Abstract
The SLRP opticin (OPTC) has been demonstrated to be produced and degraded in osteoarthritic (OA) human cartilage. Here, we investigated the in vivo effect of OPTC deficiency in OA cartilage. OA was induced in 10-week-old Optc −/− and Optc +/+ mice. Ten weeks post-surgery, cartilage was processed for histology and immunohistochemistry. SLRP expression was determined in non-operated mouse cartilage. OA Optc −/− demonstrated significant protection against cartilage degradation. Data revealed that in non-operated Optc −/− cartilage, expression of SLRPs lumican and epiphycan was up-regulated at day 3 and in 10-week-olds (p ≤ 0.039), and fibromodulin down-regulated in 10-week-olds (p = 0.001). Immunohistochemistry of OA mice showed a similar pattern. In OA Optc −/− cartilage, markers of degradation and complement factors were all down-regulated (p ≤ 0.038). In OA Optc −/− cartilage, collagen fibers were thinner and better organized (p = 0.038) than in OA Optc +/+ cartilage. The protective effect of OPTC deficiency during OA results from an overexpression of lumican and epiphycan, known to bind and protect collagen fibers, and a decrease in fibromodulin, contributing to a reduction in the complement activation/inflammatory process. This work suggests that the evaluation of the composition of the different SLRPs in OA cartilage could be applied as a new tool for OA prognosis classification.
Introduction
Articular cartilage degeneration is the main feature of arthritic joint diseases such as osteoarthritis (OA). The process involves the degradation of extracellular matrix components including the major macromolecules type II collagen and proteoglycans. Proteoglycans are heavily glycosylated proteins that can be divided into large hyaluronan-binding proteoglycans like aggrecan, and small proteoglycans including the small leucine-rich repeat protein (SLRP) superfamily1. The latter is divided into five classes (class I-V) of structurally and genetically related molecules2,3. Some members of this family have emerged as being capable of binding and regulating collagen fibril assembly and growth4, protecting the collagen fibers by surface coating avoiding the access of catabolic factors including proteases5,6, while others have been involved in collagen mineralization7, and complement regulation8,9.
Although not all the functions of the SLRPs have yet been elucidated, several studies demonstrate that the interruption of the normal balance through altered expression of some of them can influence the tissue properties10–12. Studies have described the rescue and/or compensation mechanisms by SLRPs when a single member is lacking or modified13–16, suggesting a role of the SLRPs in the regulation of the extracellular matrix architecture.
One of the SLRPs, opticin (OPTC; class III), first found associated with vitreous humor collagen fibrils in the eye17,18, was further found to be of interest in the human OA process19,20. Hence, our group was the first to demonstrate the OPTC expression/production in human articular cartilage and its degradation during OA19,20. Moreover, in addition to the chondrocyte, OPTC is also produced by other articular cells: synoviocytes and osteoblasts19, and in other tissues: ligaments and skin17. OPTC can also be digested by several proteases implicated in OA development20.
However, the in vivo effect of the absence of OPTC in OA cartilage remains to be clarified. We hypothesized that in vivo lack of OPTC would be detrimental to the articular cartilage structure. To this end, we investigated the effect of OPTC deficiency in OA using a mouse model.
Materials and Methods
Mice
We used a transgenic mouse in which the Optc gene has been deleted using a targeting vector that encompasses exon 1 to exon 421. For further details see Supplementary Material, Materials and Methods and Supplementary Material Fig. S1. All procedures involving animals were performed according to regulations of the Canadian Council on Animal Care and were approved by the Animal Care Committee of the University of Montreal Hospital Centre. All mice were maintained under a 12-hour light/dark cycle. Food and water were available ad libitum.
Mouse weight and length were measured on day 5 (P05) and day 16 (P16).
Surgically Induced OA Mouse Model
OA was surgically induced in 10-week-old male mice (Optc +/+ and Optc −/−) for 10 weeks by destabilization of the medial meniscus (DMM) of the right knee as described22,23. For further details see Supplementary Material, Materials and Methods.
Evaluation of knee joint swelling
The knee diameter was measured in the mediolateral plane at baseline (day 0) and every 3 days, for 15 days, using a digital caliper (model 2071M; Mitutoyo), as described24.
Extraction of total RNA, reverse transcriptase (RT) PCR, and gene expression determination
Articular cartilage from the knees of P03 and 10-week-old Optc +/+ and Optc −/− mice was processed for RNA extraction. Femoral and humeral heads were carefully dissected and placed in TRIzol (Invitrogen, Carlsbad, CA, USA). Tissue was homogenized with a Polytron homogenizer (Kinematica, Bohemia, NY, USA) and total RNA was extracted according to the manufacturer’s specifications and treated with the RapidOut DNA Removal Kit (Thermo Fisher, Waltham, MA, USA) to ensure complete removal of chromosomal DNA. This extraction method yields undegraded RNA as observed by agarose/SYBR Safe nucleic acid stain (Invitrogen) gel electrophoresis and reproducible Ct values of the housekeeping gene. Purity and quantitation of total RNA were assessed with NanoDrop (Thermo Fisher).
The RT reactions were primed with random hexamer primers and messenger RNA (mRNA) levels were quantified by real-time PCR (qPCR), performed in the GeneAmp 5700 Sequence Detection System (Applied Biosystems, Foster City, CA, USA) with QuantiTect SYBR Green PCR Master Mix (Qiagen, Valencia, CA, USA), and used according to the manufacturer’s specifications. To evaluate the effect of Optc −/−, the expression level of Optc +/+ studied genes was assigned an arbitrary value of 1, and Optc −/− cartilage expression evaluated as fold change over Optc +/+ and calculated as 2ΔΔCt. Primer efficiencies for the genes under study were the same as those for the housekeeping gene, RPL19. The primer sequences were as described in Supplementary Material Fig. S2. Data were collected and processed with Rotor-Gene Q 6 software version 6.1 (Corbett Research, Mortlake, Australia). qPCR was performed in duplicate for each sample and gene studied.
