Abstract
The accurate measurement of blood pressure in pregnancy is essential to guide medical decision making that affects both mother and fetus. The aim of this systematic review was to determine the accuracy of ambulatory, home, and clinic blood pressure measurement devices in pregnant women. We searched Ovid MEDLINE, The Cochrane Library, EMBASE, CINAHL EBSCO, Clinicaltrials.gov, International Clinical Trials Registry Platform, and dabl from inception through August 3, 2017 for articles that assessed the validity of an upper arm blood pressure measurement device against a mercury sphygmomanometer in pregnant women. Two independent investigators determined eligibility, extracted data, and adjudicated protocol violations. From 1,798 potential articles identified, 41, that assessed 28 devices, met the inclusion criteria. Most articles (N= 32) followed a standard or modified American National Standards Institute/Association for the Advancement of Medical Instrumentation/International Organization for Standardization, British Hypertension Society, or European Society of Hypertension validation protocol. Several articles described the results of validation studies performed on more than one device (N=7) and/or in more than one population of pregnant women (N=12), comprising 64 pairwise validity assessments. The device was validated in 61% (32 of 52) of studies which used a standard or modified protocol. Only 34% (11 of 32) of the studies wherein the device was successfully validated were performed without a protocol violation. Given the implications of inaccurate blood pressure measurement in pregnant women, healthcare providers should be aware of and try to use the blood pressure measurement devices which have been properly validated in this population.
Keywords: pregnancy; pre-eclampsia; hypertension; blood pressure monitoring, ambulatory; blood pressure monitoring, home; validation studies
Introduction
Hypertension (HTN) is one of the most common medical disorders complicating pregnancy, occurring in up to 10% of pregnancies.1 Hypertensive disorders of pregnancy include chronic HTN, gestational HTN, preeclampsia, and preeclampsia superimposed on chronic HTN.1 Complications associated with HTN in pregnancy include placental abruption, preterm delivery, fetal growth restriction, stillbirth, maternal death secondary to stroke and eclampsia, as well as future risk of HTN, diabetes, and cardiovascular disease.1-6 Blood pressure (BP) control is recommended to help prevent maternal-fetal adverse outcomes.1, 7 However, the optimal BP goal for pregnant women with HTN is uncertain.8 Prior and ongoing randomized trials are investigating the effect of more intensive BP control in pregnant women with HTN.7,9 Because hemodynamic and vascular changes occur during pregnancy, guidelines recommend validating BP measurement devices in pregnant women.10, 11
The Association for the Advancement of Medical Instrumentation (ANSI/AAMI/ISO), British HTN Society (BHS), and European Society of HTN (ESH) have published protocols to validate BP measurement devices and ensure that their accuracy is comparable to the reference standard, a mercury sphygmomanometer.10-16 These protocols were developed to standardize the procedures for validating BP devices,17, 18 and strict adherence to an individual protocol is necessary for accuracy and statistical validity.19
Given the importance of measuring BP accurately in pregnancy, we undertook a systematic review of published studies assessing the validity of ambulatory, home, and clinic BP measurement devices in pregnant women to evaluate the methodology and quality of the published validation data.
Methods
The authors declare that all supporting data are available within the article [and its online supplementary files]. We followed the guidelines from the Cochrane Handbook for Systematic Reviews 20 and the PRISMA (Preferred Reporting Items for Systematic Review and Meta-Analysis) guidelines.21 All methods and inclusion/exclusion criteria were specified in advance and documented in a study protocol as described below.
Inclusion and Exclusion Criteria
Articles that assessed the validity of an upper arm brachial BP measurement device compared to a traditional mercury sphygmomanometer in pregnant women were included. Articles were excluded if they examined devices that measured BP from an anatomic site other than the upper arm, if they used intra-arterial comparisons, random-zero sphygmomanometers, or other devices as the reference standard. If studies included both pregnant and non-pregnant women, they were excluded if they did not report results for pregnant participants separately from non-pregnant participants. Commentaries, meeting abstracts, editorials, book chapters, and review articles were also excluded. There was no restriction on language.
Literature Search Strategy
The following databases were searched from inception through August 3, 2017: Ovid MEDLINE, The Cochrane Library, EMBASE, CINAHL EBSCO, Clinicaltrials.gov, and International Clinical Trials Registry Platform. The search strategies are provided in the online supplemental material (Supplemental Methods). To supplement the database searches, a PubMED similar articles search and a cited reference search through the ISI Web of Science were conducted using articles identified from the first set of results. The dabl Educational Trust Website (http://www.dableducational.org/) was searched manually. A manual search was also performed using the reference lists from the included articles, and from review articles produced by the Ovid MEDLINE search.
Study Selection
Article eligibility was determined by two investigators (NAB and JJW) who independently reviewed the title and abstract of all identified articles. If an article appeared to meet the inclusion criteria upon reviewing the abstract, the full text version of the article was retrieved for review. In the event the two investigators (NAB and JJW) disagreed on an article's eligibility, a third investigator (DS) resolved the discrepancy.
Data Extraction
Two reviewers (NAB and JJW) independently abstracted all data using standardized data abstraction forms. The data extraction results were compared and discrepancies were resolved by a third investigator (DS). Information was extracted on sample size, trimester of pregnancy, systolic and diastolic BP (SBP and DBP) at study entry, and arm circumference, as well as the validation protocol(s) used and specific procedures followed during the protocol, including details on: a) the BP device being evaluated; b) the arm used for measurement by the device and reference; c) the sequence of device and reference measurements; d) the number of BP comparisons between the device and reference obtained; e) the timing of observer comparisons (sequential vs. simultaneous); and f) final SBP and DBP validation grades for the device. The device type (ambulatory, home, clinic, home/clinic) was based on the authors' reported description. If the authors did not specify the device type, it was categorized based on other authors' classification or the device manufacturer's specification. If an article reported the validation of more than one device, or one device in more than one population of pregnant women (e.g., those with and without preeclampsia), the results are reported separately for each device and/or population. For the purposes of this systematic review, a “population” is defined as a unique group of pregnant women described in an article which had data on BP and validation grade. A “study” is defined as the testing of a BP device within a population.
Definition of SBP and DBP
SBP by auscultation is defined by convention as the first appearance of clear, repetitive sounds for at least two consecutive beats (K1). There has been controversy and a lack of consensus regarding whether K4 or K5 should be used to define DBP in pregnancy.22 Consistent with the National High Blood Pressure Education Program Working Group Report on High Blood Pressure in Pregnancy, we chose to use K5, the point at which all Korotkoff sounds disappear, to define DBP in the current study.23 Several articles report both K4 and K5 as DBP. In this situation, DBP is presented as K5. There were two studies in which only K4 was reported as DBP.43,61
Assessment of Methodological Quality and Protocol Violations
Adherence was assessed for each study published in articles that used protocols proposed by the AAMI (1987), BHS (original 1990 or revised 1993 version), and/or the ESH-international protocol (IP) (original 2002 version or revised 2010 version) to perform the validation study. In addition to extracting the authors' reported SBP and DBP grade for the devices, we independently determined the SBP and DBP grades for each device using data in the published articles. Similar to the classification system used to examine BP devices in other reviews, protocol violations were adjudicated and classified as major or minor, as defined in Supplemental Table S1.18, 24, 25 In some articles, the authors report additional grades based on a second validation protocol. In this situation, we extracted the reported SBP and DBP grade for each device and independently adjudicated grades. For articles that used a non-standard protocol to perform the validation study including two for which only K4 was reported for DBP, 43,61 no grades or violations are reported.
