Abstract
The present study aimed at the potential role of selenium in providing protection to plants subjected to chromium toxicity. The study was carried out on 15-day-old seedlings of Brassica juncea raised in the solutions of Cr (300 µM) and Se (2, 4 and 6 µM), both alone and in combinations under controlled laboratory environment. The effects were studied on growth, plant metabolites (involved in osmotic homeostasis and stress protection), and essential elements. The results showed that the exposure of B. juncea seedlings to 300 µM Cr led to an increase in the contents of total sugars, reducing sugars, non-reducing sugars, total phenols and flavonoids. However, a significant decline in growth characteristics, the contents of proteins and free amino acids was observed. The essential elements (Na, K, Ca, Mg, C, H, N) also decreased in response to Cr. Se application in binary combinations, on the other hand, aided in improving seed germination (19%), root (88.3%) and shoot (18.2%) lengths. It also helped to increase the contents of sugars [total (16.3%), reducing (21.6%) and non-reducing (15.2%)], phenols (36.7%) and flavonoids (27.4%), thereby aiding in alleviating the phytotoxicity of Cr. The profiling of polyphenols and amino acids, and histological study of phenols supported the above results. The contents of essential elements also showed a significant increase, while Cr uptake was observed to decline by Se supplementation. The observations from the present study indicate that Se has the ability to influence primary and secondary metabolism, improve mineral nutrition and reduce Cr uptake in B. juncea seedlings to combat the Cr phytotoxicity and enhance the tolerance against stress.
Keywords: Brassica juncea, Selenium, Chromium, Metabolites, Essential elements, Phytotoxicity
Introduction
Selenium (Se), belonging to chalcogen group of elements, has a strong physico-chemical resemblance with sulphur (S). This property of Se gives it a capability to be taken up by the plants and substitute S in the biomolecules. The importance of Se pertaining to its benefits as an essential nutrient came to light with the studies carried out by Schwarz and Foltz (1957). Since then, a wide array of work has proved its imperative role in improving plant and animal health. In plants, Se at low concentrations has been frequently reported to be beneficial in their growth and development (Malik et al. 2011; Hawrylak-Nowak 2013). Apart from growth, Se is also known to promote seed germination (Han et al. 2010; Pukacka et al. 2011), enhance yield (Soleimanzadeh 2012; Teimouri et al. 2014), delay senescence (Moussa and Ahmed 2010; Pezzarossaa et al. 2014), improve photosynthetic efficiency (Yao et al. 2011; Diao et al.2014) and increase accumulation of metabolites (Bansal et al. 2012; Owusu-Sekyere et al. 2013). Such an activity is attributed to its ability to strengthen antioxidative potential and decrease lipid peroxidation (Hajiboland and Keivanfar 2012; Handa et al. 2017; Alyemeni et al. 2017). Lately, the role of Se against the damaging effects of various biotic and abiotic stresses on plants has also garnered interest. Studies conducted on plants subjected to drought, salinity, high temperatures, UV-B radiations and heavy metals suggest its significance in counteracting the stressful conditions and improving the plant growth.
Exposure of plants to several abiotic stresses is unavoidable due to their sedentary nature (Ahmad et al. 2012). Environmental pollution caused by heavy metals, in recent years, is on the rise due to increased industrialization and other anthropogenic activities. The excessive use of fertilizers and pesticides has also led to the entry of heavy metals in the soil (Belouchrani et al. 2016). The presence of heavy metals in soils and waters has led to their accumulation in plants, and further making entry into the food chain (Demim et al. 2013b). These bioaccumulating metals further lead to health hazards in plant, animal and human life due to several biochemical and physiological changes (Sharma et al. 2010; Demim et al. 2013a, 2014; Kanwar et al. 2015). Chromium (Cr) is a non-essential heavy metal for living systems which is considered to be one of the most toxic elements that has detrimental effects on both plants and animals. In nature, it exists in various oxidation states ranging from Cr(−II) to Cr(+VI). Out of these, Cr(III) and Cr(VI) are the most stable forms that are easily taken up by plants. Cr(VI) is considered to be the most toxic and is placed in Group A carcinogens (USEPA 1999). The release of Cr(VI) to the environment occurs mainly through industries like leather, cement, paints and pigments, pulp and paper, electroplating, timber processing and finishing (Zayed and Terry 2003; Singh et al. 2013; Cheballah et al. 2015). Several earlier studies conducted on plants like Triticum aestivum, Oryza sativa and Brassica juncea have demonstrated the adverse effects of Cr on plants (Datta et al. 2011; Nagarajan and Ganesh 2014; Handa et al. 2017). In plants, Cr(VI) can produce various toxicity symptoms that can cause complete damage. The exposure of plants to Cr(VI) can result in over-production of reactive oxygen species (ROS) which further leads to oxidative damage (Panda and Patra 2000; Ali et al. 2013). The plants exposed to high levels of Cr(VI) show symptoms of acute chlorosis and necrosis along with many morphological and anatomical defects (Samantaray et al. 1998).
Brassica juncea L. is a major oil yielding crop grown mostly in northern parts of India. It is a fast growing plant which yields a good biomass. Also, out of the various members of the family Brassicaceae, B. juncea has been classified as a primary accumulator of Se (Hasanuzzaman et al. 2010). Therefore, B. juncea can be used as a model plant to study the effects of Se and Cr in unary and binary combinations. The present investigation was intended to understand the probable role of Se in alleviating Cr stress in B. juncea by assessing growth and biochemical parameters. To test this, the effects were observed on sugars, which are not only primary metabolites, but also act as osmoregulators. The variations in phenols and flavonoids were also tested as they are not only important constituents of secondary metabolism, but also participate in metal chelation and ROS scavenging. Thirdly, the effect on those mineral nutrients was studied which are either involved in various biochemical processes or are components of various biomolecules.
Methodology
Study material, treatments and growth conditions
Certified seeds of B. juncea (var. RLC 1) were procured from Punjab Agricultural University, Ludhiana, India, which served as the experimental material for the present study. The seeds were surface sterilized with 0.01% HgCl2 and washed with distilled water several times. The sterilized seeds were then soaked in distilled water for 2 h and then germinated in autoclaved Petri dishes lined with Whatman No. 1 filter paper. The solutions containing Se(VI) in the form of Na2SeO4 (2, 4 and 6 μM) and Cr(VI) in the form of K2CrO4 (300 μM) were prepared in half-strength Hoagland’s nutrient medium, either alone or in combination, and poured in Petri dishes. Preliminary trials were conducted to select the 50% inhibitory concentration of Cr (IC50), and most stimulatory concentrations of Se for B. juncea. For first 72 h, the Petri dishes were covered to allow germination, and then 16 h of light (175 μmol m−2 s−1 intensity) and 8 h of darkness were provided. A temperature of 25 ± 0.5 °C was maintained inside the seed germinator and seedlings were harvested after 15 days for analysis. The treatments were given in triplicates and the experiment was conducted thrice.
Growth characteristics
Percentage germination was estimated by counting the number of germinated seeds. The root and shoot lengths of the 15-day-old seedlings were also recorded.
Total sugars
The method described by Hedge and Hofreiter (1962) was used to estimate the content of total sugars in the seedlings of B. juncea. The dried seedlings (100 mg) were powdered and hydrolysed in 2.5 N HCl in water bath for 3 h at 100 °C. The mixture was cooled and Na2CO3 was added until no effervescence occurred. Distilled water was added to this reaction mixture and the final volume was made up to 100 mL. Anthrone reagent (4 mL), freshly prepared from 200 mg anthrone and 100 mL 95% H2SO4, was added to 1 ml aliquot of the above reaction mixture, boiled for 8 min and then cooled. The absorbance was noted at 630 nm and the content was calculated by a standard curve obtained from glucose.
Reducing sugars and non-reducing sugars
The estimation of reducing sugars was done using the method proposed by Miller (1972). Five mL of 80% ethanol and 3 mL 3,5-dinitrosalicylic acid were added to 100 mg of dried seedlings and the reaction mixture was boiled. This was followed by adding 40% potassium sodium tartrate and the absorbance of the cooled reaction mixture was observed at 510 nm. Glucose was used to obtain a standard curve to calculate reducing sugars. For the estimation of non-reducing sugars, the following formula given by Loomis and Shull (1937) was used:
Protein content
The method by Lowry et al. (1951) was used to determine the content of proteins in 15-day-old seedlings of B. juncea and calculations were made from a standard curve obtained using bovine serum albumin as a standard.
Flavonoids
The method given by Zhishen et al. (1999) was used to estimate the content of flavonoids. The plant sample (100 mg) was extracted with 4 mL absolute methanol and then filtered to obtain the extract, and 1 mL of this extract was diluted with 4 mL distilled water. To this, 5% NaNO2 and 10% AlCl3 were added and incubated for 5 min. To this reaction mixture, 4% NaOH (2 mL) and 2.4 mL of distilled water were added and the absorbance was measured at 510 nm. Rutin was used as a standard to estimate flavonoid content.
Total phenols
The content of total phenols was estimated by following the protocol proposed by Singleton and Rossi (1965). To 400 mg of dried seedlings of B. juncea, 40 mL of 60% ethanol was added and heated for 10 min. After filtering the extract, the residue was re-extracted and the filtrates were combined. The volume of the filtrate was made up to 100 mL by adding more 60% ethanol. An aliquot of 2 mL of filtrate was taken and 10 mL of Folin–Ciocalteau reagent was added to it followed by the addition of Na2CO3 after 8 min. After 2 h of incubation, the absorbance was read at 765 nm. Gallic acid was used as a standard to calculate the content of total phenols.
