Abstract
Background
Breast cancer patients’ misunderstanding of their systemic cancer recurrence risk has consequences on decision-making and quality of life. Little is known about how women derive their risk estimates.
Methods
Using Los Angeles and Georgia’s SEER registries (2014–2015), a random sample of early-stage breast cancer patients was sent surveys about 2–3 months after surgery (N=3930, RR 68%). We conducted inductive thematic analysis of open-ended responses about why women chose their risk estimates in a uniquely large sub-sample (N=1754). Clinician estimates of systemic recurrence risk were provided for patient subgroups with DCIS and with low, intermediate, and high-risk invasive disease. Women’s perceived risk of systemic recurrence (0–100%) was categorized as overestimation, reasonably accurate estimation, or underestimation (0% for invasive disease) and was compared across identified factors and by clinical presentation.
Results
Women identified 9 main factors related to their clinical experience (e.g., diagnosis and testing; treatment) and non-clinical beliefs (e.g., uncertainty; spirituality). Women who mentioned at least one clinical experience factor were significantly less likely to overestimate their risk (12% vs. 43%, p<.001). Most women who were influenced by “communication with a clinician” had reasonably accurate recurrence estimates (68%). “Uncertainty” and “family and personal history” were associated with overestimation, particularly for women with DCIS (75%; 84%). “Spirituality, religion, and faith” was associated with underestimation of risk (63% vs. 20%, p<.001).
Limitations
The quantification of our qualitative results is subject to any biases that may have occurred during the coding process despite rigorous methodology.
Conclusions
Patient-clinician communication is important to breast cancer patients’ understanding of their numeric risk of systemic recurrence. Clinician discussions about recurrence risk should address uncertainty and relevance of family and personal history.
Introduction
Women diagnosed with breast cancer almost always want information about their risk of systemic disease recurrence following treatment.1–3 Since early-stage breast cancer therapy is designed to prevent an incurable, distant metastatic recurrence, reasonable understanding of recurrence risk affects patients’ ability to participate in shared decision-making during the treatment phase4–6 and also impacts their quality of life during survivorship.4, 7, 8 Patient-clinician discussions about systemic recurrence risk can be challenging, especially for women with lower health literacy, lower numeracy skills, or considerable anxiety or worry post-diagnosis.7, 9–12 Previous research has shown that many women overestimate their recurrence risk after treatment,7, 11, 12 while other women underestimate their risk, including a substantial minority of women with invasive disease who believe they have zero risk.11, 12
Although many studies have documented that women do not understand their risk after treatment, few have examined what influences women’s perceptions of their recurrence risk. Of these, one focused on local recurrence and found beliefs were primarily related to weight and lifestyle behaviors (e.g., diet, exercise and tobacco use).13 In studies focused on recurrence more generally, women mentioned estrogen replacement therapy as a possible contributor to recurrence,14, 15 while prayer, weight, lifestyle behaviors, and a positive attitude were thought to help prevent recurrence.16–19 Unfortunately, these studies have been limited by relatively small sample sizes in non-diverse populations and have not specifically focused on what influences the development of numeric risk estimates. A better understanding of the factors that influence these estimates would provide a foundation for improved patient-clinician risk communication interventions.
To provide a more comprehensive understanding of how women derive their recurrence risk perceptions, this study used inductive thematic analysis to identify factors that influenced early-stage breast cancer patients’ numeric risk estimates in a large, diverse population-based sample. We then explored whether the factors women mentioned differed by clinical presentation and/or by understanding of recurrence risk.
Methods
Study Population
The iCanCare Study, a large, diverse, population-based survey study of women with favorable prognosis breast cancer, accrued women aged 20–79 with newly diagnosed breast cancer (ductal carcinoma in situ, DCIS and stages I–II) as identified by rapid reporting systems from the Surveillance Epidemiology and End Results (SEER) registries of Georgia and Los Angeles County in 2014–15. Black, Asian, and Hispanic women were oversampled in Los Angeles.20
Data Collection
Patients were sent surveys approximately 2–3 months after surgery. The median time between SEER date of diagnosis and receipt of the survey was 7 months. We provided a $20 cash incentive and used a modified Dillman method for patient recruitment, as done in prior work.20, 21 All materials were sent in English and Spanish to those with Spanish surnames.20 Survey responses were merged with clinical data from SEER. The study was approved by the Institutional Review Boards of the University of Michigan, University of Southern California, Emory University, the Committee for the Protection of Human Subjects, and the California Cancer Registry. Please note that our funding source, the National Cancer Institute (NCI), had no role in this study.
Questionnaire Design and Content
Patient questionnaire content was guided by a conceptual framework, research questions, and hypotheses. We chose established measures when available and developed new measures, when necessary, drawing from the literature and our prior research.22–24 We used standard techniques to assess content validity, including expert review, cognitive pre-testing, and pilot studies in clinic populations.25, 26
The iCanCare survey was divided into a number of sections including items/measures on quality of life, diagnosis, testing and treatment for breast cancer, decision-making, communication, support from others, thoughts and feelings, family history, and demographics. This paper focuses on items from the section entitled “thoughts and feelings.” This section was devoted to patient perception of risk of recurrence, doctor communication about risk, and worry about recurrence. Previous papers from our group have focused on doctor communication11 and worry.7 Measures specific to this current sub-study are listed below.
Measures
Perceived risk of systemic recurrence
As part of the survey, women were asked two questions about their perceptions of their numeric recurrence risk. First, they were asked to give a numeric estimate from 0 to 100% in response to the following question: “After receiving all the planned treatments, what do you think is the chance that your cancer will spread to other parts of your body within 10 years?” Secondly, women were asked an open-ended follow-up question, “Why did you pick this number?” Some women had completed all primary treatment when the survey was sent to them, while others were still receiving radiation and/or chemotherapy. As a result, we chose to include the phrase “after receiving all the planned treatments” in the first question so that all women were using the same context in making their recurrence risk estimate.