Results
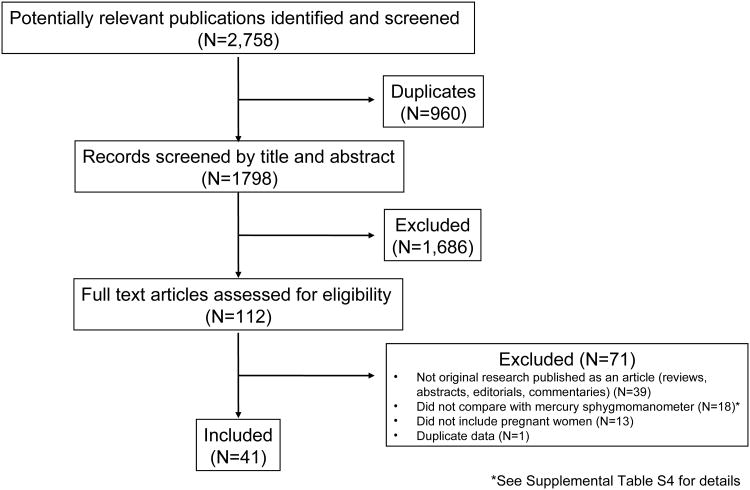
A total of 2,758 articles were identified and screened for inclusion (Figure 1). Of these, 960 duplicates were excluded leaving 1798 unique articles. Another 1,686 articles were excluded based on abstract review, and the remaining 112 articles were assessed for eligibility based on full-text review. Of the 112 articles, 41 met the inclusion criteria for the current analysis.26-66 The 41 articles included more than 2,000 pregnant women with sample sizes ranging from 10 to 170 (Supplemental Table S2). The majority of articles (N=18) included women in the latter two trimesters of pregnancy, and 10 articles included women in all trimesters. Supplemental Table S3 displays the devices, sample population, reference mean BP, and mean device-reference BP difference for each article. Twelve articles30-34, 37, 40, 42, 49, 61-63 described the results of device validation in more than one population of pregnant women (e.g. normotensive women, hypertensive women, and/or women with preeclampsia).
Figure 1.
Process of Study Selection
Overall, the 41 included articles examined 28 different devices; 5 were designated by the authors as ambulatory devices (Table 1), 5 as home devices (Table 2), and 14 as clinic devices (Table 3). Four devices (Omron T9P,29 Omron MIT Elite,31 Microlife 3BTO-A and Omron M756) were designated as appropriate for use in both the clinic and home settings by the authors; these studies are listed in both Tables 2 and 3. The Spacelabs 90207 ambulatory BP monitor was tested most often (N=10 articles).28, 37, 39, 46, 52, 53, 58, 64-66 The majority of devices (N=23) examined used the oscillometric method, and the remainder of devices (N=5) used the auscultatory method. Of the 41 included articles, 31 used a standard validation protocol or a modification of one (Tables 1 to 3): 1 used the AAMI protocol, 27 used the BHS (7 used the 1990 version and 2 used a modification of it, and 18 utilized the 1993 version), 3 used the ESH-IP (2 used the 2002 version and 1 used the 2010 version). With one exception,45 the authors' published grades for the devices matched our adjudicated grades.
Table 1. Validation Studies of Ambulatory Devices.
| Device | First Author (Year) | Protocol(s) | Population (N) | BHS Grade (SBP/DBP) | ESH Grade (SBP/DBP) | AAMI Grade (SBP/DBP) | Violations | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Pub | Adj | Pub | Adj | Pub | Adj | Major | Minor | ||||
| BP Lab | Bartosh (2006) | ESH 02 | Normotensive (without preeclampsia) and hypertensive (with and without preeclampsia) (N=33) | - | - | P/P | P/P | - | - | none | none |
| Dorogova (2015) | BHS 93 | Normotensive and hypertensive (N=30) | A/A | A/A | - | - | - | - | none | Circ | |
| DisetronicProfilomat | Franx (1997) | BHS 90* Additional: AAMI | Pregnancy (N=55) | B/C | B/C | - | - | P/P | P/P | N, Comp | SBP, DBP |
| Oxford Medilog | Franx (1994) | BHS 90 Additional: AAMI | Pregnancy (N=30) | C/C | NG | - | - | P/P | P/P | N, Comp | SBP, DBP |
| Spacelabs 90207 | Brown (1998) | Non-standard | Normotensive and hypertensive (N=79) | - | - | - | - | - | - | - | - |
| Elvan-Taspinar (2003) | BHS 93 Additional: AAMI | Normotensive, hypertensive, and preeclampsia (N=123) | B/B | B/B | - | - | P/P | P/P | Obs | none | |
| Normotensive (N=54) | A/A | A/A | - | - | P/P | P/P | Obs | none | |||
| Preeclampsia (N=31) | B/C | B/C | - | - | F/F | F/F | Obs | none | |||
| Hypertensive (N=38) | B/A | B/A | - | - | P/P | P/P | Obs | none | |||
| Franx (1997) | BHS 90* Additional: AAMI | Pregnancy (N=55) | B/C | B/C | - | - | P/P | P/P | N*, Comp* | SBP, DBP | |
| Koenen (1998) | BHS 90 | Pregnancy (N=10) | NR | NG | - | - | - | - | N, Comp | SBP, DBP | |
| Montan (1995) | Non-standard | Preeclampsia (N=20) | - | - | - | - | - | - | - | - | |
| Natarajan (1999) | BHS 93 Additional: AAMI | Preeclampsia (N=30) | D/D | D/D | - | - | F/F | F/F | none | Circ, SBP, DBP | |
| Obrien (1993) | BHS 90 Additional: AAMI | Pregnancy (N=86 recruited, N=81 included in the analysis) | A/C | A/C | - | - | P/F | P/F | N, Comp | SBP, DBP | |
| Shennan (1993) | BHS 90 Additional: AAMI | Pregnancy (N=122) | B/B | B/B | - | - | P/P | P/P | none | N, Comp, SBP, DBP | |
| Shennan (1996) | BHS 90 | Preeclampsia (N=30) | C/C | NG | - | - | - | - | N, Comp | SBP, DBP | |
| Tape (1994) | AAMI | Pregnancy (N=59) | - | - | - | - | P/P | NG | N | SBP, DBP | |
| Welch Allyn QuietTrak | Natarajan (1999) | BHS 93 Additional: AAMI | Preeclampsia (N=30) | D/D | D/D | - | - | F/F | F/F | none | Circ, SBP, DBP |
| Penny (1996) | BHS 93 Additional: AAMI | Pregnancy (N=85) | B/B | B/B | - | - | F/F | F/F | none | none | |
Note: When more than one protocol is listed, the first protocol is the methodology followed for the validation study and additional protocols are listed when their grading criteria were applied to the data.
Individual studies are indicated by bold type in the population column. Shaded rows indicate studies that passed the primary protocol.
Protocol Modification: Authors state they used BHS 90 criteria to enable comparison with prior studies of Spacelabs device but fewer participants because the BHS 93 allows for ≥30.
Abbreviations: Pub= published grade; Adj= adjudicated grade; A= age, T=trimester, SBP= systolic blood pressure range, DBP= diastolic blood pressure range, Circ= arm circumference, N= number of participants, Comp= number of BP comparisons, Timing= Device v. Mercury simultaneous/sequential, Obs= observer measurementssimultaneous/sequential, G= final grade; NR= not reported; NG= not able to adjudicate a grade.
