Abstract
Background:
We counsel our triple-negative breast cancer (TNBC) patients that the risk of recurrence is highest in the first 5 years after diagnosis. However, there are limited data with extended follow-up on the frequency, characteristics, and predictors of late events.
Methods:
We queried the MD Anderson Breast Cancer Management System database to identify patients with stage I–III TNBC who were disease free at 5 years from diagnosis. The Kaplan–Meier method was used to estimate yearly recurrence-free interval (RFI), recurrence-free survival (RFS), and distant relapse-free survival (DRFS), as defined by the STEEP criteria. Cox proportional hazards model was used to compute hazard ratios (HRs) and 95% confidence intervals (CIs).
Results:
We identified 873 patients who were disease free at least 5 years from diagnosis with median follow-up of 8.3 years. The 10-year RFI was 97%, RFS 91%, and DRFS 92% the 15-year RFI was 95%, RFS 83%, and DRFS 84%. On a subset of patients with oestrogen receptor and progesterone receptor percentage recorded, low hormone receptor positivity conferred higher risk of late events on multivariable analysis for RFS only (RFI: HR=1.98, 95% CI=0.70–5.62, P-value=0.200; RFS: HR=1.94, 95% CI=1.05–3.56, P-value=0.034; DRFS: HR=1.72, 95% CI=0.92–3.24, P-value=0.091).
Conclusions:
The TNBC survivors who have been disease free for 5 years have a low probability of experiencing recurrence over the subsequent 10 years. Patients with low hormone receptor-positive cancers may have a higher risk of late events as measured by RFS but not by RFI or DRFS.
Keywords: breast cancer, triple negative, survivorship, survivors, hormone receptor, recurrence
A total of 10–20% of newly diagnosed early breast cancers are triple-negative breast cancers (TNBCs), a term used to describe breast cancers that do not express oestrogen receptor (ER) or progesterone receptor (PR) and lack overexpression of human epidermal growth factor receptor 2 (HER-2/neu) (Foulkes et al, 2010). Several large studies have demonstrated that patients with TNBC have worse clinical outcomes and a unique pattern of recurrence compared with hormone receptor-positive (HR+) and HER-2/neu receptor-positive (HER2+) breast cancer patients (Dent et al, 2007; Liedtke et al, 2008; Lin et al, 2012). Patients with TNBC have been shown to have the highest rate of recurrence within the first 5 years after diagnosis, with a significant decrease and plateauing of the recurrence rate afterwards. Compared with patients with HR+ tumours, distant recurrence tends to occur more frequently in visceral organs, including the brain, liver, and lungs, and less frequently in bone (Liedtke et al, 2008). Furthermore, post-recurrence survival is decreased compared with that in patients with HR+ tumours. Our research group previously published a large study of TNBC patients after neoadjuvant chemotherapy; in addition to highlighting this unique pattern of recurrence, importantly, we demonstrated that patients who do not achieve a pathologic complete response (pCR) have a poor outcome relative to patients with HR+ disease (Liedtke et al, 2008).
Although we counsel our TNBC patients that the recurrence rate is highest in the first 5 years after diagnosis, there are limited data with extended follow-up, in particular of TNBC survivors who survive ⩾5 years from diagnosis. Published studies on this topic have a median follow-up of <5 years (Liedtke et al, 2008; Lin et al, 2012) or have a relatively small population of TNBC 5-year disease-free survivors (Cortazar et al, 2014). In addition, they have incomplete receptor information and only classify tumours as ER negative (Saphner et al, 1996; Brewster et al, 2008; Dignam et al, 2009) or do not present specific hormone receptor percentage to distinguish <1% ER and PR tumours from low hormone receptor-positive (ER and/or PR 1–9%) tumours (Saphner et al, 1996; Dent et al, 2007; Brewster et al, 2008; Liedtke et al, 2008; Dignam et al, 2009; Lin et al, 2012; Cortazar et al, 2014). Several of these are older publications and do not necessarily include contemporary anthracycline-based regimens (Saphner et al, 1996; Dignam et al, 2009), lack specific information on the timing and type of chemotherapy (Dent et al, 2007; Brewster et al, 2008; Lin et al, 2012), or lack information on pCR when patients receive neoadjuvant chemotherapy (Dent et al, 2007; Lin et al, 2012). It is critical to obtain more specific information on long-term outcomes, particularly the frequency and pattern of late recurrences, in TNBC patients to accurately inform patient counseling. In addition, identifying the predictors of recurrence may help us identify high-risk patients who we can offer potential investigative therapeutic strategies to reduce the risk of late relapse. Notably, we do not know how late outcomes differ on the basis of the old definition of TNBC and the new definition established in 2010 by ASCO/CAP (Hammond et al, 2010) that requires <1% ER and PR expression instead of the <10% commonly used cutoff in earlier studies. The University of Texas MD Anderson Cancer Center (Houston, TX, USA) Breast Cancer Management System (BCMS) provides a large data set of TNBC patients, including survivors with long-term follow-up data. In this retrospective study, we queried this database to identify the long-term (>5 years) recurrence rates, patterns, and predictors of late recurrence in TNBC patients.
