Abstract
Background: High flow nasal cannula therapy (HFNC) has been widely adopted for respiratory distress, and evidence suggests that purging dead space of the upper airway improves gas fractions in the lung. This study tests the hypothesis that HFNC with room air could be as effective as low flow oxygen in chronic obstructive pulmonary disease (COPD).
Methods: Thirty-two COPD patients prescribed 1 - 2 L/min of oxygen were studied. The conditions tested consisted of a control (CTRL; no therapy), then in random order HFNC and prescribed low flow oxygen (LFO). HFNC was the highest flow tolerated up to 35 L/min without supplemental oxygen. Arterial blood gases (ABGs), respiratory rate (RR), heart rate (HR) and tidal volume (VT) were measured at the end of each condition.
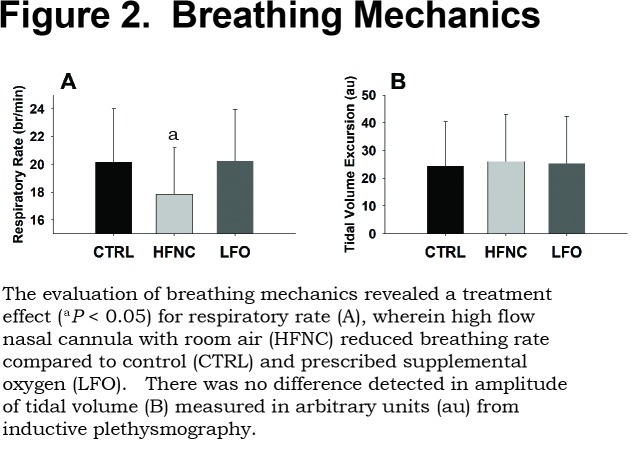
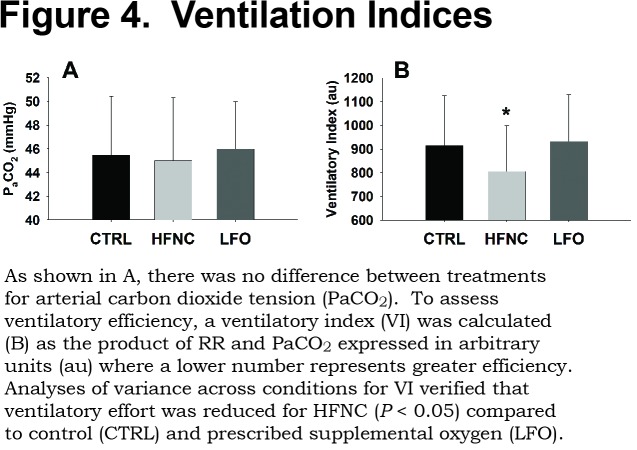
Results: Arterial oxygen (PaO2) was greater (p < 0.001) for LFO than both HFNC and CTRL (CTRL=57.4±6.1mmHg, HFNC=58.6±8.3mmHg, LFO=72.6±10.2mmHg). HFNC reduced RR by 11% (p<0.05) from CTRL and LFO (CTRL=20.2±3.8br/min, HFNC=17.9±3.3br/min, LFO=20.2±3.7br/min) with no differences in VT. There were no differences between arterial carbon dioxide (PaCO2) (CTRL=45.5±4.9mmHg, HFNC=45.0±5.3mmHg, LFO=46.0±3.9mmHg).
Conclusions: HFNC resulted in a clinically relevant reduction in ventilatory effort with no change in ABG indicating a gas equilibrium effect of purging anatomical dead space.