Table 2. Validation Studies of Home Devices.
| Device | First Author (Year) | Protocol(s) | Population (N) | BHS Grade (SBP/DBP) | ESH Grade (SBP/DBP) | AAMI Grade (SBP/DBP) | Violations | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Pub | Adj | Pub | Adj | Pub | Adj | Major | Minor | ||||
| Microlife 3BTO-A | Nouwen (2012)∼ | ESH 02 Additional: BHS 93$ | Hypertensive preeclampsia (N=33) | B/B | B/B (BHS 93) C/C (BHS 90) | F/F | F/F | - | - | none | A, SBP, DBP |
| Reinders (2005) | BHS 93 Additional: AAMI | Pregnancy (N=105) | A/B | A/B | - | - | P/P | P/P | none | Circ | |
| Normotensive (N=35) | A/B | A/B | - | - | P/P | P/P | none | Circ | |||
| Hypertensive (N=35) | B/B | B/B | - | - | P/P | P/P | none | Circ | |||
| Preeclampsia (N=35) | A/B | A/B | - | - | P/P | P/P | none | Circ | |||
| Microlife WatchBP Home | Chung (2009) | BHS 93 Additional: AAMI | Pregnancy (N=30) | A/A | A/A | - | - | P/P | P/P | none | none |
| Preeclampsia (N=15) | B/A | B/A | - | - | P/P | P/P | none | N, Comp | |||
| Omron HEM 705 CP | Brown (1998) | Non-standard | Pregnancy (N=50) | - | - | - | - | - | - | - | - |
| Gupta (1997) | BHS 93 Additional: AAMI | Pregnant w/o preeclampsia (N=85) | B/B | B/B | - | - | F/F | F/F | none | Circ, SBP, DBP | |
| Preeclampsia (N=42) | C/D | C/D | - | - | F/F | F/F | none | Circ, SBP, DBP | |||
| Lo (2002) | Non-standard | Healthy pregnancy (N=101) | - | - | - | - | - | - | - | - | |
| Preeclampsia (N=45) | - | - | - | - | - | - | - | - | |||
| Omron-M7 | De Greeff (2009) | BHS 93 Additional: AAMI | Pregnancy & preeclampsia (N=45) | A/A | A/A | - | - | P/P | P/P | none | Circ ^ |
| Normotensive (N=30) | A/A | A/A | - | - | P/P | P/P | none | Circ ^ | |||
| Preeclampsia (N=15) | B/B | B/B | - | - | P/P | F/P | N, Comp | Circ ^ | |||
| Nouwen (2012)∼ | ESH 02 Additional: BHS 93$ | Hypertensive preeclampsia (N=33) | B/A | B/A (BHS 93) C/B (BHS 90) | F/P | F/P | - | - | none | A, SBP, DBP | |
| Omron-MIT | De Greeff (2009) | BHS 93 Additional: AAMI | Pregnancy & preeclampsia (N=45) | A/A | A/A | - | - | P/P | P/P | none | none |
| Normotensive (N=30) | A/A | A/A | - | - | P/P | P/P | none | none | |||
| Preeclampsia (N=15) | A/A | A/A | - | - | P/P | P/P | N, Comp | none | |||
| Omron-MIT Elite | Chung (2012)∼ | BHS 93 Additional: AAMI | Pregnancy & preeclampsia (N=45) | A/A | A/A | - | - | P/P | P/P | none | SBP, DBP |
| Normotensive (N=30) | A/A | A/A | - | - | P/P | P/P | none | none | |||
| Preeclampsia (N=15) | A/A | A/A | - | - | P/P | P/P | none | N, Comp | |||
| James (2017)† | BHS 93 | Pregnancy with arm circumference >= 32 cm (N=46) | D/D | C/D | - | - | - | - | none | none | |
| Omron T9P | Brown (2011)∼ | BHS 90 Additional: AAMI | Pregnancy (N=85) | A/A* | A/A (BHS 93) B/B (BHS 90) | - | - | P/P | P/P | G* | SBP, DBP |
| Takeda UA-751 | Brown (1991) | Non-standard | Pregnancy (N=79) | - | - | - | - | - | - | - | - |
| Withings BP-800 | Hay (2016) | BHS 93 | Normotensive, hypertensive, and preeclampsia (N=47) | C/B | C/B | - | - | - | - | none | none |
Note: When more than one protocol is listed, the first protocol is the methodology followed for the validation study and additional protocols are listed when their grading criteria were applied to the data.
Individual studies are indicated by bold type in the population column. Shaded rows indicate studies that passed the primary protocol.
authors state device can be used for home or clinical use; †authors do not specify device type, so prior authors' designation followed (data is also found in Table 3).
Protocol Modification: BHS 90 protocol cited in references, but BHS 93 grading was used.
Protocol Modification: BHS 90 protocol was followed, but BHS 93 grading was used.
two devices were tested MIT can only accommodate an arm circumference of 22-32 cm M7 can accommodate up to 42cm. Only women with an arm circumference 22-32 cm were included.
Abbreviations: Pub= published grade; Adj= adjudicated grade; A= age, T=trimester, SBP= systolic blood pressure range, DBP= diastolic blood pressure range, Circ= arm circumference, N= number of participants, Comp= number of BP comparisons, Timing= Device v. Mercury simultaneous/sequential, Obs= observer measurementssimultaneous/sequential, G= final grade; NR= not reported; NG= not able to adjudicate a grade.
Table 3. Validation Studies of Clinic Devices.
| Device | First Author (Year) | Protocol(s) | Population (N) | BHS Grade (SBP/DBP) | ESH Grade (SBP/DBP) | AAMI Grade (SBP/DBP) | Violations | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Pub | Adj | Pub | Adj | Pub | Adj | Major | Minor | ||||
| Acutorr III | Quinn (1994) | Non-standard | Normotensive (N=40) | - | - | - | - | - | - | - | - |
| Preeclampsia (N=17) | - | - | - | - | - | - | - | - | |||
| A&D UM-101 | Davis (2015) | BHS 93 | Normotensive (N=85) | A/A | A/A | - | - | - | - | none | none |
| Hypertensive (N=85) | A/A | A/A | - | - | - | - | none | none | |||
| Dinamap 1846 | Hasan (1993) | Non-standard | Pregnancy (N==28) | - | - | - | - | - | - | - | - |
| Dinamap 1846SX | Franx (1994) | BHS 90 Additional: AAMI | Pregnancy (N=32) | C/D | NG | - | - | F/F | F/F | N, Comp, Timing | SBP,DBP |
| Green (1996) | AAMI | Pregnancy (N=81) | - | - | - | - | F/NR | F/NG | N, comp | SBP,DBP | |
| Marx (1991) | Non-standard | Pregnancy (N=20) | - | - | - | - | - | - | - | - | |
| Marx (1993) | Non-standard | Pregnancy (N=12) | - | - | - | - | - | - | - | - | |
| Dinamap 1846SX/p | Pomini (2001) | BHS 90 | Normotensive (N=not specified) | C/C | C/C | - | - | - | - | N | SBP,DBP |
| Dinamap ProCare 400 | De Greeff (2010) | BHS 93 Additional: AAMI | Pregnancy without preeclampsia (N=30) | A/A | A/A | - | - | P/P | P/P | none | none |
| Preeclampsia (N=15) | A/B | A/B | - | - | P/F | P/F | N, Comp | none | |||
| Microlife 3AS1-2 | Nathan (2015) | BHS 93 Additional: AAMI | Pregnancy & preeclampsia (N=45) | B/A | B/A | - | - | P/P | P/P | none | SBP,DBP |
| Nathan (2015) | BHS 93 Additional: AAMI | Hypotensive (N=30) | A/A | A/A | - | - | P/P | P/P | none | SBP,DBP | |
| Microlife 3BTO-A | Nouwen (2012)∼ | ESH 02 Additional: BHS 93$ | Hypertensive preeclampsia (N=33) | B/B | B/B (BHS 93)C/C (BHS 90) | F/F | F/F | - | - | none | A, SBP, DBP |
| Nissei DS-400 | De Greeff (2015) | BHS 93 Additional: AAMI | Pregnancy & preeclampsia (N=45) | A/A | A/A | - | - | P/P | P/P | none | none |
| Omron HEM-907 | Davis (2015) | BHS 93 | Normotensive (N=85) | A/A | A/A | - | - | - | - | none | none |
| Hypertensive (N=85) | A/A | A/A | - | - | - | - | none | none | |||
| Omron HEM-7200 | Lan (2014) | Non-standard | Pregnancy (N= 89) | - | - | - | - | - | - | - | - |
| Omron M7 | Nouwen (2012)∼ | ESH 02 Additional: BHS 93$ | Hypertensive preeclampsia (N=33) | B/A | B/A (BHS 93) C/B (BHS 90) | F/P | F/P | - | - | none | A, SBP, DBP |