Free amino acids
Free amino acids were assayed by the protocol given by Lee and Takahashi (1966). The plant extract was prepared by extracting 100 mg dried seedling samples with 80% ethanol. To 0.2 mL extract, 3.8 mL of 1% ninhydrin reagent was added that was prepared in 0.5 M citrate buffer (pH 5.5). It was followed by heating of reaction mixture for 12 min in boiling water bath, and blue colour was developed when it was cooled to room temperature. The absorbance was recorded at 570 nm. For computing the content of free amino acids, standard solution of glycine was used.
Polyphenol profiling
The profiling of polyphenols was carried out on fresh 15-day-old seedlings of B. juncea in ultra-performance liquid chromatography (UPLC). The methanolic extracts of B. juncea seedlings were used and analysed in Shimadzu UPLC Nexera System. The UPLC had C-18 column (150 × 4.6 mm) with pore size of 5 µm and flow rate of 1 mL min−1 at 280 nm. It was coupled with a photodiode array detector. The standards used for calibration and quantitative analysis were gallic acid, catechin, chlorogenic acid, epicatechin, caffeic acid, umbelliferone, coumaric acid, rutin, ellagic acid, quercetin and kaempferol which were procured from Sigma Aldrich.
Amino acid profiling
Moore and Stein’s (1963) method, with some modifications, was used for the profiling of amino acids in Amino Acid Analysis System (Shimadzu). The methanolic extracts of B. juncea seedlings were hydrolysed by adding 6 N HCl and kept at 110 °C in oven for 24 h. The hydrolysed samples were mixed with 0.12 M citrate buffer. To 0.3 mL of the sample, 0.7 mL of 0.1 N HCl was added. This was finally injected in amino acid analyser having C18G column (150 × 4.6 mm) with pore size of 120 Å and flow rate of 1 mL min−1 at 245 nm.
Histological study of phenols
The phenols were also localized in the roots of 15-day-old B. juncea seedlings using the method given by Gahan (1984). The dye used for tagging the phenols was fast blue BB that was prepared in acetate buffer and the roots were viewed under light microscope (Magnus MLXi).
Elemental analysis
The dried seedling samples were digested by following the method proposed by Allen et al. (1976) and were used to estimate Na, K and Cr uptake using atomic absorption spectrophotometer (AA240 FS, Agilent Technologies). The contents of Ca and Mg were determined using EDTA titration method by Allen et al. (1976). The contents of C, H, N and S were also analysed using CHNS/O Analyser (Flash 2000, Thermo Scientific). The weighed dried samples were used to estimate the percentage of the elements.
Statistical analysis
The data obtained were first subjected to Shapiro–Wilk Normality Test (Shapiro and Wilk 1965), and then analysed statistically using one-way analysis of variance (ANOVA) and Tukey’s HSD (honestly significant difference). The linear multiple regression analysis was applied to understand the interaction between the two elements (Sokal and Rholf 1981; Bailey 1995). The model used for multiple regression for binary combination was:
where Y is the parameter under study, X1 and X2 are the two elements in binary combinations, b1 and b2 are the partial regression coefficients due to the effects of X1 and X2, respectively, and β1 and β2 are the β-regression coefficients due to X1 and X2, respectively.
The statistical analysis was done using self-coded programs in Microsoft Excel.
Results
Growth characteristics
Exposure to Cr led to a lower seed germination percentage (19.2%), root lengths (56.3%) and shoot lengths (10.9%) as compared to the untreated controls. However, Se at 4 µM concentration in binary combination with Cr resulted in a significant increase of 19% in seed germination, 88.3% in root length and 18.2% in shoot length, when compared to only Cr-treated seedlings. The β-regression coefficients for Cr were negative for all the three parameters, which confirmed its growth inhibiting effects. For Se, the coefficients were positive which signified its growth promoting effects (Table 1).
Table 1.
Changes in the growth characteristics of 15-day-old B. juncea seedlings subjected to binary combinations of Cr and Se
| Concentrations (µM) | Percentage germination | Root length (cm) | Shoot length (cm) | |
|---|---|---|---|---|
| Cr | Se | |||
| 0 | 0 | 86.67 ± 2.875ab | 11.52 ± 1.207a | 4.74 ± 0.181b |
| 0 | 2 | 88.33 ± 2.875ab | 10.93 ± 0.599a | 4.88 ± 0.23b |
| 0 | 4 | 95.0 ± 4.988a | 11.88 ± 0.279a | 5.39 ± 0.165a |
| 0 | 6 | 90.0 ± 4.988ab | 11.17 ± 0.336a | 4.84 ± 0.09b |
| 300 | 0 | 70.0 ± 4.988c | 5.03 ± 0.686c | 4.22 ± 0.211c |
| 300 | 2 | 78.33 ± 2.875bc | 9.27 ± 0.604b | 4.56 ± 0.068bc |
| 300 | 4 | 83.3 ± 7.621ab | 9.47 ± 0.296b | 4.99 ± 0.165ab |
| 300 | 6 | 81.66 ± 2.875ab | 8.73 ± 0.227b | 4.78 ± 0.062b |
| F ratio(df 7,16) | 8.571** | 39.866** | 13.456** | |
| HSD | 12.912 | 1.058 | 0.449 | |
| Multiple regression analysis | ||||
|---|---|---|---|---|
| Parameters (Y) | Multiple regression equations | r | β 1 | β 2 |
| Percentage germination | Y = 85.75 − 0.0389 X1 + 1.4167 X2 | 0.817*** | − 0.718 | 0.390 |
| Root length | Y = 10.538 − 0.0109 X1 + 0.2802 X2 | 0.814*** | − 0.759 | 0.292 |
| Shoot length | Y = 4.744 − 0.0011 X1 + 0.0732 X2 | 0.679*** | − 0.479 | 0.481 |
Data presented in mean ± SD of three replicates. Means followed by the same letter are not significantly different using Tukey’s HSD test
µM micromole, cm centimetre, X1 µM Cr, X2 µM Se, r correlation coefficient, β1 β-regression coefficient for Cr, β2 β-regression coefficient for Se
Significant at ***p ≤ 0.001, **p ≤ 0.01, *p ≤ 0.05
Total sugars, reducing sugars and non-reducing sugars
As compared to the control seedlings, those grown in Cr containing media showed 18.6% increase in the content of total sugars, 26% in reducing sugars, and 16.4% in non-reducing sugars (Table 2). However, when Se was supplemented with Cr, further increase in the contents was observed as compared to the seedlings treated only with Cr. The Se application of 4 µM in binary combination with Cr resulted in further enhancement in the contents of total sugars by 16.3%, reducing sugars by 21.6% and non-reducing sugars by 15.2%. Multiple linear regression and β-regression coefficients showed positive effects of Cr and Se on total sugar, reducing and non-reducing sugars (Table 2).
Table 2.
Changes in the contents of total sugars, reducing sugars, non-reducing sugars, proteins, flavonoids, total phenols and free amino acids in 15-day-old B. juncea seedlings under the influence of Cr and Se
| Concentrations (µM) | Total sugars (µg mg−1 DW) | Reducing sugars (µg mg−1DW) | Non-reducing sugars (µg mg−1 DW) | Proteins (mg g−1 FW) | Flavonoids (µg mg−1 DW) | Total phenols (mg g−1 DW) | Free amino acids (µg g−1 DW) | |
|---|---|---|---|---|---|---|---|---|
| Cr | Se | |||||||
| 0 | 0 | 112.14 ± 1.928de | 17.82 ± 0.443e | 90.18 ± 0.882d | 3.76 ± 0.078ab | 1.15 ± 0.085d | 12.86 ± 0.385 g | 6.12 ± 0.319a |
| 0 | 2 | 117.73 ± 1.237d | 18.8 ± 0.563de | 93.98 ± 1.019d | 3.92 ± 0.041a | 1.23 ± 0.147d | 15.29 ± 0.346f | 6.47 ± 0.435a |
| 0 | 4 | 108.64 ± 5.182e | 17.65 ± 0.511e | 86.43 ± 4.723d | 3.66 ± 0.103abc | 1.21 ± 0.066d | 17.77 ± 0.331e | 6.60 ± 0.416a |
| 0 | 6 | 114.5 ± 2.835de | 19.74 ± 0.554d | 90.02 ± 2.983d | 3.64 ± 0.085bc | 1.71 ± 0.092c | 17.55 ± 0.312e | 3.97 ± 0.525b |
| 300 | 0 | 133.02 ± 0.275c | 22.53 ± 0.376c | 104.97 ± 2.549c | 3.03 ± 0.166e | 2.47 ± 0.180b | 20.64 ± 0.258d | 2.37 ± 0.319c |
| 300 | 2 | 143.39 ± 3.642b | 24.53 ± 0.466b | 112.91 ± 3.139b | 3.42 ± 0.095 cd | 2.96 ± 0.139a | 22.07 ± 0.400c | 3.76 ± 0.731b |
| 300 | 4 | 154.7 ± 3.596a | 27.4 ± 0.405a | 120.93 ± 2.986a | 3.56 ± 0.089bcd | 3.15 ± 0.215a | 24.76 ± 0.365b | 5.84 ± 0.319a |
| 300 | 6 | 144.73 ± 1.739b | 24.99 ± 0.513b | 113.76 ± 1.528ab | 3.31 ± 0.061cd | 2.57 ± 0.097b | 28.23 ± 0.385g | 6.19 ± 0.551a |
| F-ratio(df 7,16) | 96.579** | 168.88** | 68.636** | 25.377** | 109.3** | 567.08** | 33.56** | |
| HSD | 8.771 | 1.394 | 7.787 | 0.272 | 0.388 | 1.034 | 1.334 | |
| Multiple regression analysis | ||||
|---|---|---|---|---|
| Parameters (Y) | Multiple regression equations | r | β 1 | β 2 |
| Total sugars | Y = 109.92 + 0.102 X1 + 1.111 X2 | 0.934*** | 0.922 | 0.149 |
| Reducing sugars | Y = 17.388 + 0.0212 X1 + 0.372 X2 | 0.944*** | 0.913 | 0.238 |
| Non-reducing sugars | Y = 88.177 + 0.0766 X1 + 0.659 X2 | 0.926*** | 0.918 | 0.118 |
| Proteins | Y = 3.717 − 0.0014 X1 + 0.0091 X2 | 0.767*** | − 0.763 | 0.074 |
| Flavonoids | Y = 1.161 + 0.0049 X1 + 0.0543 X2 | 0.949*** | 0.936 | 0.155 |
| Total phenols | Y = 12.738 + 0.0267 X1 + 1.043 X2 | 0.983*** | 0.849 | 0.495 |
| Free amino acids | Y = 5.247 − 0.0042 X1 + 0.181 X2 | 0.488* | − 0.409 | 0.264 |
Data presented in mean ± SD of three replicates. Means followed by the same letter are not significantly different using Tukey’s HSD test
µM micromole, µg microgram, mg milligram, g gram, DW dry weight, FW fresh weight, X1 µM Cr, X2 µM Se, r correlation coefficient, β1 β-regression coefficient for Cr, β2 β-regression coefficient for Se
Significant at ***p ≤ 0.001, **p ≤ 0.01, *p ≤ 0.05
Protein content
The Cr application showed a significant negative effect on the content of proteins in 15-day-old B. juncea seedlings. When compared to the untreated control seedlings, a decrease of 19.4% was observed with Cr metal treatment. Se aided in reducing the damaging effect of Cr. In binary combination with Cr, the maximum effect was observed at a concentration of 4 µM Se which helped to increase the content of proteins by 17.5% followed by 2 µM which caused an increase of 12.87% when compared to seedlings raised in only Cr solutions (Table 2). The phytotoxic effect of Cr was confirmed by a negative value of β-regression coefficient (Table 2).
