Abstract
Ambient air pollution has been associated with adverse respiratory outcomes, especially among children with asthma. This study reports on associations between daily ambient air pollutant concentrations and the respiratory symptoms of schoolchildren living in Durban, South Africa. This city is Africa’s busiest port and a key hub for imported crude oil and exported refined petroleum and petrochemical products, and it experiences a mixture of air pollutants that reflects emissions from industry, traffic and biomass burning. Children in four communities in the highly industrialized southern portion of the city were compared to children of similar socio-economic profiles living in the north of the city. One school was selected in each community. A total of 423 children were recruited. Symptom logs were completed every 1.5 to 2 hours over 3-week period in each of four seasons. Ambient concentrations of NO2, NO, SO2, CO, O3, PM2.5 and PM10 were measured throughout the study. Generalized estimating equation (GEE) models were used to estimate odds ratios (ORs) and assess lag effects (1 to 5 days) using single pollutant (single lags or distributed lags) models. Concentrations of SO2 and NOx were markedly higher in the south, while PM10 did not vary. Significant increase in the odds ratios of cough were identified for the various lags analyzed. The OR of symptoms was further increased among those living in the south compared to the north. In conclusion, in this analysis of over 70000 observations, we provide further evidence that exposure to PM10, SO2, NO2 and NO is associated with significantly increased occurrence of respiratory symptoms among children. This was evident for cough, shortness of breath, and chest tightness, across the four pollutants and for different lags of exposure. This is the first study describing these changes in sub-Saharan Africa.
Keywords: ambient pollution, respiratory symptoms, distributive lags, asthma, schoolchildren
Graphical abstract

Introduction
Ambient air pollution has been associated with a range of adverse respiratory outcomes. Among children, these include pulmonary function decrements (Weinmayr et al., 2010), visits to emergency departments and hospital admissions (Nastos et al, 2010; Villeneuve et al., 2008), increase in symptoms presentations (Zora et al., 2013; Mann et al, 2010; Liu et al, 2009) and increased medication use (Lewis et al., 2013). Asthmatic children or those with pre-existing respiratory disease are most at risk for experiencing adverse pollutant-related outcomes. (Esposito et al., 2014; Samoli et al., 2011; Epton et al, 2008; O’Connor et al., 2008). Pollutants associated with these outcomes include particulate matter (PM) (Graveland et al, 2011; Berhane et al, 2011); ozone (Strickland et al, 2010; Lee et al, 2010); sulfur dioxide (SO2) (Pan et al, 2010; Dales et al, 2009) and oxides of nitrogen (McConnell et al, 2010; Patel et al, 2010; Pan et al, 2010). Associations with traffic factors (e.g., distance from major roads, traffic intensity or traffic type) (Jung et al., 2015; Lindgren et al., 2013) and industry factors (distance from industry generally or specific types, e.g. refineries, mills and chemical factories) (Rovira et al., 2014).
A key challenge in understanding the relationship between ambient pollution and health outcomes is disentangling the effects of individual pollutants and the combined effects of multipollutant exposures (Oakes et al., 2014; Gass et al., 2015; Winquist et al., 2014). Most populations are subjected to simultaneous exposures from multiple pollutants. The nature of pollutant interactions, which are largely unknown, may range from antagonistic through to synergistic effects. While studies focusing on single pollutants lack the ability to investigate co-pollutant effects, multiple pollutant studies face the challenge of appropriate statistical approaches to tease out these health outcome associations given the presence of multicollinearity and other interactions. These require innovative modelling and a combination of techniques to describe spatial and temporal variations in exposure (Coull et al., 2015; Park et al., 2015; Sun et al., 2013).
Cross sectional studies lack the ability to investigate the association of acute respiratory responses with pollutant fluctuations. Panel studies have the advantage of controlling for individual and behavioral factors known to affect respiratory health, especially among asthmatic children. Another advantage, particularly in the context of the limited understanding between mechanisms of exposure and effect, is that these repeated measures-type studies can investigate lag effects of exposure. Panel studies of asthmatic children have shown pollutant-related effects with short or acute exposure (Li et al., 2012; Weinmayr et al., 2010; Epton et al., 2008). Population-based panel studies of schoolchildren are few, and our understanding of the effects of acute pollution exposure on children without pre-existing respiratory disorders is limited.
Key factors that drive ambient pollutant exposure among communities include proximity to industry, traffic and other air pollution sources, emission rates from these sources, and the prevailing meteorological conditions that affect pollutant dispersion. Exposure conditions can vary considerably, particularly between developed and developing countries (Cheng et al., 2016). South Africa is in a transitional stage regarding traffic and industrial emissions, with important differences compared to Europe and North America. In developed countries, in part due to improved regulation and enforcement of industrial or “point source” emissions, exposure to traffic-related air pollutants has become a priority. Levels of these pollutants can vary considerably, reflecting differences in fleet mix (trucks, utility vehicles and cars), engine types and fuels (e.g., petrol and diesel), traffic activity, and requirements and penetration of control technology like diesel particulate traps and catalytic converters (Cames and Helmers, 2013). The policy and regulatory frameworks in place in North America and Europe (Font et al., 2016; Rojas-Rueda, 2016; Leem et al., 2014) are either absent or only weakly enforced in developing countries, allowing manufacturers great flexibility with their products, and contributing to the geographical variation in traffic-related pollution (Torjesen, 2014). The variation of exposures between developing and developed countries provides strong reasons for investigation of health outcomes in countries such as South Africa.
Durban, South Africa’s third largest city, is Africa’s busiest port and primary route for imported crude oil and exported refined petroleum and petrochemical products. The primary study area in the southern portion of the city is highly industrialized, with two oil refineries, a paper mill, a sewage treatment plant, a major highway, landfill sites, and many processing/manufacturing industries. Through rigorous monitoring and enforcement, industrial emissions of SO2 have been reduced from 107 tons per day in 2000 to 61 tons per day in 2005. Prevailing meteorological conditions, complex topography, short emission stacks, and fugitive emissions all contribute to high pollutant concentrations. Over 400 000 people live in close proximity to industrial sources (eThekwini Health, 2007). High prevalence rates of asthma have been reported among children at a primary school in south Durban, and short-term concentrations of PM10, NO2 and SO2 were significantly associated with increased respiratory symptoms and decrements of pulmonary function (Kistnasamy et al., 2008).
Our objective was to investigate and compare associations between daily respiratory symptoms and daily variability in ambient pollutant exposures among a population based panel of schoolchildren, with and without asthma, living in the industrialized south of the city with those of similar socio-economic profiles living north of the city. This is the first repeated measures study conducted in sub-Saharan Africa with multiple ambient exposures across several locations and across all seasons.
Methods
Selection of Communities and Student Recruitment
We have described the selection of communities and schools and student recruitment elsewhere (Naidoo et al., 2013). In summary, four communities (Merebank, Wentworth/Austerville, Bluff and Lamontville) in the intensely industrialized south Durban were compared to three communities with similar socio-economic profiles in the less industrialized north (Newlands East, Newlands West and KwaMashu) (see fig. 4 for the location of the schools). One school was selected in each community, based on geography, location to point and mobile sources of pollution, and limited busing of students from outside communities.
Figure 4.
