Abstract
Biodegradable porous biomaterial scaffolds play a critical role in bone regeneration. In this study, the porous nano-hydroxyapatite/collagen/poly(lactic-co-glycolic acid)/graphene oxide (nHAC/PLGA/GO) composite scaffolds containing different amount of GO were fabricated by freeze-drying method. The results show that the synthesized scaffolds possess a three-dimensional porous structure. GO slightly improves the hydrophilicity of the scaffolds and reinforces their mechanical strength. Young’s modulus of the 1.5 wt% GO incorporated scaffold is greatly increased compared to the control sample. The in vitro experiments show that the nHAC/PLGA/GO (1.5 wt%) scaffolds significantly cell adhesion and proliferation of osteoblast cells (MC3T3-E1). This present study indicates that the nHAC/PLGA/GO scaffolds have excellent cytocompatibility and bone regeneration ability, thus it has high potential to be used as scaffolds in the field of bone tissue engineering.
Electronic supplementary material
The online version of this article (10.1186/s11671-018-2432-6) contains supplementary material, which is available to authorized users.
Keywords: Graphene oxide, Poly(lactic-co-glycolic acid), Collagen, Nano-hydroxyapatite, Biodegradable porous scaffold, Bone tissue engineering
Background
Bone tissue engineering combining three-dimensional porous scaffolds and bone cells has been widely studied as an attractive approach in the treatment of malfunctioning or lost tissue [1]. Biodegradable scaffolds, which mimic the nature of the bone, play an important role for accommodating cells, controlling cell adhesion and proliferation, and facilitating bone regeneration [2]. Till now, various methods including electrospinning, integration of computational topology design (CTD) and solid free-form fabrication (SFF), and freeze-drying have been applied to fabricate different three-dimensional (3D) porous structures [3–7]. Electrospinning is able to make nanofibrous or microfibrous scaffolds with complex structures (aligned, spring-like fiber) and compositions [7]. However, the production efficiency of it is a bit low. The integration of CTD and SFF allows design of 3D anatomic scaffolds with porous architecture and better mechanical property. But this method requires strong professional knowledge [4]. Compared to these two methods, freeze-drying method allows fabricating porous structures with a much simpler process by the sublimation of frozen liquid phase under vacuum to fabricate a porous structure [8].
Nature bones possess complex hierarchical architecture with two main components, collagen and hydroxyapatite [9–11]. In bone tissue engineering, fabricating an ideal biomimicry of the bone extracellular matrix accommodating for cell adhesion and proliferation for the treatment of malfunction is still a challenge [12]. Nano-hydroxyapatite/collagen (nHAC)-based biodegradable scaffolds which are mimic of the natural bone could provide better biocompatibility, cell affinity, and bioresorbability [13]. However, the drawbacks of collagen, including the poor mechanical and rapid degrading properties, remain an obstacle for its application in bone tissue engineering [14]. Biodegradable aliphatic polymers, such as poly(lactic-co-glycolic) acid (PLGA), with high mechanical strength, outstanding biocompatibility, biodegradability, and good solubility in organic solvents, are ideal compensated material constructing 3D porous scaffolds for bone tissue engineering [15, 16]. A hybrid porous scaffold containing collagen and synthetic polymers combines the advantages of collagen and polymers and overcome their weaknesses, which is extensively used for bone repair and regeneration [17–19]. For instance, Liao et al. have developed a bone scaffold prepared by nHAC and poly(lactic acid) (PLA) to promote bone regeneration [17]. Niu et al. have fabricated nHAC/poly(L-lactic acid)/chitosan microspheres composite scaffolds for enhancing osteoblast proliferation [19].
Recently, graphene oxide (GO), a novel carbon sheet with one-atom thickness [20, 21], have attracted great interest in biological field because it owns good biocompatibility. The GO hybridized scaffolds are able to enrich both the mechanical property of the scaffold and the cellular behaviors, such as cell spreading and proliferation [22, 23]. Luo et al. reported that the incorporation of GO into PLGA nanofibrous enhanced proliferation and osteogenic differentiation of mesenchymal stem cells (MSCs) [20]. Jing et al. reported that the addition of 1.0 wt% GO into the thermoplastic polyurethane could facilitate the Swiss mouse fibroblasts cell proliferation [24]. Compared with adding the chemical cross-linking agents (genipin, glutaraldehyde, carbodiimide, etc.) [25, 26], which have certain cytotoxicity, to improve the mechanical property of composite scaffolds, the small amount of GO hybridized scaffolds show good biocompatibility. Therefore, the hybridization of the GO and the nHAC/PLGA could be a novel artificial scaffold for bone tissues.
In this study, the porous nano-hydroxyapatite/collagen/poly(lactic-co-glycolic acid) /graphene oxide (nHAC/PLGA/GO) scaffolds, which contains different weight ratio of GO (0.0, 0.5, 1.0, and 1.5 wt%) have been fabricated and characterized. The hybridization scaffolds show porous structures. The addition of GO modifies the hydrophilic property and the mechanical property of the hybridization scaffolds. To investigate the effect of the nHAC/PLGA/GO scaffold on bone tissue engineering, the MC3T3-E1 cells were cultured on the porous hybridization scaffolds. The results show that the 1.5 wt% GO-doped hybridization scaffolds facilitate cell adhesion, growth, and proliferation, further indicating nHAC/PLGA/GO scaffold can be considered as a promising candidate in bone tissue engineering.
Results and Discussion
Structure of nHAC/PLGA/GO Composite Scaffolds
Figure 1 illustrates the fabrication process of nHAC/PLGA/GO scaffolds. The details of the fabrication process are shown in the experimental section. The nHAC was synthesized prior to fabricate the nHAC/PLGA/GO composite scaffolds. The scanning electron microscope (SEM) image of the nHAC powder shows its nanostructure. The corresponding energy-dispersive X-ray spectroscopy (EDS) spectra of nHAC is also shown (Additional file 1: Figure S1), which reveals the presence of Ca, Cu, P, C, and O. Copper signals should be contributions of the supporting samples. Thus, the nHAC is composed of Ca, P, C, O, and the Ca:P molar ratio of nHAC powder is 1.41, which is lower than that of hydroxyapatite (HA) (1.66). This indicates that the synthesized HA is calcium deficiency type [27], which will lead to reduction in the hardness, elastic modulus, and toughness in nHAC. To increase the mechanical properties of the composite scaffold, the PLGA and GO were added into the nHAC powder. The optical overview of the fabricated nHAC/PLGA/GO scaffolds with different amount of GO are shown in Fig. 2a. The sample is a cylinder with a diameter of 14 mm. It is apparent that the composite scaffolds without GO is in white color. As the increasing of the GO, the composite scaffolds become more and more dark. The detailed morphologies of different nHAC/PLGA/GO scaffolds are revealed by SEM (Fig. 2b–e). It significantly shows that all the scaffolds form porous structures and the surfaces of four different scaffolds are pretty rough. In order to characterize the information of these holes, we used an automatic surface area and porosity tester to evaluate. The results of the hole distribution was shown in Fig. 2f. The size of the four scaffold holes is between 0 and 200 nm. And the number of the holes which dozens of nanometers are more than those with a few hundred nanometers in four scaffolds. It has been reported that porosity of biomaterial scaffolds is non-trivial for bone formation in vitro and in vivo [28]. To optimize integration into surrounding tissue, scaffolds for osteogenesis should mimic bone morphology, structure, and function [4]. Thus, the 3D porous structure of nHAC/PLGA/GO composite scaffolds is critical for bone regeneration. The large-scale SEM images of the four composite scaffolds are also shown (Additional file 1: Figure S2), which illustrates the overview structures of different surfaces.
