Abstract
Objective
To determine prognostic value of tumor size and metabolic activity on survival for patients with early stage NSCLC receiving SBRT.
Methods
We retrospectively evaluated patients who underwent PET/CT scan prior to SBRT treatment. Tumor diameter, tumor volume, SUVmax, SUV average, and SUV volume were obtained. Cox regression analyses were performed to determine associations between tumor characteristics and survival.
Results
Patients with large tumors and high SUVmax have worse survival than small tumors with low SUVmax (hazard ratio = 3.47; p=0.007). Patients with small tumors and high SUVmax (HR=1.80; p=0.24) and large tumors and low SUVmax (HR = 1.55; p=0.43) had increased risk of death compared to small tumors and low SUVmax..
Conclusion
Both increased tumor size and metabolic activity are associated with increased risk of death. Combining size and metabolic activity together is superior for predicting 2-year survival and identifying patients for whom survival is statistically worse.
Keywords: CT, PET/CT, lung cancer, stereotactic body radiation therapy
Introduction
Non-small cell lung cancer (NSCLC) remains the most common cancer worldwide and lung cancer is responsible for about one in four cancer deaths1. Surgical resection is the standard treatment for NSCLC, but its aggressive nature and often-late presentation can limit surgical resection effectiveness. With the increase in the aging population and the more liberal use of CT, more patients are being diagnosed with lung cancer that are not medically fit for operation. Patients who are declared medically inoperable or who refuse surgical resection are candidates for stereotactic body radiation therapy (SBRT), which delivers optimally positioned high dose radiation beams to minimize surrounding normal tissue damage2. SBRT has been shown to be a successful, if not equivalent, alternative treatment for non-resectable early stage NSCLC3. Because SBRT is becoming a more widely accepted alternative standard of treatment, more and more patients with expanded life expectancy will be encountered. In this group, there may be individuals that would benefit from additional therapy in a similar paradigm to those who receive adjuvant therapy following surgery.
[F18]-Fluorodeoxyglucose positron emission tomography (FDG-PET) is an imaging modality that takes advantage of tumor properties such as increased glucose metabolism and is reflective of intrinsic metastatic and proliferative potential4. FDG-PET is a valuable tool for initial staging and subsequent assessments, and has been suggested to be a prognostic indicator in the treatment of NSCLC. Previous studies have shown that tumors with a higher maximum standardized uptake value (SUVmax) have a poorer overall prognosis with shorter progression-free and overall survival5–8, although the relationship between SUVmax and survival is inconsistent9,10. In order to improve survival in higher risk individuals, it may be helpful to modify treatment plans by including different dosing of radiotherapy or by adding ablative therapies, adjuvant immunotherapy, or chemotherapy. As all of the options are associated with additional risks, knowledge of the optimal population for alternative strategies is important. In this study, we retrospectively evaluated the prognostic value of the initial tumor size and metabolic activity on overall two-year survival for patients with early stage NSCLC receiving SBRT with curative intent. Our hypothesis was that a combination of factors to include both size and tumor metabolic activity would better predict survival at two years than either factor alone.
Materials and Methods
This retrospective HIPAA compliant study was done under a waiver of consent from our local institutional review board.
Subjects
We performed a retrospective review of all patients who underwent thoracic SBRT for the treatment of lung cancer over a 5-year period from June 2008 through June 2013. During this period, 240 unique patients were treated. Patients were excluded if the PET/CT was not available, performed at another institution, or performed greater than 2 months prior to SBRT, or if the patient was treated for metastatic lesions or with palliative intent, leaving a total of 100 subjects. All subjects were considered to be Stage I or II (T1-3N0M0) based on the 7th edition of the American Joint Committee on Cancer lung cancer staging handbook. 2-year survival outcome was documented via the electronic medical record. For those subjects that could not be confirmed as alive or deceased at their two-year anniversary from the medical record, a search of the National Death Index (NDI) was performed to determine vital status.