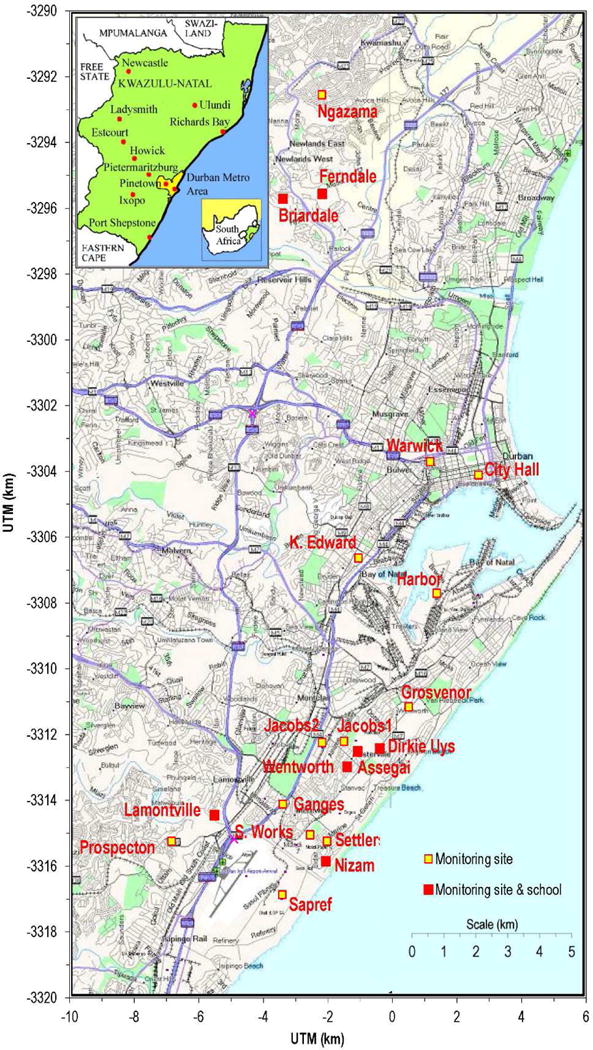
Map of the city of Durban, showing the location of the participating schools and additional monitoring sites.
In order to recruit enough asthmatic children for the study, we used a “sample enrichment strategy’ that resulted in selecting a random sample of 4th graders and all asthmatic children from grades 3, 5 and 6. There were on average 1,400 children in the 4th grades (average of 50 children per grade, two 4th grades for each of the 7 selected schools) and 93 asthmatic children in grades 3, 5 and 6. We targeted to recruit a random sample of 30% (n=420) of 4th graders and all 93 asthmatic children in grades 3, 5 and 6 were invited to participate. The final sample was composed of 342 4th graders (81.4%) randomly selected from one or two classes in each of the seven schools and of 81 children, having known or probable persistent asthma, based on responses to a screening questionnaire, from grades 3, 5 and 6 (87.1%).
Interview Data
Trained interviewers conducted interviews using standardized instruments, translated and back-translated into the languages spoken by caregivers of the participants. Validated questions used in previous international studies on childhood asthma (Lewis et al., 2004), and previously field tested in the participating communities (Kistnasamy et al., 2008) were used. These included questions relating to presence and severity of respiratory symptoms, demographics, past respiratory history and exposure to cigarette smoke. Final categorization of having moderate to severe persistent, mild persistent, mild intermittent or no asthma, among the children, was based on the guidelines established by the U.S. National Asthma Education and Prevention Program (NAEPP, 1991).
Symptoms Data
Previously validated symptom logs were completed by all participants for the full school week over a three week period in each of four seasons (Kistnasamy et al., 2008). On each of the five schooldays during the week, students were asked to complete the symptom/activity log every 1.5 to 2 hours (four times per 5.5 hour school day: approximately 8:00, 9:45, 11:30 and 13:20). Each of these four bi-hourly measurements was considered a repeated measure of the outcome of interest. Participants in all seven schools completed these logs at approximately the same time on the same days. In these logs, students recorded symptoms, odor detection and information about location and activity over the previous two-hour interval. Study personnel provided intensive individualized training to the participants prior to the first three-week intensive data collection and refresher training was provided prior to each subsequent phases. Study personnel supervised the completion of these bihourly assessments in each classroom during the three week cycles.
Assessment of Atopy Status
Students underwent skin prick testing at school for allergic sensitization. Antigens tested included mixed cockroach, mixed dust mite, mold mix (Aspergillus, Cladosporium and Penicillium), cat, dog, mouse, rat, mixed grasses, plus histamine as a positive control and saline as a negative control. Standardized methods for testing were employed. A positive test was defined as wheal 2 mm or greater than saline control.
Air Quality Monitoring
Ambient pollutants, including NO2, NO, SO2, CO, O3 and PM10, were measured throughout the study period. Monitoring sites were established at the seven schools for SO2, PM10 and CO. SO2 was monitored continuously by ultraviolet fluorescence spectrometry. Daily PM10 samples were collected continuously during the study intensive periods and on a 6-day cycle otherwise. CO was monitored using either infrared absorption (API 300 analyzer) or electrochemical sensors (Hobo H11-001 CO Loggers, Onset Computer Corporation). In addition to the school sites, ambient air pollutants were monitored at Durban (eThekwini) municipality sites (eThekwini, 2007) (see Fig 4 for the location of these sites): NO2 and NO were continuously monitored at eight sites using chemiluminescence (Monitor Europe, model ML 9841B); O3 at two sites (in north and south Durban) using non-dispersive ultraviolet spectroscopy; and PM10 at seven sites including several (City Hall, Warwick, Ganges School) at high traffic areas and two (Jacobs 1 and 2) in industrial areas. Most measurement types (e.g., NO2, O3, SO2) used US EPA Federal Reference Methods. Quality assurance (QA) activities included written protocols, regular calibrations using certified materials, and use of an environmentally-controlled laboratory for gravimetric determinations of PM10 filters.
Multiple imputation (MI) methods were used to impute missing values of pollutants, using a Markov Chain Monte Carlo (MCMC) method with multiple chain option. Imputation models included meteorological variables, e.g., barometric pressure, daily rainfall, relative humidity, temperature, vertical temperature difference (delta T), wind direction by sector (8 total), and wind speed, as well as pollutant variables at all available sites for the same pollutant being imputed. After optimizing and evaluating performance, m = 5 values were generated for each pollutant and site for use in the health model.
Pollutant exposures were estimated for north and south Durban using the school and eThekwini data considered to be most representative of population exposure. Where feasible, multisite averages were used to increase the representativeness of the data and improve the data completeness. In south Durban, NOx measurements at four sites (Wentworth, Jacobs, Southern Works, Ganges) were selected to reflect a mixture of industrial and vehicular sources. Concentrations at these sites were similar and highly correlated in time. Although the Ganges site was originally considered to be traffic-impacted site, traffic influence was judged as moderate. The downtown and highly traffic-impacted sites (Warwick, City Hall, King Edward) were excluded. In north Durban, the centrally located Ferndale site was used to represent NOx exposures. 24-hr exposures (midnight-to-midnight) were estimated from the continuous data.
Statistical Analysis
Initially, we assessed the frequency distribution of the primary outcomes (reported bi-hourly symptoms including cough, wheezing, shortness of breath and chest tightness or heaviness). We used the generalized estimating equation (GEE) approach with log link and exchangeable correlation structure of the log odds to assess the pollution-symptoms associations. Possible lag effects (1 to 5 days) were determined, using single pollutant (single lags or distributed lags) and multi-pollutants models. Each bi-hourly symptoms report was considered as a separate event in the multivariate longitudinal with within-day repeated measures model. Each of these four measures was matched to the daily average exposure and their lags. Covariates examined for entry into regression models included age, gender, race/ethnicity, school, previous history of respiratory disease, education level of primary caregiver, smoking history of caregiver, atopy status, annual household income and season. Positive atopy status was defined as a positive skin prick test to any of the eight common allergens tested. The location of the child’s residence, classified as south=1 and north=0, was considered as effect modifier.
