Abstract
Mechanical forces due to fetal movements play an important role in joint shape morphogenesis, and abnormalities of the joints relating to abnormal fetal movements can have long-term health implications. While mechanical stimulation during development has been shown to be important for joint shape, the relationship between the quantity of mechanical stimulation and the growth and shape change of developing cartilage has not been quantified. In this study, we culture embryonic chick limb explants in vitro in order to reveal how the magnitude of applied movement affects key aspects of the developing joint shape. We hypothesise that joint shape is affected by movement magnitude in a dose-dependent manner, and that a movement regime most representative of physiological fetal movements will promote characteristics of normal shape development. Chick hindlimbs harvested at seven days of incubation were cultured for six days, under either static conditions or one of three different dynamic movement regimes, then assessed for joint shape, cell survival and proliferation. We demonstrate that a physiological magnitude of movement in vitro promotes the most normal progression of joint morphogenesis, and that either under-stimulation or over-stimulation has detrimental effects. Providing insight into the optimal level of mechanical stimulation for cartilage growth and morphogenesis is pertinent to gaining a greater understanding of the etiology of conditions such as developmental dysplasia of the hip, and is also valuable for cartilage tissue engineering.
Keywords: Joint morphogenesis, Joint shape, Explant culture, Chick knee (Stifle) joint, Mechanobiology
1. Introduction
Each type of synovial joint has a highly specialised shape, and alterations or abnormalities in the development of these shapes can compromise their functionality. Reduced fetal movements are implicated in musculoskeletal conditions of impaired joint shape development, such as development dysplasia of the hip and arthrogryposis (reviewed in Nowlan (2015)). However, it is unclear how the quantity, timing and type of mechanical stimulation due to fetal movements influence joint shape morphogenesis. This question is relevant to lifelong musculoskeletal health, as developmental joint abnormalities can affect the joint׳s range of motion and the transmission of mechanical loads, increasing the risk of degenerative joint diseases such as osteoarthritis later in life (Sandell, 2012). Furthermore, a better understanding of how mechanical stimulation directs or determines cartilage growth during prenatal development is highly relevant to cartilage tissue engineering, in which the aim is to recapitulate the developmental processes occurring in fetal rudiments.
Previous studies have explored the influence of mechanical stimuli on skeletal development using animal models of reduced, absent or abnormal fetal movements (reviewed in Nowlan et al. (2010b)). While many of these studies focused on cavitation or ossification rather than joint morphogenesis, we do have some understanding of the effects of immobility on joint shape development. In immobilised chicks embryos, articular joints are often fused across the joint site, and normal interlocking joint shapes are lost (Drachman and Sokoloff, 1966, Hall and Herring, 1990, Hosseini and Hogg, 1991, Nowlan et al., 2014, Osborne et al., 2002, Roddy et al., 2011b). Paralysis of zebrafish embryos leads to alterations in jaw joint shape and inhibition of normal joint function (Brunt et al., 2015). Failure to produce joint cavities and abnormal joint shapes have also been observed in ‘muscle-less’ mice embryos (Kahn et al., 2009, Nowlan et al., 2010a) along with signs of irregular joint shape development in ‘reduced muscle’ mice embryos (Kahn et al., 2009). Early studies used in vitro culture methods to investigate the role of movement on joint development. Explants from four day old embryos failed to form a complete knee (stifle) joint after six days of static culture in vitro, using the “watch glass” technique (Fell and Canti, 1934). However, when six or seven day old embryonic chick knee explants were cultured for six days by Lelkes (1958), manual manipulation of the explants led to cavitation and development of articular surfaces between the femur and tibia (or tibiotarsus). Since these pioneering papers were published, in vitro culture methods have improved dramatically. Modern bioreactors enable repeatable cultivation of tissue and application of controlled mechanical stimulation in ways that are not possible in vivo (Cohen et al., 2005, Pörtner et al., 2005). In vitro culture of embryonic chick hindlimb elements has been shown to be a versatile model for studying skeletal development (Smith et al., 2013), and a bioreactor system has been used to apply cyclic hydrostatic pressure to promote bone growth and mineralisation in embryonic chick femurs (Henstock et al., 2013). A recent feasibility study showed the whole chick hindlimb could be cultured whilst applying flexion and extension movements to the knee joint (Rodriguez and Munasinghe, 2016). However, the quantitative relationship between mechanical stimulation and joint morphogenesis has not been described, a deficit that is addressed in this current study.
In this study, a novel 3D explant culture system is used to investigate the development of the embryonic chick knee joint under a range of flexion movement regimes, with the aim of characterising the relationship between the magnitude of applied movements and key aspects of fetal joint morphogenesis. It was hypothesised that joint shape development would be affected by movement magnitude in a dose-dependent manner, and that the most physiological movement regime would lead to a joint with the most normal progression of shape morphogenesis.
2. Methods
2.1. Characterisation of physiological knee morphology
To evaluate the progression of joint shape development in cultured explants, we first analysed the morphology of the knee joint over 7 to 9 days of incubation, a period of dramatic shape change for the joint. Limbs were processed for 3D shape and size analysis as described below.
2.2. Preparation of explants for culture
Fertilised white DeKalb eggs (Henry Stewart & Co, UK) were incubated at 37 °C under humidified conditions for seven days. Hindlimbs were harvested, the digits removed, and the soft tissues surrounding the rudiments removed as described by Henstock et al. (2013). Preliminary experiments demonstrated that this step of soft tissue removal increased the duration of time that the explant could be viably maintained in vitro (data not shown).
