Abstract
Imagination and creative cognition are often associated with the brain's default network (DN). Recent evidence has also linked cognitive control systems to performance on tasks involving imagination and creativity, with a growing number of studies reporting functional interactions between cognitive control and DN regions. We sought to extend the emerging literature on brain dynamics supporting imagination by examining individual differences in large‐scale network connectivity in relation to Openness to Experience, a personality trait typified by imagination and creativity. To this end, we obtained personality and resting‐state fMRI data from two large samples of participants recruited from the United States and China, and we examined contributions of Openness to temporal shifts in default and cognitive control network interactions using multivariate structural equation modeling and dynamic functional network connectivity analysis. In Study 1, we found that Openness was related to the proportion of scan time (i.e., “dwell time”) that participants spent in a brain state characterized by positive correlations among the default, executive, salience, and dorsal attention networks. Study 2 replicated and extended the effect of Openness on dwell time in a correlated brain state comparable to the state found in Study 1, and further demonstrated the robustness of this effect in latent variable models including fluid intelligence and other major personality factors. The findings suggest that Openness to Experience is associated with increased functional connectivity between default and cognitive control systems, a connectivity profile that may account for the enhanced imaginative and creative abilities of people high in Openness to Experience.
Keywords: brain dynamics, creativity, default network, imagination, individual differences, personality
1. INTRODUCTION
Recent neuroimaging research has sought to identify cognitive functions associated with the interaction of large‐scale functional brain networks (Braun et al., 2015; Douw, Wakeman, Tanaka, Liu, & Stufflebeam, 2016; Kucyi, Hove, Esterman, Hutchison, & Valera, 2017; Medaglia, Lynall, & Bassett, 2015). Of particular interest has been the brain's default network (DN), a set of cortical midline, medial temporal, and inferior parietal regions that activate during the resting‐state and during cognitive processes that involve self‐generated thought, such as mind‐wandering, episodic memory retrieval, future imagination, mentalizing, and creative cognition (Andrews‐Hanna, Smallwood, & Spreng, 2014; Buckner, Andrews‐Hanna, & Schacter, 2008; Raichle, 2015). Functional imaging studies indicate that the DN supports specific types of self‐generated thought, such as imagination and creativity, through its interactions with brain systems associated with cognitive control (Beaty, Benedek, Silvia, & Schacter, 2016a; Christoff, Irving, Fox, Spreng, & Andrews‐Hanna, 2016; Zabelina & Andrews‐Hanna, 2016), suggesting that brain network flexibility supports cognitive flexibility (cf., Braun et al., 2015; Shine et al., 2016). Here, we aimed to extend research on brain networks underlying imaginative thought by assessing dynamic fluctuations of resting‐state network interactions in relation to individual differences in Openness to Experience, a personality trait epitomized by imagination and creativity (Oleynick et al., 2017; Saucier, 1992). This approach allowed us to investigate brain network function associated with high imaginative ability.
2. IMAGINATION AND BRAIN DYNAMICS
Imaginative thinking has consistently been associated with engagement of the DN (Zabelina & Andrews‐Hanna, 2016). The DN shows consistent activation in the absence of external task demands, a phenomenon that has largely been attributed to mind‐wandering or the spontaneous generation of thought that is independent of sensory input (O'Callaghan, Shine, Lewis, Andrews‐Hanna, & Irish, 2015; Smallwood et al., 2016, 2013). Critically, however, recent work has shown that the DN is not merely a task‐negative system (Spreng, 2012) but rather reflects active internal processing that contributes to goal‐directed task performance (Andrews‐Hanna et al., 2014; Buckner et al., 2008; Christoff et al., 2016). For example, the DN shows robust activity during episodic memory retrieval, a constructive process of extracting and recombining episodic details to form representations of past events (Schacter & Addis, 2007). Consistent with this constructive function, the DN has been shown to support episodic future thinking, the imagination of possible future experiences that have not yet occurred (Schacter et al., 2012).
The neural basis of imagination has recently been studied in the context of individual differences in personality traits linked to imaginative ability. One particularly relevant trait is Openness to Experience, a Big Five personality factor characterized by the tendency to engage in imaginative, creative, and abstract cognitive processes (DeYoung, 2014). “Imagination” was originally considered as a possible label for the trait that was ultimately labeled “Openness,” and it continues to be a defining description of those high in Openness to Experience (DeYoung, Grazioplene, & Peterson, 2012; Oleynick et al., 2017; Saucier, 1992). Openness is also referred to as the “creativity trait” because it strongly predicts performance on creative thinking tasks (Dollinger, Urban, & James, 2004; Silvia et al., 2008), frequency of real‐world creative achievements (Kaufman, 2013; Kaufman et al., 2016), and engagement in everyday creative behaviors (Silvia, Nusbaum, Berg, Martin, & O'Conner, 2009). Contemporary personality models distinguish between two facets of the higher‐order trait: Openness (a tendency to engage with fantasy and aesthetics) and Intellect (a tendency to engage in abstract thinking and problem solving). Although moderately correlated (DeYoung, 2014), Openness and Intellect tend to predict different behavioral outcomes: Openness is associated more with artistic behavior and creative thinking, whereas Intellect is associated more with scientific achievement and cognitive abilities (e.g., intelligence; Kaufman et al., 2016).
Neuroimaging research has shown that Openness is associated with individual variation in the structure and function of specific DN regions (Adelstein et al., 2011; Beaty et al., 2016b; Li et al., 2014; Passamonti et al., 2015). Recently, Beaty et al. (2016b) assessed the contribution of Openness to DN functional connectivity using graph theoretical analysis of resting‐state fMRI data. Across two studies, the authors found that Openness predicted increased global efficiency within a network comprised of DN nodes and edges, indicating that people high in Openness show greater efficiency of information processing within the DN. Another resting‐state fMRI study found that Openness is related to increased functional connectivity between DN hubs and regions associated with cognitive control (Adelstein et al., 2011), consistent with task‐based fMRI studies reporting functional interactions among these brain regions during tasks involving imagination and creativity (Zabelina & Andrews‐Hanna, 2016).
An increasing number of studies have examined how the DN interacts with other brain networks during tasks involving imagination. Research on creative cognition has found that the DN interacts with brain systems associated with cognitive control during tasks requiring the generation and evaluation of novel ideas (Beaty, Benedek, Kaufman, & Silvia, 2015; Beaty, Christensen, Benedek, Silvia, & Schacter, 2017a; Ellamil, Dobson, Beeman, & Christoff, 2012; Mayseless, Eran, & Shamay‐Tsoory, 2015). In a recent study of divergent thinking, for example, Beaty et al. (2015) found that core default regions, e.g., the posterior cingulate cortex, showed increased functional connectivity with regions of the executive control network (ECN; right dorsolateral prefrontal cortex) and the salience network (SN; bilateral insula). The ECN, comprised of lateral prefrontal and anterior inferior parietal regions, activates during goal‐directed cognition and executive functioning, such as working memory and pre‐potent response inhibition (Seeley et al., 2007). The SN, comprised of bilateral insula and anterior cingulate cortex, contributes to the detection of behaviorally relevant stimuli and facilitates interactions of the ECN and DN (Uddin, 2015). Researchers have hypothesized that DN‐ECN coupling reflects the dynamic interplay between spontaneous and controlled modes of thought, with the DN contributing to idea generation and the ECN constraining DN activity to meet specific task goals (Beaty et al., 2016a; Chen et al., 2014; Christoff et al., 2016; Jung, Mead, Carrasco, & Flores, 2013; McMillan, Kaufman, & Singer, 2013; Pinho, Ullén, Castelo‐Branco, Fransson, & de Manzano, 2016).