Clinical Trial Registration: ClinicalTrials.gov ID: NCT00990210
Keywords: high flow nasal cannula, high velocity nasal insufflation, work of breathing, dead space, ventilatory efficiency, oxygenation, ventilation
Introduction
Heated, humidified high-flow nasal cannula therapy (HFNC) has been widely adopted over the past decade as a therapeutic intervention for patients in respiratory distress. The operating premise is that by approximating a patient’s inspiratory flow demand with the flow of respiratory gas from a nasal cannula, the patient will inhale the intended gas mixture without dilution via the entrainment of room air and provide for washout of nasopharyngeal dead space.1 However, the technical aspect is that HFNC devices must be able to heat and humidify the delivered gas to at or near body temperature to avoid drying and possible injury to the nasal mucosa2,3 and subsequent infections.4
The purging of the anatomical dead space contributes to improved fractions of alveolar oxygen and carbon dioxide (CO2) independent of any other respiratory support mechanism. In this regard, the end-expiratory gas destined to be re-breathed is replaced with inspiratory gas that is higher in oxygen and lower in CO2.5-7 It is plausible that, based on this mechanism, in the absence of a change in breathing pattern, arterial oxygenation (PaO2) would improve and arterial CO2 (PaCO2) would be reduced.
In previous studies, HFNC has been shown to provide respiratory assistance particularly with respect to dyspnea.8,9 Studies in acute care settings have established the superiority of HFNC over conventional means of oxygen therapy, with respect to both oxygenation and ventilation (respiratory rate) outcomes.10,11 However, little has been done to manipulate the application of HFNC to distinguish specific mechanisms of action. Whereas animal data show that the dead space flush is a principle mechanism of action,6 most human studies have not been mechanistic in nature.
The objective of the current study was to measure the effect of HFNC with room air on blood gas parameters and respiratory patterns in patients with stable chronic obstructive pulmonary disease (COPD) who are currently prescribed continuous use of 1-2 liters of oxygen per minute at rest. We hypothesized that because of nasopharyngeal flush, HFNC with room air would result in the equivalent arterial oxygen tension and hemoglobin saturation (SaO2) as with low flow oxygen cannula. Secondary endpoints were related to ventilatory indices such as arterial CO2 tension, breathing pattern and dyspnea.
Methods
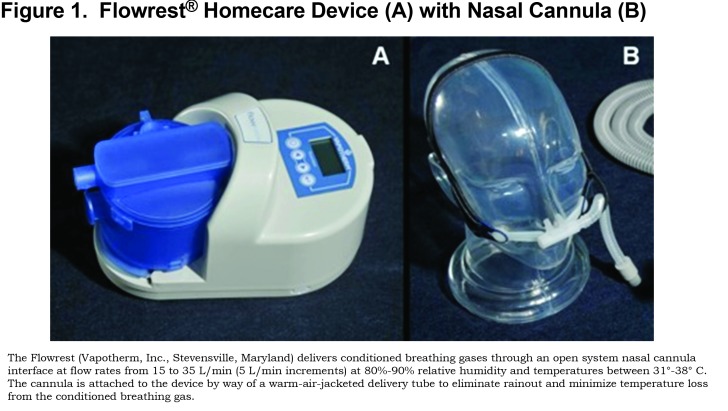
Thirty-two patients were studied under 3 conditions in a repeated-measures experimental design. Participants were first evaluated with neither oxygen nor HFNC support (control; CTRL), and then randomized to receive heated, humidified HFNC with room air (Flowrest®, Vapotherm, Inc., Stevensville, Maryland; Figure 1) and their prescribed low flow oxygen therapy (LFO). Supplemental oxygen was not used with HFNC because the study was intended to identify if purging the upper airway between breaths (i.e., replacing expiratory gas that is approximately 16% oxygen with room air that is 21% oxygen) can achieve an equivalent increase in alveolar, and subsequently arterial, PO2 compared to low flow oxygen supplementation. Participants were stabilized on room air for 20 min between each arm of the study. The study protocol was approved by the Veterans Administration Pittsburgh Healthcare System’s Institutional Review Board.
Patient Selection Criteria
Inclusion criteria were set such that participants must be greater than 40 years of age, have a greater than 10 pack years smoking history for cigarettes, carry a diagnosis of COPD, and have been prescribed continuous oxygen at 1 to 2L/min at rest.