Multipollutant models exhibited multicollinearity and thus standard errors were inflated. Single exposure distributed lags models are presented here. Using reported cough as an example the models we considered were,
| (1) |
Where p=P(cough=1) and ‘Conc’ is the pollutant concentration tested by the model (PM10, SO2, NO2 or NO). The null hypothesis, H0: L’β= 0, is the key research question.
Model (1) was modified to address the effect modification of location by adding an interaction term for pollutant lags and location. Models were fitted on the basis of multiple imputed datasets using SAS software for windows.
Ethics
The legal guardians of participating children gave written informed consent, and children participated voluntarily and had the right to withdraw at any stage. Ethical approvals were obtained from the Institutional Review Board of the University of Michigan and the Ethics Committee of the University of KwaZulu-Natal, Durban.
Results
Participation
Of the total sample of 423 students in the sample, participation rates varied across the seven schools. The expected number of observations for the total sample was 101 520 (423 × 4 times per day × 15 days of observation × 4 seasons), and of these, we achieved a 73.1% success rate (74 260 observations). Generally, the reasons for the missing observations was absence of pupils from school during the assessments.
Pollutant Exposure
Pollutant levels during the intensive periods of the study are summarized in Table 1. PM10 concentrations showed strong seasonality, with the highest levels in winter (March through August), but only modest spatial variation. These trends reflect the widespread dispersion of combustion and fugitive emissions throughout the Durban area, increased burning of vegetation during the dry winter period, and meteorological influences such as temperature inversions and recirculating winds that are common in the winter. Based on collocated measurements at three sites (Nizam, Wentworth and Ferndale), PM10 and PM2.5 levels were moderately correlated (0.3 < R2 < 0.8, depending on site). PM2.5 concentrations at the three sites were also very similar, e.g., the southern sites (Wentwork and Nizam) averaged 20.7 μg/m3 and the northern Ferndale site average 20.0 μg/m3. In contrast, concentrations of NOx and SO2 showed considerable spatial variation, anticipated given the much greater density of industry and traffic in south Durban. For NO2 and NO, concentrations in the south (average at Wentworth, Jacobs, Southern Works and Ganges sites) were 58% and 85% higher, respectively, than levels in the north (average at Ferndale), and the highest concentrations were observed in the winter and the lowest in summer. Of the monitored pollutants, SO2 showed the strongest spatial contrasts, e.g., concentrations were near background levels at the three schools in the north (average of 1–3 ppb), while the influence of industrial sources was apparent at the four schools in the south (6–13 ppb). SO2 showed similar seasonal patterns to the other pollutants, as well as trends by wind direction and time of day that showed local site influence.
Table 1.
School and geographic averages for the criteria pollutants measured in the study
| Schools in the South | Average for Schools | Schools in the North | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Assegai | Dirkie Uys | Nizam | Enthuthukweni | in the South | in the North | Briardale | Ferndale | Ngazana | |
| Particulate matter (PM10)a mean (ug/m3) | 59,4 | 46,3 | 50,1 | 51,9 | 51,8 | 47,7 | 40,9 | 44,4 | 57,4 |
| SD | 33,8 | 23,8 | 25,2 | 26,4 | 27,2 | 30,1 | 22,5 | 27,8 | 39,5 |
| range | 10.4–208.0 | 13.9–170.6 | 14.0–179.4 | 9.6–173.8 | 12.1–182.8 | 6.1–162.4 | 7.6–124.9 | 4.5–178.7 | 6.5–181.2 |
| Sulphur Dioxideb mean (ppb) | 12,6 | 6,9 | 7,5 | 5,8 | 8,7 | 1,9 | 1,5 | 2,7 | 1,2 |
| SD | 9,1 | 6,1 | 6,9 | 7,2 | 7,4 | 1,6 | 1,3 | 2,1 | 1,2 |
| range | 0.6–65.6 | 0–36.7 | 0–45.2 | 0–56.7 | 0–50.5 | 0–9.47 | 0–9.1 | 0–12.7 | 0–6.1 |
| Nitrogen Dioxideb* mean (ppb) | – | – | – | – | 17,2 | 10,9 | – | – | – |
| SD | – | – | – | – | 8,8 | 6,2 | – | – | – |
| range | – | – | – | – | 3.7–63.8 | 0–47.5 | – | – | – |
| Nitrogen Oxideb* mean (ppb) | – | – | – | – | 40,9 | 22,1 | – | – | – |
| SD | – | – | – | – | 30,5 | 22,2 | – | – | – |
| range | – | – | – | – | 3.2–192.2 | 0–115.7 | – | – | – |
these pollutants were monitored only at single sites in the north and south respectively
Significant South vs North mean difference 0.001<p-value <0.05
Significant South vs North mean difference p-value <0.001
Sample Demographics and Prevalence of Health Outcomes
The mean age of the children was 10.5 years (SD=0.9 years), with the majority being female (57.2%). Most participants were of African origin (44.2%), and 28.2% of households earned less than US$ 1 250 per month (Table 2). Just over half of households were English speaking (50.7%). Among the randomly sampled schoolchildren, the prevalence of asthma and asthma severity (based on symptoms) was higher in the south compared with the north, e.g., prevalence rates for moderate-to-severe persistent asthma were 9.1% and 5.0% in south and north, respectively, persistent asthma of any severity were 15.4 and 9.4%, and mild intermittent asthma were 20.6 and 18.7% (not shown in tables)
Table 2.
Demographics of the sample (N=423)
| Variable Name | N | Statistics1 | |
|---|---|---|---|
| Age, Mean(SD) | 423 | 10.5(0.9) | |
| Gender,% | Female | 238 | 57,2 |
| Race,% | African | 159 | 44,2 |
| Mixed race | 82 | 22,8 | |
| Indian | 99 | 27,5 | |
| White | 20 | 5,6 | |
| Caregiver education,% | Less than high school | 129 | 39 |
| High school Graduate | 144 | 43,5 | |
| More than High | |||
| School | 56 | 16,9 | |
| Refused | 2 | ||
| Total annual household income (US$),% | <1,500 | 57 | 28,2 |
| 1,501–4,500 | 54 | 26,8 | |
| 4,501–8,000 | 43 | 21,3 | |
| 8,001 and above | 48 | 23,7 |
Mean and standard deviation for continuous outcomes and % for categorical outcomes
In the first season of the three week period of repeated symptoms logs, the prevalence rates of symptoms varied across the geographic areas and, with the exception of chest tightness, rates were higher in the south than the north. The largest differences were seen for wheezing (21.9% vs 9.5%) (Table 3). Given the large sample sizes in these analyses, p-values were 0.01 or smaller, and even small differences in proportions were highly significant.
Table 3.
Baseline Reported Symptoms (n=423 children)1
| Full sample | Average for Schools | Chi-square3 test | |||
|---|---|---|---|---|---|
| (unweighted) | in the South | in the North | |||
| Symptoms2 | % | % | % | p-value | |
| Cough | 10248 | 54.1 | 56.1 | 53.7 | <0.001 |
| Wheezing | 2975 | 15.7 | 21.9 | 9.5 | <0.001 |
| Chest tightness or heavyness | 4968 | 26.2 | 25.9 | 28.1 | 0.01 |
| Shortness of breath | 3359 | 17.8 | 20.2 | 16.6 | <0.001 |
| Any Symptoms | 11812 | 57.4 | 59.6 | 58.1 | 0.01 |
Each child was expected to report symptoms 4 times a day during 15 days each seasonal data collection period, a total of n=60 (4*15) person day observation each season
Number of repeated measure per child at baseline.
Given the large sample size used in Chi-square test p-value are most likely to be low, thus identifying small differences in proportions as highly significant.