18F-FDG-PET/CT Technique
FDG-PET scans were acquired after the patient had fasted for 6 hours and had a confirmed blood glucose of lower than 200 mg/dl. 18F-FDG 3.5 MBq/kg body weight was administered via intravenous injection. The PET/CT was acquired using a (GE Discovery ST PET/CT, GE Health Care, Waukesha, WI) 45 minutes after FDG administration. The CT portion was performed during quiet breathing with the following parameters: kVp 120, automated tube current modulation with mA range 10–180, noise index 18, tube rotation time 0.8 sec., pitch 1.75, 50 cm field of view with 3.75mm slice thickness from the eyes to mid-thigh. Iodinated contrast was not administered. PET data was acquired following the CT. PET images were acquired in 3D mode and iteratively reconstructed by using 21 subsets and 4 iterations. The attenuation correction images fused with the CT Image data were displayed in axial, coronal, and sagittal views.
Image Analysis
For each study, the acquired data was retrieved from the PET/CT data archives and reviewed on a GE AW1 workstation. Measurements were then carried out by a single observer (blind). After the tumor was identified on CT attenuation correction images, automatically segmented boundaries were constructed to encompass the primary tumor and the tumor diameter and volume were obtained. The diameter was recorded as the average of the long axis and perpendicular measure in the short axis. Manual adjustments to the volume were not made. Following the CT measurements, the tumor was identified again using both the attenuation correction and fused images and the following measurements were then recorded: SUVmax, SUV average, and the SUV volume. SUVmax was recorded as the maximum SUV pixel value within the tumor volume, SUV average was defined as the average SUV measurement within a 1 cm3 volume surrounding SUVmax, and SUV volume was defined as average SUV activity within the entire tumor volume.
SBRT Methods
Treatment simulation was initiated by a four dimensional (4D) CT through the thorax and subdivided into 10 bins through the respiratory phase. Images were then delivered to the treatment planning system (TPS) (PINNACLE 9.0; Phillips Healthcare). The internal target volume (ITV) was planned by a radiation oncologist to delineate and contain all respiratory motion of the tumor. A set up margin of 5 mm was added to the ITV resulting in the planning target volume (PTV).
Prescription doses were either 50 Gy in five fractions or 48 Gy in four fractions with each fraction separated by 2–3 days and no more than two fractions in one week. Initial radiographic follow-up occurred between 1–3 months following completion of treatment either with CT or PET/CT. This was followed by CT at 6 month intervals for a total of two years.
Evaluation of Patient Outcomes
For the purpose of our study, the overall survival was assessed at two years post the start of SBRT. For all patients in whom a definitive outcome at two years could not be ascertained from the medical record, a search of the National Death Index (NDI) was performed. The recorded date of death was used in survival analysis. Those who could not be confirmed as deceased were presumed to be alive at the two-year anniversary.
Statistical Analysis
Summary statistics, including mean or median, standard deviation, and range were used to describe distributions of continuous variables. Categorical variables were tabulated and reported using percentages. The primary outcome was overall survival, defined as time from SBRT to death (or censored at the last known date the patient was alive). Kaplan-Meier estimation was used to calculate median survival and two-year survival rates with 95% confidence intervals. Comparisons of survival curves were performed using log rank tests and Cox regression. Comparisons were based on binary versions of tumor size and PET/CT where a small vs. large tumor was defined as ≤2 cm vs. > 2 cm diameter; tumors were categorized as low metabolizing if SUVmax ≤5 vs. SUVmax>5. We also considered a tumor diameter cutoff of 3cm, but opted for the 2cm cutoff as described in the Results section. The final Cox regression model included main effects of both SUVmax and tumor size, as well as their interaction. Proportional hazards were tested using graphical displays and was found to adequately meet the assumption. Inferences were based on hazard ratios with an alpha level set at 0.05.