Data Analysis
Part I: Identifying factors that influenced women’s numeric estimates of systemic recurrence risk
A primary analyst with qualitative research training thematically coded27 women’s responses to the question, “Why did you pick this number?” (N=1754).28 The responses were coded without knowledge of the numeric response that women provided in the preceding question. Each woman’s response could be assigned to more than one code, as some women mentioned multiple reasons for selecting their numeric estimates. A second analyst independently reviewed 1000 randomly selected open-ended survey responses. There was high concordance between the codes identified by the primary and secondary data analysts; the secondary analyst did not identify any new codes and small discrepancies were resolved though discussion. A study co-investigator with training in qualitative methodology held regular meetings with the two analysts to discuss the emerging factors identified by women and to make adjustments to the codes as needed.
Once there was a consensus that theoretical saturation had been reached, a final codebook was decided upon, and the primary analyst completed the coding/recoding utilizing the following factor codes: diagnosis and testing; treatment; uncertainty; personality traits and emotions; communication with a clinician; current and future health; spirituality, religion, and faith; family and personal history; statistics, research, and stories of others; and other (Table 2 provides factor code descriptions and examples). Two of the codes had valence that should be noted. “Personality traits and emotions” included responses that indicated both positive and negative traits and emotions; women may have mentioned something positive (e.g., “positive thinking”), something neutral (e.g., “just a feeling”), or something negative, including mentions of fear of recurrence (e.g., “just worry about it coming back”). “Family and personal history” included both respondents who mentioned having the presence of a family and/or personal history of cancer and those who mentioned the absence of a family and/or personal history of cancer. Additionally, there were two different subcategories of “uncertainty.” It included women who were uncertain due to a lack of knowledge regarding recurrence risk (e.g., “I don’t know,” “I’m unsure”) and women who were uncertain due to their attitude that recurrence, or life in general, is unpredictable (e.g., “always a chance,” “50/50”).
Table 2.
Findings from Qualitative Analysis: Final Code Descriptions and Representative Examples
| CODE | DESCRIPTION | EXAMPLES |
|---|---|---|
| Diagnosis and Testing | Characteristics of the respondent’s breast cancer and tests used to better understand the breast cancer or the respondent’s risk of cancer | “Cancer was very small.” “I feel that my breast cancer was diagnosed very early.” “Oncotype DX.” “I had DCIS Stage 0.” |
| Treatment | Respondent’s perception of cancer treatments’ effectiveness or of healthcare providers’ abilities in regard to providing treatment | “I think chemo + radiation took care of all the cancer.” “Because it was fully removed.” “I had clear margins” “Because I trust my Drs.” |
| Uncertainty | Respondent is uncertain herself, perceives cancer or life to be unpredictable or uncertain, or believes there is a 50/50 chance | “I don’t know.” “Just a guess.” “Always a chance.” “50/50 chance.” |
| Personality Traits and Emotions | Respondent’s personality, emotions, or attitude | “Positive thinking.” “I am trying to be as optimistic as possible.” “I hope it doesn’t spread.” “Just worry about it coming back.” |
| Communication with a Clinician | Clinicians’ opinions or comments about the respondent’s cancer or information provided by clinicians | “It is what I was told.” “% the doctor said it could come back.” “What I was given by oncologist.” “Information from my doctor.” |
| Current and Future Health | How the respondent currently feels as well as her lifestyle or surveillance/prevention behaviors | “I have made lifestyle changes.” “Because I am generally healthy.” “Because of my age.” “I get regular check-ups and mammograms.” |
| Spirituality, Religion, and Faith | Any mention of God, religion, or faith | “Because of my faith in God.” “Faith.” “I pray to God that it does not come back.” “‘I believe in God’s healing.” |
| Family and Personal History | Past family or personal experiences with cancer or the lack thereof, or personal experiences that the respondent feels are related to her breast cancer | “Family history.” “No family history.” “Because on my mother’s side of the family there have been several kinds of cancer.” “Get cancer once, you have a higher risk of getting elsewhere.” |
| Statistics, Research, and Stories of Others | Sources of information not explicitly said to be from healthcare providers: statistics, research, and stories of friends and other non-family members | “Based on research I have done.” “After reading information.” “I have known people that it has come back in different places.” “That is what stats showed in comparable situation.” |
| Other | A response that is uncommon and does not warrant the creation of a new code, and/or does not allow clear placement into a code | “Because it’s low.” “Because that’s what I think.” (Other miscellaneous responses that were even less common) |
As a final step to test for interrater reliability, the secondary analyst independently coded a random selection of 100 responses using the final codebook. There was 91% interrater agreement after comparison. As percent agreement may overestimate coding consistency between two raters,29, 30 we used further discussion about the coding process to confirm shared interpretation of the codes. Afterwards, minor modifications were made where necessary, and coding adjusted if relevant for all respondents.
Part II: Assessing the relationship between identified factors and patient understanding of risk
Clinicians’ estimates of systemic recurrence risk
According to practice guidelines and the published literature,31 and in consultation with breast cancer physician co-investigators (R.J., A.W.K.), we used stage, histology, and biology from SEER to estimate systemic recurrence risk for patient sub-groups following treatment (surgery, radiation, chemotherapy). Women were considered to have almost no risk of systemic recurrence if SEER data indicated stage 0 (DCIS); low risk of systemic recurrence (<10% risk of systemic recurrence) if SEER data indicated stage IA, estrogen receptor (ER)-positive, HER2-negative, tumor grade 1–2, and tumor genomic profiling with the 21-gene recurrence score (RS) either not done or RS 0–10 32; intermediate risk of systemic recurrence (<20% risk of systemic recurrence) if SEER data indicated stage IA, ER-positive, HER2-negative, tumor grade 1–2, and RS recurrence score >10; or stage IA, ER-positive, HER2-negative, and tumor grade 3+; or stage IB or IIA, ER-positive, HER2-negative, with any tumor grade and any RS status; or high risk of systemic recurrence (<40% risk of systemic recurrence) if SEER data indicated stage IIB, ER-negative, and/or HER2-positive.