Pollutant-related Health Outcomes
Statistically significant pollutant-related odds ratios (ORs) were seen for multiple pollutants and symptoms, particularly cough, across the various lags analyzed. Covariate-adjusted ORs for an interquartile range increase in the pollutants were evident for the various outcomes across the different lags: cough was particularly strong for lag 0, 1.07 (95% CI: 1.02; 1.13) for PM10; 1.11 (1.04; 1.17) for SO2; 1.14 (1.06; 1.22) for NO2; and 1.09 (1.02; 1.16) for NO. A significant OR for cough was consistently seen for all lags for SO2 with estimates from 1.05 – 1.11. With the exception of wheeze, most symptoms showed a significant OR at lag 0 for PM10, SO2 and NO2. NO2 was associated with most symptoms, but only for specific lags: cough (lags 0 and 5); wheeze (lag 2); SOB (lags 0 and 3), and chest tightness (lags 0 and 4). (Figs. 1a–d)
Figure 1.
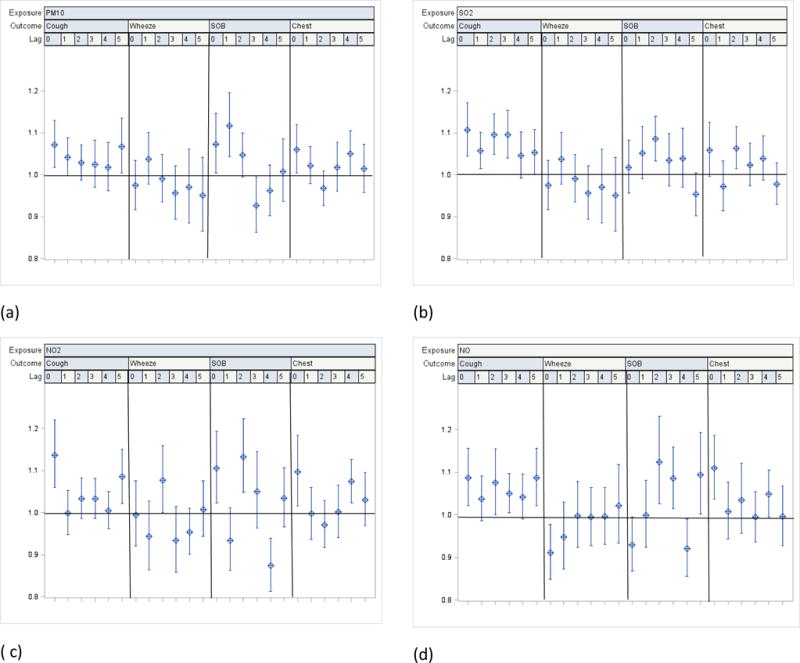
a–d. Odds ratios for reported symptoms and 95% CI associated with one interquartile range increase in ambient levels of PM10, SO2, NO2 and NO from single pollutant distributed lags covariate-adjusted linear regression models among all participating children (n=432)
Notes:
1 Interquartile range (IQR): PM10: 29.35ug/m3; SO2: 6.98 ppb; NO2: 8.19 ppb; NO: 29.7 ppb.
2 Pollution levels used in regression models combine measured and imputed values
3 Covariates in each model: age, gender, race, school, caregiver smokes, caregiver’s education, household income, phase (season), asthma severity, interactions between asthma severity and exposure
Residence location modified the pollutant-related ORs for symptoms. As for the main effects model, most pollutants were associated with an increased risk of cough; the OR was further increased among those living in the south compared to the north. For example, at lag 0, the OR for PM10 when living in the south was 1.12 (95% CI: 1.04; 1.21) compared to 1.02 (95% CI: 0.96; 1.10) when living in the north. For children living in the south, estimates for other symptoms consistently exceeded 1, however, in most instances, ORs failed to reach statistical significance. This contrasts with children living in the north, where, with exception of cough, chest tightness and shortness of breath, ORs were generally less than 1. In addition, models for residents in the north were less robust, e.g., confidence intervals were far wider than those in the south (Fig 2 (a)–(d) and Fig 3 (a)–(d)).
Figure 2.
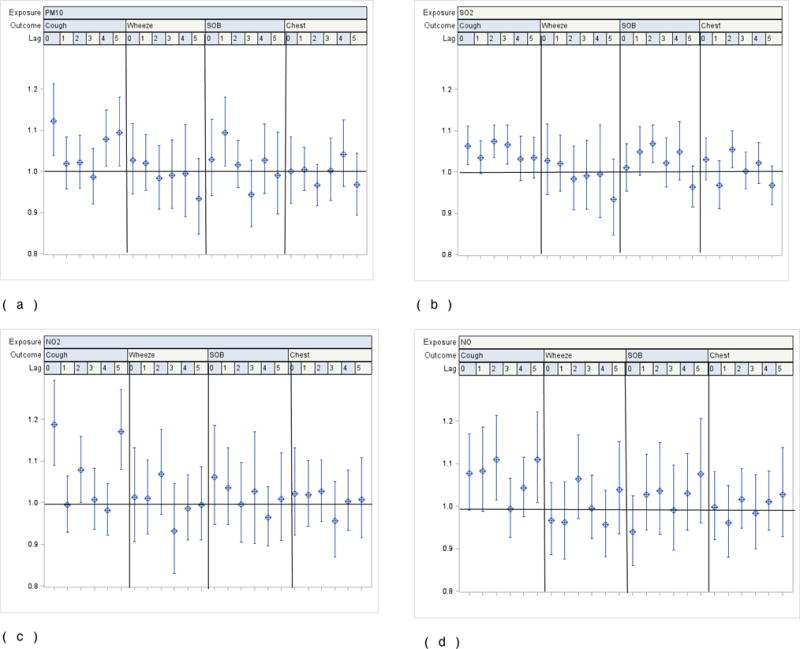
a–d. Odds ratios for reported symptoms and 95% CI associated with one interquartile range increase in ambient levels of PM10, SO2, NO2 and NO from single pollutant distributed lags covariate-adjusted linear regression models by location among all participating children living in the South (n=218)
1 Interquartile range (IQR): PM10: 29.35ug/m3; SO2: 6.98 ppb; NO2: 8.19 ppb; NO: 29.7 ppb.
2 Pollution levels used in regression models combine measured and imputed values
3 Covariates in each model: age, gender, race, school, caregiver smokes, caregiver’s education, household income, phase (season), asthma severity, interactions between asthma severity and exposure
Figure 3.
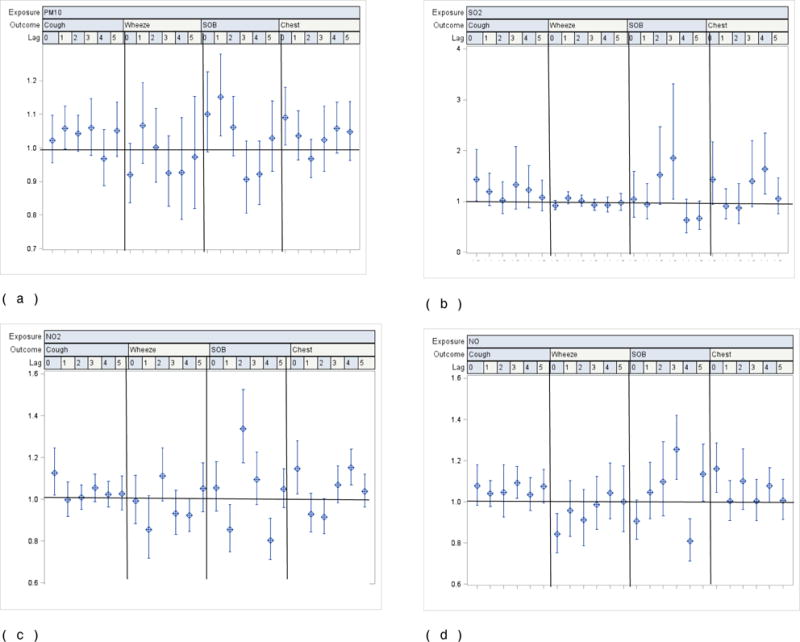
a–d. Odds ratios for reported symptoms and 95% CI associated with one interquartile range increase in ambient levels of PM10, SO2, NO2 and NO from single pollutant distributed lags covariate-adjusted linear regression models by location among all participating children living in the North (n=205)