Flavonoids
The content of flavonoids was observed to increase in seedlings that were grown in solution containing 300 µM Cr. Se in binary combination with Cr caused a further increase in its content by 27.4% at 4 µM concentration followed by 19.8% at 2 µM concentration. The β-regression coefficients for both Se and Cr were positive (Table 2).
Total phenols and polyphenol profiling
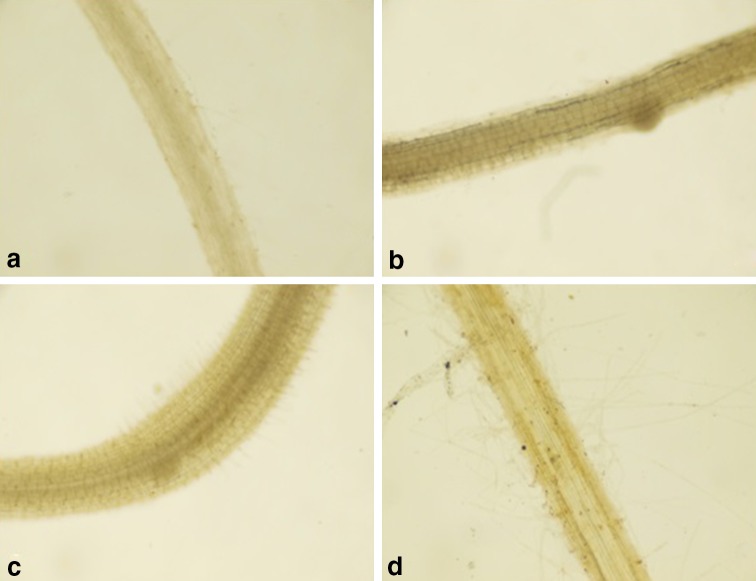
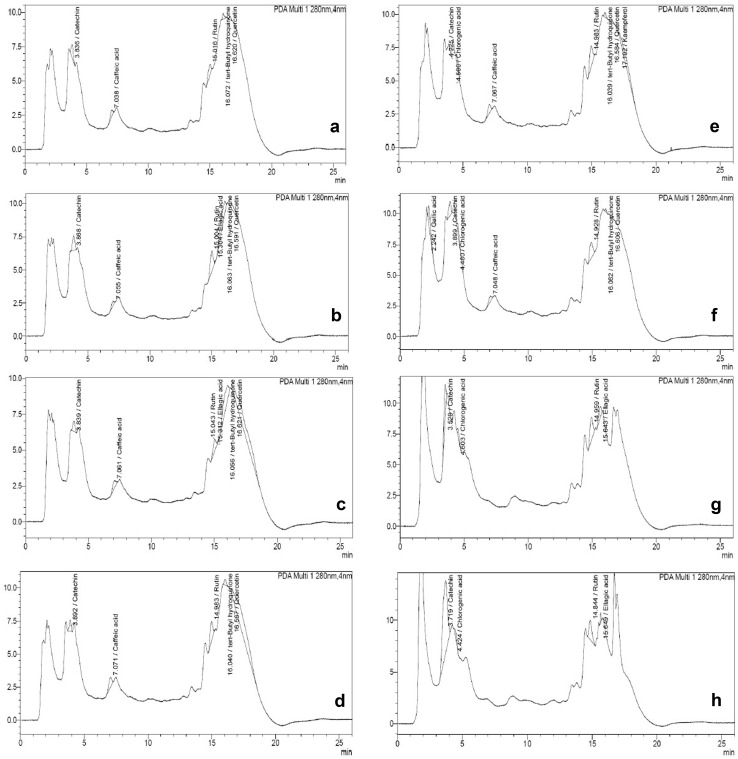
The 15-day-old B. juncea seedlings showed an increased content of total phenols with Cr application. Se, when applied alone, also resulted in an enhanced content of phenols. The binary combination of Se with Cr resulted in a further increase in the content of phenols. The maximum effect was observed at 6 µM concentration of Se in binary combination with Cr that resulted in a 36.7% increase in the content of phenols in the seedlings. The concentration of 4 µM Se in combination with Cr caused an increase of 19.9% in the content of phenols (Table 2). The β-regression coefficients for Se as well as Cr confirmed the positive influence of both the elements on total phenols (Table 2). The histological localization of phenols in the roots of B. juncea also confirmed the above results (Fig. 1). The characterization of polyphenols by UPLC in the 15-day-old seedlings of B. juncea revealed the presence of gallic acid, catechin, chlorogenic acid, caffeic acid, rutin, ellagic acid, tert-butyl hydroquinone, quercetin and kaempferol (Table 3, Fig. 2). The chromatograms showed the presence of catechin and rutin in all eight methanolic extracts of B. juncea. Caffeic acid, tert-butyl hydroquinone and quercetin were present in all extracts except for extracts made from seedlings grown in binary treatments of 300 µM Cr with 4 µM Se and 6 µM Se, respectively. Kaempferol and gallic acid were found in extracts prepared from seedlings treated with 300 µM Cr alone, and Cr in binary combination with 2 µM Se, respectively. The presence of ellagic acid was seen in four samples viz. extracts prepared from seedlings treated with 2 µM Se, 4 µM Se and binary combinations of Cr with 4 µM Se and 6 µM Se. Chlorogenic acid was present only in seedlings raised either in 300 µM Cr alone, or in combination with Se.
Fig. 1.
Effect of binary combinations of Se and Cr on localization of phenols in the roots of 15-day-old B. juncea seedlings. a Control, b 6 µM Se, c 300 µM Cr, d 300 µM Cr and 6 µM Se
Table 3.
Changes in the contents of various polyphenols under the influence of Cr and Se in 15-day-old B. juncea seedlings
| Concentrations (µM) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Cr | 0 | 0 | 0 | 0 | 300 | 300 | 300 | 300 |
| Se | 0 | 2 | 4 | 6 | 0 | 2 | 4 | 6 |
| Polyphenols (µg g−1 FW) | ||||||||
| Gallic acid | – | – | – | – | – | 4.72 | – | – |
| Catechin | 19.81 | 24.78 | 13.98 | 14.22 | 20.98 | 24.51 | 54.66 | 288.2 |
| Chlorogenic acid | – | – | – | – | 3.79 | 3.69 | 3.004 | 3.48 |
| Caffeic acid | 1.78 | 1.44 | 2.60 | 3.43 | 2.91 | 2.18 | – | – |
| Rutin | 12.82 | 26.11 | 13.68 | 22.24 | 26.65 | 22.79 | 30.35 | 30.08 |
| Ellagic acid | – | 0.452 | 0.700 | – | – | – | 11.19 | 4.29 |
| Tert-butyl hydroquinone | 2.56 | 24.09 | 25.35 | 2.92 | 2.02 | 1.22 | – | – |
| Quercetin | 1.42 | 4.93 | 43.11 | 57.04 | 1.11 | 1.15 | – | – |
| Kaempferol | – | – | – | – | 52.72 | – | – | – |
Fig. 2.