Exclusion criteria included the following: COPD exacerbation in the previous 2 months, abnormal Allen's test, an unstable comorbidity, or women who are pregnant, have not been through menopause (either natural or surgical) or are not using birth control.
Study Procedures
After obtaining informed consent participants were enrolled for an approximately 3-hour session in the sleep disorders laboratory. As a first step, all participants completed baseline spirometry using the EasyOne portable spirometer (Niche Medical, Sydney, Australia) to provide a lung function profile for each patient. Following spirometry, participants were fitted with monitoring equipment including respiratory inductive plethysmography belts, electrocardiogram (ECG) leads and pulse oximetry probes (Viasys sleep data acquisition system, CareFusion, San Diego, California). Once connected, and prior to the initiation of testing, the dyspnea scales were explained to the participant so that perceived respiratory effort could be assessed at later points.
Patients were tested at CTRL, and then at HFNC and LFO in random order. During the 30 min at each condition, heart rate (HR), respiratory rate (RR), change in tidal volume excursion and pulse oximetry (SPO2) were monitored continuously and recorded by a modified respiratory montage using the sleep laboratory acquisition system. Additionally, SPO2 was recorded every 5 min from a bedside monitor. Criteria were set that if the participant's SPO2 fell below 80% for 15 min then the study for that participant would be halted. In the last 5 min of each condition an arterial blood sample was drawn from the radial artery by trained staff, and analyzed for respiratory gas composition.
In the last minute of each condition, the Borg and the modified Medical Research Council (mMRC) scales were used to assess perceived dyspnea. The Borg Dyspnea scale is a scale of numbers from 0 to 10, where 0 is associated with no dyspnea and 10 being maximal dyspnea. The participant is asked how much difficulty their breathing is causing them right now. ThemmRC dyspnea scale is a scale from 0 to 4 where each number is associated with a statement about the participant's breathlessness. A score of 0 represents breathlessness only during strenuous activity and 4 represents extreme breathlessness during minimal activity.
For the HFNC treatment condition, participants were started on the therapy at the lowest setting of 15L/min of nasal cannula flow and increased by 5L/min increments every 5 min as tolerated by the participant. The objective was to use the greatest flow rate that was comfortable for the patient. For the LFO treatment condition, participants wore conventional oxygen cannulae with their prescribed resting liter flow of oxygen.
HR and RR were determined by counting each R wave from the EKG and each inspiratory waveform signal for a 2 min period from the first 5 min of the recording for each condition, and a 2 min period from the last 5 min of the recording. The reason for having an early versus late group was to examine the possibility of change in cardiac or respiratory parameters from the beginning of the 30 min experimental condition to the end of it.
To determine if there was a change in ventilatory efficiency, we calculated a ventilatory index (VI), where VI is the product of RR and PaCO2 expressed in arbitrary units (au). The reason for this determination was to allow us to look for changes in ventilatory efficiency of the respiratory system as a whole (i.e., a change in RR without a concomitant change in CO2 elimination, or vice versa). Thus, a change in VI would be indicative of a change in efficiency of the system where a lower value represents greater efficiency.
Relative changes in VT were determined from fractional change in amplitude of thoraco-abdominal excursion waveforms using the respiratory inductance plethysmography belts of the data acquisition system. The respiratory inductance plethysmography belts were applied carefully at the beginning of the session and remained unperturbed during data acquisition for the 3 conditions. This allows us to look across conditions for relative changes in respiratory excursion, which would represent changes in VT. Mean amplitude was measured in arbitrary units (au) from a consecutive 1 min series of VT waveforms during the last 5 min of each condition.
Data Analysis
The study was powered to detect a 10% difference in PaO2 between conditions. For each outcome parameter, analyses of variance were performed to identify differences as a function of treatment and treatment by time interactions where applicable. Pearson correlation analyses were performed between change in respiratory parameters with HFNC and baseline spirometry measures to determine if baseline lung function was predictive of treatment results. Significance was accepted at P<0.05.