Categorizing patients’ understanding of systemic recurrence risk
We then compared women’s perceived recurrence risk with their clinically estimated recurrence risk, categorizing each woman into one of three perceived risk sub-groups: underestimation, reasonably accurate estimation, and overestimation of systemic risk of recurrence after treatment. Any woman with invasive disease who responded that her risk of systemic recurrence was 0% was placed in the underestimation risk group, as 0% represents substantial underestimation of recurrence risk.31 The overestimation risk group included women with DCIS who reported perceived risk as greater or equal to 10%, women with low-risk invasive breast cancer who reported perceived risk as greater or equal to 20%, women with intermediate-risk invasive breast cancer who reported perceived risk as greater or equal to 30%, and women with high-risk invasive breast cancer who reported perceived risk as greater or equal to 50%. All remaining women were put in the reasonably accurate risk group; this included women with DCIS who wrote their risk of systemic recurrence was 0%, as these women have almost no systemic recurrence risk. Women in the intermediate-risk and high-risk invasive groups were subsequently combined into a “higher-risk invasive” group as their findings yielded similar patterns.
Identifying factors to compare by understanding of risk and clinical presentation
After comparing women’s understanding of risk by which factor (final code) influenced their estimates, we selected the factors that had the strongest associations with underestimation, reasonably accurate estimation, or overestimation to be further examined by clinical presentation.
All statistical comparisons were conducted on the qualitative results using chi-squared tests.
Results
We identified 3930 women with breast cancer from the SEER registries: 258 were later considered ineligible due to a prior breast cancer diagnosis or stage III or IV disease; residing outside the SEER registry area; or being deceased, too ill, or unable to complete a survey in Spanish or English. Of the 3672 eligible women, 2502 (68%) patients responded, and 1172 refused to participate or did not return mailed surveys even after follow-up efforts. Of the 2502 respondents, 748 women were excluded from this sub-study: 478 women did not answer both survey questions about numeric risk of systemic recurrence, 87 women had insufficient information to determine their understanding of risk due to missing clinical information from SEER, and 183 women had a very high recurrence risk, which made them outside of the primary focus of the iCanCare Study, which aims to observe women with favorable prognoses. Thus, the final study sample was 1754 women.
The study design oversampled invasive cancer patients and nonwhite patients, and had significantly lower response rates from nonwhite patients and Georgia residents. The 478 women who did not answer the questions about numeric recurrence risk were significantly more likely to be older, nonwhite, and with lower educational status. Weights were developed to account for these differences and all analyses were repeated using these weights. All significant comparisons below remained significant when weights were used.
Table 1 shows characteristics of the study sample. The majority of women were 50 years or older (80%) and had some college education (71%). Additionally, nearly half were non-white: 9% were Asian, 17% non-Hispanic Black, and 18% Latina. Most women had no family history of breast cancer (96%), and 22% had a diagnosis of DCIS (stage 0) while 78% had invasive disease (stages I–II). Almost two-thirds of women had lumpectomy (63%), with the remainder divided equally between unilateral and bilateral mastectomy. Approximately half of the sample had radiation and about one quarter had received chemotherapy.
Table 1.
Characteristics of Study Sample, N=1754
| Variables | Na (%) |
|---|---|
| Sociodemographic Factors | |
| Age | |
| Under 50 | 352 (20%) |
| 50–65 | 846 (48%) |
| 65 and over | 556 (32%) |
| Race | |
| Asian | 163 (9%) |
| Non-Hispanic White | 956 (55%) |
| Non-Hispanic Black | 295 (17%) |
| Latina | 316 (18%) |
| Other/Unknown/Missing | 24 (1%) |
| Education | |
| High school diploma or less | 480 (27%) |
| Some college or more | 1240 (71%) |
| Clinical Factors | |
| Family History | |
| No family history of breast cancer | 1679 (96%) |
| 1 or more family member with breast cancer | 73 (4%) |
| SEER Stage | |
| 0 | 382 (22%) |
| I | 978 (56%) |
| II | 392 (22%) |
| Surgery Type | |
| Lumpectomy | 1096 (63%) |
| Unilateral mastectomy | 311 (18%) |
| Bilateral mastectomy | 316 (18%) |
| Radiation Therapy | |
| No | 797 (45%) |
| Yes | 927 (53%) |
| Chemotherapy | |
| No | 1322 (75%) |
| Yes | 397 (23%) |
| Comorbidities | |
| None | 1202 (69%) |
| 1 or more | 552 (31%) |
N values for education, family history, SEER stage, surgery type, radiation therapy, and chemotherapy do not sum to the analytic sample N due to missingness; accordingly, their percentages may not sum to 100%
Table 2 provides descriptions of the 9 main factors identified by women and “other” (the final codes) and representative examples of answers to “why did you pick this number?” (N=1754). 68% of women mentioned one factor, 24% mentioned two factors, and 8% mentioned three or more factors. Each factor was mentioned with similar frequency across the clinical presentation sub-groups (see Supplementary Table 1).
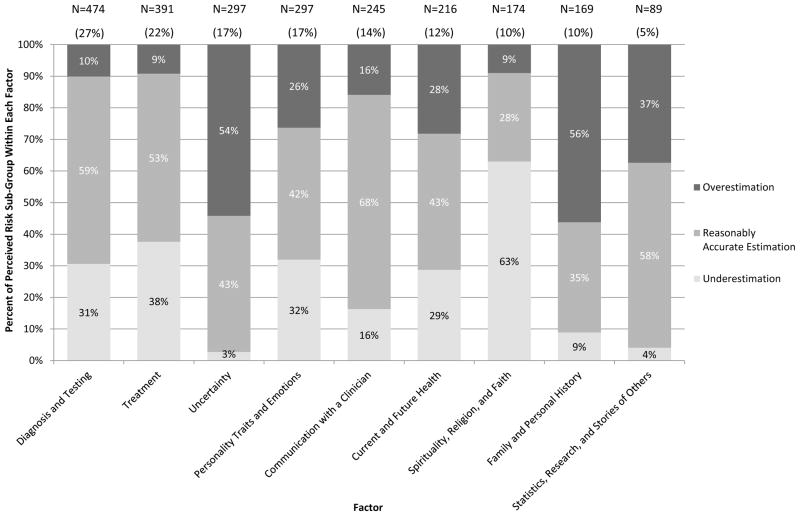
Figure 1 displays how women’s understanding of their risk of systemic recurrence varied by the factors used to derive risk estimates. Because women could have mentioned more than one reason for their estimates, the sum of the number of women who mentioned each factor is greater than the analytic sample. The two most common factors were “diagnosis and testing” and “treatment,” which influenced 27% and 22% of women, respectively; they were the two most common factors mentioned irrespective of clinical presentation. Note that “other” is not included in Figure 1 as 96% of women who mentioned something coded as “other” also mentioned one of the 9 main factors.
