Abstract
Several epidemiological studies have found that low vitamin D levels are associated with worse prognosis and poorer outcomes in patients with breast cancer (BCa), although some studies have failed to find this association. In addition, prior research has found that BCa patients with vitamin D deficiency have a more aggressive molecular phenotype and worse prognostic biomarkers. As vitamin D deficiency is common in patients diagnosed with BCa, elucidating the cause of the association between poor outcomes and vitamin D deficiency promises to have a significant impact on improving care for patients with BCa including enabling the development of novel therapeutic approaches. Here we review our recent findings in this area, including our data revealing that reduction of the expression of the vitamin D receptor (Vdr) within BCa cells accelerates primary tumor growth and enables the development of metastases, demonstrating a tumor autonomous effect of vitamin D signaling to suppress BCa metastases. We believe that these findings are likely relevant to humans as we discovered evidence that a mechanism of VDR regulation identified in our mouse models is conserved in human BCa. In particular, we identified a negative correlation between serum 25(OH)D concentration and the level of expression of the tumor progression factor ID1 in primary tumors from patients with breast cancer.
Keywords: vitamin D, Vitamin D receptor, calcitriol, breast cancer, ID1, metastasis, stromal cells, microenvironment
1. Introduction
Vitamin D is the precursor to the potent steroid hormone, 1α, 25-dihydroxyvitamin D3 (calcitriol). Vitamin D, induced by sunlight or ingested in the diet, is readily converted in the liver to 25(OH)D, which is then converted into calcitriol by the mitochondrial enzyme 25-hydroxyvitamin D 1α-hydroxylase (CYP27B1) in the kidney or extra-renal sites (Fig. 1) [1–3]. In order to maintain calcium and phosphorus homeostasis, one of the major physiological roles of calcitriol, renal calcitriol synthesis is tightly regulated by other key calciotropic hormones, parathyroid hormone and fibroblast growth factor [2,3].
Figure 1. Tumor autonomous and non-autonomous vitamin D action in BCa.
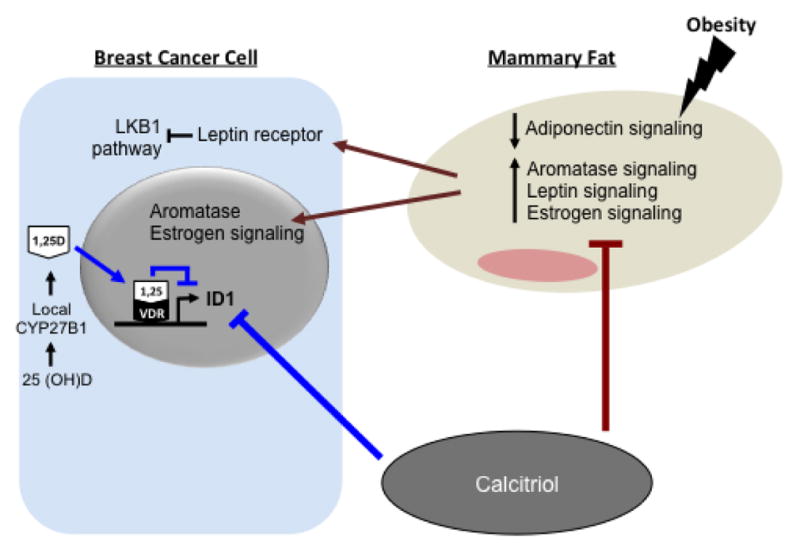
Schematic representation of tumor autonomous (blue) and non- autonomous (brown) effects of vitamin D signaling on BCa cells discussed in this manuscript (For clarity, not all vitamin D actions are shown). Vitamin D is converted to active calcitriol (1,25D) by CYP27B1 either in the kidneys (systemic) or in the BCa tumor (local). Within the BCa, 1,25D binds to the vitamin D receptor (VDR) which promotes occupancy of a negative VDRE that inhibits transcription of Inhibitor of Differentiation 1 (Id1) expression. 1,25D also inhibits ER expression and COX-2 synthesis of prostaglandins. Adipocytes in the mammary tissue promote proliferation of BCa cells by increasing aromatase, leptin, and estrogen signaling, and decreasing adiponectin. Calcitriol mitigates the adverse effects of obesity on BCa by acting on pathways both within BCa cells and the adjacent non-tumor breast tissue that influence BCa growth and metastasis.