| Omron MIT | Golara (2002) | BHS 93 Additional: AAMI | Normotensive (N=38) | B/A | B/A | - | - | P/P | P/P | none | Circ, T, SBP, DBP |
| Preeclampsia (N=15) | B/A | B/A | - | - | P/P | P/P | N, comp | Circ, T, SBP, DBP | |||
| Omron-MIT Elite | Chung (2012)∼ | BHS 93 Additional: AAMI | Pregnancy & preeclampsia (N=45) | A/A | A/A | - | - | P/P | P/P | none | SBP, DBP |
| Normotensive (N=30) | A/A | A/A | - | - | P/P | P/P | none | none | |||
| Preeclampsia (N=15) | A/A | A/A | - | - | P/P | P/P | none | N, Comp | |||
| James (2017)† | BHS 93 | Pregnancy with arm circumference >= 32 cm (N=46) | D/D | C/D | - | - | - | - | none | none | |
| Omron T9P | Brown (2011)∼ | BHS 90 Additional: AAMI | Pregnancy (N=85) | A/A* | A/A (BHS 93) B/B (BHS 90) | - | - | P/P | P/P | G* | SBP,DBP |
| Terumo ES-H51 | Kewk (1998) | BHS 90 | Pregnancy (N=87) | A/A | A/A | - | - | - | - | none | SBP,DBP |
| Welch Allyn Vital Signs | Reinders (2003) | BHS 93 Additional: AAMI | Normotensive (N=31) | A/A | A/A | - | - | P/P | P/P | none | Circ |
| Preeclampsia (N=17) | D/B | D/B | - | - | F/F | F/F | N, Comp | Circ | |||
| Welch Allyn Vital Signs 300 | Nzelu (2017) | ESH 10 | Normotensive, hypertensive, and preeclampsia (N=33) | - | - | F/F | F/F | - | - | A, SBP,DBP | |
Note: When more than one protocol is listed, the first protocol is the methodology followed for the validation study and additional protocols are listed when their grading criteria were applied to the data.
Individual studies are indicated by bold type in the population column. Shaded rows indicate studies that passed the primary protocol.
authors state device can be used for home or clinical use; †authors do not specify device type, so prior authors' designation followed (data is also found in Table 2).
Protocol Modification: BHS 90 protocol was followed, but BHS 93 grading was used.
Protocol Modification: BHS 90 protocol cited in references, but BHS 93 grading was used.
Abbreviations: Pub= published grade; Adj= adjudicated grade; A= age, T=trimester, SBP= systolic blood pressure range, DBP= diastolic blood pressure range, Circ= arm circumference, N= number of participants, Comp= number of BP comparisons, Timing= Device v. Mercury simultaneous/sequential, Obs= observer measurements simultaneous/sequential, G= final grade; NR= not reported; NG= not able to adjudicate a grade.
Ambulatory Devices
The devices passed validation in 6 of the 16 studies in which ambulatory BP monitoring devices were examined using a standard protocol (Table 1-shaded rows indicate passing, individual studies are indicated by bold type in the population column). Among the 16 studies, 2 had no protocol violations,26, 59 3 had at least one minor violation,36, 53, 64 2 had at least one major violation,37 and 7 had major and minor violations.38, 39, 46, 58, 65, 66 Of the 5 ambulatory devices, 3 (BP Lab, Spacelabs 90207, Welch Allyn QuietTrak) passed at least one standard validation protocol. The Disetronic Profilomat39 and Oxford Medilog38 each failed one standard validation protocol. The BP Lab passed validation in 2 of 2 studies26, 36; 1 of the 2 studies had no protocol violations.26 The Spacelabs 90207 passed in 3 of 10 studies; all 3 studies had at least one major or minor protocol violation.37, 64 The Welch Allyn QuietTrak passed in 1 of 2 studies without a protocol violation.53, 59
Home Devices
Of the 18 studies in which home BP measurement devices were examined using a standard protocol (Table 2), the devices passed validation in 13 studies, 3 of which had no protocol violations,30, 31, 33 7 had at least one minor violation,30, 31, 33, 42, 63 1 had at least one major violation,33 and 2 had major and minor violations.29, 33 The Microlife WatchBP Home and the Omron MIT, and T9P passed in all studies; 2 of these 5 (40%) studies had no violations.30, 31, 33
Clinic Devices
Of the 24 studies in which clinic BP measurement devices were examined using a standard protocol (Table 3), the devices passed the validation in 17 studies, 7 of which had no protocol violations,31, 32, 35 7 had at least one minor violation,31, 40, 47, 54-56, 62 1 had at least one major violation,34 and 2 had major and minor violations.29, 40
Overall, among pregnant women, 61% of devices passed the stated validation protocol in at least one study, and 34% of those devices passed without a protocol violation (Table 4).
Table 4. Blood Pressure Measurement Devices Successfully Validated without Violation in At Least One Study of Pregnant Women.
| Device Type | Device | Study Population |
|---|---|---|
|
| ||
| Ambulatory | ||
|
| ||
| BP Lab26 | Normotensive (without preeclampsia) and Hypertensive (with and without preeclampsia) | |
| Welch Allyn QuietTrak59 | Normotensive and Hypertensive | |
|
| ||
| Home | ||
|
| ||
| MicrolifeWatchBP Home30 | Normotensive and Hypertensive, without Preeclampsia | |
| Omron MIT33 | Normotensive and Hypertensive, without Preeclampsia | |
|
| ||
| Clinic | ||
|
| ||
| A&D UM-10132 | Normotensive (without preeclampsia) and Hypertensive (with and without preeclampsia) | |
| DinamapProCare 40034 | Normotensive and Hypertensive, without Preeclampsia | |
| Nissei DS-40035 | Normotensive and Hypertensive, with and without Preeclampsia | |
| Omron HEM-90732 | Normotensive (without preeclampsia) and Hypertensive (with and without preeclampsia) | |
|
| ||
| Home/Clinic | ||
|
| ||
| Omron MIT Elite31 | Normotensive and Hypertensive, without Preeclampsia | |
Discussion
In this systematic review, we found that the majority of BP measurement devices passed a validation protocol in pregnant women, but that only one third of these devices did so without any protocol violations. The most common major protocol violation was the inclusion of too few pregnant women when a population was examined, so that too few comparisons were obtained between the device and the reference standard. The most common minor violations were related to arm circumference and SBP and DBP ranges. Of the 11 categories of protocol violations, only 2 did not occur: the use of the same/opposite arm (major) for device and reference standard, and inclusion of at least 10 women in each of the second and third trimesters of pregnancy (minor). While not considered a violation, in one article29, the authors followed a modified BHS 1990 protocol,16 but evaluated the results using grading criteria from the BHS 1993 protocol.10, 15 The BHS 1993 protocol is more lenient than the 1990 BHS protocol, requiring fewer participants, examining a narrower range of BP, and requiring less stringent grading criteria to pass validation. Additionally, all studies which undertook the 1993 BHS protocol10 reported a letter grade for SBP and DBP despite the recommendation that grading should not be attempted in special groups such as pregnant women.
