Abstract
Inflammation in adipose tissue is recognized as a causative factor in the development of type II diabetes. Adipocyte hypertrophy as well as bacterial and environmental factors have been implicated in causing inflammation in mature adipocytes. Exposure to persistent organic pollutants such as polychlorinated biphenyls (PCBs) has been associated with the development of type II diabetes. We show here that the PCB126, a dioxin-like PCB, activates a robust proinflammatory state in fat cell precursors (preadipocytes). The response was found to be dependent on aryl hydrocarbon receptor (AhR) activation, although induction of the response was delayed compared to upregulation of CYP1A1, a classic AhR responsive gene. Treatment of preadipocytes with an NF-κB inhibitor partially attenuated the PCB126 induced inflammatory response and partly, but not completely, ameliorated disruption of adipogenesis caused by PCB126. Our results indicate a role for PCB126 in mediating an inflammatory response through AhR in preadipocytes that interferes with adipogenesis.
Keywords: Adipocytes, Preadipocytes, Fat, PCB, Inflammation, Diabetes, AhR
INTRODUCTION
The incidence of type II diabetes has been increasing in the past several decades (Patel et al. 2013a). While a large part of this rise is associated with diet and lack of exercise, a number of studies indicate that exposure to persistent organic pollutants (POPs) is associated with the development of diabetes (Ruzzin et al. 2010, Hectors et al. 2013, Lee et al. 2014). These compounds are ubiquitous in the environment and many build up in fat tissue. The mechanisms by which POPs cause diabetes are not completely clear.
Polychlorinated biphenyls (PCBs) were originally manufactured for numerous industrial applications and were useful for their insulating and flame retardant properties as well as providing a base for many paints and dyes (Beyer et al. 2009). While PCB production was discontinued in the late 1970s, inadvertent production still occurs and, importantly, many PCBs have accumulated in the environment because of their extreme stability. Contamination is prevalent not only in regions where they were manufactured but also in old buildings, particularly in schools (Herrick et al. 2011, Ampleman et al. 2015). PCB mixtures contain up to 209 different congeners, many with very different properties. Toxic effects of PCBs can vary widely depending on chlorination patterns of the congeners. PCB126 is dioxin-like and is known to bind to and activate the aryl hydrocarbon receptor (AhR)(Chen et al. 2004). Recent epidemiologic studies link PCB exposure to the development of insulin resistance and diabetes (Everett et al. 2011, Lee et al. 2011, Persky et al. 2011, Everett et al. 2012, Kim et al. 2014). Higher serum levels of PCB126 are associated with insulin resistance (Everett et al. 2012). The mechanisms by which PCBs may cause diabetes are unclear. For dioxin-like PCBs, a role for AhR activation seems likely.
AhR is a ligand-activated transcriptional regulator that responds to many different endogenous and exogenous factors (Labrecque et al. 2013). When activated, AhR translocates to the nucleus and binds to ARNT (aryl hydrocarbon receptor nuclear translocator). AhR/ARNT binds to xenobiotic response elements (XREs) in gene promoters to regulate transcription of genes such as CYP1A1/2 (cytochrome p450, family 1, subfamily A, polypeptides 1 and 2). While many genes are transcriptionally activated by AhR/ARNT, recent expression data and ChIP (chromatin immunoprecipitation) analyses indicates that expression of a significant subset of genes is repressed by AhR/ARNT (Sartor et al. 2009, Lo et al. 2012).
Adipocytes are emerging as key players in the development of type II diabetes (Guilherme et al. 2008, Mlinar et al. 2011, Patel et al. 2013b, Rosen et al. 2014). These cells do much more than just storing fat, and “healthy” adipocytes are critical for normal metabolism. Mature white adipocytes secrete numerous hormones including leptin and adiponectin. Dysfunctional adipocytes secrete reduced levels of hormones such as adiponectin and become proinflammatory (Suzawa et al. 2003, Xu et al. 2003, Dunmore et al. 2013, Ye et al. 2013, Fisman et al. 2014). This can lead to release of free fatty acids (FFAs) and glycerol, which can affect other tissues to blunt insulin signaling and glucose uptake. In addition, increased FFAs can cause fat accumulation in other tissues including the liver (i.e. steatosis) (Wree et al. 2011). Long-term increases in FFAs can also inhibit insulin production and secretion from pancreatic beta-cells (Tanabe et al. 2011, Prentki et al. 2012). Because of normal cell death processes (apoptosis, hypertrophy, etc.), approximately 10% of adipocytes in the human body are replaced every year (Tchkonia et al. 2010). This replacement relies on adipocyte precursor cells called preadipocytes, which originate from mesenchymal stem cells (MSCs). Because adipocytes need to be replaced, disruption of adipogenesis can also therefore lead to dysfunctional adipose tissue.
Previous studies have demonstrated that AhR agonists can disrupt adipogenesis (Phillips et al. 1995, Brodie et al. 1996a, Alexander et al. 1998, Olsen et al. 1998, Li et al. 2008b, Hsu et al. 2010, Kim et al. 2012). Early work focused on the role of 2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD), also referred to as dioxin, in activating AhR and causing inhibition of adipogenesis in the mouse adipocyte precursor cells (Phillips et al. 1995, Brodie et al. 1996a, Alexander et al. 1998, Olsen et al. 1998). AhR activation by dioxin was found to be associated with downregulation of PPARγ (peroxisome proliferator-activated receptor gamma), the key transcriptional activator of adipogenesis (Brodie et al. 1996b, Liu et al. 1996, Alexander et al. 1998, Cho et al. 2004). The mechanism by which AhR activation by dioxin inhibits PPARγ and adipogenesis is not completely clear, although activation of the ERK (extracellular signal-regulated kinase) pathway has been implicated (Hanlon et al. 2003, Cimafranca et al. 2004, Hanlon et al. 2005). It has also been demonstrated that dioxin causes a proinflammatory response that is associated with inhibition of adipogenesis in mouse adipocytes (Kern et al. 2002, Li et al. 2008a). Using adipose derived human stem cells, it was also shown that the AhR agonists such as dioxin and PCB126 activate a proinflammatory response and that is associated with inhibition of adipocyte differentiation (Li et al. 2008b, Kim et al. 2012). In apparent contradiction to these findings, another study demonstrated that the AhR agonist PCB77 induces a proinflammatory response but instead of inhibiting adipogenesis and increase in adipocyte differentiation was observed (Arsenescu et al. 2008). Further studies are needed to help define and characterize the apparent link between AhR agonists, inflammation, and adipogenesis.
In our previous research using human preadipocytes, we demonstrated that PCB126 inhibited adipogenesis (Gadupudi et al. 2015). Given that AhR agonists have been associated with a proinflammatory response in adipocytes and that adipose tissue inflammation is now considered to be an important event in the development of metabolic disruption, we wanted to further explore the nature and role of a proinflammatory response in the inhibition of adipogenesis by PCB126. We show that PCB126 causes a robust proinflammatory response in preadipocytes, much more so than in mature adipocytes. This proinflammatory response was found to be dependent on AhR but was significantly delayed compared to a classical AhR response or LPS-induced inflammation. An inhibitor of NF-κB (nuclear factor kappa-light-chain-enhancer of activated B cells) partially protected preadipocytes from the effects of PCB126. These studies, using human cells, point to a potential mechanism by which PCB126 can disrupt metabolism through its effects on adipose tissue.
MATERIALS AND METHODS
Cell Culture and Treatments
The immortal human preadipocyte subcutaneous NPAD-B (normal preadipocyte-clone B) line was derived from subcutaneous preadipocytes from a non-diabetic donor (Vu et al. 2013), and its use in various studies has been described previously (Gadupudi et al. 2015, Littlejohn et al. 2016, Zhang et al. 2017). This human cell line is stable and can be consistently differentiated to mature and functional adipocytes that accumulate lipid droplets. Cells were cultured and passaged routinely as preadipocytes in preadipocyte growth media, PGM2 (Lonza), a proprietary rich media with high buffering capacity supplemented with glutamine (2mM), and 10% FBS, along with gentamycin (30 ug/ml) and fungizone (15 ng/ml) according to the manufacturer’s recommendations (Lonza).