Calcitriol and its analogs bind to and activate the vitamin D receptor (VDR), a member of the steroid nuclear receptor family. This activation of the VDR leads to heterodimerization with the retinoid X receptor (RXR) and occupancy of vitamin D response elements (VDREs) in the DNA. The occupancy of VDREs initiates the recruitment of other co-factors to form a regulatory complex that modulates the expression of target genes [4, 5]. This mode of activation enables vitamin D to have complex and context-specific actions. Indeed, over the past decades, a large body of work in numerous laboratories has established that vitamin D action extends far beyond its role in calcium homeostasis to participate in a variety of physiological processes from hair growth to metabolism. In addition, perturbations in vitamin D action have been connected to the pathogenesis of a number of diseases [6]. Many studies have shown that vitamin D deficiency is common in the United States, even using the less rigorous cut-point of 20 ng/mL, especially in people of color [7]. In this manuscript, we will review the connections between vitamin D signaling and cancer with a primary concentration on two studies that examined tumor-autonomous actions as well as vitamin D actions on the tumor microenvironment in breast cancer (BCa).
2. Vitamin D action in the breast
Although the kidney is the principal site of CYP27B1 activity regulating the generation of circulating calcitriol, this enzyme is also expressed in multiple extra-renal sites along with the VDR leading to local synthesis and paracrine actions [2, 8, 9]. These findings suggest that there are multiple local tissue-specific vitamin D actions. For example, CYP27B1 is highly expressed in breast tissue [10–12]. Consistent with a role for vitamin D action in the breast tissue, the VDR and CYP27B1 are expressed in both normal breast and BCa tissue [11, 13]. Mice that have the VDR gene knocked-out have accelerated development and increased branching of the ducts in the breast compared to wild-type controls [14], confirming an important role for vitamin D action in breast tissue physiology and development.
3. Vitamin D action in breast cancer
Mounting evidence supports the concept that vitamin D has anti-cancer actions that may be beneficial in the prevention and/or treatment of several types of cancers including prostate, colon and breast [15]. These studies indicate that calcitriol has effects on cell proliferation, differentiation, and apoptosis. Furthermore, increased expression of VDR in BCa tumors is associated with a reduced risk of death [16, 17]. Recent studies have also shown that increased vitamin D action suppresses BCa cell migration and metastasis [15, 18–22]. Together these, and multiple additional studies have raised optimism that increasing the levels of vitamin D could have beneficial effects against BCa in humans.
In additional to potential benefits of the pharmacological use of vitamin D and calcitriol, some (but not all) epidemiological studies suggest an inverse association between BCa risk and physiological vitamin D stores [20, 23–25]. For example, some studies examining the association between serum 25(OH)D levels and BCa prognostic indicators report that patients with vitamin D deficiency [low serum 25(OH)D] have a more aggressive molecular phenotype of BCa and worse prognostic indicators [26–29]. If confirmed, these results indicate that vitamin D action has a physiological role in tumor suppression and would support increasing efforts to reduce the prevalence of vitamin D deficiency. Furthermore, given the widespread prevalence of vitamin D deficiency in the population in general and in cancer patients in particular [30], understanding the relationship between vitamin D deficiency and BCa progression could have broad implications and impact.
3.1 Tumor-autonomous effects of vitamin D action in BCa
Breast tissue is composed of multiple cell types, as well as an intricate extracellular matrix, and all of these components are known to have important roles in both physiology as well as the pathogenesis of BCa [31–33]. Therefore, it is of both scientific and medical interest to localize various actions of vitamin D within BCa and, in particular, specify which compartments and actions are responsible for any anti-tumor vitamin D activity.