Materials and methods
Patient selection
Patients were identified from the BCMS, a database housed in the Department of Breast Medical Oncology at MD Anderson that includes all patients with a diagnosis of breast cancer assessed and treated at the institution since January 1997. Included in this study were all female patients with a history of stage I–III primary TNBC who survived to 5 years from diagnosis without disease recurrence or development of second primary breast cancer and had been seen in the breast centre or cancer survivorship clinic at MD Anderson from 1 January 1997 when the database was started until 7 April 2015 when the analysis for this project was begun. To minimise referral selection bias, we limited our study population to patients who had presented to MD Anderson within 3 months of diagnosis. The study was approved by the institutional review board that granted a waiver of informed consent for the study.
TNBC was defined as ER-negative or <10% if the percentage was specified, PR-negative or <10% if the percentage was specified, and HER-2/neu status 0 or 1+ by immunohistochemistry analysis or 2+ with negative fluorescence in situ hybridisation (HER2/CEP17 ratio of <2 or HER2 gene copy number <4). Patients with any missing receptor information or a missing pathology report were excluded from the analysis; however, patients were included in this study if receptor status was defined as ‘negative’ even if percentage was missing. The MD Anderson electronic medical record was reviewed to verify the receptor status of all the patients included in this study. Patients with concurrent non-TNBC and/or who developed a second primary breast cancer even after 5 years of disease-free survival were also excluded to minimise competing risks.
Variable and outcome definitions
The database was used to gather information regarding patient demographics (age, race, and body mass index), cancer stage (including TNM stage), tumour characteristics (receptor information and tumour histologic type and grade), type of therapy (surgery, chemotherapy, radiation therapy, or endocrine therapy), dosing and administration details of chemotherapy, and pathologic and clinical outcomes. Clinical outcomes included local recurrence, distant recurrence, breast cancer-related death, non-breast cancer-related death, and death from unspecified cause. These clinical end points were translated to the outcomes of interest on the basis of the STEEP criteria. Recurrence-free interval (RFI) was measured from the date of diagnosis of primary cancer to the date of first invasive ipsilateral breast tumour recurrence, local or regional invasive recurrence, distant recurrence, or death documented because of breast cancer. Recurrence-free survival (RFS) was measured from the date of diagnosis of primary cancer to the date of the first invasive ipsilateral breast tumour recurrence, local or regional invasive recurrence, distant recurrence, or death from any cause. Distant relapse-free survival (DRFS) was measured from the date of diagnosis of primary cancer to the date of first distant recurrence or death from any cause (Hudis et al, 2007). Patients were censored at the date of their last follow-up for those who did not have an event.
Statistical analysis
Patient characteristics were described by their frequency and percentage for categorical variables and mean and s.d. for continuous variables. The median follow-up time was calculated on the basis of the ‘reverse Kaplan–Meier’ method (Schemper and Smith, 1996). The Kaplan–Meier method was used to estimate survival functions. Potential predictors were assessed using univariate Cox proportional hazards models. Variables that were significant at the 0.25 significance level in univariate analysis were considered as candidates for multivariable model (Hosmer et al, 2013). A backward selection method was used for multivariable model building and P<0.05 was considered for statistical significance in multivariable modelling. The data analysis was conducted using SAS software, version 9.4 (SAS Institute, Cary, NC, USA), and STATA software, version 12 (Statacorp, College Station, TX, USA).