Results
100 NSCLC patients (37 female/63 male) were included in this study with an average age of 75.8 years (range 51–95). Tumors ranged in size from 0.5–8.0 cm with a median size of 2.2 cm with tumor size as follows: 46 nodules were less than 2 cm, 24 were between 2 and 3 cm (70% were T1 tumors) and 30 were > 3 cm. With regard to outcomes, 11 subjects did not have definitive documentation of vital status at the 2-year anniversary. The majority of subjects had a tissue diagnosis of lung cancer (n=96). In cases where tissue could not be safely obtained, patients were discussed at a lung cancer multi-disciplinary tumor board and treatment decision was made based on a very high likelihood of cancer. In 3 of these 4 cases the SUVmax was >5. Demographic and tumor characteristics are listed in Tables 1 and 2.
Table 1.
Demographic and tumor characteristics. (N=100)
| Age (years), mean (SD) [Range] | 75.8 (9.6) [51, 95] |
| SUVmax, median (SD) [Range] | 7.6 (6.4) [1.1, 38.5] |
| SUV average, median (SD) [Range] | 5.1 (5.2) [0.7, 31.3] |
| SUV volume, median (SD) [Range] | 4.6 (4.2) [0.6, 24.3] |
| Nodule diameter, median (SD) [Range] | 2.2 (1.5) [0.5, 8.0] |
| Nodule volume, median (SD) [Range] | 5.95 (16.6) [0.9, 97.9] |
| Histology, N (%) | |
| NSCLC | 32 (32%) |
| Adenocarcinoma | 33 (33%) |
| Squamous cell | 31 (31%) |
| Not specified | 4 (4%) |
Table 2.
Distribution of nodule diameters.
| Nodule Diameter | ≤ 2 cm | 2.1 – 3 cm | 3.1 – 5 cm | 5.1 – 7 cm | > 7 cm |
| Nodules, N (%) | 46 (46%) | 24 (24%) | 24 (24%) | 3 (3%) | 3 (3%) |
Tumor size vs. survival
Tumor size was related to outcome as tumors with a diameter of ≤ 2 cm had a median overall survival (OS) of 40 months and a two-year OS of 74% (95% confidence interval (CI): 0.62, 0.80; Figure 1) after SBRT treatment, while tumors with a diameter of > 2 cm had a median survival of 27 months and a two-year OS of 59% (95%CI: 0.47, 0.74). The estimated hazard ratio (HR) for tumors with diameter greater than 2 cm vs. less than or equal to 2 cm was 1.85 (p=0.039). Increasing the tumor size cut point to 3 cm resulted in a similar median OS of 34.7 months and 2 year survivals of 70% and 57%, respectively, but the differences were not statistically significant (HR= 1.22, p=0.52). Because of this, a size cut point of 2 cm was carried forward for further analysis.
Figure 1.
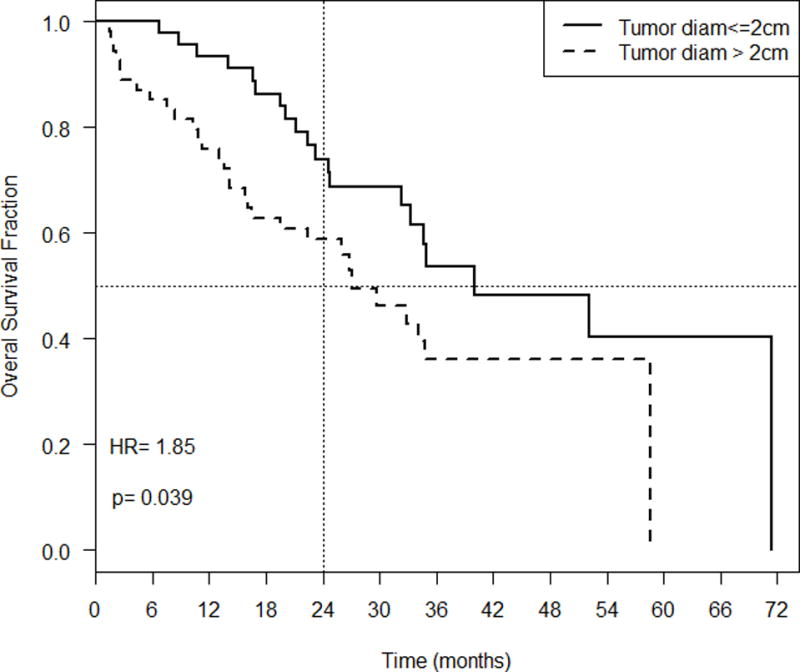
Overall survival fraction over time (months) based on tumor diameter.
