Abstract
Reference genes are essential for gene expression analysis when using real-time quantitative PCR (RT-qPCR). Xenopus laevis is a popular amphibian model for studying vertebrate embryogenesis and development. Further, X. laevis is ideal for studying thyroid signaling due to its thyroid dependent metamorphosis, a stage comparable to birth in humans. When using PCR based studies, a primary concern is the choice of reference genes. Commonly used references are eef1a1, odc1, rpl8, and actnB, although there is a lack of ad hoc reference genes for X. laevis. Here, we used previously published RNA-seq data on different X. laevis stages and identified the top 14 candidate genes with respect to their expression levels as a function of developmental stage and degree of variation. We further evaluated the stability of these and other candidate genes using RT-qPCR on various stages including the unfertilised eggs, whole embryos during early development and brains during late development. We used four different statistical software packages: deltaCT, geNorm, NormFinder and BestKeeper. We report optimized reference gene pair combinations for studying development (early whole embryos), brains at later stages (metamorphosis and adult), and thyroid signalling. These reference gene pairs are suitable for studying different aspects of X. laevis development and organogenesis.
Introduction
Several features make the African clawed frog, Xenopus laevis, an outstanding tool in biomedical research and vertebrate development. Since the 1930s, they have been used by doctors as a simple test for pregnancy. Egg production in female frogs is stimulated by the chorionic gonadotropin hormone found in pregnant woman’s urine. Biologists have utilized this same method to induce female frogs to lay eggs and then fertilise in vitro for synchronised development1. In turn, the synchronised development has been indispensable for developmental biologists to study regulatory and interactions networks that direct embryogenesis. The embryos develop externally and are free from direct maternal influences. This allows for embryos to be easily manipulated e.g. microinjections for genetic knockouts and knockdowns, germ layer dissections, tissue transplantations, etc. Rapid embryo growth means that a tadpole has fully functional set of organs within a couple of days. Coupled with a high number of brood size, X. laevis allows for large-scale genetic and chemical screens. Finally, the X. laevis genome has been fully sequenced and showed a remarkable structural similarity with the human genome2,3.
Real time quantitative polymerase chain reaction (RT-qPCR) is a widely popular technique for relative gene expression analysis, particularly during development. Reference genes within a RT-qPCR serve as internal controls for standard correction or normalisation4. Ideal reference genes should be stable throughout the diverse experimental conditions. Their expression is expected to be under different regulatory mechanisms/pathways than the ones being investigated. However, no single reference genes have been shown to be universally constant during development. Furthermore, many studies have shown that traditionally used popular housekeeping genes used to normalize gene expression vary considerably under different conditions5–8. There is currently only one study reporting the expression of reference genes for X. laevis 7 and another for its close relative, Xenopus tropicalis 8. In both cases, the reference genes characterised were the top most commonly used orthologues of genes found stably expressed in mammal tissues or were identified by systematic comparisons with traditional reference genes in mammals. These include the genes, glyceraldehyde-3-phosphate dehydrogenase (gadph), beta-actin (actb), H4-histone protein (h4), elongation factor eEF-1 alpha (eef1a1), and ornithine decarboxylase (odc1). However, several reports demonstrate that these choices can lead to inadequate normalization of gene expression. gadph, actb and h4 have been shown to be differentially expressed during the pre- and post-natal periods in mammals9–11. The latter two, eef1a1 and odc1, also exhibit varying expression levels before and after the mid-blastula (MBT) stage (shown below). There is, therefore, a need for identification and validation of alternative reference genes.
Deep RNA sequencing (RNA-seq) has become a powerful tool in high-throughput transcriptomic studies with its high resolution, large data set, and sensitivity. Recently, this technique has been used to identify novel reference genes for model systems such as the human cell cultures, zebrafish, mice and plants12–15. In this study, we used the recent RNA-seq data from Session et al., to identify the most stably expressed genes during X. laevis development3. Their expression stability was further validated using RT-qPCR. We used four statistical algorithms, delta-CT16, geNorm17, BestKeeper18 and NormFinder19. According to their stability, we identified the best reference genes candidates during different stages of X. laevis development. We further identified and validated the reference gene candidates for stage NF48 X. laevis brains when studying thyroid hormone signalling and its disruption.
Material and Methods
Animal husbandry and collection
The Xenopus laevis strains (outbred, wildtype, Centre de Ressources Biologiques Xénopes Rennes) were maintained in accordance with institutional and European guidelines (2010/63/UE Directive 2010). All procedures and experiments performed were approved and in accordance by the local ethic committee (Museum National d’Histoire Naturelle, Project approval N°68.039). X. laevis eggs were obtained from females after injection with 500–800 units of human chorionic gonadotrophin hormone, hCG (MSD Santé Animale, France). Tadpoles were obtained by in vitro fertilisation and “de-jellied” using 2% cysteine solution (pH 7.8) in Marc’s Modified Ringer’s (MMR, 0.1 M NaCl, 2.0 mM KCl, 1 mM MgSO4, 2 mM CaCl2, 5 mM HEPES (pH 7.8), 0.1 mM EDTA). The animals were reared in 0.1x MMR (pH7.4) at 23°C. The tadpoles were staged according to the Xenopus table of development and collected20.