Results
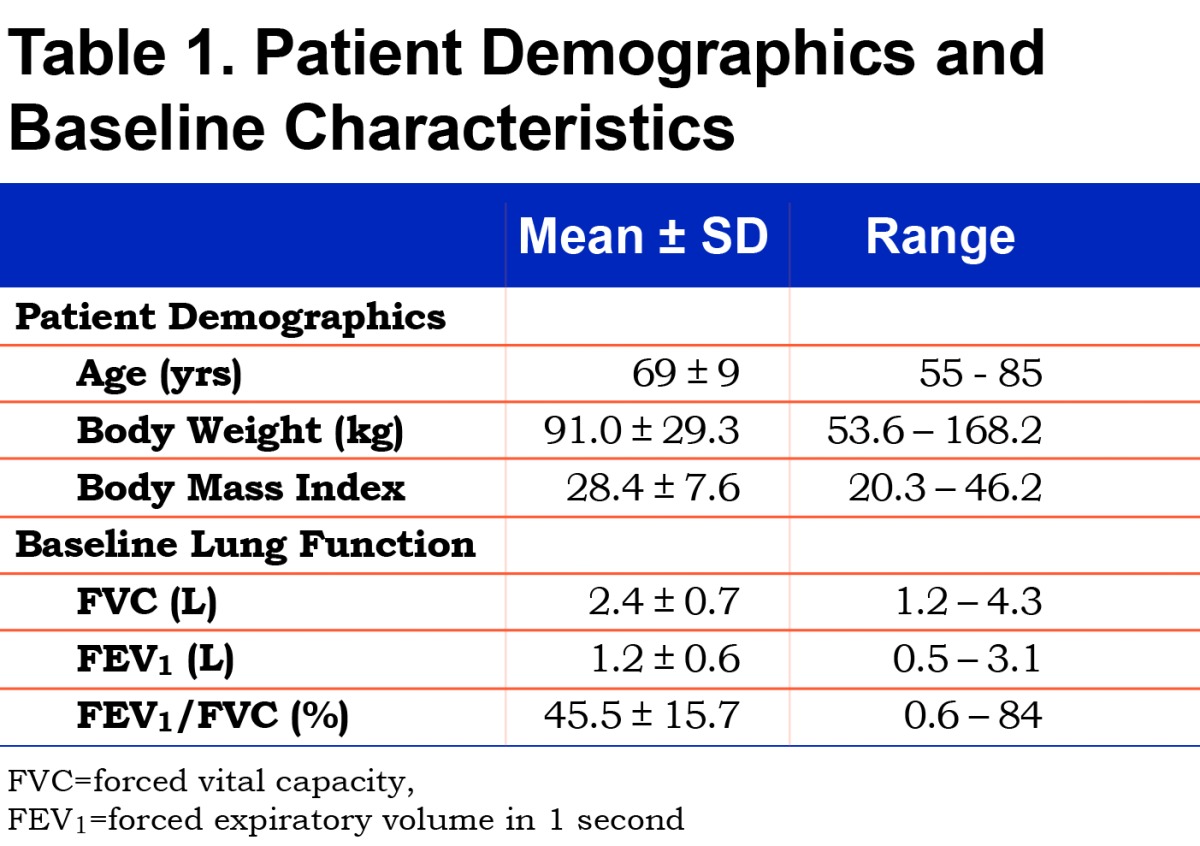
Participants were all males with a mean age of 69±9 yrs with an age range of 55 to 85 years. All participants had clinically confirmed COPD and were prescribed either 1 or 2 liters oxygen at rest. Spirometric testing demonstrated that the participants had significant airflow obstruction wherein the mean forced expiratory volume in 1 second (FEV1) was 1.16±0.60 L (range=0.28 to 3.06 L) and the mean FEV1/FVC ratio was 45.5±15.7%.