2.3. Explant culture setup
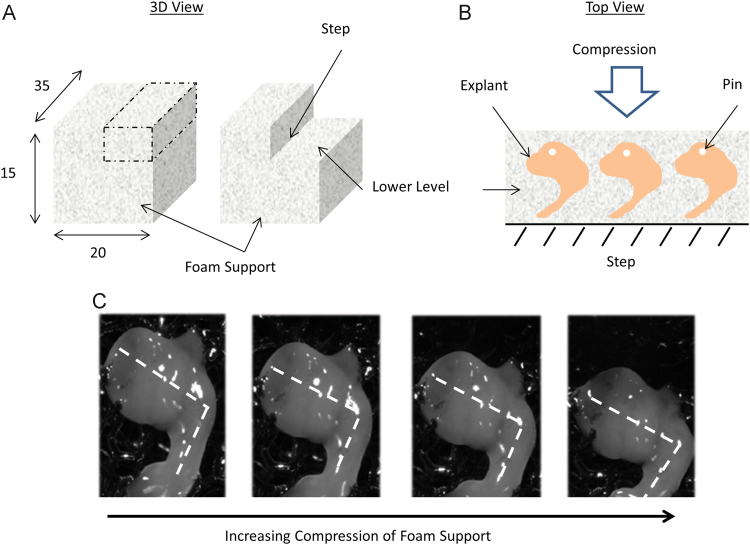
Rectangular pieces (35×20×15 mm3) of polyurethane foam (Sydney Heath & Son, UK) were used to support the hindlimb explants during culture. The foam support was cut to create a step running horizontally along the top surface (Fig. 1A). Each hindlimb was positioned, medial side down, onto the lower level and oriented with the distal end nearest the step (Fig. 1B). Six specimens were placed on each support (Fig. 1B). Once positioned, each explant was pinned to the support using a 27G needle through the superior part of the pelvis to secure the limb. The foam supports were transferred into a uniaxial compression bioreactor (Ebers TC-3, Spain) and filled with basal culture media (alpha – minimum essential media (α-MEM GlutaMAX, Gibco) supplemented with 1% pen/strep, 1% Amphotericin B and 100 µM Ascorbic Acid (Sigma, UK)). The explants were maintained at an air–liquid interface, and the culture medium was replenished every 24 h.
Fig. 1.
Dynamic explant culture setup. A) A polyurethane foam support was used to support the hindlimb explants during culture. The rectangular piece of foam was cut to create a step along the horizontal axis. Measurements are in mm. B) Explants were positioned onto the lower level of the foam and pinned into place. Explants were orientated medial side down with the hip joint furthest away from the step. C) Uniaxial compression of the foam support caused the hindlimb to bend at the knee joint mimicking a flexion motion of the knee joint.
2.4. Application of in vitro movement regime
The bioreactor was programmed to apply compressive displacement regimes to the foam supports which caused the angle between the thigh and shank to decrease, mimicking a flexion motion of the knee joint (Fig. 1C). A range of compressive displacements were applied during calibration to quantify the amount of flexion experienced at the knee joint. Six explants were subjected to 1–4 mm of compression and their movement patterns recorded using a high-definition camera (HV-5080, HAVIT, China). The change in the joint angle was calculated using ImageJ (Schneider et al., 2012) to give the flexion magnitudes for each applied displacement. An average-flexion movement regime was designed in order to mimic normal flexion of the chick embryo, and two additional dynamic regimes of lower and higher magnitudes were also included. The three movement regimes were termed reduced-flexion, average-flexion and over-flexion where 2, 3 and 4 mm of compressive displacement were applied respectively, resulting in flexion magnitudes of 9±3, 14±4.0 and 22±5.0 (mean±SD) degrees. Observations of chick motility in ovo report an approximate mean knee flexion of 12 degrees in 9–10 day old embryos (Watson and Bekoff, 1990). All dynamically stimulated explants were loaded with a sinusoidal waveform at an average rate of 4 mm/s for 2 h, three times per day, replicating aspects of activity-inactivity cycles observed in ovo (Chambers et al., 1995, Hamburger et al., 1965), and similar to stimulation patterns used in in vitro micromass cultures (Bian et al., 2011, Elder et al., 2000). Limbs cultured under the same conditions but without dynamic stimulation were used as controls. Six days was chosen as the duration of the culture, as this allowed for sustained viability of the explants and enabled sufficient time for shape morphogenesis to progress in vitro. (Supplementary Video 1 shows the culture system in action).
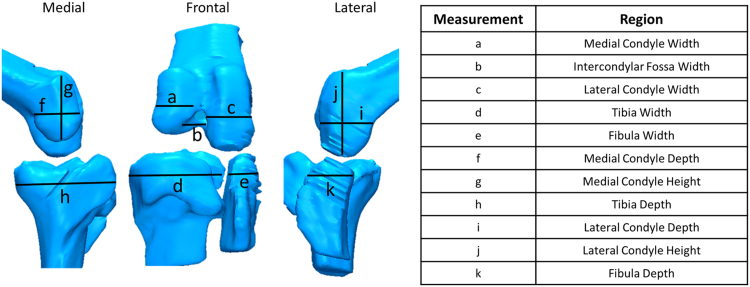
Fig. 2.
Joint regions measured. Eleven joint regions were defined and analysed. Medial, frontal and lateral views of the knee joint were used to visualise the anatomical locations of each joint region.
Supplementary material related to this article can be found online at doi:10.1016/j.jbiomech.2016.09.029.
The following is Supplementary material related to this article Video 1.
Video to show the culture system in action. Hindlimbs are experiencing 3 mm of compressive displacement as per the average-flexion group.