To test the effect of vitamin D deficiency on BCa tumor progression, we recently performed studies using two different mouse models of vitamin D deficiency [21]: First, we used the classic model of diet-induced vitamin D deficiency that occurs when mice are fed low vitamin D diet (25 IU vitamin D3/kg) (or controls with standard chow containing 500 IU vitamin D3/kg) for 10 weeks. Once vitamin D deficiency was established, we orthotopically implanted BCa cells into the mammary fat pad. We found that mice with diet-induced vitamin D deficiency had increased rate of BCa growth, with tumors becoming palpable ~1 week prior to mice with sufficient vitamin D levels [21].
While these results are highly intriguing, indicating that the association between vitamin D deficiency and worse BCa outcomes may result from cause-and-effect mechanisms, the studies did not resolve the question of whether this effect of vitamin D deficiency was occurring because of tumor-autonomous or non-autonomous changes. To address this important question, we established, a second model, using non-metastatic 168FARN BCa cells [34]. From this parental line, we established cells with VDR levels knocked-down (168FARN-Vdr KD) and subsequently rescued with a Vdr expression plasmid (control cells) in order to control for any off-target effects. We propose that knocking-down the VDR decreases vitamin D signaling in a manner analogous to vitamin D deficiency, as there is reduced ligand/receptor activation, but the method enables uncoupling of vitamin D deficiency in tumor cells from the local and systemic extra-tumor environments. Therefore, we could evaluate the tumor-autonomous effects of vitamin D deficiency by transplanting these cells into mice that were systemically vitamin D sufficient to resolve the site of relevant activity. Tumors established with the 168FARN-Vdr KD cells grew significantly faster compared to tumors from control cells. Strikingly, we also discovered that 60% of the 168FARN-Vdr KD tumors were metastatic while none of the tumors established with the control cells metastasized. This finding indicates that tumor-autonomous vitamin D action in BCa is a critical and sufficient regulator of metastasis and might explain the worse prognosis in patients that have vitamin D deficiency [21].
In order to understand the molecular mechanisms that govern these effects, we performed unbiased expression profiling in the tumors harvested from the mice and identified Inhibitor of Differentiation 1 (Id1) as differentially expressed in 168FARN-Vdr KD versus control cells. Using chromatin immunoprecipitation and luciferase assays, we identified a negative VDRE in the Id1 gene that was occupied by calcitriol/VDR, establishing Id1 as a direct target gene.
3.1.1 Vitamin D deficiency results in dysregulation of Id1
ID1 belongs to a family proteins that act as dominant negative regulators of the basic helix-loop-helix family of transcription factors, regulating cell cycle and tumorigenesis [35] and promoting epithelial-to-mesenchymal transition, angiogenesis, invasion and metastasis [36, 37]. Id1 over-expression increases BCa metastasis in mouse models [38]. Importantly, an increase in Id1 expression has been associated with more aggressive disease and poor clinical outcomes in BCa patients [39]. Therefore, we hypothesized that dysregulation of Id1 expression in 168FARN-Vdr KD tumors was responsible for the acquisition of metastatic potential. Indeed, we also found that knocking-down the overexpression of Id1 in 168FARN-Vdr KD cells restored their rate of migration to control levels in transwell assays [21].
In order to begin to test if this pathway is conserved in humans, we obtained data from a clinical trial to establish the relationship between serum 25(OH)D levels and ID1 expression in breast tumor samples from patients undergoing BCa resections. Using archived de-identified BCa specimens from this clinical trial, we demonstrated an inverse correlation between serum 25(OH)D levels and ID1 expression in breast tumor indicating that systemic vitamin D deficiency/insufficiency is associated with higher ID1 mRNA levels with BCa tumors in humans [21].