Substantial hemodynamic changes occur during pregnancy, including increased blood volume, stroke volume, heart rate, and consequently cardiac output along with a decrease in peripheral vascular resistance67, 68 Thus, guidelines recommend BP devices intended for use in pregnant women should be validated in this population.10, 11 Accurate measurement of BP enables the timely diagnosis, monitoring, and treatment of hypertensive disorders of pregnancy.68 However, it is important to note that although validation protocols provide reassurance that a device has been validated for use in a population, the device is not necessarily accurate in all individuals. When possible, devices should be tested against traditional sphygmomanometer in individual patient to confirm accuracy before clinical use.69
Obstetric guidelines1 suggest frequent monitoring of BP in the clinic and at home for pregnant women with poorly controlled BP and those at high risk of developing preeclampsia. Preeclampsia, the concurrent development of elevated BP after 20 weeks of gestation accompanied by proteinuria or organ dysfunction, is a significant contributor to maternal-fetal morbidity and mortality.23 In light of the evidence that the treatment of preeclampsia can reduce maternal-fetal morbidity and mortality, the US Preventive Services Task Force recently updated its preeclampsia screening guideline to recommend BP measurements be obtained during each prenatal care visit (Grade B).70, 71 Several devices have undergone validation studies (N=17) among pregnant women with preeclampsia. These included 4 studies of ambulatory BP monitors,37, 53, 65 8 studies of home BP devices,30, 31, 33, 42, 56, 63 and 5 studies of clinic BP devices31, 34, 40, 56, 62. Of these studies, no ambulatory device passed validation; 5 of the home devices (Microlife 3BTO-A,63 Microlife WatchBP Home,30 Omron M7 and MIT,33 and Omron MIT Elite31), and 3 clinic devices (Dinamap ProCare 400,34 Omron MIT,33 and Omron MIT Elite31), the latter two of which are also recommended for home use, passed the validation criteria. None of the 17 studies testing ambulatory, home or clinic BP devices among pregnant women with preeclampsia was performed without a protocol violation.
The finding that many studies wherein devices were validated had protocol violations is consistent with published studies conducted in non-pregnant women and men.18, 25, 72, 73 In a systematic review of BP devices validated using the ESH-IP, Stergiou et al. found protocol violations in 23 of 78 studies.18 Similarly, Hodgkinson et al. found that of 28 validation studies of ambulatory devices performed in a general adult population, 42%, 11% and 0% that followed the ESH-IP, AAMI, BHS, respectively, adhered to the specified protocol without violations.25 The proper execution of a BP device validation study with strict adherence to the specified protocol is a complex undertaking with several opportunities for violation which may lead to improper performance and result in a detrimental effect on the study's power.74 All violations, whether classified as major or minor,18, 25 have the potential to effect the results of a validation study, and when noted the results of a validation study should be interpreted with caution.
Standardized validation protocols (AAMI, BHS, ESH-IP) were developed to demonstrate statistical equivalence between new devices and the gold-standard mercury sphygmomanometer.19 Although the ESH-IP was designed to simplify validation studies and has been the most widely used protocol since 2006 among non-pregnant women and men,18 some have questioned whether it is sufficiently powered to demonstrate equivalence.18, 19 In the current systematic review, 3 articles (testing 4 devices)26, 56, 57 used the ESH-IP as the primary validation protocol. The BPLab ambulatory monitor26 was the only one of the four devices to pass the ESH-IP in pregnant women. The Microlife 3BTO-A, Omron M7, and Welch Allyn Vital Signs 300 failed the ESH-IP validation.56, 57 Ongoing efforts by an AAMI-ESH-ISO collaboration to create a standardized sufficiently powered “universal protocol” that will replace all previous protocols should simplify the performance and analysis of future device validation studies among both non-pregnant and pregnant populations.74
A strength of the current study is the use of a comprehensive search strategy of multiple databases and websites and the manual review of reference lists of all included articles, without limitations on language. Additionally, we evaluated the available validation data on ambulatory, home, and clinic devices. Data from systematic reviews of the validation of BP devices in pregnant women are sparse, and the results of our study address important knowledge gaps in this area. However, the current review has several known and potential limitations. First, we excluded studies (N=18) that utilized a reference device other than a standard mercury sphygmomanometer (Supplemental Table S4). While this approach may exclude potentially important studies, the majority of validation protocols require mercury sphygmomanometer as the reference standard,10, 11, 15, 16 with the exception being the ANSI/AAMI/ISO which has an alternative direct intra-arterial reference option.12-14 Second, we cannot exclude the possibility of publication bias. Although we found many published studies describing device validation failures, there may be situations in which validation failures were not published. Additionally, although several studies tested the same device, we did not attempt to perform a meta-analysis due to the small number of studies in which these devices were validated without protocol violations. Lastly, for the 11 studies that followed a non-standard validation protocol27, 28, 43, 48-52, 61 we were unable to adjudicate grades or assess violations and cannot comment on the validity of the devices examined. The majority of devices included in this review were oscillometric and utilize proprietary algorithms to calculate SBP and DBP. Without knowledge of the algorithms, further evaluation of the strengths and weaknesses of the performance of an individual device compared to another is limited.
Perspectives
In the current systematic review, a majority of validation studies examining BP measurement devices in pregnancy had violations. Of the 28 devices examined, 2 ambulatory devices (BP Lab,26 Welch Allyn QuietTrak59), 2 home devices (Microlife WatchBP Home,30 Omron MIT33), 4 clinic BP devices (A&D UM-101,32 Dinamap ProCare 400,34 Nissei DS-400,35 Omron HEM90732) and 1 home/clinic device (Omron MIT-Elite31) passed a validation study in at least one population of pregnant women without any protocol violations. As results from validation studies not adhering to the protocol specifications or those without sufficient power cannot be assumed to be valid, future validation studies of devices in pregnant women are needed to ensure the accurate measurement of BP in pregnancy. Given the potential consequences of inaccurate BP measurement in pregnant women, healthcare providers should use BP devices that have been proven accurate and valid in this population.
Supplementary Material
Novelty and Significance.
What is New?
We have systematically reviewed and summarized the published validation data for ambulatory, home, and clinic blood pressure measurement devices in pregnancy.
What is Relevant?
The use of properly validated devices are essential for the accurate measurement of blood pressure and the provision of care to pregnant women at risk for and diagnosed with hypertension.
As results from validation studies not adhering to the protocol specifications or those without sufficient power cannot be assumed to be valid, healthcare providers should use blood pressure measurement devices that have been proven accurate and valid in this population.
Summary.
Of the 28 devices examined, 2 ambulatory devices (BP Lab, Welch Allyn QuietTrak), 2 home devices (Microlife WatchBP Home, Omron MIT), 4 clinic BP devices (A&D UM-101, Dinamap ProCare 400, Nissei DS-400, Omron HEM907) and 1 home/clinic device (Omron MIT-Elite) passed a validation study in at least one population of pregnant women without any protocol violations. The availability of validated blood pressure measurement devices is increasingly important as the prevalence of hypertensive disorders of pregnancy continues to rise and specialty societies are increasingly recognizing the importance of close monitoring and follow-up of these women.
Acknowledgments
None.
Sources of Funding: This work was supported by the National Center for Advancing Translational Sciences, National Institutes of Health (NIH) UL1TR001873 (NB), K24-HL125704 from the NIH/National Heart Lung and Blood Institute (DS) and 15SFRN2390002 from the American Heart Association (SO, PM, DS).
Footnotes
Conflicts of Interest/Disclosures: DS is a consultant for Abbott Vascular and Novartis Pharmaceuticals Corporation.