Chromatograms of polyphenols showing effect of binary combinations of Se and Cr in 15-day-old seedlings of B. juncea. a Control, b 2 µM Se, c 4 µM Se, d 6 µM Se, e 300 µM Cr, f 300 µM Cr and 2 µM Se, g 300 µM Crand 4 µM Se, h 300 µM Cr and 6 µM Se
Free amino acids and amino acid profiling
A significant decline in amino acid content was observed in the seedlings raised in Cr containing solutions. The Cr application caused a decrease of 61.3% in the content when compared to the control seedlings. The Se application in combination with Cr helped to enhance the free amino acid content and the maximum effect was observed at 6 µM concentration subsequently followed by 4 µM Se (Table 2). The damaging effects of Cr, and stress ameliorative properties of Se were also verified by regression analysis. In linear model of multiple regression, the β-regression coefficient for Cr was negative while for Se, it was positive (Table 2).
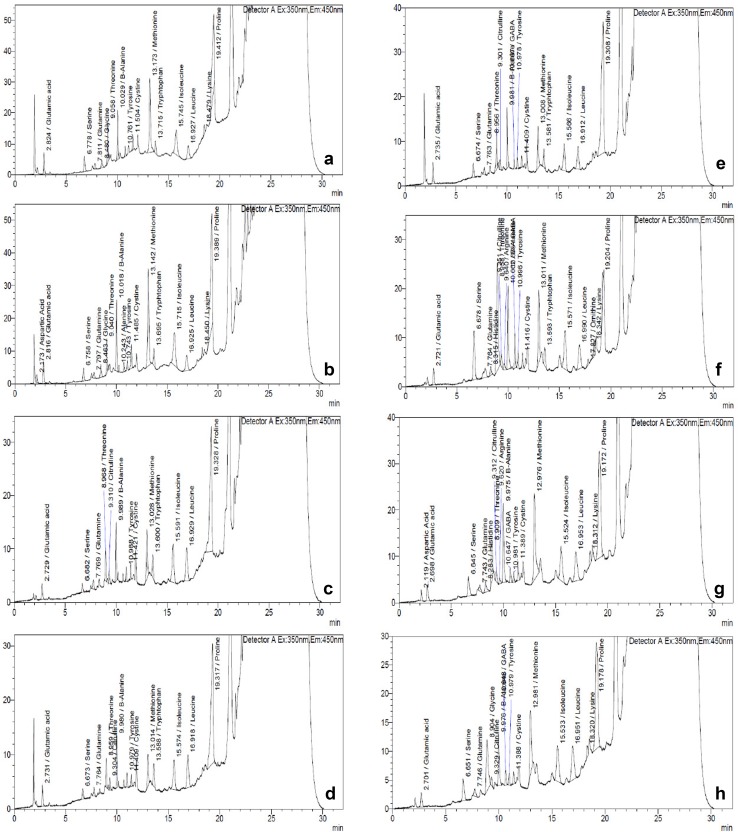
Amino acid profiling showed the presence of various amino acids and their quantities in the extracts of different B. juncea samples (Table 4, Fig. 3). Some amino acids were detected in all the samples of B. juncea seedling extracts. In the samples prepared from the seedlings raised in Cr solutions, the contents of glutamic acid, serine, glutamine, β-alanine, tyrosine, cystine, methionine and isoleucine were observed to decrease in comparison to the control seedlings. On the other hand, the extracts of seedlings raised in the binary combinations of Cr and Se showed an increase in these amino acid contents as compared to metal-treated seedlings (Table 4). The contents of leucine and proline, however, were observed to increase in seedlings cultured in Cr solution in comparison to untreated seedlings. In binary combination of the two elements, leucine showed a further increase in its content, while proline was observed to decrease. Threonine was detected in all samples, except in the samples raised in binary combination of 300 µM Cr and 6 µM Se. With the application of Cr, the seedlings showed a reduced content of threonine in comparison to control seedlings, while the binary combination of Se and Cr caused an increase in the content of threonine. The presence of citrulline was also observed in all the samples except for control seedlings and seedling raised in 2 µM Se solution. The content of citrulline decreased in seedlings raised in 300 µM Cr when compared to Se-treated seedlings, and an increase in the content was observed in seedlings raised in binary combination of Cr and Se. The presence of gamma-aminobutyric acid (GABA) was seen only in seedlings grown in medium containing Cr either alone or in binary combination with Se. The content of GABA was lowest in seedlings grown only in Cr solutions but the content enhanced in binary treatments. Apart from these amino acids, aspartic acid, histidine, glycine and alanine were also detected in a few samples of B. juncea seedlings.
Table 4.
Changes in the contents of various amino acids under the influence of Cr and Se in 15-day-old B. juncea seedlings
| Treatments | Concentrations (µM) | |||||||
|---|---|---|---|---|---|---|---|---|
| Cr | 0 | 0 | 0 | 0 | 300 | 300 | 300 | 300 |
| Se | 0 | 2 | 4 | 6 | 0 | 2 | 4 | 6 |
| Amino acids (µg g−1 FW) | ||||||||
| Aspartic acid | – | 12.41 | – | – | – | – | 13.14 | – |
| Glutamic acid | 63.88 | 67.12 | 34.40 | 40.52 | 47.19 | 43.17 | 47.53 | 34.20 |
| Serine | 17.38 | 14.57 | 5.97 | 6.45 | 10.44 | 48.83 | 20.64 | 18.67 |
| Glutamine | 98.89 | 98.31 | 84.59 | 78.83 | 80.65 | 236.1 | 94.0 | 98.51 |
| Histidine | – | – | – | – | – | 39.26 | 69.31 | – |
| Glycine | 9.61 | 15.02 | – | – | – | – | – | 61.45 |
| Threonine | 59.33 | 47.16 | 22.38 | 22.27 | 45.39 | 198.1 | 69.14 | – |
| Citrulline | – | – | 12.48 | 11.88 | 11.54 | 15.21 | 16.83 | 11.68 |
| Arginine | – | – | – | – | – | 273.2 | 285.1 | – |
| β-Alanine | 84.63 | 114.4 | 52.99 | 47.96 | 65.56 | 130.5 | 134.7 | 74.56 |
| Alanine | – | 45.45 | – | – | – | – | – | – |
| GABA | – | – | – | – | 4.84 | 21.53 | 9.792 | 8.02 |
| Tyrosine | 13.22 | 11.57 | 9.60 | 9.56 | 8.73 | 15.53 | 10.92 | 10.54 |
| Cystine | 17.32 | 30.09 | 10.13 | 7.82 | 7.74 | 14.29 | 20.51 | 11.43 |
| Methionine | 2812.8 | 2592.9 | 667.3 | 415.76 | 687.9 | 1285.4 | 2208.4 | 1123.8 |
| Isoleucine | 98.23 | 124.2 | 71.18 | 55.06 | 57.31 | 91.51 | 91.85 | 81.11 |
| Leucine | 45.60 | 53.91 | 62.78 | 62.79 | 51.95 | 62.83 | 91.47 | 85.81 |
| Ornithine | – | – | – | – | – | 6.59 | – | – |
| Lysine | 12.87 | 11.53 | – | – | – | 14.95 | 13.02 | 9.53 |
| Proline | 209.9 | 279.1 | 191.6 | 202.2 | 275.0 | 18.57 | 231.1 | 188.0 |
Fig. 3.
Chromatograms of amino acids showing effect of binary combinations of Se and Cr in 15-day-old seedlings of B. juncea. a Control, b 2 µM Se, c 4 µM Se, d 6 µM Se, e 300 µM Cr, f 300 µM Cr and 2 µM Se, g 300 µM Cr and 4 µM Se, h 300 µM Cr and 6 µM Se
Elemental analysis
The Cr uptake was estimated in both roots and shoots of B. juncea seedlings. Maximum accumulation of Cr was seen in seedlings grown in medium containing only Cr. Roots, however, showed higher Cr accumulation as compared to the shoots. The binary combinations of Cr and Se showed reduced Cr uptake in the seedlings (Table 5). The β-regression coefficients for Cr in linear model for multiple regression showed a positive value for both roots and shoots, while for Se, the values were negative which indicated that Se aided in reducing Cr uptake (Table 5).
Table 5.