SUV vs. survival
A similar outcome was noted with SUVmax, SUVavg, SUVvol where lower values were associated with better outcomes (Figure 2). A SUVmax of ≤ 5 had a median OS of 58.6 months and two-year OS of 79% (95% CI: 0.66, 0.94) whereas a SUVmax of > 5 had a median survival of 27.0 months and two-year survival of 60% (95% CI 0.48, 0.72). The HR comparing patients with high SUVmax vs. those with low SUVmax was 2.07 (p=0.025). Estimates of median and two-year survival were similar for SUVavg, SUVvol and SUVmax when a cutoff of 5 was used (results not shown). As there was no difference in associations between OS and SUVmax, SUVavg and SUVvol, SUVmax was carried forward for further analysis.
Figure 2.
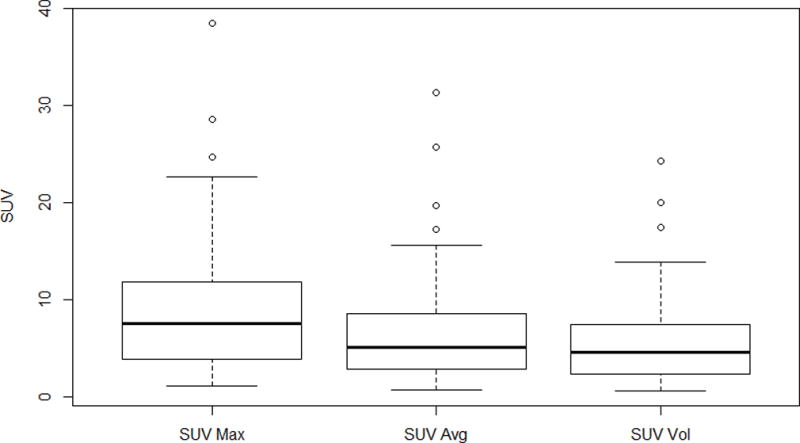
Pretreatment SUV characteristics. SUVmax, SUV average, and SUV volume median, standard deviation, and range are depicted.
Combining size and SUV vs. survival
Results from the Cox regression demonstrate that the interaction term was not significant (p= 0.7), implying that the effect of high metabolism on OS is similar among patients with small tumors and those with large tumors. Combining tumor size and SUVmax showed that small tumors regardless of SUVmax and large tumors with SUVmax ≤ 5 had similar survival distributions, while large tumors with SUVmax > 5 had worse outcomes (Figure 3). Using small tumors (≤ 2cm) with low SUVmax (≤ 5) as a reference, the HR for large tumors with low SUVmax was 1.55 (p=0.43). Comparing small tumors with high SUVmax to small tumors with low SUVmax, the HR was 1.80 (p=0.24). Only large tumors with high SUVmax were statistically significant from small tumors with low SUVmax with a HR of 3.47 (p=0.007).
Figure 3.