References
- 1.Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists' Task Force on Hypertension in Pregnany. Obstet Gynecol. 2013;122:1122–1131. doi: 10.1097/01.AOG.0000437382.03963.88. [DOI] [PubMed] [Google Scholar]
- 2.Harper LM, Biggio JR, Anderson S, Tita AT. Gestational Age of Delivery in Pregnancies Complicated by Chronic Hypertension. Obstet Gynecol. 2016;127:1101–1109. doi: 10.1097/AOG.0000000000001435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roberts JM, Pearson GD, Cutler JA, Lindheimer MD. Summary of the NHLBI Working Group on Research on Hypertension During Pregnancy. Hypertens Pregnancy. 2003;22:109–127. doi: 10.1081/PRG-120016792. [DOI] [PubMed] [Google Scholar]
- 4.Berg CJ, Mackay AP, Qin C, Callaghan WM. Overview of maternal morbidity during hospitalization for labor and delivery in the United States: 1993-1997 and 2001-2005. Obstet Gynecol. 2009;113:1075–1081. doi: 10.1097/AOG.0b013e3181a09fc0. [DOI] [PubMed] [Google Scholar]
- 5.Callaghan WM, Creanga AA, Kuklina EV. Severe maternal morbidity among delivery and postpartum hospitalizations in the United States. Obstet Gynecol. 2012;120:1029–1036. doi: 10.1097/aog.0b013e31826d60c5. [DOI] [PubMed] [Google Scholar]
- 6.Creanga AA, Berg CJ, Syverson C, Seed K, Bruce FC, Callaghan WM. Pregnancy-related mortality in the United States, 2006-2010. Obstet Gynecol. 2015;125:5–12. doi: 10.1097/AOG.0000000000000564. [DOI] [PubMed] [Google Scholar]
- 7.Magee LA, von Dadelszen P, Rey E, Ross S, Asztalos E, Murphy KE, et al. Less-Tight versus Tight Control of Hypertension in Pregnancy. N Engl J Med. 2015;372:407–417. doi: 10.1056/NEJMoa1404595. [DOI] [PubMed] [Google Scholar]
- 8.SMFM Publications Committee. SMFM Statement: benefit of antihypertensive therapy for mild-to-moderate chronic hypertension during pregnancy remains uncertain. Am J Obstet Gynecol. 2015 Jul;213:3–4. doi: 10.1016/j.ajog.2015.04.013. [DOI] [PubMed] [Google Scholar]
- 9.Chronic Hypertension and Pregnancy (CHAP) Project. Retrieved Jun 30, 2017 from https://clinicaltrials.gov/ct2/show/NCT02299414?term=chap&rank=3.
- 10.O'Brien E, Petrie J, Littler WA, De Sweit M, Padfield PL, Altman D, Bland M, Coats A, Atkins N. The British Hypertension Society Protocol for the evaluation of blood pressure measuring devices. J Hypertens. 1993;11:S43–S63. doi: 10.1097/00004872-199306000-00013. [DOI] [PubMed] [Google Scholar]
- 11.O'Brien E, Atkins N, Stergiou G, Karpettas N, Parati G, Asmar R, Imai Y, Wang J, Mengden T, Shennan A. Working Group on Blood Pressure Monitoring of the European Society of H. European Society of Hypertension International Protocol revision 2010 for the validation of blood pressure measuring devices in adults. Blood Press Monit. 2010;15:23–38. doi: 10.1097/MBP.0b013e3283360e98. [DOI] [PubMed] [Google Scholar]
- 12.Association for the Advancement of Medical Instrumentation. American National Standard Electronic or automated sphygmomanometers ANSI/AAMI SP10-1987. Arlington, VA: Association for the Advancement of Medical Instrumentation; 1987. [Google Scholar]
- 13.Association for the Advancement of Medical Instrumentation. American National Standard Manual, electronic or automated sphygmomanometers ANSI/AAMI SP10-2002/A1. Arlington, VA: Association for the Advancement of Medical Instrumentation; 2003. [Google Scholar]
- 14.Association for the Advancement of Medical Instrumentation. American National Standard ANSI/AAMI/ISO 81060-2:2013 Non-invasive sphygmomanometers -Part 2: Clinical investigation of automated measurement type. Arlington, VA: Association for the Advancement of Medical Instrumentation; 2013. [Google Scholar]
- 15.O'Brien E, Petrie J, Littler W, de Swiet M, Padfield PL, Altman DG, Bland M, Coats A, Atkins N. An outline of the revised British Hypertension Society protocol for the evaluation of blood pressure measuring devices. J Hypertens. 1993;11:677–679. doi: 10.1097/00004872-199306000-00013. [DOI] [PubMed] [Google Scholar]
- 16.O'Brien E, Petrie J, Littler W, de Swiet M, Padfield PL, O'Malley K, Jamieson M, Altman D, Bland M, Atkins N. The British Hypertension Society protocol for the evaluation of automated and semi-automated blood pressure measuring devices with special reference to ambulatory systems. J Hypertens. 1990;8:607–619. doi: 10.1097/00004872-199007000-00004. [DOI] [PubMed] [Google Scholar]
- 17.O'Brien E. Proposals for simplifying the validation protocols of the British Hypertension Society and the Association for the Advancement of Medical Instrumentation. Blood Pres Monit. 2000;5:43–45. [PubMed] [Google Scholar]
- 18.Stergiou GS, Karpettas N, Atkins N, O'Brien E. European Society of Hypertension international protocol for the validation of blood pressure monitors: A critical review of its application and rationale for revision. Blood Pres Monit. 2010;15:39–48. doi: 10.1097/MBP.0b013e3283360eaf. [DOI] [PubMed] [Google Scholar]
- 19.Friedman BA, Alpert BS, Osborn D, Prisant LM, Quinn DE, Seller J. Assessment of the validation of blood pressure monitors: a statistical reappraisal. Blood Press Monit. 2008;13:187–191. doi: 10.1097/MBP.0b013e3283071a64. [DOI] [PubMed] [Google Scholar]
- 20.Higgins JPT, Green S, editors. The Cochrane Collaboration. 2011. Cochrane Handbook for Systematic Reviews of Interventions Version 5.10 [updated March 2011] [Google Scholar]
- 21.Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, Shekelle P, Stewart LA. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4:1. doi: 10.1186/2046-4053-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Helewa ME, Burrows RF, Smith J, Williams K, Brain P, Rabkin SW. Report of the Canadian Hypertension Society Consensus Conference: 1. Definitions, evaluation and classification of hypertensive disorders in pregnancy. CMAJ. 1997;157:715–725. [PMC free article] [PubMed] [Google Scholar]
- 23.Report of the National High Blood Pressure Education Program Working Group on High Blood Pressure in Pregnancy. Am J Obstet Gynecol. 2000;183:S1–S22. [PubMed] [Google Scholar]
- 24.O'Brien E, Atkins N, Staessen J. State of the market. A review of ambulatory blood pressure monitoring devices. Hypertension (Dallas, Tex : 1979) 1995;26:835–842. doi: 10.1161/01.hyp.26.5.835. [DOI] [PubMed] [Google Scholar]