Early stage embryos including unfertilised oocytes (NF0), NF1, NF10, NF21, NF24, NF37, NF41 and NF50 were anesthetized with 0.01% MS-222, rinsed with sterile water, placed in 1.5 ml Sorenson tubes containing 100 μl lysis buffer (provided in RNAqueous micro kit (Ambion)) 3 embryos per tube, flash frozen in liquid nitrogen and stored at −80 °C until RNA extraction. For brain collection at stages, NF41, NF50, NF54, NF56, NF57, NF61 and NF66, the tadpoles were anesthetised in 0.01% MS-222, rinsed with sterile water, and their brains dissected on ice under sterile RNase-free conditions. A pool of 3 brains were placed in 1.5 ml Sorenson tubes containing 100 μl lysis buffer (provided in RNAqueous micro kit (Ambion)), flash frozen in liquid nitrogen and stored at −80 °C until RNA extraction. For each developmental stage, three biological replicates were collected.
Screening for thyroid signalling alterations with T3 (Sigma-Aldrich, Saint-Quentin Fallavier, France), NH3 (T3 antagonist synthesized by AGV Discovery (France)21 or Triclosan (Sigma-Aldrich), was carried out as previously described22. In summary, fifteen tadpoles were placed per well in 6 well plates (TPP Switzerland), containing either control solvent (DMSO) or chemical (T3, NH3 or Triclosan). DMSO concentration was 0.01% in all treatments. Plates were placed at 23 °C for 3 days. The water (and solutions) were renewed every day, at regular 24 h intervals. At 72 hours, brains were dissected for RNA extraction as described above.
RNA extraction and cDNA synthesis
The RNA was extracted using the RNAqueous™-Micro Total RNA Isolation Kit (AMBION, ThermoFischer) using manufacturer’s instructions. In summary, the collected embryos were homogenised for 1 min at 30 Hz with a tissue disruptor, TissueLyser II (Qiagen, Netherlands). Lysis solution (provided in RNA extraction kit) was added to homogenised mix and vortexed. Samples were centrifuged at 12,000 g for 10 min at 4°C. The sample/lysate was passed through the Micro Filter Cartridge (RNA extraction kit), washed 3 times and eluted with 10 µl of pre heated elution solution. 1/10 of 10x DNase 1 buffer and 1 µl of Dnase 1 (provided in RNA extraction kit) was added to RNA elution and incubated for 20 mins at 37°C. 1/10 volume of DNase inactivation reagent (RNA extraction kit) was added and incubated for 2 mins at room temperature. The RNA elution was centrifuged for 90 secs at max speed to pellet the DNase Inactivation reagent, RNA transferred to RNase-free tubes and stored at −80°C until use. RNA concentrations were determined using a spectrophotometer (NanoDrop ThermoScientific, Rockford, IL) and RNA quality verified using BioAnalyzer (Agilent) where we only used samples with RNA Integrity Number (RIN) >7.5.
500 ng of RNA was used for reverse transcription using a High Capacity cDNA RT kit (Applied BioSystem, Foster City, CA) using manufacturer’s instructions in a total 20 µl reaction. The random primers used ensure that the first strand synthesis includes total RNA, including mRNA and rRNA. The cDNAs were stored at −20°C until use.
Primer design
RT-qPCR primers for the novel candidate reference genes were designed using Primer3plus23 (Supplementary table 1). Primers for eef1a1.S, odc1.L, dio1, dio2, dio3, thrα (tr-alpha), and thrβ (tr-beta) were used from previously published data22,24. The primers were designed to target the exon-exon region where possible to avoid genomic DNA amplification. The specificity of each primer set was checked used Xenbase Blast25 and by manual alignment of the small and large copy of both genes. Transcript information of genes was based on Xenopus laevis 9.1 genome primary transcripts by JGI. Primers were ordered from Eurofins, Germany. Melting curves were analysed to ensure amplification specificity and null primer-dimer formation. Primer PCR efficiency was evaluated using serial dilutions of cDNA sample (1:10, 1:100, 1;1,000 & 1:10,000) in Expression Suite Software (Applied Biosystem). The amplification efficiency (E) of the primers are; clta.L (93.599%), cfl1.S (102.668%), mlf2.S (104.01%), tm9sf4.L (93.713%), lpcat3.S (121.422%), slc35b1.L (97.972%), mtch2.L (100.694%), ube2m.S (91.468%), ppp1ca.L (92.59%), sub1.L (97.972%), mcts1.L (116.719%), ralb.S (107.707%), cox7b.S (96.615%), and prcp.S (95.233%). The correlation coefficients (R2) of the standard curve varied from 0.932 to 0.996. For full primers sequences, please refer to Supplementary Table 1.
RT-qPCR and data analysis
Real time quantitative PCR (RT-qPCR) was carried out using QuantStudio 6 flex (Life technologies) on 384 well-plates, with a standard reaction per well containing 1/20 diluted cDNA as template (1 μl per well) plus 5 μl of mix (Power SyBR mix, Applied BioSystem). The RT-qPCR reaction for each sample conducted in duplicates (technical replicate). Water and no-template controls were used as negative controls for each primer set.
The RT-qPCR data were analysed using the QuantStudio™ 6 and 7 Flex Real-Time PCR System (Life technologies). Cycle threshold (Ct) values were obtained using auto baseline and applied to all amplicons of the same primer set. Duplicates with the Ct value different of more than 0.5 were not considered and removed from analysis. qBase+ 2.6 was used for geNorm analysis. BestKeeper, NormFinder and delta-CT values were calculated using respective excel sheets provided by the authors16–18.