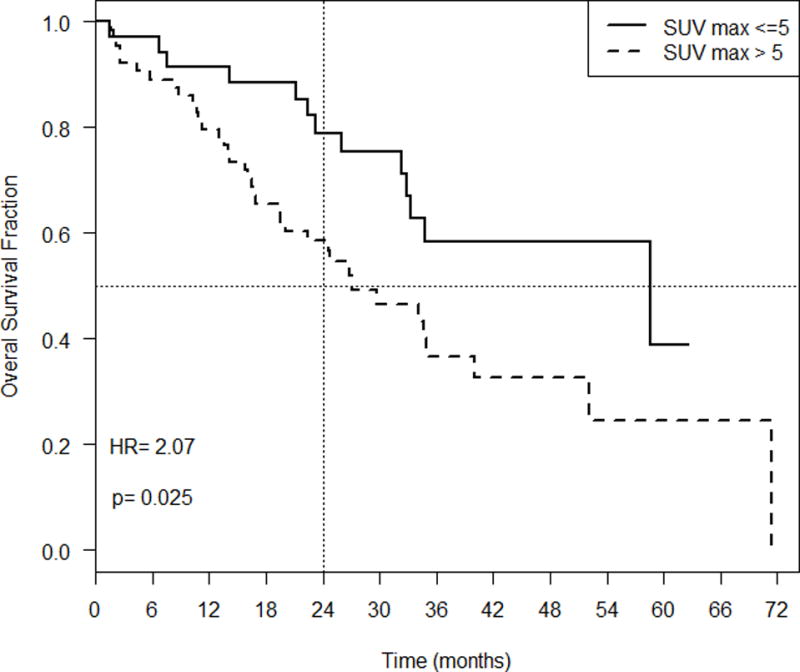
Overall survival fraction over time (months) based on SUVmax.
Discussion
While tumor size is often used to guide the need for adjuvant therapy for surgically resected tumors, there is no such guidance for patients treated with SBRT. As many of these are medically inoperable due to advanced age and comorbidities, they are often not candidates for systemic therapy. Nonetheless, advances in chemotherapy and immunotherapy raise the hope that appropriate individuals may benefit from adjuvant therapy beyond SBRT. Metabolic activity can also play a role in prognosis, although its utility is less well–defined owing to measurement variability and a lack of generalizability across sites. However, with a lack of consistent evidence demonstrating any concrete association between metabolic activity and prognosis with SBRT, the extent of this PET-CT measurement has yet to be fully explored and defined.
Our results show that patients with the combination of both large size and high metabolic activity are at greater risk of death than patients with neither or a single risk factor. Using both tumor characteristics in combination was shown to be a superior predictive value than either alone. The findings are important as they define a subgroup of patients who may benefit from adjuvant therapy in a paradigm similar to those treated surgically. Whether survival can be improved with adjuvant therapies in this group remains to be determined.
Several studies to date have shown that SUVmax does demonstrate a statistically significant prognostic value in the treatment of early stage NSCLC with SBRT6,7,11,12. Horne et al found that the SUVmax is a statistically significant predictor of overall survival and progression free survival in early stage NSCLC, while other studies also found that SUVmax is a significant prognosticator for overall survival5,6,12. Also, a study performed by Takeda et al demonstrated that SUVmax was a significant predictor of disease-free survival, overall survival, as well as local progression8. Yamamoto et al sought to improve pretreatment SUVmax values by correcting for partial volume effect and motion artifact10. Despite registering higher SUVmax when correcting for these factors, the corrected SUVmax did not improve local control prediction, and tumor size remained more relevant.
More recently, Satoh et al assessed the prognostic value of tumor volume, attenuation, and SUVmax, and found that both volume ≤ 2.5 cm3 and mean attenuation ≥ −120 Hounsfield units were better predictors of overall survival13. They did not, however, assess whether using a combination of these features would predict a unique group that might benefit from additional therapy.
Despite this, some studies are not able to produce the same conclusions regarding the relevance of SUVmax values9. A meta-analysis in 2009 found that SUVmax on preoperative PET-CT was a poor prognostic indicator, and another meta-analysis in 2008 also concluded that the primary tumor SUVmax was a suboptimal prognosticator in stages I-III NSCLC14,15. They found it difficult to draw concrete conclusions because of the number of heterogeneous factors including SUVmax thresholds and research designs that had been utilized. In addition, it does not appear that a SUVmax threshold is necessarily generalizable across different institutions.
