Abstract
Giant cell arteritis is a large- and medium-vessel vasculitis that has been described as a systemic disease process with disseminated vessel involvement. Advances in vascular imaging techniques have demonstrated that involvement of the large vessels of the upper and lower limbs may be more prevalent than was once thought, although the clinical implications of this are unknown. Isolated lower extremity claudication without systemic or classic cranial symptoms, especially as a primary manifestation of giant cell arteritis, is rare. We present the case of a patient with isolated bilateral limb claudication that rapidly progressed to critical limb ischemia requiring urgent surgical intervention after steroid therapy. Our patient has consented to the publication of this report.
Case report
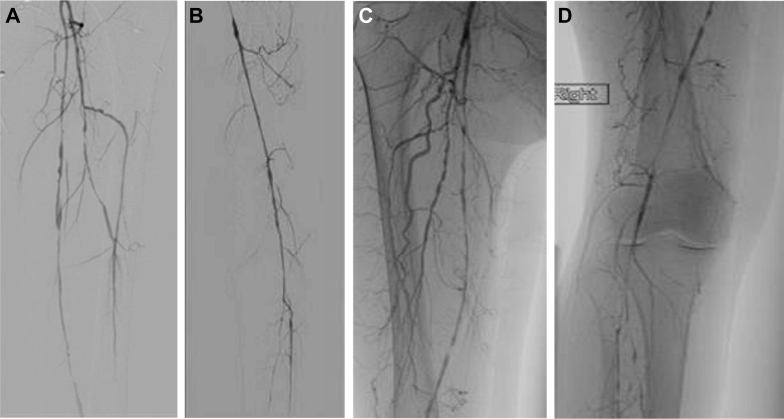
Our patient, a 67-year-old woman with significant cardiac history, was seen in the emergency department with a 3-week history of bilateral lower extremity rest pain, with the left being worse than the right. The patient had never smoked. Her cardiac history was significant for a two-vessel coronary artery bypass graft (CABG) 2 years earlier. Her cholesterol level had always been controlled with statins. On physical examination of her lower extremities, femoral pulses were palpable bilaterally; however, her tibial arteries had only weak Doppler signals. Her preoperative ankle-brachial indices were 0.42 on the left and 0.32 on the right. She was sent to the vascular surgery clinic for further workup and treatment. Computed tomography angiography showed multifocal disease of bilateral lower extremity arteries. Her angiograms (Fig 1) also showed significant disease of the superficial femoral arteries (SFAs), with her right lower extremity involving the popliteal artery. Based on her clinical presentation and imaging results, inflammatory markers such as erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) concentration were checked. The patient had elevated ESR (50 mm/h). CRP concentration was 13 mg/L. She was started on steroids (prednisone 60 mg/day orally) and monitored closely until her inflammatory markers improved. ESR and CRP determinations were repeated in 48 hours, when her ESR was 36 mm/h and CRP concentration was 6 mg/L. After resolution of her inflammatory state, she underwent a right common femoral artery (CFA) endarterectomy along with a femoral to posterior tibial artery bypass with a reverse saphenous vein graft. There was some atherosclerotic disease underlying the vasculitis. We believed it was necessary to provide the patient with the best revascularization possible and therefore performed the endarterectomy. We bypassed to the CFA, which we thought did not appear to be inflamed. However, there was some atherosclerotic disease, and therefore we performed an endarterectomy to ensure the best outcome. The CFA specimen was not sent for pathologic examination. A biopsy specimen of the right SFA was sent for permanent pathology section. Her right lower extremity rest pain symptoms resolved, and she had a palpable posterior tibial artery pulse on examination after the operation.
Fig 1.
A, Angiogram of left superficial femoral artery (SFA) and left profunda artery. B, Angiogram of left SFA, popliteal artery, and tibial arteries. C, Angiogram of right SFA and left profunda artery. D, Angiogram of right SFA, popliteal artery, and tibial arteries.
Five days after her initial surgery, the patient underwent an external iliac, femoral, profunda femoris endarterectomy with bovine patch angioplasty and a Viabahn (W. L. Gore & Associates, Flagstaff, Ariz) stent-assisted angioplasty of the left SFA. Although the use of Viabahn is not ideal, we believed that it was a short-segment stenosis of the SFA on the left lower extremity. Furthermore, her left lower extremity great saphenous vein had been harvested previously for CABG. She had an uncomplicated postoperative course and was discharged to home on an antiplatelet agent and steroid therapy. Her postoperative ankle-brachial indices were 0.81 on the left and 0.88 on the right. Before discharge, the patient had palpable dorsalis pedis and posterior tibial arteries on examination. The patient has been seen in the vascular surgery clinic for her 6-month follow-up visit. Her symptoms have resolved; she has palpable pedal pulses and is currently taking a low-dose prednisone.