Figure 1.
Distribution of factors by patient understanding of risk of systemic recurrence.
Across the entire sample we found that four factors—“communication with a clinician,” “spirituality, religion, and faith,” “uncertainty,” and “family and personal history” — had very strong associations with women’s understanding of risk (i.e., with either reasonably accurate estimation, underestimation, or overestimation). For example, women who mentioned being influenced by “communication with a clinician” were significantly more likely to have a reasonably accurate understanding of their recurrence risk (68% vs. 46%, p<.001). Women who were influenced by “spirituality, religion, and faith,” were more likely to underestimate their risk as zero percent (63% vs. 20%, p<.001). Conversely, women who were influenced by “uncertainty” and/or “family and personal history” were more likely to overestimate their risk (54% vs. 22%, p<.001 and 56% vs. 24%, p<.001, respectively). Given that we had used a numeric scale (0 to 100%), we further explored the 158 women out of 1754 (9%) who said 50%. When we re-analyzed our results with these 158 women removed from the sample, all significant findings remained significant. Two-thirds of women who estimated 50% as their recurrence risk wrote a response as to “why” that was coded “uncertainty” (N=95, 42%) and/or “family and personal history” (N=53, 24%).
As “family and personal history” had valence, we completed a sensitivity analysis of all women who mentioned it in their response. Among women who wrote in their response to “why did you pick this number” that they had the presence of a family or personal history N=143, 90 (63%) overestimated their risk, 43 (30%) were reasonably accurate, and 10 (7%) underestimated. Among women who wrote a response indicating the absence of a family or personal history N=26, 4 (15%) overestimated, 17 (65%) were reasonably accurate, and 5 (19%) underestimated their clinically determined risk. Overestimation was significantly more likely for women with the presence a family or personal history compared to all other women (p<.001). There was not a significant difference for women with the absence of a family or personal history compared to all other women (p=.302), though this was a small group of women.
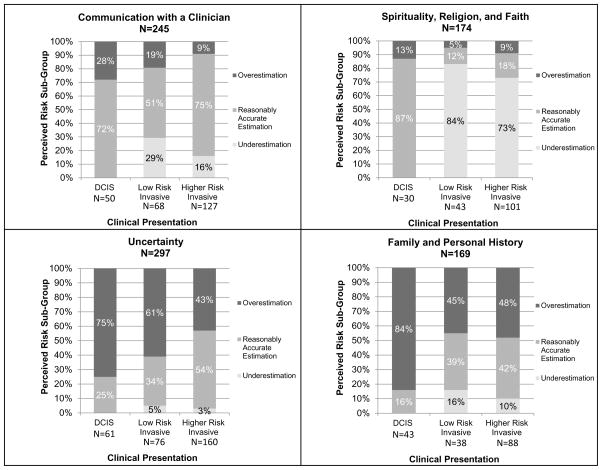
We further examined the four factors with very strong associations with women’s understanding of risk by clinical presentation to separately observe their influence for women with DCIS, low-risk invasive disease, and higher-risk invasive disease (Figure 2).
Figure 2.
Factors that exhibited strong associations with a perceived risk subgroup across the entire sample delineated by clinical presentation.
Communication with a Clinician: For women with DCIS and higher-risk invasive disease, those who specifically mentioned they were influenced by “communication with a clinician” were very likely to have a reasonably accurate estimation of their risk (72% and 75%, respectively). This relationship between patient-clinician communication and understanding of risk was also strong among women with low-risk invasive disease (51%), although a substantial minority (29%) of these women underestimated their risk of recurrence.
Spirituality, Religion, and Faith: Over 70 percent of the 144 women with invasive disease who were influenced by spirituality, religion, and faith underestimated their risk as zero percent. In contrast, most (87%) of women with DCIS who were influenced by spirituality, religion, and faith were reasonably accurate estimators. A majority of these women actually also reported zero percent risk (83%), however, as noted earlier, this was categorized as reasonably accurate estimation, as women with DCIS do essentially have zero risk of systemic recurrence.
Uncertainty and 4. Family and Personal History: “Uncertainty” and “family and personal history” were associated with considerable overestimation of risk for women with all types of clinical presentation, though women with DCIS who mentioned these factors were far more likely to overestimate their numeric risk of systemic recurrence (75% and 84%) compared to women with low-risk invasive disease (61% and 45%, p<.001) or with higher-risk invasive disease (43% and 48%, p<.001).
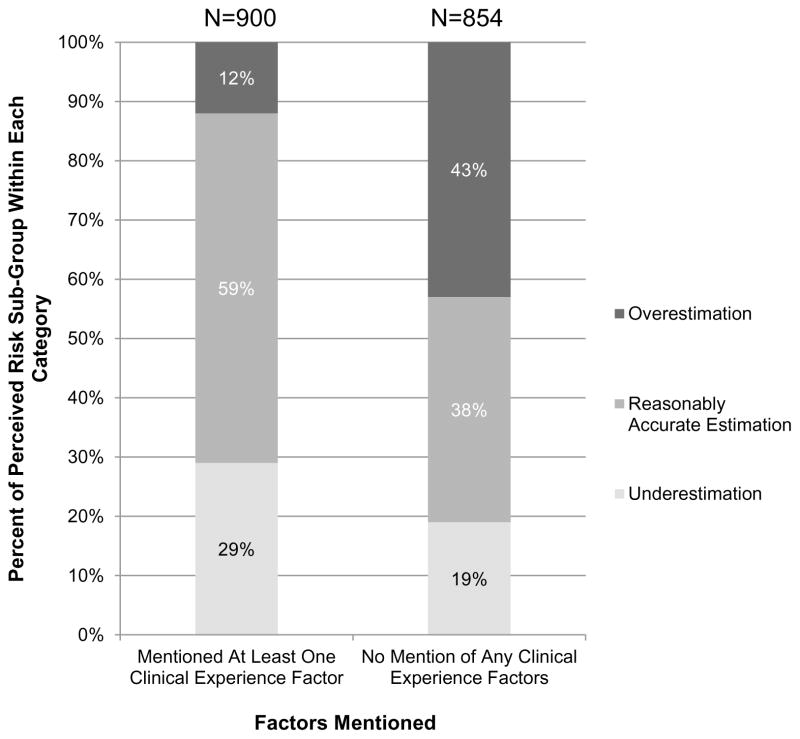
For final analyses, we collapsed the clinical presentation sub-groups and dichotomized the factors women used to derive risk estimates into 2 categories: (1) responses that related to some clinical aspect of the current cancer experience (i.e., communication with a clinician, diagnosis and testing, and/or treatment) versus (2) no mention of any of these factors (Figure 3). About half of the women in our sample included some mention of their current cancer experience as an influence in their risk perception. Those women who did not mention any aspect of their communication with clinicians, clinical diagnosis, testing, or treatment were less likely to have a reasonably accurate understanding of their risk of recurrence (38% vs. 59%, p<.001) and were substantially more likely to overestimate their risk (43% vs. 12%, p<.001).