Participant demographic and lung function data are presented in Table 1. No participant experienced an adverse event during the testing procedures. The flow rates used for HFNC ranged from 15L/min to 35L/min.
There was no difference in HR as a function of treatment (Table 2), nor was there a change in amplitude of VT (CTRL=24±16au, HFNC=26±17au, LFO=25±17au). However, HFNC had a significant impact on RR, reducing frequency by 11% from both CTRL and LFO (p < 0.05; Table 2). RR for CTRL compared to LFO was not different. RR and VT comparisons are presented in Figure 2.

Oxygenation parameters from the 3 treatment conditions are shown in Figure 3. HFNC was not different from CTRL, where HFNC PaO2 was 58.6±8.3mmHg with a SaO2 of 90.4±3.4%, and CTRL PaO2 was 57.4±6.1mmHg with a SaO2 of 89.8±3.2%. LFO was greater than both HFNC and CTRL (p<0.001) with a PaO2 that was 72.6±10.2mmHg and a SaO2 of 94.0±3.4%.
There were no significant differences between the groups for PaCO2. The mean values for the CTRL, HFNC, and LFO were 45.5±4.9mmHg, 45.0±5.3mmHg, and 46.0±3.9mmHg, respectively. The analysis of VI revealed a significant effect of treatment where ventilation was reduced 12% with HFNC compared to CTRL and reduced 14% with HFNC compared to LFO (p<0.05; CTRL=914±210au, HFNC=805±192au, LFO=932±197au). Ventilation indices are presented in Figure 4.
Table 2 shows the data for trending HR and RR over time. For HR there was no impact associated with time on therapy. For RR there was a significant increase over time for the HFNC condition from 16.4±3.2 br/min to 17.7±3.5 br/min (p<0.01) but not for the control or LFO conditions.
Correlations of patient responsiveness to HFNC (percent decrease in VI from CTRL) with baseline spirometry measures showed a moderate-low level of association for FVC (r=0.43; p<0.05), but non-significant associations for FEV1 (r=0.31), and FEV1/FVC (r=0.18). The correlation between ventilatory responsiveness to HFNC and FVC is shown in Figure 5.
Discussion
This is the first study to examine mechanisms of action of HFNC therapy in a clinically important population: elderly patients with advanced COPD. Our findings confirm that HFNC therapy provides a clinically meaningful effect on respiratory parameters as well as showing that the main effect on the respiratory system was a reduction in RR during the acute application of HFNC therapy. The reduction in RR likely equates to a reduction in work of breathing by way of a decreased RR without a change in PaCO2 or VT. Our data supports the concept that HFNC flushes the upper airway dead space of CO2 and thereby allows for inhalation of a lower inspired fraction of CO2.6,7 In this regard, the current study represents the in-human translational component to complement prior animal studies that demonstrated the ventilatory mechanisms of dead space purge by high flow nasal cannula.6
Neither arterial oxygen tension nor SaO2 improved from the CTRL condition with HFNC, whereas it did rise significantly with oxygen therapy. However, the most remarkable finding of the study was a reduction in RR for HFNC compared to both CTRL and oxygen therapy, which occurred without a concomitant reduction in PaO2. Therefore, it can be inferred that there was in fact an improvement in alveolar fraction of oxygen with HFNC that counterbalanced the reduction in minute ventilation associated with a concomitant improvement in CO2 ventilation.