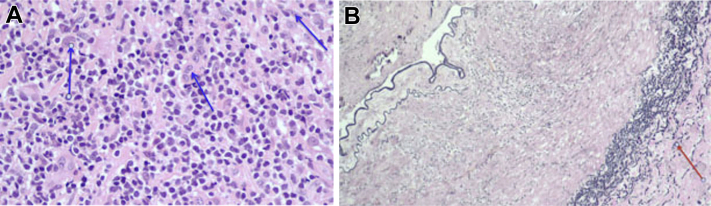
The patient's SFA biopsy specimen was analyzed in two cross sections (Fig 2). One cross section contained a partially to nearly completely occluded lumen with evidence of chronic inflammation, fibrin, and fibrosis. The mural chronic inflammatory cell infiltrate was composed of lymphocytes and mononuclear cells with scattered rare multinucleated giant cells. There were also multifocal small collections of calcification. Elastin stain highlighted a fragmented elastic lamina. The microscopic findings were consistent with vasculitis and suggested a diagnosis of giant cell arteritis (GCA).
Fig 2.
A, Cross section of right superficial femoral artery (SFA). There is a mural chronic inflammatory cell infiltrate composed of lymphocytes and mononuclear cells with scattered rare multinucleated giant cells (arrows). The findings are consistent with a vasculitis and may represent giant cell arteritis (GCA). B, An elastin stain highlights a fragmented elastic lamina (arrow). The findings are consistent with a vasculitis and may represent GCA.
Discussion
GCA, a large- and medium-vessel vasculitis, is the most common form of systemic vasculitis in adults, with a prevalence of between 1.47 and 200 per 100,000 people older than 50 years.1, 2 The large vessels it most commonly involves are those of the proximal aorta, including the subclavian, axillary, and brachial arteries.3 Upper extremity lesions predominate; however, they rarely progress to ischemic complications because of sufficient collateral blood supply. In contrast, lower extremity vasculitis has been reported to present as rapidly progressive claudication resulting in limb ischemia that can warrant urgent surgical intervention.4, 5
The prevalence of lower extremity GCA findings according to the largest series of histologically documented extracranial GCA is 18%.6 Numerous case series acknowledge the SFA and popliteal artery as being most frequently affected.4, 7, 8, 9, 10 Whereas limb claudication may be present on initial diagnosis of GCA in conjunction with systemic signs of inflammation, classic cranial manifestations, or pre-existing diagnosis of polymyalgia rheumatic, even more rare are patients presenting with isolated lower extremity claudication or ischemia preceding the diagnosis of GCA.
Our case is unique in that our patient's rapidly progressive limb ischemia necessitated surgery; in addition, although the diagnosis of a vasculitis was suspected with her history of rapidly progressive claudication, an elevated ESR, and characteristic imaging findings, definitive diagnosis of GCA was based on intraoperative biopsy of the occluded SFA that required bypass. Treatment with steroids was started on initial evaluation secondary to elevated inflammatory markers. There was a slight improvement in the patient’s rest pain symptoms with the start of steroids. However, there was no change on pulse examination.
Because lower extremity claudication is not uncommon in the elderly population, GCA vasculitis can be mistakenly attributed to atherosclerotic disease.7, 8, 11 Several reports describe surgical intervention preceding the diagnosis of GCA because of presumed atherosclerosis.8, 9 Important distinguishing characteristics of lower extremity GCA can be gleaned from clinical presentation, evaluation of risk factors, inflammatory markers, and imaging studies. In contrast to atherosclerotic disease, ischemic symptoms develop and progress rapidly within a period of several weeks.7 This was observed in our patient, who had patent arteries on initial imaging and complete occlusion about 2 months later. This patient had no prior imaging secondary to never having claudication or rest pain-like symptoms. The only history provided was that she had palpable pedal arteries during her previous hospital admission for CABG. Multiple studies have demonstrated the absence or limited presence of peripheral vascular disease risk factors in lower extremity GCA patients.8, 9 Our patient had a history of coronary artery disease that complicated appropriate diagnosis; however, her rapidly progressive claudication, suggestive ESR of 50 mm/h, and classic imaging findings increased our suspicion of a vasculitic entity. Atherosclerosis results from an intimal injury causing an inflammatory process whereby macrophages ingest oxidized low-density lipoprotein, forming foam cells. This eventually leads to the formation of atheroma and wall thickening. Vasculitis results from an inflammatory infiltrate composed of lymphocytes and mononuclear cells with scattered rare multinucleated giant cells.
Because limb-restricted GCA is rarely described, there is no consensus on its medical and surgical management. Steroid therapy is well known to treat constitutional symptoms associated with GCA; however, reports vary as to whether steroids prevent progression and aid in resolution of occlusive lesions or whether these lesions remain refractory to therapy.2, 12 In our review, patients have been shown to develop ischemic complications after initiation of steroid therapy.4, 8, 9, 13
Surgical intervention for limb-restricted vasculitis is even rarer than medical therapy, and the best interventional technique has not yet been determined. The limited instruction provided in the literature indicates that bypass grafts tend to occlude if they are placed during active disease, and it is suggested that surgery should await improvement in inflammation. When urgent surgery is required, it is proposed that grafts should be attached to adjacent normal tissue and steroid therapy should be initiated immediately.12 Surgical techniques reported in the literature include venous bypass grafting, endarterectomy, and angioplasty with variable success rates, with venous bypass grafting being most common.4 Restenosis is a feared common complication, seen in one of the four patients in our literature review requiring surgical intervention who had undergone endarterectomy.9 There are few data on the long-term patency of bypass.2 Although prompt diagnosis and treatment initiation are important, morbidity despite treatment remains high.
