Abstract
Background and Purpose
Subjective cognitive complaints (SCCs) are very common in patients with Parkinson's disease (PD). However, the relationship between SCCs and objective cognitive impairment is still unclear. This study aimed to determine whether SCCs are correlated with objective cognitive performance in patients with PD.
Methods
Totals of 148 cognitively normal patients, 71 patients with mild cognitive impairment (MCI), and 31 demented patients were recruited consecutively from a movement-disorders clinic. Their SCCs and cognitive performances were evaluated using the Cognitive Complaints Interview (CCI) and a comprehensive neuropsychological battery.
Results
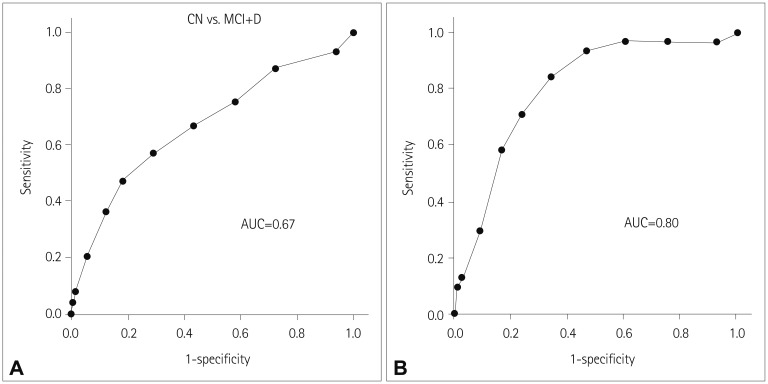
The CCI score increased with age, duration of PD, and depression score, and was inversely correlated with cognitive performance. The association between CCI score and performance remained significant after adjustment for the depression score, age, and duration of PD. The CCI score could be used to discriminate patients with dementia from cognitively normal and MCI patients [area under the receiver operating characteristics curve (AUC) of 0.80], but not patients with MCI or dementia from cognitively normal patients (AUC of 0.67).
Conclusions
SCCs as measured by the CCI are strongly correlated with objective cognitive performance in patients with PD. The CCI can also be used to screen for dementia in patients with PD.
Keywords: Parkinson's disease, cognition, cognitive dysfunction, dementia
INTRODUCTION
Parkinson's disease (PD) is diagnosed based on its motor symptoms, but cognitive impairment is actually the most disabling complication in patients with PD.1 A quarter of newly diagnosed patients with PD have mild cognitive impairment (MCI),2,3 and approximately 90% of PD patients eventually develop dementia.4
Subjective feelings of cognitive impairment subjective cognitive complaints (SCCs) are very common in the elderly, and the clinical importance of SCCs is increasing. The subjective feeling of memory impairment subjective memory complaints (SMCs) was considered an important component in the early concept of MCI due to Alzheimer's disease (AD), and SMCs have received attention as a possible predictor for subsequent cognitive impairment even in cognitively normal individuals.
Patients with PD also frequently report SCCs,5 but their clinical meaning is still unclear. One of the fundamental questions is whether SCCs are related to objective cognitive impairment. Several studies that have explored this relationship have produced conflicting results. Three studies found an association between subjective complaints and poorer cognitive performances,5,6,7 whereas others failed to show clear relationship8 or found that cognitive scores were higher in patients with SCCs than in those without SCCs.9
These studies used various methods to define SCCs, with some assessing SCCs using a simple yes/no question,6,8,9 and others using questionnaires focusing on memory symptoms only.5,7 However, because the pattern of cognitive impairment in patients with PD is not identical to that of AD, other tools employing questions covering various cognitive symptoms beyond memory complaints are needed.
The Cognitive Complaints Interview (CCI) is a validated questionnaire for assessing SCCs that includes questions about subjective complaints on memory, language, and visuospatial function, daily activity, and mood. We therefore considered the CCI to be suitable for assessing SCCs in patients with PD.
The aim of this study was to determine the association between SCCs as measured by the CCI and objective cognitive performance, and to verify the ability of the CCI to detect objective cognitive impairment in patients with PD.
METHODS
Subjects
We consecutively recruited patients with PD from a movement-disorders clinic. All of the included patients were diagnosed with PD based on the criteria of the UK Parkinson's Disease Society Brain Bank.10 Patients who had a focal lesion in the basal ganglia revealed by magnetic resonance imaging or abnormalities in laboratory tests including thyroid function tests, vitamin B12 level, folic acid level, or the syphilis test were excluded from this study. Patients who experienced visual hallucinations or had dementia that developed before or within 1 year after the onset of motor symptoms were also excluded. Because several of the cognitive tests are not applicable to individuals with a low level of education, only patients who had received at least 9 years of education (middle-school graduate) were enrolled in this study.
