Abstract
Severe drought stress (water deficit) in finger millet (Eleusine coracana L. Gaertn.) plants significantly reduced total leaf chlorophyll and relative water content in shoots and roots, whereas electrolyte leakage, concentrations of proline and hydrogen peroxide, as well as caspase-like activity were significantly increased. The role of spermidine in plant defence to water-stress was investigated after subjected to various drought treatments. Three weeks of daily spermidine sprays (0.2 mM) at early flowering stage significantly changed shoot and root growth, in both fresh and dry weights terms. At 75% of water deficit stress, leaves accumulated twice as much proline as unstressed plants, and roots accumulated thrice. Plants treated with spermidine under water stress showed lower electrolyte leakage, hydrogen peroxide and caspase-like activity than unstressed and untreated control.
Keywords: Drought stress, Finger millet, Physiological parameters, Polyamines, Spermidine
Introduction
Drought is the worst natural menace, causing devastating impacts on all organisms worldwide. The tropics of Asia and Africa may enter a period of remarkable change in the prevalence of development, occurrence and the intensity of droughts (IPCC 2007; Singh et al. 2014). Under field conditions, plants are consistently subjected to a complex of different abiotic stresses (Zhou and Shao 2008). Water shortage, in terms of both low vapor pressure deficit and low soil water potential, occurs at the same time as high temperatures and high light (Pollastrini et al. 2014). The metabolic and molecular responses of plants to the combination of drought and salinity are distinct and cannot be responsively concluded from the equivalent response of plants to each of these varied stresses when applied individually (Slama et al. 2015). Efforts to develop crop performance against drought stress were un-successful due to (i) incomplete understanding of underlying essential mechanisms of stress tolerance and (ii) lack of awareness of the interactions among various stresses (Hussain et al. 2011). It is consequently essential to understand the mechanisms of abiotic stress tolerance in plants and to categorize the genetic resources that can result in high levels of tolerance for various stresses (Ahmed et al. 2014).
Drought stress impedes plant growth and development, and hence goes through disturbing various physiological and biochemical processes (Shah et al. 2011). Environmental stresses, including drought can augment an oxidative stress at a cellular level by overproducing reactive oxygen species (ROS) such as hydrogen peroxide (H2O2), singlet oxygen (1O2), superoxide () and hydroxyl radicals (Mostofa et al. 2014). ROS are exceedingly reactive to cellular components, thus critically affecting plant metabolism, nutrient uptake and limiting their development and yield (Mittler 2002). Plant cells usually respond to ROS using different antioxidants including superoxide dismutase (SOD), glutathione peroxidase, catalase, and ascorbate peroxidase which shows the major line of enzymatic protection against ROS or catalyzes the dismutation of superoxide to H2O2 in the cells (Roxas et al. 2000; Mostofa et al. 2014). In natural field environments, stress can be transient and the ability of the plant to complete its entire progression is directly associated with its capacity to improve after the stress period. According to Lutts et al. (2004) several cytological or biochemical injuries may occur during or even after re-watering in previously drought-exposed plants. However, the coordinating role of the antioxidative systems in imparting drought tolerance in small millets, especially finger millet has not been extensively studied.
Finger millet (Eleusine coracana L. Gaertn.), cultivated worldwide in more than four million hectares, is a staple food for millions in less developed countries of Africa and Asia (Satish et al. 2015, 2016a, b, c, d, 2017). The nutritional qualities of finger millet are outstanding to that of other major cereals since it includes well-known invaluable amino acids viz., tryptophan, cysteine, tyrosine and methionine, and is rich in microelements including iron, calcium and phosphorus, in addition sprouted finger millet seeds are convincingly consumable (Ceasar and Ignacimuthu 2011; Satish et al. 2016d, 2017). The calcium content of finger millet is 5–30 times higher than the other cereals and it contains on an average of 7.3% protein and 44.7% of the essential amino acids of total amino acids (Nirgude et al. 2014). It is a recommended nutritious food for children and diabetic patients; in addition, its grains may also be powdered and provided as a healthful food for infants as it is digested easily (Ignacimuthu and Ceasar 2012; Satish et al. 2016b). It is also renowned for its health benefits such as hypo-cholesterolemic, hypo-glycemic and anti-ulcerative characteristics (Chethan and Malleshi 2007). With the increasing interest in improvement of highly nutritious finger millet, trait improvement to abiotic and biotic resistance is an important research direction. In the past, limited efforts have been made in this crop with reverence to its anti-oxidative potential aligned with drought stress (Bartwal and Arora 2017). In addition, the relationship among drought stress tolerance and genetic expression of anti-oxidative enzymes is not well understood. Major problem associated with conventional breeding is that, if the gene is present in a wild relative of the crop, there is difficulty in transferring it to the domesticated cultivar, due to reproductive barriers and linkage drag (Rahman et al. 2014).
Polyamines (spermidine, spermine and putrescine) are aliphatic amines that are ubiquitously present in all organisms, and are required for the normal development of both prokaryotes and eukaryotes (Zhang et al. 2003; Satish et al. 2016c). Polyamines are not only involved in essential cellular processes, for example cell proliferation, differentiation, and programmed cell death, but also in adaptive responses to environmental stresses including acid stress, heavy metal stress, osmotic stress, UV radiation, and salinity and drought stress due to their polycationic nature (Kusano et al. 2007; Yin et al. 2014; Li et al. 2016). Extensive reports of exogenous polyamine application suggest that a possible involvement of polyamines in plant adaptation to several environmental stresses (Alcazar et al. 2006). In recent years, research on polyamines has increased tremendously and has provided clues to improve plant developmental processes (Satish et al. 2016c). Extensive studies of exogenous polyamine application recommend a promising involvement of polyamines in plant adaptation to various environmental stresses including, drought and salinity (Yin et al. 2014). A spermidine-deficient Arabidopsis mutant showed hypersensitivity to salinity stress (Yamaguchi et al. 2007), but the query as to whether spermidine plays a defensive role against drought stress in finger millet plants and has received little attention to date. Although spermidine is involved in abiotic stress response and is a complex process that unites several metabolic processes, this study focused on the influence of spermidine on finger millet plants during drought stress with respect to physiological parameters, caspase like activity and H2O2.
The present study describes the effect of water-stressed conditions and the influence of spermidine on biomass, growth parameters, relative water content (RWC), total chlorophyll (Chl) content, electrolyte leakage (EL) and proline content in high yielding and widespread Indian finger millet cultivar ‘CO(Ra)-14’ plants. This report also showed a few biochemical basis of oxidative stress, such as H2O2 and caspase-like activity in finger millet at water-stressed conditions with and without spermidine application. Studying the mechanism and pathway involved in finger millet drought tolerance might aid in better understanding of plant growth and development under water deficient conditions and therefore allow the existing use of breeding, genomic or genetic methods to develop drought tolerance in important crops.