Fig. 1.
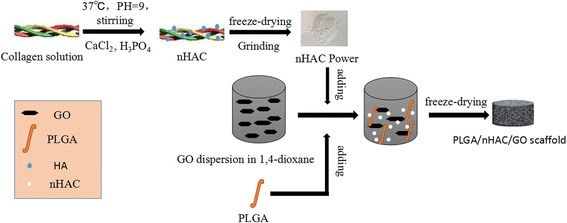
Schematic diagram of the fabrication process for nHAC/PLGA/GO scaffolds
Fig. 2.
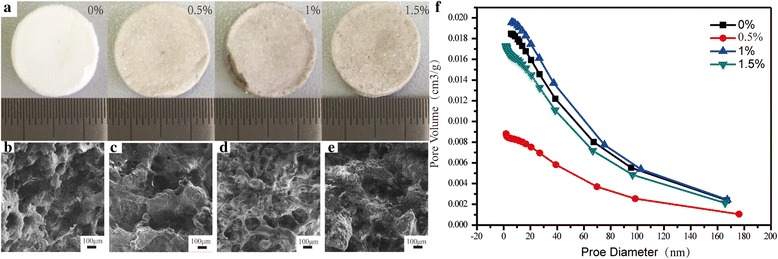
a Optical image of the synthesized nHAC/PLGA/GO scaffolds with different amount of GO. b–e SEM images of b nHAC/PLGA, c nHAC/PLGA/GO (0.5 wt%), d nHAC/PLGA/GO (1.0 wt%), and e nHAC/PLGA/GO (1.5 wt%) scaffolds. f Hole distribution of nHAC/PLGA/GO(0, 0.5, 1.0, and 1.5 wt%)
Physicochemical and Mechanical Characterizations of nHAC/PLGA/GO Composite Scaffolds
The mechanism of the synthesize process can be revealed by the X-ray diffraction (XRD) and Fourier transform infrared spectroscopy (FT-IR) spectra of various single substance and composites (Fig. 3). As indicated in Fig. 3a, the inorganic phase was determined as HA according to the powder diffraction file (PDF card no. 09–0432) since no peaks from other Ca-P materials were present in the XRD pattern. Compared with nHA, the broaden diffraction peaks of nHAC implied a small grain size and low crystallinity. Similar to nHAC, the pattern of nHAC/PLGA/GO with different amount of GO also had low crystallinity. However, the peak of GO did not appear in the nHAC/PLGA/GO composites, which might because of the few amount of GO compared to the bulk. Figure 3b shows the FT-IR spectra of various single substance and composites. From Fig. 3b, the typical bands for collagen can be observed, such as N–H stretching at 3336 cm−1 for amide A; C–H stretching at 3079 cm−1 for amide B; C=O stretching at 1656 cm−1 for the amide I; N–H deformation at 1548 cm−1 for the amide II and absorption peak at 1238 cm−1 for amide III. As the formation of nHAC, the amide A moves from 3336 cm−1 to around 3411 cm−1, the amide B was weakened, the amide I, amide II, amide III move from 1656, 1548, and 1238 cm−1 to 1654, 1542, and 1240 cm−1, respectively. Thus, it confirms the chemical reaction between collagen and HA. Additionally, the peaks at 1033, 601, and 563 cm−1 are the typical peaks for (PO4)3− group, which indicates the newly formation of HA on the collagen because of the merchandized HA only possessing characteristic peaks of (PO4)3− at 1033, 603, and 565 cm−1. The characterized peaks of PLGA around 2996 and 2944 cm−1 were assigned to −CH2, 1752 cm−1 was assigned to C=O, 1183 and 1093 cm−1 were assigned to C–O, are clearly seen. Compared with PLGA scaffold, the peaks of nHAC/PLGA scaffold move from 1752 and 1183 cm−1 to 1760 and 1187 cm−1, respectively, which demonstrate the chemical reaction between PLGA and nHAC power. Compared with nHAC/PLGA scaffold, the peaks of GO-doped nHAC/PLGA scaffolds moved from 1760 to 1762 cm−1; there is a red shift occurred which demonstrates the chemical reaction between GO and nHAC/PLGA.
Fig. 3.
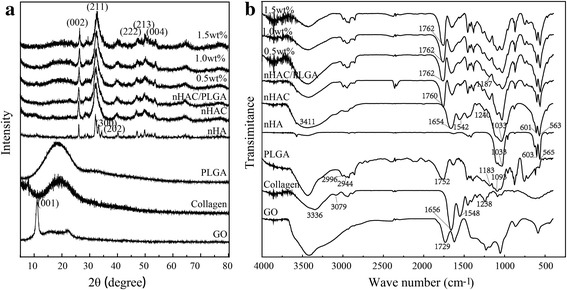
a XRD and b FT-IR spectra of different components
The nanostructure and mechanical property of the nHAC/PLGA/GO scaffolds with different GO amount were characterized by quantitative nano-mechanical atomic force microscope (QNM-AFM) [29–34], which is able to provide the morphology and the stiffness spontaneously and is widely used to detect the mechanical properties of various materials, including bone [30], teeth [35], cornea [36], etc. Figure 4a–d shows the tomography of four kinds of composite scaffolds. Due to the limitation of the scanning areas, AFM images only shows local surface structure. Thus, the porous structure is not obvious. However, the AFM images also show rough surface morphology similar with the SEM images. Roughness has important effect on cell proliferation and differentiation. The surface with rough surface was beneficial to the cell proliferation and differentiation [37–39]. The line profiles (Fig. 4e–h) derived from the morphology (Fig. 4a–d) show the maximum height differences alone different line direction. It is clearly shown that the maximum height differences range from ~ 200 to ~ 600 nm. The corresponding stiffness distribution (Fig. 4i) shows the Young’s modulus of four different scaffolds are 7.53 ± 1.25, 8.34 ± 1.00, 9.15 ± 0.85, and 10.20 ± 1.28 GPa, respectively. To clearly show the stiffness differences, the corresponding bar chart is also given (Fig. 4j). Although the Young’s modulus of the nHAC/PLGA/GO scaffolds with a few GO amount difference are not significantly different, for example, the nHAC/PLGA/GO with the GO amount of 0.0 and 0.5 wt% (7.53 ± 1.25 and 8.34 ± 1.00 GPa), of 0.5 and 1.0 wt% (8.34 ± 1.00 and 9.15 ± 0.85 GPa), of 1.0 and 1.5 wt% (9.15 ± 0.85 and 10.20 ± 1.28 GPa), the Young’s modulus of the nHAC/PLGA/GO scaffolds with a bit large GO amount difference (0.0 wt% and 1.5 wt%) are significantly different (7.53 ± 1.25 and 10.20 ± 1.28 GPa). This indicates that the mechanical property of the nHAC/PLGA/GO scaffold increase with the increasing GO amount.
Fig. 4.