An interesting aspect regarding the decrease in RR is the lack of significant change in the PaCO2, which was verified by the calculation of VI. These findings suggest that the reduction in RR is either offset by an increased VT or that effective exhalation of CO2 is increased such that the mixed dead space CO2 fraction is less. The respiratory inductive plethysmography data indicates that there was no change in VT excursion. Thus, it is evident that RR was reduced in response to more efficient ventilation, despite persistent elevated PaCO2 in these advanced COPD patients. These data are in agreement with a recent study of HFNC on patients presenting with acute respiratory failure, wherein, along with dyspnea, RR was significantly reduced compared to oxygen therapy via face mask.10
Braunlich and colleagues demonstrated similar ventilatory outcomes administering HFNC to severe, hospitalized COPD patients.12 RR was reduced with HFNC, independent of flow rate above 20L/min, and despite a reduced minute volume hypercapnia, was reduced in a flow dependent manner with 20L/min and then 30L/min. These trials agree on the influence of HFNC on CO2 removal (ventilatory efficiency), however, the lack of reduction in PaCO2 or increase in VT in the current study may be attributed to the stability of the COPD patients evaluated here. Similarly, in a prior study by Braunlich and colleagues HFNC increased VT in hospitalized COPD patients, but not in healthy volunteers, which the authors reason is related to the need for the COPD patients to achieve more ventilatory work.13
In a study of COPD patients during rest and exercise with HFNC versus conventional oxygen therapy, Chatila and colleagues also compared oxygenation and ventilation indices.9 These authors compared low flow oxygen to HFNC at 20L/min wherein they controlled inspired oxygen fraction (FIO2) by blending the HFNC gas composition to what would be reaching the lung in the low flow condition considering entrainment of room air during inhalation. Under these circumstances, the authors found that during both rest and exercise, PaO2 and SPO2 were significantly improved with HFNC versus low flow oxygen, again with a decrease in RR. However, a critical difference in design of these 2 studies is that in the current study supplemental oxygen was not given with the HFNC condition. It is therefore likely that the reason why PaO2 during HFNC did not increase was due to the reduction in RR, and therefore, in minute ventilation.
Recent studies of HFNC in adult in hypoxemic respiratory failure indicate that HFNC may be a more effective treatment for hypoxemia compared to non-invasive positive pressure ventilation.14,15 While these studies report positive outcomes for oxygenation support, they focused specifically on patients with stable ventilatory parameters and did not show differences in ventilatory indices; note, Stephan et al present PaCO2 and breathing frequency data without significant and clinically meaningful reductions.15 The current study shows a ventilatory effect, owing to a decreased minute ventilation without an effect on PaCO2, on patients with a consistent and stable oxygen response. Mechanistic evidence is suggesting that the ventilatory effect may be attributed to properties of the cannula, and subsequently the flow parameters. Frizzola and colleague showed in an animal model of respiratory failure that a cannula design optimizing the flush potential results in improved physiologic ventilation outcomes compared to a cannula that was more occlusive of the nares.6 Subsequent computation fluid modeling work demonstrated that cannula flow velocity plays a major role, in that greater velocity adds turbulent energy and accelerates the vortices formed on the nasal cavity.16 In this regard, the current trial was performed using the Vapotherm Flow Rest device platform that has a remarkably smaller cannula prong tip diameter compared to the Optiflow™ Cannulae (Fisher and Paykel Healthcare) used in the Frat and Stephan trials (FlowRest approximately=3.36mm ID; adult Optiflow Cannula approximately=5.72mm ID).14,15 This difference in cannula prong tip diameters results in a 189% difference, a 3-fold increase, in exit flow velocity for the cannula used in this study (normalized to 30L/min of volumetric flow typically used in this study: Flow rest approximately=28.21 m/sec; Adult Optiflow approximately 9.75 m/sec). Therefore, at the lower flow rates used in the current trial compared to the large clinical evaluations, produced a much greater velocity and dynamic energy in the extrathoracic space. Cannula diameter was noted as 4.9mm in the study by Braunlich and colleagues demonstrating a ventilation effect in COPD patients,12 but it is not specified whether this is the ID or OD. Nonetheless, the cannula ID is assumed to be in-between the dimensions discussed above.
HFNC may have provided a mild additional pressure in the upper airway, thus allowing for lower resistance in the upper airway and a lower respiratory rate. It is known from previous work that HFNC therapy can pressurize the upper airway up to 4 cmH2O. 9,17,18 In our study, we used a wide range of HFNC flow rates and saw benefits in patients who did not receive relatively high flows. Nonetheless, while the pressure effect may have been present it cannot explain discontinuity between minute ventilation and arterial blood gas values; i.e., ventilation decreased without a rise in PaCO2 or a fall in PaO2.