Data analysis
The RNA-seq data set was obtained from Sessions et al., 20163. Average TPM (transcript per million) value of the 14 developmental stages of individual gene was calculated. Standard deviation (SD) between the 14 developmental stages of each individual genes was then calculated. The genes were then ranked from least deviation, among the 14 developmental stages, to highest. Only genes which had an average value of more than 50 TPM were selected (see Supplementary Dataset 1 & 2, and Fig. 1). The Heatmap, box plots, and line graphs were generated using GraphPad Prism 7.0. Relative concentrations of cDNA for analysing relative changes in gene expression were calculated using 2−ΔΔCt method32.
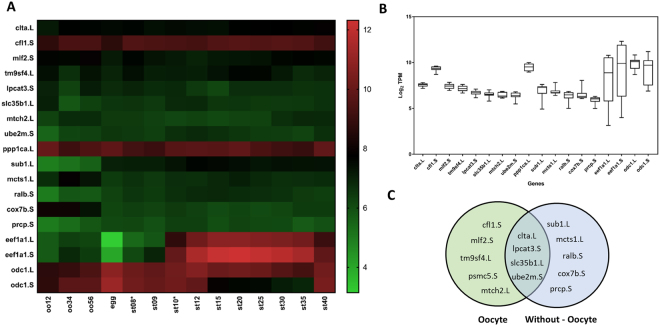
Figure 1.
RNA-seq expression of 14 candidate reference genes and four previously used reference genes. (A) Heatmap of 18 candidate genes’ total RNA expression (TPM, transcript per million) during the 14 developmental stages of Xenopus laevis development. (B) Variation of 18 candidate genes’ total RNA expression (TPM) at the different stages as a box plot. (C) Venn diagram outlining the two different classes of top ranking genes, with oocyte and without oocyte.
Results
Identification of candidate reference genes from Xenopus laevis RNA-seq data
In this study, RNA-seq data from 14 developmental X. laevis stages (including the unfertilised oocyte) by Session et al., was used to search and sort for candidate reference genes (45,831 genes)3. According to their paper, total RNA was extracted using Isogen (Nippon Gene) and the cDNA libraries were constructed using Illumina Truseq RNA sample prep kit V2 (Illumina), with the standard non-strand specific mRNA library preparation protocol. Our first step was to divide the RNA-seq data-set into two categories, with and without the oocyte stage (Fig. 1C). The genes in the two data sets were ranked and sorted according to two main criteria. First, a minimum mean expression across the stages was defined to be no lower than 50 TPM (transcripts per millions of read). Second, the genes were ranked according the least variation in expression in TPM among the different stages (see material and methods). Significant different rankings were observed for top genes for the two different data-sets (Table 1 and Supplementary Figure 1). We then selected the top 14 overlapping candidate genes for comparative expression analysis (Table 1 and Fig. 1). Note, X. laevis is an allotetraploid and contains two copies of certain genes (large (L) and small (S) chromosomes. We therefore differentiated and selected for one copy of the gene according to its ranking.
Table 1.
Table of the 14 candidate reference genes and four previously used reference genes.
| Gene | Name | Rank (Without Oocyte) | Rank (Oocytes) | Function |
|---|---|---|---|---|
| clta.L | Clathrin light chain A | 1 | 1 | Main structural component of the lattice-type cytoplasmic face of coated pits and vesicles which entrap specific macromolecules during receptor-mediated endocytosis |
| sub1.L | SUB1 Homolog, Transcriptional Regulator | 2 | 588 | General coactivator that functions cooperatively with TAFs and mediates functional interactions between upstream activators and the general transcriptional machinery |
| mcts1.L | Malignant T-Cell Amplified Sequence 1 | 3 | 135 | Anti-oncogene that plays a role in cell cycle regulation; decreases cell doubling time and anchorage-dependent growth; shortens the duration of G1 transit time and G1/S transition |
| lpcat3.S | Lysophosphatidylcholine Acyltransferase 3 | 4 | 7 | Involved in the pathway phospholipid metabolism, which is part of Lipid metabolism |
| ralb.S | RAS Like Proto-Oncogene B | 5 | 28682 | GTP-binding protein that belongs to the small GTPase superfamily and Ras family of proteins |
| slc35b1.L | Solute Carrier Family 35 Member B1 | 6 | 8 | A nucleotide sugar transporter |
| ube2m.S | Ubiquitin Conjugating Enzyme E2 M | 7 | 10 | Encodes a member of the E2 ubiquitin-conjugating enzyme family |
| cox7b.S | Cytochrome C Oxidase Subunit 7B | 8 | 3881 | Terminal component of the mitochondrial respiratory chain, catalyzes the electron transfer from reduced cytochrome c to oxygen |
| prcp.S | Prolylcarboxypeptidase | 9 | 44 | Member of the peptidase S28 family of serine exopeptidases. An activator of the cell matrix-associated prekallikrein |
| cfl1.S | Cofilin 1 | 19 | 3 | Polymerize and depolymerize F-actin and G-actin in a pH-dependent manner. Involved in the translocation of actin-cofilin complex from cytoplasm to nucleus. |
| mlf2.S | Myeloid Leukemia Factor 2 | 21 | 4 | Unclear |
| tm9sf4.L | Transmembrane 9 Superfamily Member 4 | 49 | 5 | Associates with proteins harbouring glycine-rich transmembrane domains and ensures their efficient localization to the cell surface |
| mtch2.L | Mitochondrial Carrier 2 | 58 | 9 | Nuclear-encoded transporter, localized in the inner mitochondrial membrane. Thought to play a regulatory role in adipocyte differentiation and biology |
| odc1.L | Ornithine Decarboxylase 1 | 241 | 371 | Rate-limiting enzyme of the polyamine biosynthesis pathway which catalyzes ornithine to putrescine |
| eef1a1.S | Eukaryotic Translation Elongation Factor 1 Alpha 1 | 3833 | 2580 | Responsible for the enzymatic delivery of aminoacyl tRNAs to the ribosome |
| eef1a1.L | Eukaryotic Translation Elongation Factor 1 Alpha 1 | 3911 | 2690 | Responsible for the enzymatic delivery of aminoacyl tRNAs to the ribosome |
| odc1.S | Ornithine Decarboxylase 1 | 3925 | 2677 | Rate-limiting enzyme of the polyamine biosynthesis pathway which catalyzes ornithine to putrescine |
The rank of each candidate gene was calculated by the least variation of expression in transcript per million (TPM) during mean expression <50 TPM between the 14 developmental stages from RNA-seq data.