Results
Patient population
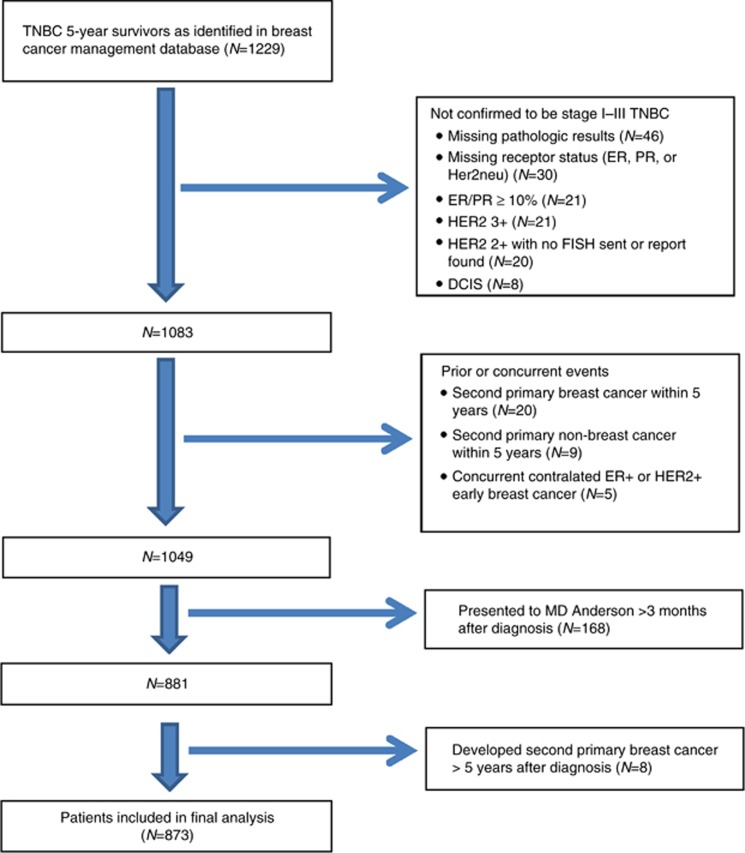
We identified 873 TNBC patients (Figure 1) who were disease free 5 years after diagnosis and met selection criteria for study inclusion, with a median follow-up of 8.3 years (range, 6.8–10.4 years) from initial diagnosis. Table 1 shows the patient demographic, tumour characteristics, treatment, and pathological outcome variables. The mean age at diagnosis was 51.4 years. Most patients had stage 2 cancer (51.7%), grade 3 disease (88.4%), and invasive ductal histology (90.8%). The other histologic types, in the order of decreasing frequency, were lobular, sarcomatoid, papillary, medullary, and adenocystic. More than 80% of patients had received anthracycline-based chemotherapy. Approximately one-third of patients had received neoadjuvant chemotherapy, and 40.6% of these patients had achieved a pCR. The primary tumour was treated with adjuvant endocrine therapy in 4.5% of patients, predominantly because of either low hormone receptor disease (1–9%) or concurrent HR+ ductal carcinoma in situ. A mastectomy was performed in 59.3% of patients, and 72.2% received adjuvant radiation therapy. Of the 623 (71.3%) patients for whom ER and PR percentage was documented, 76.4% met the current definition of TNBC (ER and PR <1%).
Figure 1.
Flowchart of study population selection. ER=oestrogen receptor; Her2neu=human epidermal growth factor receptor 2; PR=progesterone receptor; TNBC=triple-negative breast cancers.
Table 1. Patient, tumour, and treatment characteristics, n=873.
| Characteristic | N (%) |
|---|---|
|
Age at diagnosis | |
| Mean (s.d.) | 51.4 (11.4) |
| Median (interquartile range) | 51 (44–59) |
| BMI,a mean (s.d.) | 28.6 (6.9) |
|
Race/ethnicity | |
| White | 583 (66.8) |
| Black | 141 (16.2) |
| Spanish/Hispanic | 109 (12.5) |
| Other | 40 (4.6) |
|
Menopausal status | |
| Post | 544 (62.3) |
| Pre | 304 (34.8) |
| Peri/unknown | 25 (2.9) |
|
Stage | |
| I | 302 (34.6) |
| II | 451 (51.7) |
| III | 120 (13.8) |
|
Grade | |
| I | 5 (0.6) |
| II | 87 (10.0) |
| III | 772 (88.4) |
| Unknown | 9 (1.0) |
|
Histology | |
| Ductal | 793 (90.8) |
| Lobular | 12 (1.4) |
| Mixed | 5 (0.6) |
| Other | 63 (7.2) |
|
ER/PR%b | |
| ER and PR <1% | 476 (76.4) |
| ER and/or PR 1–9% | 147 (23.6) |
|
Chemotherapy | |
| A+T | 554 (63.5) |
| A | 174 (19.9) |
| Other | 34 (3.9) |
| Not received | 111 (12.7) |
|
Hormone therapy | |
| No | 834 (95.5) |
| Yes | 39 (4.5) |
|
Surgery | |
| Mastectomy | 517 (59.3) |
| Lumpectomy | 347 (39.8) |
| Axillary lymph node dissection | 4 (0.5) |
| Unknown | 5 (0.6) |
|
Radiation | |
| No | 243 (27.8) |
| Yes | 630 (72.2) |
|
pCRc | |
| Obtained | 133 (40.6) |
| Not obtained | 188 (57.3) |
| Unknown | 7 (2.1) |
Abbreviations: A=anthracycline; A+T=anthracycline+taxane; BMI =body mass index; ER=oestrogen receptor; pCR=pathological complete response; PR=progesterone receptor.