Materials and methods
Plant material and experimental design
A high-yielding finger millet cultivar ‘CO(Ra)-14’ obtained from Tamil Nadu Agricultural University, Coimbatore, India was used in this study. Prior to germination, seeds were surface sterilized (Satish et al. 2015): pre-treated with sterile distilled water (SDW) for 30 min to remove the husk. Dehusked seeds were subjected to surface disinfestations with 70% (v/v) ethanol for 1 min and washed with SDW twice for 1 min. Seeds were later disinfected in 0.1% mercuric chloride (HgCl2) solution for 5 min followed by five washes with SDW. Then, the seeds were soaked in SDW for 24 h to initiate germination. Germinated seeds were sown in a tray, filled with Soilrite™ (Keltech, India), vermiculate and red soil at 2:1:1 ratio, grown for 20 days moved to soil pots (30 cm height × 25 cm upper diameter × 20 cm lower diameter) and maintained up to early flowering stage (50 days). Plants were irrigated regularly by Yoshida (Yoshida et al. 1976) solution (pH 5.8) and maintained at 26 °C day/16 °C night with a 16 day/8 night h photoperiod with a relative humidity of 70%.
After fully grown, the plants with uniform sizes (Early flowering stage/50-day-old plants) in seven soil pots each with three plants were allocated for stress treatments. All experiments were accomplished in a completely randomized design with two major experimental setups. In experiment 1, all the sample pots were treated with four different soil water conditions (one is well-watered state (control) and remaining three are in various water-stressed conditions viz., 25, 50 and 75% of field capacity). Similarly, in experiment 2 all the sample pots were treated with four different spermidine treatments (one is well-watered state sprayed with distilled water alone (control) and in remaining three plants were foliar sprayed with 0.2 mM (in 100 ml of water) spermidine at 25, 50 and 75% water-stressed conditions, individually) for ‘CO(Ra)-14’ finger millet cultivar at early flowering stage. Data of experiment 1, i.e., water-stressed conditions (without spermidine) was considered as negative controls to compare the results obtained in experiment 2, i.e., water-stressed conditions (with 0.2 mM spermidine sprays). There were no separate controls maintained for each independent spermidine concentration, as shown above. The rationale for the use of 0.2 mM spermidine was according to Yin et al. (2014), proved that 0.2 mM spermidine helped to maintain the positive effects on antioxidant systems in Cerasus humilis. All treatments were rejuvenated for every 2 days up to 3 weeks, samples were collected for all the treatments and prepared at the end of the dried up time.
Growth measurements
Plants were collected from all the pots individually, cleaned with SDW to remove the surface dust and soil particles and growth was measured. After washing, the water molecules from the samples were drained using Whatman no. 3 filter papers and plants were used to measure mean length of shoots and roots. Further the plants were divided into shoots and roots and measured fresh weight and then the samples was oven dried for 72 h at 70 °C to determine the dry weight in response to water deficit and spermidine sprayed conditions.
Determination of relative water content
The fresh weight (WF) of harvested leaves and roots from all treatments viz., water deficit (25, 50, 75% and control) and water deficit of similar concentrations along with 0.2 mM spermidine was analyzed. The harvested tissues of leaves and roots were dipped in deionized water to overnight for swelling, and then shortly air dried and weighed to determine the swelling weight (WS). These samples were oven dried at 70 °C for 48 h and dry weights (WD) was measured according to Barrs and Weatherley (1962)in finger millet. The relative water content (RWC) was calculated as:
Changes in total chlorophyll
Total chlorophyll (Chl) (a, b, and a + b) content were determined according to Lichtenthaler (1987). At the end of the experiment, about 100 mg of fresh leaves was collected from each treatment and covered in an aluminum foil to prevent degradation of pigments in the light. Leaf samples were finely ground in a cold mortar and pestle and extracted using 5 mL absolute ethanol (99%). Extracts were filtered through Whatman no. 1 filter paper (Whatman®, Sigma-Aldrich, India) and absorbance of centrifuged filtrates was measured by UV–Vis spectrophotometer (UV-2450, Shimadzu Scientific Instruments, Japan) at 661.6 and 646.8 nm, respectively.
Determination of proline content
Plant leaf samples each 500 mg in fresh weight was randomly collected from water deficit (25, 50 and 75%), conditions and controls with and without spermidine (0.2 mM) spray, and quantified for free proline content according to Bates et al. (1973). Plant leaf tissues were gently sliced and homogenized in 10 mL of 3% aqueous sulphosalicylic acid and filtered the homogenate through Whatman no. 2 filter paper. Acid ninhydrin and glacial acetic acid (1 mL each) was added to 1 mL of filtrate and the reaction mixture was boiled (100 °C for 1 h) followed by an ice bath for 1 min. The reaction mixture was then collected from all treatments and 2 mL of toluene was added followed by 30 s vortex. Proline content was evaluated using UV–Vis spectrophotometer at 520 nm and l-proline (Hi-Media, Mumbai, India) calibration values, and expressed as mmol proline gram−1 of leaf fresh weight.
Determination of electrolyte leakage
Electrolyte leakage (EL) was determined with 100 mg of leaves from the various treatments. Samples were sliced (1–2 cm), and gently washed with deionized water and removed the surface bound electrolytes which are released during cutting of leaves. Leaf slices were submerged in 20 mL of deionized water and incubated in a desiccator under a negative force of ≈ 10−1 bar for 1 h. The primary electrical conductivity (E1) of the sample was measured using a conductivity/TDS/ °C gauge (Cyberscan200, Eutech Instruments, Singapore). All the sample tubes were then boiled at 121 °C for 2 h to discharge all electrolytes, frozen to 25 °C, and the total electrical conductivity (ET) of the sample was calculated. The E1 value was expressed as a percentage of electrolytes leaked from slice cells compared to the total electrolyte pool (ET) in the solution (Tabot and Adams 2013),
Determination of caspase-like activity
The caspase-like activity in finger millet was analyzed according to del Pozo and Lam (1998). Young leaves (the second youngest) and roots were collected from all treated plants. From each treatment, 10 mg of leaf and root tissues were frozen in liquid nitrogen, powdered and homogenized with 2 mL of assay buffer containing 10% (v/v) glycerol, 5 mM MgCl2, 100 mM Tris–HCl (pH 7.2), 10 mM β-mercaptoethanol, 1 mM phenyl methyl sulfonyl fluoride (PMSF) and 2 mM EDTA. The samples were centrifuged (13,000 rpm for 30 min at 4 °C), the supernatant was carefully discarded and the tissue extract was used for this assay. For caspase-like activity, 20 μL of the tissue extract was mixed with 70 µL of assay buffer and incubated at 37 °C for 5 min, followed by addition of 10 µL of 5 mM N-acetyl-Asp-Glu-Val-Asp-p-nitroanilide (Ac-DEVD-pNA; dissolved in dimethyl sulfoxide) as substrate to a final concentration of 0.5 mM and incubated at 37 °C for 60 min. A control reaction was set as blank in which Ac-DEVD-pNA was mixed with 10 µL of dimethyl sulfoxide. The caspase-like activity was measured by absorbance at 405 nm every 20 min during the incubation period of 60 min.