A limitation to this study in determining if HFNC can improve oxygenation in COPD patients is the time course of the protocol. It may be that 20 min is too short a time for HFNC to have an equilibration effect on oxygenation, as well as a downward trend in chronic hypercapnia. Another intriguing aspect of longer-term studies is the impact of ideally conditioned gas, as provided by HFNC systems, to improve mucocilliary clearance. Research is beginning to emerge suggesting a significant and clinically relevant improvement in mucocilliary function and secretion management.19,20 The present study was not designed to examine the effect of humidification on COPD patients.
In conclusion, our study does not support HFNC with room air as a successful stand-alone therapy for the purpose of oxygenating hypoxemic COPD patients who already require supplemental oxygen. However, HFNC therapy appears to improve respiratory efficiency through its effects on clearance of anatomic dead space CO2 and reductions in RR. Thus, HFNC therapy with small amounts of titrated oxygen may do more for COPD patients than supplemental oxygen alone, although this will require further study.
Abbreviations
high flow nasal cannula, HFNC; chronic obstructive pulmonary disease, COPD; control condition, CTRL; low flow oxygen treatment condition, LFO; arterial blood gas analysis, ABG; respiratory rate, RR; heart rate, HR; tidal volume, VT; carbon dioxide, CO2; arterial oxygen oxygenation, PaO2; arterial carbon dioxide oxygenation, PaCO2; electrocardiogram, ECG; pulse oximetry, SpO2; modified Medical Research Council, mMRC; ventilatory index, VI; arbitrary units, au; forced expiratory volume in 1 second, FEV1; forced vital capacity, FVC
Funding Statement
This study was sponsored by Vapotherm, Inc., Exeter, New Hampshire and conducted at the Veterans Administration Pittsburgh Healthcare System, Pittsburgh, Pennsylvania.
References
- 1.Dysart K,Miller TL,Wolfson MR,Shaffer TH.Research in high flow therapy: mechanisms of action. Resp Med. 2009;103(10):1400-1405. doi: https://doi.org/10.1016/j.rmed.2009.04.007 [DOI] [PubMed] [Google Scholar]
- 2.Williams R,Rankin N,Smith T,Galler D,Seakins P.Relationship between the humidity and temperature of inspired gas and the function of the airway mucosa. Crit Care Med. 1996;24(11):1920-1929. doi: https://doi.org/10.1097/00003246-199611000-00025 [DOI] [PubMed] [Google Scholar]
- 3.Kelly MG,McGarvey LP,Heaney LG,Elborn JS.Nasal septal perforation and oxygen cannulae. Hosp Med. 2001;62(4):248. [PubMed] [Google Scholar]
- 4.Kopelman AE,Holbert D.Use of oxygen cannulas in extremely low birthweight infants is associated with mucosal trauma and bleeding, and possibly with coagulase-negative staphylococcal sepsis. J Perinatol. 2003;23(2):94-97. doi: https://doi.org/10.1038/sj.jp.7210865 [DOI] [PubMed] [Google Scholar]
- 5.Dewan NA,Bell CW.Effect of low flow and high flow oxygen delivery on exercise tolerance and sensation of dyspnea. A study comparing the transtracheal catheter and nasal prongs. Chest. 1994;105(4):1061-1065. doi: https://doi.org/10.1378/chest.105.4.1061 [DOI] [PubMed] [Google Scholar]