Conclusions
Increased awareness of the different manifestations of GCA is crucial for diagnosis of patients with isolated lower extremity vasculitis. It is imperative that GCA be considered in the differential diagnosis of unexplained lower extremity claudication, especially in patients with few risk factors for atherosclerotic disease, rapidly progressing disease, elevated inflammatory markers, and characteristic findings on imaging. GCA classification criteria require revision to include the subgroup of patients presenting with isolated extremity claudication or ischemia. With advances in imaging identifying a higher prevalence of limb involvement than previously thought, consideration must be given to systemic screening methods to assess for limb vasculitis with the potential for rapid progression to critical limb ischemia. Further studies with systemic imaging and longer follow-up are necessary to clarify optimal imaging studies and treatment methods in limb-restricted GCA.
Footnotes
Author conflict of interest: none.
The editors and reviewers of this article have no relevant financial relationships to disclose per the Journal policy that requires reviewers to decline review of any manuscript for which they may have a conflict of interest.
References
- 1.Salvarani C., Cantini F., Hunder G.G. Polymyalgia rheumatica and giant-cell arteritis. Lancet. 2008;372:234–245. doi: 10.1016/S0140-6736(08)61077-6. [DOI] [PubMed] [Google Scholar]
- 2.Tato F., Hoffmann U. Giant cell arteritis: a systemic vascular disease. Vasc Med. 2008;13:127–140. doi: 10.1177/1358863x07085499. [DOI] [PubMed] [Google Scholar]
- 3.Lie J.T. Aortic and extracranial large vessel giant cell arteritis: a review of 72 cases with histopathologic documentation. Semin Arthritis Rheum. 1995;24:422–431. doi: 10.1016/s0049-0172(95)80010-7. [DOI] [PubMed] [Google Scholar]
- 4.Assie C., Janvresse A., Plissonnier D., Levesque H., Marie I. Long-term follow-up of upper and lower extremity vasculitis related to giant cell arteritis: a series of 36 patients. Medicine (Baltimore) 2011;90:40–51. doi: 10.1097/MD.0b013e318206af16. [DOI] [PubMed] [Google Scholar]
- 5.Muratore F., Kermani T.A., Crowson C.S., Green A.B., Salvarani C., Matteson E.L. Large-vessel giant cell arteritis: a cohort study. Rheumatology (Oxford) 2015;54:463–470. doi: 10.1093/rheumatology/keu329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lie J.T., Failoni D.D., Davis D.C., Jr. Temporal arteritis with giant cell aortitis, coronary arteritis, and myocardial infarction. Arch Pathol Lab Med. 1986;110:857–860. [PubMed] [Google Scholar]
- 7.Czihal M., Tato F., Rademacher A., Kuhlencordt P., Schulze-Koops H., Hoffmann U. Involvement of the femoropopliteal arteries in giant cell arteritis: clinical and color duplex sonography. J Rheumatol. 2012;39:314–321. doi: 10.3899/jrheum.110566. [DOI] [PubMed] [Google Scholar]
- 8.Kermani T.A., Matteson E.L., Hunder G.G., Warrington K.J. Symptomatic lower extremity vasculitis in giant cell arteritis: a case series. J Rheumatol. 2009;36:2277–2283. doi: 10.3899/jrheum.090269. [DOI] [PubMed] [Google Scholar]
- 9.Le Hello C., Levesque H., Jeanton M., Cailleux N., Galateau F., Peillon C. Lower limb giant cell arteritis and temporal arteritis: followup of 8 cases. J Rheumatol. 2001;28:1407–1412. [PubMed] [Google Scholar]
- 10.Tato F., Hoffmann U. Clinical presentation and vascular imaging in giant cell arteritis of the femoropopliteal and tibioperoneal arteries. Analysis of four cases. J Vasc Surg. 2006;44:176–182. doi: 10.1016/j.jvs.2006.02.054. [DOI] [PubMed] [Google Scholar]
- 11.Kermani T.A., Warrington K.J. Lower extremity vasculitis in polymyalgia rheumatica and giant cell arteritis. Curr Opin Rheumatol. 2011;23:38–42. doi: 10.1097/BOR.0b013e3283410072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Joyce J.W. The giant cell arteritides: diagnosis and the role of surgery. J Vasc Surg. 1986;3:827–833. doi: 10.1067/mva.1986.avs0030827b. [DOI] [PubMed] [Google Scholar]
- 13.Loo Z.Y., Thwaites S., Kyaw P. Giant cell arteritis presenting as critical lower limb ischemia. Vasc Endovascular Surg. 2013;47:660–662. doi: 10.1177/1538574413503563. [DOI] [PubMed] [Google Scholar]