1 Interquartile range (IQR): PM10: 29.35ug/m3; SO2: 6.98 ppb; NO2: 8.19 ppb; NO: 29.7 ppb.
2 Pollution levels used in regression models combine measured and imputed values
3 Covariates in each model: age, gender, race, school, caregiver smokes, caregiver’s education, household income, phase (season), asthma severity, interactions between asthma severity and exposure
Discussion
In this, the first panel study of ambient pollution and respiratory outcomes in sub-Saharan Africa, with a panel of 423 schoolchildren, providing in excess of 70 000 observations, we provide further evidence that exposure to PM10, SO2, NO2 and NO is associated with significantly increased occurrence of respiratory symptoms. This was particularly evident for cough, shortness of breath, and chest tightness. These associations were evident across the four pollutants and for different lags of exposure. Our findings are particularly striking, given our sample of mostly non-asthmatic children.
Only a few panel studies have described acute pollutant-related symptoms in developing countries. We identified studies from Thailand, Korea and Mexico. PM concentrations in our study tend to be either higher or much higher than levels reported in most European or North American studies, reflecting poorly controlled industrial emissions in populated areas, large number of diesel vehicles, and widespread burning of agricultural waste in the Durban area. For PM10, annual average levels across Durban (41 – 59 μg/m3) considerably exceed levels reported in Spain (30 μg/m3; Roviro et al., 2014) and Detroit, Michigan (26 μg/m3; Lewis et al., 2013). However, PM10 concentrations in Durban fell below levels reported in Thailand (83 – 104 μg/m3; Vichit-Vadakan et al., 2001) and Seoul (66 μg/m3; Lee et al., 2005). For NOx, Durban’s long term averages (33 and 58 ppb in the south and north, respectively), considerably exceed levels reported in Sweden near roads (11 ppb; Lindgren et al., 2013), while NO2 levels in Durban (11 – 17 ppb) were comparable to levels in Spain (7 – 14 ppb; Roviro et al., 2014).
Pollutant-Outcome Relationships
We found ORs for the symptoms across the different lags and pollutants that were comparable to those reported in previous panel studies. For example, our pollutant related cough risk estimates (ORPM10: 1.02–1.07; ORSO2: 1.05–1.11; ORNO2: 1.00–1.14; ORNO: 1.04–1.09) are similar to those in the review of panel studies by Li et al (2012) and other more recent panel studies (Ranzi et al., 2015). The similarities with other studies are notable, given the mix of asthmatics and non-asthmatics in our sample, compared to most panel study reports which focus on outcomes among asthmatic children (Roemer et al., Sarnat et al., 2012; Ranzi et al., 2015). We found no consistent increased risk for wheeze. This finding differs from the “asthmatics only” studies, where ORs range from 1.01 to 1.18 (Ward and Ayres, 2002; Ranzi et al., 2015). Our findings for wheeze were similar to “mixed” sample studies where no increased risk of pollutant-related wheeze were reported among non-asthmatics (Lee et al., 2005; Escamillo-Nunez et al., 2008; Epton et al., 2008).
Among studies in other developing countries, limited significant associations have been reported across the various pollutants and respiratory symptoms (sAekplakorn et al., 2003; Lee et al., 2005). Significant associations for lower respiratory symptoms (ORPM10 = 1.22) and upper respiratory symptoms (ORPM10 = 1.31) were described among children from Bangkok (Vichit-Vadakan et al., 2001)
Although modest, a definite exposure-related effect was evident, even in the absence of a clinical diagnosis of asthma, and varied across the lags. Because of our lack of knowledge about the biological effect of pollutants on the respiratory system, the lag between exposure and outcome remains poorly understood. Panel studies provide an opportunity to explore these lag effects. However, across the various studies, there is a lack of consistency for the lag of greatest effect. It is reasonable to assume however, that lag 0 is unlikely to be the only important lag of interest.
Geographical Variation of Pollutant and Health Outcomes
The study region showed strong geographical differences in pollutant exposures and the associated risks of respiratory symptoms. Concentrations of NO2 and SO2 varied substantially between north and south, a result of emissions from intense industrial activity, as well as higher traffic in the vicinity of the schools in the south. In contrast, particulate matter showed little variability across northern and southern portions of Durban, a result of more dispersed emission sources (including agricultural burning) as well as greater atmospheric residence time. The higher ORs obtained for Durban south, which resulted from effect modification associated with the geographic region of residence, were consistent with odds ratios from similar interactive single pollutant models. The simultaneous adjustment for pollutant concentration and residence location in studies of respiratory and symptom health outcomes appears to be novel. Previous panel studies showing geographical location-related outcomes have used residence location as a proxy for exposure; moreover, these studies could not investigate different pollutants (Ranzi et al., 2015; Ranzi et al., 2004; Roemer et al., 1998; Van der Zee et al., 1999). Our findings suggest that additional and unmeasured factors may influence acute health outcomes. While unrelated to the covariates used in the models, these factors are likely to be related to other environmental factors. Other studies have reported school-specific risks (Sarnat et al., 2012). While noted in our sample, such risks were not substantial when grouped by geographic region. Exposure characterization at the participant’s residence may have provided a better understanding of individual exposure. However, our study did not have the resources for this level of assessment.
Exposure Characterisation
Because PM10, SO2, NO2 and NO concentrations were correlated, (with correlations ranging between 0.67 for PM10 and NO2 and 0.80 between NO2 and NO), multipollutant modelling was not possible. In addition, the variance inflation factor analysis indicated that by simultaneously including these four exposures, their estimated variance would increase by a factor of two or larger. This strongly suggested collinearity was present in the multipollutant analysis. It was therefore, not possible to evaluate which pollutant was more likely to be responsible for observed effects. Exposures of PM10 and NOx levels, particularly in south Durban, are in the higher range reported in the literature (Ranzi et al., 2015; Zora et al., 2013; Sarnat et al., 2012; Weimayr et al., 2010). Despite the temporal correlation, pollutant exposures can vary within each study region, especially for SO2 which tends to be highly localized. While school-based monitoring captures some of the variability, we could not monitor homes and other areas frequented by children. Averaging exposure over the two study regions may suppress some of the exposure variability, but has the advantage of potentially increasing the representativeness of the exposure estimates.
Although not being able to assess simultaneous multipollutant effects, the Durban analysis highlights the significant exposures contributed by industrial, traffic, and community sources, which differs somewhat from the contemporary North American and European situation. Industrial point emissions of SO2 continue to affect large populations in countries such as South Africa. In contrast, SO2 emissions have been greatly reduced in the developed economies. Due in part to policy initiatives in European countries, the number of light diesel powered vehicles has risen, as compared to a decrease in Japan, and slow uptake in the US (Cames and Helmers, 2013; Torjesen, 2014). In countries such as South Africa, the number of diesel vehicles has gradually increased over the last 15 years (Ratshomo and Nebahe, 2013), but these vehicles are not subject to similar controls as in Europe. Although an emissions-based purchase tax policy exists (Vosper and Mercure, 2016), and a cleaner fuels strategy and vehicle emission standards are in place, enforcement of vehicular emissions is non-existent and the vehicle fleet is aging, which tends to increase emissions. Overall, Durban residents experience high exposures to traffic-related pollutants (e.g., CO, NOx, PM2.5, VOCs), the southern portions of the city experiences high levels of industrial pollutants (especially SO2 and H2S), and PM2.5 levels are often high over the entire region due to dust entrainment, agricultural burning and regional transport.