Figure 3.
Patient understanding of risk of systemic recurrence based on mention of clinical experience factors. Clinical experience factors included “communication with a clinician,” “diagnosis and testing,” and “treatment.”
Discussion
Within this large, diverse population-based sample of women with newly diagnosed DCIS and early-stage invasive breast cancer, women mentioned a wide variety of clinical and non-clinical factors that they used to derive their perception of their systemic recurrence risk after treatment. While previous studies have also found that many women with breast cancer are not accurate in their numeric recurrence risk estimates7, 11–12 and have noted that women use factors that are not clinically relevant to recurrence to estimate its likelihood,13–19 current literature, to our knowledge, has not explored the connections between these two elements with an inductive qualitative methodology. Our application of this methodology and a large sample size allowed us (1) to comprehensively identify multiple factors that influence women’s development of their numeric risk estimates about systemic recurrence and (2) to explore the effects of these factors on understanding of risk.
This paper’s findings suggest that clinicians should discuss the true factors that influence recurrence risk with their patients. Across all women in this study, those who were influenced by a least one factor related to their clinical experience, “diagnosis and testing”; “treatment”; “communication with a clinician,” were much more likely to have a reasonably accurate understanding of their risk of systemic recurrence than women who were solely influenced by other factors. Specifically, mention of “spirituality, religion, and faith” had the strongest association with underestimation in our sample of women with DCIS and early-stage invasive disease, and “uncertainty” and “family and personal history” had the strongest associations with overestimation.
One factor, “communication with a clinician” played a particularly positive role for women at the extremes of the risk spectrum: those with DCIS or higher-risk invasive disease. Over 70% of women in each group who mentioned “communication with a clinician” had reasonably accurate estimates, consistent with our previous work.11 A considerable percentage of women with low-risk invasive disease who mentioned “communication with a clinician” actually underestimated their risk. It may be that if clinicians use only qualitative terms such as “low risk,” some patients may incorrectly interpret this to mean “no chance” of recurrence.33, 34 Clinician explanations that include both a number as a frame of reference along with words may be particularly important for women with low health literacy and/or numeracy.11
Women who were influenced by “spirituality, religion, and faith” considered their risk of systemic recurrence to not only be low but to be “zero percent,” irrespective of clinical presentation. Specifically, for women with invasive cancer, this meant substantially underestimating recurrence risk. Other research has found that some women believe that prayer and God’s will might help to prevent cancer recurrence.16, 35 Because underestimation may be linked to negative behaviors, such as non-adherence to adjuvant endocrine therapy or annual mammographic screening, our results suggest that clinicians carefully review the importance of closely following survivorship recommendations with women, irrespective of religious orientation, explaining that the recommendations have relevance regardless of one’s own perceived risk.36–38
Prior research has also shown that some women voice a sense of “uncertainty” about their risk of breast cancer or breast cancer recurrence.18, 39 We found that women who mentioned uncertainty overwhelmingly overestimated their recurrence risk, especially women with DCIS. Others have also reported that women with DCIS may be particularly vulnerable to overestimating their risk,7, 12, 40 and our findings provide further insight that uncertainty may contribute to this misunderstanding. Additionally, the different forms of “uncertainty” reported by women in our study (e.g., “I don’t know,” “always a chance,” “50/50”) support the inherent variability of the construct. Mishel’s theory of uncertainty in illness as well as work by Han et al. discuss how patients’ uncertainty can relate to the illness and/or the health care system and may be due to a lack of knowledge or the unpredictability of the situation.41, 42 In our sample, women were more likely to overestimate their risk regardless of which subcategory of uncertainty they expressed. When uncertainty is due to a lack of knowledge, it may be effectively reduced by simplifying and reorganizing the risk information.42 Some uncertainty due to an illness may be irreducible, as even clinicians must rely on estimates.41, 42 Clinicians should help women accept the uncertainty inherent in predicting disease recurrence without letting it lead to marked inaccuracy in perceptions of the magnitude of risk.42, 43
Finally, our finding that family and personal history influences women’s risk estimates is consistent with prior studies.44–48 We previously reported that women who had a family history of breast cancer were more likely to overestimate their systemic recurrence risk,7 and our findings are similar in the present study. However, we expand prior work through finding overestimation is more likely not only if women have a family history7 but also if women state that their family history influenced the development of their recurrence risk estimate. When women have a family or personal history, it may naturally generate feelings of increased risk, which for some may be warranted, but for many less so, as family history is likely related to patients’ risk of a new cancer not their risk of systemic recurrence.49, 50 Focused discussions of how family and personal cancer history are relevant to a woman’s current cancer experience may help to sort out this complex dynamic.7 Special consideration should be given to women with DCIS, as risk overestimation was present in a remarkably large percentage (84%) of these women who mentioned family and personal cancer history, and while overestimation of recurrence risk may lead to positive behaviors, such as exercising, there is also strong evidence that it may be harmful—breast cancer survivors’ overestimation has been associated with worry, anxiety, and a worse quality of life.7,12 Thus, clinicians should aim to correct for overestimations of risk while encouraging women to develop and/or maintain healthy behaviors. Future research might use the factors we have identified in this qualitative analysis to develop closed-ended survey questions in order to (1) gain a better understanding of how commonly these factors influence women’s estimates and (2) why these factors influence women’s numeric risk estimates.