.
2.5. Analysis
2.5.1. Specimen numbers
Three knee joints from 7, 8 and 9 day non-experimental control embryos were analysed for physiological joint shape and size. For the in vitro culture study, a total of 72 chicks were harvested at day 7. For each embryo, the right hindlimb was subjected to movement and the left hindlimb was kept under static conditions. Explants selected for dynamic stimulation were split evenly across the three experimental groups. A total of four cultures were performed for each group, with static controls performed alongside each culture. Explants were pooled together for the three experimental groups and static controls to give four groups for analysis. This yielded a total of 24 explants cultured for each experimental group and a total of 72 static control explants. During media changes, each culture chamber was visually inspected for any signs of abnormal growth or infection. Contaminated or infected cultures were discarded and repeated. Successfully cultured explants were used either for 3D joint analysis or for cellular level analysis. Explants damaged during processing were discarded. A total of 26, 7, 12 and 11 knee joints were analysed for shape and size in the static, reduced-flexion, average-flexion and over-flexion groups respectively. An additional two explants per group were used for cellular level activity assays, and within these samples, two sections from each of the two explants were used to quantify proliferation rates and cell viability.
2.5.2. 3D shape and size analysis
To visualise the cartilaginous skeletal rudiments each explant was stained with 0.055% Alcian blue for 5 h and cleared in 1% KOH for 2 h at room temperature. Samples were prepared for Optical Projection Tomography (OPT) (Sharpe et al., 2002) using protocols outlined in Quintana and Sharpe (2011). 3D models of the limbs were rendered using Mimics (Materialise, Belgium). Models were virtually orientated into a flexed resting position, where the tibia and fibula were aligned vertically and the femur was flexed to align the ventral tip of the lateral condyle with the dorsal surface of the fibula. Frontal, lateral and medial views of the joint were traced to produce shape profiles for each specimen. Shape profiles for each rudiment (femur, tibia and fibula) were grouped and analysed independently. Eleven measurements were made of the distal femur, proximal tibia and fibula, as listed in Fig. 2.
2.5.3. Immunohistochemistry analysis
Limbs were fixed in 4% PFA immediately after culture for 2 h at room temperature. Explants were prepared for cryopreservation by incubating in 15% and 30% sucrose overnight at 4 °C and then embedded and frozen in an OCT-30% Sucrose (50:50) medium over dry ice. All explants were sectioned at 10 µm at −18 °C. Proliferating cells were identified using the mitosis marker phospho-Histone H3 (pHH3), similarly to Roddy et al. (2011a). Cryosections were blocked (5% normal goat serum, 1% DMSO) for 2 h at room temperature before being incubated with anti-phospho-Histone H3 from rabbit ser10 (Merck Millipore, Germany) diluted 1:50 overnight at 4 °C. Cryosections were then incubated with goat anti-rabbit secondary antibody, Cy3 conjugate (Invitrogen, USA) diluted 1:200 at room temperature for 1 h. Sections were counter stained and mounted using a DAPI fluoroshield medium (Sigma, UK). Cell viability was assessed using a terminal deoxynucleotidyl transferase (TdT) dUTP nick-end labelling (TUNEL) detection kit (APO-BRDU-IHC, Bio-Rad Laboratories, USA) using the recommended protocols (AbD Serotec, 2016) to identify cells undergoing apoptosis or necrosis. Dead cells were stained brown and live cells were counter stained green. All sections were imaged at 100x magnification. The number of proliferating and total number of cells were counted using the particle analysis function in ImageJ (U.S. National Institutes of Health, USA). The number of dead cells was counted manually.
2.6. Statistical analysis
For all measured variables and quantities, 1-way ANOVA tests were performed to investigate if significant differences existed between the four groups. If significant differences existed, a post-hoc Tukey-Kramer pairwise comparison was performed to identify specifically which groups were significantly different from each other. p-values less than 0.05 were considered statistically significant.
3. Results
3.1. Physiological chick knee shape development
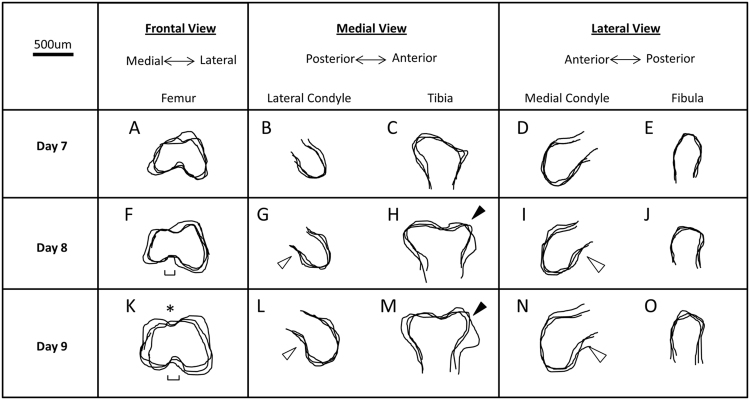
In the distal femur at 7 days, both condyles can be identified as simple outgrowths, with the size of the lateral condyle exceeding that of the medial condyle by 60% in anterior–posterior depth (Fig. 3A, B, D). At this timepoint, the proximal fibula and tibia shapes resemble a simple rounded rod and in the case of the tibia the rounded end appears to protrude posteriorly since the anterior tibial crest is yet to develop (Fig. 3C and E). At 8 days, the lateral condyle remains bigger than the medial condyle (Fig. 3F), but has developed a posterior curl which can be seen in the sagittal plane (Fig. 3I) which also leads to the intercondylar fossa between the femoral condyles becoming more distinct (Fig. 3F). The fibula remains rounded in shape but is 40% larger than at day 7 (Fig. 3J). At this point, the anterior tibial crest first becomes obvious, creating a concave tibial plateau in the sagittal plane (Fig. 3H). At 9 days, the femoral medial condyle appears similar to the lateral condyle, both exhibiting posterior curls seen in the sagittal profiles (Fig. 3K, L and N), with the lateral condyle remaining slightly larger (16%) in anterior–posterior depth than the medial condyle. At this stage, the condyles are clearly separated by the intercondylar fossa on the ventral side, and a channel like groove on the dorsal surface (Fig. 3K). The articular end of the fibula has flattened and curled slightly in the posterior direction (Fig. 3O). The anterior tibial crest has further matured to lengthen the concave shape of the tibial plateau.