Changes in the content of Cr in roots and shoots of 15-day-old B. juncea seedlings subjected to binary combinations of Cr and Se
| Concentrations (µM) | Roots (mg g−1 DW) | Shoots (mg g−1 DW) | |
|---|---|---|---|
| Cr | Se | ||
| 0 | 0 | 0.0299 ± 0.0008e | 0.0201 ± 0.0005e |
| 300 | 0 | 1.55 ± 0.0031a | 0.257 ± 0.0012a |
| 300 | 2 | 1.29 ± 0.0081c | 0.243 ± 0.0006b |
| 300 | 4 | 1.23 ± 0.0025d | 0.188 ± 0.0012d |
| 300 | 6 | 1.34 ± 0.004b | 0.239 ± 0.0012c |
| F-ratio (df 4,10) | 55,238.4** | 31,784.9** | |
| HSD | 0.0119 | 0.0025 | |
| Multiple regression analysis | ||||
|---|---|---|---|---|
| Parameters (Y) | Multiple regression equations | r | β 1 | β 2 |
| Cr content in roots | Y = 0.0299 + 0.0048 X1 − 0.035 X2 | 0.988*** | 1.057 | − 0.150 |
| Cr content in shoots | Y = 0.0201 + 0.00076 X1 − 0.005 X2 | 0.972*** | 1.039 | − 0.146 |
Data presented in mean ± SD of three replicates. Means followed by the same letter are not significantly different using Tukey’s HSD test
µM micromole, mg milligram, g gram, DW dry weight, X1 µM Cr, X2 µM Se, r correlation coefficient, β1 β-regression coefficient for Cr, β2 β-regression coefficient for Se
Significant at ***p ≤ 0.001, **p ≤ 0.01, *p ≤ 0.05
The contents of Na, K, Ca and Mg were studied in the 15-day-old B. juncea seedlings. Significant decline in the contents of these elements were observed in the seedlings raised in the media containing Cr. When compared to the untreated control seedlings, the contents of Na, K, Ca and Mg declined by 48.8, 68.9, 61.6 and 50.5%, respectively, in Cr-treated seedlings. Se proved to be beneficial in improving the content of these elements and, thus, minimising the effect of Cr on plants. The application of 2 µM Se with Cr increased the content of Na and K by 86.7 and 68.9%, respectively. Ca and Mg contents, however, showed maximum increase (139.6 and 93.8%, respectively) at 4 µM Se in combination with Cr (Table 6). The linear multiple regression equation and β-regression coefficient for Cr for Na, K, Ca and Mg were negative. For Se, the β-regression coefficients were negative for Na and K, while for Ca and Mg, the β-regression coefficients were positive (Table 6).
Table 6.
Changes in the contents of Na, K, Ca, Mg, C, H, N and S in 15-day-old B. juncea seedlings under the influence of Cr and Se
| Concentrations (µM) | Sodium (mg g−1 DW) | Potassium (mg g−1 DW) | Calcium (mg g−1DW) | Magnesium (mg g−1 DW) | Carbon (%) | Hydrogen (%) | Nitrogen (%) | Sulphur (%) | |
|---|---|---|---|---|---|---|---|---|---|
| Cr | Se | ||||||||
| 0 | 0 | 138.9 ± 0.321b | 38.0 ± 0.519a | 156.3 ± 31.49c | 81.22 ± 9.29bc | 44.09 ± 0.279ab | 5.95 ± 0.168a | 5.65 ± 0.093bc | 0.059 ± 0.014d |
| 0 | 2 | 177.03 ± 1.557a | 37.63 ± 1.848a | 200.4 ± 30.06b | 97.46 ± 6.09ab | 46.68 ± 3.12a | 6.01 ± 0.134a | 5.81 ± 0.156ab | 0.081 ± 0.018d |
| 0 | 4 | 122.8 ± 0.090d | 25.63 ± 1.595b | 250.5 ± 10.02a | 109.6 ± 6.09a | 43.12 ± 0.338bc | 6.12 ± 0.071a | 6.17 ± 0.192a | 0.131 ± 0.024 cd |
| 0 | 6 | 103.2 ± 1.172e | 17.1 ± 2.252 cd | 156.9 ± 20.85c | 62.89 ± 6.98d | 41.85 ± 0.514bcd | 5.92 ± 0.209a | 5.62 ± 0.169bc | 0.184 ± 0.031cd |
| 300 | 0 | 71.2 ± 1.30 h | 11.83 ± 0.352de | 60.1 ± 10.02e | 42.64 ± 6.09e | 38.34 ± 0.465e | 5.11 ± 0.027c | 4.90 ± 0.329d | 0.264 ± 0.087bc |
| 300 | 2 | 132.9 ± 0.666c | 17.23 ± 3.647c | 113.6 ± 20.86d | 69.03 ± 7.04 cd | 39.91 ± 0.587cde | 5.29 ± 0.065bc | 5.28 ± 0.093 cd | 0.374 ± 0.013ab |
| 300 | 4 | 92.23 ± 0.850f | 12.7 ± 1.058cde | 143.6 ± 5.8c | 77.16 ± 9.31 cd | 41.34 ± 0.591bcde | 5.67 ± 0.371ab | 5.439 ± 0.063bc | 0.505 ± 0.92a |
| 300 | 6 | 75.6 ± 0.889g | 10.7 ± 1.609e | 83.5 ± 15.31e | 58.88 ± 3.51de | 39.24 ± 0.219de | 5.19 ± 0.081bc | 5.232 ± 0.039cd | 0.376 ± 0.041ab |
| F-ratio (df 7,16) | 3662.3** | 105.45** | 28.037** | 28.008** | 16.343** | 15.945** | 16.372** | 30.716** | |
| HSD | 2.891 | 5.333 | 56.881 | 19.88 | 3.339 | 0.494 | 0.471 | 0.142 | |
| Multiple regression analysis | ||||
|---|---|---|---|---|
| Parameters (Y) | Multiple regression equations | R | β 1 | β 2 |
| Sodium | Y = 149.66 − 0.142 X1 − 4.721 X2 | 0.710*** | − 0.636 | − 0.316 |
| Potassium | Y = 35.79 − 0.055 X1 − 2.066 X2 | 0.894*** | − 0.779 | − 0.437 |
| Calcium | Y = 179.63 − 0.303 X1 + 3.807 X2 | 0.773*** | − 0.759 | 0.142 |
| Magnesium | Y = 86.75 − 0.0862 X1 + 0.351 X2 | 0.619** | − 0.619 | 0.038 |
| Carbon | Y = 44.401 − 0.014 X1 − 0.155 X2 | 0.778*** | − 0.768 | − 0.126 |
| Hydrogen | Y = 5.949 − 0.0023 X1 + 0.0158 X2 | 0.853*** | − 0.848 | 0.088 |
| Nitrogen | Y = 5.704 − 0.0019 X1 + 0.036 X2 | 0.798*** | − 0.771 | 0.206 |
| Sulphur | Y = 0.047 + 0.00089 X1 + 0.022 X2 | 0.913*** | 0.855 | 0.320 |
Data presented in mean ± SD of three replicates. Means followed by the same letter are not significantly different using Tukey’s HSD test
µM micromole, mg milligram, g gram, DW dry weight, % percentage, X1 µM Cr, X2 µM Se, r correlation coefficient, β1 β-regression coefficient for Cr, β2 β-regression coefficient for Se
Significant at ***p ≤ 0.001, **p ≤ 0.01, *p ≤ 0.05
The percentage of C, H and N in the seedlings of B. juncea also decreased in seedlings grown under Cr supplementation. Cr caused a decrease of 13.1, 14.1 and 13.2% in the percentage of C, H and N, while the percentage of S was observed to enhance under the effect of Cr in B. juncea seedlings. Se supplementation caused an improvement in C, H and N by 7.82, 10.9 and 10.95%, respectively, as compared to Cr-treated seedlings. The percentage of S was further enhanced in binary combination of Cr and Se (Table 6). The damaging effects of Cr on C, H and N were also indicated by negative β-regression coefficients, while the ameliorative property of Se was supported by positive β-regression coefficients for C, H and N. For S, however, the β-regression coefficients for both Cr and Se were positive (Table 6).
Discussion
The study showed significant damaging effects of Cr on seed germination, root and shoot lengths of B. juncea. Cr is reported to trigger the activity of proteases which further cause reduction in germination (Zeid 2001). Also, in the present study, the toxic effects of Cr were more significant on roots than on shoots. Cr can inhibit the cell division and cell elongation which could the reasons for reduced root lengths (Singh et al. 2013). Further, the underdeveloped roots are unable to absorb and translocate adequate amount to water and mineral nutrients to the shoots, which leads to reduced shoot growth (Singh et al. 2013). The study is confirmed by reports on T. aestivum and Glycine max, (Datta et al. 2011; Amin et al. 2014; Ghani et al. 2015). Various strategies have been adopted in earlier studies to combat abiotic stresses (Sharma et al. 2015) but Se application is a novel and recent approach to encounter metal stress. Se application to B. juncea helped in improving all the growth characteristics. The ability of Se to trigger carbohydrate metabolism could be the reason for its growth promoting effects (Malik et al. 2012). Similar results were also reported in Phaseolus aureus and T. aetivum (Malik et al. 2012; Teimouri et al. 2014).
The present study showed enhanced contents of total sugars, reducing sugars and non-reducing sugars when the B. juncea seedlings were subjected to Cr stress. It has been hypothesized that heavy metal toxicity might hinder the metabolic pathway of carbohydrates or it might play a role in the accumulation of photoassimilates because of reduced loading of veins (Rauser and Samarakoon 1980). Also, a study conducted on Spinicia oleracea by Gopal et al. (2009) established that Cr stress in plants leads to reduced availability of water that further causes water stress like conditions due to reduced water potential. Therefore, water stress induces the accumulation of osmolytes that also include various types of sugars (Smirnoff 1993). The findings of the study are in conformity with the previous studies on different plants subjected to stress by Cr as well as other heavy metals. Similar results were reported in Azolla caroliniana and Salvinia minima which showed enhanced contents of carbohydrates in response to Cr toxicity (Wilson and Al-Hamdani 1997; Nichols et al. 2000). Another study in S. minima by Prado et al. (2010) confirmed the accumulation of sucrose due to toxic effects of Cr. However, Se in the present study caused an additional increase in the contents of sugars, non-reducing and reducing sugars. Se-induced increased activity of amylases was established in rye grains and these enzymes are responsible for hydrolysis of starch to simple sugars (Malik et al. 2011). The soluble sugars start accumulating under stressful conditions and play an important role in maintaining osmotic homeostasis which further results in maintaining the integrity of various biomolecules and membranes (Dubey and Singh 1999). Eichhornia crassipes, at low doses of Se, showed enhanced contents of total carbohydrates and starch (Mane et al. 2011). Likewise, in P. aureus, exogenous Se application led to an increase in sucrose, reducing sugars and starch (Malik et al. 2011). Similarly, Brassica napus showed an increase in soluble sugars and starch in both roots and shoots after foliar application of Se (Hajiboland and Keivanfar 2012). The drought-stressed T. aestivum also showed enhanced contents of total soluble sugars (Nawaz et al. 2015).