This study’s strengths include its large, diverse population-based sample and in-depth, inductive qualitative methodology. However, there are some potential limitations. Caution is necessary when generalizing these findings to regions dissimilar to the two areas from which our patients were drawn: Los Angeles and Georgia. Additionally, despite our use of investigator triangulation,51 the quantification of the results from our qualitative analysis is subject to any biases that remained during the coding process.
We also note limitations with the survey questions. The first question’s use of the term “planned treatments” may have been a confounder, and some patients might conflate true cancer recurrence (the failure to fully eradicate the cancer, such that it is able to spread) with new primary cancer development (eradication of the existing cancer but development of an entirely new cancer that spreads). Also, our findings are limited by our use of a numeric risk estimate of systemic recurrence, which may not fully represent respondents’ underlying understanding of their likelihood of recurrence. Past research has demonstrated that some respondents have difficulty generating responses to such questions because they have lower numeracy skills. As we have discussed above, some people answer these questions with a 50% estimate when what they mean by that response is a qualitative sense of uncertainty about an uncertain event (i.e., it might happen, or it might not).52 Even among more numerate respondents, there is a tendency for individuals to estimate numeric risk in clusters, perhaps focusing on the “gist” meaning of a numeric scale (i.e., low versus high) rather than truly estimating the underlying likelihood.53 Alternate approaches to measuring risk perceptions include providing respondents with a probability scale to use when making a numeric estimate,54 focusing on relative risk perceptions (e.g., Dillard)55 or using verbal terms (e.g., Berry),56 although the research shows that each of these approaches have their own drawbacks.57–58 Thus, while we can conclude that many breast cancer survivors misunderstand their numeric recurrence risk, we cannot claim that the only reasons behind the underestimation and overestimation shown in this study are the influencing factors that we identified, as some of the inaccuracy may have been due to the effects of the numeric scale. Future research to evaluate the associations between the factors identified here and numeric estimates provided through the use of a probability scale, gist estimates, or relative estimates of risk (e.g., questions asking respondents for perceptions of how their risk compares to that of the average patient) may yield further insights.
Conclusion
This study of more than 1700 women enabled a greater understanding of what factors influence breast cancer patients’ perceptions of their systemic recurrence risk. To our knowledge, this has been the largest study to qualitatively analyze reasons for reporting risk of recurrence estimates. Patient-reported communication with a clinician about disease recurrence was most commonly associated with patients’ having a reasonable understanding of risk. These findings emphasize the importance of cancer clinicians educating patients about their systemic cancer recurrence risks, given the documented negative consequences of overestimation and underestimation of risk. It also reinforces the need to clarify with patients what factors actually do and do not influence recurrence risk, including asking patients about how they derive their risk estimates. Priorities moving forward include informing clinicians about best practices to present risk information in the challenging circumstances of a cancer diagnosis, with a specific focus on uncertainty and family and personal cancer history, especially among women with DCIS.
Supplementary Material
Acknowledgments
Financial support for this study was provided entirely by a grant (P01 CA163233) from the National Cancer Institute (NCI) of the National Institutes of Health. The funding agreement ensured the authors’ independence in designing the study, interpreting the data, writing, and publishing the report.
We acknowledge the work of our project staff (Mackenzie Crawford, M.P.H. and Kiyana Perrino, M.P.H. from the Georgia Cancer Registry; Jennifer Zelaya, Pamela Lee, Maria Gaeta, Virginia Parker, B.A. and Renee Bickerstaff-Magee from USC; Rebecca Morrison, M.P.H., Alexandra Jeanpierre, M.P.H., Stefanie Goodell, B.S., and Rose Juhasz, Ph.D. from the University of Michigan). We acknowledge with gratitude the breast cancer patients who responded to our survey.
The collection of Los Angeles County cancer incidence data used in this study was supported by the California Department of Public Health pursuant to California Health and Safety Code Section 103885; Centers for Disease Control and Prevention’s (CDC) National Program of Cancer Registries, under cooperative agreement 5NU58DP003862-04/DP003862; the National Cancer Institute’s Surveillance, Epidemiology and End Results Program under contract HHSN261201000140C awarded to the Cancer Prevention Institute of California, contract HHSN261201000035C awarded to the University of Southern California, and contract HHSN261201000034C awarded to the Public Health Institute. The ideas and opinions expressed herein are those of the author(s) and endorsement by the State of California, Department of Public Health, the National Cancer Institute, and the CDC or their Contractors and Subcontractors is not intended nor should be inferred. The collection of cancer incidence data in Georgia was supported by contract HHSN261201300015I, Task Order HHSN26100006 from the NCI and cooperative agreement 5NU58DP003875-04-00 from the CDC. The ideas and opinions expressed herein are those of the author(s) and endorsement by the States of California and Georgia, Department of Public Health the National Cancer Institute, and the Centers for Disease Control and Prevention or their Contractors and Subcontractors is not intended nor should be inferred.
Footnotes
Disclosure: the authors have no conflicts of interest to report.
Requests for data or other resources related to the iCanCare study can be directed to the corresponding author.