Interactions between the DN and ECN have been reported during other tasks that involve imagination and goal‐directed cognition. Several studies have reported increased functional connectivity between the DN and ECN during autobiographical future planning, a goal‐directed process of constructing mental representations about a future event (Gerlach, Spreng, Madore, & Schacter, 2014; Spreng, Gerlach, Turner, & Schacter, 2015; Spreng, Stevens, Chamberlain, Gilmore, and Schacter, 2010). Spreng et al. (2010) also found that visual‐spatial planning is associated with increased coupling of the ECN and dorsal attention network (DAN; see also, Spreng & Schacter, 2012), a system comprised of the frontal eye fields and superior parietal cortices that supports externally oriented attention and cognition (Fox, Corbetta, Snyder, Vincent, & Raichle, 2006). Moreover, a recent study using dynamic functional connectivity analysis reported variable interactions between the DAN and subsystems of the DN at rest and during naturalistic cognitive states (Dixon et al., 2017), building on prior work reporting negative associations between the DAN and global DN (e.g., Fox et al., 2005) by employing new methods to assess variation in spatiotemporal network dynamics. Other research has implicated interactions among the DN and ECN in the context of mind‐wandering, including experimental work on meta‐awareness of mind‐wandering during task performance (Christoff, Gordon, Smallwood, Smith, & Schooler, 2009; Schooler et al., 2011) as well as resting‐state research reporting an association between DN‐ECN coupling and individual differences in the tendency to engage in intentional (but not unintentional) mind‐wandering (Golchert et al., 2016).
3. THIS RESEARCH
Recent evidence suggests that imagination and creativity are supported by functional interactions among regions of the default and cognitive control networks (Beaty et al., 2016a; Christoff et al., 2016). This observation has received further support from individual differences research on Openness to Experience indicating that the imaginative mind is marked by enhanced functional connections among regions of these networks (Adelstein et al., 2011; Beaty et al., 2016b). In this research, we sought to extend research on the neural basis of imagination by examining the contribution of Openness to variation in dynamic functional connectivity between default and cognitive control networks, building on past work exploring static connections between individual brain regions in relation to Openness. This approach allowed us to determine whether people high in Openness are more likely to simultaneously engage default and control networks, a connectivity profile that is linked to imagination (Christoff et al., 2016), cognitive flexibility (Douw et al., 2016), and creative problem solving (Zabelina & Andrews‐Hanna, 2016).
We examined variation in dynamic functional network connectivity (dFNC) in two large samples of participants recruited from the United States and China. To assess imaginative ability, we administered the Openness/Intellect subscale of the Big Five Aspect Scale (BFAS; DeYoung, Quilty, & Peterson, 2007). We then examined associations between Openness/Intellect and dynamic fluctuations of intrinsic functional connectivity networks. In light of recent evidence linking imagination and brain network connectivity, we hypothesized that Openness would be associated with enhanced functional coupling among the DNs and other networks associated with cognitive control, including the salience, executive, and DANs.
4. STUDY 1
Our first study examined the extent to which Openness/Intellect is associated variation in temporal “brain states”—recurring patterns of correlation between networks—characterized by default and cognitive control network interaction. We thus obtained personality and resting‐state fMRI data from a sample of healthy young adults from the United States. Independent component analysis (ICA) was used to identify intrinsic connectivity networks previously associated with imagination and related cognitive processes. Dynamic functional connectivity analysis assessed interactions among these networks using a sliding window method. Consistent with past work (Damaraju et al., 2014), we anticipated that in addition to yielding brain states showing variable patterns of positive and negative correlation, the dynamic connectivity analysis would reveal a brain state characterized by positive correlations among the networks of interest. We further hypothesized that Openness/Intellect would relate to the proportion of time that participants spent in this positively correlated brain state.
5. METHOD
5.1. Participants
The sample consisted of 117 young adults from the University of North Carolina at Greensboro (UNCG; 78 females, mean age = 21.39, age range: 18–34). All participants were right‐handed with normal or corrected‐to‐normal vision, and reported no history of neurological disorder, cognitive disability, or medication that affects the central nervous system. The study was approved by the UNCG Institutional Review Board.
5.2. Behavioral assessment
Personality was assessed with the Openness/Intellect subscale of the BFAS (DeYoung et al., 2007). The scale measures two facets of the higher‐order factor: Openness to Experience and Intellect. Openness is characterized by fantasy proneness and aesthetic sensitivity, and is assessed with items such as “I seldom daydream” (reverse scored). Intellect is characterized by a tendency to engage in problem solving and abstract thought, and is assessed with items such as “I like to solve complex problems.” Past research has shown that Openness and Intellect are correlated but separable facets (DeYoung, Shamosh, Green, Braver, & Gray, 2009) that tend to predict distinct behavioral and neural markers (Kaufman et al., 2016). Participants used a 1 (strongly disagree) to 5 (strongly agree) scale to indicate their extent of agreement with the trait statements.
5.3. MRI data acquisition and preprocessing
Resting‐state functional imaging data were acquired for five minutes as participants relaxed awake in the scanner with eyes closed. Whole‐brain imaging was performed on a 3T Siemens Magnetom MRI system (Siemens Medical Systems, Erlangen, Germany) using a 16‐channel head coil. BOLD‐sensitive T2*‐weighted functional images were acquired using a single shot gradient‐echo EPI pulse sequence (TR = 2,000 ms, TE = 30 ms, flip angle = 78°, 32 axial slices, 3.5 × 3.5 × 4.0 mm, distance factor 0%, FoV = 192 × 192 mm, interleaved slice ordering) and corrected online for head motion. A high‐resolution T1 scan was acquired for anatomical normalization.
Imaging data were preprocessed using the Statistical Parametric Mapping (SPM) 8 package (Wellcome Trust Centre for Neuroimaging, London). The first 2 volumes from each subject's functional data were discarded to account for steady‐state magnetization. Functional volumes were then slice‐time corrected, realigned, coregistered, resliced to a voxel size of 3 mm³, normalized to the MNI template brain (Montreal Neurological Institute), and smoothed with an 8 mm3 isotropic Gaussian kernel.
5.4. Independent component analysis
Intrinsic functional connectivity networks were identified using the GIFT toolbox in MATLAB. In a first step, pre‐processed functional images were entered into a principal component analysis to reduce the data to 120 principal components (Calhoun, Adali, Pearlson, & Pekar, 2001). The concatenated volumes were then decomposed into 20 independent components, in line with past work demonstrating that a 20 network parcellation is sufficient for identifying intrinsic functional connectivity networks (Abou‐Elseoud et al., 2010; Ray et al., 2013; Smith et al., 2009). Next, we applied a back‐reconstruction procedure via the GICA1 algorithm (Erhardt et al., 2011) using the individual time courses and spatial maps. The analysis yielded 20 components corresponding to established intrinsic connectivity networks (e.g., default, salience, and executive) and others representing functional imaging artifact (e.g., cerebral spinal fluid). Group‐level intrinsic connectivity networks were identified via visual inspection and compared to spatial templates from past work to confirm their network affiliation (Smith et al., 2009). We then extracted the independent components corresponding to the cognitive networks of interest—default, salience, executive, and dorsal attention—for dFNC analysis.