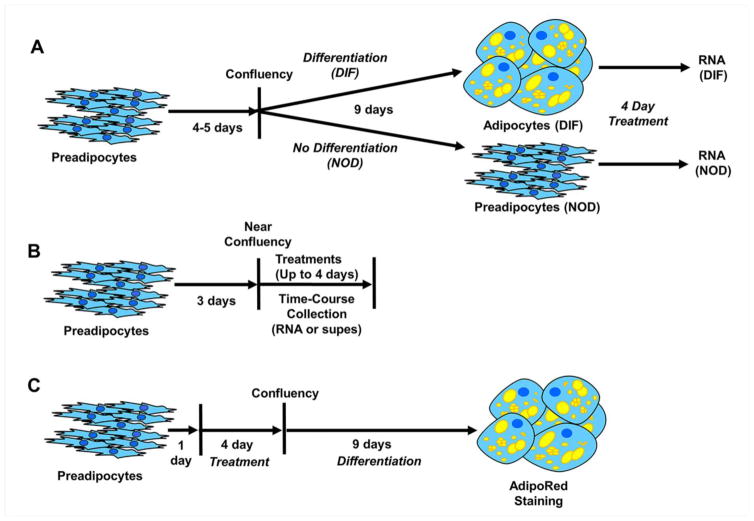
Depending on what parameters were being assessed, preadipocytes and/or adipocytes were subjected to different treatment regimens. Diagrams to explain the different treatments are provided in figure 1 and are also referred to in the Results section when describing the results of the experiments. For PCB126 treatments, only doses that did not result in cytotoxicity (0.5 to 10 uM), as measured in our previous study (Gadupudi et al. 2015), were utilized, with a dose of 10 uM used to maximize effects in the time-course experiments. All doses, including zero, were administered in the same amount of DMSO (usually 0.1% v/v).
Fig. 1.
Schematic diagrams of different treatment protocols used in this study. A. Exposure of differentiated (DIF) or non-differentiated (NOD) cells to PCB126 or vehicle. Preadipocytes were grown to confluence and then subjected to differentiating agents or regular media as described in the Materials and Methods. DIF or NOD cells were treated for 4 days before collection of RNA for Q-RT-PCR. B. Exposure of preadipocytes to treatments to assess effects on proinflammatory response. Preadipocytes were grown to near confluence and subjected to treatments followed by collection of RNA and/or supernatants at different time points or at the end of 4 days treatment. C. Testing effects on adipogenesis. Preadipocytes were plated and treated the day after with test compounds or vehicle until confluent (4 days) at which time differentiation media was added. After 9 days differentiation, cultures were stained with AdipoRed, a lipid dye.
To compare the proinflammatory response between nondifferentiated (NOD) and differentiated (DIF) cells (see diagram, figure 1a), cells were plated in 35mm dishes at 80,000 cells per dish in PGM2 media and allowed to grow to confluency over 4 days. Cells were then media changed with PDM2 (preadipocyte differentiation media) or nondifferenting media, PGM2. PDM2 (Lonza) is PGM2 media with added differentiation components from a Bullet Kit that consists of dexamethasone (final concentration of 1 uM, IBMX (final concentration of 0.5 mM), insulin (final concentration of 15 ug/ml), and indomethacin (final concentration of 0.2 mM), all according to the manufacturer’s instructions. For differentiation, cells were kept in PDM2 media for 9 days as recommended by Lonza and as described previously (Gadupudi et al. 2015). The nondifferentiated cells were maintained in PGM2. Media in the NOD and DIF cultures was replaced on day 5 with PGM2 or PDM2, respectively. At the end of 9 days, the cells were media changed with PGM2 (for consistency) and treated with PCB126 or vehicle (DMSO) for 4 days before cells were collected for RNA. Four days of treatment was used because pilot studies indicated that this amount of time gave maximum upregulation of proinflammatory genes in preadipocytes (data not shown).
For time course experiments using different agents (PCB126, LPS, Bay11-7082) on preadipocytes (see diagram, figure 1b), cells were plated in 35 mm dishes at 80,000 cells/dish in PGM2 and allowed to become 90–100% confluent (3 days growth), as visually judged by microscopy, before treatments. This consistent level of confluency was used to limit any potential effects on growth and to obtain reproducible results. Supernatants and RNA were collected at different time points up to 4 days.
To assess the effects of different treatments on adipogenesis, we use a protocol (see diagram, figure 1c) that has been described previously for PCB126 treatments (Gadupudi et al. 2015). Cells were plated at 80,000 cells/well in 6-well plates and treated with test compound or vehicle the next day. Treatments were removed at the time of addition of differentiation media (4 days after treatment). Differentiation media (PDM2) was kept on cells for 9–10 days after which lipid accumulation was assessed by AdipoRed staining (see below).
To assess how the NF-κB inhibitor, Bay-11-7082, affected the proinflammatory response and inhibition of adipogenesis by PCB126, experiments were performed using a dose of Bay-11 (4 uM) that was determined to not significantly inhibit the growth (as assessed by Trypan Blue staining and cell counting) of preadipocytes over a 4 day treatment period (data not shown). For these experiments, a PCB126 dose of 2 uM was used since this was a dose that was known from previous studies to cause significant inhibition of adipogenesis and it was reasoned that any effects of Bay-11-7082 would be more apparent at a dose of PCB126 lower than the high dose of 10 uM. Experiments to assess effects on adipogenesis were done as described above for and as shown in figure 1c. To test how Bay-11 inhibited a proinflammatory response in preadipocytes by PCB126, cells were treated and collected similar to was described above for the time-course experiments but only at the day 4 time point (see diagram, figure 1b).
Knockdown by shRNA
ShRNA were designed using the method described by Wang X et al (Wang et al. 2009). ShRNA duplex targeting AhR sequence (sense GAATGGAGTTTTAAATGAA) and ShSCR control (sense TGGTTTACATGTCGACTAA) were cloned into a pSUPER-puro vector (OligoEngine) according to the manufacturer’s manual. Replication defective retroviruses were generated using the Phoenix packaging cell line and target preadipocytes were transduced with virus and selected in puromycin at 1 ug/ml as described (Westin et al. 2011).
Quantitative RT-PCR
Preadipocyte were homogenized in 1ml of TRIzol Reagent (Invitrogen). Total RNA from the aqueous phase was further purified using RNeasy Column (Qiagen). cDNA was synthesized using MMLV Reverse Transcriptase and random Decamers (Invitrogen).
Quantitative PCR using SYBR-green master mix (Applied Biosystems) were run on ABI-PRISM sequence Detection System (model 7900HT). After verifying its suitability, GAPDH (glyceraldehyde 3 phosphate dehydrogenase) was used to normalize gene expression. Primer pairs that were used are presented in Supplementary Table 1. Fold changes over untreated controls were calculated using the 2−DDCt method as described (Livak et al. 2001).
ELISAs
Supernatants were collected from treated cells and analyzed for IL-6 or IL-8 by ELISA using kits from R&D Systems. ELISAs were performed according to the manufacturer’s instructions. A standard curve was run with each ELISA for an estimation of concentration. Graphed values represent a ratio of the concentration detected in the treated samples over the vehicle controls.
Microscopy
Differentiated cultures from various treatment groups were visualized by microscopy for lipid droplet formation. The cells were fixed with 10% formalin and stained with AdipoRed™ (Lonza, MD) for 15 min. as described by the manufacturer. AdipoRed™ is a fluorescent dye that binds to triglycerides. The wavelength with highest emission is 572 nm, which is in the green part of the spectrum (thus green color instead of red). The photomicrographs of the stained cells were taken using a fluorescent microscope (Nikon) at 200X magnification. The images post-acquisition were processed and quantified using the Image J software package. Four replicate images were used to quantify staining.