Sample collection and preparation
DMM and sham operated mice were euthanized at 10 weeks after surgery and the non-operated mice at 20 weeks old. Right knee joints were dissected free of tissue.
Histology
Samples were fixed in 4% paraformaldehyde, pH 7.4 for 16 hours at 4 °C (Sigma-Aldrich, Oakville, ON, Canada), decalcified in 10% EDTA pH 7.3 for 12 days (Wisent, St-Bruno, QC, Canada), and embedded in paraffin, as described25. Sections (5 µm) were deparaffinized in xylene followed by a graded series of alcohol washes prior to staining. Sections were stained with Safranin O–fast green (Sigma-Aldrich, Oakville).
The severity of the OA cartilage lesions was determined using the Osteoarthritis Research Society International (OARSI) scoring method25.
Subchondral bone plate histomorphometry was done on the medial compartment as described23,26 using a Leitz Diaplan microscope coupled to a personal computer as above using three Safranin-O/fast green stained sections. To measure the subchondral bone plate thickness, the bone volume/total volume ratio (%BV/TV) and trabecular thickness, a box with a fixed width (1,000 μm) and variable length was created with the upper limit at the calcified cartilage–subchondral bone junction and the lower limit at the subchondral bone–trabecular bone junction. The mean distance between the upper and lower limit was calculated automatically by the software as well as the trabecular thickness.
Histomorphometric quantitative analysis of the anterior synovial membrane thickness was performed by capturing images at 63X with a Leitz Diaplan microscope (Leica Microsystems, Wetzlar, Germany) coupled to a personal computer and data determined with Bioquant OSTEO Image Analysis Software version 12.5.60 MIR (Bioquant, Nashville, TN); data are expressed as micrometers.
Collagen organization
Cartilage collagen organization was evaluated on 5 µm paraffin sections following picrosirius red staining, as described27. In brief, each slide was evaluated under polarized light microscopy using a scale of 0–2, where 0 = no birefringence, completely disorganized collagen; 1 = birefringence on 25%-50% of the total cartilage thickness, collagen partially disorganized; and 2 = bright birefringence on more than 50% of total cartilage thickness, organized collagen network. Three areas for each slide were evaluated and the scores summed (maximum score 6).
Immunohistochemistry
Immunohistochemical analysis was performed on 5 µm paraffin sections as described24. The primary antibodies were against epiphycan, fibromodulin, lumican, opticin, C5b-9, CCL2, C terminal peptide of aggrecan G1 domain (VDIPEN), type II collagen primary cleavage site (Col2-3/4Cshort), type X collagen, MMP-3, and MMP-13. For further details see Supplementary Material, Materials and Methods. After incubation with an anti-rabbit IgG secondary antibody, slides were incubated with Vectastain ABC kit (Vector Laboratories, Burlingame, CA, USA) following the manufacturer’s instructions. The color was developed with 3,3′-diaminobenzidine containing hydrogen peroxide, and slides were counterstained with methyl green.
Control procedures were performed according to the same experimental protocol as follows: (1) omission of the primary antibody, (2) substitution of the primary antibody with a non-specific IgG from the same host as the primary antibody (Santa Cruz Biotechnology), and (3) a third control for type X collagen was performed by adsorption with the peptide YNRQQHYDPRSGIFTCKIPGIYYFSYGGC (provided by Dr. E. Lee, Shriners Hospital for Children, McGill University Hospital Centre, Montreal, Quebec, Canada) at 10-fold. Controls only showed background staining.
For each specimen, positive cells were quantified as previously described23. In brief, for each section, 1 microscopic field at 250X captured with a Leitz Diaplan microscope (Leica Microsystems) was assigned and chondrocytes staining positive were quantified following the determination of the total number of cells and of those staining positive for the antigen. Final results were expressed as the percentage of cells staining positive for the antigen with the maximum score being 100% for the entire cartilage, except for lumican which was expressed according to the upper (first half) or deep (second half) cartilage zone.
For type II collagen (Col2-3/4Cshort antibody) and VDIPEN, the matrix staining was assessed as follows. For type II collagen staining, images were captured at 250X with a Leitz Diaplan microscope connected to BIOQUANT OSTEO II Image Analysis software23. Surface area of positively stained extracellular cartilage matrix was measured and data expressed as % of positive stained area over total area. VDIPEN staining was graded on a scale of 0–3, where 0 = no staining; 1 = minor staining; 2 = marked staining; and 3 = maximal staining, as described previously23. Each slide was examined and scored by 2 independent observers who were blinded to group allocation.
Transmission electron microscopy (TEM)
Right knees from 10-week-old Optc +/+ and Optc −/− mice were used. For further details see Supplementary Material, Materials and Methods. Ultra-thin (100 nm) sections from the zone of interest were obtained using a microtome (Leica Microsystems UCT ultramicrotome, Vienna, Austria) and coated with 1–2 nm platinum. From each specimen, images were taken at magnification X1200 and X30000.
Statistical analysis
Statistical significance was assessed by the Mann-Whitney and one sample t test where P values < 0.050 were considered significant.
Data availability
The datasets generated and/or analysed during the current study are available from the corresponding author on reasonable request. Data generated or analysed during this study are included in this published article and its Supplementary Material files or are noted as data not shown.
Results
Confirmation of the lack of Optc expression in Optc−/− mice
Confirmation of the lack of Optc expression in the Optc −/− mouse cartilage was performed using genomic DNA from 10-week-old mice. The signal observed at 350 bp in the samples obtained from Optc −/− mice confirmed the deletion in the region studied. Furthermore, expression of Optc mRNA extracted at day 3 (P03) from articular cartilage and processed for qPCR analysis as well as immunohistochemistry of 20-week-old DMM mice were done for the confirmation of OPTC protein deficiency in the cartilage. Results showed no signal in the chondrocytes or the extracellular matrix, suggesting the absence of OPTC (Supplementary Material Fig. S3).