In addition, both copies of the two commonly used developmental reference genes eef1a and odc1 were selected and ranked (Table 1 and Fig. 1). Compared to the top 14 candidate reference genes, eef1a1 and odc1 ranked much lower (<2000) compared to candidate genes in both data-sets (Table 1). Expression heatmap of the 14 candidate genes revealed stable and consistent expression throughout the stages (Fig. 1A,B). In comparison, eef1a1 and odc showing varying expression during the pre-and post mid blastula stages (Fig. 1A,B).
Expression profile of candidate reference genes
Different rankings were observed for the isoforms of the candidate reference genes i.e. large vs small chromosome (Supplementary Table 1). Due to this, primer design for RT-qPCR was limited to just one copy of the gene (see material and methods). The performance of each primer pair was tested using a pool of cDNA for different stages and organs from the total RNA (see material and methods).
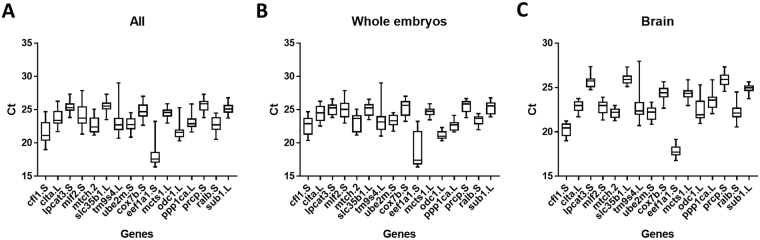
The expression profiles candidate reference genes during the different stages of X. laevis development and brain were investigated. The expression levels of the candidate reference genes were determined by Ct (cycle threshold) values through RT-qPCR (Fig. 2). For each stage, the RT-qPCR was plotted with and without oocytes (Fig. 2 and Supplementary 2). For brain, the metamorphic stages from NF 41 onwards until Juvenile were used. Different ranges of the Ct values were found for all genes with varying standard deviations (Fig. 2 and Supplementary 2). In whole stages with oocytes, odc1.L had the least deviation (21.16 ± 0.5408) while eef1a1.S had the most (18.76 ± 2.596) (Supplementary Fig. 2A). In all stages without oocytes, odc1.L had the least deviation (21.08 ± 0.535) while eef1a1.S had the most (18.12 ± 2.085) (Supplementary Fig. 2A). In metamorphic brains, sub1.L had the least deviation (24.93 ± 0.4564) while tm9sf4.L had the most (22.64 ± 1.488) (Supplementary Fig. 2A).
Figure 2.
Ct values of 16 reference genes during different developmental stages. Variation of 16 candidate genes’ RNA expression (Ct) assessed using RT-qPCR in (A) whole embryos during early developmental stages and brain during metamorphic stages, (B) whole embryos during early developmental stages and in (C) brain during metamorphic stages.
Expression stability of candidate reference genes
To evaluate stability of expression of the candidate reference genes, we initially used geNorm17. The geNorm algorithm is one of the most commonly used statistical analysis for ranking reference genes. It is based on the expression stability “M” value, derived from the average pairwise variation of a potential reference gene set with all other genes under investigation. Therefore, lower the M value, the higher the expression stability of the gene. We classified the various whole embryo and brain development stages into ten different developmental series (Fig. 3A and B). Series A encompasses stages from unfertilized eggs (NF0) until premetamorphic (NF50). Series B differs from Series A by exclusion of unfertilised eggs (NF0). Series C, D and G highlight developmental windows starting from unfertilised and fertilised eggs, and gastrulation (NF21) respectively and ending prior to thyroid gland formation (NF41). Series E focusses NF1 until gastrulation NF10 whereas series F and H are the stages adjacent to before and after the thyroid gland formation. Series AA and AB correspond to brain tissues during different developmental periods i.e. AA; Brain tissue from early developmental period, metamorphosis and Juvenile (NF41–NF66), and AB; Brain tissue from metamorphic and[H1] juvenile stages (NF50–NF66).