Statistical Analyses
Our statistical analyses fall under the generalized linear mixed modeling (GLMM) framework. Univariate, bivariate, or bivariate interaction models were fit in order to accurately obtain estimates for the desired comparisons between factor levels. For outcome variables that followed a right-skewed distribution (Figures 2–5), we used a log link function so that the modeling assumptions are appropriately satisfied by the data. All other outcomes (Figure 6) used an identity link. For each outcome, multiple comparisons between factor levels were estimated. We obtained corresponding p-values and used them to determine statistical significance by comparing to the cutoff value α = 0.05/n, where n is the number of comparisons made within a fitted model. This is often referred to as the Bonferroni correction and, accordingly, p<0.01 is considered significant. All estimates and p-values were generated using SAS 9.4. Results with error bars (standard error of the mean) were graphed using GraphPad Prism. Statistical significance in graphs is designated by the conventional use of asterisks where **=p<0.01; ***=p<0.001.
RESULTS
PCB126 Induces a Proinflammatory Response in Preadipocytes
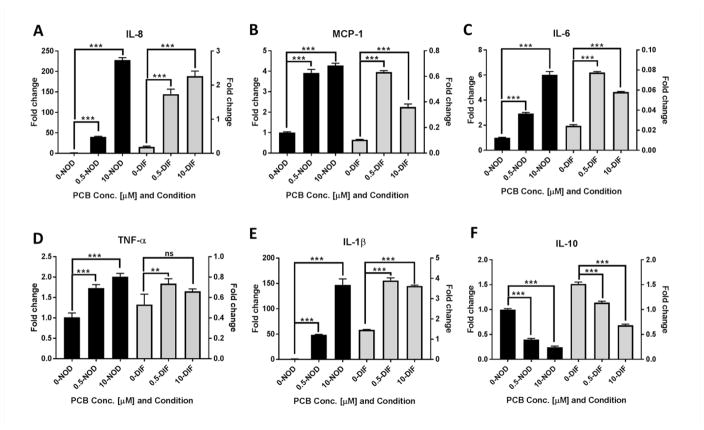
A number of studies have linked inflammation with improper adipogenesis (Suzawa et al. 2003, Fuentes et al. 2013). Furthermore PCB126 exposure has been associated with induction of proinflammatory genes (Hennig et al. 2002, Imbeault et al. 2012, Kim et al. 2012). Our previous study demonstrated that PCB126 treatment of preadipocytes activated AhR, causing induction of AhR-responsive genes such as CYP1A1, and concomitantly inhibited their ability to properly differentiate into mature adipocytes (Gadupudi et al. 2015). In initial experiments, we wanted to assess how nondifferentiated (NOD) preadipocytes and differentiated (DIF) mature adipocytes responded to PCB126 with regard to induction of proinflammatory cytokines. Preadipocytes were kept in nondifferentiating or differentiating conditions as described in the Materials and Methods and as diagrammed in figure 1a. NOD or DIF cells were treated with doses of PCB126 at 0, 0.5 and 10 μM and a time period of 4 days, a regimen that was previously found to be non-cytotoxic (Gadupudi et al. 2015). Quantitative RT-PCR assays using RNA collected after 4 days indicated that levels of the proinflammatory cytokines IL-8 (interleukin-8), MCP-1 (monocyte chemoattractant protein-1), IL-6 (interleukin-6), TNFα (tumor necrosis factor alpha), and IL1-β (interleukin-1 beta) were significantly induced by PCB126 in NOD cultures, much more so that the same treatment of DIF cultures (figure 2a–e). Transcript levels of the anti-inflammatory cytokine IL-10 (interleukin-10), on the other hand, were significantly reduced in both NOD and DIF cultures (figure 2f). These results suggest that undifferentiated preadipocytes are sensitive to the proinflammatory effects of PCB126 after four days treatment, more so than mature adipocytes.
Fig. 2.
PCB126 induces a proinflammatory response in human preadipocytes. Non-differentiated (NOD) preadipocytes (black bars) or differentiated (DIF) adipocytes (gray bars) were treated with 0, 0.5, or 10 μM PCB126 for 4 days and RNA was isolated (see diagram, figure 1a). Transcript levels of different cytokines were assessed by quantitative RT-PCR. All values represent the fold change (calculated as described in the Materials and Methods) compared to vehicle-treated NOD preadipocytes. Three assay replicates were performed and averaged. To more completely show the data and to demonstrate the differences between NOD and DIF, the left-hand y-axis indicates the calculated fold-change for NOD and the right-hand axis indicates the fold change for DIF. (A–E) inflammatory cytokines IL-8, MCP-1, IL-6, TNFα, and IL-1β. (F) Anti-inflammatory cytokine IL-10. Statistics comparing PCB-treated to vehicle-treated were performed as described in the Materials and Methods. **=p<0.01; ***=p<0.001; ns=non-significant.
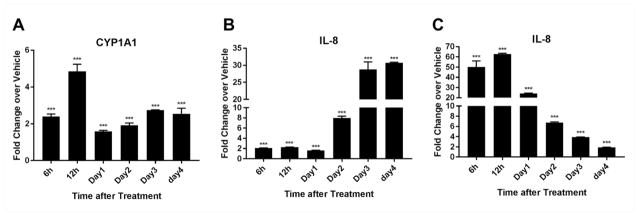
To begin to characterize the nature of the of the proinflammatory response induced by PCB126, specifically in preadipocytes, a time-course of induction was performed preadipocytes (see diagram, figure 1b). We focused on IL-8 as a surrogate marker of inflammation since it was the cytokine gene that exhibited the highest upregulation with PCB126 treatment and because its upregulation has been associated with adipose tissue inflammation (Chung et al. 2006, Hoch et al. 2008, Meijer et al. 2011, Vu et al. 2013). At the same time, we assessed levels of CYP1A1 as a measurement of AhR activation. A high PCB126 dose of 10 μM was used with the idea of generating a strong response, also knowing that this dose does not cause cytotoxicity (Gadupudi et al. 2015). CYP1A1 induction was observed to be induced within 6–12 hours treatment of preadipocytes with PCB126 (figure 3a). This tapered off somewhat at day 1 post-treatment and began to rise again at later time points, a response that is similar to what has been reported previously for AhR agonists such as dioxin (Kim et al. 2009). Increases in levels of IL-8 transcript occurred at significant but lower levels at early time points but did not begin to increase dramatically until day 2, with maximal levels at days 3 and 4 (figure 3b). Previous studies have shown that our NPAD-B preadipocyte cell line responds normally to bacterial toxins such as LPS (lipopolysaccharide) with regard to induction of proinflammatory cytokines such as IL-8 (Vu et al. 2013, Vu et al. 2015). To verify these results and to determine when IL-8 transcript was maximally induced, we treated the preadipocytes with LPS and followed expression of IL-8 with time (figure 3c). As expected, LPS caused a rapid induction of IL-8 transcript levels within hours, indicating that the cells have a normal response to this classic proinflammatory agent. Overall, these results indicate that a robust proinflammatory response to PCB126 is delayed compared to LPS and does not occur until day 2 post-treatment.
Fig. 3.
Time-course of IL-8 and CYP1A1 induction in preadipocytes. Preadipocytes at near confluency were treated with PCB126 and RNA was collected over a time-course of 6 hours to 4 days (see diagram, figure 1b). Transcript levels of (A) CYP1A1 and (B) IL-8 were assessed by quantitative RT-PCR. (C) Transcript levels of IL-8 in LPS treated (5 ng/ml) preadipocytes over the same time course as in A and B. Values represent the fold-change over vehicle-treated control (calculated as described in the Materials and Methods). Three assay replicates were averaged. Statistics comparing PCB-treated over vehicle treated were performed as described in the Materials and Methods. **=p<0.01; ***=p<0.001.