Mouse morphometric assessment
No significant differences were observed in growth (weight and length) between Optc −/− and Optc +/+ mice as reported21 (Supplementary Material Fig. S4).
Knee joint swelling
Data showed that the initial joint swelling (day 3 post-surgery) receded similarly in both Optc −/− and Optc +/+ mice (Supplementary Material Fig. S5).
Optc−/− mice exhibited a protective effect on cartilage degradation
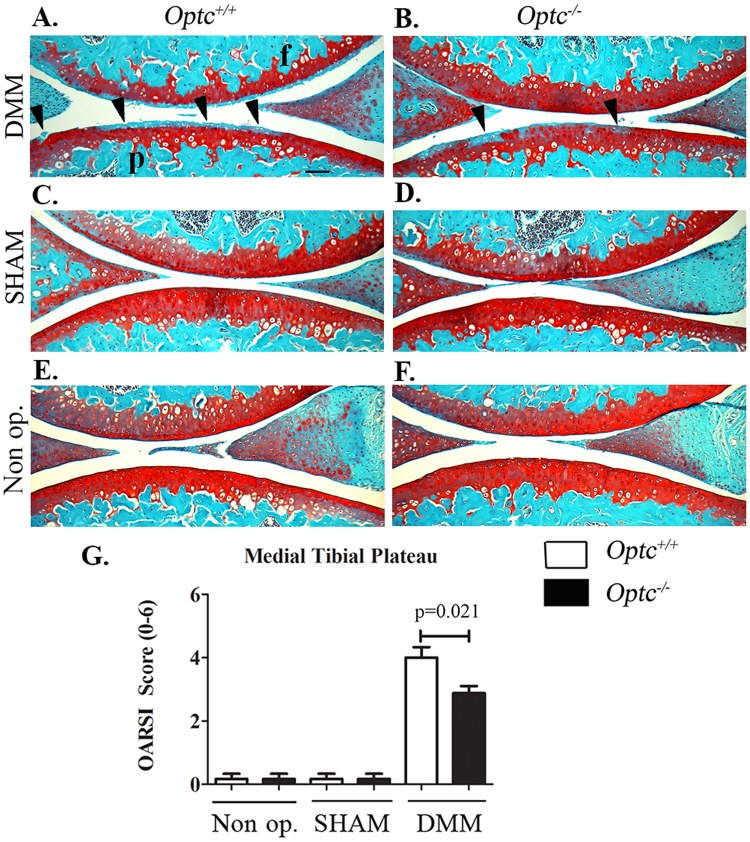
The cartilage integrity in the medial tibial plateau in non-operated, sham and DMM (OA) mice was assessed histologically (Fig. 1). No significant differences among the control groups (20-week-old non-operated and sham) of Optc −/− and Optc +/+ mice were found (Fig. 1). Comparison between DMM-Optc +/+ and -Optc −/− mice (Fig. 1A,B) revealed a decreased loss of cartilage integrity in the Optc −/− mice, including Safranin O–fast green staining, cellularity, thinning of the cartilage, and fibrillation. These observations were corroborated by the OARSI scores (Fig. 1G), which showed significantly lower histologic scores in the DMM-Optc −/− mice (p = 0.021).
Figure 1.
Histology of articular cartilage. Photomicrographs of representative histological sections of the (A,p) medial tibial plateaus and (A,f) femoral condyles obtained from (A,C,E) opticin Optc +/+ and (B,D,F) Optc −/− mice at 10 weeks after (A,B) DMM, (C,D) sham (control) surgery, and (E,F) non-operated (Non op.; control). Black arrowheads in (A and B) indicate areas of fibrillation, with loss of cartilage ultrastructure. Bar in (A) = 100 μm. Original magnification X100. (G) Representative histograms of the Osteoarthritis Research Society International (OARSI) scores in the medial tibial plateau. Values are the mean ± SEM of Non op. (Optc +/+, n = 4; Optc −/−, n = 7), sham (n = 6/group), DMM-Optc +/+ (n = 9) and DMM-Optc −/− (n = 8). p values were determined by Mann-Whitney test; only significant p values are shown except for those between Non op./sham with respective DMM groups in medial tibial plateau which are < 0.010.
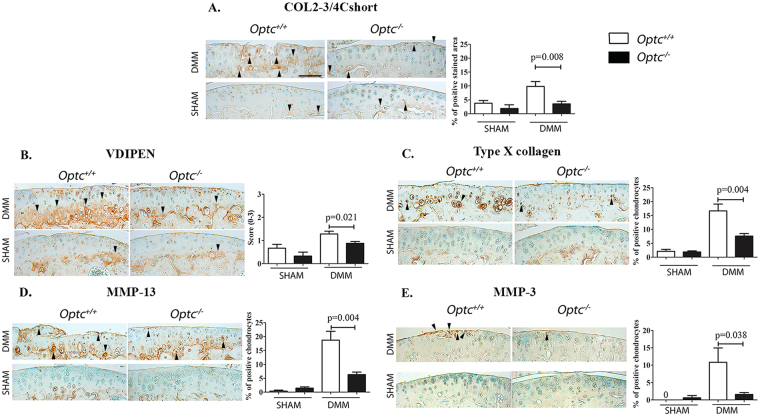
Less cartilage degradative markers in DMM-Optc−/−
According to the histology, DMM-Optc −/− compared to -Optc +/+ mice demonstrated significantly reduced levels of α1 chain of type II collagen (Col2-3/4Cshort; p = 0.008) (Fig. 2A); aggrecan degradation products (VDIPEN; p = 0.021) (Fig. 2B); type X collagen (p = 0.004) (Fig. 2C), MMP-13 (p = 0.004) (Fig. 2D) and MMP-3 (p = 0.038) (Fig. 2E).
Figure 2.
