Abstract
We present a case of a 70-year-old man who was admitted with rupture of an abdominal aneurysm 4 years after endovascular aneurysm repair. He was compliant with yearly follow-up computed tomography angiography. One month earlier, his computed tomography angiogram showed perfect exclusion of the aneurysm and no endoleak. We explanted the stent graft and confirmed effective sealing, and the graft was intact. We found no signs of infection during 2 years of follow-up. This rupture is nonpredictable and unexplained and illustrates that unremarkable imaging does not guarantee prevention of rupture. This case shows that the ultimate failure of endovascular aneurysm repair cannot be prevented despite surveillance protocols.
The ultimate goal of elective endovascular aneurysm repair (EVAR) is to prevent aneurysm rupture. To ensure that post-EVAR rupture incidence is as low as possible, strict imaging follow-up is recommended by global guidelines. These intensive surveillance programs are successful in preventing ruptures, but their efficiency is the subject of debate as surveillance results in high costs and is a huge burden for patients and hospitals.1 To try to increase efficiency, patients with exceptionally low risk of post-EVAR complications could be identified.2 Even in these low-risk groups, unexpected rupture could not be completely prevented, but a clear cause was then detected on imaging at hospital admittance, usually infection of the endograft, and preceded by clinical manifestations. We present a case of completely unexpected post-EVAR rupture, 1 month after follow-up computed tomography angiography (CTA) showing an optimal result, in which no cause was detected preoperatively, postoperatively, or during long-term follow-up, and it serves to emphasize our incomplete understanding of post-EVAR processes.
Consent for publication was obtained from the patient.
Case report
In 2010, we performed elective EVAR in a 70-year-old man for a 58-mm infrarenal aneurysm. The anatomy was inside instructions for use for all available stent grafts. The infrarenal neck was 24 mm wide and 35 mm in length. The right iliac diameter was 19 mm and the left 17 mm. There was only minimal calcification at the level of the neck, no thrombus, and 54 degrees of angulation. The patient was in good clinical condition and used a statin, an angiotensin-converting enzyme inhibitor, and an inhaler for his chronic obstructive pulmonary disease. A 28-mm C3 Gore Excluder (W. L. Gore & Associates, Flagstaff, Ariz) with iliac extensions of 20 mm (left) and 23 mm (right) was implanted successfully. At that time, the distal descending aorta was ectatic (43 mm diameter) but returned to normal diameter 2 cm above the renal arteries. The first postoperative CTA scan within 30 days (Fig 1) showed a good 19-mm proximal seal length, a 21-mm right distal seal length, and a 13-mm left distal seal length with a type II endoleak from a lumbar artery. For surveillance of the total aorta, yearly CTA imaging was performed, which showed steady shrinkage of the abdominal aortic aneurysm sac to 38 mm after 4 years with continuous good proximal and distal seal (proximal seal length of 19 mm, right and left distal seal length of 19 mm after 4 years) without dilation at the sealing zones. The type II endoleak showed only on the 30-day CTA scan but not on any of the following scans. Over time, the suprarenal aneurysm grew slowly in diameter but not in length. In 2012, the patient was admitted with an ischemic right leg because of an occlusion of the right EVAR limb. A thrombectomy was performed, and he was prescribed a vitamin K antagonist. His last CTA scan was performed 4 years after EVAR (Fig 2) and showed no endoleak and almost complete shrinkage of the aneurysm. One month later, he presented to the emergency department, hemodynamically stable, with acute onset of abdominal pain. A new CTA scan (Fig 3) showed a rupture of the abdominal aneurysm with a large retroperitoneal hematoma. The diameter of the aneurysm had increased to 52 mm with continuous good proximal and distal seal but with recurrence of the type II endoleak. The suprarenal aneurysm was 52 mm in diameter and was intact. Through a median laparotomy, the aorta was exposed, and a rupture was clearly seen at the right lateral ventral side of the aneurysm. Before clamping, the aneurysm sac was opened to reveal the possible cause of rupture. Good proximal and distal seal was found, also after manipulation, and except for minimal backbleeding from lumbar arteries, no other endoleak was found despite a physiologic blood pressure. A standard tube graft was implanted, and after careful inspection of the explanted endograft, no irregularities were found. The patient recovered uneventfully. No signs of infection were found then or during the following 2 years. We extensively studied conformational changes of the endograft during the complete follow-up period using three-dimensional software and did not notice any differences over time. In 2016, his suprarenal aneurysm had increased to 69 mm, which was successfully treated with a fenestrated and branched endograft. DNA testing has been done, but no genetic predisposition was found for him or his family.