We received approval for human experimentation from the Institutional Review Board at the hospital (Approval No. CR315007), and written informed consent was obtained from all subjects participating in this study.
Cognitive assessment
The cognitive assessments were conducted by experienced neuropsychologists using the following tests in order to fulfill the Level II criteria for PD-MCI11 proposed by the Movement Disorders Society (MDS): forward digit span12 and trail-making test type A (TMT-A)12 for the attention domain, the Korean version of the Boston Naming Test (K-BNT)12 and similarity test of the Wechsler Adult Intelligence Scale-Fourth Edition13 for language function, the copying task of the Rey-Osterrieth Complex Figure Test (RCFT)12 and clock copying (CLOX2)14 for visuospatial function, 20-min delayed recall using the Seoul Verbal Learning Test (SVLT)12 and 20-min delayed recall using the RCFT12 for the memory domain, semantic fluency (animal) using the Controlled Oral Word Association Test,12 and the clock-drawing test for executive function. The Korean version of the Mini Mental State Examination (K-MMSE),15 Korean version of the Montreal Cognitive Assessment (K-MoCA),16 and self-rated Beck Depression Inventory (BDI) were used to assess general cognition and depressive symptoms in the subjects.
Assessment of SCCs using the CCI
The subjective self-perceived cognitive function of the subjects was assessed using the CCI17 just before they completed a comprehensive neuropsychological assessment. The questions from the CCI were translated into Korean by the investigators and provided to subjects on paper. The subjects were asked to read the questions and answer as either “yes” or “no.” The CCI score was calculated as the number of questions to which the subjects responded “yes” to.
Diagnosis of MCI and dementia
MCI was diagnosed according to the following Level II criteria for PD-MCI proposed by the MDS:11 1) performance in at least 2 of the 10 subtests was lower than the mean minus 1.5×standard deviations of the normative data corrected for age and level of education and 2) activities of daily living (ADL) were not impaired.
Dementia was diagnosed according to the following criteria proposed by the MDS:18 1) the mean z score of two tests for each cognitive domain was lower than -1.5 in at least two domains and 2) impaired ADL.
Statistical analysis
One-way analysis of variance and the chi-square test were used to compare groups, with the Bonferroni method used for post hoc analyses. The Kuder-Richardson Formula 20 (KR-20) coefficient was calculated to verify the internal consistency of the CCI. We used multivariate linear regression models and calculated partial correlation coefficients to adjust for the confounding factors influencing the relationship between the CCI score and cognitive performance. Receiver operating characteristics (ROC) curves were drawn to test the discriminative value of the CCI score. Statistical analyses were performed using SPSS Statistics (version 23, IBM Corp., Armonk, NY, USA), and p<0.05 was considered significant.
RESULTS
Subjects and baseline demographics
In total, 250 patients with PD (150 males and 100 females) were recruited for this study, and they were classified into cognitively normal (CN, n=148), MCI (n=71), and dementia (D, n=31) groups based on neuropsychological assessments. The subjects in group D were older and had a longer duration of PD and higher Unified Parkinson's Disease Rating Scale (UPDRS) motor score than those in group CN, and both groups MCI and D had a higher levodopa equivalent daily dose (LEDD) and BDI score than group CN (Table 1). There were no differences in sex, age at onset of PD, or level of education among the groups.
Table 1. Demographic characteristics of subjects classified according to cognitive status.
| Variables | CN (n=148) | MCI (n=71) | D (n=31) | p | Significant differences |
|---|---|---|---|---|---|
| Sex (male/female) | 84/64 | 45/26 | 21/10 | 0.415 | |
| Age (years) | 68.2±7.9 | 70.0±8.7 | 73.6±7.2 | 0.003 | CN<D |
| Age at onset of PD (years) | 64.4±8.7 | 64.9±10.8 | 67.7±9.0 | 0.215 | |
| Duration of PD (years) | 3.8±3.6 | 5.1±4.8 | 5.9±4.9 | 0.008 | CN<D |
| UPDRS motor score | 22.3±10.8 | 27.6±10.1 | 32.6±11.8 | 0.004 | CN<D |
| LEDD (mg/day) | 298.6±298.7 | 642.2±535.4 | 736.8±342.1 | <0.001 | CN<MCI, D |
| Level of education (years) | 12.3±2.9 | 12.8±3.0 | 13.3±3.3 | 0.171 | |
| BDI score | 11.1±7.9 | 15.7±10.3 | 18.3±11.2 | <0.001 | CN<MCI, D |
Data are mean±standard-deviation values.