Immunohistochemistry of articular cartilage 10 weeks after DMM surgery. Representative sections and histograms of the percentage of positive chondrocytes or matrix staining of cartilage degradation markers in medial tibial plateaus (A–E) from Optc +/+ and Optc −/− mice at 10 weeks after sham (control) and DMM surgery, counterstained with methyl green. Black arrowheads indicate stained area (A,B) or positive-stained cells (C,D,E). Bar in (A) = 100 μm. Original magnification X250. Values are the mean ± SEM of sham (control) (n = 6/group), DMM-Optc +/+ (n = 9) and DMM-Optc −/− (n = 8). p values were determined by Mann-Whitney test; only significant p values are shown except for those between sham and their respective DMM groups which are < 0.050. Negative controls are shown in Supplementary Material Fig. S10.
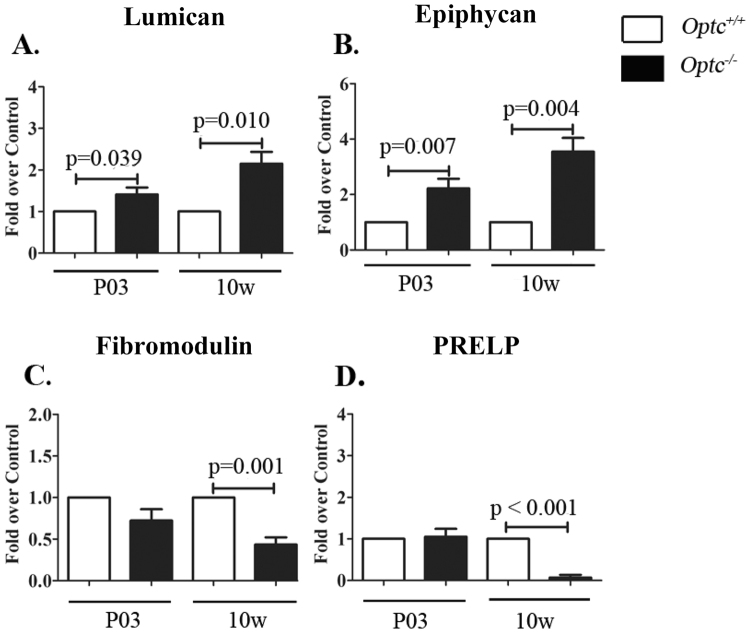
Optc−/− present different SLRP expression/production in articular cartilage
Analysis of the expression (mRNA) of SLRP members from each class revealed that biglycan (class I), osteomodulin (class II), osteoglycin (class III), tsukushi and nyctalopin (class IV), and podocan (class V) expression showed no significant difference between non-operated Optc +/+ and Optc −/− mice at either day 3 (P03) or at 10 weeks of age in articular cartilage (data not shown). Interestingly, lumican (class II) (Fig. 3A) and epiphycan (Fig. 3B), a member of the same class as OPTC (class III), were significantly overexpressed at both P03 (p ≤ 0.039) and at 10 weeks of age (p ≤ 0.010) in Optc −/− compared to Optc +/+ mice. Moreover, compared to Optc +/+, in Optc −/− mice fibromodulin (class II) (Fig. 3C) and PRELP (class II) (Fig. 3D) had similar values at P03, but were significantly down-regulated at 10 weeks of age (p < 0.001) (Fig. 3C,D).
Figure 3.
Expression of small leucine-rich proteoglycans (SLRPs) in articular cartilage. Relative gene expression (mRNA) (A–D) in articular cartilage in day 3 (P03) and 10-week-old (10 w) Optc +/+ and Optc −/− mice. Values are the mean ± SEM of (n = 6/group) mice. p values were determined by one-sample t test with Optc +/+ value of 1.
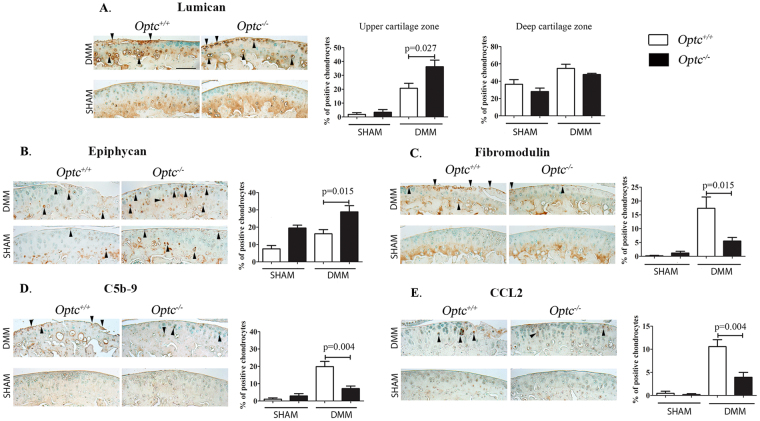
Complementary analysis comparing cartilage immunohistochemistry of DMM-Optc +/+ and DMM-Optc −/− mice showed significantly increased levels in chondrocyte staining of lumican in the upper cartilage zone (p = 0.027) (Fig. 4A) and epiphycan (p = 0.015) (Fig. 4B), but a significant decrease for fibromodulin (p = 0.015) (Fig. 4C), concurring with the expression findings (Fig. 3). On the other hand, PRELP levels did not show differences between DMM-Optc −/− and -Optc +/+ mouse cartilage (data not shown).
Figure 4.
Immunohistochemistry of articular cartilage 10 weeks after DMM surgery. Representative images and histograms of the percentage of positive chondrocytes of SLRPs (A–E) from Optc +/+ and Optc −/− mice at 10 weeks after sham (control) and DMM surgery, counterstained with methyl green. Controls performed with non-specific IgG and in addition for type X collagen, by adsorption with the peptide are shown in Supplementary Material Fig. S10. Black arrows indicate positive-stained cells. Bar in (A) = 100 μm. Original magnification X250. Values are the mean ± SEM of sham (n = 6/group), DMM-Optc +/+ (n = 9) and DMM-Optc −/− (n = 8). p values were determined by Mann-Whitney test; only significant p values are shown except for those between sham and their respective DMM groups which are < 0.010 except for epiphycan and C5b-9 between sham and DMM-Optc −/−. Negative controls are shown in Supplementary Material Fig. S10.