Fig. 3.
Distinct shape changes occur in the knee joint shape of normal controls between 7 and 9 days of incubation. Shape profiles of 7 (A–E), 8 (F–J) and 9 (K–0) day old chick embryo knee joints were outlined and overlaid (n=3). Hollow arrows: posterior curl of the condyles; ⁎ dorsal groove between the two femoral condyles; single edged bars: intercondylar fossa; filled arrows: emergence of anterior tibial crest.
3.2. 3D joint region shapes and sizes
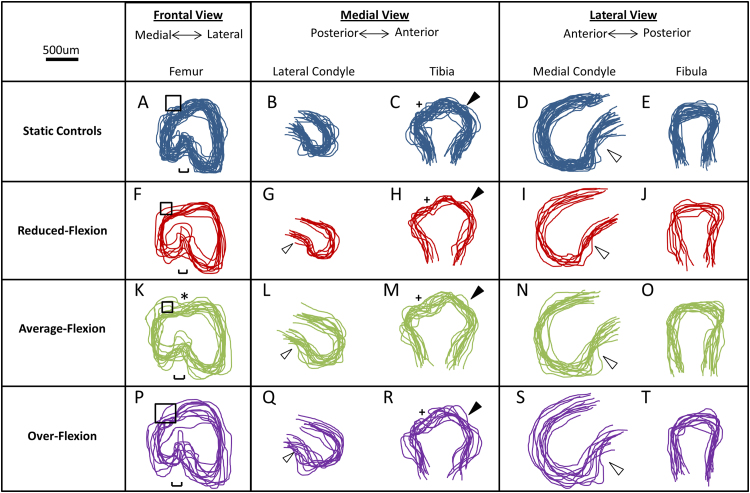
The characteristics of physiological shape development described above were used as indicators to assess the normality of joints developed in vitro. Under this classification system, the joint shapes of explants cultured under static conditions were less developed than any of the dynamically moved explants. In the distal femur of statically cultured limbs, the medial condyle remained substantially smaller than the lateral condyle, measuring 41% smaller on average in condyle depth (Fig. 4A, B and D). The shape of the medial condyle was abnormal, appearing rounded and missing the posterior curl seen in normal shape development (Fig. 4B). In contrast, the lateral side of the statically cultured knee joints exhibited features of normal development, in particular a posterior curl of the lateral condyle (Fig. 4D) and flattening of the proximal fibula (Fig. 4E). While emergence of the anterior tibial crest was detectable in static controls, the tibial plateau exhibited only a slight concave profile in the medial view (Fig. 4C).
Fig. 4.
The average-flexion movement regime led to more normal progression of morphogenesis than other movement regimes or static culture. Shape profiles of the 3D reconstructed joint regions were outlined and overlaid for each cultured group. Squares: dominance of the lateral condyle over the medial condyle; hollow arrows: posterior curl of condyles seen in moved explants; ⁎ dorsal groove between the two femoral condyles; single edged bars: intercondylar fossa; + concave curve of the tibial plateau; filled arrows: anterior tibial crest.
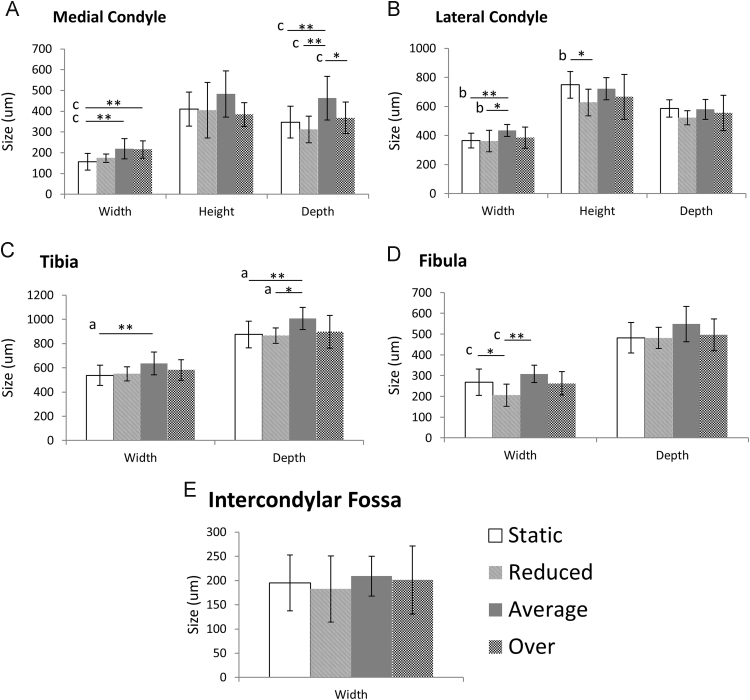
Joints cultured under dynamic movement regimes displayed more features of physiological shape development than seen in the static controls. Of the three dynamic stimulation groups, the average-flexion regime led to joint shapes which most closely approximated normal shape development. In the average-flexion group, both femoral condyles developed a posterior curl (Fig. 4L and N) and were most comparable in size. While the lateral condyle was still bigger than the medial condyle (as seen in the frontal view of the distal femur) (Fig. 4K), the medial condyle was only 20% less deep on average than its opposing lateral condyle, compared to the 41% difference between the condyles reported above for the static controls. The medial condyle was significantly larger in width and depth following average-flexion compared to static controls (Fig. 5A). No significant differences were found in femoral condyle height or lateral condyle depth (Fig. 5A and B) between average-flexion and static groups. The lateral condyle width was significantly wider following average-flexion compared to static controls (Fig. 5B), as were the depth and width of the tibia (Fig. 5C).