Hydrogen peroxide content
The hydrogen peroxide (H2O2) content was analyzed after 3 weeks of water deficit treatments with and without spermidine. Shoots and root samples (1 cm2 sections, to a total fresh weight of 100 mg) were collected from all water deficit-treated plants and H2O2 was determined according to del Pozo and Lam (1998): tissues were fine powdered using liquid nitrogen and homogenized in 5% (w/v) of 800 μL cold trichloroacetic acid. The homogenate was centrifuged (30 min at 12,000 rpm 4 °C) and the supernatant was used to determine the H2O2 content. Finally, 50 μL of the extract was incubated with 0.5 M KI and 5 mM K2HPO4 (pH 5.0) for 20 min at 25 °C and read the values at 390 nm using a UV–Vis spectrophotometer. The H2O2 content was considered through comparing its OD value with the standard graph.
Data analysis
All experiments were performed in triplicates, using 100 mg of plant samples. Plant tissues were collected from the individual pots of each treatment after 3 weeks. The values (n = 3) provided in the text and table stipulate mean ± standard error. Differences between water deficit stress, alone and along with 0.2 mM spermidine treatments, were analyzed by one-way ANOVA. The significant levels of differences between means were persistent at p ≤ 0.05 according to Duncan’s multiple range test using a software SPSS 17.0 version (IBM, SPSS Statistics).
Results
Growth measurements
Finger millet plants growth was determined based on the shoot and root biomass. Severe water deficit stress and water deficit along with 0.2 mM spermidine sprays on finger millet plants at early flowering stage revealed significant differences of the shoot and root growth after 3 weeks of treatments (Table 1). Similarly, fresh and dry weights of shoots and roots showed significant variation by the effects of water deficit condition and as well as water deficit with spermidine spray treatments (Table 1). In 50 and 75% of water deficit stress an outstanding reduction of the shoot and root development was observed, wherein similar stress (50 and 75%) conditions along with spermidine spray treatments alleviated the water deficient mediated growth reduction in both shoots and roots. Up to 3 weeks of treatment with 0.2 mM spermidine showed increased growth parameters compared to all (25, 50 and 75%) water deficit treatments (Table 1). Since it was expected, the maximum values of both parameters were recorded in control and spermidine-treated control conditions. In water deficit treatments in terms of fresh and dry weights were significantly different when compared to spermidine sprays and controls. Only 25% of water deficit treatment moderately affected the finger millet growth (length of shoots and roots, and biomass of shoots and roots) when compared to 50 and 75% water deficit conditions (Table 1).
Table 1.
Plant growth parameters in 50-day-old finger millet plants exposed to spermidine at 25, 50 and 75% of drought stress
| Water stress conditions (%) | Fresh weight (g) (mean ± SE) | Dry weight (g) (mean ± SE) | Length (cm) (mean ± SE) | Chlorophyll content (mg g−1 fresh weight) (mean ± SE) | |||||
|---|---|---|---|---|---|---|---|---|---|
| Shoot | Root | Shoot | Root | Shoot | Root | Chl a | Chl b | Total Chl a + b | |
| Control | 9.37 ± 0.5d | 3.98 ± 0.6d | 1.61 ± 0.5d | 0.69 ± 0.5d | 35.1 ± 0.8d | 20.7 ± 0.7d | 5.1 ± 0.7d | 2.1 ± 0.2c | 7.2 ± 0.9d |
| 25 | 6.63 ± 0.3c | 2.03 ± 1.0c | 1.07 ± 0.5c | 0.51 ± 0.2c | 27.8 ± 0.4c | 16.9 ± 0.6c | 3.1 ± 0.2c | 1.1 ± 0.9b | 4.2 ± 0.3c |
| 50 | 4.03 ± 0.9b | 1.19 ± 0.6b | 0.75 ± 0.4b | 0.38 ± 0.3b | 22.9 ± 0.7b | 12.8 ± 0.8b | 2.2 ± 0.1b | 0.1 ± 0.1b | 2.3 ± 0.2b |
| 75 | 1.66 ± 0.8a | 0.68 ± 0.6a | 0.4 ± 0.5a | 0.2 ± 0.4a | 18.7 ± 0.4a | 9.7 ± 0.6a | 1.3 ± 0.2a | 0.5 ± 0.2a | 1.8 ± 0.2a |
| Control + SPM | 9.41 ± 0.3d | 4.02 ± 0.6d | 1.67 ± 0.9d | 0.7 ± 0.2d | 36.4 ± 0.9d | 21.8 ± 0.7d | 5.6 ± 0.3d | 2.4 ± 0.4c | 8.0 ± 0.7d |
| 25 + SPM | 7.87 ± 0.8c | 2.84 ± 1.3c | 1.31 ± 0.6c | 0.6 ± 0.2c | 31.9 ± 0.7c | 18.4 ± 0.7c | 3.8 ± 0.1c | 1.6 ± 0.1b | 5.4 ± 0.2c |
| 50 + SPM | 6.08 ± 1.0b | 1.95 ± 0.5b | 0.87 ± 0.3b | 0.48 ± 0.3b | 26.3 ± 0.9b | 15.8 ± 0.8b | 2.8 ± 0.2b | 1.2 ± 0.1a | 4.0 ± 0.3b |
| 75 + SPM | 3.44 ± 0.8a | 1.09 ± 0.6a | 0.55 ± 0.3a | 0.32 ± 0.7a | 24.0 ± 1.0a | 12.6 ± 0.8a | 1.9 ± 0.1a | 0.9 ± 0.2a | 2.8 ± 0.3a |
Measurements were made 3 weeks after water deficit along with spermidine sprays. Data are given as the mean ± SE of three independent experiments. Means followed the same column that have the same letter are significantly different at p ≤ 0.05 based on Duncan’s multiple range test
SPM Spermidine
Effects of exogenous spermidine on relative water content under water deficit stress
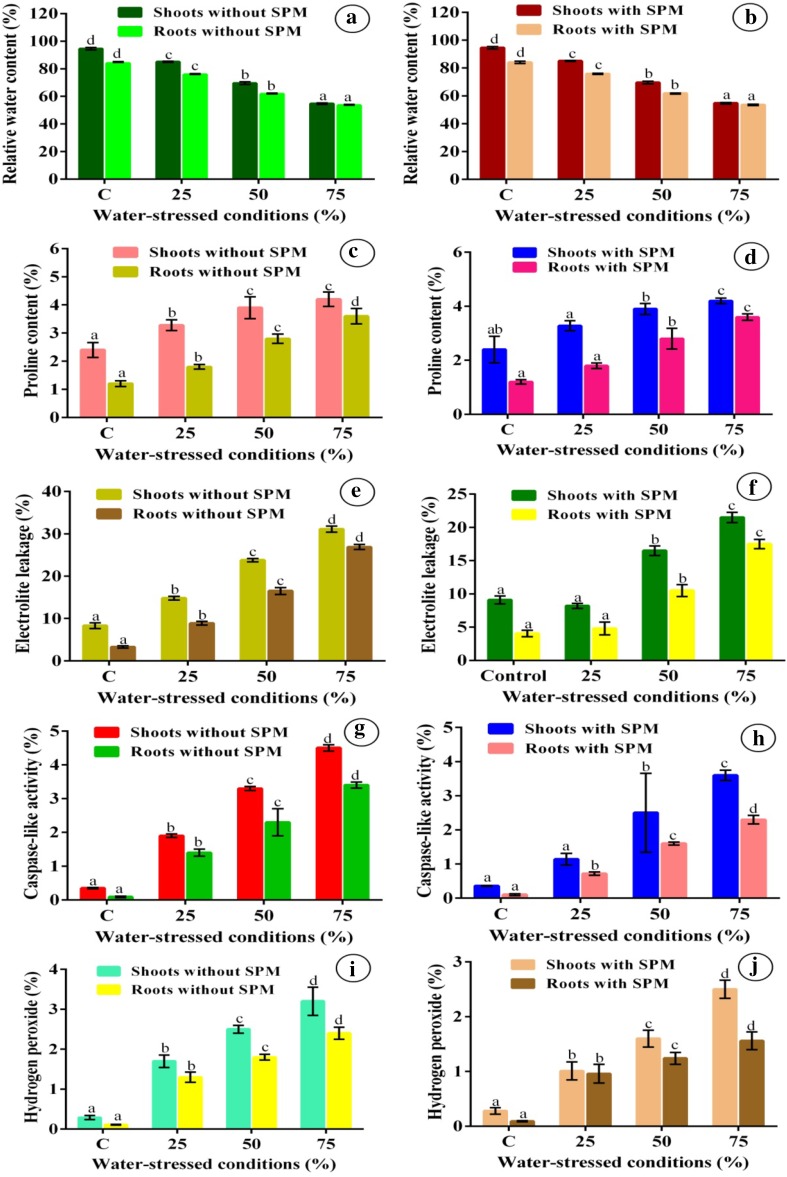
RWC was influenced in all water-stressed conditions in this study. A significant reduction was found between water deficient stress alone and water deficient stress with 0.2 mM spermidine spray treatments (Fig. 1a, b). Finger millet plants exposed to 25, 50 and 75% of water-stressed conditions showed decreased RWC in shoots and roots compared to water stressed with spermidine sprayed and controls after 3 weeks of treatment (Fig. 1a, b). The RWC in the leaf and root samples obtained from all the water-stressed plants was progressively decreased by the application of spermidine (0.2 mM). But, the level of RWC was significantly higher in both leaf and root samples obtained from the plants undergone water stress alone without any spermidine application (control).