As fibromodulin contributes to an increase in the complement activation/inflammatory process, we further looked at the levels of two factors of the complement activation, C5b-9 and CCL2. Data revealed that for both factors, DMM-Optc −/− mice demonstrated a significantly decreased level of positive chondrocytes (p = 0.004) compared to -Optc +/+ mice (Fig. 4C,D).
Collagen fibers of Optc−/− mice were thinner and better organized than Optc+/+mice
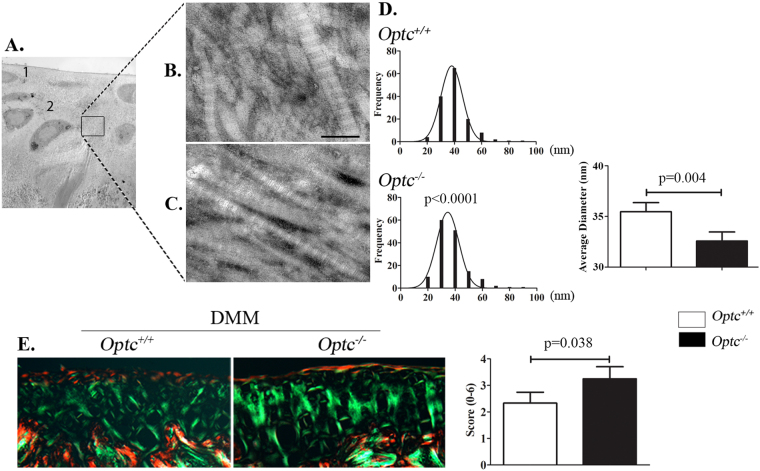
Fibril diameter distributions were analyzed by TEM in the upper and deep zones of the cartilage (Fig. 5A–C). Data showed similar findings for both zones studied and only data of the deep zone are illustrated (Fig. 5B,C). Findings revealed that Optc −/− mice had a significantly higher frequency of cartilage collagen fibers of smaller diameter compared to Optc +/+ mice (Fig. 5D). TEM images also showed that in Optc −/− mice, type II collagen fibers were more tightly packed than in Optc +/+ mice. This was further substantiated by the use of the collagen birefringence methodology (Fig. 5E), in which collagen fibers in the DMM-Optc +/+ mouse cartilage had a significantly lower birefringence intensity (p = 0.038) than in the -Optc −/− mice, indicating a higher disorganization of the collagen fibers in the Optc +/+ mice.
Figure 5.
Ultrastructure of type II collagen fibers. Representative images obtained with transmission electron microscopy (TEM) of (A) cartilage, in which 1 indicates the upper zone and 2 the deep zone, and collagen fibers from (B) Optc +/+ and (C) Optc −/− mice at 10 weeks of age. Bar in (B) = 100 μm. Magnifications: (A) X1200 and (B,C) X30000. (D) Histograms represent the frequency of each fiber’s diameter and the average diameter of Optc +/+ and Optc −/− mice. (E) Picrosirius red staining of mouse cartilage from DMM-Optc +/+ and DMM-Optc −/− mice and the histogram representing the birefringence score. Values in graphs in (D,E) are mean ± SEM. p values were determined by Mann-Whitney test.
Optc−/− effect on two other articular tissues
As OPTC was also found present in both osteoblasts and synoviocytes19, we further looked at the effect of Optc deficiency in both subchondral bone and synovial membrane. Histomophometric evaluations of the subchondral bone: plate thickness, percentage of bone volume/total volume and trabecular thickness, demonstrated similar values for the DMM-Optc +/+ and -Optc −/− mice (Supplementary Material Fig. S6). However, synovial membrane thickness comparison of DMM-Optc +/+ with -Optc −/− revealed a significant increase (p = 0.036) (Supplementary Material Fig. S7).
Discussion
Our group previously reported that OPTC is expressed and produced in articular cartilage and degraded during human OA19,20. Because SLRPs contribute to the maintenance and integrity of articular cartilage, OPTC reduction/cleavage in OA was suggested to predispose cartilage to degeneration. Here, we further investigated the in vivo effect of the lack of OPTC during OA, using an Optc-null mouse model.
Although mice with a deficiency of one or more SLRPs have been shown to develop premature OA11,13, unexpectedly and in contrast to our hypothesis, the present study showed that OA Optc-null mice exhibited a cartilage protective effect. The lack of OPTC in OA mice resulted in significantly better cartilage histology features and lower expression markers of catabolic processes. We then further explored the relevant in vivo mechanisms implicated in OA cartilage associated with the OPTC absence. Data revealed that this ensues from an overexpression of two other members of the SRLP super-family, lumican and epiphycan, combined with a down-regulation of fibromodulin, a factor involved in activation of the complement/inflammatory process. These changes are associated with a better preservation of the collagen fibril organization observed in the Optc-null mice.
Our observation of the cartilage protection in OA Optc −/− mice, together with the literature demonstrating that the lack of some members of the SLRP super-family is compensated by an overexpression of other members12,16,28,29, led us to investigate the expression of SLRPs from each class on articular mouse cartilage at birth and in adults. Data revealed that in Optc −/− mice, lumican and epiphycan are overexpressed at birth (P03) and in adults (10 weeks old), and fibromodulin down-regulated in adult mice. Such overexpression of lumican in adult mice has also been reported in the tendons when fibromodulin is absent15, and agrees with our data on the significant decrease in fibromodulin in adult Optc −/− mice. The epiphycan overexpression (SLRP belonging to the same class as OPTC) also concurs with cartilage protection during OA, as this SLRP was shown to play an important role in maintaining joint integrity and its absence (knockout mice) resulted in OA development with aging13. In addition, interaction between SLRP members has also been documented; for example, epiphycan/biglycan double-deficient mice present a more severe OA than only epiphycan knock-out mice13.