In the study, the protein content in the seedlings decreased in response to Cr application. It has been suggested by Nag et al. (1981) that reduction in protein content might be due to reduced nitrogen content which is the precursor for the synthesis of amino acids. The current work showed a significant reduction in free amino acids and nitrogen contents. The study is in conformity with a study conducted by Datta et al. (2011) on different cultivars of T. aestivum treated with Cr which showed a dose-dependent decrease in the protein content. The studies carried out in Raphanus sativus also suggested lowered protein content and reduced enzyme activities under metal stress (Sharma et al. 2011). Also, in two varieties of Catharanthus roseus, a decrease in the protein content was reported in response to Cr (Rai et al. 2014). The phytotoxic effects of Cr were, however, observed to be alleviated by Se application in the present work. The B. juncea seedlings showed a significant increase in the contents of proteins, amino acid and nitrogen when Se was applied along with Cr. The S-metabolism has direct effect on N-metabolism; therefore, it can be assumed that Se might have an effect on biosynthesis of amino acids and proteins (Malagoli et al. 2015). The results of the current study also show enhanced accumulation of N which might be the reason for increased amino acid content and consequently enhanced protein content. The results of amino acid quantification also showed similar results and suggested the ameliorative role of Se. Similar observations were also reported in E. crassipes, Allium sativum, T. aestivum (Mane et al. 2011; Kapoor et al. 2012; Yao et al. 2012; Nawaz et al. 2015).
The increase in total phenols and flavonoid contents in response to Cr may be attributed to the fact that phenols have the ability to form chelates with metals and can also scavenge ROS (Brown et al. 1998; Lavid et al. 2001), while flavonoids can form complexes with heavy metals (Brown et al. 1998; Aherne and O’Brien 2000; Soczynska-Kordala et al. 2001; Michalak 2006; Korkina 2007). Therefore, these two metabolites have ameliorative properties against the heavy metal stress. The histological study carried out on roots of B. juncea seedlings also showed an increase in the intensity of dye in roots treated with Cr. Studies on B. napus and Lactuca sativa in response to waste water containing heavy metals (Hassanein et al. 2013), Hypoxis hemerocallidea in response to Cd and Al (Okem et al. 2015) and Lycopersicon esculentum in response to Cu (Chakraborty et al. 2015) showed enhanced contents of total phenols and flavonoids. Se application, in the present work, led to an additional increase in the contents of phenols and flavonoids. Se has the ability to influence N-containing secondary compounds (phenolic compounds) which have the capacity to scavenge free radicals produced as a result of stress (Malagoli et al. 2015). Se also affects the synthesis of many amino acids including phenylalanine which is a precursor of phenolic compounds (Malagoli et al. 2015). The results are supported by reports on T. aestivum in which Se application increased phenol and flavonoid contents in UV-B stressed plants (Yao et al. 2011). Similarly, Cd-stressed Lepidium sativum also showed increased contents of phenolic compounds when subjected to Se application (Elguera et al. 2013).
The elemental analysis showed that the content of Cr was higher in roots than in shoots. Na, K, Ca, Mg, C, H and N contents in the seedlings decreased with Cr application. It has been postulated that Cr can reduce the uptake of essential elements by roots due to the structural similarity leading to competition in movement and absorbance (Kabata-Pendias and Pendias 2001; Najafian et al. 2012). It has been suggested by Kabata-Pendias and Pendias (2001) that Cr has the ability to compete with elements such as Ca, Mg, Mn, Fe, K, P and N that leads to their reduced absorption and uptake. The decrease of Na and K can also result from an efflux of these elements due to membrane damage as heavy metals cause excess lipid peroxidation leading to increased permeability and reduced selectivity of the membranes (Janas et al. 2010). A study on B. napus reported a decreased content of Ca and K with Cr application (Najafian et al. 2012). In a study conducted on O. sativa, Cr treatment was reported to reduce the contents of Ca, Mg, K and N (Nagarajan and Ganesh 2014). Also, Cr-treated T. aestivum plants showed enhanced Na content, while decreased contents of K and Ca (Ghani et al. 2015). In this study, however, the percentage of S increased with Cr concentration. This might be because heavy metals cause an increase in various S containing compounds like metallothionins, phytochelatins and thiols.
In binary combinations, the stress ameliorative effect of Se was observed as it reduced the Cr uptake but enhanced the contents of Na, K, Ca, Mg, C, H, N and S. Our earlier study on Cr-stressed B. juncea showed significant role of Se in reducing the membrane damage by lowering lipid peroxidation, superoxide anion production and H2O2 contents (Handa et al. 2017). Therefore, lesser membrane damage due to Se could be the probable reason for accumulation of mineral nutrients. The results are supported by studies on Zea mays and T. aestivum which also showed accumulation of essential elements in response to Se application (Hawrylak-Nowak 2008; Zembala et al. 2010).
Conclusion
The study indicates that Se application to the plants at low doses can enhance their potential to combat Cr stress. The secondary metabolites like phenols and flavonoids, which are important antioxidants as well as metal chelators, are enhanced under the effect of Se to reduce the phytotoxic effects of Cr. These secondary metabolites scavenge ROS and also reduce membrane lipid peroxidation. Reduced membrane damage further aids in accumulation of essential micronutrients. From the results, it can be concluded that Se has an important role in maintaining the balance of the total sugars, reducing and non-reducing sugars which are involved in the osmotic system of the plants as osmolytes. It ultimately leads to osmotic homeostasis which supports various vital processes of plants. The proteins and amino acids were severely affected by Cr toxicity, but the ameliorative properties of Se aided in the recovery of these metabolites. The application of Se also showed its potential to reduce the uptake of Cr that results in lowering of oxidative stress. Se, therefore, through various physiological processes, can counter damaging effects of Cr and protect the plants from metal stress. Further studies on mechanisms of Se pertaining to metal amelioration can provide a better understanding of stress tolerance strategies in plants.
Acknowledgements
The authors extend their appreciation to the Deanship of Scientific Research at King Saud University, Riyadh, Saudi Arabia for funding this research group no (RG-1438-039). The authors also acknowledge UGC-UPE and DRS-SAP (II) program of University Grants Commission, India.
Compliance with ethical standards
Conflict of interest
The authors declare no conflict of interest.
Contributor Information
Renu Bhardwaj, Email: renubhardwaj82@gmail.com.
Parvaiz Ahmad, Email: parvaizbot@yahoo.com.