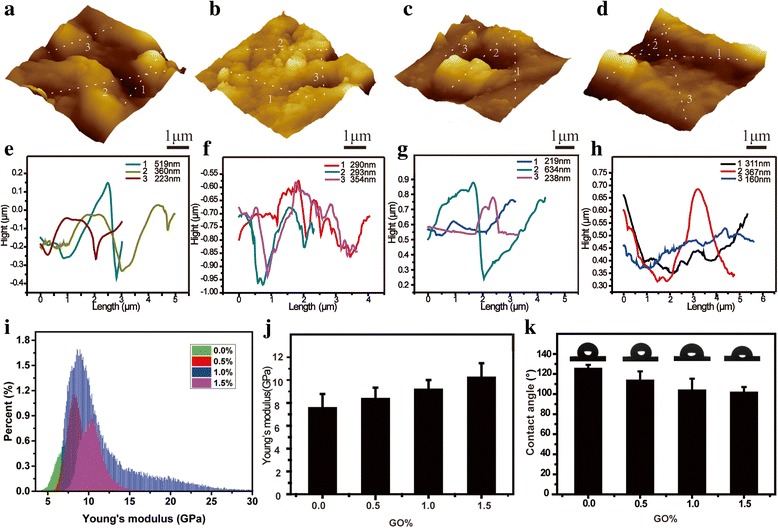
AFM images of a nHAC/PLGA, b nHAC/PLGA/GO (0.5 wt%), c nHAC/PLGA/GO (1.0 wt%), and d nHAC/PLGA/GO (1.5 wt%) scaffolds. e–h Line profiles derived from the morphology images. i Stiffness distribution of the four different scaffolds measured by QNM-AFM. j The bar chart of Young’s modulus versus the GO amount. k The corresponding contact angles of four kinds of scaffolds measured by the sessile drop method
The hydrophilicity of the scaffolds plays a key role in interacting with cells. The addition of GO not only increases the mechanical property of the composite scaffolds, but also changes the hydrophobicity of four kinds of scaffolds. Figure 4f shows the contact angles of different nHAC/PLGA/GO scaffolds. The contact angles of the nHAC/PLGA scaffolds was ~ 125.1° while for nHAC/PLGA/GO with different GO amount (0.5. 1.0, and 1.5 wt%) are ~ 113.4°, ~ 103.4°, and ~ 101.4°, respectively. As the increasing of the GO amount, the contact angles of the composite scaffolds decrease slightly because of both the hydroxyl groups and the negatively charged groups, such as carboxylic acid groups on the GO surface [40]. Thus, GO can provide remarkable bioactivity to 3D nHAC/PLGA scaffolds.
In general, scaffolds for tissue engineering not only require exhibition of biocompatible morphology and properties, but also porous structure and physical strength [41]. The freeze-dried 3D nHAC/PLGA/GO scaffolds possess porous structure because of the sublimation of solvent. The functional groups, including hydroxyl (OH), epoxy (C-O-C), and carboxyl (COOH) species on scaffolds surfaces [40] induces good hydrophilicity. The addition of PLGA and GO provides sufficient physical strength. Thus, the 3D nHAC/PLGA/GO scaffolds could be a promising candidate for tissue engineering.
Cell Culture
It is well known that the scaffolds used for bone tissue should be biocompatible, cell proliferative, and exclusive from immune response [21]. The nHAC/PLGA/GO, which contains the components of natural bone (collagen and HA) and possesses suitable mechanical property and hydrophilicity, should be an ideal candidate for bone tissue engineering. To investigate the cell proliferation of these scaffolds, the MC3T3-E1 osteoblast cells were cultured in this work. Figure 5 shows the cell viability versus culture time evaluated by cell counting kit-8 (CCK-8) assay. The proliferation of cells was consistently increased during the whole culture period for all groups. More specifically, the cell proliferation of MC3T3-E1 on nHAC/PLGA/GO (0.5 and 1.0 wt%) scaffolds are significantly decreased at day 1, while that on nHAC/PLGA/GO (1.5 wt%) scaffolds is similar to that on nHAC/PLGA scaffolds. As time increases, the cell proliferation of MC3T3-E1 on nHAC/PLGA/GO (1.5 wt%) scaffolds is significantly increased at days 3, 5, and 7. However, the cell proliferation of MC3T3-E1 on nHAC/PLGA/GO (0.5 and 1.0 wt%) scaffolds is not significantly different compared to that on nHAC/PLGA scaffolds.
Fig. 5.
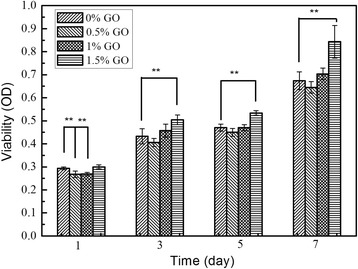
Comparison of the MC3T3-E1 cells on different scaffolds surfaces; double asterisks indicate p < 0.01, number of samples N = 4
The evidence of cell growth, proliferation on different scaffolds was also captured by SEM. Figure 6 shows the surface morphology of osteoblast cells on four different scaffolds after been cultured for 1, 3, 5, 7 days, respectively. At day 1, all the cells are evenly and isolated distributed on four different scaffolds. As time continues (days 3, 5, and 7), all groups of cells grow, proliferated, and started to integrate on different scaffolds, forming a large layer of cells. Compared with the cell morphologies on different scaffolds, the cells on nHAC/PLGA/GO scaffolds surfaces were much larger and stretched than that on the surface of nHAC/PLGA scaffolds. There is no significant difference in the situation of cells spreading, adhesion between the cells on nHAC/PLGA/GO with different amount (0.5, 1.0, and 1.5 wt%) according to the SEM images.
Fig. 6.
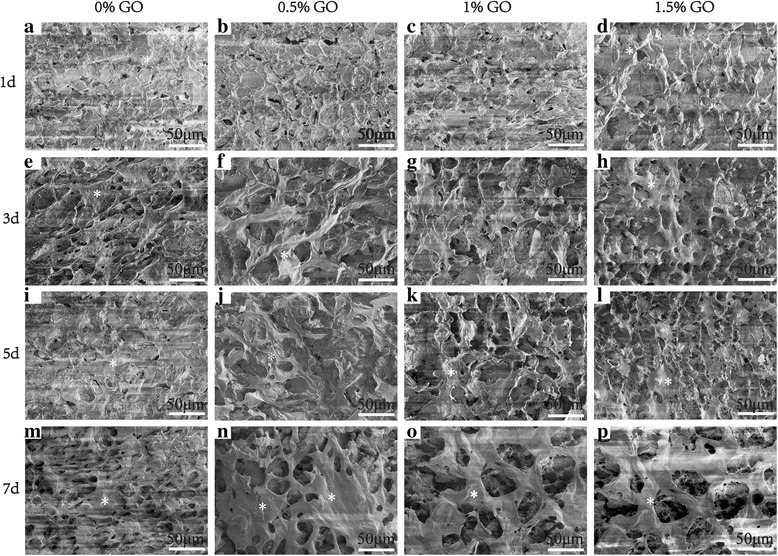
a–p SEM images of MC3T3-E1 cells cultured on four different scaffolds for 1, 3, 5, and 7 days. Scale bars are 50 μm in all images.The white asterisk represents the MC3T3-E1 osteoblast cells
Cytotoxicity Test
The cytotoxicity of GO is an essential concern, for its application in biology field. So we evaluate the cytotoxicity of the four scaffolds in the time of 24 h. The results were shown in Fig. 7. The cell vitality of fibroblasts cells (NIH-3T3) in nHAC/PLGA contain 0.5,1, and 1.5% GO is 99,101.11, and 97.86% relate to nHAC/PLGA, which have no significantly difference than nHAC/PLGA, indicating that the increase in graphene oxide is safe at 0–1.5%.
Fig. 7.