3.2 Non-tumor-autonomous effects of vitamin D in BCa
While our [15, 21] and other’s (i.e. [40]) studies established critical tumor-autonomous effects of vitamin D action, this does not exclude the possibility that non-tumor-autonomous actions are also relevant. For example, studies indicate that the tumor microenvironment could be an important contributor to the protective effects of vitamin D in cancer [32]. In pancreatic ductal adenocarcinoma progression, for instance, VDR in tumor stromal cells modulate tumor volumes and impacts survival in humans [41]. In a recent nested case-control study, serum 25(OH)D levels during summer negatively correlated not only with tumor risk but also elevated stromal VDR levels [42]. Similarly, in colon cancer, the level of VDR expression and induced gene signature in the fibrous stroma play a predictive role in determining the clinical outcome [43]. In the breast microenvironment, both the stromal and tumor cells produce estrogen, a known driver of BCa growth, particularly in postmenopausal women [44, 45]. In an obese setting, the adipose stroma is inflamed leading to an unfavorable microenvironment, with increased production of estrogen, thereby promoting BCa progression [46]. Therefore, we examined the effects of vitamin D in mitigating the adverse effects of BCa in obesity [21]. We injected VDR-expressing Mmtv-Wnt1 BCa cells isolated from mammary tumors that spontaneously developed in Mmtv-wnt1 mice, into ovariectomized (OVX) wild-type mice that were standard weight on a standard chow diet as well as into diet-induced obese wild-type mice on a high-fat diet and placed them in cohorts that were treated with a vitamin D supplemented diet, calcitriol injections or controls on a standard diet that received mock injections. After allowing for tumors to establish and grow, the mice were sacrificed and the tumors and surrounding tissues were analyzed. We discovered that vitamin D and calcitriol mitigated the adverse effects of obesity on BCa progression by acting on pathways that were dysregulated by obesity in both the tumor cells, as well as the surrounding breast adipose tissue. Specifically, vitamin D suppressed estrogen synthesis and signaling, enhanced pAMPK signaling and restored the adipokine profile (suppressed leptin signaling and stimulated adiponectin signaling) that were dysregulated in response to diet-induced obesity in both the tumor cells and the surrounding mammary adipose tissue.
4. Conclusions
Prior epidemiological data suggest an association between vitamin D deficiency and increased risk of BCa development, as well as a poor prognosis. Our studies contribute to the increasing molecular evidence that vitamin D action has a cause-and-effect role in BCa progression. In particular, our mouse models indicate that VDR target genes within BCa cells become dysregulated as a result of vitamin D deficiency and the tumor-autonomous dysregulation of Id1 expression with vitamin D deficiency is sufficient to promote metastatic spread. Our results from pilot studies using clinical samples support that this pathway is conserved in humans and builds on the foundation that avoiding vitamin D deficiency may have broad benefits including protection from and/or improved prognosis with BCa. Other data from our labs and others indicate that indirect vitamin D actions on the tumor microenvironment may also play an important role in mediating vitamin D actions and determining the behavior of the tumor and the clinical outcome. It is possible that these anti-tumor actions may benefit tumors that are VDR negative due to effects mediated via actions on VDR positive cells in the stromal microenvironment. Thus both tumor-autonomous and indirect actions on the tumor microenvironment and elsewhere in the body combined might mediate the total effect of vitamin D on the course of tumor growth.
Highlights.
Epidemiological data suggests an inverse correlation between vitamin D deficiency and breast cancer risk
Tumor-autonomous effects of vitamin D signaling suppress breast cancer metastases
Tumor-autonomous dysregulation of Id1 expression with vitamin D deficiency is sufficient to promote metastatic spread
Acknowledgments
We are grateful to the patients that volunteered to participate in the clinical trial and to the D. Feldman laboratory team that carried out the clinical trial. We also thank the members of the Breast Cancer Connections advocacy group who provided input and feedback on our projects. A.A. is supported by the Stanford Child Health Research Institute and the Stanford NIH- NCATS-CTSA grant (UL1 TR001085). The studies were supported by grants from the National Institutes of Health, The California Breast Cancer Research Program (19IB-0103), The Stanford Cancer Institute and the Harrari Fund. B.J.F. is the Bechtel Endowed Faculty Scholar.