- 25.Hodgkinson JA, Sheppard JP, Heneghan C, Martin U, Mant J, Roberts N, McManus RJ. Accuracy of ambulatory blood pressure monitors: a systematic review of validation studies. J Hypertens. 2013;31:239–250. doi: 10.1097/HJH.0b013e32835b8d8b. [DOI] [PubMed] [Google Scholar]
- 26.Bartosh LF, Dorogova JV, Kuznecova TN, Krylova AV. The testing of BPLab Ambulatory Blood Pressure Monitor on the pregnant in conformity with International Protocol of the European Society of Hypertension (ESH-2001) Arterial Hypertension TOM. 2006;12:3–6. [Google Scholar]
- 27.Brown MA, Adsett D. Automated Blood-Pressure Recording in Pregnancy. Clin Exp Hypertens. 1991;10:7–19. [Google Scholar]
- 28.Brown MA, Robinson A, Buddle ML. Accuracy of automated blood pressure recorders in pregnancy. Aust N Z J Obstet Gynaecol. 1998;38:262–265. doi: 10.1111/j.1479-828x.1998.tb03062.x. [DOI] [PubMed] [Google Scholar]
- 29.Brown MA, Roberts L, Davis G, Mangos G. Can we use the Omron T9P automated blood pressure monitor in pregnancy? Hypertens Pregnancy. 2011;30:188–193. doi: 10.3109/10641955.2010.507854. [DOI] [PubMed] [Google Scholar]
- 30.Chung Y, de Greeff A, Shennan A. Validation and compliance of a home monitoring device in pregnancy: microlife WatchBP home. Hypertens Pregnancy. 2009;28:348–359. doi: 10.1080/10641950802601286. [DOI] [PubMed] [Google Scholar]
- 31.Chung Y, Brochut MC, de Greeff A, Shennan AH. Clinical accuracy of inflationary oscillometry in pregnancy and pre-eclampsia: Omron-MIT Elite. Pregnancy Hypertens. 2012;2:411–415. doi: 10.1016/j.preghy.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 32.Davis GK, Roberts LM, Mangos GJ, Brown MA. Comparisons of auscultatory hybrid and automated sphygmomanometers with mercury sphygmomanometry in hypertensive and normotensive pregnant women: parallel validation studies. J Hypertens. 2015;33:499–505. doi: 10.1097/HJH.0000000000000420. discussion 505-506. [DOI] [PubMed] [Google Scholar]
- 33.de Greeff A, Beg Z, Gangji Z, Dorney E, Shennan AH. Accuracy of inflationary versus deflationary oscillometry in pregnancy and preeclampsia: OMRON-MIT versus OMRON-M7. Blood Press Monit. 2009;14:37–40. doi: 10.1097/MBP.0b013e32831e305d. [DOI] [PubMed] [Google Scholar]
- 34.De Greeff A, Ghosh D, Anthony J, Shennan A. Accuracy assessment of the Dinamap ProCare 400 in pregnancy and preeclampsia. Hypertens Pregnancy. 2010;29:198–205. doi: 10.3109/10641950902968650. [DOI] [PubMed] [Google Scholar]
- 35.de Greeff A, Shennan AH. Clinical accuracy of a low cost portable blood pressure device in pregnancy and pre-eclampsia: the Nissei DS-400. Trop Doct. 2015;45:168–173. doi: 10.1177/0049475515581542. [DOI] [PubMed] [Google Scholar]
- 36.Dorogova IV, Panina ES. Comparison of the BPLab(R) sphygmomanometer for ambulatory blood pressure monitoring with mercury sphygmomanometry in pregnant women: validation study according to the British Hypertension Society protocol. Vascular Health Risk Manag. 2015;11:245–249. doi: 10.2147/VHRM.S82381. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 37.Elvan-Taspinar A, Uiterkamp LA, Sikkema JM, Bots ML, Koomans HA, Bruinse HW, Franx A. Validation and use of the Finometer for blood pressure measurement in normal, hypertensive and pre-eclamptic pregnancy. J Hypertens. 2003;21:2053–2060. doi: 10.1097/00004872-200311000-00014. [DOI] [PubMed] [Google Scholar]
- 38.Franx A, van der Post JA, Elfering IM, Veerman DP, Merkus HM, Boer K, van Montfrans GA. Validation of automated blood pressure recording in pregnancy. Br J Obstet Gynaecol. 1994;101:66–69. doi: 10.1111/j.1471-0528.1994.tb13013.x. [DOI] [PubMed] [Google Scholar]
- 39.Franx A, van der Post JA, van Montfrans GA, Bruines HW. Comparison of an Auscultatory versus an Oscillometric Ambulatory Blood Pressure Monitor in Normotensive, Hypertensive, and Preeclamptic Pregnancy. Hypertens Pregnancy. 1997;16:187–202. [Google Scholar]
- 40.Golara M, Benedict A, Jones C, Randhawa M, Poston L, Shennan AH. Inflationary oscillometry provides accurate measurement of blood pressure in pre-eclampsia. BJOG. 2002;109:1143–1147. doi: 10.1111/j.1471-0528.2002.01487.x. [DOI] [PubMed] [Google Scholar]
- 41.Green LA, Froman RD. Blood pressure measurement during pregnancy: auscultatory versus oscillatory methods. J Obstet Gynecol Neonatal Nurs. 1996;25:155–159. doi: 10.1111/j.1552-6909.1996.tb02419.x. [DOI] [PubMed] [Google Scholar]
- 42.Gupta M, Shennan AH, Halligan A, Taylor DJ, de Swiet M. Accuracy of oscillometric blood pressure monitoring in pregnancy and pre-eclampsia. Br J Obstet Gynaecol. 1997;104:350–355. doi: 10.1111/j.1471-0528.1997.tb11467.x. [DOI] [PubMed] [Google Scholar]
- 43.Hasan MA, Thomas TA, Prys-Roberts C. Comparison of automatic oscillometric arterial pressure measurement with conventional auscultatory measurement in the labour ward. Br J Anaesth. 1993;70:141–144. doi: 10.1093/bja/70.2.141. [DOI] [PubMed] [Google Scholar]
- 44.Hay A, Ayis S, Nzelu D, James L, Kametas NA. Validation of the Withings BP-800 in pregnancy and impact of maternal characteristics on the accuracy of blood pressure measurement. Pregnancy Hypertens. 2016;6:406–412. doi: 10.1016/j.preghy.2016.09.005. [DOI] [PubMed] [Google Scholar]
- 45.James L, Nzelu D, Hay A, Shennan A, Kametas NA. Validation of the Omron MIT Elite blood pressure device in a pregnant population with large arm circumference. Blood Pressure Monit. 2017;22:109–111. doi: 10.1097/MBP.0000000000000239. [DOI] [PubMed] [Google Scholar]
- 46.Koenen SV, Franx A, Oosting H, Bonsel GJ, Bruinse HW, Visser HA. Within-subject variability of differences between conventional and automated blood pressure measurements in pregnancy. Eur J Obstet Gynecol Reprod Biol. 1998;80:79–84. doi: 10.1016/s0301-2115(98)00096-7. [DOI] [PubMed] [Google Scholar]
- 47.Kwek K, Chan YG, Tan KH, Yeo GS. Validation of an oscillometric electronic sphygmomanometer in an obstetric population. Am J Hypertens. 1998;11:978–982. doi: 10.1016/s0895-7061(98)00093-4. [DOI] [PubMed] [Google Scholar]
- 48.Lan PG, Clayton PA, Hyett J, Gillin AG. Measuring blood pressure in pregnancy and postpartum: assessing the reliability of automated measuring devices. Hypertens Pregnancy. 2014;33:168–176. doi: 10.3109/10641955.2013.843007. [DOI] [PubMed] [Google Scholar]
- 49.Lo C, Taylor RS, Gamble G, McCowan L, North RA. Use of automated home blood pressure monitoring in pregnancy: is it safe? Am J Obstet Gynecol. 2002;187:1321–1328. doi: 10.1067/mob.2002.126847. [DOI] [PubMed] [Google Scholar]