BDI: Beck Depression Inventory, CN: cognitively normal, D: dementia, LEDD: levodopa equivalent daily dose, MCI: mild cognitive impairment, PD: Parkinson's disease, UPDRS: Unified Parkinson's Disease Rating Scale.
Neuropsychological performance
The findings for the neuropsychological performance of the subjects are presented in Supplementary Table 1 (in the online-only Data Supplement). Groups CN and MCI showed better performance in all tests compared to group D. Groups CN and MCI performed comparably in the TMT-A, while group CN performed better than MCI group in all of the other tests.
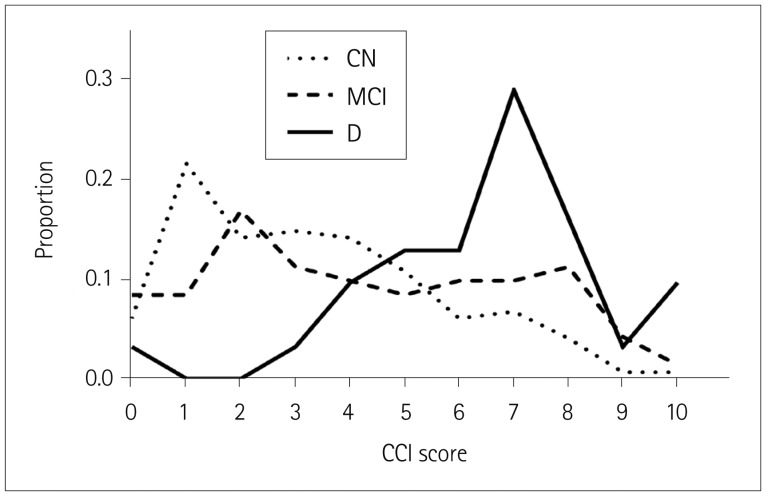
The CCI score was lowest in group CN and highest in group D (Fig. 1). Significant differences were observed among the groups.
Fig. 1. Proportions of CCI scores in the three cognitive groups. CCI: Cognitive Complaints Interview, CN: cognitively normal, D: dementia, MCI: mild cognitive impairment.
Internal consistency of the CCI
The KR-20 coefficient of the CCI was 0.78, and the coefficients for corrected item-total correlation were greater than 0.25 (p<0.001) for all items of the CCI.
Correlation between the CCI score and neuropsychological performance
The CCI score was strongly correlated with the scores in all of the neuropsychological performance tests (Table 2), and also correlated with other factors including age (r=0.145, p=0.022), duration of PD (r=0.158, p=0.013), LEDD (r=0.338, p=0.003), and BDI score (r=0.527, p<0.001).
Table 2. Correlation between CCI score and neuropsychological performance.
| Variables | Pearson's correlation | Model 1 | Model 2 | |||
|---|---|---|---|---|---|---|
| r | p | r | p | r | p | |
| Age | 0.145 | 0.022 | ||||
| Age at onset of PD | 0.056 | 0.374 | ||||
| Duration of PD | 0.158 | 0.013 | ||||
| UPDRS motor score | 0.142 | 0.166 | ||||
| LEDD | 0.338 | 0.003 | ||||
| Level of education | −0.084 | 0.188 | ||||
| K-MMSE score | −0.370 | <0.001 | −0.270 | <0.001 | −0.221 | 0.001 |
| K-MoCA score | −0.339 | <0.001 | −0.223 | <0.001 | −0.168 | 0.008 |
| Forward digit span | −0.220 | <0.001 | −0.120 | 0.058 | −0.078 | 0.227 |
| TMT-A score | 0.258 | <0.001 | 0.191 | 0.003 | 0.150 | 0.020 |
| K-BNT score | −0.345 | <0.001 | −0.239 | <0.001 | −0.205 | 0.001 |
| Word similarity | −0.164 | 0.010 | −0.011 | 0.863 | 0.025 | 0.693 |
| Copying task of RCFT | −0.386 | <0.001 | −0.271 | <0.001 | −0.236 | <0.001 |
| Clock copying (CLOX2) | −0.206 | 0.001 | −0.125 | 0.050 | −0.089 | 0.169 |
| Delayed recall in SVLT | −0.352 | <0.001 | −0.239 | <0.001 | −0.179 | 0.005 |
| Delayed recall in RCFT | −0.345 | <0.001 | −0.212 | 0.001 | −0.166 | 0.009 |
| Semantic fluency (animal) | −0.230 | <0.001 | −0.106 | 0.095 | −0.043 | 0.507 |
| Clock-drawing test | −0.311 | <0.001 | −0.253 | <0.001 | −0.220 | 0.001 |
| BDI | 0.527 | <0.001 | ||||
Model 1: adjusted for BDI score, Model 2: adjusted for BDI score, age, sex, duration of PD, and level of education, r: correlation coefficient.