5.5. Dynamic functional network connectivity
We examined dynamic brain states using temporal dFNC in the GIFT toolbox. For each participant, a sliding window method was used to sample brief segments of the resting‐state time series. We used a window size of 30 TRs sliding in steps of 1 TR convolved with a Gaussian window alpha value of 3 TRs. Additional pre‐processing steps included detrending, despiking, and filtering (0.15 Hz) of the timecourses. The k‐means clustering algorithm was then used to separate the temporal network windows into clusters or brain states (k), reflecting recurring correlational patterns among the cognitive networks of interest. We specified a k of 5, in line with past work (Allen et al., 2014), using the city distance function with 150 repetitions. The covariance matrices of each participant's dFNC values were standardized via Z‐transformation. The dFNC analysis yielded parameters for each participant associated with the five brain states, including the brain state “dwell time,” that is, the proportion of time participants spent in each of the five brain states.
To test whether participant head motion correlated with personality, we computed mean framewise displacement (FD; Power, Barnes, Snyder, Schlaggar, & Petersen, 2012) and correlated mean FD with personality values. Results revealed nonsignificant associations between mean FD and the higher‐order Openness/Intellect factor (r = .01, p = 97) as well as the lower‐order facets (Openness, r = .05, p = .58; Intellect, r = –.09, p = .31), indicating that the behavioral measures of interest were unrelated to movement during resting‐state imaging.
5.6. Structural equation modeling (SEM)
Multivariate SEM was employed to assess the effects of Openness/Intellect on dwell time within the five brain states. SEM models error variance separately from true measurement variance, providing a more robust estimate of effect size (Kline, 2004). Openness/Intellect was modeled as a higher‐order latent variable indicated by the two lower‐order facets (i.e., Openness to Experience and Intellect). For model identification, the paths of the two indicators were constrained to equality and the variance of the latent variable was fixed to 1. We also specified a model with the lower‐order Openness and Intellect variables to determine the relative contribution of each facet to state dwell time. All regression weights reported below are standardized. Note that goodness‐of‐fit indices are not reported in Study 1 as the model is “just identified” with two indicators of a single latent variable.
6. RESULTS
6.1. ICA and dynamic functional connectivity
The ICA revealed several clusters corresponding to established intrinsic connectivity networks, including several cognitive networks: anterior and posterior default, left and right executive, dorsal attention, and salience. A dFNC analysis of these six networks revealed variable patterns of functional connectivity across the five brain states (see Figure 1). Consistent with prior work, the dFNC revealed a brain state characterized by positive correlations between the six networks of interest (i.e., state 2; see Figure 2a). The four other brain states were characterized by positive and negative correlations among the networks.
Figure 1.
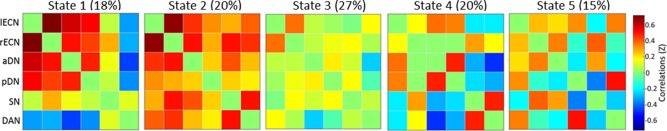
Brain states identified using dFNC in Study 1. Proportion of scan time spent in each state is shown in parenthses next to the state number. aDN = anterior default network; DAN = dorsal attention network; lECN = left executive control network; pDN = posterior default network; rECN = right executive control network; SN = salience network [Color figure can be viewed at http://wileyonlinelibrary.com]
Figure 2.
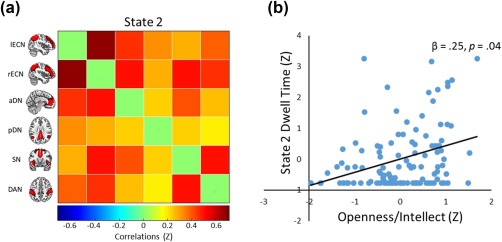
(a) Heat map depicting the positively correlated brain state from Study 1 (i.e., State 2) with ICA components representing the six cognitive networks on the x‐axis and y‐axis. (b) Scatterplot of the association between latent Openness/Intellect and dwell time in State 5, controlling for age and sex. Variables are standardized for visualization [Color figure can be viewed at http://wileyonlinelibrary.com]
6.2. Personality and brain state dwell time
Our first model tested the effect of latent Openness/Intellect on dwell time within the five states. Results revealed a significant effect of Openness/Intellect on dwell time in state 2, the brain state marked by positive correlations among the six networks: β = .28, p = .02. Openness/Intellect was not significantly related to dwell time in the other four states, but it remained a robust predictor of time spent in this correlated state in a second model including age and sex (β = .25, p = .04; see Figure 2b), which were not significantly related to time spent in the five states (see Table 1).1
Table 1.
Study 1 effects of openness/intellect and demographic variables on dwell time in the five brain states
| State 1 | State 2 | State 3 | State 4 | State 5 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β | p | 95% CI | β | p | 95% CI | β | p | 95% CI | β | p | 95% CI | β | p | 95% CI | |
| O/I | .054 | .634 | –.17, .279 | .252 | .040 | .006, .496 | –.094 | .426 | –.33, .142 | –.198 | .08 | –.428, .032 | –.040 | .669 | –.223, .143 |
| Age | .178 | .1 | –.037, .394 | .092 | .214 | –.055, .239 | –.058 | .509 | –.233, .117 | –.143 | .062 | –.3, .013 | –.080 | .28 | –.23, .069 |
| Sex | .151 | .072 | –.039, .681 | –.171 | .069 | –.737, .014 | –.014 | .879 | –.423, .363 | –.069 | .459 | –.536, .242 | .113 | .152 | –.1, .58 |
Note: O/I = openness/intellect.
To determine if the effects were driven by Openness, Intellect, or both, we specified a second model with the lower‐order facets (Openness and Intellect) predicting dwell time in the five states. At the zero‐order level, Openness and Intellect were strongly correlated (r = .46). The effects of Openness (β = .09, p = .39) and Intellect (β = .13, p = .13) on state 2 dwell time were both small and nonsignificant, indicating that the higher‐order Openness/Intellect variable accounted for the results reported in the previous models.
7. STUDY 2
Study 1 found that Openness/Intellect is associated with increased dwell time in a positively correlated brain state comprised of default and cognitive control networks. In Study 2, we sought to replicate and extend these findings in a culturally distinct sample of healthy young adults in China. We also sought evidence for incremental validity by considering several additional variables that may be related to brain state dwell time, including fluid intelligence and four of the Big 5 factors of personality not included in Study 1 (i.e., Neuroticism, Extraversion, Agreeableness, and Conscientiousness). We hypothesized that Openness/Intellect would again be related to dwell time in a correlated brain state, but that intelligence and other personality variables would be unrelated to this state.
8. METHOD
8.1. Participants
The sample consisted of 255 young adults from Southwest University, China (140 females, mean age = 19.91, SD = 1.27). The study was part of a larger project investigating individual differences in personality, creativity, and brain structure and function (Chen et al., 2014, in press; Li et al., 2014; Wei et al., 2014). All participants were right‐handed with normal or corrected‐to‐normal vision, and reported no history of neurological disorder, cognitive disability, or substance abuse. The study was approved by the Institutional Review Board of the Southwest University Brain Imaging Center.
8.2. Behavioral assessment
Personality was assessed with a Chinese‐translated version of the BFAS (DeYoung et al., 2007), which included all five personality factors: Neuroticism, Extraversion, Openness/Intellect, Agreeableness, and Conscientiousness. Intelligence was assessed with the Combined Raven's Test (CRT). The CRT is a widely used measure of fluid reasoning ability with documented evidence of reliability and validity (Wang, 2007). Similar to the Raven's Advanced Progressive Matrices, the CRT presents a series of matrices that change based on specific rules. Participants must discover the rule by completing a missing segment of the matrix based on a set of six or eight answer choices (72 items). Participant scores are derived by summing the number of correct responses.