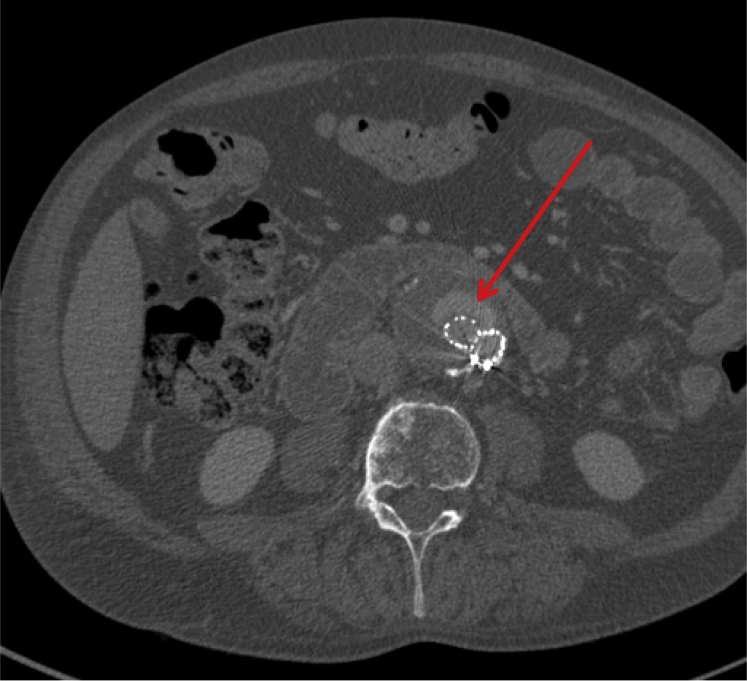
Fig 1.
First postoperative computed tomography angiography (CTA) scan. The diameter of the aneurysm is 58 mm. The arrow points out the type II endoleak.
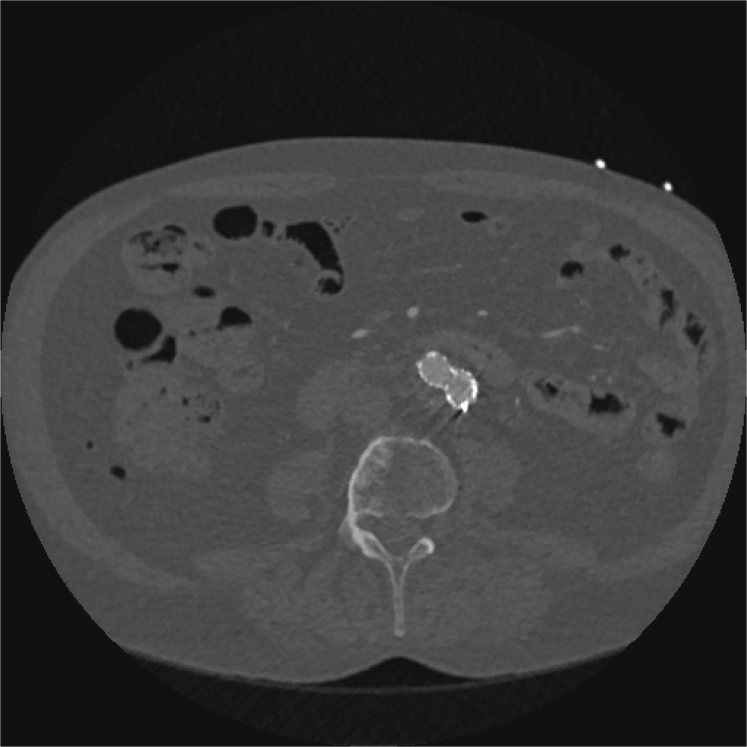
Fig 2.
