphy KE, Becker DA, Davis WC, Kelly WR, Long SE, Sieber JR. Standard Reference Materials® (SRMs) for measurement of inorganic environmental contaminants. Anal Bioanal Chem. 2006;386:1137–1151. doi: 10.1007/s00216-006-0785-7. [DOI] [PubMed] [Google Scholar]
- 18.Julious SA. Using confidence intervals around individual means to assess statistical significance between two means. Pharm Stat. 2004;3:217–222. [Google Scholar]
- 19.Payton ME, Greenstone MH, Schenker N. Overlapping confidence intervals or standard error intervals: What do they mean in terms of statistical significance? J Insect Sci. 2003;3:34. doi: 10.1093/jis/3.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mittmann B, Ungerer P, Klann M, Stollewerk A, Wolff C. Development and staging of the water flea Daphnia magna (Straus, 1820; Cladocera, Daphniidae) based on morphological landmarks. Evodevo. 2014;5:12. doi: 10.1186/2041-9139-5-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.