8.3. MRI data acquisition and preprocessing
Resting‐state fMRI data were acquired for eight minutes. Whole‐brain imaging was performed on a 3T Siemens Trio MRI system (Siemens Medical Systems, Erlangen, Germany) using an 8‐channel head coil. BOLD‐sensitive T2*‐weighted functional images were acquired using a single shot gradient‐echo EPI pulse sequence (TR = 2,000 ms, TE = 30 ms, flip angle = 90°, 32 axial slices, 3.4 × 3.4 × 4.0 mm, FoV = 220 × 220 mm, interleaved slice ordering, 242 volumes) and corrected online for head motion. During functional imaging, participants were asked to keep their eyes closed, remain awake, and not think about anything in particular. A high resolution T1 scan was also acquired for anatomic normalization.
Imaging data were preprocessed using the SPM 8 package (Wellcome Trust Centre for Neuroimaging, London). The first 10 volumes from each subject's functional imaging data were discarded to account for steady‐state magnetization, resulting in 232 volumes for subsequent analysis. Functional data were then slice‐time corrected, realigned, coregistered, resliced to a voxel size of 3 mm³, normalized to the MNI template brain (Montreal Neurological Institute), and smoothed with an 8 mm3 isotropic Gaussian kernel. The ICA and dFNC followed the same procedure as Study 1.
To test whether participant head motion correlated with personality, we computed mean FD (Power et al., 2012) and correlated mean FD with personality values. Results revealed nonsignificant associations between mean FD and the higher‐order Openness/Intellect factor (r = .07, p = 31) as well as the lower‐order facets (Openness, r = .06, p = .29; Intellect, r = .01, p = .94), indicating that the behavioral measures of interest were unrelated to movement during resting‐state imaging.
8.4. Structural equation modeling
Multivariate SEM was employed to estimate effects of personality and fluid intelligence on brain state dwell time. The five factors of personality were modeled as latent variables, indicated by the two facets of their respective higher‐order variable. Consistent with the model specifications of Study 1, the paths of the lower‐order facets were constrained to be equal and the latent variables’ variances were fixed to 1. The paths between the personality variables were also fixed to zero for model identification. All regression weights reported below are standardized.
9. RESULTS
9.1. ICA and dynamic functional connectivity
As in Study 1, the ICA yielded several clusters corresponding to known resting‐state networks, including two DN clusters (anterior and posterior), two executive networks (left and right), a DAN, and a SN. A dFNC analysis with these six networks revealed five brain states (see Figure 3), including a state characterized by predominantly positive correlations among networks (i.e., state 2). Note that although this state was comparable to the positively correlated state in Study 1, it showed a small negative correlation between the aDMN and DAN and near‐zero correlation between the SN and aDMN and lECN (see Figure 4a). Similar to Study 1, the other four brain states showed variable patterns of positive and negative correlation among the six resting‐state networks.
Figure 3.
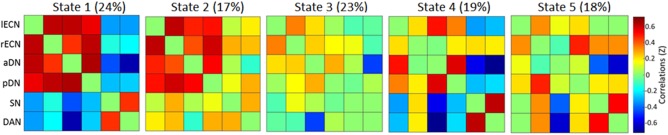
Brain states identified using dFNC in Study 2. Proportion of scan time spent in each state is shown in parenthses next to the state number. aDN = anterior default network; DAN = dorsal attention network; lECN = left executive control network; pDN = posterior default network; rECN = right executive control network; SN = salience network [Color figure can be viewed at http://wileyonlinelibrary.com]
Figure 4.
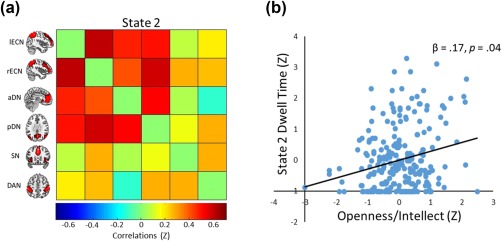
(a) Heat map depicting the positively correlated brain state from Study 2 (i.e., State 2) with ICA components representing the six cognitive networks on the x‐axis and y‐axis. (b) Scatterplot of the association between latent Openness/Intellect and dwell time in State 5, controlling for fluid intelligence, age, sex, and four other factors of personaltiy (i.e., neuroticism, extraversion, agreeableness, and conscientiousness). Variables are standardized for visualization [Color figure can be viewed at http://wileyonlinelibrary.com]
9.2. Personality and brain state dwell time
The first model estimated the effects of the latent Openness/Intellect variable on dwell time within the five brain states, controlling for age and sex. Results revealed a significant effect of Openness/Intellect on the brain state characterized by positive correlations among networks (β = .21, p = .006). We then examined the unique effects of Openness and Intellect on brain state dwell time. Consistent with Study 1, the two variables were highly correlated (r = .49). Regression analysis revealed nonsignificant effects of Openness (β = .10, p = .18) and Intellect (β = .10, p = .15) on dwell time spent in the correlated brain state, suggesting that the effect was again driven by the higher‐order latent factor. We also found a negative effect of age on dwell time in this state (β = –.16, p = .03); age and sex showed significant effects on dwell time in other brain states (see Table 2).
Table 2.
Study 2 effects of personality, fluid intelligence, and demographic variables on dwell time in the five brain states
| State 1 | State 2 | State 3 | State 4 | State 5 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β | p | 95% CI | β | p | 95% CI | β | p | 95% CI | β | p | 95% CI | β | p | 95% CI | |
| O/I | –.104 | .239 | –.278, .071 | .175 | .045 | .001, .348 | –.034 | .717 | –.218, .15 | –.165 | .109 | –.379, .046 | .164 | .051 | –.008, .343 |
| N | –.084 | .311 | –.249, .08 | .082 | .278 | –.065, .228 | –.013 | .869 | –.166, .14 | .041 | .576 | –.104, .187 | –.009 | .905 | –.158, .14 |
| A | .168 | .212 | –.099, .437 | –.002 | .987 | –.255, .251 | –.377 | .016 | –.693, –.058 | .166 | .232 | –.108, .444 | .089 | .534 | –.196, .377 |
| C | –.04 | .698 | –.243, .163 | –.032 | .76 | –.236, .172 | .041 | .696 | –.164, .245 | .255 | .008 | .056, .46 | –.237 | .018 | –.452, –.03 |
| E | .041 | .678 | –.154, .237 | .089 | .357 | –.101, .279 | –.105 | .267 | –.29, .081 | .081 | .453 | –.134, .299 | –.09 | .377 | –.298, .115 |
| Gf | .01 | .868 | –.117, .138 | .087 | .131 | –.026, .208 | –.067 | .333 | –.211, .072 | .037 | .554 | –.092, .17 | –.051 | .475 | –.204, .096 |
| Age | –.04 | .441 | –.143, .062 | –.137 | .09 | –.293, .021 | –.03 | .577 | –.135, .075 | .06 | .262 | –.044, .165 | .147 | .019 | .024, .276 |
| Sex | .164 | .007 | .081, .583 | –.011 | .868 | –.271, .229 | –.208 | .001 | –.663, –.171 | .263 | .000 | .297, .775 | –.203 | .001 | –.673, –.158 |
Note. O/I = openness/intellect; N = Neuroticism; A = Agreeableness; C = Conscientiousness; E = Extraversion; Gf = fluid intelligence.