References
- 1.Janz NK, et al. Emotional well-being years post-treatment for breast cancer: prospective, multi-ethnic, and population-based analysis. J Cancer Surviv. 2014;8(1):131–42. doi: 10.1007/s11764-013-0309-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kelly KM, et al. Perception of cancer recurrence risk: more information is better. Patient Educ Couns. 2013;90(3):361–6. doi: 10.1016/j.pec.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 3.Tan AS, et al. Evolving information needs among colon, breast, and prostate cancer survivors: results from a longitudinal mixed-effects analysis. Cancer Epidemiol Biomarkers Prev. 2015;24(7):1071–8. doi: 10.1158/1055-9965.EPI-15-0041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hawley ST, et al. Social and Clinical Determinants of Contralateral Prophylactic Mastectomy. JAMA Surg. 2014;149(6):582–9. doi: 10.1001/jamasurg.2013.5689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.DeFrank JT, Carey LA, Brewer NT. Understanding how breast cancer patients use risk information from genomic tests. Journal of behavioral medicine. 2013;36(6) doi: 10.1007/s10865-012-9449-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fagerlin A, et al. An informed decision? Breast cancer patients and their knowledge about treatment. Patient Educ Couns. 2006;64(1–3):303–12. doi: 10.1016/j.pec.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 7.Hawley ST, et al. Recurrence risk perception and quality of life following treatment of breast cancer. Breast Cancer Res Treat. 2017;161(3):557–565. doi: 10.1007/s10549-016-4082-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaptein AA, et al. Illness perceptions in women with breast cancer-a systematic literature review. Curr Breast Cancer Rep. 2015;7(3):117–126. doi: 10.1007/s12609-015-0187-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Halbach SM, et al. Health literacy and fear of cancer progression in elderly women newly diagnosed with breast cancer--A longitudinal analysis. Patient Educ Couns. 2016;99(5):855–62. doi: 10.1016/j.pec.2015.12.012. [DOI] [PubMed] [Google Scholar]
- 10.Peters E. Numeracy and the perception and communication of risk. Ann N Y Acad Sci. 2008;1128:1–7. doi: 10.1196/annals.1399.001. [DOI] [PubMed] [Google Scholar]
- 11.Janz NK, et al. The impact of doctor-patient communication on patients’ perceptions of their risk of breast cancer recurrence. Breast Cancer Res Treat. 2017;161(3):525–535. doi: 10.1007/s10549-016-4076-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu Y, et al. Accuracy of perceived risk of recurrence among patients with early-stage breast cancer. Cancer Epidemiol Biomarkers Prev. 2010;19(3):675–80. doi: 10.1158/1055-9965.EPI-09-1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burris JL, et al. Breast cancer recurrence risk reduction beliefs in breast cancer survivors: prevalence and relation to behavior. Psychooncology. 2012;21(4):427–35. doi: 10.1002/pon.1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Couzi RJ, Helzlsouer KJ, Fetting JH. Prevalence of menopausal symptoms among women with a history of breast cancer and attitudes toward estrogen replacement therapy. J Clin Oncol. 1995;13(11):2737–44. doi: 10.1200/JCO.1995.13.11.2737. [DOI] [PubMed] [Google Scholar]
- 15.Vassilopoulou-Sellin R, Zolinski C. Estrogen replacement therapy in women with breast cancer: a survey of patient attitudes. Am J Med Sci. 1992;304(3):145–9. doi: 10.1097/00000441-199209000-00001. [DOI] [PubMed] [Google Scholar]
- 16.Costanzo ES, Lutgendorf SK, Roeder SL. Common-sense beliefs about cancer and health practices among women completing treatment for breast cancer. Psychooncology. 2011;20(1):53–61. doi: 10.1002/pon.1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ansa B, et al. Beliefs and Behaviors about Breast Cancer Recurrence Risk Reduction among African American Breast Cancer Survivors. Int J Environ Res Public Health. 2015;13(1):46. doi: 10.3390/ijerph13010046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stewart DE, et al. Attributions of cause and recurrence in long-term breast cancer survivors. Psychooncology. 2001;10(2):179–83. doi: 10.1002/pon.497. [DOI] [PubMed] [Google Scholar]
- 19.Costanzo ES. Post-treatment adjustment and behavior change among women with breast cancer. 2006 [Google Scholar]
- 20.Hamilton AS, et al. Latinas and breast cancer outcomes: population-based sampling, ethnic identity, and acculturation assessment. Cancer Epidemiol Biomarkers Prev. 2009;18(7):2022–9. doi: 10.1158/1055-9965.EPI-09-0238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dillman D, Smyth J, Christian L. Internet, mail, and mixed-mode surveys: the tailored design method. 3. Hoboken, NY: John Wiley & Sons; 2009. [Google Scholar]
- 22.Jagsi R, et al. Concerns about cancer risk and experiences with genetic testing in a diverse population of patients with breast cancer. J Clin Oncol. 2015;33(14):1584–91. doi: 10.1200/JCO.2014.58.5885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Janz NK, et al. Correlates of worry about recurrence in a multiethnic population-based sample of women with breast cancer. Cancer. 2011;117(9):1827–36. doi: 10.1002/cncr.25740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hawley ST, et al. Decision involvement and receipt of mastectomy among racially and ethnically diverse breast cancer patients. J Natl Cancer Inst. 2009;101(19):1337–47. doi: 10.1093/jnci/djp271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Willis GB. Cognitive Interviewing: A tool for improving questionnaire design. Thousand Oaks, CA: Sage Publication; 2005. [Google Scholar]
- 26.Fowler FJ. Improving survey questions: Design and evaluation. Vol. 38. Sage; 1995. [Google Scholar]
- 27.Braun V, Clarke V. Using thematic analysis in psychology. Qualitative research in psychology. 2006;3(2):77–101. [Google Scholar]
- 28.NVivo qualitative data analysis Software. QSR International Pty Ltd; 2012. Version 10. [Google Scholar]
- 29.Hruschka DJ, et al. Reliability in coding open-ended data: Lessons learned from HIV behavioral research. Field methods. 2004;16(3):307–331. [Google Scholar]