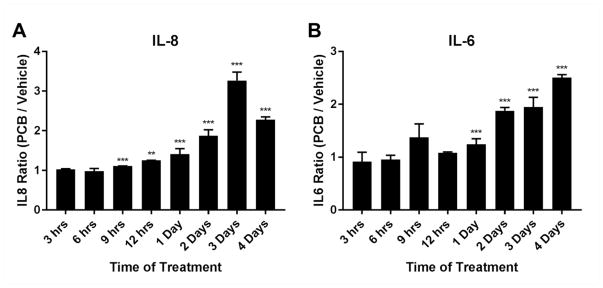
To determine whether inflammatory cytokine secretion levels followed a pattern that was similar to transcript levels, we performed ELISAs for IL-6 and IL-8 from supernatants derived from the PCB126 time-course treatments. Although not identical to RNA transcript levels, both IL-6 and IL-8 were detected at the highest levels in media from PCB126 treated cells at the later time points (days 2–4) as compared to non-treated controls (figure 4).
Fig. 4.
Secretion of proinflammatory cytokines induced by PCB126. PCB126 (10 uM) or vehicle control were applied to confluent cells and supernatants were collected at different time points (see diagram, figure 1b). ELISAs for (A) IL-8 and (B) IL-6 were performed using commercially available kits with standards (see Material and Methods). Values represent the ratio of PCB126-treated cells over vehicle controls at the same time points. Two biological replicates were performed and averaged. Statistics comparing PCB-treated over vehicle-treated were performed as described in the Materials and Methods. **=p<0.01; ***=p<0.001.
Inhibition of Adipogenesis is Dependent on AhR
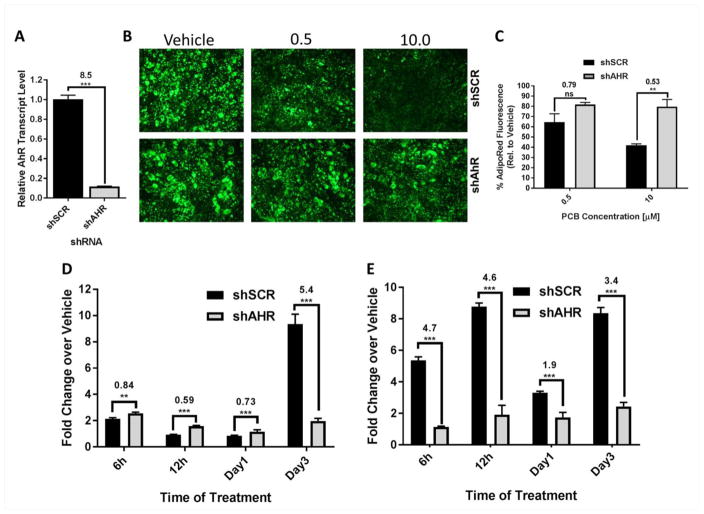
Because we observed a potential disconnect between the timing of induction of CYP1A1 and IL-8 levels in PCB126 treated cells, we wanted to determine whether the proinflammatory response was dependent on AhR. To test this, we performed shRNA mediated knockdown of AhR utilizing a recombinant retroviral vector. Stable knockdown of AhR of approximately 90% was achieved as compared to cells expressing a scrambled (shSCR) control (figure 5a). Using a treatment protocol of preadipocytes that we previously reported (Gadupudi et al. 2015)(see diagram, figure 1c), we tested whether knockdown of AhR blocked the inhibitory effects of PCB126 (0.5 and 10 uM) on adipogenesis. We found that AhR knockdown partially prevented PCB126 from inhibiting adipogenesis (statistical significance for 10 uM treatments), as demonstrated by staining with AdipoRed, a stain that preferentially marks lipid droplets (figures 5b and 5c). To determine whether AhR was involved in PCB126 induced inflammation in preadipocytes, we treated shAhR and shSCR preadipocytes with PCB126 (10 uM) over a time course (see diagram, figure 1b). While AhR knockdown preadipocytes were not completely refractory to PCB126-mediated induction of IL-8 at early time points compared to shSCR cells, they exhibited significantly less IL-8 induction at the later day 3 time point (figure 5d) indicating that this response was dependent on AhR. As expected, AhR knockdown also inhibited activation of CYP1A1by PCB126 treatment as compared to shSCR control cells (figure 5e).
Fig. 5.
AhR knockdown cells are refractory to PCB126 induced inhibition of adipogenesis and IL-8 induction. (A) Quantitative RT-PCR demonstrated that AhR was knocked down to by shRNA (shAhR) to a level that was ~90% that of scrambled control (shSCR). The calculated fold change compares transcript levels of shSCR to shAhR cells. (B) Preadipocytes expressing scrambled control shSCR or shAhR were treated with different doses of PCB126 and cells were differentiated (see diagram, figure 1c) and stained with AdipoRed. (C) AdipoRed fluorescence was quantified in four different fields using ImageJ software. Percentage is relative to vehicle-treated cells. (D) Preadipocytes were treated with PCB126 at 0, 0.5 or 10 uM (see diagram, figure 1b) and quantitative RT-PCR was used to assess IL-8 transcript levels in PCB126 treated preadipocytes over a time course. Values represent fold changes over vehicle controls. Three assay replicates were performed and averaged. (E) Quantitative RT-PCR of CYP1A1 transcript levels in the same samples as in “D”. Statistics comparing shSCR versus shAhR were performed as described in the Materials and Methods. Values over statistics bars represent the fold-difference of shSCR over shAhR. **=p<0.01; ***=p<0.001.
NF-κB Inhibitor Dampens Effects of PCB126 on Adipogenesis
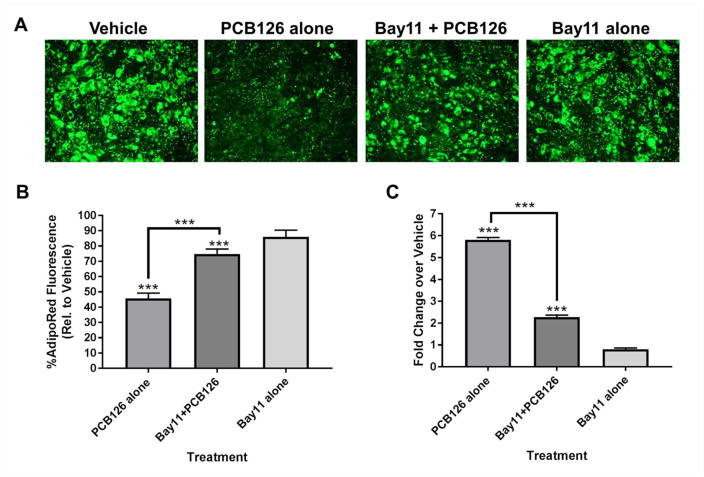
After finding that AhR was necessary for the proinflammatory response, we wanted to determine whether the proinflammatory response was responsible for inhibiting the ability of the PCB126 treated preadipocytes to properly differentiate. To assess this, we used a well-known NF-κB inhibitor called Bay11-7082 (Mori et al. 2002). Preadipocytes were treated with Bay11-7082 and PCB126 concomitantly for a 4 day period followed by removal of the treatments and addition of differentiation media (see diagram, figure 1c). Interestingly, Bay11-7082 partially, but not completely, blocked the effects of PCB126 in inhibiting adipogenesis (figure 6a and 6b). To confirm that Bay11-7082 repressed the proinflammatory response in PCB126 treated cells, preadipocytes were treated as above (see diagram, figure 1b) and IL-8 transcript levels were measured. As expected, Bay11-7082 significantly inhibited IL-8 induction caused by PCB126 (figure 6c). Overall, these results indicate that the effects of PCB126 on adipogenesis are at least partially mediated by a proinflammatory response.
Fig. 6.
The NF-κB inhibitor Bay11-7082 partially ameliorates the effects of PCB126 on adipogenesis. (A) Preadipocytes were treated for 4 days in vehicle only, PCB126 only, Bay11-7082 and PCB126, or Bay11-7082 only and then differentiated for 9 days (see diagram, figure 1c). Differentiated cultures were fixed and stained with AdipoRed, which has the highest fluorescence and lowest background in a green wavelength. Fluorescent photos were taken at 200X. (B) Fluorescence was quantified in four different fields using ImageJ software. Percentage is relative to vehicle-treated cells. (C) Preadipocytes were treated with indicated compounds and collected for RNA (see diagram, figure 1b) followed by assessment of IL-8 transcript. Fold-changes over vehicle-treated cells were calculated as described in the Materials and Methods. Three assay replicates were averaged. Statistics comparing treatments were performed as described in the Materials and Methods. **=p<0.01; ***=p<0.001.