Fig. 5.
Significant differences in joint region measurements were most pronounced between the average-flexion regime and static culture. Mean size of joint regions measured for static, reduced-flexion, average-flexion and over-flexion explants. Error bars represent standard deviation. ⁎p-values<0.05; ⁎⁎p-values<0.01. ‘a’ indicates a difference of 10–15%, ‘b’ indicates a difference of 16–20% and ‘c’ indicates a difference of 21–35%, where differences represent how much smaller the regions measured relative to the larger joint.
Differences in joint shape profiles and joint region measurements were also observed between moved groups. The average-flexion regime promoted the most normal progression of shape morphogenesis when compared to reduced-flexion or over-flexion groups. The relative size differences between the depths of the femoral condyles were more pronounced in the reduced-flexion (40% on average) and the over-flexion groups (34% on average) than in the average-flexion group (20%, as reported above). Evidence of a dorsal groove between the femoral condyles was only observable in explants following average-flexion (Fig. 4K), but not in any of the other groups (Fig. 4A, F and P). The intercondylar fossa was clearly distinguishable following average-flexion, but was distorted in both the reduced-flexion and over-flexion groups (Fig. 4F, K and P). While no significant differences were found between the intercondylar fossa widths between the different groups (Fig. 5E), there was greater variation in this joint region size in the reduced-flexion and over-flexion groups than in the average-flexion group (Fig. 5E). On average, reduced-flexion joints were smaller than average-flexion joints, measuring significantly smaller lateral femoral condyle and fibula width and medial femoral condyle and tibia depth. Measurements of joint regions in the over-flexion group were not significantly different to those of the average-flexion group, except for the medial condyle depth, which was significantly smaller by 21% in over-flexion joints compared with average-flexion (Fig. 5A). However, joint shape profiles in the over-flexion group were less consistent when overlaid showing some variability in joint shape development following the larger movements (Fig. 4P–T). In particular, measurements of the lateral condyle depth exhibited a large standard deviation of 22% compared to 12% in the average-flexion group (Fig. 5B). A 3D representation of each experimental group is included in Supplementary materials (Supplementary Videos 2–5).
The following is Supplementary material related to this article Video 2, Video 3, Video 4, Video 5.
Static control 3D representation. The femur is shown in green. The fibula is shown in blue. The tibia is shown in yellow. No scale.
Reduced-flexion 3D representation. The femur is shown in green. The fibula is shown in blue. The tibia is shown in yellow. No scale.
Average-flexion 3D representation. The femur is shown in green. The fibula is shown in blue. The tibia is shown in yellow. No scale.
Over-flexion 3D representation. The femur is shown in green. The fibula is shown in blue. The tibia is shown in yellow. No scale.
.
3.3. Cell viability and proliferation
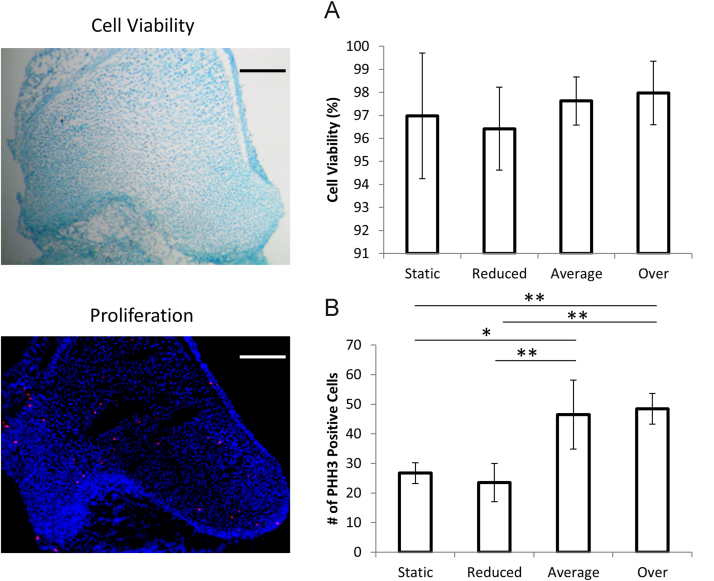
Cell viability within the distal femur was greater than 95% in all groups, demonstrating that high cellular survival was maintained during the culture period, and there were no significant differences between groups for cell viability (Fig. 6A). However, average-flexion and over-flexion regimes led to significantly more proliferation than the static or reduced-flexion regimes (Fig. 6B), with almost double the number of proliferating cells in the average-flexion regime compared to the static or reduced-flexion groups.
Fig. 6.