Fig. 1.
Physiological parameters in shoots and roots of finger millet plants exposed to different drought stress conditions with and without spermidine treatment. a, b Relative water content, c, d Proline content, e, f Electrolyte leakage, g, h Caspase-like activity, i, j Hydrogen peroxide. Data analyzed for all the parameters 3 weeks after water deficit treatment with and without spermidine sprays. Data are given as the mean ± SE of three independent experiments. Means followed the same bar that have the same letter are significantly different at p ≤ 0.05 based on Duncan’s multiple range test. SPM, Spermidine. Every column in each graph represents the mean ± SE of three replicates
Influence of exogenous spermidine on chlorophyll content
Water-stressed conditions and spermidine treatments significantly affected the total Chl content in finger millet (Table 1). All three water deficit stresses significantly reduced the control levels of Chl (a, b and a + b). However, the Chl content in the leaf samples obtained from water deficit treatments with spermidine sprays was high compared to the Chl readings of leaf samples obtained from water deficit treatments alone (without spermidine spray) (Table 1). The damaging effect of water deficit severity on Chl content was intensified over the course of the experiment. In 75% of water deficit stress condition total Chl content was reduced to ~ 30% of controls after 3 weeks of treatment. The decrease of total Chl content in plant leaves resulted in chlorosis by degrading Chl inducing the destruction of the chloroplast structure.
Proline accumulation
Water deficit treatments (25, 50 and 75%) induced considerable proline accumulation in plant shoot and root samples (Fig. 1c). At 75% of water deficit stress, the shoots accumulated twice as much proline (4.2 mol g−1 FW) where root samples accumulated threefold higher level (3.6 mol g−1 FW) than control samples. These increases were more significant in control plants and water deficit treatments with 0.2 mM spermidine. Spermidine application, the most effective treatment, significantly controlled proline concentration in all treatments. In water deficit treatments (25, 50 and 75%) along with 0.2 mM spermidine shoots and roots proline was present at only 3.4 and 2.7 mol g−1 FW, respectively (Fig. 1d). In all the stress treatments proline accumulation was higher in plant shoots than roots since it might be to maintain the photosynthesis in plants and it has been extensively advocated for use as a parameter of selection for drought stress tolerance (Fig. 1c, d). The proline content was significantly enhanced at 50 and 75% of drought stress in both shoots and roots. However, it was significantly controlled in the plant samples sprayed with spermidine.
Electrolyte leakage
All water deficit treatments (25, 50 and 75%) damaged finger millet plants causing a significant increase in EL (Fig. 1e, f). The highest level of EL was evident in 75% water deficit-treated plants. A severe damage was observed even at 25 and 50% of water deficit treatments in both shoots and root samples compared to the EL level in control (well-watered condition) samples (Fig. 1e). A significant positive correlation was found between EL in shoot and root samples in 50-day-old plants treated at 25, 50 and 75% of water deficit conditions. Plants treated with 0.2 mM spermidine along with 25, 50 and 75% of water deficit environment shoot and root samples showed lowest levels of EL compared to control and water deficit treatments without spermidine sprays (Fig. 1e, f). Thus, 0.2 mM spermidine spray on 50-day-old finger millet plants during water deficit stress significantly reduced the EL in both shoot and root samples.
Caspase-like activity
Water deficit treatments from 25 to 75% induced significant increases in the total caspase-like activity in the finger millet plant leaves and roots (Fig. 1g). Lower activity was found in 50-day-old plant leaves and root samples of 0.2 mM spermidine sprayed during water deficit treatments from 25 to 75% and control. Treatment with 75% water deficit stress significantly increased caspase-like activity four-eightfold above controls and spermidine treatments for both leaves and roots in 50-day-old plants (Fig. 1g, h).
Effect of exogenous spermidine on hydrogen peroxide accumulation
Finger millet plant samples (leaves and roots) with water deficit treatments of 25, 50 and 75% exhibited a significant increase in H2O2 content over control (Fig. 1i). Above fivefold higher H2O2 content in leaves and roots was found compared to controls at 75% water deficit stress (Fig. 1i). Minimum H2O2 contents in leaves and roots were recorded in control, unstressed plants with and without spermidine spray treatments (Fig. 1i, j).
Influence of spermidine spray on phenotypic appearance of finger millet
Spermidine spray affected the appearance of water deficit-stressed plants in terms of freshness: leaf wrinkles, chlorosis and burning observed under water stress were avoided after spraying.
Discussion
Drought causes aggressive effects on plant growth (biomass production), development and yield (Sapeta et al. 2013; Yin et al. 2014). In this study, growth reduction in shoots and roots was observed under moderate and severe water deficit conditions. Water deficit treatments (25, 50 and 75%) decreased the length as well as fresh and dry weight of shoots and roots, RWC and total Chl (a + b) content in 50-day-old finger millet plants. During drought stress plants exhibited leaf wilting, yellowing and progressive senescence (data not shown). Under severe water deficit conditions (50 and 75%) leaf Chl contents frequently decline due to degradation of leaf Chl (Anjum et al. 2011; Sapeta et al. 2013). Polyamines are involved in plant defence to environmental stresses (Bouchereau et al. 1999). To our knowledge, this is the first report on the influence of exogenous spermidine in finger millet plants at early flowering stage. The results suggest that exogenous application of spermidine might turn out to be a promising step to overcome the damage induced by drought stress in finger millet plants at early flowering stage and it was found that 0.2 mM spermidine is more potent against ROS effect. These results are consistent with studies showing that spermidine is a more active scavenger of peroxy radicals (Yin et al. 2014). Spermidine plays an important role under environmental stress, especially drought, and it may accumulate in response to water deficiency. Polyamines are constituents of the protective appliance, being involved in regulating antioxidant enzyme activities (Groppa et al. 2001). Spermidine sprays enhance plant reproductive health under osmotic stress condition in Glycine max pods as well as seeds (Radhakrishnan and Lee 2013).