Abbreviations
- calcitriol
1α, 25-dihydroxyvitamin D3
- 25(OH)D
25-hydroxyvitamin vitamin D
- CYP27B1
1α-hydroxylase
- VDR
vitamin D receptor
- RXR
retinoid X receptor
- VDRE
vitamin D response element
- ID1
Inhibitor of Differentiation 1
- OVX
ovariectomized
- BCa
breast cancer
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Feldman D, Krishnan AV, Swami S. Vitamin D: biology, action and clinical implications. In: Marcus R, Feldman D, Dempster D, Luckey M, Cauley J, editors. Osteoporosis. 4. Vol. 1. San Diego: Elsevier Academic Press; 2013. pp. 283–329. [Google Scholar]
- 2.Jones G, Prosser DE, Kaufmann M. Cytochrome P450-mediated metabolism of vitamin D. Journal of Lipid Research. 2014;55(1):13–31. doi: 10.1194/jlr.R031534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adams JS, Rafison B, Witzel S, Reyes RE, Shieh A, Chun R, Zavala K, Hewison M, Liu PT. Regulation of the extrarenal CYP27B1-hydroxylase. The Journal of Steroid Biochemistry and Molecular Biology. 2014;144(Pt A):22–27. doi: 10.1016/j.jsbmb.2013.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haussler MR, Whitfield GK, Kaneko I, Haussler CA, Hsieh D, Hsieh JC, Jurutka PW. Molecular mechanisms of vitamin D action. Calcified Tissue International. 2013;92(2):77–98. doi: 10.1007/s00223-012-9619-0. [DOI] [PubMed] [Google Scholar]
- 5.Pike JW, Meyer MB, Lee SM, Onal M, Benkusky NA. The vitamin D receptor: contemporary genomic approaches reveal new basic and translational insights. The Journal of Clinical Investigation. 2017;127(4):1146–1154. doi: 10.1172/JCI88887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feldman D, Pike JW, Bouillon R, Giovannucci E, Goltzman D, Hewison M. Vitamin D. 4. San Diego: Elsevier/Academic Press; 2017. [Google Scholar]
- 7.Forrest KY, Stuhldreher WL. Prevalence and correlates of vitamin D deficiency in US adults. Nutrition Research (New York, NY) 2011;31(1):48–54. doi: 10.1016/j.nutres.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 8.Hewison M, Zehnder D, Bland R, Stewart PM. 1alpha-Hydroxylase and the action of vitamin D. Journal of Molecular Endocrinology. 2000;25(2):141–148. doi: 10.1677/jme.0.0250141. [DOI] [PubMed] [Google Scholar]
- 9.Adams JS, Hewison M. Extrarenal expression of the 25-hydroxyvitamin D-1-hydroxylase. Archives of Biochemistry and Biophysics. 2012;523(1):95–102. doi: 10.1016/j.abb.2012.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kemmis CM, Salvador SM, Smith KM, Welsh J. Human mammary epithelial cells express CYP27B1 and are growth inhibited by 25-hydroxyvitamin D-3, the major circulating form of vitamin D-3. Journal of Nutrition. 2006;136(4):887–892. doi: 10.1093/jn/136.4.887. [DOI] [PubMed] [Google Scholar]
- 11.Townsend K, Banwell CM, Guy M, Colston KW, Mansi JL, Stewart PM, Campbell MJ, Hewison M. Autocrine metabolism of vitamin D in normal and malignant breast tissue. Clinical Cancer Research: An official journal of the American Association for Cancer Research. 2005;11(9):3579–3586. doi: 10.1158/1078-0432.CCR-04-2359. [DOI] [PubMed] [Google Scholar]