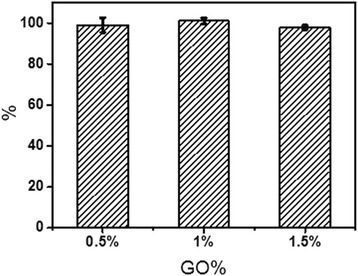
The relative activity of HIH-373 cells in nHAC/PLGA(0.5, 1, 1.5 wt%) relate to nHAC/PLGA
Table 1 summarized the mechanical properties and cell culture properties of four kinds of composite scaffolds. As the increasing of the GO, the Young’s modulus of the scaffolds increase accordingly. However, only the mechanical properties of nHAC/PLGA and nHAC/PLGA/GO (1.5 wt%) are distinctly different. The cell viability of four kinds of scaffolds show the same trend with the mechanical property, that is to say the OD values of all groups increase with the increasing cell culture time, but only the nHAC/PLGA and nHAC/PLGA/GO (1.5 wt%) groups show significant difference. This indicates that the mechanical property of the scaffolds is closely related to the cell culture property. The results may be because that tissue cells can feel and respond to the stiffness of their substrates [42–45]. Tuning the mechanical properties of the substrates can promote cellular responses affecting cell-surface interactions along with cell growth and viability [46–49]. Haugh et al. found that the stiffness of the scaffolds enhanced the activity of MC3T3-E1 cells (cell proliferation and migration) [50]. Engler et al. demonstrated that an important physical factor in the response of many cell types was substrate stiffness [51]. They found that smooth muscle cells derive from rat aorta (A7R5 line), like other anchorage dependent cells, spread more and organize their cytoskeleton and focal adhesions much more on ‘stiff’ substrates than on ‘soft’ substrates. The mechanical property not only affects the cell behaviors but also the tissue activities. Duncan et al. studied the mechanotransduction and the mechanical strain of functional response on bone. They found that mechanical loading can inhibit bone resorption and increase bone formation in vivo [52]. Therefore, the stiffest nHAC/PLGA/GO (1.5 wt%) scaffolds could promote the proliferation of MC3T3-E1 cells.
Table 1.
Young’s modulus and OD values of different PLGA/nHAC/GO scaffolds
| Young’s modulus and OD values | PLGA/nHAC | PLGA/nHAC/GO (0.5 wt%) | PLGA/nHAC/GO (1.0 wt%) | PLGA/nHAC/GO (1.5 wt%) |
|---|---|---|---|---|
| Young’s modulus | 7.53 ± 1.25 (GPa) | 8.34 ± 1.00 (GPa) | 9.25 ± 0.85 (GPa) | 10.20 ± 1.28 (GPa) |
| OD (1 day) | 0.294 ± 0.006 | 0.268 ± 0.014 | 0.269 ± 0.007 | 0.300 ± 0.008 |
| OD (3 days) | 0.433 ± 0.033 | 0.406 ± 0.018 | 0.458 ± 0.028 | 0.505 ± 0.021 |
| OD (5 days) | 0.470 ± 0.015 | 0.450 ± 0.017 | 0.470 ± 0.013 | 0.534 ± 0.010 |
| OD (7 days) | 0.674 ± 0.039 | 0.645 ± 0.025 | 0.704 ± 0.026 | 0.843 ± 0.071 |
The cytotoxicity of GO is an essential concern for its application in biology field. Till now, two arguments have been arisen. One claim the GO would induce cytotoxicity and its effect is concentration dependent. For example, Chatterjee, et al. reported the toxic response with differential dose dependency for GO [53]. Pinto, et al. reported that only low concentrations of GO may be incorporated safety in PLA to facilitate cell adhesion and proliferation [6]. The others state that even higher amount of GO would have good biocompatibility and enhance both mechanical properties of the substrates and the cellular behaviors. Shin, et al. studied the C2C12 skeletal myoblasts were enhanced on PLGA-GO-collagen hybrid matrices than PLGA, PLGA-collagen matrices [54]. And Luo, et al. reported the GO-doped PLGA nanofiber scaffolds can enhance the osteogenic differentiation of MSCs [22]. In this study, the GO was selected based on the first argument. The limited amount is added into the composite scaffolds for non-cytotoxicity and enhanced mechanical property. The conjugation of GO into nHAC/PLGA scaffolds significantly enhanced cell growth, proliferation. Although the cell number on both nHAC/PLGA and nHAC/PLGA with small amount of GO, for example, nHAC/PLGA/GO (0.5 wt%), is more or less the same, the number of cells on nHAC/PLGA/GO (1.5 wt%) scaffold was higher than that on the nHAC/PLGA scaffolds. These results indicate the nHAC/PLGA/GO scaffolds are biofunctional with the ability of enhancing the growth and proliferation of MC3T3-E1 cells. Therefore, the excellent biocompatibility and biofunctionality allows nHAC/PLGA/GO to be employed as effective scaffolds for bone regeneration.
The nature of the biomaterial and the fabrication process are very important to scaffold properties [28]. So far, the biomaterials have been extensively studied, including metals [55], ceramics [56], glass [57], chemically synthesized polymers [58], natural polymers [59], and combinations of these materials to form composites [60]. Changing components of composite scaffolds will induce the scaffolds property. For instance, to fabricate the biomimic scaffold of natural bone, the type I collagen is been used in this study. Currently, the collagen family includes more than 20 different types of collagen existing in the skin, bone, cartilage, etc. Replacing type I collagen with other types, it is possible to fabricate different composite scaffolds for different purpose. For example, collagen type II is one of the fibril-forming collagens and the predominant type of collagen in cartilage. Coordinating collagen type II into the scaffolds may be able to facilitate cartilage bone regeneration [61]. In addition, the collagen with proper annealing may further strength the scaffolds, which may induce a new composite material with functional structures. Besides the nature of biomaterials, the processing also determines the functional of scaffolds, such as different processing methods. Material chemistry and processing determines the maximum functional properties as well as how cells interact with the scaffold. Scaffolds of properties and requirements in bone tissue engineering have been extensively investigated, including degradation [62], mechanical properties [63], cytokine delivery [64], and combinations of scaffolds and cells [65].
Conclusions
In summary, nHAC/PLGA/GO scaffolds with different amount of GO (0.0, 0.5, 1.0, and 1.5 wt%) were fabricated by freeze-drying method. The fabricated nHAC/PLGA/GO scaffolds show porous structure. Furthermore, the mechanical property and the hydrophilicity of the scaffolds are enhanced because of the addition of PLGA and GO. The in vitro study shows the porous scaffolds facilitate the cell adsorption, growth, and proliferation. These nHAC/PLGA/GO scaffolds could be a promising candidate for bone tissue applications.
Methods
Materials
The purified lyophilized type 1 collagen was obtained from Tianjin Saining Biological Engineering Technology Co., Ltd. PLGA with lactide:glycolide ratio of 75:25 and Mw of 95,000 g/mol was purchased from Shandong Medical Appliance Factory (China). GO was purchased from Shanghai Aladdin biochemical Polytron Technologies Inc. MC3T3-E1 osteoblast cells were provided by cell bank of Shanghai Chinese Academy of Sciences. Fetal bovine serum (FBS), antibiotic-antimycotic, CCK-8, and Dulbecco’s modified Eagle media (DMEM) were accessed from Tianjin Nobuo Letter Technology Co., Ltd. 1,4-dioxane, phosphate buffered saline (PBS, 0.1 M, PH 7.4), and all other chemicals were analytical grade and used as received with no further purification.