Average-flexion or over-flexion led to significant increases in cell proliferation, while movement regime did not affect cell viability. Stained sections are shown from the average-flexion group for live/dead and proliferating cells. Viability staining shows dead cells in brown and live cells in green. Proliferation staining shows background cells in blue and proliferating cells in red. Scales bars=200 m. A) shows live cell count as a percentage of total number of cells for each group. B) shows the total number of proliferating cells in the distal femur for each group. Error bars represent standard deviation. ⁎p-values<0.05; ⁎⁎p-values<0.01. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
4. Discussion
This research used in vitro culture of embryonic limb explants to quantify, for the first time, the effects of movement magnitude on morphogenesis of developing joints. The effects of three dynamic movement regimes and one static regime on chick knee joint morphogenesis were compared and supported our hypothesis that joint shape development would be affected by movement magnitude in a dose-dependent manner. The average-flexion regime, which was most similar to physiological movement patterns in ovo, showed the most progression of normal joint shape development when compared to the sequence of physiological shape changes seen in normal embryos. Following average-flexion, the distal femur also contained significantly more proliferative cells than explants which experienced reduced-flexion or no movements, suggesting that the size and shape changes in this group were due to an upregulation in mechanically-mediated proliferation rates. The average-flexion regime also produced the largest joints with both femoral condyles significantly larger than statically-cultured explants. The medial femoral condyle was the most consistently affected region by mechanical stimulation, with differences found in both shape and size between moved and unmoved explants. In the chick, the medial condyle articulates with the tibia and the lateral condyle with the fibula (Roddy et al., 2009). Since the majority of the significant differences between the static and average-flexion regimes were on the medial condyle and tibia, this suggests that average-flexion conditions preferentially promoted development of the medial side of the joint as compared to statically cultured explants. These results highlight the importance of movement during joint development and reveal that the magnitude of movement is a critical mechanical factor in driving joint morphogenesis. This study suggests that movements lower in extent than those seen physiologically can still advance joint morphogenesis but fail to promote proliferation and growth. The research also suggests that movement greater in extent than the physiological range can promote proliferation and growth but can cause irregularities and distortions in joint shape. Analysis of these three magnitudes supports our hypothesis that the most physiological movement regime would lead to a joint shape that more closely approximated normal development and further reveals a non-linear relationship between magnitude of movement and joint shape normality.
The aim of this study was to compare the effects of different movement magnitudes on joint morphogenesis, rather than to recreate physiological joint shapes in vitro. Whilst joints developed in vitro and joints developed in ovo are not directly comparable, the relative comparisons made between the different groups of joints developed in this study do provide an insight into the quantitative relationship between mechanical stimulation and joint morphogenesis, which would not be possible with in vivo experiments. The forces experienced within the joint explant due to the applied movement regimes were not quantified due to the complexity of the culture system, but can be assumed to increase with increased movement magnitudes. We believe that movement, rather than load, is a better parameter to quantify the level of mechanical stimulation, as it is more directly comparable to physiological conditions in vivo. A computational model would be ideal to investigate the role of loading during joint morphogenesis and predict how the compressive load applied to the foam support is transferred to the developing explants. Conditioned medium can significantly increase chondrocyte differentiation, proliferation and ECM synthesis (Elder, 2002, Kanczler et al., 2012) and could potentially amplify the differences found in this study. However, variance in serum performance and biochemical activity would be a possible confounding factor, hence a serum-free medium was used in this study.
While there are limited studies with which to directly compare our findings to, aspects of the shape changes found for in vitro cultured explants correlate with results found in in vivo models. In pharmacologically immobilised chicks, similar abnormal shape features found in statically cultured limbs were reported in the knee joint, including a smaller medial condyle and a flatter, more simplified distal femur (Roddy et al., 2011b). The only recent description of the effects of applying mechanical stimulation in vitro to developing limb explants did not report definitive findings on joint shape (Rodriguez and Munasinghe, 2016). This current study is the first to explore the effects of mechanical stimulation on chondrocyte viability and proliferation in embryonic joint explants cultured in vitro. Previous studies have explored the relationship between mechanical stimulation and proliferation using cellular micro-mass deformation culture models, but conflicting results have been reported (Elder et al., 2000, Juhász et al., 2014, Klumpers et al., 2015). The data from the current study suggests that mechanical stimulation affects proliferation and that a threshold for the level of stimulation exists, above which proliferation is enhanced. This finding agrees with a recent meta-analysis of in vitro cultivation of chondrocytes, which found that a threshold of loading exists for mechanically-mediated enhanced cartilage proliferation and differentiation (Natenstedt et al., 2015). For our experimental model, this threshold lies somewhere between the levels of stimulation applied in the reduced-flexion and average-flexion groups. Our findings that proliferation is down-regulated in cases of static or reduced-stimulation agrees with Roddy et al. (2011b) who showed a significant decrease in cell proliferation in the distal femur of immobilised chick embryos. Tissue engineers are currently trying to develop functional cartilage from stem cells or progenitor cell populations and it has been proposed that replicating the effects of mechanical stimulation during embryogenesis may be required to achieve this aim (Foster et al., 2015). This study provides evidence to support this theory, and shows that physiological magnitudes of mechanical stimulation in vitro are most beneficial.
In conclusion, we have shown that joint morphogenesis of the embryonic chick knee joint is affected by the magnitude of movement experienced in vitro. The most developed joint shapes were observed under a movement regime similar to those observed in ovo. Above this, the joint shape was seen to vary and even deteriorate, whilst reduced-flexion produced shapes more similar to those of statically cultured explants. This study reveals for the first time, how the magnitudes of fetal movements direct and determine aspects of prenatal joint morphogenesis, with important implications for understanding the etiology of congenital joint abnormalities related to abnormal fetal movements. Furthermore, this research provides useful cues to the cartilage tissue engineering field, in demonstrating that replicating the magnitude of physiological fetal movements leads to the most normal progression of cartilage growth.