A total of 860 patients had available information on BMI.
A total of 623 patients had % ER and PR documented in medical record.
A total of 328 patients received neoadjuvant chemotherapy.
Frequency and characterisation of late events
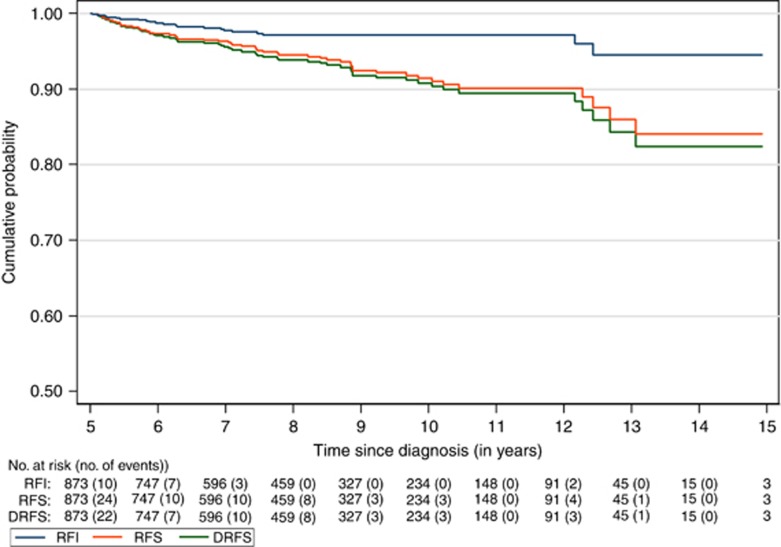
Figure 2 illustrates the cumulative probabilities of remaining free from event for RFI, RFS, and DRFS. The 10-year RFI was 97%, RFS 91%, and DRFS 92%. The 15-year RFI was 95%, RFS 83%, and DRFS 84%. Of the 873 patients, 22 had late recurrences. Sixteen (72.7%) of the recurrences were distant. Sites of distant metastases included the lungs/pleura (50.0%), distant lymph nodes (36.4%), bones (27.3%), liver (13.6%), central nervous system (13.6%), pancreas (4.5%), and distant skin (4.5%). Six patients initially presented with local recurrence only, with 5 presenting with ipsilateral breast masses and the other with regional lymph node recurrence. Of the 22 patients who had recurrences, 16 died, with a median time to death after recurrence of 1.2 years (range, 0.7–2.6 years). There were 57 deaths: 28.1% were attributed to breast cancer, 63.2% to other, and 8.8% to unknown causes in the absence of documented recurrence. Supplementary Tables 1–3 show comparison of patient, tumour, and treatment characteristics between patients who had an event and those who did not for each of the three end points in this study.
Figure 2.
Recurrence-free interval (RFI), recurrence-free survival (RFS), and distant relapse-free survival (DRFS) of triple-negative breast cancer 5-year survivors as function of time from diagnosis.
Predictors of late events
Table 2 shows the univariate analysis of patient demographic, tumour, and treatment variables and their association with RFI, RFS, and DRFS. Based on a predetermined selection criteria (P-value <0.25 on univariate analysis), the following variables were included in the multivariate model for the entire cohort (n=873): age at diagnosis (for RFI, RFS, DRFS), chemotherapy received (for RFI, RFS, DRFS), race (for DRFS), stage (for RFI), and grade (for DRFS). Of note, given that menopausal status and age were tightly correlated, menopausal status was not incorporated into our multivariable model. Age remained the only variable to maintain significance on multivariable analysis, with older age at diagnosis being associated with worse RFS and DRFS but not RFI (RFI: hazard ratio (HR)=0.96, 95% confidence interval (CI)=0.93–1.00, P-value=0.074; RFS: HR=1.04, 95% CI=1.02–1.07, P-value<0.001; DRFS: HR=1.06, 95% CI=1.04–1.08, P-value<0.001). As shown in Table 2, low hormone receptor positivity (ER and/or PR 1–9%) and not achieving a pathological complete response were associated with worse outcomes on univariate analyses. Because of relatively smaller sized cohorts of patients with ER and PR percentage information available (n=623) and patients who received neoadjuvant chemotherapy (n=328), separate multivariable analyses were conducted within these subsets in order to determine whether these variables were predictive of outcomes. Low hormone receptor positivity maintained significance for RFS only (RFI: HR=1.98, 95% CI=0.70–5.62, P-value=0.200; RFS: HR=1.94, 95% CI=1.05–3.56, P-value=0.034; DRFS: HR=1.72, 95% CI=0.92–3.24, P-value=0.091), while achieving a pathological complete response did not maintain significance for any end points.