Polyamines are small polycationic complexes which also plays a well-known role in plant growth, metabolism, senescence, development and also in biotic and abiotic stress responses (Alcazar et al. 2006; Satish et al. 2016d). During the last decade, understanding on polyamine mechanism and functions has been achieved using genetically modified plants by changing the polyamine levels (Marco et al. 2011). A prominent level of polyamines have been found in various plant species in response to multiple stresses such as drought, salinity, heat, chilling, heavy metal toxicity, ozone, hypoxia, UV-B, UV-C, herbicide treatment and mechanical wounding (reviewed in Bouchereau et al. 1999; Groppa et al. 2001; Alcazar et al. 2006; Marco et al. 2011). However, the distinct mechanism of action of polyamines, which could safeguard the plants from challenging abiotic stress conditions, remains unclear and molecular studies are necessary to understand the role of polyamines in stress tolerance (Alcazar et al. 2006). Several groups were keenly focused on this aspect and during the last decade, various genes involved in polyamine metabolism (including spermidine synthesis) have been cloned from many species and their expression profiles under different stress conditions including abiotic stress and developmental stages have been analyzed (Kakkar and Sawhney 2002). The importance of such metabolic pathway in the plant against abiotic stress has been approached very recently with the use of Arabidopsis as a model plant since it allows global approaches to understand the major role of polyamine metabolism and other stress-signalling pathways (Ferrando et al. 2004; Alcazar et al. 2006).
Improved resistance to different abiotic stresses has also been accomplished by overexpressing the genes involved in spermidine biosynthesis. Polyamines were shown to enhance tolerance and recovery from abiotic stress, and recent results consign spermidine is most closely associated with stress tolerance in plants with a leading position for this effect (Li et al. 2016). In plants, increase in S-adenosylmethionine decarboxylase (SAMDC) gene levels conferred tolerance against various stresses and constitutive overexpression of human SAMDC in tobacco gave rise to an increment in putrescine and spermidine levels that lead to a major tolerance to salt and osmotic stress (Waie and Rajam 2003). Spermidine is synthesized from putrescine by the transfer of aminopropyl groups from SAMDC. Arabidopsis plants overexpressing spermidine synthase cDNA from Cucurbita ficifolia exhibited significant increases in spermidine synthase activity and the transformed lines have showed increased tolerance to various abiotic stresses, including drought, salinity, chilling, freezing and hyper osmosis (Kasukabe et al. 2004). Exogenous spermidine sprays did not change the polyamine contents through regulation of polyamine degrading enzymes, and an increase in polyamine biosynthetic enzyme levels was observed in Zoysia japonica during abiotic stress (Li et al. 2016). In addition, exogenous application of spermidine dramatically reversed the observed cinnamic acid-induced effects on spermidine and partially re-established the polyamine ratio in plant leaves, and also it was found to affect systemic glucosylsalicylic acid levels and arginine decarboxylase gene expression in Nicotiana tabacum leaves (Lazzarato et al. 2009). Polyamine metabolism response to abiotic stress changes with various exogenous spermidine concentrations, plant species and interactions between other stress factors (Gill and Tuteja 2010). Exogenous spermidine inhibits the accumulation of free putrescine and promotes the accumulation of free spermidine and spermine as well as both soluble conjugated and insoluble bound putrescine, spermidine and spermine (Li et al. 2016). However, further studies regarding homeostatic polyamine mechanisms such as degradation and conjugation are required to establish a comprehensible general view of polyamine metabolism in various stress responses.
Drought stress adversely affects plant growth and development (Mostofa et al. 2014). In this study, water deficit stress caused a severe decline in finger millet plant biomass, including shoot and root length and FW and DW of the shoot and root samples indicating that these plants dried up because of water stress injury. However, spermidine mediated protection of plant biomass is attributed to its ability to resist water deficit stress in these plants. Chl is the major source of ROS in plants under both water stresses with and without spermidine treatments (Mostofa et al. 2014). Therefore, destruction of the Chl and its loss likely take place because of accumulation of ROS during water stress injury (Xu et al. 2006). In these experiments, Chl content decreased considerably during water deficit stress, signifying that the photosynthesis pathway was injured irreparably and incapable to synthesize sufficient Chl. In contrast, spermidine sprays reduced the injury to Chl, thereby maintaining a reasonable level of Chl in water deficit stressed finger millet plants which are consistent with the findings of Mostofa et al. (2014) in Oryza sativa.
Osmoprotectants such as proline accumulate in several plant species cultivated under salinity or drought stresses and are considered as a stress metabolite. Proline acts as a membrane protectant and owing to zwitterion nature, accumulates to prominent concentration in cell cytoplasm during stress stipulation without interfering with cellular metabolism or organization (Goyal and Asthir 2010; Satish et al. 2016a). Drought exposure increased the proline accumulation in O. sativa seedlings (Mostofa et al. 2014). Similarly, in this study, proline content was increased significantly in shoots and roots exposed to all water deficit stress treatments (25, 50 and 75%) which might be an adaptive effect to control the water loss. Excessive proline accumulation was injurious to A. thaliana during heat stress (Lv et al. 2011). In the last decade, it was reported that overexpression of spermidine synthase improved the tolerance to multiple environmental stresses and up-regulated the expression of different stress-regulated genes in transgenic A. thaliana (Kasukabe et al. 2004). Similarly, variation of the polyamine biosynthetic pathway in O. sativa transgenic lines confers tolerance of drought stress (Capell et al. 2004). Application of spermidine (0.2 mM) to the plants reduced the water deficit stress-induced proline synthesis, suggesting that drought-imposed stress might be moderately tolerated without proline accumulation to high levels. This is supported by Jimenez-Bremont et al. (2006) and Mostofa et al. (2014) who reported that exogenous polyamines including spermidine reduced the proline accumulation in Phaseolus vulgaris and O. sativa during salinity and heat stress, respectively.
EL is a criterion to plant tissues injury due to exposure to any kind of stress. It has been confessed as a simple, valid, reproducible and quantitative method for assessing cell viability after the stress, including drought, salt or cold stresses (Busaidi and Farag 2015). Pre-treatment of Cucumis sativus seedlings with polyamines viz., putrescine and spermidine diminished the increased EL caused by chilling the leaves of C. sativus cultivars (Zhang et al. 2009). The polyamines reversed the salinity-induced reductions in seedling growth and biomass accumulation and increased EL in leaf tissues of Brassica juncea (Verma and Mishra 2005). In this study, spermidine sprays during water deficit stress for 3 weeks balanced the EL levels in shoots and roots of finger millet plants. The results suggest that spermidine play a vital role in the tolerance of finger millet plants against water deficit stress, which is most likely attained by acting as oxidative machinery against drought stress injury.