We then specified a second model to test whether the effect of the latent Openness/Intellect variable was robust to the addition of fluid intelligence in a model with age and sex predicting state dwell time. Results showed a significant effect of Openness/Intellect on dwell time in the correlated brain state (β = 19, p = .01). Intelligence showed a small but nonsignificant effect on dwell time in this state (β = .09, p = .11), and the age effect from the previous model was similar in magnitude but nonsignificant (β = –.14, p = .08).
A third model assessed the effects of the five latent personality factors on dwell time within the five brain states: χ2(65 df) = 474.077, p = .0000; CFI = .841; RMSEA = .157 (90% CI: .144, .171); SRMR = .143. Of the five factors, Openness/Intellect was the only significant predictor of dwell time within the correlated brain state (β = .18, p = .03). Agreeableness and Conscientiousness were significantly related to dwell time in other brain states, including a large negative effect of Agreeableness on state three dwell time (β = –.39, p = .01; see Table 2).
Finally, we specified a model with the five latent personality variables—along with intelligence, age, and sex—predicting state dwell time in the five brain states: χ2(95 df) = 496.542, p = .0000; CFI = .837; RMSEA = .134 (90% CI: .122, .145); SRMR = .125. Results were largely similar to the previous model: Openness/Intellect remained a robust predictor of dwell time in the correlated brain state (β = .17, p = .04; see Figure 4b). The effects of Agreeableness and Conscientiousness were comparable to the previously specified model; age and sex similarly predicted dwell time in other brain states.
10. DISCUSSION
In two studies, we showed that the personality trait Openness to Experience—a Big Five factor epitomized by imaginative and creative thought—is associated with a pattern of resting‐state activity characterized by positive correlations among large‐scale cognitive brain systems. Study 1 established the effect of Openness on dwell time in the correlated brain state and further demonstrated the specificity of this effect, with Openness not significantly related to time spent in other brain states. Study 2 replicated and extended these findings in a large sample of Chinese young adults, and provided evidence for the robustness of the effect in latent variable models including fluid intelligence and other personality factors. Taken together, the current findings indicate that the imaginative personality is associated with default and cognitive control network cooperation, consistent with the growing literature on brain dynamics supporting imagination and creativity (Beaty et al., 2016a; Christoff et al., 2016; Zabelina & Andrews‐Hanna, 2016).
This study extends recent work on the neural correlates of Openness to Experience (Adelstein et al., 2011; Beaty et al., 2016b; Li et al., 2014; Passamonti et al., 2015). For example, Adelstein et al. (2011) reported a positive correlation between Openness and resting‐state functional connectivity between discrete regions of the default and control networks. The current findings build on research exploring static connections between individual brain areas by examining dynamic shifts in whole‐brain intrinsic connectivity networks—including the default, salience, executive, and DANs—and demonstrate that people high in Openness are more likely to simultaneously engage these distributed brain systems. Critically, Study 2 replicated and extended the findings reported in Study 1, using data from a culturally distinct sample of Chinese young adults. To our knowledge, this study is the first to assess cross‐cultural variation in brain dynamics in relation to Openness to Experience.
Our study also extends the recent work of Beaty et al. (2016b), who reported an association between Openness and global efficiency of the DN. Considered in the context of the current findings, we suspect that increased DN functioning may support dynamic and efficient cooperation with other large‐scale brain systems during imagination and creative cognition. This interpretation remains tentative, however, as we did not assess brain dynamics linked to performance on cognitive tasks assessing imagination and creativity. Another potential limitation of the present study regards the generalizability of the results. Like most behavioral and neuroimaging studies, our data came from younger, college‐educated participants who likely differ from the general population in terms of personality and cognitive ability. Future studies should include a broader age‐range of participants from the community to determine whether the current findings extend to a more representative sample.
Our results indicate that people high in Openness spend more time in a correlated brain state at rest, a connectivity profile that may support enhanced imaginative and creative cognitive processes that distinguish people high in Openness. A growing body of neuroimaging investigations have reported positive correlations between regions of the default and control networks, including experimental studies showing network interactions during creative thinking tasks (Beaty et al., 2015; Beaty, Silvia, & Benedek, 2017b; Pinho et al., 2016) as well as individual differences studies showing increased resting‐state network coupling associated with creative thinking ability (Beaty et al., 2014; Zhu et al., 2017) and a tendency to engage in deliberate mind‐wandering (Golchert et al., 2016). Recent work suggests that default‐control network coupling broadly reflects goal directed, self‐generated thought, with the control network directing spontaneous default activity to meet higher‐order goals (Beaty et al., 2016a; Pinho et al., 2016; Spreng et al., 2015, 2010). The tendency to engage multiple brain systems thus may correspond to a relative advantage of people high in Openness to dynamically reconfigure relevant brain networks when thinking flexibly and creatively, consistent with the notion that neural flexibility supports cognitive flexibility (cf., Braun et al., 2015; Douw et al., 2016). It remains unclear, however, whether Openness is associated with enhanced network coupling during cognitive tasks, an open and promising question for future research.
Recent methodological studies have raised important concerns regarding the proper statistical estimation of dynamic FC (Hindriks et al., 2016; Laumann et al., 2016; Lehmann, White, Henson, Can, & Geerligs, 2017). For example, Lehmann et al. found that non‐dynamic properties of resting‐state fMRI data can account for observed group differences in network dynamics (e.g., HRF shape and measurement noise). However, a growing literature has provided evidence for the validity of this approach and potential benefits compared with static FC. For example, recent studies have linked dynamic FC to cognitive task performance (Shine et al., 2016) and fluctuations in attentional states (Kucyi et al., 2017; Mooneyham et al., 2016). Other work has directly tested the predictive power of statistic versus dynamic FC in classifying psychiatric disorders such as schizophrenia, with evidence indicating that dynamic FC may classify schizophrenia via resting‐state fMRI data better than static FC (Rashid et al., 2016). This research extends this emerging literature by demonstrating robust and reliable effects of major personality traits on dynamic FC states, providing further evidence for the utility of dynamic FC in assessing important psychological constructs. Nevertheless, understanding the extent to which individual differences in dynamic FC track meaningful psychological variables (e.g., personality and cognitive ability) versus non‐dynamic factors (e.g., artefacts and neural autocorrelation; Lehmann et al., 2017) remains an open and central question. Future research should continue to investigate the benefits and limitations of dynamic FC, with a focus on examining cognitive and behavioral correlates related to temporal shifts in large‐scale network interactions.
This work has as few limitations worth noting. One potential issue concerns the relatively shorter scanning duration in Study 1 (5 min) compared with Study 2 (8 min). Although effects of personality replicated across studies, a longer scanning sequence is generally optimal for characterizing time‐varying connectivity differences across individuals. Another limitation concerns whether individual differences in Openness/Intellect and dynamic FC were driven in part by variation in static FC between specific brain networks. Future work should assess the stability and replicability of associations among personality and resting‐state network dynamics, with a focus on isolating individual differences related to static and dynamic FC. One promising direction would be to collect multiple resting‐state scans within the same individual, and assess personality at each time point. Because personality factors remain relatively stable across time, a reasonable hypothesis would be that personality stability corresponds to relative stability in dynamic connectivity. In the context of this study, we would expect that openness would predict time spent in a positively correlated brain state during resting‐state fMRI. However, longitudinal work has shown that openness declines with age (McCrae, 1987), so perhaps time spent in this correlated brain state tracks age‐related declines in openness, likely corresponding to a relative loss of cognitive flexibility. However, openness to experience acts as a buffer against cognitive decline (Ziegler, Cengia, Mussel, & Gerstorf, 2015), so age‐related declines in openness—and potential corresponding decreases in time spent in a positively correlated brain state—might be mitigated in aging adults that are high in openness (Voytek & Knight, 2015). We think that such longitudinal analyses have the potential to provide greater clarity on the replicability and stability of dynamic connectivity measures (cf., Abrol et al., 2017).