Findings of the OA Optc −/− cartilage protective effect also concur with those of the markers of the cartilage degradation, including products of type II collagen and aggrecan, as well as chondrocyte hypertrophy, MMP-3 and MMP-13, which were all significantly and markedly reduced in the DMM-Optc −/− compared to DMM-Optc +/+ mice.
SLRPs are encoded in 18 different genes clustered on 7 chromosomes, suggesting duplication to generate functional redundancy during evolution2. Interestingly, OPTC and fibromodulin share the same gene cluster, suggesting that OPTC could have a regulatory role in fibromodulin transcription18. This could explain, at least in part, the decrease in fibromodulin expression in Optc −/− mouse cartilage. In addition, it could be that fibromodulin degradation by proteases is high in OA cartilage or at least higher than other SLRPs. Indeed, previous data reported that although MMP-13, a major MMP involved in collagen degradation, is able to cleave both lumican and fibromodulin, fibromodulin appears to be the preferred substrate30.
Collagen fibrillogenesis during normal cartilage development is finely regulated and involves multiple steps. Type II collagen fibrils impart a strength and compressibility to the cartilage matrix and permit this tissue to resist large deformations in shape, allowing joints to absorb shocks. During the first steps of fibrillogenesis, a population of collagen molecules is assembled into quarter staggered arrays forming short small-diameter fibril intermediates31 (Supplementary Material Fig. S8). After the molecular assembly phase, fibrils start growing and become longer and larger in diameter and the stabilization of the assembled fibrils is mediated by interactions with fibril-associated molecules such as SLRPs29, more specifically by lumican and fibromodulin, two SLRPs differently regulated in the Optc −/− mice. Lumican and fibromodulin belong to the same SLRP subfamily (class II) and show a 47% sequence identity. Additionally, these SLRPs are similar in their post-translational modifications. Both carry tyrosine sulfate residues in their N-terminal domains and are substituted with carbohydrates in a similar manner32. They bind to the same binding site of type I collagen fibrils, although fibromodulin has a higher affinity than lumican33. Even though their binding characterization to type II collagen has not been studied, one could speculate that these two SLRPs could also compete for binding in this collagen type. Moreover, data from tendon development studies in lumican-, fibromodulin-, and double-deficient mice suggest a collagen fibrillogenesis model where fibromodulin would displace lumican at the fibril maturation stage29 (Supplementary Material Fig. S8). At the beginning of the collagen fibril growth phase, lumican promotes the lateral fibril growth by fusion of intermediate subunits and, as fibrillogenesis progresses, the lumican level decreases to a barely detectable level, while the fibromodulin level increases significantly and is essential for diameter growth of the mature fiber at later stages29.
These data, together with our findings on the SLRP expression/production and the cartilage collagen analysis, and further experiments in which the Optc +/+ lumican, epiphycan and fibromodulin fold change expression (mRNA) in 10-week-old mice over P03 mice was investigated and found down-regulated at 10 weeks old (Supplementary Material Fig. S9), are suggestive of a collagen fibrillogenesis model in the absence of OPTC (Supplementary Material Fig. S8).The decreased level of fibromodulin is translated by a compensatory overexpression of lumican over time. As a result, the collagen fibers of the Optc −/− mice are over-coated by lumican and epiphycan molecules during the fibrillogenesis process, preventing the final steps of the fibril process from developing and the fibers to fuse laterally, thus remaining thinner and tightly packed, and therefore less susceptible to degradation. This scheme is strengthened by the picrosirius red data that showed that OA-Optc −/− mice maintained a better organization of the cartilage collagen network than OA-Optc +/+ mice.
In turn, the decrease in fibromodulin in Optc −/− mice could be responsible for less catabolic activity in the cartilage. Indeed, fibromodulin, in addition to its structural role in the cartilage matrix, is known to activate the classical complement pathway9. Unbalanced complement activation has been shown to play a central role in OA cartilage34. The complement system consists of pathways that converge at the formation of the C3 and C5 convertases, enzymes that mediate activation, among others, of the formation of membrane attack complex (MAC), comprising the complement effector C5b-935. The levels of C5b-9 have been found elevated in OA synovial fluid when compared to healthy individuals34,36. Interestingly, MAC co-localized with MMP-13 around human OA chondrocytes and was shown to play a critical role in the pathophysiology of this disease, in which in addition to inducing cell lysis, it increases the chondrocytes’ expression of multiple genes including MMPs, inflammatory cytokines and complement effectors34. We then looked at the effect of the lack of OPTC on two representative complement factors, C5b-9 and CCL2 (also referred to as monocyte chemoattractant protein 1 [MCP-1]), which have been found significantly increased in serum and synovial fluid of human OA patients34,36,37. Data revealed that the levels of these two factors were significantly decreased in OA-Optc −/− compared to -Optc +/+ mice contributing to a reduction in the inflammatory/catabolic processes during the disease process.
Although the hallmark of OA is the progressive degeneration of articular cartilage, it is acknowledged that the subchondral bone and synovial membrane alterations are also components of the disease process. An interdependence between subchondral bone alterations and cartilage degeneration, as well as inflammatory factors released from the synovial membrane, is gaining strong support. As data showed that OPTC deficiency imparts a protective effect on the synovial membrane thickness, but not on the subchondral bone, the question arises as to whether a protective effect on the synovial membrane alterations could also be beneficial to the cartilage in this OA mouse model. Although more research should be performed to that effect, in this OA model it is noteworthy that inflammation of the synovial membrane is not the main feature38 and that OA alterations in this tissue are more related to non-inflammatory progressive fibrosis. However, it could be that the synovial membrane preservation occurs via a direct effect of the cartilage preservation, as less degradation products will be released from the cartilage.
In summary, this is the first report showing that the in vivo lack of OPTC destabilizes the natural balance of SLRP members in the cartilage, which is translated into a protective environment in OA cartilage. Such effect is a result of different events comprising an overexpression of lumican and epiphycan expression/production at birth and over time and therefore a protection of the collagen fibers by their surface deposition, enabling the fibrils to better resist catabolic factors. In addition, the protective effect also reflects the decrease in fibromodulin, which will result in a reduction of the classical complement pathway activation during the progression of the disease, favoring production of less catabolic factors, thus less degradation.