Preparation of the nHAC Power and nHAC/PLGA/GO Composite Scaffolds
The method of compositing nHAC powder has been reported previously [66–68]. Briefly, collagen was dissolved in acetic acid (0.5 mol/L) forming a solution with the concentration of 4 g/L. The CaCl2 and H3PO4 (Ca/P = 1.66) solutions were then added separately by drops. The dropping rate is 100 drops per minute. The solution was gently stirred and titrated at 37 °C with ammonia solution to pH 9. After 24 h, the nHAC deposition was harvested by centrifugation and freeze-drying. For the preparation of nHAC/PLGA/GO composite scaffolds, GO was evenly dispersed in dioxane by using an ultrasonic cell crusher, forming a final concentration of 0.0, 0.5, 1.0, and 1.5 g/L, respectively. The PLGA was then added into GO solutions, forming a final concentration of 10% (m/v). The GO/PLGA solutions were then stirred gently at room temperature for 12 h. The final solution was formed by adding the nHAC power into the GO/PLGA solution at a 1:1 nHAC:PLGA weight ratio. The formed nHAC/PLGA/GO solution was then stirred and ultrasonicated for 4 h. After frozen at − 20 °C overnight, the nHAC/PLGA/GO composite scaffolds were obtained by lyophilizing to remove dioxane.
Characterizations
The composite scaffolds were coated with gold and were observed under a SEM (JSM-7100F). We spray gold for 20 s for preparation of electron microscopy samples. The topography and the mechanical properties of the matrices were characterized by atomic force microscopy (AFM, Multimode VIII, Bruker, Germany) in air. Image analysis was performed using Gwyddion and Nanoscope Analysis Software. Compositional analysis of the nHAC/PLGA/GO composite scaffolds was performed by a FT-IR spectrophotometer (VECTOR22, Bruker, Germany). All spectra were recorded in absorption mode in the wavelength range of 1000–2200 cm−1 with a resolution of 4.0 cm−1 and 16-times scanning. The contact angles of the samples were measured using a contact angle measurement system by the sessile drop method (EasyDrop, model DAS30, kruss, Germany). The XRD patterns were measured using the X-ray diffractometer (D8 DISCOVER). The Cu-Kα radiation (λ = 0.154 nm) is 40 kV and 30 mA. The scan rate of the measurements is 8°min−1 over the 2θ range of 5–80° at RT. The porosity of the scaffolds were measured by an automatic surface area and porosity analyzer (ASAP 2460, Micromeritics, GA, USA).
Cell Culture
MC3T3-E1 osteoblast cells were incubated in DMEM supplemented with 10% FBS and 3% antibiotic-antimycotic solution at 37 °C and 5% CO2 in a cell incubator. The initial attachment and proliferation were tested by using CCK-8 according to the manufacturer’s instruction, in which the number of viable cells was directly proportional to the metabolic reaction products obtained in the CCK-8 assay [69]. Briefly, the MC3T3-E1 osteoblast cells were seeded at a density of 2.5 × 104 cells per well on the nHAC/PLGA, nHAC/PLGA/GO (0.5 wt%), nHAC/PLGA/GO (1.0 wt%), and nHAC/PLGA/GO (1.5 wt%) matrices embedded in 48-well cell culture plate. The cells were incubated with the CCK-8 solution in the last 2 h of the culture periods (1, 3, 5, and 7 days) for the proliferation at 37 °C in the dark. The absorbance was measured at the wavelength of 450 nm using an ELISA reader (DNM-9602).
The cell samples for SEM measurement were fixed with formaldehyde, and the specimens were then dehydrated through a graded series of ethanol (30, 50, 75, 95, and 100%) for 15 min at each concentration. Then, the samples were drying by critical point drying was allowed to occur with a carbon dioxide analyzer (Hitachi, HCP-2). Finally, the samples with gold coating were observed by SEM.
Cytotoxicity Test
The fibroblasts cells concentration was adjusted to 1 × 104/ml and was inoculated into 96-well plates at 200 ul per well. Then, the well plates were incubated at 37 °C in a 5% CO2 incubator for 24 h. The samples (nHAC/PLGA, nHAC/PLGA/GO (0.5 wt%), nHAC/PLGA/GO (1.0 wt%) and nHAC/PLGA/GO (1.5 wt%)) were powdered to make a 100 mg/ml suspension. The experiment group with 100 ul suspension and control group with equal volume of DMEM complete medium were incubated for 24 h and were further incubated for 4 h after the CCK-8 was added to the incubator. The cell viability was obtained by measuring the absorbance at the wavelength of 450 nm using an ELISA reader. The cell viability was calculated by using the following formula,
Where A (experiment) represents absorbance of wells with cells, CCK-8 solution and power samples solution; A (blank) represents absorbance of wells with medium and CCK-8 solution without cells and A (control) represents absorbance of wells with cells, CCK-8 solution without power samples solution.
Statistical Analysis
Quantitative results were expressed as the mean value from at least triplicate samples ± standard deviation (SD). Student’s t test was used to the statistical analysis. A value of p < 0.05 was considered statistically significant. Data are marked ** to indicate p < <0.01.
Acknowledgments
Funding
The authors gratefully acknowledge the support from the National Natural Science Foundation of China (No. 31600753), from the Natural Science Foundation of Tianjin (No. 16JCYBJC43400) and from the National Science Foundation of Hebei (No. C2017202206) and from Hebei Province Foundation for Returned Overseas Chinese Scholars (CL201711). CL acknowledges the financial support by the National Natural Science Foundation of China (Project Nos. 51201056, 51171058, 51401146), Natural Science Foundation of Hebei Province of China (Project Nos. E2013202021, E2013202022, and E2015202037), Outstanding Youth Foundation of Hebei Province of China (Project No. E2015202282), and Science and Technology Correspondent Project of Tianjin (Nos. 14JCTPJC00496, 15JCYBJC29900).
Availability of Data and Materials
The authors declare that materials, data, and associated protocols are promptly available to readers without undue qualifications in material transfer agreements.
All date generated or analyzed during this study are included in this article.
Abbreviations
- 3D
Three-dimensional
- AFM
Atomic force microscopy
- CCK-8
Cell counting kit-8
- CTD
Computational topology design
- DMEM
Dulbecco’s modified Eagle media
- EDS
X-ray spectroscopy
- FBS
Fetal bovine serum
- FT-IR
Fourier transform infrared spectroscopy
- GO
Graphene oxide
- HA
Hydroxyapatite
- MC3T3-E1
Osteoblast cells
- MSCs
Mesenchymal stem cells
- nHAC
Nano-hydroxyapatite/collagen
- NIH-3T3
Fibroblast cells
- PBS
Phosphate-buffered saline
- PLA
Poly(lactic acid)
- PLGA
poly(lactic-co-glycolic acid)
- QNM-AFM
Quantitative nano-mechanical atomic force microscope
- SD
Standard deviation
- SEM
Scanning electron microscope
- SFF
Olid free-form fabrication
- XRD
X-ray diffraction
Additional file
a Surface morphology of the nHAC. b EDS spectra of the nHAC. Figure S2 SEM images of a nHAC/PLGA; b nHAC/PLGA/GO (0.5 wt%). c nHAC/PLGA/GO (1.0 wt%); d nHAC/PLGA/GO (1.5 wt%) scaffolds. (DOCX 2689 kb)
Authors’ Contributions
YL, GY, and LL carried out the experiments and analysis. XZ, HW, and DX supervised the writing of the manuscript and created the figures. YL supervised the whole work. All the authors have read and approve the final manuscript.
Competing Interests
The authors declare that have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Footnotes
Chunyong Liang and Yongchao Luo contributed equally to this work.