Future research may also examine whole‐brain dynamic connectivity in relation to personality factors. In this study, we analyzed brain states comprised of specific functional networks that have previously been linked to attention and cognition (Zabelina & Andrews‐Hanna, 2016). It remains unclear, however, whether Openness relates to whole‐brain dynamic connectivity or other intrinsic networks not considered in the current analysis (e.g., subcortical and sensorimotor networks). Indeed, past work has reported associations between Openness and functional connectivity within mesocortical networks (Passamonti et al., 2015), consistent with studies linking Openness to enhanced functioning of dopaminergic circuits (Oleynick et al., 2017). We thus encourage future research to examine dynamic connectivity of sensory, limbic, and whole‐brain networks to determine whether Openness and other personality factors relate to dynamic connectivity of states comprised of these cortical systems.
1Because the distributions of dwell times are not normally distributed the implications of the distributional assumptions of structural equation models are worth considering. The structural equation models reported in the text were estimated using maximum likelihood with robust standard errors (MLR), which use the Huber‐White sandwich estimators to correct for deviations from normality. Nevertheless, to explore this issue further, we estimated the model again using normal maximum likelihood with bootstrapped standard errors (5,000 bootstrap samples). The models yielded essentially identical effects. For the primary effect (the effect of Openness on dwell time in state 5), for example, the MLR model (β = .25, p = .040) and bootstrapped model (β = .25, p = .045) yielded similar coefficients and p‐values.
In addition, to explore a fully nonparametric approach, we estimated the model with Bayesian methods (Lee, 2007) using Marcov Chain Monte Carlo and Gibbs sampling (4 chains, minimum 5000 iterations), and the results were evaluated for consistency across a range of random seed values and starting values (Lynch, 2007). The estimated effects (i.e., the median of the MCMC‐derived posterior distribution of effects) were again essentially the same as in the other models (e.g., for openness and state 5 dwell time, β = .24, p = .048).
Because the results are consistent across a range of estimation methods, including approaches using resampling and Bayesian methods, the non‐normality of the dwell times do not appear to bias the conclusions that we draw from our analyses.
ACKNOWLEDGMENT
R.E.B. and P.J.S. were supported by grant RFP‐15‐12 from the Imagination Institute (http://www.imagination-institute.org), funded by the John Templeton Foundation. The opinions expressed in this publication are those of the authors and do not necessarily reflect the view of the Imagination Institute or the John Templeton Foundation. Q.C. and J.Q. were supported by grants from the. D.L.S was supported by National Institute of Mental Health R01 MH060941.
Beaty RE, Chen Q, Christensen AP, Qiu J, Silvia PJ, Schacter DL. Brain networks of the imaginative mind. Hum Brain Mapp. 2018;39:811–821. 10.1002/hbm.23884
Funding information Grant RFP‐15‐12 from the Imagination Institute (http://www.imagination-institute.org); the John Templeton Foundation; National Science Foundation of China, Grant/Award Numbers: 31571137 and 31470981; National Institute of Mental Health, Grant/Award Number: R01 MH060941
REFERENCES
- Abou‐Elseoud, A. , Starck, T. , Remes, J. , Nikkinen, J. , Tervonen, O. , & Kiviniemi, V. (2010). The effect of model order selection in group PICA. Human Brain Mapping, 31, 1207–1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abrol, A. , Damaraju, E. , Miller, R. L. , Stephen, J. M. , Claus, E. D. , Mayer, A. R. , & Calhoun, V. D. (2017). Replicability of time‐varying connectivity patterns in large resting state fMRI samples. bioRxiv, 172866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adelstein, J. S. , Shehzad, Z. , Mennes, M. , DeYoung, C. G. , Zuo, X. N. , Kelly, C. , … Milham, M. P. (2011). Personality is reflected in the brain's intrinsic functional architecture. PloS ONE, 6, e27633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen, E. A. , Damaraju, E. , Plis, S. M. , Erhardt, E. B. , Eichele, T. , & Calhoun, V. D. (2014). Tracking whole‐brain connectivity dynamics in the resting state. Cerebral cortex, 24, 663–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews‐Hanna, J. R. , Smallwood, J. , & Spreng, R. N. (2014). The default network and self‐generated thought: Component processes, dynamic control, and clinical relevance. Annals of the New York Academy of Sciences, 1316, 29–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaty, R. E. , Benedek, M. , Kaufman, S. B. , & Silvia, P. J. (2015). Default and executive network coupling supports creative idea production. Scientific Reports, 5, 10964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaty, R. E. , Benedek, M. , Silvia, P. J. , & Schacter, D. L. (2016a). Creative cognition and brain network dynamics. Trends in Cognitive Sciences, 20, 87–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaty, R. E. , Benedek, M. , Wilkins, R. W. , Jauk, E. , Fink, A. , Silvia, P. J. , … Neubauer, A. C. (2014). Creativity and the default network: A functional connectivity analysis of the creative brain at rest. Neuropsychologia, 64, 92–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaty, R. E. , Christensen, A. P. , Benedek, M. , Silvia, P. J. , & Schacter, D. L. (2017a). Creative constraints: Brain activity and network dynamics underlying semantic interference during idea production. Neuroimage, 148, 189–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaty, R. E. , Kaufman, S. B. , Benedek, M. , Jung, R. E. , Kenett, Y. N. , Jauk, E. , … Silvia, P. J. (2016b). Personality and complex brain networks: The role of Openness to Experience in default network efficiency. Human Brain Mapping, 37, 773–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaty, R. E. , Silvia, P. J. , & Benedek, M. (2017b). Brain networks underlying novel metaphor production. Brain and Cognition, 111, 163–170. [DOI] [PubMed] [Google Scholar]
- Braun, U. , Schäfer, A. , Walter, H. , Erk, S. , Romanczuk‐Seiferth, N. , Haddad, L. , … Bassett, D. S. (2015). Dynamic reconfiguration of frontal brain networks during executive cognition in humans. Proceedings of the National Academy of Sciences, 112, 11678–11683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner, R. L. , Andrews‐Hanna, J. R. , & Schacter, D. L. (2008). The brain's default network. Annals of the New York Academy of Sciences, 1124, 1–38. [DOI] [PubMed] [Google Scholar]
- Calhoun, V. D. , Adali, T. , Pearlson, G. D. , & Pekar, J. J. (2001). A method for making group inferences from functional MRI data using independent component analysis. Human Brain Mapping, 14, 140–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, Q. , Yang, W. , Li, W. , Wei, D. , Li, H. , Lei, Q. , … Qiu, J. (2014). Association of creative achievement with cognitive flexibility by a combined voxel‐based morphometry and resting‐state functional connectivity study. Neuroimage, 102, 474–483. [DOI] [PubMed] [Google Scholar]