Computed tomography angiography (CTA) scan 1 month before rupture showing complete shrinkage of the aneurysm sac with a diameter of 23 mm.
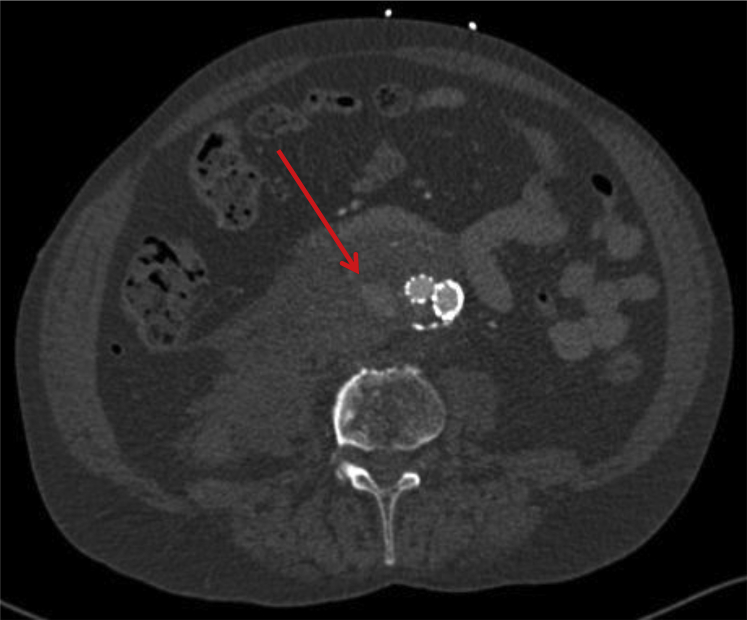
Fig 3.
Computed tomography angiography (CTA) scan at time of rupture showing the retroperitoneal hematoma. The diameter of the aneurysm is 52 mm. The arrow points out the type II endoleak.
Discussion
Rupture after EVAR is rare, with a contemporary risk estimated to be below 1% per year. Intensive follow-up is done to detect complications like direct endoleaks, sac enlargement, or anything else that warrants a secondary intervention to minimize the chance of rupture. This strategy is well supported by current literature1, 3, 4, 5 but remains inefficient as the majority of patients do not benefit from routine imaging. This is costly and consumes resources.6 Also relevant is the fact that complications frequently arise without warning, even in patients with recent imaging, as in our case.5, 7 On the other hand, if there is clear shrinkage of the sac, adequate proximal and distal seal, and no signs of a type I or type III endoleak or persistent type II endoleak, we are confident that the aneurysm is optimally excluded, and surveillance, especially with imaging, may be restricted at least during the midterm.2, 6
Here we present a case of rupture after EVAR with all the signs of a long-lasting successful aneurysm exclusion during follow-up. To our knowledge, this rupture is nonpredictable and unexplained and serves to emphasize our incomplete understanding of post-EVAR processes. In the absence of infection, with demonstrated aneurysm shrinkage of 35 mm during 4 years, confirmed intraoperative effective proximal and distal seal, and an intact graft, the underlying cause of rupture is still undetermined. The type II endoleak that showed on the CTA scan at the time of rupture was not present on the CTA scan 1 month earlier. It is highly unlikely if not impossible that this endoleak could have caused growth of the aneurysm to its original size within a month and led to a rupture. The fact that rupture occurred only 1 month after apparently unremarkable CTA is particularly worrisome. As previously pointed out by Dias et al,7 ruptures may occur after examinations that show no signs of concern, which raises more doubts about the utility of routine imaging follow-up after EVAR.