References
- Aherne SA, O’Brien NM. Mechanism of protection by the flavonoids, quercetin and rutin, against tertbutylhydroperoxide- and menadione-induced DNA single strand breaks in Caco-2 cells. Free Rad Biol Med. 2000;29:507–514. doi: 10.1016/S0891-5849(00)00360-9. [DOI] [PubMed] [Google Scholar]
- Ahmad P, Bhardwaj R, Tuteja N. Plant signaling under abiotic stress environment. In: Ahmad P, Prasad MNV, editors. Environmental adaptations and stress tolerance of plants in the era of climate change. New York: Springer; 2012. pp. 297–323. [Google Scholar]
- Ali S, Farooq MA, Yasmeen T, Hussain S, Arif MS, Abbas F, Bharwana SA, Zhang G. The influence of silicon on barley growth, photosynthesis and ultra-structure under chromium stress. Ecotoxicol Environ Saf. 2013;89:66–72. doi: 10.1016/j.ecoenv.2012.11.015. [DOI] [PubMed] [Google Scholar]
- Allen SE, Grimshaw HM, Parkinson JA, Quarmby C, Roberts JD. Chemical Analysis. In: Chapmanm SB, editor. Methods in plant ecology. Oxford: Blackwell Scientific Publications; 1976. pp. 424–426. [Google Scholar]
- Alyemeni MN, Ahanger MA, Wijaya L, Alam P, Bhardwaj R, Ahmad P. Selenium mitigates cadmium-induced oxidative stress in tomato (Solanum lycopersicum L.) plants by modulating chlorophyll fluorescence, osmolyte accumulation, and antioxidant system. Protoplasma. 2017 doi: 10.1007/s00709-017-1162-4. [DOI] [PubMed] [Google Scholar]
- Amin H, Arain BA, Amin F, Surhio MA. Analysis of growth response and tolerance index of Glycine max (L.) Merr. under hexavalent chromium stress. Adv Life Sci. 2014;1(4):231–241. [Google Scholar]
- Bailey NTJ. Statistical methods in biology. Cambridge: Cambridge University Press; 1995. [Google Scholar]
- Bansal A, Sharma S, Dhillon SK, Dhillon KS. Selenium accumulation and biochemical composition of Brassica grains grown in selenate or selenite treated alkaline sandy loam soil. Commun Soil Sci Plant Anal. 2012;43:1316–1331. doi: 10.1080/00103624.2012.666306. [DOI] [Google Scholar]
- Belouchrani AS, Mameri N, Abdi N, Grib H, Lounici H, Drouiche N. Phytoremediation of soil contaminated with Zn using Canola (Brassica napus L) Ecol Eng. 2016;95:43–49. doi: 10.1016/j.ecoleng.2016.06.064. [DOI] [Google Scholar]
- Brown JE, Khodr H, Hider RC, Rice-Evans CA. Structural dependence of flavonoid interactions with Cu2+ ions: implications for their antioxidant properties. Biochem J. 1998;330:1173–1178. doi: 10.1042/bj3301173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty N, Chandra S, Acharya K. Sublethal heavy metal stress stimulates innate immunity in tomato. Sci World J. 2015;2015:1–7. doi: 10.1155/2015/208649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheballah K, Sahmoune A, Messaoudi K, Drouiche N, Lounici H. Simultaneous removal of hexavalent chromium and COD from industrial wastewater by bipolar electrocoagulation. Chem Eng Process. 2015;96:94–99. doi: 10.1016/j.cep.2015.08.007. [DOI] [Google Scholar]
- Datta JK, Bandhyopadhyay A, Banerjee A, Mondal NK. Phytotoxic effect of chromium on the germination, seedling growth of some wheat (Triticum aestivum L) cultivars under laboratory condition. J Agric Technol. 2011;7(2):395–402. [Google Scholar]
- Demim S, Drouiche N, Aouabed A, Benayad T, Dendene-Badache O, Semsari S. Cadmium and nickel: assessment of the physiological effects and heavy metal removal using a response surface approach by L. gibba. Ecol Eng. 2013;61:426–435. doi: 10.1016/j.ecoleng.2013.10.016. [DOI] [Google Scholar]
- Demim S, Drouiche N, Aouabed A, Semsari S. CCD study on the ecophysiological effects of heavy metals on Lemna gibba. Ecol Eng. 2013;57:302–313. doi: 10.1016/j.ecoleng.2013.04.041. [DOI] [Google Scholar]
- Demim S, Drouiche N, Aouabed A, Benayad T, Couderchet M, Semsari S. Study of heavy metal removal from heavy metal mixture using the CCD method. J Ind Eng Chem. 2014;20(2):512–520. doi: 10.1016/j.jiec.2013.05.010. [DOI] [Google Scholar]
- Diao M, Ma Long, Jianwei W, Jinxia C, Aifei F, Huiying L. Selenium promotes the growth and photosynthesis of tomato seedlings under salt stress by enhancing chloroplast antioxidant defense system. J Plant Growth Regul. 2014;33:671–682. doi: 10.1007/s00344-014-9416-2. [DOI] [Google Scholar]
- Dubey RS, Singh AK. Salinity induces accumulation of soluble sugars and alters the activity of sugar metabolising enzymes in rice plants. Biol Plant. 1999;42:233–239. doi: 10.1023/A:1002160618700. [DOI] [Google Scholar]
- Elguera JCT, Barrientos EY, Wrobel K, Wrobel K. Effect of cadmium (Cd(II)), selenium (Se(IV)) and their mixtures on phenolic compounds and antioxidant capacity in Lepidium sativum. Acta Physiol Plant. 2013;35:431–441. doi: 10.1007/s11738-012-1086-8. [DOI] [Google Scholar]
- Gahan PB. Plant histochemistry and cytochemistry: an introduction. London: Academic Press; 1984. [Google Scholar]
- Ghani A, Khan I, Umer S, Ahmed I, Mustafa I, Mohammed N. Response of wheat (Triticum aestivum) to exogenously applied chromium: effect on growth, chlorophyll and mineral composition. J Environ Anal Toxicol. 2015;5:273. [Google Scholar]
- Gopal R, Rizvi AH, Nautiyal N. Chromium alters iron nutrition and water relations of spinach. J Plant Nutr. 2009;32(9):1551–1559. doi: 10.1080/01904160903094313. [DOI] [Google Scholar]
- Han GQ, Li J, Song MM, Liu HY. Effects of selenium on the germination of tomato seeds and protective system against active oxygen under salt stress. J Shihezi Univ Nat Sci. 2010;28:422–426. [Google Scholar]
- Handa N, Kohli SK, Thukral AK, Arora S, Bhardwaj R. Role of Se (VI) in counteracting oxidative damage in Brassica juncea L. under Cr(VI) stress. Acta Physiol Plant. 2017;39:51. doi: 10.1007/s11738-017-2352-6. [DOI] [Google Scholar]
- Hasanuzzaman M, Hossain MA, Fujita M. Selenium in higher plants: physiological role, antioxidant metabolism and abiotic stress tolerance. J Plant Sci. 2010;5:354–375. doi: 10.3923/jps.2010.354.375. [DOI] [Google Scholar]
- Hajiboland R, Keivanfar N. Selenium supplementation stimulates vegetative and reproductive growth in canola (Brassica napus L.) plants. Acta Agric Slov. 2012;19:13–19. [Google Scholar]
- Hassanein RA, Hashem HA, El-Deep MH, Shouman A. Soil contamination with heavy metals and its effect on growth, yield and physiological responses of vegetable crop plants (turnip and lettuce) J Stress Physiol Biochem. 2013;9:145–162. [Google Scholar]
- Hawrylak-Nowak B. Effect of selenium on selected macronutrients in maize plant. J Elementol. 2008;13(4):513–519. [Google Scholar]
- Hawrylak-Nowak B. Comparative effects of selenite and selenate on growth and selenium accumulation in lettuce plants under hydroponic conditions. Plant Growth Regul. 2013;70:149–157. doi: 10.1007/s10725-013-9788-5. [DOI] [Google Scholar]
- Hedge JE, Hofreiter BT. Methods in carbohydrate chemistry. New York: Academic Press; 1962. [Google Scholar]
- Janas KM, Zielińska-Tomaszewskaa J, Rybaczek D, Maszewskib J, Posmyk MM, Amarowiczc R, Kosińska A. The impact of copper ions on growth lipid peroxidation and phenolic compound accumulation and localization in lentil (Lens culinaris Medic.) seedlings. J Plant Physiol. 2010;167:270–276. doi: 10.1016/j.jplph.2009.09.016. [DOI] [PubMed] [Google Scholar]
- Kabata-Pendias A, Pendias H. Trace elements in soils and plants. 3. Boca Raton, Florida, USA: CRC Press LLC; 2001. [Google Scholar]
- Kanwar MK, Poonam, Bhardwaj R. Arsenic induced modulation of antioxidative defense system and brassinosteroids in Brassicajuncea L. Ecotoxicol Environ Saf. 2015;115:119–125. doi: 10.1016/j.ecoenv.2015.02.016. [DOI] [PubMed] [Google Scholar]
- Kapoor R, Nasim SA, Dhir B, Mahmooduzzafar Mujib A. Selenium treatment alters phytochemical and biochemical activity of in vitro-grown tissues and organs of Allium sativum L. In Vitro Cell Dev Biol Plant. 2012;48:411–416. doi: 10.1007/s11627-012-9456-x. [DOI] [Google Scholar]
- Korkina LG. Phenylpropanoids as naturally occurring antioxidants: from plant defense to human health. Cell Mol Biol. 2007;53:15–25. [PubMed] [Google Scholar]
- Lavid N, Schwartz A, Yarden O, Tel-Or E. The involvement of polyphenols and peroxidase activities in heavy metal accumulation by epidermal glands of the waterlily (Nymphaeaceae) Planta. 2001;212:323–331. doi: 10.1007/s004250000400. [DOI] [PubMed] [Google Scholar]
- Lee YP, Takahashi T. An improved colorimetric determination of amino acids with the use of ninhydrin. Anal Biochem. 1966;14:71–77. doi: 10.1016/0003-2697(66)90057-1. [DOI] [Google Scholar]
- Loomis WE, Shull CA. Methods in plant physiology, a laboratory manual and research handbook. New York: Mc Graw-Hill Publication in Botanical Science; 1937. [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL. Protein measurement with the folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Malagoli M, Schiavon M, Pilon-Smits EA. Effects of selenium biofortification on crop nutritional quality. Front Plant Sci. 2015;6:280. doi: 10.3389/fpls.2015.00280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik JA, Kumar S, Thakur P, Sharma S, Kaur N, Kaur R, Pathania D, Bhandhari K, Kaushal N, Singh K, Shrivasatva A, Nayyar H. Promotion of growth in mungbean (Phaseolusaureus Roxb.) by selenium is associated with stimulation of carbohydrate metabolism. Biol Trace Elem Res. 2011;143(1):530–539. doi: 10.1007/s12011-010-8872-1. [DOI] [PubMed] [Google Scholar]