Comparison of Asthma Prevalence with previous studies
Rates of symptom-defined asthma prevalence in our Durban-based sample were high: 32% with “any” asthma and 12% with persistent asthma. These rates varied geographically. These rates are consistent with those reported in other studies using similar symptoms-based definitions. Considering physician-diagnosed asthma, community-based samples have reported prevalence rate of 5 to 6% in India (Singh et al., 2016), 1.4 to 4% in in the Belarus countries (Brozek et al., 2016); and 3.8% in northern Portugal (Branco et al., 2015). In higher risk communities, prevalence rates are higher: South American studies show rates from 3.9 to 33.1%, (Mallol et al., 2010), and rates for children from lower socio-economic communities in the USA ranged from 6.2 to 15.2%. (Joseph et al., 2010). Elsewhere in South Africa, schoolchildren aged 7 to 8 years from Cape Town had an asthma diagnosis prevalence rate of 10.8%, as reported by parents (Ehrlich et al., 1995). In our sample, we previously reported that the physician-diagnosed asthma prevalence rate was 14.8% (Naidoo et al., 2013). This was considerably lower than the symptoms-based definition as well as our objective assessments (spirometry and methacholine challenge testing), with at least 26.8% having some evidence of bronchial hyperresponsiveness.
Strengths and Limitations
This study has several important strengths. We had a rich and unusually large dataset to investigate daily pollutant-related acute respiratory symptoms given the panel size of 423 children, four cycles/seasons of intensive symptoms, and the use of repeated measures (four times daily for 15 school days each season), which resulted in over 70 000 health observations. The intensive ambient air monitoring of PM10 and SO2 at each school and accessing the NO2, NO, O3 and PM10 monitored at Durban (eThekwini) municipality sites provided reasonably robust exposure measures as well as both spatial and temporal coverage of key pollutants. These factors increased the power to investigate small increases in risk and the opportunity to investigate lag effects. We used standardized and well-validated instruments, used previously in Detroit, USA (Lewis et al., 2004) and subsequently modified and tested in Durban, South Africa (Kistnasamy et al., 2008). In addition to the usual measures of asthma diagnosis (e.g., reports of physician-diagnosed asthma), we used the NAEPP categories of intermittent and persistent asthma. Among communities in which we have previously described under-diagnosis of childhood asthma (Kistnasamy et al., 2008), these outcome measures strengthened our investigation into pollutant associated relationships. Completion of the daily symptoms logs under direct supervision of fieldworkers ensured maximum response by participants with a high degree of accuracy.
We recognize certain limitations in the study. A panel study can benefit greatly from personal exposure monitoring, although this is burdensome and compromises the sample size. We opted for a reasonable sample size, which meant that we had to rely on area monitoring at the schools. Although bussing at the selected schools was limited, our assumption that all pupils lived within a short radius of the monitoring station at the school is unlikely to be valid for the full sample. Equipment failures resulted in missing monitoring data, particularly for SO2. However, the extensive network of monitors allowed the use of imputation techniques to develop a more complete exposure dataset. Still, the possibility of exposure misclassification always exists, especially given the localized nature of SO2 and NO concentrations, unknown exposures at homes and other non-school locations, and the lack of monitoring for pollutants other than SO2, NO2, NO, and PM10. The outcomes of interest, although relatively imprecise (self-reported) endpoints with somewhat limited reliability, have been widely used in epidemiologic studies of children. It is possible that more accurate classification of individuals based on objective hallmarks of asthma, such as pulmonary measures, would strengthen the observed associations. We report on these measures in subsequent manuscripts of this study.
Conclusion
In conclusion, our study of a panel of 423 schoolchildren from seven schools with similar socio-economic profiles across the north and south of the city of Durban, provided evidence for pollutant-related acute respiratory symptoms. These acute responses were immediate (lag 0) as well as delayed (lags 1 – 5). Our findings were evident in models which combined both asthmatics and non-asthmatics, suggesting that even in the absence of underlying respiratory diseases, children are likely to present with symptoms.
STUDY HIGHLIGHTS.
The first repeated measures study conducted in sub-Saharan Africa
Multiple ambient exposures across all seasons assessed with over 70 000 observations
New findings for context with higher industrial pollution and different vehicle types
An interquartile range pollutant increase was associated with increased symptom risk
Residence location modified the pollutant-related ORs for symptoms.
Acknowledgments
This project was supported by grants from the eThekwini Municipality, the South African Medical Research Council, and the US National Institutes of Health/Fogarty International Center (grant D43 TW000812). The authors are grateful for support given by the Pollution Control Unit of the eThekwini Department of Health and the Durban University of Technology. We express our appreciation to Yogan Gounden, Thabang Molefi, Pamela Nasirumbi, Mary Lou Thompson, and the staff and students of all the participating schools. We also thank the research fieldworkers, our research administrator Jenny Pillay, and the staff of the Department of Occupational and Environmental Health at the University of KwaZulu-Natal.
The funders played no role in the study design, collection, analysis or interpretation of the data, the writing of the reports, or the decision to submit for publication.
Main Finding
Short term exposure to ambient pollutants in a population based sample of asthmatic and non-asthmatic schoolchildren in South Africa, is associated with increased respiratory symptom risk.
Declaration
The data used in this analysis and the presentation of findings have all been conducted in compliance with the Declaration of Helsinki and the principles of ethical practice in public health. The study was approved by the University of KwaZulu-Natal’s Biomedical Research Ethics Committee and the University of Michigan’s Institutional Review Board. These approvals are attached. This project was supported by grants from the eThekwini Municipality (South Africa), the South African Medical Research Council, and the US National Institutes of Health/Fogarty International Center (grant D43 TW000812). These funders played no role in the study design, data collection, analysis, manuscript preparation or decisions on publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
We have no known conflicts of interest to disclose. All of the authors have read and approved the paper and it has not been published previously nor is it being considered by any other peer-reviewed journal.