Total lesion glycolysis (TLG) has been advocated as a better measure of metabolic activity by multiplying the average SUV within the entire tumor by the tumor volume and has been shown to help predict outcome in both surgically resected patients16 and those treated with SBRT11. Despite having the advantage of combining metabolic activity and size into one measure, TLG has not been shown to be superior to SUVmax in predicting outcome. Only in a subset of tumors > 3 cm treated with SBRT did TLG better predict survival11. This corroborates our finding that the combination of larger tumor size associated with higher metabolic activity represents a distinct risk for recurrence. We chose not to perform TLG in our population owing to the inherent difficulties in obtaining an accurate volume measurement on the attenuation correction CT. We believe the combination of diameter and SUVmax is a reasonable surrogate for TLG, while being both easier and faster to obtain.
Our study has several limitations. This is a retrospective study conducted over several years and we cannot account for effects of SBRT improvements and experience over time. Because we were not always able to account for vital status at two years, we performed an NDI search in an effort to improve the endpoint. This is an imperfect strategy that may not account for all events and it is possible that some deaths were not captured. The SUV values were all calculated for the same PET/CT scanner and therefore optimal cut points may differ from site to site. Unfortunately, our sample size is relatively small. Another limitation that we encountered was in the acquisition of SUV values for tumors of ≤ 2 cm. We do not routinely use respiratory gating which may result in an underestimation of SUV. While the use of respiratory gating would undoubtedly give higher SUV values, we believe this would simply change cut-off thresholds rather than provide additional information. And finally, due to patients’ medical conditions, many did not undergo formal mediastinal staging. While it is possible that some subjects were therefore under-staged, this may be mitigated by the incidental prophylactic nodal radiation that occurred during SBRT17. In a recent study by Giuliani et al, they found that regional recurrence was associated with higher SUVmax value in the primary tumor, and therefore suggested that routine nodal sampling with endoscopic bronchial ultrasound (EBUS) should be considered in these higher risk patients18.
In conclusion, both tumor size and tumor metabolic activity alone are associated with increased risk of death. A model that accounts for tumor diameter and metabolic activity is superior for predicting 2-year survival and identifying a subgroup of patients for whom both overall survival and 2-year survival are statistically worse. The results suggest a subgroup population that may benefit from clinical trials evaluating the need for radiation dose escalation approaches or adjuvant chemo or immune therapy.
Figure 4.
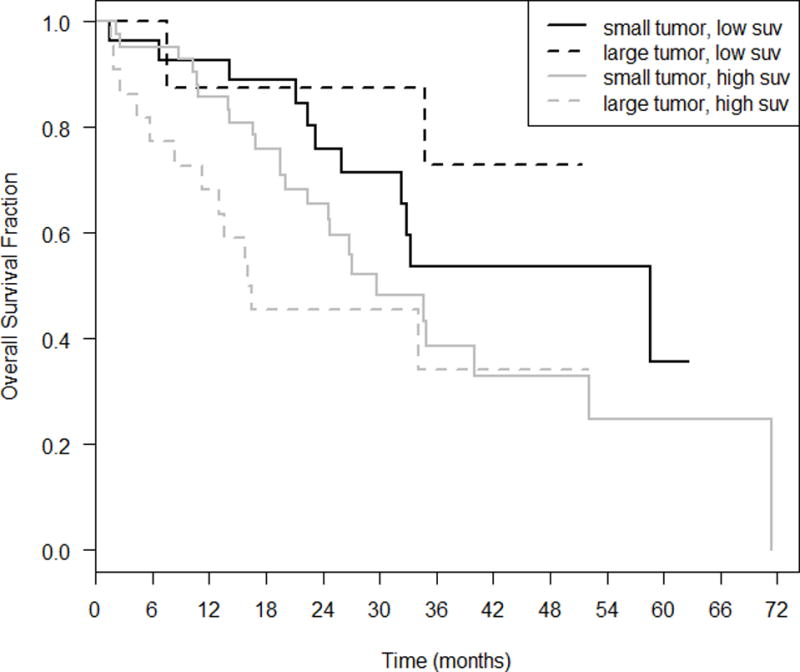
Multiple regression model with both nodule diameter (≤ 2 cm vs. > 2 cm) and SUV.