DISCUSSION
Here, we have shown that human preadipocytes exhibit a robust proinflammatory response when exposed to PCB126, suggesting that preadipocytes may be targets for PCBs to cause adipose tissue dysfunction through inflammation. The inflammatory response was found to be dependent on AhR and was associated with disruption of adipogenesis. A time-course study demonstrated that the response significantly lagged behind upregulation of CYP1A1, a gene that is known to be transcriptionally regulated by AhR binding at its promoter, indicating that the inflammatory response may be regulated by a mechanism that is different from that which activates CYP1A1. An inhibitor of NF-κB, a transcriptional mediator of many proinflammatory response genes, partially ameliorated the effects of PCB126 on adipogenesis. These results indicate that the proinflammatory response caused by PCB126 is at least partly responsible for disrupting adipogenesis. These findings may have implications for understanding how exposure to PCBs causes metabolic disruption.
The finding that PCB126 causes a proinflammatory response in preadipocytes is not entirely new but a time course of activation and dependence on AhR has not been previously demonstrated. Earlier studies showed that PCB126 and other dioxin-like PCBs activate NF-κB responsive genes in endothelial cells (Hennig et al. 2002). Dioxin and PCB126 have also been shown to turn on proinflammatory response pathway genes in adipocytes or mesenchymal stem cells (MSCs) (Li et al. 2008a, Kim et al. 2012). In concordance with our findings here, a more robust proinflammatory response was observed in the MSCs as compared to adipocytes (Kim et al. 2012), supporting the idea that precursor cells may be more responsive to PCB126 than mature adipocytes. This is of interest since most recent studies have focused on the role of mature adipocytes and surrounding macrophages in mediating the inflammatory response in adipose tissue (Kohlgruber et al. 2015). Our studies suggest that more attention should be given to the preadipocyte population.
The mechanism that is responsible for the delayed induction of the proinflammatory pathways after PCB126 treatment and AhR activation is unknown. Previous reports have suggested that AhR can inhibit, rather than induce, inflammation through its ability to bind to NF-κB components (Tian 2009, Vogel et al. 2011, Ovrevik et al. 2014, Vogel et al. 2014). One possibility is that the initial activation of AhR in preadipocytes inhibits NF-κB and it is not until after AhR signaling goes down that NF-κB can be activated to turn on proinflammatory genes. It is interesting to note that LPS treatment of our preadipocytes causes a rapid induction of IL-8 within 3 hours. The PCB126 response is therefore likely mediated through mechanisms that are different than that induced by LPS. Future studies will be needed to address these mechanisms.
With regard to how AhR activation inhibits adipogenesis, a variety of possibilities exist. Our results with the Bay-11 inhibitor would suggest that at least part of the inhibition is mediated by the proinflammatory response. This would also be supported by other studies that indicate that inflammation disrupts adipogenesis (Chao et al. 2008, Gustafson et al. 2009, Mlinar et al. 2011), although there is evidence that adipocyte inflammation might also be important for healthy adipose tissue (Wernstedt Asterholm et al. 2014). Our previous studies showed that PCB126 inhibited induction of PPARγ2 (Gadupudi et al. 2015). NF-κB and PPARγ2, the main transcription factor inducing adipogenesis, are antagonistic in nature and NF-κb is known to downregulate PPARγ2 whereas PPARγ2 is known to turn down NF-κB pathway components (Chung et al. 2006). How the effects of a proinflammatory response in preadipocytes are sustained after PCB126 is removed and the cells are differentiated is unknown but may involve a mechanism that inhibits transcriptional activation of PPARγ2. That the Bay-11 inhibitor did not completely ameliorate the effects of PCB126 indicates that NF-κB activation is not the full story with regard to inhibition of adipogenesis by PCB126. However, the idea that anti-inflammatory agents play a role in ameliorating the effects of inflammatory agents such as PCB126 on preadipocytes is appealing. Could inhibition of inflammation also prevent problems such as insulin resistance that often are initiated or exacerbated by adipocyte dysfunction? Further studies will be needed to address this issue. It should be noted that a fine balance exists between the negative and positive effects of NF-κB activation. In many cases, NF-κB activation is anti-apoptotic (James et al. 2006, Prasad et al. 2010), which would promote cell survival. Use of any drug that inhibits NF-κB would need to take this and other caveats into account.
The role of dioxin-like compounds such as PCB126 in causing metabolic dysfunction is currently being studied in animal models. Recent results in mice have demonstrated that dioxin-like PCB77 causes insulin resistance (Baker et al. 2013, Baker et al. 2015). PCB126 has also been implicated in causing insulin resistance in mice (Baker et al. 2013). Mice that are knocked out for AhR are protected from the effects of PCB77 (Baker et al. 2015). How the PCBs affect adipogenesis in vivo and how this leads to metabolic dysfunction is still under debate. Disruption of adipogenesis by PCB126 could put significant stress on existing adipocytes, particularly those from the subcutaneous depot, and cause them to be dysfunctional and/or put stress on visceral fat depots. There is evidence that PCB77 induces adipocyte differentiation of mouse 3T3-L1 cells and promotes obesity in mice (Arsenescu et al. 2008). One possibility is that different doses of exposure may cause different effects with regard to adipogenesis, although we have not observed this with PCB126 in our human cell system. We have also recently demonstrated that PCB126 causes hepatic steatosis in rats through disruption of the gluconeogenesis pathway, most likely through downregulation of genes such as PEPCK (phosphoenolpyruvate carboxykinase) through dampening of CREB (cAMP response element binding) phosphorylation (Gadupudi et al. 2016a, Gadupudi et al. 2016b). These studies, along with epidemiologic data indicating that PCB exposure and higher serum levels of certain PCBs, such as PCB126, are associated with the development of diabetes, strongly supports the idea that exposure to PCBs cause or exacerbate metabolic problems in humans.
Recently, other compounds have been found to activate or repress AhR (Labrecque et al. 2013, Jin et al. 2014, Esser et al. 2015, Hubbard et al. 2015). Aside from organic pollutants, some of these chemicals are endogenous, such as kynurenin, and bacterial or microbiome-derived metabolites such as indoxyl sulfate have been shown to activate AhR (Hubbard et al. 2015). It is possible that these agents play a role in regulating adipogenesis and, if so, may also be important for the development of obesity and diabetes.
In summary, our results provide evidence that PCB126 causes a delayed proinflammatory response in preadipocytes and demonstrate that this response is mediated by AhR. Chemical inhibition of the proinflammatory response may mitigate certain effects of PCB126 and other dioxin-like compounds on adipocyte function, which could be important for development of insulin response. Finally, AhR is emerging as key player in immune response and our results suggest that other agents, both exogenous and endogenous, that interact with AhR might be important for regulating metabolism.
Supplementary Material
Supplemental Table 1: Primers used or quantitative RT-PCR
Acknowledgments
FUNDING INFORMATION
This work was supported by a seed grant from the University of Iowa Center for Health Effects of Environmental Contamination (CHEEC), a pilot grant from the University of Iowa Environmental Health Sciences Research Center (grant number P30 ES05605), and a Fraternal Order of Eagles Diabetes Research Center Award given to AJK; and the Iowa Superfund Research Program (grant number P42 ES 013661) awarded to LWR.
We thank Dr. Patrick Ten Eyck at the Institute for Clinical and Translational Science, University of Iowa for statistical analysis. We also thank Hans Joachim-Lehmler of the University of Iowa Superfund Synthesis Core for supplying PCB126, Anna Chaly for technical support, and Gopi Gadupudi for advice.