Table 2. Univariate analysis.
|
RFI |
RFS |
DRFS |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Characteristic | HR | P-value | 95% CI | HR | P-value | 95% CI | HR | P-value | 95% CI |
| Age at diagnosisa | 0.96 | 0.074 | (0.93–1.00) | 1.04 | 0.000 | (1.02–1.07) | 1.06 | 0.000 | (1.04–1.08) |
| BMIb | 1.03 | 0.336 | (0.97–1.09) | 1.01 | 0.467 | (0.98–1.05) | 1.00 | 0.845 | (0.97–1.04) |
| Race/ethnicity | |||||||||
| White | Ref. | Ref. | Ref. | ||||||
| Black | 0.87 | 0.819 | (0.25–2.99) | 0.74 | 0.454 | (0.33–1.64) | 0.57 | 0.231 | (0.22–1.44) |
| Others | 1.06 | 0.919 | (0.35–3.19) | 1.31 | 0.378 | (0.72–2.40) | 1.31 | 0.395 | (0.70–2.46) |
| Menopausal status | |||||||||
| Post | Ref. | Ref. | Ref. | ||||||
| Pre | 2.11 | 0.080 | (0.91–4.90) | 0.49 | 0.018 | (0.27–0.89) | 0.41 | 0.008 | (0.21–0.79) |
| Stage | |||||||||
| I | Ref. | Ref. | Ref. | ||||||
| II | 2.64 | 0.085 | (0.87–7.96) | 0.94 | 0.822 | (0.54–1.63) | 0.85 | 0.572 | (0.48–1.51) |
| III | 1.85 | 0.422 | (0.41–8.26) | 1.2 | 0.628 | (0.58–2.47) | 1.14 | 0.735 | (0.54–2.42) |
| Gradec | |||||||||
| I–II | Ref. | Ref. | Ref. | ||||||
| III | 0.8 | 0.718 | (0.24–2.70) | 0.69 | 0.280 | (0.35–1.35) | 0.61 | 0.157 | (0.31–1.21) |
| Histology | |||||||||
| Ductal | Ref. | Ref. | Ref. | ||||||
| Other | 1.55 | 0.481 | (0.46–5.24) | 0.86 | 0.754 | (0.35–2.15) | 0.97 | 0.943 | (0.39–2.42) |
| Hormone receptord | |||||||||
| ER and PR, <1% | Ref. | Ref. | Ref. | ||||||
| ER/PR, 1–9 % | 1.86 | 0.245 | (0.65–5.26) | 1.89 | 0.040 | (1.03–3.45) | 1.69 | 0.099 | (0.91–3.17) |
| Chemotherapy | |||||||||
| A+T | Ref. | Ref. | Ref. | ||||||
| A | 0.35 | 0.160 | (0.08–1.52) | 1 | 0.990 | (0.52–1.95) | 1.05 | 0.889 | (0.52–2.11) |
| Other | 1.13 | 0.906 | (0.15–8.52) | 2.82 | 0.031 | (1.10–7.25) | 2.57 | 0.077 | (0.90–7.33) |
| Not received | 0.94 | 0.927 | (0.27–3.24) | 2.29 | 0.010 | (1.22–4.29) | 2.62 | 0.003 | (1.38–4.97) |
| Hormone therapy | |||||||||
| No | Ref. | Ref. | |||||||
| Yes | 0.89 | 0.913 | (0.12–6.66) | 0.88 | 0.823 | (0.27–2.80) | 0.63 | 0.528 | (0.15–2.61) |
| Surgerye | |||||||||
| Lumpectomy | Ref. | Ref. | |||||||
| Mastectomy | 0.89 | 0.787 | (0.37–2.11) | 0.83 | 0.470 | (0.49–1.39) | 0.90 | 0.689 | (0.52–1.54) |
| Radiation | |||||||||
| No | Ref. | Ref. | |||||||
| Yes | 1.21 | 0.704 | (0.45–3.29) | 1.24 | 0.477 | (0.68–2.24) | 1.20 | 0.561 | (0.65–2.23) |
| pCRf | |||||||||
| Obtained | Ref. | Ref. | Ref. | ||||||
| Not obtained | 1.07 | 0.928 | (0.25–4.53) | 2.64 | 0.082 | (0.88–7.89) | 3.26 | 0.061 | (0.95–11.28) |
Abbreviations: A=anthracycline-based chemotherapy; A+T=anthracycline- and taxane-based chemotherapy; BMI=body mass index; CI=confidence interval; DRFS=distant relapse-free survival; ER=oestrogen receptor; HR=hazard ratio; pCR=pathological complete response; PR=progesterone receptor; RFI=recurrence-free interval; RFS=recurrence-free survival; Ref.=reference value.