Table 3.
Cox regression results with small tumor (≤ 2 cm) and low SUVmax (<5).
| Hazard Ratio | 95% CI | p-value | |
|---|---|---|---|
| Small tumor, low suv (n=21) | 1.0 (reference) | ||
| Large tumor, low suv (n=14) | 1.55 | 0.51, 4.64 | 0.43 |
| Small tumor, high suv (n=25)_ | 1.80 | 0.68, 4.74 | 0.24 |
| Large tumor, high suv (n=40) | 3.47 | 1.39, 8.61 | 0.007 |
Acknowledgments
Supported in part by the Biostatistics Shared Resource, Hollings Cancer Center, Medical University of South Carolina (P30 CA138313)
Footnotes
There are no financial disclosures to report.
References
- 1.Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359–386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.Senthi S, Lagerwaard FJ, Haasbeek CJ, Slotman BJ, Senan S. Patterns of disease recurrence after stereotactic ablative radiotherapy for early stage non-small-cell lung cancer: a retrospective analysis. The Lancet Oncology. 2012;13(8):802–809. doi: 10.1016/S1470-2045(12)70242-5. [DOI] [PubMed] [Google Scholar]
- 3.Zheng X, Schipper M, Kidwell K, et al. Survival outcome after stereotactic body radiation therapy and surgery for stage I non-small cell lung cancer: a meta-analysis. Int J Radiat Oncol Biol Phys. 2014;90(3):603–611. doi: 10.1016/j.ijrobp.2014.05.055. [DOI] [PubMed] [Google Scholar]
- 4.Kligerman S, Digumarthy S. Staging of non-small cell lung cancer using integrated PET/CT. AJR Am J Roentgenol. 2009;193(5):1203–1211. doi: 10.2214/AJR.09.3193. [DOI] [PubMed] [Google Scholar]
- 5.Horne ZD, Clump DA, Vargo JA, et al. Pretreatment SUVmax predicts progression-free survival in early-stage non-small cell lung cancer treated with stereotactic body radiation therapy. Radiat Oncol. 2014;9:41. doi: 10.1186/1748-717X-9-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kohutek ZA, Wu AJ, Zhang Z, et al. FDG-PET maximum standardized uptake value is prognostic for recurrence and survival after stereotactic body radiotherapy for non-small cell lung cancer. Lung Cancer. 2015;89(2):115–120. doi: 10.1016/j.lungcan.2015.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kwon W, Howard BA, Herndon JE, Patz EF., Jr FDG Uptake on Positron Emission Tomography Correlates with Survival and Time to Recurrence in Patients with Stage I Non-Small-Cell Lung Cancer. J Thorac Oncol. 2015;10(6):897–902. doi: 10.1097/JTO.0000000000000534. [DOI] [PubMed] [Google Scholar]
- 8.Takeda A, Sanuki N, Fujii H, et al. Maximum standardized uptake value on FDG-PET is a strong predictor of overall and disease-free survival for non-small-cell lung cancer patients after stereotactic body radiotherapy. J Thorac Oncol. 2014;9(1):65–73. doi: 10.1097/JTO.0000000000000031. [DOI] [PubMed] [Google Scholar]
- 9.Lin MY, Wu M, Brennan S, et al. Absence of a relationship between tumor (1)(8)F-fluorodeoxyglucose standardized uptake value and survival in patients treated with definitive radiotherapy for non-small-cell lung cancer. J Thorac Oncol. 2014;9(3):377–382. doi: 10.1097/JTO.0000000000000096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yamamoto T, Kadoya N, Shirata Y, et al. Formula corrected maximal standardized uptake value in FDG-PET for partial volume effect and motion artifact is not a prognostic factor in stage I non-small cell lung cancer treated with stereotactic body radiotherapy. Annals of nuclear medicine. 2015;29(8):666–673. doi: 10.1007/s12149-015-0991-5. [DOI] [PubMed] [Google Scholar]