References
- Alexander DL, Ganem LG, Fernandez-Salguero P, Gonzalez F, Jefcoate CR. Aryl-hydrocarbon receptor is an inhibitory regulator of lipid synthesis and of commitment to adipogenesis. J Cell Sci. 1998;111(Pt 22):3311–3322. doi: 10.1242/jcs.111.22.3311. [DOI] [PubMed] [Google Scholar]
- Ampleman MD, Martinez A, DeWall J, Rawn DF, Hornbuckle KC, Thorne PS. Inhalation and dietary exposure to PCBs in urban and rural cohorts via congener-specific measurements. Environ Sci Technol. 2015;49:1156–1164. doi: 10.1021/es5048039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arsenescu V, Arsenescu RI, King V, Swanson H, Cassis LA. Polychlorinated biphenyl-77 induces adipocyte differentiation and proinflammatory adipokines and promotes obesity and atherosclerosis. Environ Health Perspect. 2008;116:761–768. doi: 10.1289/ehp.10554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker NA, Karounos M, English V, Fang J, Wei Y, Stromberg A, Sunkara M, Morris AJ, Swanson HI, Cassis LA. Coplanar polychlorinated biphenyls impair glucose homeostasis in lean C57BL/6 mice and mitigate beneficial effects of weight loss on glucose homeostasis in obese mice. Environ Health Perspect. 2013;121:105–110. doi: 10.1289/ehp.1205421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker NA, Shoemaker R, English V, Larian N, Sunkara M, Morris AJ, Walker M, Yiannikouris F, Cassis LA. Effects of Adipocyte Aryl Hydrocarbon Receptor Deficiency on PCB-Induced Disruption of Glucose Homeostasis in Lean and Obese Mice. Environ Health Perspect. 2015;123:944–950. doi: 10.1289/ehp.1408594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyer A, Biziuk M. Environmental fate and global distribution of polychlorinated biphenyls. Rev Environ Contam Toxicol. 2009;201:137–158. doi: 10.1007/978-1-4419-0032-6_5. [DOI] [PubMed] [Google Scholar]
- Brodie AE, Azarenko VA, Hu CY. 2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD) inhibition of fat cell differentiation. Toxicol Lett. 1996a;84:55–59. doi: 10.1016/0378-4274(95)03537-0. [DOI] [PubMed] [Google Scholar]
- Brodie AE, Manning VA, Hu CY. Inhibitors of preadipocyte differentiation induce COUP-TF binding to a PPAR/RXR binding sequence. Biochem Biophys Res Commun. 1996b;228:655–661. doi: 10.1006/bbrc.1996.1713. [DOI] [PubMed] [Google Scholar]
- Chao LC, Bensinger SJ, Villanueva CJ, Wroblewski K, Tontonoz P. Inhibition of adipocyte differentiation by Nur77, Nurr1, and Nor1. Mol Endocrinol. 2008;22:2596–2608. doi: 10.1210/me.2008-0161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G, Bunce NJ. Interaction between halogenated aromatic compounds in the Ah receptor signal transduction pathway. Environ Toxicol. 2004;19:480–489. doi: 10.1002/tox.20053. [DOI] [PubMed] [Google Scholar]
- Cho YC, Jefcoate CR. PPARgamma1 synthesis and adipogenesis in C3H10T1/2 cells depends on S-phase progression, but does not require mitotic clonal expansion. J Cell Biochem. 2004;91:336–353. doi: 10.1002/jcb.10743. [DOI] [PubMed] [Google Scholar]
- Chung S, Lapoint K, Martinez K, Kennedy A, Boysen Sandberg M, McIntosh MK. Preadipocytes mediate lipopolysaccharide-induced inflammation and insulin resistance in primary cultures of newly differentiated human adipocytes. Endocrinology. 2006;147:5340–5351. doi: 10.1210/en.2006-0536. [DOI] [PubMed] [Google Scholar]
- Cimafranca MA, Hanlon PR, Jefcoate CR. TCDD administration after the pro-adipogenic differentiation stimulus inhibits PPARgamma through a MEK-dependent process but less effectively suppresses adipogenesis. Toxicol Appl Pharmacol. 2004;196:156–168. doi: 10.1016/j.taap.2003.12.005. [DOI] [PubMed] [Google Scholar]
- Dunmore SJ, Brown JE. The role of adipokines in beta-cell failure of type 2 diabetes. J Endocrinol. 2013;216:T37–45. doi: 10.1530/JOE-12-0278. [DOI] [PubMed] [Google Scholar]
- Esser C, Rannug A. The aryl hydrocarbon receptor in barrier organ physiology, immunology, and toxicology. Pharmacol Rev. 2015;67:259–279. doi: 10.1124/pr.114.009001. [DOI] [PubMed] [Google Scholar]
- Everett CJ, Frithsen I, Player M. Relationship of polychlorinated biphenyls with type 2 diabetes and hypertension. J Environ Monit. 2011;13:241–251. doi: 10.1039/c0em00400f. [DOI] [PubMed] [Google Scholar]
- Everett CJ, Thompson OM. Associations of dioxins, furans and dioxin-like PCBs with diabetes and pre-diabetes: is the toxic equivalency approach useful? Environ Res. 2012;118:107–111. doi: 10.1016/j.envres.2012.06.012. [DOI] [PubMed] [Google Scholar]
- Fisman EZ, Tenenbaum A. Adiponectin: a manifold therapeutic target for metabolic syndrome, diabetes, and coronary disease? Cardiovasc Diabetol. 2014;13:103. doi: 10.1186/1475-2840-13-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuentes E, Fuentes F, Vilahur G, Badimon L, Palomo I. Mechanisms of chronic state of inflammation as mediators that link obese adipose tissue and metabolic syndrome. Mediators Inflamm. 2013;2013:136584. doi: 10.1155/2013/136584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadupudi G, Gourronc FA, Ludewig G, Robertson LW, Klingelhutz AJ. PCB126 inhibits adipogenesis of human preadipocytes. Toxicol In Vitro. 2015;29:132–141. doi: 10.1016/j.tiv.2014.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadupudi GS, Klaren WD, Olivier AK, Klingelhutz AJ, Robertson LW. PCB126-Induced Disruption in Gluconeogenesis and Fatty Acid Oxidation Precedes Fatty Liver in Male Rats. Toxicol Sci. 2016a;149:98–110. doi: 10.1093/toxsci/kfv215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadupudi GS, Klingelhutz AJ, Robertson LW. Diminished Phosphorylation of CREB Is a Key Event in the Dysregulation of Gluconeogenesis and Glycogenolysis in PCB126 Hepatotoxicity. Chem Res Toxicol. 2016b;29:1504–1509. doi: 10.1021/acs.chemrestox.6b00172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilherme A, Virbasius JV, Puri V, Czech MP. Adipocyte dysfunctions linking obesity to insulin resistance and type 2 diabetes. Nat Rev Mol Cell Biol. 2008;9:367–377. doi: 10.1038/nrm2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafson B, Gogg S, Hedjazifar S, Jenndahl L, Hammarstedt A, Smith U. Inflammation and impaired adipogenesis in hypertrophic obesity in man. Am J Physiol Endocrinol Metab. 2009;297:E999–E1003. doi: 10.1152/ajpendo.00377.2009. [DOI] [PubMed] [Google Scholar]
- Hanlon PR, Ganem LG, Cho YC, Yamamoto M, Jefcoate CR. AhR- and ERK-dependent pathways function synergistically to mediate 2,3,7,8-tetrachlorodibenzo-p-dioxin suppression of peroxisome proliferator-activated receptor-gamma1 expression and subsequent adipocyte differentiation. Toxicol Appl Pharmacol. 2003;189:11–27. doi: 10.1016/s0041-008x(03)00083-8. [DOI] [PubMed] [Google Scholar]
- Hanlon PR, Zheng W, Ko AY, Jefcoate CR. Identification of novel TCDD-regulated genes by microarray analysis. Toxicol Appl Pharmacol. 2005;202:215–228. doi: 10.1016/j.taap.2004.06.018. [DOI] [PubMed] [Google Scholar]