Electronic supplementary material
The online version of this article (10.1186/s11671-018-2432-6) contains supplementary material, which is available to authorized users.
Contributor Information
Chunyong Liang, Email: liangchunyong@hebut.edu.cn.
Yongchao Luo, Email: 201531804041@stu.hebut.edu.cn.
Guodong Yang, Email: yangguodong423@163.com.
Dan Xia, Email: xiad@hebut.edu.cn.
Lei Liu, Email: liul@ujs.edu.cn.
Xiaomin Zhang, Email: zxm15510922072@163.com.
Hongshui Wang, Email: kingflood@hebut.edu.cn.
References
- 1.Langer R. Tissue engineering. Science. 1993;260:920–926. doi: 10.1126/science.8493529. [DOI] [PubMed] [Google Scholar]
- 2.Liu X, Miller AL, Waletzki BE, Yaszemski MJ, Lu L. Novel biodegradable poly(propylene fumarate)-co-poly(l-lactic acid) porous scaffolds fabricated by phase separation for tissue engineering applications. RSC Adv. 2015;5:21301–21309. doi: 10.1039/C5RA00508F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hutmacher DW. Scaffolds in tissue engineering bone and cartilage. Biomaterials. 2000;21:2529–2543. doi: 10.1016/S0142-9612(00)00121-6. [DOI] [PubMed] [Google Scholar]
- 4.Hollister SJ. Porous scaffold design for tissue engineering. Nat Mater. 2005;4:518–524. doi: 10.1038/nmat1421. [DOI] [PubMed] [Google Scholar]
- 5.Pinto AM, Moreira S, Gonçalves IC, Gama FM, Mendes AM, Magalhães FD. Biocompatibility of poly(lactic acid) with incorporated graphene-based materials. Colloid Surface B. 2013;104:229–238. doi: 10.1016/j.colsurfb.2012.12.006. [DOI] [PubMed] [Google Scholar]
- 6.Wang C, Shen H, Tian Y, Xie Y, Li A, Ji L, Niu Z, Wu D, Qiu D. Bioactive nanoparticle-gelatin composite scaffold with mechanical performance comparable to cancellous bones. ACS Appl Mater Inter. 2014;6:13061–13068. doi: 10.1021/am5029582. [DOI] [PubMed] [Google Scholar]
- 7.Zhao G, Zhang X, Lu TJ, Xu F. Recent advances in electrospun nanofibrous scaffolds for cardiac tissue engineering. Adv Fun Mater. 2015;25:5726–5738. doi: 10.1002/adfm.201502142. [DOI] [Google Scholar]
- 8.Sultana N, Wang M. PHBV/PLLA-based composite scaffolds fabricated using an emulsion freezing/freeze-drying technique for bone tissue engineering: surface modification and in vitro biological evaluation. Biofabrication. 2012;4:015003. doi: 10.1088/1758-5082/4/1/015003. [DOI] [PubMed] [Google Scholar]
- 9.Nudelman F, Pieterse K, George A, Bomans PH, Friedrich H, Brylka LJ, Hilbers PAJ, With GD, Sommerdijk NAJM. The role of collagen in bone apatite formation in the presence of hydroxyapatite nucleation inhibitors. Nat Mater. 2010;9:1004–1009. doi: 10.1038/nmat2875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nair AK, Gautieri A, Chang SW, Buehler MJ. Molecular mechanics of mineralized collagen fibrils in bone. Nat Commun. 2013;4:1724. doi: 10.1038/ncomms2720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boskey AL. Bone composition: relationship to bone fragility and antiosteoporotic drug effects. Bonekey Rep. 2015;4:710. doi: 10.1038/bonekey.2015.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mitra J, Tripathi G, Sharma A, Basu B. Scaffolds for bone tissue engineering: role of surface patterning on osteoblast response. RSC Adv. 2013;3:11073–11094. doi: 10.1039/c3ra23315d. [DOI] [Google Scholar]
- 13.Rodrigues SC, Salgado CL, Sahu A, Garcia MP, Fernandes MH, Monteiro FJ. Preparation and characterization of collagen-nanohydroxyapatite biocomposite scaffolds by cryogelation method for bone tissue engineering applications. J Biomed Mater Res A. 2013;101:1080–1094. doi: 10.1002/jbm.a.34394. [DOI] [PubMed] [Google Scholar]
- 14.Wang X, Wang G, Liu L, Zhang D. The mechanism of a chitosan-collagen composite film used as biomaterial support for MC3T3-E1 cell differentiation. Sci Rep. 2016;6:39322. doi: 10.1038/srep39322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang W, Shi X, Ren L, Du C, Wang Y. PHBV microspheres—PLGA matrix composite scaffold for bone tissue engineering. Biomaterials. 2010;31:4278–4285. doi: 10.1016/j.biomaterials.2010.01.059. [DOI] [PubMed] [Google Scholar]
- 16.Lao L, Wang Y, Zhu Y, Zhang Y, Gao C. Poly(lactide-co-glycolide)/hydroxyapatite nanofibrous scaffolds fabricated by electrospinning for bone tissue engineering. J Mater Sci- Mater M. 2011;22:1873–1884. doi: 10.1007/s10856-011-4374-8. [DOI] [PubMed] [Google Scholar]
- 17.Liao SS, Cui FZ, Zhang W, Feng QL. Hierarchically biomimetic bone scaffold materials: nano-HA/collagen/PLA composite. J Biomed Mater Res B. 2004;69:158–165. doi: 10.1002/jbm.b.20035. [DOI] [PubMed] [Google Scholar]
- 18.Liu Y, Sun Q, Wang S, Fan J, Chen A, Wu W. Preparation of SF/PLGA scaffold with microporous-nano/microfibrous architecture by thermal induced phase separation. Sci Adv Mater. 2015;7:2380–2387. doi: 10.1166/sam.2015.2386. [DOI] [Google Scholar]
- 19.Niu X, Feng Q, Wang M, Guo X, Zheng Q. Porous nano-HA/collagen/PLLA scaffold containing chitosan microspheres for controlled delivery of synthetic peptide derived from BMP-2. J Control Release. 2009;134:111–117. doi: 10.1016/j.jconrel.2008.11.020. [DOI] [PubMed] [Google Scholar]
- 20.Luo Y, Shen H, Fang Y, Cao Y, Huang J, Zhang M, Dai J, Shi X, Zhang Z. Enhanced proliferation and osteogenic differentiation of mesenchymal stem cells on graphene oxide-incorporated electrospun poly(lactic-co-glycolic acid) nanofibrous mats. ACS Appl Mater Inter. 2015;7:6331–6339. doi: 10.1021/acsami.5b00862. [DOI] [PubMed] [Google Scholar]
- 21.Shao W, He J, Sang F, Wang Q, Chen L, Cui S, Ding B. Enhanced bone formation in electrospun poly(l-lactic-co-glycolic acid)-tussah silk fibroin ultrafine nanofiber scaffolds incorporated with graphene oxide. Mat Sci Eng C Mater. 2016;62:823–834. doi: 10.1016/j.msec.2016.01.078. [DOI] [PubMed] [Google Scholar]
- 22.Shin YC, Lee JH, Jin L, Min MJ, Kim YJ, Hyun YJ, Jung TG, Hong SW, Han DW. Stimulated myoblast differentiation on graphene oxide-impregnated PLGA-collagen hybrid fibre matrices. J Nanobiotechnol. 2015;13:21. doi: 10.1186/s12951-015-0081-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li Y, Umer R, Samad YA, Zheng L, Liao K. The effect of the ultrasonication pre-treatment of graphene oxide (GO) on the mechanical properties of GO/polyvinyl alcohol composites. Carbon. 2013;55:321–327. doi: 10.1016/j.carbon.2012.12.071. [DOI] [Google Scholar]