- Christoff, K. , Gordon, A. M. , Smallwood, J. , Smith, R. , & Schooler, J. W. (2009). Experience sampling during fMRI reveals default network and executive system contributions to mind wandering. Proceedings of the National Academy of Sciences, 106, 8719–8724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christoff, K. , Irving, Z. C. , Fox, K. C. , Spreng, R. N. , & Andrews‐Hanna, J. R. (2016). Mind‐wandering as spontaneous thought: a dynamic framework. Nature Reviews Neuroscience, 17, 718–731. [DOI] [PubMed] [Google Scholar]
- Damaraju, E. , Allen, E. A. , Belger, A. , Ford, J. M. , McEwen, S. , Mathalon, D. H. , … Turner, J. A. (2014). Dynamic functional connectivity analysis reveals transient states of dysconnectivity in schizophrenia. Neuroimage Clin, 5, 298–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeYoung, C. G. (2014). Openness/Intellect: A dimension of personality reflecting cognitive exploration In Cooper M. L. & Larsen R. J. (Eds.), APA Handbook of Personality and Social Psychology: Personality Processes and Individual Differences (Vol. 4, pp. 369–399). Washington, DC: American Psychological Association. [Google Scholar]
- DeYoung, C. G. , Grazioplene, R. G. , & Peterson, J. B. (2012). From madness to genius: The Openness/Intellect trait domain as a paradoxical simplex. Journal of Research in Personality, 46, 63–78. [Google Scholar]
- DeYoung, C. G. , Quilty, L. C. , & Peterson, J. B. (2007). Between facets and domains: 10 aspects of the Big Five. Journal of Personality and Social Psychology, 93, 880. [DOI] [PubMed] [Google Scholar]
- DeYoung, C. G. , Shamosh, N. A. , Green, A. E. , Braver, T. S. , & Gray, J. R. (2009). Intellect as distinct from Openness: Differences revealed by fMRI of working memory. Journal of Personality and Social Psychology, 97, 883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon, M. L. , Andrews‐Hanna, J. R. , Spreng, R. N. , Irving, Z. C. , Mills, C. , Girn, M. , & Christoff, K. (2017). Interactions between the default network and dorsal attention network vary across default subsystems, time, and cognitive states. Neuroimage, 147, 632–649. [DOI] [PubMed] [Google Scholar]
- Dollinger, S. J. , Urban, K. K. , & James, T. A. (2004). Creativity and openness: Further validation of two creative product measures. Creativity Research Journal, 16, 35–47. [Google Scholar]
- Douw, L. , Wakeman, D. G. , Tanaka, N. , Liu, H. , & Stufflebeam, S. M. (2016). State‐dependent variability of dynamic functional connectivity between frontoparietal and default networks relates to cognitive flexibility. Neuroscience, 339, 12–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellamil, M. , Dobson, C. , Beeman, M. , & Christoff, K. (2012). Evaluative and generative modes of thought during the creative process. Neuroimage, 59, 1783–1794. [DOI] [PubMed] [Google Scholar]
- Erhardt, E. B. , Rachakonda, S. , Bedrick, E. J. , Allen, E. A. , Adali, T. , & Calhoun, V. D. (2011). Comparison of multi‐subject ICA methods for analysis of fMRI data. Human Brain Mapping, 32, 2075–2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golchert, J. , Smallwood, J. , Jefferies, E. , Seli, P. , Huntenburg, J. M. , Liem, F. , … Margulies, D. S. (2016). Individual variation in intentionality in the mind‐wandering state is reflected in the integration of the default‐mode, fronto‐parietal, and limbic networks. Neuroimage, 146, 226–235. [DOI] [PubMed] [Google Scholar]
- Fox, M. D. , Corbetta, M. , Snyder, A. Z. , Vincent, J. L. , & Raichle, M. E. (2006). Spontaneous neuronal activity distinguishes human dorsal and ventral attention systems. Proceedings of the National Academy of Sciences of the United States of America, 103, 10046–10051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox, M. D. , Snyder, A. Z. , Vincent, J. L. , Corbetta, M. , Van Essen, D. C. , & Raichle, M. E. (2005). The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proceedings of the National Academy of Sciences of the United States of America, 102, 9673–9678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerlach, K. D. , Spreng, R. N. , Madore, K. P. , & Schacter, D. L. (2014). Future planning: Default network activity couples with frontoparietal control network and reward‐processing regions during process and outcome simulations. Social Cognitive and Affective Neuroscience, 9, 1942–1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hindriks, R. , Adhikari, M. H. , Murayama, Y. , Ganzetti, M. , Mantini, D. , Logothetis, N. K. , & Deco, G. (2016). Can sliding‐window correlations reveal dynamic functional connectivity in resting‐state fMRI? Neuroimage, 127, 242–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung, R. E. , Mead, B. S. , Carrasco, J. , & Flores, R. A. (2013). The structure of creative cognition in the human brain. Frontiers in Human Neuroscience, 7, 330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman, S. B. (2013). Opening up openness to experience: A four‐factor model and relations to creative achievement in the arts and sciences. The Journal of Creative Behavior, 47, 233–255. [Google Scholar]
- Kaufman, S. B. , Quilty, L. C. , Grazioplene, R. G. , Hirsh, J. B. , Gray, J. R. , Peterson, J. B. , & DeYoung, C. G. (2016). Openness to experience and intellect differentially predict creative achievement in the arts and sciences. Journal of Personality, 82, 248–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucyi, A. , Hove, M. J. , Esterman, M. , Hutchison, R. M. , & Valera, E. M. (2017). Dynamic brain network correlates of spontaneous fluctuations in attention. Cerebral Cortex, 27, 1831–1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline, R. B. (2004). Principles and practice of structural equation modeling (2nd ed.). New York: Guilford. [Google Scholar]
- Laumann, T. O. , Snyder, A. Z. , Mitra, A. , Gordon, E. M. , Gratton, C. , Adeyemo, B. , … McCarthy, J. E. (2016). On the stability of BOLD fMRI correlations. Cerebral Cortex, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, S. Y. (2007). Structural equation modeling: A Bayesian perspective. Hoboken, NJ: Wiley. [Google Scholar]
- Lehmann, B. C. L. , White, S. R. , Henson, R. N. , Can, C. , & Geerligs, L. (2017). Assessing dynamic functional connectivity in heterogeneous samples. Neuroimage, 157, 635–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, W. , Li, X. , Huang, L. , Kong, X. , Yang, W. , Wei, D. , … Liu, J. (2014). Brain structure links trait creativity to openness to experience. Social Cognitive and Affective Neuroscience, 10, 191–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch, S. M. (2007). Introduction to applied Bayesian statistics and estimation for social scientists. New York: Springer. [Google Scholar]
- Mayseless, N. , Eran, A. , & Shamay‐Tsoory, S. G. (2015). Generating original ideas: The neural underpinning of originality. Neuroimage, 116, 232–239. [DOI] [PubMed] [Google Scholar]
- McCrae, R. R. (1987). Creativity, divergent thinking, and openness to experience. Journal of Personality and Social Psychology, 52, 1258–1265. [Google Scholar]
- McMillan, R. , Kaufman, S. B. , & Singer, J. L. (2013). Ode to positive constructive daydreaming. Frontiers in Psychology, 4, 626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medaglia, J. D. , Lynall, M. E. , & Bassett, D. S. (2015). Cognitive network neuroscience. Journal of Cognitive Neuroscience, 27, 1471–1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooneyham, B. W. , Mrazek, M. D. , Mrazek, A. J. , Mrazek, K. L. , Phillips, D. T. , & Schooler, J. W. (2016). States of mind: Characterizing the neural bases of focus and mind‐wandering through dynamic functional connectivity. Journal of Cognitive Neuroscience, 29, 495–506. [DOI] [PubMed] [Google Scholar]