HR expressed per additional year.
Available for 860 patients.
Available for 864 patients.
Available for 623 patients.
Available for 864 patients.
Available for 328 patients.
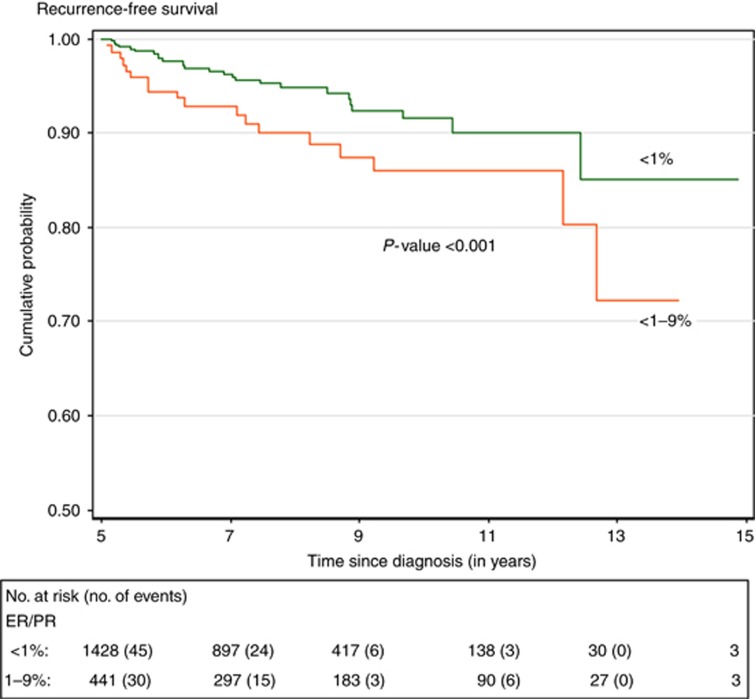
The Kaplan–Meier survival curve by hormone receptor percentage is illustrated in Figure 3 for RFS. Of note, given that 28.6% of patients had missing percentage information, we performed a comparison of the baseline demographic, tumour-specific, and treatment variables between patients with missing information and those with a specified hormone receptor percentage and found no significant differences.
Figure 3.
Recurrence-free survival of triple-negative breast cancer 5-year survivors as function of hormone receptor positivity and time from diagnosis. ER=oestrogen receptor; PR=progesterone receptor.
Discussion
To our knowledge, this is the first large study with extended follow-up to quantify the frequencies of late events in 5-year TNBC survivors (including HER2-negative disease and not just hormone receptor negative (HR−) disease reported in several prior reports) and to identify predictors of late event risk. In clinical practice, we generally reassure our patients and their families that the risk of breast cancer recurrence is minimal once they have survived for 5 years without disease. Although this is generally true, we found that 5% of these survivors will have a breast cancer recurrence within the subsequent 10 years. This quantification of late events is important to better educate our patients about what to expect once they have transitioned to survivorship and to emphasise the importance of continued follow-up even after this transition.
These low late recurrence rates are in stark contrast to what has historically been seen in patients with HR+ breast cancer. The Early Breast Cancer Trialists’ Collaborative Group presented data on long-term recurrence risks after use of 5 years of endocrine therapy in 46 000 patients with HR+ breast cancer who were alive and disease free at 5 years (Pan, 2016). Continued increased risk was seen up to 20 years from diagnosis even in those with T1N0 disease, with distance recurrence rates between years 5 and 20 from diagnosis ranging from 14% for T1N0 disease to 47% for T2N4-9 disease. These results are supported by contemporary randomised trials, including MA-17 and ATAC, that show continued risk even with 10 years of adjuvant endocrine therapy, with an ∼5–10% recurrence risk between years 5 and 10 from diagnosis (Ingle et al, 2008; Cuzick et al, 2010). The results of our study, in comparison with these recurrence rates of patients with HR+ breast cancer, have key implications for counseling patients, surveillance monitoring, and also importantly for design of clinical trials. Although it is a longstanding observation that HR− disease has lower recurrence rates in later years post diagnosis than HR+ disease, this study incorporates HER2 receptor status to demonstrate that this same observation holds true in a TNBC population. In addition to evaluating a truly HR− TNBC population, based on a subset analysis this study also shows that late recurrence rates in low HR+ disease are also not comparable to ⩾10% HR+ disease.