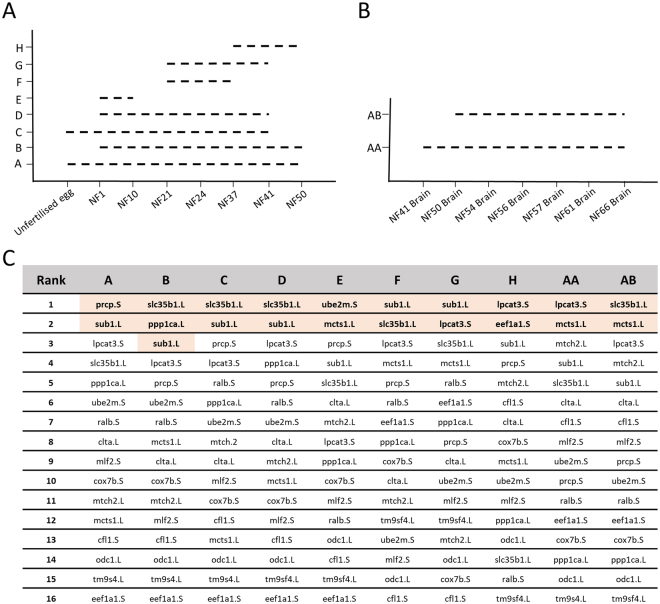
Figure 3.
Stability of genes during different developmental periods. Gene expression and stability calculated using geNorm (qBase+). Different developmental series in (A) whole embryos during early developmental stages and (B) brain during metamorphic stages. The significance of the different developmental series are, A; whole embryo developmental stages including unfertilised egg (NF0–NF50), B; whole embryo developmental stages (NF1–NF50), C; whole embryo developmental stages prior to thyroid gland formation including unfertilised egg (NF0–NF41), D; whole embryo developmental stages prior to thyroid gland formation (NF1–NF41), E; whole embryo developmental stages from 1 cell to mid blastula (NF1–NF10), F; whole embryo developmental stages after gastrulation and prior to thyroid gland formation (NF21–NF37), G; whole embryo developmental stages after gastrulation and thyroid gland formation (NF21–NF41), H; whole embryo developmental stages during thyroid gland formation (NF37–NF50), AA, Brain tissue from early developmental period, metamorphosis and Juvenile (NF41–NF66), and AB; Brain tissue from metamorphosis and Juvenile stages (NF50–NF66). (C) Different series and their corresponding genes ranked using geNorm M. Highlighted genes are minimum combination of high ranking reference genes required in the series using geNorm V.
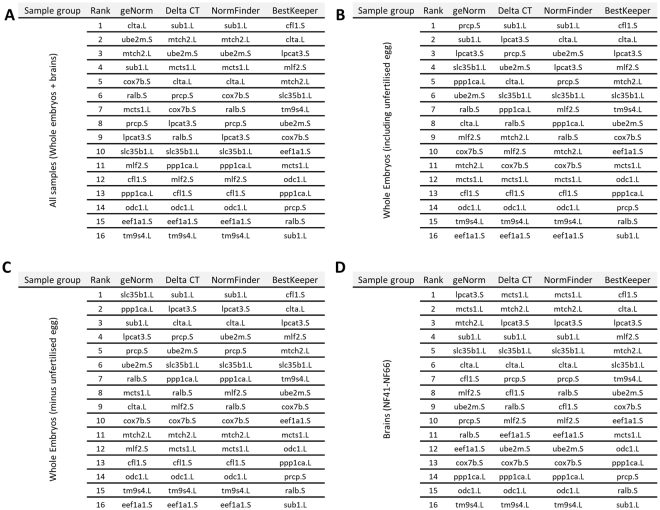
Using geNorm, we identified the top ranked candidate reference genes (Fig. 3C). Using the geNorm analysis of pairwise variation “V” value (Vn/n + 1), we determined the optimal number of reference genes required for normalisation (Fig. 3 and Supplementary 3s). The genes sub1.L and slc35b1.L, were consistently the top ranked genes for early whole embryonic stages, while lpcat1.S and sub1.L were preferable for later stages. For brain during metamorphosis, mcts1.L and slc35b1.L were the top two minimum genes required. We further ranked and compared the candidate reference genes using delta-CT, BestKeeper and NormFinder with the geNorm (Fig. 4). delta-CT ranks candidate reference genes based on their delta-CT values where the genes with lowest delta-CT values have the most stable expression16. BestKeeper evaluates each candidate reference gene in terms of its coefficient of correlation to an index (geometric mean of all candidate genes). It also calculates both the SD and coefficient of variation (CV) of the original CT values. Candidate genes with the greatest stability possess the lowest calculated variations (CV ± SD). NormFinder is a model-based approach which ranks the candidate reference genes according to their expression stability19. The expression stability is calculated from linear scale expression transformed by delta-CT method where the lowest ranking represents lowest variation and most stable expression. We observed varying rankings for the top candidate genes with the four independent algorithms (Fig. 4 and Supplementary 4).
Figure 4.
Comparison of four different statistical algorithms used to calculate reference gene stability. Four different statistical algorithms, geNorm, delta-CT, NormFinder and BestKeeper, were used to compare the gene expression and stability of the 16 candidate reference genes. Different developmental series (A) All samples including whole embryos and brain during metamorphosis, (B) whole embryos including unfertilised oocyte, (C) whole embryos without the unfertilised oocyte, and (D) brains during metamorphosis.