- 30.Cohen J. A coefficient of agreement for nominal scales. Educational and psychological measurement. 1960;20(1):37–46. [Google Scholar]
- 31.Gradishar WJ, et al. Invasive Breast Cancer Version 1.2016, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2016;14(3):324–54. doi: 10.6004/jnccn.2016.0037. [DOI] [PubMed] [Google Scholar]
- 32.Sparano JA, et al. Prospective Validation of a 21-Gene Expression Assay in Breast Cancer. N Engl J Med. 2015;373(21):2005–14. doi: 10.1056/NEJMoa1510764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hildon Z, Allwood D, Black N. Impact of format and content of visual display of data on comprehension, choice and preference: a systematic review. International Journal for Quality in Health Care. 2011;24(1):55–64. doi: 10.1093/intqhc/mzr072. [DOI] [PubMed] [Google Scholar]
- 34.Lipkus IM. Numeric, verbal, and visual formats of conveying health risks: suggested best practices and future recommendations. Med Decis Making. 2007;27(5):696–713. doi: 10.1177/0272989X07307271. [DOI] [PubMed] [Google Scholar]
- 35.Costanzo ES, et al. Cancer attributions, distress, and health practices among gynecologic cancer survivors. Psychosom Med. 2005;67(6):972–80. doi: 10.1097/01.psy.0000188402.95398.c0. [DOI] [PubMed] [Google Scholar]
- 36.Katapodi MC, et al. Predictors of perceived breast cancer risk and the relation between perceived risk and breast cancer screening: a meta-analytic review. Prev Med. 2004;38(4):388–402. doi: 10.1016/j.ypmed.2003.11.012. [DOI] [PubMed] [Google Scholar]
- 37.Schapira MM, McAuliffe TL, Nattinger AB. Underutilization of mammography in older breast cancer survivors. Med Care. 2000;38(3):281–9. doi: 10.1097/00005650-200003000-00005. [DOI] [PubMed] [Google Scholar]
- 38.Fink AK, et al. Patient beliefs and tamoxifen discontinuance in older women with estrogen receptor--positive breast cancer. J Clin Oncol. 2004;22(16):3309–15. doi: 10.1200/JCO.2004.11.064. [DOI] [PubMed] [Google Scholar]
- 39.Waters EA, et al. “I don’t know” my cancer risk: implications for health behavior engagement. Annals of Behavioral Medicine. 2016;50(5):784–788. doi: 10.1007/s12160-016-9789-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ruddy KJ, et al. Long-term risk perceptions of women with ductal carcinoma in situ. Oncologist. 2013;18(4):362–8. doi: 10.1634/theoncologist.2012-0376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mishel MH. Uncertainty in illness. Journal of Nursing Scholarship. 1988;20(4):225–232. doi: 10.1111/j.1547-5069.1988.tb00082.x. [DOI] [PubMed] [Google Scholar]
- 42.Han PK, Klein WM, Arora NK. Varieties of uncertainty in health care: a conceptual taxonomy. Med Decis Making. 2011;31(6):828–38. doi: 10.1177/0272989X11393976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Charles C, Gafni A, Whelan T. Shared decision-making in the medical encounter: What does it mean? (or it takes at least two to tango) Social Science & Medicine. 1997;44(5):681–692. doi: 10.1016/s0277-9536(96)00221-3. [DOI] [PubMed] [Google Scholar]
- 44.Lipkus IM, Rimer BK, Strigo TS. Relationships among objective and subjective risk for breast cancer and mammography stages of change. Cancer Epidemiol Biomarkers Prev. 1996;5(12):1005–11. [PubMed] [Google Scholar]
- 45.Neise C, et al. Risk perception and psychological strain in women with a family history of breast cancer. Onkologie. 2001;24(5):470–5. doi: 10.1159/000055128. [DOI] [PubMed] [Google Scholar]
- 46.Panjari M, et al. Breast cancer survivors’ beliefs about the causes of breast cancer. Psychooncology. 2012;21(7):724–9. doi: 10.1002/pon.1949. [DOI] [PubMed] [Google Scholar]
- 47.Thomson AK, et al. Beliefs and perceptions about the causes of breast cancer: a case-control study. BMC Res Notes. 2014;7:558. doi: 10.1186/1756-0500-7-558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Spector D, et al. Breast cancer risk perception and lifestyle behaviors among White and Black women with a family history of the disease. Cancer Nurs. 2009;32(4):299–308. doi: 10.1097/NCC.0b013e31819deab0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ahern TP, et al. Family history of breast cancer, breast density, and breast cancer risk in a U.S. breast cancer screening population. Cancer Epidemiol Biomarkers Prev. 2017 doi: 10.1158/1055-9965.EPI-16-0801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Eccles BK, et al. Family history and outcome of young patients with breast cancer in the UK (POSH study) British Journal of Surgery. 2015;102(8):924–935. doi: 10.1002/bjs.9816. [DOI] [PubMed] [Google Scholar]
- 51.Denzin NK. The research act: A theoretical introduction to sociological methods. Transaction publishers; 1973. [Google Scholar]
- 52.Bruine de Bruin W, et al. Verbal and numerical expressions of probability: ‘It’s a fifty-fifty chance’. Organizational Behavior and Human Decision Processes. 2000;81(1):115–131. doi: 10.1006/obhd.1999.2868. [DOI] [PubMed] [Google Scholar]
- 53.Cameron LD, et al. Impact of genetic risk information and type of disease on perceived risk, anticipated affect, and expected consequences of genetic tests. Health Psychology. 2009;28(3):307. doi: 10.1037/a0013947. [DOI] [PubMed] [Google Scholar]
- 54.Bruine de Bruin W, et al. What number is “fifty-fifty”?: Redistributing excessive 50% responses in elicited probabilities. Risk Analysis. 2002;22(4):713–723. doi: 10.1111/0272-4332.00063. [DOI] [PubMed] [Google Scholar]
- 55.Dillard AJ, et al. The distinct role of comparative risk perceptions in a breast cancer prevention program. Annals of Behavioral Medicine. 2011;42(2):262–68. doi: 10.1007/s12160-011-9287-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Berry DC, Knapp P, Raynor DK. Provision of Information about Drug Side-Effects to Patients. Lancet. 2002;359(9309):853–54. doi: 10.1016/s0140-6736(02)07923-0. [DOI] [PubMed] [Google Scholar]
- 57.Berry DC, Hochhauser M. Verbal labels can triple perceived risk in clinical trials. Drug Information Journal. 2006;40(3):249–58. [Google Scholar]
- 58.Knapp P, Raynor DK, Berry DC. Comparison of two methods of presenting risk information to patients about the side effects of medicines. Quality and Safety in Health Care. 2004;13(3):176–80. doi: 10.1136/qshc.2003.009076. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.