In most cases, during abiotic stress in plants, spermidine-mediated protection had a close association with enhanced levels of antioxidant capacity. Initial studies seemed to impart indirect affirmation supporting the presence of caspase orthologues with various caspase-like activities found in plant extracts which confirmed them to be required for programmed cell death (Bonneau et al. 2008). The cytosolic caspase-like activity during leaf senescence leads to cell death in O. sativa plants (Wang et al. 2014). However, as in animal cell apoptosis, an attribute that seems to characterize all types of plant programmed cell death examined so far is the caspase-like activity. In this study, plants exposed to polyamine spermidine triggered caspase-like activity.
Catalase is an oxidative enzyme involved in abiotic stress. In this study, results showed that water deficit stress-induced increases in catalase activity which correlates with the levels of H2O2 (Fig. 1g–j). The rising trend in catalase activity and H2O2 content suggested that water deficit stress caused severe peroxidation in finger millet shoots and roots at the early flowering stage. H2O2 may work as structural defence signal molecule, although a high concentration of H2O2 is cytotoxic to the plants, leading to oxidative stress (Goyal and Asthir 2010). Therefore, elucidation of methods of tolerance at a specific stage of plant growth and development is essential to understand the plant responses to water deficit stress (Goyal and Asthir 2010). Under abiotic stresses, polyamines are beginning to accumulate significantly to confer adaptive and protective responses (Yang et al. 2007). The endogenous levels of polyamines are associated with the extent of stress tolerance in different plant species. Exogenous application of polyamines or genetic engineering of plants to maintain the polyamine levels might be liable for enhanced tolerance of multiple abiotic stresses (Goyal and Asthir 2010; Mostofa et al. 2014).
Conclusion
During 3 weeks of water deficit of 25 and 50% in finger millet plants, levels of final biomass, RWC and net Chl content are reduced, whereas proline, EL, caspase-like activity and H2O2 increase. Foliar spray of Spermidine (0.2 mM) restores the damages inflicted by drought stress. Theefore, biochemical consequences of water drought stress-mediated changes in plant oxidative defence system, particularly in concurrence with spermidine catabolism are venerable of research.
Acknowledgements
L. Satish thanks the University Grants Commission (UGC), New Delhi, India for financial support in the form of UGC-BSR SRF (UGC order no: F.4-1/2006 (BSR)/7-326/2011-BSR). We thank the Department of Small Millets, Millet Research Station, Tamil Nadu Agricultural University, Coimbatore, India for providing seed material used in this study. The authors also gratefully acknowledge the Bioinformatics Infrastructure Facility of Alagappa University (funded by the Department of Biotechnology, Government of India, grant no. BT/BI/25/001/2006) for providing the computational facility. The first author gratefully expresses gratitude to Prof. Arieh Zaritsky, Emeritus Professor, Faculty of Natural Sciences, Ben-Gurion University of Negev, Beer Sheva, Israel, improved the language and shape of the manuscript.
Compliance with ethical standards
Conflict of interest
All the authors declare that they have no conflict of interest.
Contributor Information
Lakkakula Satish, Email: lsatish@post.bgu.ac.il.
Manikandan Ramesh, Email: mrbiotech.alu@gmail.com.
References
- Ahmed IM, Nadira UA, Bibi N, Cao F, He X, Zhang G, Wu F. Secondary metabolism and antioxidants are involved in the tolerance to drought and salinity, separately and combined, in Tibetan wild barley. Env Exp Bot. 2014;111:1–12. doi: 10.1016/j.envexpbot.2014.10.003. [DOI] [Google Scholar]
- Alcazar R, Marco F, Cuevas J, Patron M, Ferrando A, Carrasco P, Tiburcio A, Altabella T. Involvement of polyamines in plant response to abiotic stress. Biotechnol Lett. 2006;28:1867–1876. doi: 10.1007/s10529-006-9179-3. [DOI] [PubMed] [Google Scholar]
- Anjum SA, Xie X, Wang LC, Saleem MF, Man C, Lei W. Morphological, physiological and biochemical responses of plants to drought stress. Afr J Agri Res. 2011;6:2026–2032. [Google Scholar]
- Barrs HD, Weatherley PE. A re-examination of the relative turgidity techniques for estimating water deficits in leaves. Austr J Biol Sci. 1962;15:413–428. doi: 10.1071/BI9620413. [DOI] [Google Scholar]
- Bartwal A, Arora S. Drought stress-induced enzyme activity and mdar and apx gene expression in tolerant and susceptible genotypes of Eleusine coracana (L.) In Vitro Cell Dev Biol Plant. 2017;53:41–49. doi: 10.1007/s11627-016-9787-0. [DOI] [Google Scholar]
- Bates LS, Waldren RP, Teare ID. Rapid determination of free proline for water-stress studies. Plant Soil. 1973;39:205–207. doi: 10.1007/BF00018060. [DOI] [Google Scholar]
- Bonneau L, Ge Y, Drury GE, Gallois P. What happened to plant caspases? J Exp Bot. 2008;59:491–499. doi: 10.1093/jxb/erm352. [DOI] [PubMed] [Google Scholar]
- Bouchereau A, Aziz A, Larher F, Martin-Tanguy J. Polyamines and environmental challenges: recent development. Plant Sci. 1999;140:103–125. doi: 10.1016/S0168-9452(98)00218-0. [DOI] [Google Scholar]
- Busaidi KTSA, Farag KM. The use of electrolyte leakage procedure in assessing heat and salt tolerance of Ruzaiz date palm (Phoenix dactylifera L.) cultivar regenerated by tissue culture and offshoots and treatments to alleviate the stressful injury. J Horti Forestry. 2015;7:104–111. doi: 10.5897/JHF2014.0378. [DOI] [Google Scholar]
- Capell T, Bassie L, Christou P. Modulation of the polyamine biosynthetic pathway in transgenic rice confers tolerance to drought stress. Proc Natl Acad Sci USA. 2004;101:9909–9914. doi: 10.1073/pnas.0306974101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceasar SA, Ignacimuthu S. Agrobacterium-mediated transformation of finger millet (Eleusine coracana (L.) Gaertn.) using shoot apex explants. Plant Cell Rep. 2011;30:1759–1770. doi: 10.1007/s00299-011-1084-0. [DOI] [PubMed] [Google Scholar]
- Chethan S, Malleshi NG. Finger millet polyphenols: optimization of extraction and the effect of pH on their stability. Food Chem. 2007;105:862–870. doi: 10.1016/j.foodchem.2007.02.012. [DOI] [Google Scholar]