References
- Aekplakorn W, Loomis D, Vichit-Vadakan N, Bangdiwala S. Heterogeneity of daily pulmonary function in response to air pollution among asthmatic children. The Southeast Asian journal of tropical medicine and public health. 2004;35(4):990–8. [PubMed] [Google Scholar]
- Berhane K, Zhang Y, Linn WS, Rappaport EB, Bastain TM, Salam MT, et al. The effect of ambient air pollution on exhaled nitric oxide in the Children’s Health Study. Eur Respir J. 2011;37(5):1029–36. doi: 10.1183/09031936.00081410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branco PT, Nunes RA, Alvim-Ferraz MC, Martins FG, Ferraz C, Vaz LG, et al. Asthma prevalence and risk factors in early childhood at Northern Portugal. Rev Port Pneumol. 2016;22(3):146–50. doi: 10.1016/j.rppnen.2015.11.001. [DOI] [PubMed] [Google Scholar]
- Brozek G, Lawson J, Shpakou A, Fedortsiv O, Hryshchuk L, Rennie D, et al. Childhood asthma prevalence and risk factors in three Eastern European countries–the Belarus, Ukraine, Poland Asthma Study (BUPAS): an international prevalence study. BMC Pulm Med. 2016;16:11. doi: 10.1186/s12890-016-0172-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cames M, Helmers E. Critical evaluation of the European diesel car boom - global comparison, environmental effects and various national strategies. Environmental Sciences Europe. 2013;25(1):15. [Google Scholar]
- Cheng Z, Luo L, Wang S, Wang Y, Sharma S, Shimadera H, et al. Status and characteristics of ambient PM25 pollution in global megacities. Environ Int. 2016;89–90:212–21. doi: 10.1016/j.envint.2016.02.003. [DOI] [PubMed] [Google Scholar]
- Coull BA, Bobb JF, Wellenius GA, Kioumourtzoglou MA, Mittleman MA, Koutrakis P, et al. Part 1. Statistical Learning Methods for the Effects of Multiple Air Pollution Constituents. Res Rep Health Eff Inst. 2015;183(Pt 1–2):5–50. [PubMed] [Google Scholar]
- Dales R, Chen L, Frescura AM, Liu L, Villeneuve PJ. Acute effects of outdoor air pollution on forced expiratory volume in 1 s: a panel study of schoolchildren with asthma. Eur Respir J. 2009;34(2):316–23. doi: 10.1183/09031936.00138908. [DOI] [PubMed] [Google Scholar]
- Ehrlich RI, Du Toit D, Jordaan E, Volmink JA, Weinberg EG, Zwarenstein M. Prevalence and reliability of asthma symptoms in primary school children in Cape Town. Int J Epidemiol. 1995;24(6):1138–45. doi: 10.1093/ije/24.6.1138. [DOI] [PubMed] [Google Scholar]
- Epton MJ, Dawson RD, Brooks WM, Kingham S, Aberkane T, Cavanagh JA, et al. The effect of ambient air pollution on respiratory health of school children: a panel study. Environ Health. 2008;7:16. doi: 10.1186/1476-069X-7-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escamilla-Nunez MC, Barraza-Villarreal A, Hernandez-Cadena L, Moreno-Macias H, Ramirez-Aguilar M, Sienra-Monge JJ, et al. Traffic-related air pollution and respiratory symptoms among asthmatic children, resident in Mexico City: the EVA cohort study. Respir Res. 2008;9:74. doi: 10.1186/1465-9921-9-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito S, Galeone C, Lelii M, Longhi B, Ascolese B, Senatore L, et al. Impact of air pollution on respiratory diseases in children with recurrent wheezing or asthma. BMC Pulm Med. 2014;14:130. doi: 10.1186/1471-2466-14-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- eThekwini Health. Produced by eThekwini Health and Norwegian Institute for Air Research. Durban: 2007. Air quality management plan for eThekwini Municipality. Available at: http://www2.nilu.no/AirQuality/data/reports/%7BB2D475E8-BCDC-BAD1-AF03-9D183B89D4B5%7D.pdf. Accessed Feb. 14, 2012. [Google Scholar]
- Font A, Fuller GW. Did policies to abate atmospheric emissions from traffic have a positive effect in London? Environ Pollut. 2016;218:463–74. doi: 10.1016/j.envpol.2016.07.026. [DOI] [PubMed] [Google Scholar]
- Gass K, Klein M, Chang HH, Flanders WD, Strickland MJ. Classification and regression trees for epidemiologic research: an air pollution example. Environ Health. 2014;13(1):17. doi: 10.1186/1476-069X-13-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graveland H, Van Roosbroeck SA, Rensen WM, Brunekreef B, Gehring U. Air pollution and exhaled nitric oxide in Dutch schoolchildren. Occup Environ Med. 2011;68(8):551–6. doi: 10.1136/oem.2010.056812. [DOI] [PubMed] [Google Scholar]
- Joseph SP, Borrell LN, Shapiro A. Self-reported lifetime asthma and nativity status in U.S. children and adolescents: results from the National Health and Nutrition Examination Survey 1999–2004. J Health Care Poor Underserved. 2010;21(2 Suppl):125–39. doi: 10.1353/hpu.0.0286. [DOI] [PubMed] [Google Scholar]
- Jung DY, Leem JH, Kim HC, Kim JH, Hwang SS, Lee JY, et al. Effect of Traffic-Related Air Pollution on Allergic Disease: Results of the Children’s Health and Environmental Research. Allergy Asthma Immunol Res. 2015;7(4):359–66. doi: 10.4168/aair.2015.7.4.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kistnasamy EJ, Robins TG, Naidoo N, et al. The relationship between asthma and ambient air pollutants among primary school students in Durban, South Africa. Int J Environment and Health. 2008;2:365–385. [Google Scholar]
- Lee BE, Ha EH, Park HS, Kim H, Lee HJ, Lee YK, et al. Air pollution and respiratory symptoms of school children in a panel study in Seoul. J Prev Med Public Health. 2005;38(4):465–72. [PubMed] [Google Scholar]
- Lee JT, Cho YS, Son JY. Relationship between ambient ozone concentrations and daily hospital admissions for childhood asthma/atopic dermatitis in two cities of Korea during 2004–2005. Int J Environ Health Res. 2010;20(1):1–11. doi: 10.1080/09603120903254033. [DOI] [PubMed] [Google Scholar]
- Leem JH, Jang YK. Increase of diesel car raises health risk in spite of recent development in engine technology. Environ Health Toxicol. 2014;29:e2014009. doi: 10.5620/eht.e2014009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis TC, Robins TG, Joseph CL, Parker EA, Israel BA, Rowe Z, et al. Identification of gaps in the diagnosis and treatment of childhood asthma using a community-based participatory research approach. J Urban Health. 2004;81(3):472–88. doi: 10.1093/jurban/jth131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis TC, Robins TG, Mentz GB, Zhang X, Mukherjee B, Lin X, et al. Air pollution and respiratory symptoms among children with asthma: vulnerability by corticosteroid use and residence area. Sci Total Environ. 2013;448:48–55. doi: 10.1016/j.scitotenv.2012.11.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Williams G, Jalaludin B, Baker P. Panel studies of air pollution on children’s lung function and respiratory symptoms: a literature review. J Asthma. 2012;49(9):895–910. doi: 10.3109/02770903.2012.724129. [DOI] [PubMed] [Google Scholar]
- Lindgren A, Stroh E, Bjork J, Jakobsson K. Asthma incidence in children growing up close to traffic: a registry-based birth cohort. Environ Health. 2013;12:91. doi: 10.1186/1476-069X-12-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Poon R, Chen L, Frescura AM, Montuschi P, Ciabattoni G, et al. Acute effects of air pollution on pulmonary function, airway inflammation, and oxidative stress in asthmatic children. Environ Health Perspect. 2009;117(4):668–74. doi: 10.1289/ehp11813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallol J, Sole D, Baeza-Bacab M, Aguirre-Camposano V, Soto-Quiros M, Baena-Cagnani C, et al. Regional variation in asthma symptom prevalence in Latin American children. J Asthma. 2010;47(6):644–50. doi: 10.3109/02770901003686480. [DOI] [PubMed] [Google Scholar]