- 50.Marx GF, Sofair DR, Winikoff SI. Blood pressure values in parturients: auscultatory versus oscillatory values. Anesth Analg. 1991;72:562–563. doi: 10.1213/00000539-199104000-00030. [DOI] [PubMed] [Google Scholar]
- 51.Marx GF, Schwalbe SS, Cho E, Whitty JE. Automated blood pressure measurements in laboring women: are they reliable? Am J Obstet Gynecol. 1993;168:796–798. doi: 10.1016/s0002-9378(12)90822-4. [DOI] [PubMed] [Google Scholar]
- 52.Montan S, Choolani M, Arulkumaran S, Ratnam SS. Automated 24-hour ambulatory blood pressure monitoring in preeclampsia. J Perinat Med. 1995;23:353–358. doi: 10.1515/jpme.1995.23.5.353. [DOI] [PubMed] [Google Scholar]
- 53.Natarajan P, Shennan AH, Penny J, Halligan AW, de Swiet M, Anthony J. Comparison of auscultatory and oscillometric automated blood pressure monitors in the setting of preeclampsia. Am J Obstet Gynecol. 1999;181:1203–1210. doi: 10.1016/s0002-9378(99)70109-2. [DOI] [PubMed] [Google Scholar]
- 54.Nathan HL, de Greeff A, Hezelgrave NL, Chappell LC, Shennan AH. An accurate semiautomated oscillometric blood pressure device for use in pregnancy (including pre-eclampsia) in a low-income and middle-income country population: the Microlife 3AS1-2. Blood Pres Monit. 2015;20:52–55. doi: 10.1097/MBP.0000000000000086. [DOI] [PubMed] [Google Scholar]
- 55.Nathan HL, de Greeff A, Hezelgrave NL, Chappell LC, Shennan AH. Accuracy validation of the Microlife 3AS1-2 blood pressure device in a pregnant population with low blood pressure. Blood Press Monit. 2015;20:299–302. doi: 10.1097/MBP.0000000000000134. [DOI] [PubMed] [Google Scholar]
- 56.Nouwen E, Snijder M, van Montfrans G, Wolf H. Validation of the Omron M7 and Microlife 3BTO-A blood pressure measuring devices in preeclampsia. Hypertens Pregnancy. 2012;31:131–139. doi: 10.3109/10641955.2010.544799. [DOI] [PubMed] [Google Scholar]
- 57.Nzelu D, Yeung F, Couderq D, Shennan A, Kametas NA. An inaccurate automated device negatively impacts the diagnosis and treatment of gestational hypertension. Pregnancy Hypertens. 2017;22:109–111. doi: 10.1016/j.preghy.2017.05.001. [DOI] [PubMed] [Google Scholar]
- 58.O'Brien E, Mee F, Atkins N, Halligan A, O'Malley K. Accuracy of the SpaceLabs 90207 ambulatory blood pressure measuring system in normotensive pregnant women determined by the British Hypertension Society protocol. J Hypertens Suppl. 1993;11:S282–S283. [PubMed] [Google Scholar]
- 59.Penny JA, Shennan AH, Rushbrook J, Halligan AW, Taylor DJ, De Sweit M. Validation of the Welch Allyn QuietTrak ambulatory blood pressure monitor in pregnancy. Hypertens Pregnancy. 1996;15:313–321. [Google Scholar]
- 60.Pomini F, Scavo M, Ferrazzani S, De Carolis S, Caruso A, Mancuso S. There is poor agreement between manual auscultatory and automated oscillometric methods for the measurement of blood pressure in normotensive pregnant women. J Matern Fetal Med. 2001;10:398–403. doi: 10.1080/714052781. [DOI] [PubMed] [Google Scholar]
- 61.Quinn M. Automated blood pressure measurement devices: a potential source of morbidity in preeclampsia? Am J Obstet Gynecol. 1994;170:1303–1307. doi: 10.1016/s0002-9378(94)70146-6. [DOI] [PubMed] [Google Scholar]
- 62.Reinders A, Cuckson AC, Jones CR, Poet R, O'Sullivan G, Shennan AH. Validation of the Welch Allyn 'Vital Signs' blood pressure measurement device in pregnancy and pre-eclampsia. BJOG. 2003;110:134–138. [PubMed] [Google Scholar]
- 63.Reinders A, Cuckson AC, Lee JT, Shennan AH. An accurate automated blood pressure device for use in pregnancy and pre-eclampsia: the Microlife 3BTO-A. BJOG. 2005;112:915–20. doi: 10.1111/j.1471-0528.2005.00617.x. [DOI] [PubMed] [Google Scholar]
- 64.Shennan AH, Kissane J, de Swiet M. Validation of the SpaceLabs 90207 ambulatory blood pressure monitor for use in pregnancy. Br J Obstet Gynaecol. 1993;100:904–908. doi: 10.1111/j.1471-0528.1993.tb15104.x. [DOI] [PubMed] [Google Scholar]
- 65.Shennan A, Halligan A, Gupta M, Taylor D, de Swiet M. Oscillometric blood pressure measurements in severe pre-eclampsia: validation of the SpaceLabs 90207. Br J Obstet Gynaecol. 1996;103:171–173. doi: 10.1111/j.1471-0528.1996.tb09671.x. [DOI] [PubMed] [Google Scholar]
- 66.Tape TG, Rayburn WF, Bremer KD, Schnoor TA. Ambulatory blood pressure monitoring during pregnancy with a new, small, easily concealed monitor. J Reprod Med. 1994;39:968–972. [PubMed] [Google Scholar]
- 67.Shennan AH, Halligan AW. Measuring blood pressure in normal and hypertensive pregnancy. Best Pract Res Clin Obstet Gynaecol. 1999;13:1–26. doi: 10.1053/beog.1999.0003. [DOI] [PubMed] [Google Scholar]
- 68.Nathan HL, Duhig K, Hezelgrave NL, Chappell LC, Shennan AH. Blood pressure measurement in pregnancy. Obstet Gynaecol. 2015;17:91–8. [Google Scholar]
- 69.Tremonti C, Beddoe J, Brown MA. Reliability of Home Blood Pressure Monitoring Devices in Pregnancy. Pregnancy Hypertens. 2017;8:9–14. doi: 10.1016/j.preghy.2017.01.002. [DOI] [PubMed] [Google Scholar]
- 70.Bibbins-Domingo K, Grossman DC, Curry SJ, Barry MJ, Davidson KW, Doubeni CA, Epling JW, Jr, Kemper AR, Krist AH, Kurth AE, Landefeld CS, Mangione CM, Phillips WR, Phipps MG, Silverstein M, Simon MA, Tseng CW. Screening for Preeclampsia: US Preventive Services Task Force Recommendation Statement. JAMA. 2017;317:1661–1667. doi: 10.1001/jama.2017.3439. [DOI] [PubMed] [Google Scholar]
- 71.Henderson JT, Thompson JH, Burda BU, Cantor A. Preeclampsia Screening: Evidence Report and Systematic Review for the US Preventive Services Task Force. JAMA. 2017;317:1668–1683. doi: 10.1001/jama.2016.18315. [DOI] [PubMed] [Google Scholar]
- 72.Al Hamarneh YN, Houle SK, Chatterley P, Tsuyuki RT. The validity of blood pressure kiosk validation studies: a systematic review. Blood Press Monit. 2013;18:167–172. doi: 10.1097/MBP.0b013e328360fb85. [DOI] [PubMed] [Google Scholar]
- 73.Wan Y, Heneghan C, Stevens R, McManus RJ, Ward A, Perera R, Thompson M, Tarassenko L, Mant D. Determining which automatic digital blood pressure device performs adequately: a systematic review. J Hum Hypertens. 2010;24:431–438. doi: 10.1038/jhh.2010.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.O'Brien E, Stergiou GS. The pursuit of accurate blood pressure measurement: A 35-year travail. J Clin Hypertens (Greenwich, Conn) 2017;19:746–752. doi: 10.1111/jch.13005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.