- 6.Frizzola M,Miller TL,Rodriguez ME,et al. High-flow nasal cannula: impact on oxygenation and ventilation in an acute lung injury model. Pediatr Pulmonol. 2011;46(1):67-74. doi: https://doi.org/10.1002/ppul.21326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moller W,Celik G,Feng S,et al. Nasal high flow clears anatomical dead space in upper airway models. J Appl Physiol. 2015;118(12):1525-1532. doi: https://doi.org/10.1152/japplphysiol.00934.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Calvano TP,Sill JM,Kemp KR,Chung KK.Use of a high-flow oxygen delivery system in a critically ill patient with dementia. Respir Care. 2008;53(12):1739-1743. [PubMed] [Google Scholar]
- 9.Chatila W,Nugent T,Vance G,Gaughan J,Criner GJ.The effects of high-flow vs low-flow oxygen on exercise in advanced obstructive airways disease. Chest. 2004;126(4):1108-1115. doi: https://doi.org/10.1378/chest.126.4.1108 [DOI] [PubMed] [Google Scholar]
- 10.Roca O,Riera J,Torres F,Masclans JR.High-flow oxygen therapy in acute respiratory failure. Respir Care. 2010;55(4):408-413. [PubMed] [Google Scholar]
- 11.Parke RL,McGuinness SP,Eccleston ML.A preliminary randomized controlled trial to assess effectiveness of nasal high-flow oxygen in intensive care patients. Respir Care. 2011;56(3):265-270. doi: https://doi.org/10.4187/respcare.00801 [DOI] [PubMed] [Google Scholar]
- 12.Braunlich J,Kohler M,Wirtz H.Nasal highflow improves ventilation in patients with COPD. Int J Chron Obstruct PulmDis. 2016;11:1077-1085. doi: https://doi.org/10.2147/COPD.S104616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Braunlich J,Beyer D,Mai D,Hammerschmidt S,Seyfarth HJ,Wirtz H.Effects of nasal high flow on ventilation in volunteers, COPD and idiopathic pulmonary fibrosis patients. Respiration. 2013;85(4):319-325. doi: https://doi.org/10.1159/000342027 [DOI] [PubMed] [Google Scholar]
- 14.Frat JP,Thille AW,Mercat A,et al. High-flow oxygen through nasal cannula in acute hypoxemic respiratory failure. New Eng J Med. 2015;372(23):2185-2196. doi: https://doi.org/10.1056/NEJMoa1503326 [DOI] [PubMed] [Google Scholar]
- 15.Stephan F,Barrucand B,Petit P,et al. High-flow nasal oxygen vs noninvasive positive airway pressure in hypoxemic patients after cardiothoracic surgery: A randomized clinical trial. JAMA. 2015;313(23):2331-2339. doi: https://doi.org/10.1001/jama.2015.5213 [DOI] [PubMed] [Google Scholar]
- 16.Miller TL,Saberi B,Saberi S.Computational fluid dynamics modeling of extrathoracic airway flush: evaluation of high flow nasal cannula design elements. J Pulm Respir Med. 2016;6(5):376. doi: https://doi.org/10.4172/2161-105X.1000376 [Google Scholar]
- 17.Parke R,McGuinness S,Eccleston M.Nasal high-flow therapy delivers low level positive airway pressure. Br J Anaesth. 2009;103(6):886-890. doi: https://doi.org/10.1093/bja/aep280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McGinley BM,Patil SP,Kirkness JP,Smith PL,Schwartz AR,Schneider H.A nasal cannula can be used to treat obstructive sleep apnea. Am J Respir Crit Care Med. 2007;176(2):194-200. doi: https://doi.org/10.1164/rccm.200609-1336OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hasani A,Chapman TH,McCool D,Smith RE,Dilworth JP,Agnew JE.Domiciliary humidification improves lung mucociliary clearance in patients with bronchiectasis. Chron Respir Dis. 2008;5(2):81-86. doi: https://doi.org/10.1177/1479972307087190 [DOI] [PubMed] [Google Scholar]
- 20.Rea H,McAuley S,Jayaram L,et al. The clinical utility of long-term humidification therapy in chronic airway disease. Respir Med. 2010;104(4):525-533. doi: https://doi.org/10.1016/j.rmed.2009.12.016 [DOI] [PubMed] [Google Scholar]