- del Pozo O, Lam E. Caspases and programmed cell death in the hypersensitive response of plants to pathogens. Current Biol. 1998;8:1129–1132. doi: 10.1016/S0960-9822(98)70469-5. [DOI] [PubMed] [Google Scholar]
- Ferrando A, Carrasco P, Cuevas JC, Altabella T, Tiburcio AF. Integrated molecular analysis of the polyamine pathway in abiotic stress signalling. In: Amancio S, Stulen I, editors. Nitrogen acquisition and assimilation in higher plants. Dordrecht: Kluwer Academic Publisher; 2004. pp. 207–230. [Google Scholar]
- Gill SS, Tuteja N. Polyamines and abiotic stress tolerance in plants. Plant Signal Behav. 2010;5:26–33. doi: 10.4161/psb.5.1.10291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyal M, Asthir B. Polyamine catabolism influences antioxidative defense mechanism in shoots and roots of five wheat genotypes under high temperature stress. Plant Growth Regul. 2010;60:13–25. doi: 10.1007/s10725-009-9414-8. [DOI] [Google Scholar]
- Groppa MD, Tomaro ML, Benavides MP. Polyamines as protectors against cadmium or copper-induced oxidative damage in sunflower leaf discs. Plant Sci. 2001;161:481–488. doi: 10.1016/S0168-9452(01)00432-0. [DOI] [Google Scholar]
- Hussain SS, Ali M, Ahmad M, Siddique KHM. Polyamines: natural and engineered abiotic and biotic stress tolerance in plants. Biotechnol Adv. 2011;29:300–311. doi: 10.1016/j.biotechadv.2011.01.003. [DOI] [PubMed] [Google Scholar]
- Ignacimuthu S, Ceasar SA. Development of transgenic finger millet (Eleusine coracana (L.) Gaertn.) resistant to leaf blast disease. J Biosci. 2012;37:135–147. doi: 10.1007/s12038-011-9178-y. [DOI] [PubMed] [Google Scholar]
- IPCC . Fourth assessment report—climate change 2007: impacts, adaptation and vulnerability. In: Parry ML, Canziani OF, Palutikof JP, van der Linden PJ, Hanson CE, editors. Contribution of working group II to the fourth assessment report of the intergovernmental panel on climate change. Cambridge: Cambridge University Press; 2007. p. 976. [Google Scholar]
- Jimenez-Bremont JF, Flora AB, Hernandez-Lucero E, Rodriguez-Kessler M, Acosta-Gallegos JA, Ramirez-Pimentel JG. Proline accumulation in two bean cultivars under salt stress and the effect of polyamines and ornithine. Biol Plant. 2006;50:763–766. doi: 10.1007/s10535-006-0126-x. [DOI] [Google Scholar]
- Kakkar RK, Sawhney VK. Polyamine research in plants—a changing perspective. Physiol Plant. 2002;116:281–292. doi: 10.1034/j.1399-3054.2002.1160302.x. [DOI] [Google Scholar]
- Kasukabe Y, He L, Nada K, Misawa S, Ihara I, Tachibana S. Overexpression of spermidine synthase enhances tolerance to multiple environmental stress and up-regulates the expression of various stress-regulated genes in transgenic Arabidopsis thaliana. Plant Cell Physiol. 2004;45:712–722. doi: 10.1093/pcp/pch083. [DOI] [PubMed] [Google Scholar]
- Kusano T, Yamaguchi K, Berberich T, Takahashi Y. Advances in polyamine research in 2007. J Plant Res. 2007;120:345–350. doi: 10.1007/s10265-007-0074-3. [DOI] [PubMed] [Google Scholar]
- Lazzarato L, Trebbi G, Pagnucco C, Franchin C, Torrigiani P, Betti L. Exogenous spermidine, arsenic and β-aminobutyric acid modulate tobacco resistance to tobacco mosaic virus, and affect local and systemic glucosylsalicylic acid levels and arginine decarboxylase gene expression in tobacco leaves. J Plant Physiol. 2009;166:90–100. doi: 10.1016/j.jplph.2008.01.011. [DOI] [PubMed] [Google Scholar]
- Li S, Jin H, Zhang Q. The effect of exogenous spermidine concentration on polyamine metabolism and salt tolerance in Zoysiagrass (Zoysia japonica Steud) subjected to short-term salinity stress. Front Plant Sci. 2016;7:1221. doi: 10.3389/fpls.2016.01221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtenthaler HK. Chlorophylls and carotenoids: pigments of photosynthetic biomembranes. Methods Enzymol. 1987;148:350–382. doi: 10.1016/0076-6879(87)48036-1. [DOI] [Google Scholar]
- Lutts S, Almansouri M, Kinet J. Salinity and water stress have contrasting effects on the relationship between growth and cell viability during and after stress exposure in durum wheat callus. Plant Sci. 2004;167:9–18. doi: 10.1016/j.plantsci.2004.02.014. [DOI] [Google Scholar]
- Lv WT, Lin B, Zhang M, Hua XJ. Proline accumulation is inhibitory to Arabidopsis seedlings during heat stress. Plant Physiol. 2011;156:1921–1933. doi: 10.1104/pp.111.175810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marco F, Alcazar R, Tiburcio AF, Carrasco P. Interactions between polyamines and abiotic stress pathway responses unraveled by transcriptome analysis of polyamine overproducers. Omics A J Integr Biol. 2011;15:775–781. doi: 10.1089/omi.2011.0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittler R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002;7:405–410. doi: 10.1016/S1360-1385(02)02312-9. [DOI] [PubMed] [Google Scholar]
- Mostofa MG, Yoshida N, Fujita M. Spermidine pretreatment enhances heat tolerance in rice seedlings through modulating antioxidative and glyoxalase systems. Plant Growth Regul. 2014;73:31–44. doi: 10.1007/s10725-013-9865-9. [DOI] [Google Scholar]
- Nirgude M, Babu BK, Shambhavi Y, Singh UM, Upadhyaya HD, Kumar A. Development and molecular characterization of genic molecular markers for grain protein and calcium content in finger millet (Eleusine coracana (L.) Gaertn.) Mol Biol Rep. 2014;41:1189–1200. doi: 10.1007/s11033-013-2825-7. [DOI] [PubMed] [Google Scholar]
- Pollastrini M, Desotgiu R, Camin F, Ziller L, Gerosa G, Marzuoli R, Bussotti F. Severe drought events increase the sensitivity to ozone on poplar clones. Env Exp Bot. 2014;100:94–104. doi: 10.1016/j.envexpbot.2013.12.016. [DOI] [Google Scholar]
- Radhakrishnan R, Lee IJ. Ameliorative effects of spermine against osmotic stress through antioxidants and abscisic acid changes in soybean pods and seeds. Acta Physiol Plant. 2013;35:263–269. doi: 10.1007/s11738-012-1072-1. [DOI] [Google Scholar]
- Rahman H, Jagadeeshselvam N, Valarmathi R, Sachin B, Sasikala R, Senthil N, Sudhakar D, Robin S, Muthurajan R. Transcriptome analysis of salinity responsiveness in contrasting genotypes of finger millet (Eleusine coracana L.) through RNA-sequencing. Plant Mol Biol. 2014;85:485–503. doi: 10.1007/s11103-014-0199-4. [DOI] [PubMed] [Google Scholar]