With the change in the definition of TNBC in the ACP-ASCO 2010 guidelines, there is an increased interest in studying the differences in outcomes, pathophysiology, and response to treatment among cancers with low HR positivity (ER and/or PR 1–9%) that were previously included in the TNBC definition and tumours that meet the current strict TNBC definition (ER and PR <1%). Recently published data from our institution found no differences in outcomes between breast cancer patients with ER and PR <1% tumours and low HR+ tumours (Yi et al, 2014). Our study extends these findings by focussing on long-term follow-up, particularly of 5-year disease-free TNBC survivors. Based on a subset analysis, our data support that the low HR+ population biologically behaves similarly to the <1% ER/PR current definition of TNBC population and is different than ⩾10% HR+ early breast cancer. We did not find a statistically significant higher risk of recurrence by RFI that, compared with our other end points, should theoretically more accurately reflect true recurrence rate as it does not include non-breast cancer-related deaths. However, because of the fact that RFI does not capture competing risk of non-breast cancer deaths and may also not be capturing additional breast cancer recurrences that were recorded as deaths from unknown cause, we performed our analyses with RFS and DRFS end points as well. Though we found an increased event rate with low HR positivity compared with TNBC with <1% ER/PR disease in RFS only, the magnitude of these event rates was still relatively low compared with historical event rates for HR+ disease; a similar trend was seen for RFI and DRFS, though as discussed did not reach statistical significance. In addition, we did not find a signal for improved outcomes with endocrine therapy in this low HR+ group, but given the low number of events, the study was underpowered to detect a clinically significant difference, should such a signal exist. These conclusions are significant for identifying a subset of patients who may be at higher risk for late breast cancer recurrences but also highlighting they behave more similar to <1% ER/PR than ⩾10% ER/PR disease.
There are several limitations to this study. First, given the retrospective nature of the analysis, there may be unmeasurable confounders, and the results need to be interpreted as correlative as opposed to cause and effect relationships. Second, the data were obtained at a large referral centre, and retrospective databases are at risk for referral selection bias. To counteract this, we limited our study population to patients who presented to MD Anderson within 3 months of diagnosis. Third, ∼30% of patients had missing ER/PR percentage information, and we were therefore limited to a subset analysis to determine effects of low hormone receptor positivity. An analysis of the baseline characteristics between those we included in our analysis and those with missing percentage information showed no significant differences; therefore, we believe that the selection bias should be minimal. Regardless, future studies with complete percentage information are needed to validate the results of this study. Finally, the sample size in this study is relatively small and therefore the power to detect differences between subgroups is limited. Analysis of larger data sets of 5-year disease-free survivors is therefore warranted in the future to build on the results of this current analysis.
This study has several key strengths, and the results could be incorporated into clinical practice and influence how we educate TNBC survivors. First, this was a large single-centre study with extended follow-up that provides important information on the frequency of late events in this patient population. The late breast cancer recurrence risk of 5% can be cited in counselling patients about their risk for late events after they transition to survivorship. In addition, we found that although patients with low hormone receptor positivity may have higher risk of late events – reaching statistical significance in our study only for RFS but not RFI or DRFS – the overall magnitude of this effect is modest, in particular compared with what is published for HR+ disease.
Overall, this study highlights that although TNBC patients have a lower late event rate than other breast cancer patients, there is still a small subset of patients who experience late recurrence; the results of this study will help us educate our patients about the magnitude of risk.
Acknowledgments
We thank the National Institute of Health (NIH) T32 Training Grant T32 CA009666 and Young Breast Cancer Survivors Program Fund 104029 as a source of funding for this research. Portions of this study were presented in abstract and poster form at the San Antonio Breast Cancer Symposium on 12 December 2015 in San Antonio, TX, USA and the ASCO Annual Meeting on 5 June 2016 in Chicago, IL, USA. This research was funded in part by the NIH/NCI Cancer Center Support Grant P30 CA016672.
Footnotes
Supplementary Information accompanies this paper on British Journal of Cancer website (http://www.nature.com/bjc)
The authors declare no conflict of interest.