Conflict of interest statement
The authors have no conflicts of interest relating to this research.
Acknowledgements
This research was funded by the European Research Council under the European Union׳s Seventh Framework Programme (ERC Grant agreement no. [336306]), and in part by the Medical Engineering Solutions in Osteoarthritis Centre of Excellence funded by the Wellcome Trust and the EPSRC (088844/Z/09/Z). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
We thank Dr Mariea Brady for her consultation with the design and optimisation of the novel explant culture system, and Ms Samantha Martin for assistance with optimisation of the histology and immunohistochemistry analyses and technical advice.
References
- AbD Serotec. 2016. A Complete Kit for Measuring Apoptosis by Dual Color ImmunoHistoChemistry.
- Bian L., Zhai D.Y., Zhang E.C., Mauck R.L., Burdick J.A. Dynamic compressive loading enhances cartilage matrix synthesis and distribution and suppresses hypertrophy in hMSC-laden hyaluronic acid hydrogels. Tissue Eng. Part A. 2011;18:715–724. doi: 10.1089/ten.tea.2011.0455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunt L.H., Norton J.L., Bright J.A., Rayfield E.J., Hammond C.L. Finite element modelling predicts changes in joint shape and cell behaviour due to loss of muscle strain in jaw development. J. Biomech. 2015;48:3112–3122. doi: 10.1016/j.jbiomech.2015.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers S., Bradley N., Orosz M. Kinematic analysis of wing and leg movements for type I motility in E9 chick embryos. Exp. Brain Res. 1995;103:218–226. doi: 10.1007/BF00231708. [DOI] [PubMed] [Google Scholar]
- Cohen I., Robinson D., Melamed E., Nevo Z. Use of a novel joint-simulating culture system to grow organized ex-vivo three-dimensional cartilage-like constructs from embryonic epiphyseal cells. Iowa Orthop. J. 2005;25:102. [PMC free article] [PubMed] [Google Scholar]
- Drachman D.B., Sokoloff L. The role of movement in embryonic joint development. Dev. Biol. 1966;14:401–420. [Google Scholar]
- Elder S.H. Conditioned medium of mechanically compressed chick limb bud cells promotes chondrocyte differentiation. J. Orthop. Sci. 2002;7:538–543. doi: 10.1007/s007760200096. [DOI] [PubMed] [Google Scholar]
- Elder S.H., Kimura J., Soslowsky L.J., Lavagnino M., Goldstein S.A. Effect of compressive loading on chondrocyte differentiation in agarose cultures of chick limb-bud cells. J. Orthop. Res. 2000;18:78–86. doi: 10.1002/jor.1100180112. [DOI] [PubMed] [Google Scholar]
- Fell H.B., Canti R.G. Experiments on the Development in vitro of the avian knee-joint. Proc. R. Soc. Lond. Ser. B, Biol. Sci. 1934;116:316–351. [Google Scholar]
- Foster N.C., Henstock J.R., Reinwald Y., El Haj A.J. Dynamic 3D culture: models of chondrogenesis and endochondral ossification. Birth Defects Res. Part C: Embryo Today: Rev. 2015;105:19–33. doi: 10.1002/bdrc.21088. [DOI] [PubMed] [Google Scholar]
- Hall B.K., Herring S.W. Paralysis and growth of the musculoskeletal system in the embryonic chick. J. Morphol. 1990;206:45–56. doi: 10.1002/jmor.1052060105. [DOI] [PubMed] [Google Scholar]
- Hamburger V., Balaban M., Oppenheim R., Wenger E. Periodic motility of normal and spinal chick embryos between 8 and 17 days of incubation. J. Exp. Zool. 1965;159:1–13. doi: 10.1002/jez.1401590102. [DOI] [PubMed] [Google Scholar]
- Henstock J.R., Rotherham M., Rose J.B., El Haj A.J. Cyclic hydrostatic pressure stimulates enhanced bone development in the foetal chick femur in vitro. Bone. 2013;53:468–477. doi: 10.1016/j.bone.2013.01.010. [DOI] [PubMed] [Google Scholar]
- Hosseini A., Hogg D.A. The effects of paralysis on skeletal development in the chick embryo. I. General effects. J. Anat. 1991;177:159–168. [PMC free article] [PubMed] [Google Scholar]
- Juhász T., Matta C., Somogyi C., Katona É., Takács R., Soha R.F., Szabó I.A., Cserháti C., Sződy R., Karácsonyi Z. Mechanical loading stimulates chondrogenesis via the PKA/CREB-Sox9 and PP2A pathways in chicken micromass cultures. Cell. Signal. 2014;26:468–482. doi: 10.1016/j.cellsig.2013.12.001. [DOI] [PubMed] [Google Scholar]
- Kahn J., Shwartz Y., Blitz E., Krief S., Sharir A., Breitel D.A., Rattenbach R., Relaix F., Maire P., Rountree R.B., Kingsley D.M., Zelzer E. Muscle contraction is necessary to maintain joint progenitor cell fate. Dev. Cell. 2009;16:734–743. doi: 10.1016/j.devcel.2009.04.013. [DOI] [PubMed] [Google Scholar]
- Kanczler J.M., Smith E.L., Roberts C.A., Oreffo R.O.C. A novel approach for studying the temporal modulation of embryonic skeletal development using organotypic bone cultures and microcomputed tomography. Tissue Eng. Part C: Methods. 2012;18:747–760. doi: 10.1089/ten.tec.2012.0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klumpers, D.D., Smit, T.H. & Mooney, D.J. 2015. The Effect of Growth-Mimicking Continuous Strain on the Early Stages of Skeletal Development in Micromass Culture. [DOI] [PMC free article] [PubMed]