Conclusions
Surveillance after EVAR is done to detect secondary complications that can be treated to prevent rupture. However, many secondary interventions are performed in the presence of symptoms, not as a result of follow-up imaging. Also, apparently unremarkable imaging does not guarantee prevention of rupture, as in the presented case, which calls into question the utility of routine image surveillance. With the development of new imaging tools like enhanced ultrasound, we might get better at identifying risk factors for rupture in the future. The 15-year follow-up of the EVAR 1 trial showed inferior survival after EVAR compared with open repair, which was mainly attributed to post-EVAR rupture.8 In young and fit patients, the choice between EVAR and open repair should therefore be carefully weighed. The lesson learned from this case is that the ultimate failure of EVAR, although a rare occurrence, may be impossible to predict even with strict surveillance protocols.
Footnotes
Author conflict of interest: H.J.M.V. is a consultant for Medtronic, W. L. Gore, Endologix, Arsenal AAA, and Philips.
The editors and reviewers of this article have no relevant financial relationships to disclose per the Journal policy that requires reviewers to decline review of any manuscript for which they may have a conflict of interest.
References
- 1.Nordon I.M., Karthikesalingam A., Hinchliffe R.J., Holt P.J., Loftus I.M., Thompson M.M. Secondary interventions following endovascular aneurysm repair (EVAR) and the enduring value of graft surveillance. Eur J Vasc Endovasc Surg. 2010;39:547–554. doi: 10.1016/j.ejvs.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 2.Bastos Goncalves F., Baderkhan H., Verhagen H.J., Wanhainen A., Björck M., Stolker R.J. Early sac shrinkage predicts a low risk of late complications after endovascular aortic aneurysm repair. Br J Surg. 2014;101:802–810. doi: 10.1002/bjs.9516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Candell L., Tucker L.Y., Goodney P., Walker J., Okuhn S., Hill B. Early and delayed rupture after endovascular abdominal aortic aneurysm repair in a 10-year multicenter registry. J Vasc Surg. 2014;60:1146–1152. doi: 10.1016/j.jvs.2014.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fransen G.A., Vallabhaneni S.R., van Marrewijk C.J., Laheij R.J., Harris P.L., Buth J. Rupture of infra-renal aortic aneurysm after endovascular repair: a series from EUROSTAR registry. Eur J Vasc Endovasc Surg. 2003;26:487–493. doi: 10.1016/s1078-5884(03)00350-2. [DOI] [PubMed] [Google Scholar]
- 5.Wyss T.R., Brown L.C., Powell J.T., Greenhalgh R.M. Rate and predictability of graft rupture after endovascular and open abdominal aortic aneurysm repair: data from the EVAR trials. Ann Surg. 2010;252:805–811. doi: 10.1097/SLA.0b013e3181fcb44a. [DOI] [PubMed] [Google Scholar]
- 6.Bastos Goncalves F., van de Luijtgaarden K.M., Hoeks S.E., Hendriks J.M., ten Raa S., Rouwet E.V. Adequate seal and no endoleak on the first postoperative computed tomography angiography as criteria for no additional imaging up to 5 years after endovascular aneurysm repair. J Vasc Surg. 2013;57:1503–1511. doi: 10.1016/j.jvs.2012.11.085. [DOI] [PubMed] [Google Scholar]
- 7.Dias N.V., Riva L., Ivancev K., Resch T., Sonesson B., Malina M. Is there a benefit of frequent CT follow-up after EVAR? Eur J Vasc Endovasc Surg. 2009;37:425–430. doi: 10.1016/j.ejvs.2008.12.019. [DOI] [PubMed] [Google Scholar]
- 8.Patel R., Sweeting M.J., Powell J.T., Greenhalgh R.M. Endovascular versus open repair of abdominal aortic aneurysm in 15-years' follow-up of the UK endovascular aneurysm repair trial 1 (EVAR trial 1): a randomised controlled trial. Lancet. 2016;388:2366–2374. doi: 10.1016/S0140-6736(16)31135-7. [DOI] [PubMed] [Google Scholar]