Supplementary Material
References
- Brewster AM, Hortobagyi GN, Broglio KR, Kau SW, Santa-Maria CA, Arun B, Buzdar AU, Booser DJ, Valero V, Bondy M, Esteva FJ (2008) Residual risk of breast cancer recurrence 5 years after adjuvant therapy. J Natl Cancer Inst 100: 1179–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortazar P, Zhang L, Untch M, Mehta K, Costantino JP, Wolmark N, Bonnefoi H, Cameron D, Gianni L, Valagussa P, Swain SM, Prowell T, Loibl S, Wickerham DL, Bogaerts J, Baselga J, Perou C, Blumenthal G, Blohmer J, Mamounas EP, Bergh J, Semiglazov V, Justice R, Eidtmann H, Paik S, Piccart M, Sridhara R, Fasching PA, Slaets L, Tang S, Gerber B, Geyer CE Jr, Pazdur R, Ditsch N, Rastogi P, Eiermann W, von Minckwitz G (2014) Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet 384: 164–172. [DOI] [PubMed] [Google Scholar]
- Cuzick J, Sestak I, Baum M, Buzdar A, Howell A, Dowsett M, Forbes JF ATAC/LATTE investigators (2010) Effect of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: 10-year analysis of the ATAC trial. Lancet Oncol 11: 1135–1141. [DOI] [PubMed] [Google Scholar]
- Dent R, Trudeau M, Pritchard KI, Hanna WM, Kahn HK, Sawka CA, Lickley LA, Rawlinson E, Sun P, Narod SA (2007) Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res 13: 4429–4434. [DOI] [PubMed] [Google Scholar]
- Dignam JJ, Dukic V, Anderson SJ, Mamounas EP, Wickerham DL, Wolmark N (2009) Hazard of recurrence and adjuvant treatment effects over time in lymph node-negative breast cancer. Breast Cancer Res Treat 116: 595–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foulkes WD, Smith IE, Reis-Filho JS (2010) Triple-negative breast cancer. N Engl J Med 363: 1938–1948. [DOI] [PubMed] [Google Scholar]
- Hammond ME, Hayes DF, Dowsett M, Allred DC, Hagerty KL, Badve S, Fitzgibbons PL, Francis G, Goldstein NS, Hayes M, Hicks DG, Lester S, Love R, Mangu PB, McShane L, Miller K, Osborne CK, Paik S, Perlmutter J, Rhodes A, Sasano H, Schwartz JN, Sweep FC, Taube S, Torlakovic EE, Valenstein P, Viale G, Visscher D, Wheeler T, Williams RB, Wittliff JL, Wolff AC American Society of Clinical Oncology, College of American Pathologists (2010) American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer (unabridged version). Arch Pathol Lab Med 134: e48–e72. [DOI] [PubMed] [Google Scholar]
- Hosmer DW, Lemeshow S, Sturdivant RX (2013) Applied Logistic Regression. Wiley: Hoboken, NJ. [Google Scholar]
- Hudis CA, Barlow WE, Costantino JP, Gray RJ, Pritchard KI, Chapman JA, Sparano JA, Hunsberger S, Enos RA, Gelber RD, Zujewski JA (2007) Proposal for standardized definitions for efficacy end points in adjuvant breast cancer trials: the STEEP system. J Clin Oncol 25: 2127–2132. [DOI] [PubMed] [Google Scholar]
- Ingle JN, Tu D, Pater JL, Muss HB, Martino S, Robert NJ, Piccart MJ, Castiglione M, Shepherd LE, Pritchard KI, Livingston RB, Davidson NE, Norton L, Perez EA, Abrams JS, Cameron DA, Palmer MJ, Goss PE (2008) Intent-to-treat analysis of the placebo-controlled trial of letrozole for extended adjuvant therapy in early breast cancer: NCIC CTG MA.17. Ann Oncol 19: 877–882. [DOI] [PubMed] [Google Scholar]
- Liedtke C, Mazouni C, Hess KR, Andre F, Tordai A, Mejia JA, Symmans WF, Gonzalez-Angulo AM, Hennessy B, Green M, Cristofanilli M, Hortobagyi GN, Pusztai L (2008) Response to neoadjuvant therapy and long-term survival in patients with triple-negative breast cancer. J Clin Oncol 26: 1275–1281. [DOI] [PubMed] [Google Scholar]
- Lin NU, Vanderplas A, Hughes ME, Theriault RL, Edge SB, Wong YN, Blayney DW, Niland JC, Winer EP, Weeks JC (2012) Clinicopathologic features, patterns of recurrence, and survival among women with triple-negative breast cancer in the National Comprehensive Cancer Network. Cancer 118: 5463–5472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan H (2016) Long-term recurrence risks after use of endocrine therapy for only 5 years: relevance of breast tumor characteristics in: American Society of Clinical Oncology Annual Meeting: Chicago. [Google Scholar]
- Saphner T, Tormey DC, Gray R (1996) Annual hazard rates of recurrence for breast cancer after primary therapy. J Clin Oncol 14: 2738–2746. [DOI] [PubMed] [Google Scholar]
- Schemper M, Smith TL (1996) A note on quantifying follow-up in studies of failure time. Control Clin Trials 17: 343–346. [DOI] [PubMed] [Google Scholar]
- Yi M, Huo L, Koenig KB, Mittendorf EA, Meric-Bernstam F, Kuerer HM, Bedrosian I, Buzdar AU, Symmans WF, Crow JR, Bender M, Shah RR, Hortobagyi GN, Hunt KK (2014) Which threshold for ER positivity? A retrospective study based on 9639 patients. Ann Oncol 25: 1004–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.