- Hectors TL, Vanparys C, Van Gaal LF, Jorens PG, Covaci A, Blust R. Insulin resistance and environmental pollutants: experimental evidence and future perspectives. Environ Health Perspect. 2013;121:1273–1281. doi: 10.1289/ehp.1307082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennig B, Meerarani P, Slim R, Toborek M, Daugherty A, Silverstone AE, Robertson LW. Proinflammatory properties of coplanar PCBs: in vitro and in vivo evidence. Toxicol Appl Pharmacol. 2002;181:174–183. doi: 10.1006/taap.2002.9408. [DOI] [PubMed] [Google Scholar]
- Herrick RF, Meeker JD, Altshul L. Serum PCB levels and congener profiles among teachers in PCB-containing schools: a pilot study. Environ Health. 2011;10:56. doi: 10.1186/1476-069X-10-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoch M, Eberle AN, Peterli R, Peters T, Seboek D, Keller U, Muller B, Linscheid P. LPS induces interleukin-6 and interleukin-8 but not tumor necrosis factor-alpha in human adipocytes. Cytokine. 2008;41:29–37. doi: 10.1016/j.cyto.2007.10.008. [DOI] [PubMed] [Google Scholar]
- Hsu HF, Tsou TC, Chao HR, Kuo YT, Tsai FY, Yeh SC. Effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin on adipogenic differentiation and insulin-induced glucose uptake in 3T3-L1 cells. J Hazard Mater. 2010;182:649–655. doi: 10.1016/j.jhazmat.2010.06.081. [DOI] [PubMed] [Google Scholar]
- Hubbard TD, Murray IA, Perdew GH. Indole and Tryptophan Metabolism: Endogenous and Dietary Routes to Ah Receptor Activation. Drug Metab Dispos. 2015;43:1522–1535. doi: 10.1124/dmd.115.064246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imbeault P, Findlay CS, Robidoux MA, Haman F, Blais JM, Tremblay A, Springthorpe S, Pal S, Seabert T, Krummel EM, Maal-Bared R, Tetro JA, Pandey S, Sattar SA, Filion LG. Dysregulation of cytokine response in Canadian First Nations communities: is there an association with persistent organic pollutant levels? PLoS One. 2012;7:e39931. doi: 10.1371/journal.pone.0039931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James MA, Lee JH, Klingelhutz AJ. Human papillomavirus type 16 E6 activates NF-kappaB, induces cIAP-2 expression, and protects against apoptosis in a PDZ binding motif-dependent manner. J Virol. 2006;80:5301–5307. doi: 10.1128/JVI.01942-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin UH, Lee SO, Sridharan G, Lee K, Davidson LA, Jayaraman A, Chapkin RS, Alaniz R, Safe S. Microbiome-derived tryptophan metabolites and their aryl hydrocarbon receptor-dependent agonist and antagonist activities. Mol Pharmacol. 2014;85:777–788. doi: 10.1124/mol.113.091165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern PA, Dicker-Brown A, Said ST, Kennedy R, Fonseca VA. The stimulation of tumor necrosis factor and inhibition of glucose transport and lipoprotein lipase in adipose cells by 2,3,7,8-tetrachlorodibenzo-p-dioxin. Metabolism. 2002;51:65–68. doi: 10.1053/meta.2002.28088. [DOI] [PubMed] [Google Scholar]
- Kim KS, Lee YM, Kim SG, Lee IK, Lee HJ, Kim JH, Kim J, Moon HB, Jacobs DR, Jr, Lee DH. Associations of organochlorine pesticides and polychlorinated biphenyls in visceral vs. subcutaneous adipose tissue with type 2 diabetes and insulin resistance. Chemosphere. 2014;94:151–157. doi: 10.1016/j.chemosphere.2013.09.066. [DOI] [PubMed] [Google Scholar]
- Kim MJ, Pelloux V, Guyot E, Tordjman J, Bui LC, Chevallier A, Forest C, Benelli C, Clement K, Barouki R. Inflammatory pathway genes belong to major targets of persistent organic pollutants in adipose cells. Environ Health Perspect. 2012;120:508–514. doi: 10.1289/ehp.1104282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Dere E, Burgoon LD, Chang CC, Zacharewski TR. Comparative analysis of AhR-mediated TCDD-elicited gene expression in human liver adult stem cells. Toxicol Sci. 2009;112:229–244. doi: 10.1093/toxsci/kfp189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohlgruber A, Lynch L. Adipose tissue inflammation in the pathogenesis of type 2 diabetes. Curr Diab Rep. 2015;15:92. doi: 10.1007/s11892-015-0670-x. [DOI] [PubMed] [Google Scholar]
- Labrecque MP, Prefontaine GG, Beischlag TV. The aryl hydrocarbon receptor nuclear translocator (ARNT) family of proteins: transcriptional modifiers with multi-functional protein interfaces. Curr Mol Med. 2013;13:1047–1065. doi: 10.2174/15665240113139990042. [DOI] [PubMed] [Google Scholar]
- Lee DH, Steffes MW, Sjodin A, Jones RS, Needham LL, Jacobs DR., Jr Low dose organochlorine pesticides and polychlorinated biphenyls predict obesity, dyslipidemia, and insulin resistance among people free of diabetes. PLoS One. 2011;6:e15977. doi: 10.1371/journal.pone.0015977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YM, Kim KS, Kim SA, Hong NS, Lee SJ, Lee DH. Prospective associations between persistent organic pollutants and metabolic syndrome: A nested case-control study. Sci Total Environ. 2014;496:219–225. doi: 10.1016/j.scitotenv.2014.07.039. [DOI] [PubMed] [Google Scholar]
- Li W, Matsumura F. Significance of the nongenomic, inflammatory pathway in mediating the toxic action of TCDD to induce rapid and long-term cellular responses in 3T3-L1 adipocytes. Biochemistry. 2008a;47:13997–14008. doi: 10.1021/bi801913w. [DOI] [PubMed] [Google Scholar]
- Li W, Vogel CF, Fujiyoshi P, Matsumura F. Development of a human adipocyte model derived from human mesenchymal stem cells (hMSC) as a tool for toxicological studies on the action of TCDD. Biol Chem. 2008b;389:169–177. doi: 10.1515/BC.2008.015. [DOI] [PubMed] [Google Scholar]
- Littlejohn NK, Keen HL, Weidemann BJ, Claflin KE, Tobin KV, Markan KR, Park S, Naber MC, Gourronc FA, Pearson NA, Liu X, Morgan DA, Klingelhutz AJ, Potthoff MJ, Rahmouni K, Sigmund CD, Grobe JL. Suppression of Resting Metabolism by the Angiotensin AT2 Receptor. Cell Rep. 2016;16:1548–1560. doi: 10.1016/j.celrep.2016.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu PC, Phillips MA, Matsumura F. Alteration by 2,3,7,8-Tetrachlorodibenzo-p-dioxin of CCAAT/enhancer binding protein correlates with suppression of adipocyte differentiation in 3T3-L1 cells. Mol Pharmacol. 1996;49:989–997. [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lo R, Matthews J. High-resolution genome-wide mapping of AHR and ARNT binding sites by ChIP-Seq. Toxicol Sci. 2012;130:349–361. doi: 10.1093/toxsci/kfs253. [DOI] [PubMed] [Google Scholar]
- Meijer K, de Vries M, Al-Lahham S, Bruinenberg M, Weening D, Dijkstra M, Kloosterhuis N, van der Leij RJ, van der Want H, Kroesen BJ, Vonk R, Rezaee F. Human primary adipocytes exhibit immune cell function: adipocytes prime inflammation independent of macrophages. PLoS One. 2011;6:e17154. doi: 10.1371/journal.pone.0017154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mlinar B, Marc J. New insights into adipose tissue dysfunction in insulin resistance. Clin Chem Lab Med. 2011;49:1925–1935. doi: 10.1515/CCLM.2011.697. [DOI] [PubMed] [Google Scholar]
- Mori N, Yamada Y, Ikeda S, Yamasaki Y, Tsukasaki K, Tanaka Y, Tomonaga M, Yamamoto N, Fujii M. Bay 11-7082 inhibits transcription factor NF-kappaB and induces apoptosis of HTLV-I-infected T-cell lines and primary adult T-cell leukemia cells. Blood. 2002;100:1828–1834. doi: 10.1182/blood-2002-01-0151. [DOI] [PubMed] [Google Scholar]