Expression profile and stability of candidate reference genes compared to thyroid signalling components
We further evaluated the expression stability of the candidate reference genes in NF48 tadpole brains after a short 3 day exposure to thyroid hormone (T3), its antagonist (NH3)21 and known thyroid signalling altering chemical, Triclosan26–28. At NF48 stage, the tadpole thyroid gland is just beginning its formation and all the thyroid hormone action is carried out by the maternally available thyroid hormone29. Furthermore, this period of brain development is one of the most critical period for thyroid hormone action22. While the RNA-seq data did not contain any thyroid hormone exposed animals, we compared the identified candidate reference genes’ expression profile and stability with the commonly used housekeeping genes; eef1a1.S and odc1.L. In addition, we also compared the expression profile and stability of genes known to play an important role in thyroid hormone signalling pathway such as the deiodinases (dio1, dio2 and dio3) and thyroid receptors alpha and beta (trα and trβ).
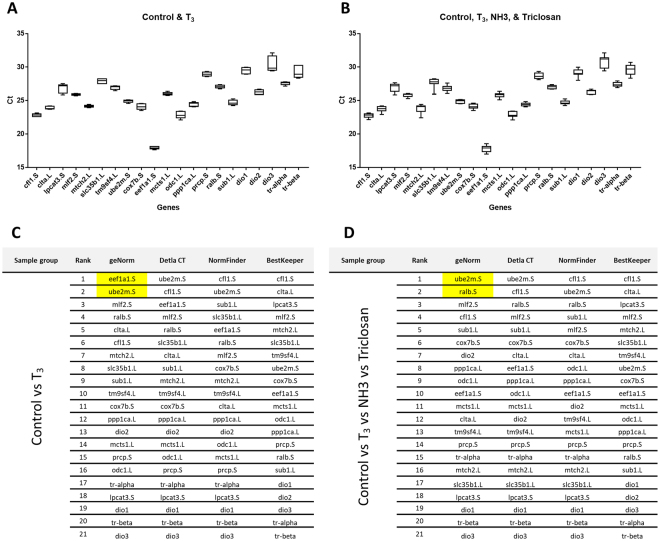
We split the experimental conditions into three main comparisons, i.e. Control vs T3, Control vs T3 vs NH3, and Control vs T3 vs NH3 vs Triclosan. As expected dio3, the inactivating enzyme of thyroid signalling, displayed the highest variation (Ct value) in all experimental conditions (30.44 ± 1.163, 30.75 ± 1.048 and 30.9 ± 0.9173 respectively) (Fig. 5 and Supplementary 5). The gene mlf2.S (Ct value) varied the least in Control vs T3 and the Control vs T3 vs NH3 exposed animals (25.9 ± 0.1287 and 25.8 ± 0.2377 respectively). Comparing controls to either T3, NH3 or Triclosan, ppp1ca.L found to vary the least (24.4 ± 0.243). geNorm algorithm ranked eef1a1.S and ube2m.S as the top ranked genes for Control vs T3 while ube2m.S and ralb.S were ranked highest for Control vs T3 vs NH3 vs Triclosan (Fig. 5C and Supplementary 5). Varying rankings were observed for the three other statistical programs in a similar fashion to the previous developmental stages.
Figure 5.
Ct values of 16 reference genes in NF48 brain exposed to thyroid (T3). Variation of 16 reference genes mRNA expression (Ct) assessed using RT-qPCR (A) Control vs T3 (B) Control vs T3 vs NH3 (T3 antagonist) vs Triclosan. Analysis using four different statistical algorithms, geNorm, delta-CT, NormFinder and BestKeeper. Series of different comparison of experimental conditions (C) Control vs T3 (D) Control vs T3 vs NH3 (T3 antagonist) vs Triclosan.
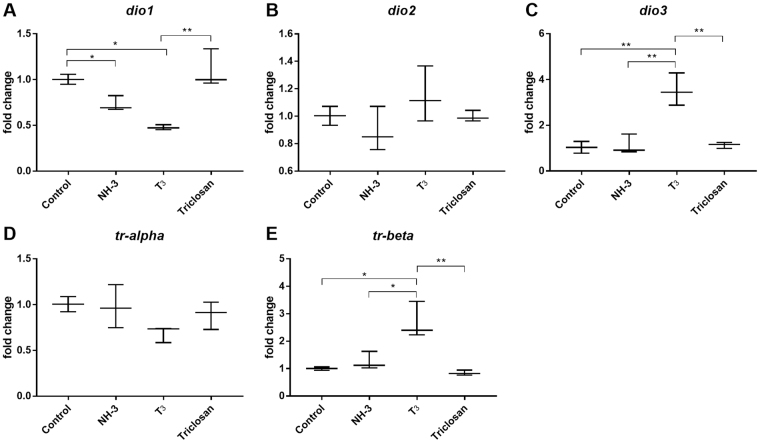
Finally, we used the two candidate reference genes identified from the Control vs T3 vs NH3 vs Triclosan group (Fig. 5D), i.e. ube2m.S and ralb.S, as normalisers and calculated the relative fold change of thyroid signalling genes (Fig. 6). As expected, altered expression levels of dio1, dio3 and tr β were observed under different experimental conditions (Fig. 6A,B and E respectively). No changes were observed for dio2 which is not surprising since dio2 is known to be regulated post translationally30,31. No change in gene expression was observed for Triclosan when compared to control. This may perhaps be due to the short exposure time (3 days) and low exposure concentration (10−7 M).