- 12.Swami S, Krishnan AV, Wang JY, Jensen K, Horst R, Albertelli MA, Feldman D. Dietary vitamin d3 and 1,25-dihydroxyvitamin d3 (calcitriol) exhibit equivalent anticancer activity in mouse xenograft models of breast and prostate cancer. Endocrinology. 2012;153(6):2576–2587. doi: 10.1210/en.2011-1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Freake HC, Abeyasekera G, Iwasaki J, Marcocci C, MacIntyre I, McClelland RA, Skilton RA, Easton DF, Coombes RC. Measurement of 1,25-dihydroxyvitamin D3 receptors in breast cancer and their relationship to biochemical and clinical indices. Cancer Research. 1984;44(4):1677–1681. [PubMed] [Google Scholar]
- 14.Narvaez CJ, Zinser G, Welsh J. Functions of 1alpha,25-dihydroxyvitamin D(3) in mammary gland: from normal development to breast cancer. Steroids. 2001;66(3–5):301–308. doi: 10.1016/s0039-128x(00)00202-6. [DOI] [PubMed] [Google Scholar]
- 15.Feldman D, Krishnan AV, Swami S, Giovannucci E, Feldman BJ. The role of vitamin D in reducing cancer risk and progression. Nature Reviews Cancer. 2014;14(5):342–357. doi: 10.1038/nrc3691. [DOI] [PubMed] [Google Scholar]
- 16.Berger U, McClelland RA, Wilson P, Greene GL, Haussler MR, Pike JW, Colston K, Easton D, Coombes RC. Immunocytochemical determination of estrogen receptor, progesterone receptor, and 1,25-dihydroxyvitamin D3 receptor in breast cancer and relationship to prognosis. Cancer Research. 1991;51(1):239–244. [PubMed] [Google Scholar]
- 17.Ditsch N, Toth B, Mayr D, Lenhard M, Gallwas J, Weissenbacher T, Dannecker C, Friese K, Jeschke U. The association between vitamin D receptor expression and prolonged overall survival in breast cancer. The Journal of Histochemistry and Cytochemistry: Official journal of the Histochemistry Society. 2012;60(2):121–129. doi: 10.1369/0022155411429155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Flanagan L, Packman K, Juba B, O’Neill S, Tenniswood M, Welsh J. Efficacy of Vitamin D compounds to modulate estrogen receptor negative breast cancer growth and invasion. The Journal of Steroid Biochemistry and Molecular Biology. 2003;84(2–3):181–192. doi: 10.1016/s0960-0760(03)00028-1. [DOI] [PubMed] [Google Scholar]
- 19.Colston KW, Hansen CM. Mechanisms implicated in the growth regulatory effects of vitamin D in breast cancer. Endocrine-Related Cancer. 2002;9(1):45–59. doi: 10.1677/erc.0.0090045. [DOI] [PubMed] [Google Scholar]
- 20.Krishnan AV, Swami S, Feldman D. The potential therapeutic benefits of vitamin D in the treatment of estrogen receptor positive breast cancer. Steroids. 2012;77(11):1107–1112. doi: 10.1016/j.steroids.2012.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Williams JD, Aggarwal A, Swami S, Krishnan AV, Ji L, Albertelli MA, Feldman BJ. Tumor Autonomous Effects of Vitamin D Deficiency Promote Breast Cancer Metastasis. Endocrinology. 2016;157(4):1341–1347. doi: 10.1210/en.2015-2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leyssens C, Verlinden L, Verstuyf A. Antineoplastic effects of 1,25(OH)2D3 and its analogs in breast, prostate and colorectal cancer. Endocrine-Related Cancer. 2013;20(2):R31–47. doi: 10.1530/ERC-12-0381. [DOI] [PubMed] [Google Scholar]