In conclusion, although OPTC, a SLRP associated with collagen fibrils, contributes to the structural stability of cartilage, its lack leads to a protection of cartilage due to an overexpression of other SLRP members throughout adult life, thus preserving the collagen fibril from degradation, and by a reduction in the complement activation/inflammatory process in this tissue during OA through the reduction of another member. This work suggests that the evaluation of the composition of the different SLRPs in human OA cartilage could be applied as a new tool for OA prognosis classification.
Electronic supplementary material
Acknowledgements
The authors thank Drs J. S. Mort and E. Lee, formerly from Shriners Hospital for Children, McGill University Hospital Centre, Montreal, Quebec, Canada for providing VDIPEN antibody and the type X collagen control peptide, respectively, and Drs H. Vali, K. Sears and J. Mui from the Facility for Electron Microscopy Research (FEMR), McGill University, Montreal, Quebec, Canada for their help with transmission electron microscopy analysis and technical assistance. We also acknowledge Drs R. Mayne, M. Takanosu and S. Henry, formerly from The University of Alabama at Birmingham, USA for producing the Optc−/− mice. The authors are also grateful to F. Paré and F. Mineau for their expert technical support and V. Wallis for her assistance with the manuscript preparation. This study was funded in part by the Osteoarthritis Research Unit of the University of Montreal Hospital Research Centre (CRCHUM), the Chair in Osteoarthritis of the University of Montreal, and the Inflammation and Cartilage Cellular Research Group, IMIM (Hospital del Mar Medical Research Institute).
Author Contributions
A.F., L.T., H.F., J.M., P.N.B., J.P.P. and J.M.P. were responsible for the study concept and design. A.F., G.V.F. and B.L. were responsible for the acquisition of data. A.F., G.V.F. and J.M.P. performed the analysis and interpretation of the data. All authors were involved in the drafting and critical revision of the manuscript for important intellectual content.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-017-18047-w.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Roughley PJ. The structure and function of cartilage proteoglycans. European cells & materials. 2006;12:92–101. doi: 10.22203/eCM.v012a11. [DOI] [PubMed] [Google Scholar]
- 2.Schaefer L, Iozzo RV. Biological functions of the small leucine-rich proteoglycans: from genetics to signal transduction. J Biol Chem. 2008;283:21305–21309. doi: 10.1074/jbc.R800020200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huxley-Jones J, Robertson DL, Boot-Handford RP. On the origins of the extracellular matrix in vertebrates. Matrix Biol. 2007;26:2–11. doi: 10.1016/j.matbio.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 4.Kalamajski S, Oldberg A. The role of small leucine-rich proteoglycans in collagen fibrillogenesis. Matrix Biol. 2010;29:248–253. doi: 10.1016/j.matbio.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 5.Pratta MA, et al. Aggrecan protects cartilage collagen from proteolytic cleavage. J Biol Chem. 2003;278:45539–45545. doi: 10.1074/jbc.M303737200. [DOI] [PubMed] [Google Scholar]
- 6.Geng Y, McQuillan D, Roughley PJ. SLRP interaction can protect collagen fibrils from cleavage by collagenases. Matrix Biol. 2006;25:484–491. doi: 10.1016/j.matbio.2006.08.259. [DOI] [PubMed] [Google Scholar]
- 7.Mochida Y, Duarte WR, Tanzawa H, Paschalis EP, Yamauchi M. Decorin modulates matrix mineralization in vitro. Biochem Biophys Res Commun. 2003;305:6–9. doi: 10.1016/S0006-291X(03)00693-4. [DOI] [PubMed] [Google Scholar]
- 8.Sjoberg AP, et al. Short leucine-rich glycoproteins of the extracellular matrix display diverse patterns of complement interaction and activation. Mol Immunol. 2009;46:830–839. doi: 10.1016/j.molimm.2008.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Happonen KE, Heinegard D, Saxne T, Blom AM. Interactions of the complement system with molecules of extracellular matrix: relevance for joint diseases. Immunobiology. 2012;217:1088–1096. doi: 10.1016/j.imbio.2012.07.013. [DOI] [PubMed] [Google Scholar]
- 10.Chakravarti S, Zhang G, Chervoneva I, Roberts L, Birk DE. Collagen fibril assembly during postnatal development and dysfunctional regulation in the lumican-deficient murine cornea. Dev Dyn. 2006;235:2493–2506. doi: 10.1002/dvdy.20868. [DOI] [PubMed] [Google Scholar]
- 11.Ameye L, Young MF. Mice deficient in small leucine-rich proteoglycans: novel in vivo models for osteoporosis, osteoarthritis, Ehlers-Danlos syndrome, muscular dystrophy, and corneal diseases. Glycobiology. 2002;12:107R–116R. doi: 10.1093/glycob/cwf065. [DOI] [PubMed] [Google Scholar]
- 12.Tasheva ES, et al. Mimecan/osteoglycin-deficient mice have collagen fibril abnormalities. Mol Vis. 2002;8:407–415. [PubMed] [Google Scholar]
- 13.Nuka S, et al. Phenotypic characterization of epiphycan-deficient and epiphycan/biglycan double-deficient mice. Osteoarthritis Cartilage. 2010;18:88–96. doi: 10.1016/j.joca.2009.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wadhwa S, Embree MC, Kilts T, Young MF, Ameye LG. Accelerated osteoarthritis in the temporomandibular joint of biglycan/fibromodulin double-deficient mice. Osteoarthritis Cartilage. 2005;13:817–827. doi: 10.1016/j.joca.2005.04.016. [DOI] [PubMed] [Google Scholar]
- 15.Svensson L, et al. Fibromodulin-null mice have abnormal collagen fibrils, tissue organization, and altered lumican deposition in tendon. J Biol Chem. 1999;274:9636–9647. doi: 10.1074/jbc.274.14.9636. [DOI] [PubMed] [Google Scholar]