- Roxas VP, Lodhi SA, Garrett DK, Mahan JR, Allen RD. Stress tolerance in transgenic tobacco seedlings that over express glutathione S-transferase/glutathione peroxidase. Plant Cell Physiol. 2000;41:1229–1234. doi: 10.1093/pcp/pcd051. [DOI] [PubMed] [Google Scholar]
- Sapeta H, Costa JM, Lourenco T, Maroco J, van der Linde P. Drought stress response in Jatropha curcas: growth and physiology. Environ Exp Bot. 2013;85:76–84. doi: 10.1016/j.envexpbot.2012.08.012. [DOI] [Google Scholar]
- Satish L, Ceasar SA, Shilpha J, Rency SA, Rathinapriya P, Ramesh M. Direct plant regeneration from in vitro-derived shoot apical meristems of finger millet (Eleusine coracana (L.) Gaertn.) In Vitro Cell Dev Biol Plant. 2015;51:192–200. doi: 10.1007/s11627-015-9672-2. [DOI] [Google Scholar]
- Satish L, Rathinapriya P, Ceasar SA, Rency AS, Pandian S, Rameshkumar R, Subramanian A, Ramesh M. Effects of cefotaxime, amino acids and carbon source on somatic embryogenesis and plant regeneration in four Indian genotypes of foxtail millet (Setaria italica L.) In Vitro Cell Dev Biol Plant. 2016;52:140–153. doi: 10.1007/s11627-015-9724-7. [DOI] [Google Scholar]
- Satish L, Rathinapriya P, Rency AS, Ceasar SA, Pandian S, Rameshkumar R, Ramesh M. Somatic embryogenesis and regeneration using Gracilaria edulis and Padina boergesenii seaweed liquid extracts and genetic fidelity in finger millet (Eleusine coracana) J Appl Phycol. 2016;28:2083–2098. doi: 10.1007/s10811-015-0696-0. [DOI] [Google Scholar]
- Satish L, Rathinapriya P, Rency AS, Ceasar SA, Prathibha M, Pandian S, Rameshkumar R, Ramesh M. Effect of salinity stress on finger millet (Eleusine coracana (L.) Gaertn): histochemical and morphological analysis of coleoptile and coleorhizae. Flora. 2016;222:111–120. doi: 10.1016/j.flora.2016.04.006. [DOI] [Google Scholar]
- Satish L, Rency AS, Rathinapriya P, Ceasar SA, Pandian S, Rameshkumar R, Rao TB, Balachandran SM, Ramesh M. Influence of plant growth regulators and spermidine on somatic embryogenesis and plant regeneration in four Indian genotypes of finger millet (Eleusine coracana (L.) Gaertn) Plant Cell Tiss Org Cult. 2016;124:15–31. doi: 10.1007/s11240-015-0870-8. [DOI] [Google Scholar]
- Satish L, Ceasar SA, Ramesh M. Improved Agrobacterium-mediated transformation and direct plant regeneration in four cultivars of finger millet (Eleusine coracana (L.) Gaertn.) Plant Cell Tiss Organ Cult. 2017;131:547–565. doi: 10.1007/s11240-017-1305-5. [DOI] [Google Scholar]
- Shah F, Huang J, Cui K, Nie L, Shah T, Chen C, Wang K. Impact of high-temperature stress on rice plant and its traits related to tolerance. J Agric Sci. 2011;149:545–556. doi: 10.1017/S0021859611000360. [DOI] [Google Scholar]
- Singh NP, Bantilan C, Byjesh K. Vulnerability and policy relevance to drought in the semi-arid tropics of Asia—a retrospective analysis. Weather Climate Extremes. 2014;3:54–61. doi: 10.1016/j.wace.2014.02.002. [DOI] [Google Scholar]
- Slama I, M’Rabet R, Ksouri R, Talbi O, Debez A, Abdelly C. Water deficit stress applied only or combined with salinity affects physiological parameters and antioxidant capacity in Sesuvium portulacastrum. Flora. 2015;213:69–76. doi: 10.1016/j.flora.2015.04.004. [DOI] [Google Scholar]
- Tabot PT, Adams JB. Early responses of Bassia diffusa (Thunb.) Kuntze to submergence for different salinity treatments. South Afr J Bot. 2013;84:19–29. doi: 10.1016/j.sajb.2012.10.002. [DOI] [Google Scholar]
- Verma S, Mishra SN. Putrescine alleviation of growth in salt stressed Brassica juncea by inducing antioxidative defense System. J Plant Physiol. 2005;162:669–677. doi: 10.1016/j.jplph.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Waie B, Rajam MV. Effect of increased polyamine biosynthesis on stress responses in transgenic tobacco by introduction of human S-adenosylmethionine gene. Plant Sci. 2003;164:727–734. doi: 10.1016/S0168-9452(03)00030-X. [DOI] [Google Scholar]
- Wang H, Zhu X, Li H, Cui J, Liu C, Chen X, Zhang W. Induction of caspase-3-like activity in rice following release of cytochrome-f from the chloroplast and subsequent interaction with the ubiquitin-proteasome system. Sci Rep. 2014;4:5989. doi: 10.1038/srep05989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu S, Li J, Zhang X, Wei H, Cui L. Effects of heat acclimation pretreatment on changes of membrane lipid peroxidation, antioxidant metabolites, and ultrastructure of chloroplasts in two cool-season turfgrass species under heat stress. Environ Exp Bot. 2006;56:274–285. doi: 10.1016/j.envexpbot.2005.03.002. [DOI] [Google Scholar]
- Yamaguchi K, Takahashi Y, Berberich T, Imai A, Takahashi T, Michael AJ, Kusano T. A protective role for the polyamine spermine against drought stress in Arabidopsis. Biochem Biophys Res Commun. 2007;352:486–490. doi: 10.1016/j.bbrc.2006.11.041. [DOI] [PubMed] [Google Scholar]
- Yang J, Zhang J, Liu K, Wang Z, Liu L. Involvement of polyamines in the drought resistance of rice. J Exp Bot. 2007;58:1545–1555. doi: 10.1093/jxb/erm032. [DOI] [PubMed] [Google Scholar]
- Yin ZP, Li S, Ren J, Song XS. Role of spermidine and spermine in alleviation of drought-induced oxidative stress and photosynthetic inhibition in Chinese dwarf cherry (Cerasus humilis) seedling. Plant Growth Regul. 2014;74:209–218. doi: 10.1007/s10725-014-9912-1. [DOI] [Google Scholar]
- Yoshida S, Forno DA, Cock JH, Gomez KA. Laboratory manual for physiological studies of rice. Manila: International Rice Research Institute; 1976. [Google Scholar]
- Zhang Z, Honda C, Kita M, Nakayama CHM, Moriguchi T. Structure and expression of spermidine synthase genes in apple: two cDNAs are spatially and developmentally regulated through alternative splicing. Mol Gen Genom. 2003;268:799–807. doi: 10.1007/s00438-002-0802-2. [DOI] [PubMed] [Google Scholar]
- Zhang W, Jiang B, Li W, Song H, Yu Y, Chen J. Polyamines enhance chilling tolerance of cucumber (Cucumis sativus L.) through modulating antioxidative system. Sci Horti. 2009;122:200–208. doi: 10.1016/j.scienta.2009.05.013. [DOI] [Google Scholar]
- Zhou Y, Shao HB. The responding relationship between plants and environment is the essential principle for agricultural sustainable development on the globe. C.R. Biologies. 2008;331:321–328. doi: 10.1016/j.crvi.2008.01.008. [DOI] [PubMed] [Google Scholar]