- 23.Bauer SR, Hankinson SE, Bertone-Johnson ER, Ding EL. Plasma vitamin D levels, menopause, and risk of breast cancer: dose-response meta-analysis of prospective studies. Medicine. 2013;92(3):123–131. doi: 10.1097/MD.0b013e3182943bc2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim Y, Franke AA, Shvetsov YB, Wilkens LR, Cooney RV, Lurie G, Maskarinec G, Hernandez BY, Le Marchand L, Henderson BE, et al. Plasma 25-hydroxyvitamin D3 is associated with decreased risk of postmenopausal breast cancer in whites: a nested case-control study in the multiethnic cohort study. BMC Cancer. 2014;14:29. doi: 10.1186/1471-2407-14-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang D, Velez de-la-Paz OI, Zhai JX, Liu DW. Serum 25-hydroxyvitamin D and breast cancer risk: a meta-analysis of prospective studies. Tumour Biology: The journal of the International Society for Oncodevelopmental Biology and Medicine. 2013;34(6):3509–3517. doi: 10.1007/s13277-013-0929-2. [DOI] [PubMed] [Google Scholar]
- 26.Kim HJ, Lee YM, Ko BS, Lee JW, Yu JH, Son BH, Gong GY, Kim SB, Ahn SH. Vitamin D deficiency is correlated with poor outcomes in patients with luminal-type breast cancer. Annals of Surgical Oncology. 2011;18(7):1830–1836. doi: 10.1245/s10434-010-1465-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peppone LJ, Rickles AS, Janelsins MC, Insalaco MR, Skinner KA. The association between breast cancer prognostic indicators and serum 25-OH vitamin D levels. Annals of Surgical Oncology. 2012;19(8):2590–2599. doi: 10.1245/s10434-012-2297-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yao S, Kwan ML, Ergas IJ, Roh JM, Cheng TD, Hong CC, McCann SE, Tang L, Davis W, Liu S, et al. Association of Serum Level of Vitamin D at Diagnosis With Breast Cancer Survival: A Case-Cohort Analysis in the Pathways Study. JAMA Oncology. 2017;3(3):351–357. doi: 10.1001/jamaoncol.2016.4188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shirazi L, Almquist M, Borgquist S, Malm J, Manjer J. Serum vitamin D (25OHD3) levels and the risk of different subtypes of breast cancer: A nested case-control study. Breast. 2016;28:184–190. doi: 10.1016/j.breast.2016.06.002. [DOI] [PubMed] [Google Scholar]
- 30.Teleni L, Baker J, Koczwara B, Kimlin MG, Walpole E, Tsai K, Isenring EA. Clinical outcomes of vitamin D deficiency and supplementation in cancer patients. Nutrition Reviews. 2013;71(9):611–621. doi: 10.1111/nure.12047. [DOI] [PubMed] [Google Scholar]
- 31.Oskarsson T. Extracellular matrix components in breast cancer progression and metastasis. Breast. 2013;22(Suppl 2):S66–72. doi: 10.1016/j.breast.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 32.Wang YY, Lehuede C, Laurent V, Dirat B, Dauvillier S, Bochet L, Le Gonidec S, Escourrou G, Valet P, Muller C. Adipose tissue and breast epithelial cells: a dangerous dynamic duo in breast cancer. Cancer Letters. 2012;324(2):142–151. doi: 10.1016/j.canlet.2012.05.019. [DOI] [PubMed] [Google Scholar]
- 33.Narvaez CJ, Matthews D, LaPorta E, Simmons KM, Beaudin S, Welsh J. The impact of vitamin D in breast cancer: genomics, pathways, metabolism. Frontiers in Physiology. 2014;5:213. doi: 10.3389/fphys.2014.00213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang J, Mani SA, Donaher JL, Ramaswamy S, Itzykson RA, Come C, Savagner P, Gitelman I, Richardson A, Weinberg RA. Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell. 2004;117(7):927–939. doi: 10.1016/j.cell.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 35.Sikder HA, Devlin MK, Dunlap S, Ryu B, Alani RM. Id proteins in cell growth and tumorigenesis. Cancer Cell. 2003;3(6):525–530. doi: 10.1016/s1535-6108(03)00141-7. [DOI] [PubMed] [Google Scholar]