- Mann JK, Balmes JR, Bruckner TA, Mortimer KM, Margolis HG, Pratt B, et al. Short-term effects of air pollution on wheeze in asthmatic children in Fresno, California. Environ Health Perspect. 2010;118(10):1497–502. doi: 10.1289/ehp.0901292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConnell R, Islam T, Shankardass K, Jerrett M, Lurmann F, Gilliland F, et al. Childhood incident asthma and traffic-related air pollution at home and school. Environ Health Perspect. 2010;118(7):1021–6. doi: 10.1289/ehp.0901232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naidoo RN, Robins TG, Batterman S, Mentz G, Jack C. Ambient pollution and respiratory outcomes among schoolchildren in Durban, South Africa. SAJCH. 2013;7(4):127–34. doi: 10.7196/sajch.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Asthma Education and Prevention Program Expert Panel Report: Guidelines for the Diagnosis and Management of Asthma. Bethesda, MD: National Institutes of Health; 1991. [Google Scholar]
- Nastos PT, Paliatsos AG, Anthracopoulos MB, Roma ES, Priftis KN. Outdoor particulate matter and childhood asthma admissions in Athens, Greece: a time-series study. Environ Health. 2010;9:45. doi: 10.1186/1476-069X-9-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakes M, Baxter L, Long TC. Evaluating the application of multipollutant exposure metrics in air pollution health studies. Environ Int. 2014;69:90–9. doi: 10.1016/j.envint.2014.03.030. [DOI] [PubMed] [Google Scholar]
- O’Connor GT, Neas L, Vaughn B, Kattan M, Mitchell H, Crain EF, et al. Acute respiratory health effects of air pollution on children with asthma in US inner cities. J Allergy Clin Immunol. 2008;121(5):1133–9.e1. doi: 10.1016/j.jaci.2008.02.020. [DOI] [PubMed] [Google Scholar]
- Pan G, Zhang S, Feng Y, Takahashi K, Kagawa J, Yu L, et al. Air pollution and children’s respiratory symptoms in six cities of Northern China. Respir Med. 2010;104(12):1903–11. doi: 10.1016/j.rmed.2010.07.018. [DOI] [PubMed] [Google Scholar]
- Park ES, Symanski E, Han D, Spiegelman C. Part 2. Development of Enhanced Statistical Methods for Assessing Health Effects Associated with an Unknown Number of Major Sources of Multiple Air Pollutants. Res Rep Health Eff Inst. 2015;183(Pt 1–2):51–113. [PubMed] [Google Scholar]
- Patel MM, Chillrud SN, Correa JC, Hazi Y, Feinberg M, Kc D, et al. Traffic-related particulate matter and acute respiratory symptoms among New York City area adolescents. Environ Health Perspect. 2010;118(9):1338–43. doi: 10.1289/ehp.0901499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranzi A, Freni Sterrantino A, Forastiere F, Sartini C, Casale G, Cavallini R, et al. Asthmatic symptoms and air pollution: a panel study on children living in the Italian Po Valley. Geospat Health. 2015;10(2):366. doi: 10.4081/gh.2015.366. [DOI] [PubMed] [Google Scholar]
- Ranzi A, Gambini M, Spattini A, Galassi C, Sesti D, Bedeschi M, et al. Air pollution and respiratory status in asthmatic children: hints for a locally based preventive strategy. AIRE study. Eur J Epidemiol. 2004;19(6):567–76. doi: 10.1023/b:ejep.0000032373.28250.84. [DOI] [PubMed] [Google Scholar]
- Nebahe Ratshomo K. Overview of petrol and diesel market in South Africa between 2002 and 2013. Department of Energy; Pretoria: 2013. Available at: http://www.energy.gov.za/files/media/explained/Overview-of-Petrol-and-Diesel-Market-in-SA-between-2002-and-2013.pdf. Last accessed: Last accessed: 30 May 2017. [Google Scholar]
- Roemer W, Hoek G, Brunekreef B, Haluszka J, Kalandidi A, Pekkanen J. Daily variations in air pollution and respiratory health in a multicentre study: the PEACE project. Pollution Effects on Asthmatic Children in Europe. Eur Respir J. 1998;12(6):1354–61. doi: 10.1183/09031936.98.12061354. [DOI] [PubMed] [Google Scholar]
- Rojas-Rueda D, Turner MC. Commentary: Diesel, Cars, and Public Health. Epidemiology. 2016;27(2):159–62. doi: 10.1097/EDE.0000000000000427. [DOI] [PubMed] [Google Scholar]
- Rovira E, Cuadras A, Aguilar X, Esteban L, Borras-Santos A, Zock JP, et al. Asthma, respiratory symptoms and lung function in children living near a petrochemical site. Environ Res. 2014;133:156–63. doi: 10.1016/j.envres.2014.05.022. [DOI] [PubMed] [Google Scholar]
- Samoli E, Nastos PT, Paliatsos AG, Katsouyanni K, Priftis KN. Acute effects of air pollution on pediatric asthma exacerbation: evidence of association and effect modification. Environ Res. 2011;111(3):418–24. doi: 10.1016/j.envres.2011.01.014. [DOI] [PubMed] [Google Scholar]
- Sarnat SE, Raysoni AU, Li WW, Holguin F, Johnson BA, Flores Luevano S, et al. Air pollution and acute respiratory response in a panel of asthmatic children along the U.S.-Mexico border. Environ Health Perspect. 2012;120(3):437–44. doi: 10.1289/ehp.1003169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh S, Sharma BB, Sharma SK, Sabir M, Singh V, investigators Ic Prevalence and severity of asthma among Indian school children aged between 6 and 14 years: associations with parental smoking and traffic pollution. J Asthma. 2016;53(3):238–44. doi: 10.3109/02770903.2015.1087558. [DOI] [PubMed] [Google Scholar]
- Strickland MJ, Darrow LA, Klein M, Flanders WD, Sarnat JA, Waller LA, et al. Short-term associations between ambient air pollutants and pediatric asthma emergency department visits. Am J Respir Crit Care Med. 2010;182(3):307–16. doi: 10.1164/rccm.200908-1201OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Z, Tao Y, Li S, Ferguson KK, Meeker JD, Park SK, et al. Statistical strategies for constructing health risk models with multiple pollutants and their interactions: possible choices and comparisons. Environ Health. 2013;12(1):85. doi: 10.1186/1476-069X-12-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torjesen I. Switch to diesel engines is significantly to blame for poor air quality in UK cities, experts say. BMJ. 2014;348:g3033. doi: 10.1136/bmj.g3033. [DOI] [PubMed] [Google Scholar]
- van der Zee S, Hoek G, Boezen HM, Schouten JP, van Wijnen JH, Brunekreef B. Acute effects of urban air pollution on respiratory health of children with and without chronic respiratory symptoms. Occup Environ Med. 1999;56(12):802–12. doi: 10.1136/oem.56.12.802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vichit-Vadakan N, Ostro BD, Chestnut LG, Mills DM, Aekplakorn W, Wangwongwatana S, et al. Air pollution and respiratory symptoms: results from three panel studies in Bangkok, Thailand. Environ Health Perspect. 2001;109(Suppl 3):381–7. doi: 10.1289/ehp.01109s3381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villeneuve PJ, Chen L, Rowe BH, Coates F. Outdoor air pollution and emergency department visits for asthma among children and adults: a case-crossover study in northern Alberta, Canada. Environ Health. 2007;6:40. doi: 10.1186/1476-069X-6-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vosper SJ, Mercure J-F. Assessing the effectiveness of South Africa’s emissions-based purchase tax for private passenger vehicles: A consumer choice modelling approach. Journal of Energy in Southern Africa. 2016;27:25–37. [Google Scholar]
- Ward DJ, Ayres JG. Particulate air pollution and panel studies in children: a systematic review. Occupational and environmental medicine. 2004;61(4):e13. doi: 10.1136/oem.2003.007088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinmayr G, Romeo E, De Sario M, Weiland SK, Forastiere F. Short-term effects of PM10 and NO2 on respiratory health among children with asthma or asthma-like symptoms: a systematic review and meta-analysis. Environ Health Perspect. 2010;118(4):449–57. doi: 10.1289/ehp.0900844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winquist A, Kirrane E, Klein M, Strickland M, Darrow LA, Sarnat SE, et al. Joint effects of ambient air pollutants on pediatric asthma emergency department visits in Atlanta, 1998–2004. Epidemiology. 2014;25(5):666–73. doi: 10.1097/EDE.0000000000000146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zora JE, Sarnat SE, Raysoni AU, Johnson BA, Li WW, Greenwald R, et al. Associations between urban air pollution and pediatric asthma control in El Paso, Texas. Sci Total Environ. 2013;448:56–65. doi: 10.1016/j.scitotenv.2012.11.067. [DOI] [PubMed] [Google Scholar]