- Lelkes G. Experiments in vitro on the role of movement in the development of joints. J. Embryol. Exp. Morphol. 1958;6:183–186. [PubMed] [Google Scholar]
- Natenstedt J., Kok A.C., Dankelman J., Tuijthof G.J. What quantitative mechanical loading stimulates in vitro cultivation best? J. Exp. Orthop. 2015;2:1–15. doi: 10.1186/s40634-015-0029-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowlan N. Biomechanics of foetal movement. Eur. Cells Mater. 2015;29:1. doi: 10.22203/ecm.v029a01. [DOI] [PubMed] [Google Scholar]
- Nowlan N.C., Bourdon C., Dumas G., Tajbakhsh S., Prendergast P.J., Murphy P. Developing bones are differentially affected by compromised skeletal muscle formation. Bone. 2010;46:1275–1285. doi: 10.1016/j.bone.2009.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowlan N.C., Chandaria V., Sharpe J. Immobilized chicks as a model system for early-onset developmental dysplasia of the hip. J. Orthop. Res. 2014;32:777–785. doi: 10.1002/jor.22606. [DOI] [PubMed] [Google Scholar]
- Nowlan N.C., Sharpe J., Roddy K.A., Prendergast P.J., Murphy P. Mechanobiology of embryonic skeletal development: Insights from animal models. Birth Defects Res. Part C: Embryo Today: Rev. 2010;90:203–213. doi: 10.1002/bdrc.20184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne A.C., Lamb K.J., Lewthwaite J.C., Dowthwaite G.P., Pitsillides A.A. Short-term rigid and flaccid paralyses diminish growth of embryonic chick limbs and abrogate joint cavity formation but differentially preserve pre-cavitated joints. J. Musculoskelet. Neuronal Interact. 2002;2:448–456. [PubMed] [Google Scholar]
- Pörtner R., Nagel-Heyer S., Goepfert C., Adamietz P., Meenen N.M. Bioreactor design for tissue engineering. J. Biosci. Bioeng. 2005;100:235–245. doi: 10.1263/jbb.100.235. [DOI] [PubMed] [Google Scholar]
- Quintana L., Sharpe J. Optical projection tomography of vertebrate embryo development. Cold Spring Harb. Protoc. 2011;2011:586–594. doi: 10.1101/pdb.top116. pdb.top116. [DOI] [PubMed] [Google Scholar]
- Roddy K.A., Kelly G.M., van Es M.H., Murphy P., Prendergast P.J. Dynamic patterns of mechanical stimulation co-localise with growth and cell proliferation during morphogenesis in the avian embryonic knee joint. J. Biomech. 2011;44:143–149. doi: 10.1016/j.jbiomech.2010.08.039. [DOI] [PubMed] [Google Scholar]
- Roddy K.A., Nowlan N.C., Prendergast P.J., Murphy P. 3D representation of the developing chick knee joint: a novel approach integrating multiple components. J. Anat. 2009;214:374–387. doi: 10.1111/j.1469-7580.2008.01040.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roddy K.A., Prendergast P.J., Murphy P. Mechanical influences on morphogenesis of the knee joint revealed through morphological, molecular and computational analysis of immobilised embryos. PLoS One. 2011:6. doi: 10.1371/journal.pone.0017526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez E.K., Munasinghe J. A chick embryo in-vitro model of knee morphogenesis. Arch. Bone Jt. Surg. 2016;4:109–115. [PMC free article] [PubMed] [Google Scholar]
- Sandell L.J. Etiology of osteoarthritis: genetics and synovial joint development. Nat. Rev. Rheumatol. 2012;8:77–89. doi: 10.1038/nrrheum.2011.199. [DOI] [PubMed] [Google Scholar]
- Schneider C.A., Rasband W.S., Eliceiri K.W. NIH image to ImageJ: 25 years of image analysis. Nat. Methods. 2012;9:671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpe J., Ahlgren U., Perry P., Hill B., Ross A., Hecksher-Sørensen J., Baldock R., Davidson D. Optical projection tomography as a tool for 3D microscopy and gene expression studies. Science. 2002;296:541–545. doi: 10.1126/science.1068206. [DOI] [PubMed] [Google Scholar]
- Smith E.L., Kanczler J.M., Oreffo R.O. A new take on an old story: chick limb organ culture for skeletal niche development and regenerative medicine evaluation. Eur. Cell Mater. 2013;26:91–106. doi: 10.22203/ecm.v026a07. discussion 106. [DOI] [PubMed] [Google Scholar]
- Watson S.J., Bekoff A. A kinematic analysis of hindlimb motility in 9- and 10-day-old chick embryos. J. Neurobiol. 1990;21:651–660. doi: 10.1002/neu.480210412. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video to show the culture system in action. Hindlimbs are experiencing 3 mm of compressive displacement as per the average-flexion group.
Static control 3D representation. The femur is shown in green. The fibula is shown in blue. The tibia is shown in yellow. No scale.
Reduced-flexion 3D representation. The femur is shown in green. The fibula is shown in blue. The tibia is shown in yellow. No scale.
Average-flexion 3D representation. The femur is shown in green. The fibula is shown in blue. The tibia is shown in yellow. No scale.
Over-flexion 3D representation. The femur is shown in green. The fibula is shown in blue. The tibia is shown in yellow. No scale.