- Olsen H, Enan E, Matsumura F. 2,3,7,8-Tetrachlorodibenzo-p-dioxin mechanism of action to reduce lipoprotein lipase activity in the 3T3-L1 preadipocyte cell line. J Biochem Mol Toxicol. 1998;12:29–39. doi: 10.1002/(sici)1099-0461(1998)12:1<29::aid-jbt5>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Ovrevik J, Lag M, Lecureur V, Gilot D, Lagadic-Gossmann D, Refsnes M, Schwarze PE, Skuland T, Becher R, Holme JA. AhR and Arnt differentially regulate NF-kappaB signaling and chemokine responses in human bronchial epithelial cells. Cell Commun Signal. 2014;12:48. doi: 10.1186/s12964-014-0048-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel P, Abate N. Body fat distribution and insulin resistance. Nutrients. 2013a;5:2019–2027. doi: 10.3390/nu5062019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel P, Abate N. Role of subcutaneous adipose tissue in the pathogenesis of insulin resistance. J Obes. 2013b;2013:489187. doi: 10.1155/2013/489187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persky V, Piorkowski J, Turyk M, Freels S, Chatterton R, Jr, Dimos J, Bradlow HL, Chary LK, Burse V, Unterman T, Sepkovic D, McCann K. Associations of polychlorinated biphenyl exposure and endogenous hormones with diabetes in post-menopausal women previously employed at a capacitor manufacturing plant. Environ Res. 2011;111:817–824. doi: 10.1016/j.envres.2011.05.012. [DOI] [PubMed] [Google Scholar]
- Phillips M, Enan E, Liu PC, Matsumura F. Inhibition of 3T3-L1 adipose differentiation by 2,3,7,8-tetrachlorodibenzo-p-dioxin. J Cell Sci. 1995;108(Pt 1):395–402. doi: 10.1242/jcs.108.1.395. [DOI] [PubMed] [Google Scholar]
- Prasad S, Ravindran J, Aggarwal BB. NF-kappaB and cancer: how intimate is this relationship. Mol Cell Biochem. 2010;336:25–37. doi: 10.1007/s11010-009-0267-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prentki M, Madiraju SR. Glycerolipid/free fatty acid cycle and islet beta-cell function in health, obesity and diabetes. Mol Cell Endocrinol. 2012;353:88–100. doi: 10.1016/j.mce.2011.11.004. [DOI] [PubMed] [Google Scholar]
- Rosen ED, Spiegelman BM. What we talk about when we talk about fat. Cell. 2014;156:20–44. doi: 10.1016/j.cell.2013.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruzzin J, Petersen R, Meugnier E, Madsen L, Lock EJ, Lillefosse H, Ma T, Pesenti S, Sonne SB, Marstrand TT, Malde MK, Du ZY, Chavey C, Fajas L, Lundebye AK, Brand CL, Vidal H, Kristiansen K, Froyland L. Persistent organic pollutant exposure leads to insulin resistance syndrome. Environ Health Perspect. 2010;118:465–471. doi: 10.1289/ehp.0901321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sartor MA, Schnekenburger M, Marlowe JL, Reichard JF, Wang Y, Fan Y, Ma C, Karyala S, Halbleib D, Liu X, Medvedovic M, Puga A. Genomewide analysis of aryl hydrocarbon receptor binding targets reveals an extensive array of gene clusters that control morphogenetic and developmental programs. Environ Health Perspect. 2009;117:1139–1146. doi: 10.1289/ehp.0800485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzawa M, Takada I, Yanagisawa J, Ohtake F, Ogawa S, Yamauchi T, Kadowaki T, Takeuchi Y, Shibuya H, Gotoh Y, Matsumoto K, Kato S. Cytokines suppress adipogenesis and PPAR-gamma function through the TAK1/TAB1/NIK cascade. Nat Cell Biol. 2003;5:224–230. doi: 10.1038/ncb942. [DOI] [PubMed] [Google Scholar]
- Tanabe K, Liu Y, Hasan SD, Martinez SC, Cras-Meneur C, Welling CM, Bernal-Mizrachi E, Tanizawa Y, Rhodes CJ, Zmuda E, Hai T, Abumrad NA, Permutt MA. Glucose and fatty acids synergize to promote B-cell apoptosis through activation of glycogen synthase kinase 3beta independent of JNK activation. PLoS One. 2011;6:e18146. doi: 10.1371/journal.pone.0018146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tchkonia T, Morbeck DE, Von Zglinicki T, Van Deursen J, Lustgarten J, Scrable H, Khosla S, Jensen MD, Kirkland JL. Fat tissue, aging, and cellular senescence. Aging Cell. 2010;9:667–684. doi: 10.1111/j.1474-9726.2010.00608.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian Y. Ah receptor and NF-kappaB interplay on the stage of epigenome. Biochem Pharmacol. 2009;77:670–680. doi: 10.1016/j.bcp.2008.10.023. [DOI] [PubMed] [Google Scholar]
- Vogel CF, Khan EM, Leung PS, Gershwin ME, Chang WL, Wu D, Haarmann-Stemmann T, Hoffmann A, Denison MS. Cross-talk between aryl hydrocarbon receptor and the inflammatory response: a role for nuclear factor-kappaB. J Biol Chem. 2014;289:1866–1875. doi: 10.1074/jbc.M113.505578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel CF, Li W, Wu D, Miller JK, Sweeney C, Lazennec G, Fujisawa Y, Matsumura F. Interaction of aryl hydrocarbon receptor and NF-kappaB subunit RelB in breast cancer is associated with interleukin-8 overexpression. Arch Biochem Biophys. 2011;512:78–86. doi: 10.1016/j.abb.2011.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vu BG, Gourronc FA, Bernlohr DA, Schlievert PM, Klingelhutz AJ. Staphylococcal superantigens stimulate immortalized human adipocytes to produce chemokines. PLoS One. 2013;8:e77988. doi: 10.1371/journal.pone.0077988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vu BG, Stach CS, Kulhankova K, Salgado-Pabon W, Klingelhutz AJ, Schlievert PM. Chronic superantigen exposure induces systemic inflammation, elevated bloodstream endotoxin, and abnormal glucose tolerance in rabbits: possible role in diabetes. MBio. 2015;6:e02554. doi: 10.1128/mBio.02554-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Wang X, Varma RK, Beauchamp L, Magdaleno S, Sendera TJ. Selection of hyperfunctional siRNAs with improved potency and specificity. Nucleic Acids Res. 2009;37:e152. doi: 10.1093/nar/gkp864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wernstedt Asterholm I, Tao C, Morley TS, Wang QA, Delgado-Lopez F, Wang ZV, Scherer PE. Adipocyte inflammation is essential for healthy adipose tissue expansion and remodeling. Cell Metab. 2014;20:103–118. doi: 10.1016/j.cmet.2014.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westin ER, Aykin-Burns N, Buckingham EM, Spitz DR, Goldman FD, Klingelhutz AJ. The p53/p21(WAF/CIP) pathway mediates oxidative stress and senescence in dyskeratosis congenita cells with telomerase insufficiency. Antioxid Redox Signal. 2011;14:985–997. doi: 10.1089/ars.2010.3444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wree A, Kahraman A, Gerken G, Canbay A. Obesity affects the liver - the link between adipocytes and hepatocytes. Digestion. 2011;83:124–133. doi: 10.1159/000318741. [DOI] [PubMed] [Google Scholar]
- Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, Chen H. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112:1821–1830. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye R, Scherer PE. Adiponectin, driver or passenger on the road to insulin sensitivity? Mol Metab. 2013;2:133–141. doi: 10.1016/j.molmet.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Xie L, Gunasekar SK, Tong D, Mishra A, Gibson WJ, Wang C, Fidler T, Marthaler B, Klingelhutz A, Dale Abel E, Samuel I, Smith JK, Cao L, Sah R. SWELL1 is a regulator of adipocyte size, insulin signalling and glucose homeostasis. Nat Cell Biol. 2017;19:504–517. doi: 10.1038/ncb3514. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 1: Primers used or quantitative RT-PCR