- 11.Satoh Y, Onishi H, Nambu A, Araki T. Volume-based parameters measured by using FDG PET/CT in patients with stage I NSCLC treated with stereotactic body radiation therapy: prognostic value. Radiology. 2014;270(1):275–281. doi: 10.1148/radiol.13130652. [DOI] [PubMed] [Google Scholar]
- 12.Paesmans M, Berghmans T, Dusart M, et al. Primary tumor standardized uptake value measured on fluorodeoxyglucose positron emission tomography is of prognostic value for survival in non-small cell lung cancer: update of a systematic review and meta-analysis by the European Lung Cancer Working Party for the International Association for the Study of Lung Cancer Staging Project. J Thorac Oncol. 2010;5(5):612–619. doi: 10.1097/JTO.0b013e3181d0a4f5. [DOI] [PubMed] [Google Scholar]
- 13.Satoh Y, Motosugi U, Nambu A, Saito A, Onishi H. Prognostic Value of Semiautomatic CT Volumetry in Patients With Stage I Non-Small Cell Lung Cancer Treated With Stereotactic Body Radiation Therapy. J Comput Assist Tomogr. 2016;40(3):343–350. doi: 10.1097/RCT.0000000000000368. [DOI] [PubMed] [Google Scholar]
- 14.Nair VS, Krupitskaya Y, Gould MK. Positron emission tomography 18F-fluorodeoxyglucose uptake and prognosis in patients with surgically treated, stage I non-small cell lung cancer: a systematic review. J Thorac Oncol. 2009;4(12):1473–1479. doi: 10.1097/JTO.0b013e3181bccbc6. [DOI] [PubMed] [Google Scholar]
- 15.Berghmans T, Dusart M, Paesmans M, et al. Primary tumor standardized uptake value (SUVmax) measured on fluorodeoxyglucose positron emission tomography (FDG-PET) is of prognostic value for survival in non-small cell lung cancer (NSCLC): a systematic review and meta-analysis (MA) by the European Lung Cancer Working Party for the IASLC Lung Cancer Staging Project. J Thorac Oncol. 2008;3(1):6–12. doi: 10.1097/JTO.0b013e31815e6d6b. [DOI] [PubMed] [Google Scholar]
- 16.Park SY, Cho A, Yu WS, et al. Prognostic value of total lesion glycolysis by 18F-FDG PET/CT in surgically resected stage IA non-small cell lung cancer. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2015;56(1):45–49. doi: 10.2967/jnumed.114.147561. [DOI] [PubMed] [Google Scholar]
- 17.Lao L, Hope AJ, Maganti M, et al. Incidental prophylactic nodal irradiation and patterns of nodal relapse in inoperable early stage NSCLC patients treated with SBRT: a case-matched analysis. Int J Radiat Oncol Biol Phys. 2014;90(1):209–215. doi: 10.1016/j.ijrobp.2014.05.006. [DOI] [PubMed] [Google Scholar]
- 18.Giuliani ME, Hope A, Mangona V, et al. Predictors and Patterns of Regional Recurrence Following Lung SBRT: A Report From the Elekta Lung Research Group. Clin Lung Cancer. 2017;18(2):162–168. doi: 10.1016/j.cllc.2016.10.006. [DOI] [PubMed] [Google Scholar]