- 16.Jepsen KJ, et al. A syndrome of joint laxity and impaired tendon integrity in lumican- and fibromodulin-deficient mice. J Biol Chem. 2002;277:35532–35540. doi: 10.1074/jbc.M205398200. [DOI] [PubMed] [Google Scholar]
- 17.Reardon AJ, et al. Identification in vitreous and molecular cloning of opticin, a novel member of the family of leucine-rich repeat proteins of the extracellular matrix. J Biol Chem. 2000;275:2123–2129. doi: 10.1074/jbc.275.3.2123. [DOI] [PubMed] [Google Scholar]
- 18.Takanosu M, et al. Structure, chromosomal location, and tissue-specific expression of the mouse opticin gene. Invest Ophthalmol Vis Sci. 2001;42:2202–2210. [PubMed] [Google Scholar]
- 19.Monfort J, et al. Identification of opticin, a member of the small leucine-rich repeat proteoglycan family, in human articular tissues: a novel target for MMP-13 in osteoarthritis. Osteoarthritis Cartilage. 2008;16:749–755. doi: 10.1016/j.joca.2007.11.007. [DOI] [PubMed] [Google Scholar]
- 20.Tio L, et al. Characterization of opticin digestion by proteases involved in osteoarthritis development. Joint Bone Spine. 2014;81:137–141. doi: 10.1016/j.jbspin.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 21.Le Goff MM, et al. The vitreous glycoprotein opticin inhibits preretinal neovascularization. Invest Ophthalmol Vis Sci. 2012;53:228–234. doi: 10.1167/iovs.11-8514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Glasson SS, Blanchet TJ, Morris EA. The surgical destabilization of the medial meniscus (DMM) model of osteoarthritis in the 129/SvEv mouse. Osteoarthritis Cartilage. 2007;15:1061–1069. doi: 10.1016/j.joca.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 23.Valverde-Franco G, et al. In vivo bone-specific EphB4 overexpression in mice protects both subchondral bone and cartilage during osteoarthritis. Arthritis Rheum. 2012;64:3614–3625. doi: 10.1002/art.34638. [DOI] [PubMed] [Google Scholar]
- 24.Amiable N, et al. Proteinase-activated receptor-2 gene disruption limits the effect of osteoarthritis on cartilage in mice: a novel target in joint degradation. J Rheumatol. 2011;38:911–920. doi: 10.3899/jrheum.100710. [DOI] [PubMed] [Google Scholar]
- 25.Glasson SS, Chambers MG, Van Den Berg WB, Little CB. The OARSI histopathology initiative - recommendations for histological assessments of osteoarthritis in the mouse. Osteoarthritis Cartilage. 2010;18(Suppl 3):S17–23. doi: 10.1016/j.joca.2010.05.025. [DOI] [PubMed] [Google Scholar]
- 26.Pelletier JP, et al. The inhibition of subchondral bone resorption in the early phase of experimental dog osteoarthritis by licofelone is associated with a reduction in the synthesis of MMP-13 and cathepsin K. Bone. 2004;34:527–538. doi: 10.1016/j.bone.2003.11.021. [DOI] [PubMed] [Google Scholar]
- 27.Drexler W, et al. Correlation of collagen organization with polarization sensitive imaging of in vitro cartilage: implications for osteoarthritis. J Rheumatol. 2001;28:1311–1318. [PubMed] [Google Scholar]
- 28.Zhang G, et al. Genetic evidence for the coordinated regulation of collagen fibrillogenesis in the cornea by decorin and biglycan. J Biol Chem. 2009;284:8888–8897. doi: 10.1074/jbc.M806590200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ezura Y, Chakravarti S, Oldberg A, Chervoneva I, Birk DE. Differential expression of lumican and fibromodulin regulate collagen fibrillogenesis in developing mouse tendons. J Cell Biol. 2000;151:779–788. doi: 10.1083/jcb.151.4.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Monfort J, et al. Degradation of small leucine-rich repeat proteoglycans by matrix metalloprotease-13: identification of a new biglycan cleavage site. Arthritis Res Ther. 2006;8:R26. doi: 10.1186/ar1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen S, Birk DE. The regulatory roles of small leucine-rich proteoglycans in extracellular matrix assembly. FEBS J. 2013;280:2120–2137. doi: 10.1111/febs.12136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grover J, Chen XN, Korenberg JR, Roughley PJ. The human lumican gene. Organization, chromosomal location, and expression in articular cartilage. J Biol Chem. 1995;270:21942–21949. doi: 10.1074/jbc.270.37.21942. [DOI] [PubMed] [Google Scholar]
- 33.Svensson L, Narlid I, Oldberg A. Fibromodulin and lumican bind to the same region on collagen type I fibrils. FEBS Lett. 2000;470:178–182. doi: 10.1016/S0014-5793(00)01314-4. [DOI] [PubMed] [Google Scholar]
- 34.Wang Q, et al. Identification of a central role for complement in osteoarthritis. Nat Med. 2011;17:1674–1679. doi: 10.1038/nm.2543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kemper C, Atkinson JP. T-cell regulation: with complements from innate immunity. Nat Rev Immunol. 2007;7:9–18. doi: 10.1038/nri1994. [DOI] [PubMed] [Google Scholar]
- 36.Gobezie R, et al. High abundance synovial fluid proteome: distinct profiles in health and osteoarthritis. Arthritis Res Ther. 2007;9:R36. doi: 10.1186/ar2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sohn DH, et al. Plasma proteins present in osteoarthritic synovial fluid can stimulate cytokine production via Toll-like receptor 4. Arthritis Res Ther. 2012;14:R7. doi: 10.1186/ar3555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Glasson SS. In vivo osteoarthritis target validation utilizing genetically-modified mice. Curr Drug Targets. 2007;8:367–376. doi: 10.2174/138945007779940061. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and/or analysed during the current study are available from the corresponding author on reasonable request. Data generated or analysed during this study are included in this published article and its Supplementary Material files or are noted as data not shown.