Figure 6.
Relative fold changes of thyroid signalling genes using reference genes in NF48 X. laevis brains. Results are presented as fold changes. The previously identified two candidate reference genes, ube2m.S and ralb.S, were used to normalise the expression of the thyroid signalling genes (A) dio1, (B) dio2, (C) dio3, (C) tr α, and (E) tr β. Statistics used one way ANOVA. Values represent means ± SD (n = 3); *P < 0.01, and **P < 0.001.
Conclusion
We have identified and characterised suitable reference genes for studying development (early whole embryo stages from NF0 to NF50), brains at later stages (including metamorphosis and adult), and thyroid signalling in X. laevis. We focused on identifying novel reference genes from the previously published RNA-seq data of 45,831 genes. We report various combinations of reference genes including sub1.L and slc35b1.L for early whole embryonic stages, and lpcat1.S and sub1.L, for latter whole embryos, and mcts1.L and slc35b1.L for brain during metamorphosis. These reference gene combinations will be of use to those studying early developmental networks during the different periods of embryogenesis. For NF48 brains exposed to thyroid hormone signalling and its antagonists/agonists, we report an optimal combination including eef1a1.S, ube2m.S and ralb.S. In addition, we further report the varying rankings by the four most common statistical algorithms used to characterise reference gene expression and stability. The differences in rankings are open to interpretation by the scientific community and their selection of reference genes according to their preferred statistical algorithm.
Electronic supplementary material
Acknowledgements
We thank Gérard Benisti for excellent animal care. We further thank Dr Nicolas Pollet and the European Amphibian Club 2017, Rennes for their insightful comments and feedback regarding our analysis. This work was supported by grants from Centre National de la Recherche Scientifique (CNRS), Muséum National d’Histoire Naturelle (MNHN), and European Union contract DEVCOM_GA N°607142.
Author Contributions
B.B.M., J.B.F. and B.D. designed the study. J.B.F., and B.M., were involved in all experiments. P.S. and M.L. helped carry out RNA extractions. J.B.F., B.M. and B.D. analysed data and wrote the paper. All authors discussed the results and commented on the manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-017-18684-1.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Barbara Demeneix, Email: bdem@mnhn.fr.
Jean-Baptiste Fini, Email: fini@mnhn.fr.
References
- 1.Gurdon JB, Hopwood N. The introduction of Xenopus laevis into developmental biology: Of empire, pregnancy testing and ribosomal genes. Int. J. Dev. Biol. 2000;44:43–50. [PubMed] [Google Scholar]
- 2.Hellsten U, et al. The Genome of the Western Clawed Frog Xenopus tropicalis. Science (80-). 2010;328:633–636. doi: 10.1126/science.1183670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Session AM, et al. Genome evolution in the allotetraploid frog Xenopus laevis. Nature. 2016;538:336–343. doi: 10.1038/nature19840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chervoneva I, et al. Selection of optimal reference genes for normalization in quantitative RT-PCR. BMC Bioinformatics. 2010;11:253. doi: 10.1186/1471-2105-11-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coulson DT, et al. Identification of valid reference genes for the normalization of RT qPCR gene expression data in human brain tissue. BMC Mol. Biol. 2008;9:46. doi: 10.1186/1471-2199-9-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Casadei R, et al. Identification of housekeeping genes suitable for gene expression analysis in the zebrafish. Gene Expr. Patterns. 2011;11:271–276. doi: 10.1016/j.gep.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 7.Šindelka R, Ferjentsik Z, Jonák J. Developmental expression profiles of Xenopus laevis reference genes. Dev. Dyn. 2006;235:754–758. doi: 10.1002/dvdy.20665. [DOI] [PubMed] [Google Scholar]
- 8.Dhorne-Pollet S, Thélie A, Pollet N. Validation of novel reference genes for RT-qPCR studies of gene expression in Xenopus tropicalis during embryonic and post-embryonic development. Dev. Dyn. 2013;242:709–717. doi: 10.1002/dvdy.23972. [DOI] [PubMed] [Google Scholar]
- 9.Moe TK, Ziliang J, Barathi a, Beuerman RW. Differential expression of glyceraldehyde-3-phosphate dehydrogenase (GAPDH), beta actin and hypoxanthine phosphoribosyltransferase (HPRT) in postnatal rabbit sclera. Curr. Eye Res. 2001;23:44–50. doi: 10.1076/ceyr.23.1.44.5420. [DOI] [PubMed] [Google Scholar]
- 10.Sarropoulou E, Nousdili D, Kotoulas G, Magoulas A. Functional Divergences of GAPDH Isoforms During Early Development in Two Perciform Fish Species. Mar. Biotechnol. 2011;13:1115–1124. doi: 10.1007/s10126-011-9375-6. [DOI] [PubMed] [Google Scholar]
- 11.Chambers SAM, Shaw BR. Levels of histone H4 diacetylation decrease dramatically during sea urchin embryonic development and correlate with cell doubling rate. J. Biol. Chem. 1984;259:13458–13463. [PubMed] [Google Scholar]