- 24.Jing X, Mi HY, Salick MR, Peng XF, Turng LS. Preparation of thermoplastic polyurethane/graphene oxide composite scaffolds by thermally induced phase separation. Polym Composite. 2014;35:1408–1417. doi: 10.1002/pc.22793. [DOI] [Google Scholar]
- 25.Huang GP, Shanmugasundaram S, Masih P, Pandya D, Amara S, Collins G, Arinzeh TL. An investigation of common crosslinking agents on the stability of electrospun collagen scaffolds. J Biomed Mater Res A. 2015;103:762–771. doi: 10.1002/jbm.a.35222. [DOI] [PubMed] [Google Scholar]
- 26.Chang Y, Tsai CC, Liang HC, Sung HW. In vivo evaluation of cellular and acellular bovine pericardia fixed with a naturally occurring crosslinking agent (genipin) Biomaterials. 2002;23:2447–2457. doi: 10.1016/S0142-9612(01)00379-9. [DOI] [PubMed] [Google Scholar]
- 27.Viswanath B, Shastry VV, Ramamurty U, Ravishankar N. Effect of calcium deficiency on the mechanical properties of hydroxyapatite crystals. Acta Mater. 2010;58:4841–4848. doi: 10.1016/j.actamat.2010.05.019. [DOI] [Google Scholar]
- 28.Karageorgiou V, Kaplan D. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials. 2005;26:5474–5491. doi: 10.1016/j.biomaterials.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 29.Zhang S, Aslan H, Besenbacher F, Dong M. Quantitative biomolecular imaging by dynamic nanomechanical mapping. Chem Soc Rev. 2014;43:7412–7429. doi: 10.1039/C4CS00176A. [DOI] [PubMed] [Google Scholar]
- 30.Zhang S, Bach-Gansmo FL, Xia D, Besenbacher F, Birkedal H, Dong M. Nanostructure and mechanical properties of the osteocyte lacunar-canalicular network-associated bone matrix revealed by quantitative nanomechanical mapping. Nano Res. 2015;8:3250–3260. doi: 10.1007/s12274-015-0825-8. [DOI] [Google Scholar]
- 31.Froning JP, Lazar P, Pykal M, Li Q, Dong M, Zbořil R, Otyepka M. Direct mapping of chemical oxidation of individual graphene sheets through dynamic force measurements at the nanoscale. Nano. 2016;9:119–127. doi: 10.1039/c6nr05799c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guo Q, Xue Q, Sun J, Dong M, Xia F, Zhang Z. Gigantic enhancement in the dielectric properties of polymer-based composites using core/shell MWCNT/amorphous carbon nanohybrids. Nano. 2015;7:3660–3667. doi: 10.1039/c4nr05264a. [DOI] [PubMed] [Google Scholar]
- 33.Jiang Z, Li Q, Chen M, Li J, Li J, Huan Y, Besenbacher F, Dong M. Mechanical reinforcement fibers produced by gel-spinning of poly-acrylic acid (PAA) and graphene oxide (GO) composites. Nano. 2013;5:6265–6269. doi: 10.1039/c3nr00288h. [DOI] [PubMed] [Google Scholar]
- 34.Li YF, Rubert M, Aslan H, Yu Y, Howard KA, Dong M, Besenbacher F, Chen M. Ultraporous interweaving electrospun microfibers from PCL–PEO binary blends and their inflammatory responses. Nano. 2014;6:3392–3402. doi: 10.1039/c3nr06197c. [DOI] [PubMed] [Google Scholar]
- 35.Xue Y, Wang L, Xia D, Li Q, Gao S, Dong M, Cai T, Shi S, He L, Hu K, Mao T, Duan X. Dental abnormalities caused by novel compound heterozygous CTSK mutations. J Dent Res. 2015;94:674–681. doi: 10.1177/0022034515573964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xia D, Zhang S, Hjortdal JØ, Li Q, Thomsen K, Chevallier J, Besenbacher F, Dong M. Hydrated human corneal stroma revealed by quantitative dynamic atomic force microscopy at nanoscale. ACS Nano. 2014;8:6873–6882. doi: 10.1021/nn5015837. [DOI] [PubMed] [Google Scholar]
- 37.Cadafalch Gazquez G, Chen H, Veldhuis SA, Solmaz A, Mota C, Boukamp BA, van Blitterswijk CA, Ten Elshof JE, Moroni L. Flexible yttrium-stabilized zirconia nanofibers offer bioactive cues for osteogenic differentiation of human mesenchymal stromal cells. ACS Nano. 2016;10:5789–5799. doi: 10.1021/acsnano.5b08005. [DOI] [PubMed] [Google Scholar]
- 38.Chen H, Huang X, Zhang M, Damanik F, Baker MB, Leferink A, Yuan H, Truckenmüller R, van Blitterswijk C, Moroni L. Tailoring surface nanoroughness of electrospun scaffolds for skeletal tissue engineering. Acta Biomater. 2017;59:82–93. doi: 10.1016/j.actbio.2017.07.003. [DOI] [PubMed] [Google Scholar]
- 39.Heweet EM, Smith K, Gall K, Boyan BD, Schwartz Z. Change in surface roughness by dynamic shape-memory acrylate networks enhances osteoblast differentiation. Biomaterials. 2016;110:34–44. doi: 10.1016/j.biomaterials.2016.08.004. [DOI] [PubMed] [Google Scholar]
- 40.Yoon OJ, Chang YJ, Sohn IY, Kim HJ, Hong B, Jhon MS, Lee NE. Nanocomposite nanofibers of poly ( d, l-lactic-co-glycolic acid) and graphene oxide nanosheets. Compos Part A- Appl S. 2011;42:1978–1984. doi: 10.1016/j.compositesa.2011.08.023. [DOI] [Google Scholar]
- 41.Salgado AJ, Coutinho OP, Reis RL. Bone tissue engineering: state of the art and future trends. Macromol Biosci. 2004;4:743–765. doi: 10.1002/mabi.200400026. [DOI] [PubMed] [Google Scholar]
- 42.Blacklock J, Vetter A, Lankenau A, Oupicky D, Mohwald H. Tuning the mechanical properties of bioreducible multilayer films for improved cell adhesion and transfection activity. Biomaterials. 2010;31:7167–7174. doi: 10.1016/j.biomaterials.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li Y, Poon CT, Li M, Lu TJ, Pingguan-Murphy B, Xu F. Chinese-noodle-inspired muscle myofiber fabrication. Adv Funct Mater. 2015;25:5999–6008. doi: 10.1002/adfm.201502018. [DOI] [Google Scholar]
- 44.Yong KW, Li Y, Huang G, Lu TJ, Safwani WK, Pingguan-Murphy B, Xu F. Mechanoregulation of cardiac myofibroblast differentiation: implications for cardiac fibrosis and therapy. Am J Physiol-Heart C. 2015;309:H532–H542. doi: 10.1152/ajpheart.00299.2015. [DOI] [PubMed] [Google Scholar]
- 45.Ma Y, Ji Y, You M, Wang S, Dong Y, Jin G, Lin M, Wang Q, Li A, Zhang X, Xu F. Labeling and long-term tracking of bone marrow mesenchymal stem cells in vitro using NaYF 4: Yb 3+, Er 3+ upconversion nanoparticles. Acta Biomater. 2016;42:199–208. doi: 10.1016/j.actbio.2016.07.030. [DOI] [PubMed] [Google Scholar]