- O'Callaghan, C. , Shine, J. M. , Lewis, S. J. , Andrews‐Hanna, J. R. , & Irish, M. (2015). Shaped by our thoughts –A new task to assess spontaneous cognition and its associated neural correlates in the default network. Brain and Cognition, 93, 1–10. [DOI] [PubMed] [Google Scholar]
- Oleynick, V. C. , DeYoung, C. G. , Hyde, E. , Kaufman, S. B. , Beaty, R. E. , & Silvia, P. J. (2017). Openness/Intellect: The core of the creative personality In Feist G. J., Reiter‐Palmon R., & Kaufman J. C. (Eds.), Cambridge handbook of creativity and personality research (pp. 9–27). New York: Cambridge University Press. [Google Scholar]
- Passamonti, L. , Terracciano, A. , Riccelli, R. , Donzuso, G. , Cerasa, A. , Vaccaro, M. G. , … Quattrone, A. (2015). Increased functional connectivity within mesocortical networks in open people. Neuroimage, 104, 301–309. [DOI] [PubMed] [Google Scholar]
- Pinho, A. L. , Ullén, F. , Castelo‐Branco, M. , Fransson, P. , & de Manzano, Ö. (2016). Addressing a paradox: dual strategies for creative performance in introspective and extrospective networks. Cerebral Cortex, 26, 3052–3063. [DOI] [PubMed] [Google Scholar]
- Power, J. D. , Barnes, K. A. , Snyder, A. Z. , Schlaggar, B. L. , & Petersen, S. E. (2012). Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage, 59, 2142–2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle, M. E. (2015). The brain's default mode network. Annual Review of Neuroscience, 38, 433–447. [DOI] [PubMed] [Google Scholar]
- Rashid, B. , Arbabshirani, M. R. , Damaraju, E. , Cetin, M. S. , Miller, R. , Pearlson, G. D. , & Calhoun, V. D. (2016). Classification of schizophrenia and bipolar patients using static and dynamic resting‐state fMRI brain connectivity. Neuroimage, 134, 645–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray, K. L. , McKay, D. R. , Fox, P. M. , Riedel, M. C. , Uecker, A. M. , Beckmann, C. F. , … Laird, A. (2013). ICA model order selection of task co‐activation networks. Frontiers in Neuroscience, 7, 237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saucier, G. (1992). Openness versus intellect: Much ado about nothing? European Journal of Personality, 6, 381–386. [Google Scholar]
- Schacter, D. L. , & Addis, D. R. (2007). The cognitive neuroscience of constructive memory: remembering the past and imagining the future. Philosophical Transactions of the Royal Society B: Biological Sciences, 362, 773–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacter, D. L. , Addis, D. R. , Hassabis, D. , Martin, V. C. , Spreng, R. N. , & Szpunar, K. K. (2012). The future of memory: Remembering, imagining, and the brain. Neuron, 76, 677–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schooler, J. W. , Smallwood, J. , Christoff, K. , Handy, T. C. , Reichle, E. D. , & Sayette, M. A. (2011). Meta‐awareness, perceptual decoupling and the wandering mind. Trends in Cognitive Sciences, 15, 319–326. [DOI] [PubMed] [Google Scholar]
- Seeley, W. W. , Menon, V. , Schatzberg, A. F. , Keller, J. , Glover, G. H. , Kenna, H. , … Greicius, M. D. (2007). Dissociable intrinsic connectivity networks for salience processing and executive control. The Journal of Neuroscience, 27, 2349–2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shine, J. M. , Bissett, P. G. , Bell, P. T. , Koyejo, O. , Balsters, J. H. , Gorgolewski, K. J. , … Poldrack, R. A. (2016). The dynamics of functional brain networks: Integrated network states during cognitive task performance. Neuron, 92, 544–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvia, P. J. , Nusbaum, E. C. , Berg, C. , Martin, C. , & O'connor, A. (2009). Openness to experience, plasticity, and creativity: Exploring lower‐order, high‐order, and interactive effects. Journal of Research in Personality, 43, 1087–1090. [Google Scholar]
- Silvia, P. J. , Winterstein, B. P. , Willse, J. T. , Barona, C. M. , Cram, J. T. , Hess, K. I. , … Richard, C. A. (2008). Assessing creativity with divergent thinking tasks: Exploring the reliability and validity of new subjective scoring methods. Psychology of Aesthetics, Creativity, and the Arts, 2, 68. [Google Scholar]
- Smallwood, J. , Karapanagiotidis, T. , Ruby, F. , Medea, B. , de Caso, I. , Konishi, M. , … Jefferies, E. (2016). Representing representation: Integration between the temporal lobe and the posterior cingulate influences the content and form of spontaneous thought. PloS One, 11, e0152272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smallwood, J. , Tipper, C. , Brown, K. , Baird, B. , Engen, H. , Michaels, J. R. , … Schooler, J. W. (2013). Escaping the here and now: Evidence for a role of the default mode network in perceptually decoupled thought. Neuroimage, 69, 120–125. [DOI] [PubMed] [Google Scholar]
- Smith, S. M. , Fox, P. T. , Miller, K. L. , Glahn, D. C. , Fox, P. M. , Mackay, C. E. , … Beckmann, C. F. (2009). Correspondence of the brain's functional architecture during activation and rest. Proceedings of the National Academy of Sciences of the United States of America, 106, 13040–13045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreng, R. N. (2012). The fallacy of a “task‐negative” network. Frontiers in Psychology, 3, 145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreng, R. N. , Gerlach, K. D. , Turner, G. R. , & Schacter, D. L. (2015). Autobiographical planning and the brain: Activation and its modulation by qualitative features. Journal of Cognitive Neuroscience, 27, 2147–2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreng, R. N. , & Schacter, D. L. (2012). Default network modulation and large‐scale network interactivity in healthy young and old adults. Cerebral Cortex, 22, 2610–2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreng, R. N. , Stevens, W. D. , Chamberlain, J. P. , Gilmore, A. W. , & Schacter, D. L. (2010). Default network activity, coupled with the frontoparietal control network, supports goal‐directed cognition. Neuroimage, 53, 303–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin, L. Q. (2015). Salience processing and insular cortical function and dysfunction. Nature Reviews Neuroscience, 16, 55–61. [DOI] [PubMed] [Google Scholar]
- Voytek, B. , & Knight, R. T. (2015). Dynamic network communication as a unifying neural basis for cognition, development, aging, and disease. Biological Psychiatry, 77, 1089–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, D. (2007). A report on the third revision of Combined Raven's Test (CRT‐C3) for children in China. Chinese Journal of Clinical Psychology, 15, 559. [Google Scholar]
- Wei, D. , Yang, J. , Li, W. , Wang, K. , Zhang, Q. , & Qiu, J. (2014). Increased resting functional connectivity of the medial prefrontal cortex in creativity by means of cognitive stimulation. Cortex, 51, 92–102. [DOI] [PubMed] [Google Scholar]
- Zabelina, D. L. , & Andrews‐Hanna, J. R. (2016). Dynamic network interactions supporting internally‐oriented cognition. Current Opinion in Neurobiology, 40, 86–93. [DOI] [PubMed] [Google Scholar]
- Zhu, W. , Chen, Q. , Xia, L. , Beaty, R. E. , Yang, W. , Guikang, C. , … Qiu, J. (2017). Common and distinct brain networks underlying verbal and visual creativity. Human Brain Mapping, 38, 2094–2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler, M. , Cengia, A. , Mussel, P. , & Gerstorf, D. (2015). Openness as a buffer against cognitive decline: The Openness‐Fluid‐Crystallized‐Intelligence (OFCI) model applied to late adulthood. Psychology and Aging, 30, 573–588. [DOI] [PubMed] [Google Scholar]