- 36.Lyden D, Young AZ, Zagzag D, Yan W, Gerald W, O’Reilly R, Bader BL, Hynes RO, Zhuang Y, Manova K, et al. Id1 and Id3 are required for neurogenesis, angiogenesis and vascularization of tumour xenografts. Nature. 1999;401(6754):670–677. doi: 10.1038/44334. [DOI] [PubMed] [Google Scholar]
- 37.Gumireddy K, Li A, Kossenkov AV, Cai KQ, Liu Q, Yan J, Xu H, Showe L, Zhang L, Huang Q. ID1 promotes breast cancer metastasis by S100A9 regulation. Molecular Cancer Research: MCR. 2014;12(9):1334–1343. doi: 10.1158/1541-7786.MCR-14-0049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gumireddy K, Li A, Gimotty PA, Klein-Szanto AJ, Showe LC, Katsaros D, Coukos G, Zhang L, Huang Q. KLF17 is a negative regulator of epithelial-mesenchymal transition and metastasis in breast cancer. Nature Cell Biology. 2009;11(11):1297–1304. doi: 10.1038/ncb1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lin CQ, Singh J, Murata K, Itahana Y, Parrinello S, Liang SH, Gillett CE, Campisi J, Desprez PY. A role for Id-1 in the aggressive phenotype and steroid hormone response of human breast cancer cells. Cancer Research. 2000;60(5):1332–1340. [PubMed] [Google Scholar]
- 40.Valrance ME, Brunet AH, Welsh J. Vitamin D receptor-dependent inhibition of mammary tumor growth by EB1089 and ultraviolet radiation in vivo. Endocrinology. 2007;148(10):4887–4894. doi: 10.1210/en.2007-0267. [DOI] [PubMed] [Google Scholar]
- 41.Sherman MH, Yu RT, Engle DD, Ding N, Atkins AR, Tiriac H, Collisson EA, Connor F, Van Dyke T, Kozlov S, et al. Vitamin D receptor-mediated stromal reprogramming suppresses pancreatitis and enhances pancreatic cancer therapy. Cell. 2014;159(1):80–93. doi: 10.1016/j.cell.2014.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Eliassen AH, Warner ET, Rosner B, Collins LC, Beck AH, Quintana LM, Tamimi RM, Hankinson SE. Plasma 25-Hydroxyvitamin D and Risk of Breast Cancer in Women Followed over 20 Years. Cancer Research. 2016;76(18):5423–5430. doi: 10.1158/0008-5472.CAN-16-0353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ferrer-Mayorga G, Gomez-Lopez G, Barbachano A, Fernandez-Barral A, Pena C, Pisano DG, Cantero R, Rojo F, Munoz A, Larriba MJ. Vitamin D receptor expression and associated gene signature in tumour stromal fibroblasts predict clinical outcome in colorectal cancer. Gut. 2016 doi: 10.1136/gutjnl-2015-310977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Howe LR, Subbaramaiah K, Hudis CA, Dannenberg AJ. Molecular pathways: adipose inflammation as a mediator of obesity-associated cancer. Clinical Cancer Research: An official journal of the American Association for Cancer Research. 2013;19(22):6074–6083. doi: 10.1158/1078-0432.CCR-12-2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Simpson ER, Brown KA. Obesity and breast cancer: role of inflammation and aromatase. Journal of Molecular Endocrinology. 2013;51(3):T51–59. doi: 10.1530/JME-13-0217. [DOI] [PubMed] [Google Scholar]
- 46.Wang X, Simpson ER, Brown KA. Aromatase overexpression in dysfunctional adipose tissue links obesity to postmenopausal breast cancer. The Journal of Steroid Biochemistry and Molecular Biology. 2015;153:35–44. doi: 10.1016/j.jsbmb.2015.07.008. [DOI] [PubMed] [Google Scholar]