- 46.Hachet E, Van Den Berghe H, Bayma E, Block MR, Auzélyvelty R. Design of biomimetic cell-interactive substrates using hyaluronic acid hydrogels with tunable mechanical properties. Biomacromolecules. 2012;13:1818–1827. doi: 10.1021/bm300324m. [DOI] [PubMed] [Google Scholar]
- 47.Zeng Y, Yi J, Wan Z, Liu K, Song P, Chau A, Wang F, Chang Z, Han W, Zheng W, Chen YH, Xiong C, Liu W. Substrate stiffness regulates B-cell activation, proliferation, class switch, and T-cell-independent antibody responses in vivo. Eur J Immunol. 2015;45:1621–1634. doi: 10.1002/eji.201444777. [DOI] [PubMed] [Google Scholar]
- 48.Zhou W, Stukel JM, Cebull HL, Willits RK. Tuning the mechanical properties of poly(ethylene glycol) microgel-based scaffolds to increase 3D Schwann cell proliferation. Macromol Biosci. 2016;16:535–544. doi: 10.1002/mabi.201500336. [DOI] [PubMed] [Google Scholar]
- 49.Discher DE, Janmey P, Wang YL. Tissue cells feel and respond to the stiffness of their substrate. Science. 2005;310:1139–1143. doi: 10.1126/science.1116995. [DOI] [PubMed] [Google Scholar]
- 50.Haugh MG, Murphy CM, Mckiernan RC, Altenbuchner C, O'Brien FJ. Crosslinking and mechanical properties significantly influence cell attachment, proliferation, and migration within collagen glycosaminoglycan scaffolds. Tissue Eng Part A. 2011;17:1201–1208. doi: 10.1089/ten.tea.2010.0590. [DOI] [PubMed] [Google Scholar]
- 51.Engler A, Bacakova L, Newman C, Hategan A, Griffin M, Discher D. Substrate compliance versus ligand density in cell on gel responses. Biophys J. 2004;86:617–628. doi: 10.1016/S0006-3495(04)74140-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Duncan RL, Turner CH. Mechanotransduction and the functional response of bone to mechanical strain. Calcified Tissue Int. 1995;57:344–358. doi: 10.1007/BF00302070. [DOI] [PubMed] [Google Scholar]
- 53.Chatterjee N, Eom HJ, Choi J. A systems toxicology approach to the surface functionality control of graphene-cell interactions. Biomaterials. 2014;35:1109–1127. doi: 10.1016/j.biomaterials.2013.09.108. [DOI] [PubMed] [Google Scholar]
- 54.Du C, Cui FZ, Zhang W, Feng QL, Zhu XD, De GK. Formation of calcium phosphate/collagen composites through mineralization of collagen matrix. J Biomed Mater Res B. 2000;50:518–527. doi: 10.1002/(SICI)1097-4636(20000615)50:4<518::AID-JBM7>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 55.Fang Y, Wang B, Zhao Y, Xiao Z, Li J, Cui Y, Han S, Wei J, Chen B, Han J, Meng Q, Hou X, Luo J, Dai J, Jing Z. Collagen scaffold microenvironments modulate cell lineage commitment for differentiation of bone marrow cells into regulatory dendritic cells. Sci Rep. 2017;7:42049. doi: 10.1038/srep42049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kokubo T. Bioactive glass ceramics: properties and applications. Biomaterials. 1991;12:155–163. doi: 10.1016/0142-9612(91)90194-F. [DOI] [PubMed] [Google Scholar]
- 57.Domingues ZR, Cortés ME, Gomes TA, Diniz HF, Freitas CS, Gomes JB, Faria AM, Sinisterra RD. Bioactive glass as a drug delivery system of tetracycline and tetracycline associated with beta-cyclodextrin. Biomaterials. 2004;25:327–333. doi: 10.1016/S0142-9612(03)00524-6. [DOI] [PubMed] [Google Scholar]
- 58.Green JJ, Elisseeff JH. Mimicking biological functionality with polymers for biomedical applications. Nature. 2016;540:386–394. doi: 10.1038/nature21005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang X, Harris RE, Bayston LJ, Ashe HL. Type IV collagens regulate BMP signalling in drosophila. Nature. 2008;455:72–77. doi: 10.1038/nature07214. [DOI] [PubMed] [Google Scholar]
- 60.Ignjatović N, Savić V, Najman S, Plavgić M, Uskoković D. A study of HAp/PLLA composite as a substitute for bone powder, using FT-IR spectroscopy. Biomaterials. 2001;22:571–575. doi: 10.1016/S0142-9612(00)00215-5. [DOI] [PubMed] [Google Scholar]
- 61.Dong M, Xu S, Bünger MH, Birkedal H, Besenbacher F. Temporal assembly of collagen type II studied by atomic force microscopy. Adv Eng Mater. 2010;9:1129–1133. doi: 10.1002/adem.200700220. [DOI] [Google Scholar]
- 62.Roy TD, Simon JL, Ricci JL, Rekow ED, Thompson VP, Parsons JR. Performance of degradable composite bone repair products made via three-dimensional fabrication techniques. J Biomed Mater Res A. 2003;66:283–291. doi: 10.1002/jbm.a.10582. [DOI] [PubMed] [Google Scholar]
- 63.Jain RA. The manufacturing techniques of various drug loaded biodegradable poly(lactide- co -glycolide) (PLGA) devices. Biomaterials. 2000;21:2475–2490. doi: 10.1016/S0142-9612(00)00115-0. [DOI] [PubMed] [Google Scholar]
- 64.Tabata Y. Tissue regeneration based on growth factor release. Tissue Eng. 2003;9:S5–15. doi: 10.1089/10763270360696941. [DOI] [PubMed] [Google Scholar]
- 65.Orban JM, Marra KG, Hollinger JO. Composition options for tissue-engineered bone. Tissue Eng. 2002;8:529–539. doi: 10.1089/107632702760240454. [DOI] [PubMed] [Google Scholar]
- 66.Du C, Cui FZ, Zhang W, Feng QL, Zhu XD, De GK. Formation of calcium phosphate/collagen composites through mineralization of collagen matrix. J Biomed Mater Res A. 2015;50:518–527. doi: 10.1002/(SICI)1097-4636(20000615)50:4<518::AID-JBM7>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 67.Wahl DA, Czernuszka JT. Collagen-hydroxyapatite composites for hard tissue repair. Eur Cells Mater. 2006;11:43–56. doi: 10.22203/eCM.v011a06. [DOI] [PubMed] [Google Scholar]
- 68.Wang RZ, Cui FZ, Lu HB, Wen HB, Ma CL, Li HD. Synthesis of nanophase hydroxyapatite/collagen composite. J Mate Sci Lett. 1995;14:490–492. doi: 10.1007/BF00665911. [DOI] [Google Scholar]
- 69.Lee EJ, Lee JH, Jin L, Jin OS, Shin YC, Sang JO, Lee J, Hyon SH, Han DW. Hyaluronic acid/poly(lactic-co-glycolic acid) core/shell fiber meshes loaded with epigallocatechin-3-O-gallate as skin tissue engineering scaffolds. J Nanosci Nanotechno. 2014;14:8458–8463. doi: 10.1166/jnn.2014.9922. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors declare that materials, data, and associated protocols are promptly available to readers without undue qualifications in material transfer agreements.
All date generated or analyzed during this study are included in this article.