- 12.Hu Y, Xie S, Yao J. Identification of novel reference genes suitable for qRT-PCR normalization with respect to the zebrafish developmental stage. PLoS One. 2016;11:1–13. doi: 10.1371/journal.pone.0149277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kudo T, et al. Identification of reference genes for quantitative expression analysis using large-scale RNA-seq data of <i>Arabidopsis thaliana</i> and model crop plants. Genes Genet. Syst. 2016;91:111–125. doi: 10.1266/ggs.15-00065. [DOI] [PubMed] [Google Scholar]
- 14.Yim AK-Y, et al. Using RNA-Seq Data to Evaluate Reference Genes Suitable for Gene Expression Studies in Soybean. PLoS One. 2015;10:e0136343. doi: 10.1371/journal.pone.0136343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheung TT, Weston MK, Wilson MJ. Selection and evaluation of reference genes for analysis of mouse (Mus musculus) sex-dimorphic brain development. PeerJ. 2017;5:e2909. doi: 10.7717/peerj.2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Silver N, Best S, Jiang J, Thein SL. Selection of housekeeping genes for gene expression studies in human reticulocytes using real-time PCR. BMC Mol. Biol. 2006;7:33. doi: 10.1186/1471-2199-7-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vandesompele, J. et al. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 3, RESEARCH0034 (2002). [DOI] [PMC free article] [PubMed]
- 18.Pfaffl MW, Tichopad A, Prgomet C, Neuvians TP. Determination of stable housekeeping genes, differentially regulated target genes and sample integrity: BestKeeper - Excel-based tool using pair-wise correlations. Biotechnol. Lett. 2004;26:509–515. doi: 10.1023/B:BILE.0000019559.84305.47. [DOI] [PubMed] [Google Scholar]
- 19.Andersen CL, Jensen JL, Ørntoft TF. Normalization of real-time quantitative reverse transcription-PCR data: A model-based variance estimation approach to identify genes suited for normalization, applied to bladder and colon cancer data sets. Cancer Res. 2004;64:5245–5250. doi: 10.1158/0008-5472.CAN-04-0496. [DOI] [PubMed] [Google Scholar]
- 20.Nieuwkoop, P. D. & Faber, J. Normal Table of Xenopus Laevis (Daudin): A Systematical & Chronological Survey of the Development from the Fertilized Egg till the End of Metamorphosis. Http://Www.Xenbase.Org/Anatomy/Alldev.Do 33, (Amsterdam: North-Holland Publishing Company. Guilders, 1994).
- 21.Lim W, Nguyen N-H, Yang HY, Scanlan TS, Furlow JD. A Thyroid Hormone Antagonist That Inhibits Thyroid Hormone Action in Vivo. J. Biol. Chem. 2002;277:35664–35670. doi: 10.1074/jbc.M205608200. [DOI] [PubMed] [Google Scholar]
- 22.Fini J-B, et al. Human amniotic fluid contaminants alter thyroid hormone signalling and early brain development in Xenopus embryos. Sci. Rep. 2017;7:43786. doi: 10.1038/srep43786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Untergasser A, et al. Primer3Plus, an enhanced web interface to Primer3. Nucleic Acids Res. 2007;35:W71–4. doi: 10.1093/nar/gkm306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dubois GM, et al. Deiodinase activity is present in Xenopus laevis during early embryogenesis. Endocrinology. 2006;147:4941–4949. doi: 10.1210/en.2006-0609. [DOI] [PubMed] [Google Scholar]
- 25.Karpinka JB, et al. Xenbase, the Xenopusmodel organism database; new virtualized system, data types and genomes. Nucleic Acids Res. 2015;43:D756–D763. doi: 10.1093/nar/gku956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Veldhoen, N. et al. Corrigendum to ‘The bactericidal agent triclosan modulates thyroid hormone-associated gene expression and disrupts postembryonic anuran development’ [Aquat. Toxicol. 80 (2006) 217–227] (10.1016/j.aquatox.2006.08.010). Aquatic Toxicology83, 84 (2007). [DOI] [PubMed]
- 27.Crofton KM, Paul KB, DeVito MJ, Hedge JM. Short-term in vivo exposure to the water contaminant triclosan: Evidence for disruption of thyroxine. Environ. Toxicol. Pharmacol. 2007;24:194–197. doi: 10.1016/j.etap.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 28.Koeppe ES, Ferguson KK, Colacino JA, Meeker JD. Relationship between urinary triclosan and paraben concentrations and serum thyroid measures in NHANES 2007–2008. Sci. Total Environ. 2013;445–446:299–305. doi: 10.1016/j.scitotenv.2012.12.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fini JB, et al. Thyroid hormone signaling in the Xenopus laevis embryo is functional and susceptible to endocrine disruption. Endocrinology. 2012;153:5068–81. doi: 10.1210/en.2012-1463. [DOI] [PubMed] [Google Scholar]
- 30.Burmeister LA, Pachucki J, St. Germain DL. Thyroid hormones inhibit type 2 iodothyronine deiodinase in the rat cerebral cortex by both pre- and posttranslational mechanisms. Endocrinology. 1997;138:5231–5237. doi: 10.1210/endo.138.12.5602. [DOI] [PubMed] [Google Scholar]
- 31.Gereben B. The mRNA Structure Has Potent Regulatory Effects on Type 2 Iodothyronine Deiodinase Expression. Mol. Endocrinol. 2002;16:1667–1679. doi: 10.1210/mend.16.7.0879. [DOI] [PubMed] [Google Scholar]
- 32.Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.