- Malik JA, Goel S, Kaur N, Sharma S, Singh I, Nayyar H. Selenium antagonises the toxic effects of arsenic on mungbean (Phaseolus aureus Roxb.) plants by restricting its uptake and enhancing the antioxidative and detoxification mechanisms. Environ Exp Bot. 2012;77:242–248. doi: 10.1016/j.envexpbot.2011.12.001. [DOI] [Google Scholar]
- Mane PC, Bhosle AB, Kulkarni PA. Biosorption and biochemical study on water hyacinth (Eichhornia crassipes) with reference to selenium. Arch Appl Sci Res. 2011;3:222–229. [Google Scholar]
- Michalak A. Phenolic compounds and their antioxidant activity in plants growing under heavy metal stress. Pol J Environ Stud. 2006;15:523–530. [Google Scholar]
- Miller GL. Use of dinitrosalicylic acid reagent for determination of reducing sugars. Anal Chem. 1972;31:426–428. doi: 10.1021/ac60147a030. [DOI] [Google Scholar]
- Moore S, Stein WH. Chromatographic amino acids determination by the use of automatic recording equipment. Method Enzymol. 1963;6:819–831. doi: 10.1016/0076-6879(63)06257-1. [DOI] [Google Scholar]
- Moussa HR, Ahmed AEM. Protective role of selenium on development and physiological responses of Vicia faba. Int J Veg Sci. 2010;16:174–183. doi: 10.1080/19315260903375137. [DOI] [Google Scholar]
- Nag P, Paul AK, Mukherjee S. Heavy metal effects in plant tissues involving chlorophyll, chlorophyllase, Hill reaction activity, and gel electrophoresis patterns of soluble proteins. Indian J Exp Biol. 1981;19:702–706. [Google Scholar]
- Nagarajan M, Ganesh KS. Effect of chromium on growth, biochemicals and nutrient accumulation of paddy (Oryza sativa L.) Int Lett Nat Sci. 2014;23:63–71. doi: 10.18052/www.scipress.com/ILNS.23.63. [DOI] [Google Scholar]
- Najafian M, Kafilzadeh F, Azad HN, Tahery Y. Toxicity of chromium (Cr6+) on growth, ions and some biochemical parameters of Brassica napus L. Am Eurasian J Agric Environ Sci. 2012;12(2):237–242. [Google Scholar]
- Nawaz F, Ashraf MY, Ahmad R, Waraich EA, Shabbir RN, Bukhari MA. Supplemental selenium improves wheat grain yield and quality through alterations in biochemical processes under normal and water deficit conditions. Food Chem. 2015;175:350–357. doi: 10.1016/j.foodchem.2014.11.147. [DOI] [PubMed] [Google Scholar]
- Nichols PB, Couch JD, Al-Hamdani SH. Selected physiological responses of Salviniaminima to different chromium concentrations. Aquat Bot. 2000;68:313–319. doi: 10.1016/S0304-3770(00)00128-5. [DOI] [Google Scholar]
- Okem A, Stirk WA, Street RA, Southway C, Finnie JF, Van Staden J. Effects of Cd and Al stress on secondary metabolites, antioxidant and antibacterial activity of Hypoxis hemerocallidea Fisch. & C.A. Mey. Plant Physiol Biochem. 2015;97:147–155. doi: 10.1016/j.plaphy.2015.09.015. [DOI] [PubMed] [Google Scholar]
- Owusu-Sekyere A, Kontturi J, Hajiboland R, Rahmat S, Aliasgharzad N, Hartikainen H, Seppanen M. Influence of selenium (Se) on carbohydrate metabolism, nodulation and growth in alfalfa (Medicago sativa L.) Plant Soil. 2013;373:541–552. doi: 10.1007/s11104-013-1815-9. [DOI] [Google Scholar]
- Panda SK, Patra HK. Does Cr(III) produce oxidative damage in excised wheat leaves? J Plant Biol. 2000;27:105–110. [Google Scholar]
- Pezzarossaa B, Rosellini I, Borghesi E, Tonutti P, Malorgio F. Effects of Se-enrichment on yield, fruit composition and ripening of tomato (Solanum lycopersicum) plants grown in hydroponics. Sci Hort. 2014;165:106–110. doi: 10.1016/j.scienta.2013.10.029. [DOI] [Google Scholar]
- Prado C, Rodrıguez-Montelongo L, Gonzalez JA, Pagano EA, Hilal M, Prado FE. Uptake of chromium by Salvinia minima: effect on plant growth, leaf respiration and carbohydrate metabolism. J Hazard Mater. 2010;177:546–553. doi: 10.1016/j.jhazmat.2009.12.067. [DOI] [PubMed] [Google Scholar]
- Pukacka S, Ratajczak E, Kalemba E. The protective role of selenium in recalcitrant Acer saccharium L. seeds subjected to desiccation. J Plant Physiol. 2011;168:220–225. doi: 10.1016/j.jplph.2010.07.021. [DOI] [PubMed] [Google Scholar]
- Rai V, Tandon PK, Khatoon S. Effect of chromium on antioxidant potential of Catharanthus roseus varieties and production of their anticancer alkaloids: vincristine and vinblastine. BioMed Res Int. 2014;2014:1–10. doi: 10.1155/2014/934182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauser WE, Samarakoon AB. Vein loading in seedlings of Phaseolus vulgaris exposed to excess cobalt, nickel and zinc. Plant Physiol. 1980;65:578–583. doi: 10.1104/pp.65.4.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samantaray S, Rout GR, Das P. Role of chromium on plant growth and metabolism. Acta Physiol Plant. 1998;20:201–212. doi: 10.1007/s11738-998-0015-3. [DOI] [Google Scholar]
- Schwarz K, Foltz CM. Selenium as an integral part of factor 3 against dietary necrotic liver degeneration. J Am Chem Soc. 1957;79(12):3292–3293. doi: 10.1021/ja01569a087. [DOI] [PubMed] [Google Scholar]
- Shapiro SS, Wilk MB. An analysis of variance test for normality (complete samples) Biometrika. 1965;52(3–4):591–611. doi: 10.1093/biomet/52.3-4.591. [DOI] [Google Scholar]
- Sharma I, Pati PK, Bhardwaj R. Regulation of growth and antioxidant enzyme activities by 28-homobrassinolide in seedlings of Raphanus sativus L. under cadmium stress. Indian J Biochem Biophy. 2010;47:172–177. [PubMed] [Google Scholar]
- Sharma I, Pati PK, Bhardwaj R. Effect of 24-epibrassinolide on oxidative stress markers induced by nickel-ion in Raphanus sativus L. Acta Physiol Plant. 2011;33(5):1723–1735. doi: 10.1007/s11738-010-0709-1. [DOI] [Google Scholar]
- Sharma I, Bhardwaj R, Pati PK. Exogenous application of 28-homobrassinolide modulates the dynamics of salt and pesticides induced stress responses in an elite rice variety Pusa Basmati-1. J Plant Growth Regul. 2015;34(3):509–518. doi: 10.1007/s00344-015-9486-9. [DOI] [Google Scholar]
- Singh HP, Mahajan P, Kaur S, Batish DR, Kohli RK. Chromium toxicity and tolerance in plants. Environ Chem Lett. 2013;11:229–254. doi: 10.1007/s10311-013-0407-5. [DOI] [Google Scholar]
- Singleton VL, Rossi JA. Colorimetry of total phenolics with phosphormolybdic phosphotungstic acid reagents. Am J Enol Vitic. 1965;16:144–158. [Google Scholar]
- Smirnoff N. The role of active oxygen in the response of plants to water deficit and desiccation. New Phytol. 1993;125(1):27–58. doi: 10.1111/j.1469-8137.1993.tb03863.x. [DOI] [PubMed] [Google Scholar]
- Soczynska-Kordala M, Bakowska A, Oszmianski J, Gabrielska J. Metal ion-flavonoid associations in bilayer phospholipidmembranes. Cell Mol Biol Lett. 2001;6:277–281. [PubMed] [Google Scholar]
- Sokal RR, Rholf FJ. Biometry: the principles and practice of statistics in biological research. San Francisco: WH Freeman and Co.; 1981. [Google Scholar]
- Soleimanzadeh H. Response of sunflower (Helianthus annus L.) to selenium application under water stress. World Appl Sci J. 2012;17(9):1115–1119. [Google Scholar]
- Teimouri S, Hasanpour J, Akbar A. Effect of selenium spraying on yield and growth indices of wheat (Triticum aestivum L.) under drought stress condition. Int J Adv Biol Biomed Res. 2014;2:2091–2103. [Google Scholar]
- USEPA (1999) Guidelines for Carcinogen Risk Assessment Review draft. NCEA-F-0644, Jul 1999. http://www.epa.gov/cancerguidelines/draft-guidelines-carcinogen-ra-1999.htm
- Wilson G, Al-Hamdani SH. Effects of chromium(VI) and humic substances on selected physiological responses of Azolla caroliniana. Am Fern J. 1997;87:17–27. doi: 10.2307/1547244. [DOI] [Google Scholar]
- Yao X, Chu J, He X, Ba C. Protective role of selenium in wheat seedlings subjected to enhanced UV-B radiation. Russ J Plant Physiol. 2011;58:283–289. doi: 10.1134/S1021443711020257. [DOI] [Google Scholar]
- Yao X, Chu J, Liang L, Geng W, Li J, Hou G. Selenium improves recovery of wheat seedlings at rewatering after drought stress. Russ J Plant Physiol. 2012;59:701–707. doi: 10.1134/S1021443712060192. [DOI] [Google Scholar]
- Zeid IM. Responses of Phaseolus vulgaris to chromium and cobalt treatments. Biol Plant. 2001;44:111–115. doi: 10.1023/A:1017934708402. [DOI] [Google Scholar]
- Zayed AM, Terry N. Chromium in the environment: factors affecting biological remediation. Plant Soil. 2003;249:139–156. doi: 10.1023/A:1022504826342. [DOI] [Google Scholar]
- Zembala M, Filek M, Walas S, Mrowiec H, Kornaś A, Miszalski Z, Hartikainen H. Effect of selenium on macro- and microelement distribution and physiological parameters of rape and wheat seedlings exposed to cadmium stress. Plant Soil. 2010;329:457–468. doi: 10.1007/s11104-009-0171-2. [DOI] [Google Scholar]
- Zhishen J, Mengcheng T, Jianming W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999;64:555–559. doi: 10.1016/S0308-8146(98)00102-2. [DOI] [Google Scholar]