BDI: Beck Depression Inventory, CCI: Cognitive Complaints Interview, LEDD: levodopa equivalent daily dose, K-BNT: Korean version of the Boston Naming Test, K-MMSE: Korean version of the Mini Mental State Examination, K-MoCA: Korean version of the Montreal Cognitive Assessment, PD: Parkinson's disease, RCFT: Rey-Osterrieth Complex Figure Test, SVLT: Seoul Verbal Learning Test, TMT-A: trail-making test type A.
When the model was adjusted for the BDI score (model 1 in Table 2), the CCI score was significantly correlated with the K-MMSE score, K-MoCA score, and performance in the TMT-A, K-BNT, copying task of the RCFT, delayed recall in both the SVLT and RCFT, and clock-drawing test. The other demographic factors including age, sex, duration of PD, and level of education (model 2 in Table 2) did not influence the significance of the correlation between the CCI and cognitive performance found in model 1.
Discriminative value of the CCI score
ROC curves were drawn to evaluate the discriminative power of the CCI score (Fig. 2).
Fig. 2. ROC curves for discriminating (A) group MCI or D from group CN, and (B) group D from group CN or MCI, AUC, area under the ROC curve. CN: cognitively normal, D: dementia, MCI: mild cognitive impairment, ROC: receiver operating characteristics.
The CCI score could be used to discriminate group D from groups CN and MCI [area under the ROC curve (AUC)=0.80, 95% confidence interval (CI)=0.72–0.88]. The optimal screening cutoff was a CCI score of ≥5 (sensitivity and specificity of 0.84 and 0.66, respectively), and the optimal diagnostic cutoff was a CCI score of ≥7 (sensitivity and specificity of 0.58 and 0.83, respectively).
However, the CCI score could not be used to discriminate group MCI or D from group CN (AUC=0.67, 95% CI=0.60–0.74).
DISCUSSION
The present results show that the severity of SCCs as measured by the CCI is correlated with objective cognitive impairment. The CCI can also be used for screening dementia in patients with PD.
SCCs were more prominent when the cognitive status was lower in this study. There were more cognitive complaints in group MCI than in group CN, and the CCI score was highest in group D. A relationship between cognitive impairment and SCCs indexes has been consistently reported in patients without PD. Recent validation studies found that scores were higher in patients with MCI or dementia due to AD than in cognitively normal subjects.19,20 Previous studies of patients with PD also found a significant relationship between SCCs and cognitive impairment. Two studies showed an inverse correlation between the SMCs score and cognitive performance in nondemented PD patients.5,7 Two other studies found a relationship between lower visuospatial or executive function and the presence of SCCs in cognitively normal patients with PD.6,21 Consistent with previous studies, the present results also demonstrated that a higher SCCs score is strongly correlated with worse performance in many cognitive subtests.
All previous studies have assessed SCCs using a simple yes/no question or questionnaire for SMCs, and so the present study is the first to assess SCCs using an ordinal scale, which revealed a significant correlation with objective cognitive performance. SCCs in patients with AD focus on memory function because memory impairment is the most common and earliest manifestation of AD. However, this is not the essential component of cognitive impairment in patients with PD–executive dysfunction is actually the characteristic feature and predictor of developing dementia in PD-MCI.22 Therefore, the CCI including questions on cognitive abilities other than memory function seems to be more suitable for assessing SCCs in patients with PD, and its scores were strongly correlated with objective cognitive performance in this study. However, the value of such questions in assessing cognitive function other than memory needs to be verified in a longitudinal study. Also, the most appropriate questions need to be determined in order to develop a better tool for measuring SCCs in patients with PD.
The average CCI score increased gradually with cognitive decline in the present study. Both the score distribution (Fig. 1) and discriminating power (Fig. 2) indicated that the difference was more prominent between patients with dementia and MCI. In previous studies that included patients with dementia and MCI due to AD, the SCCs scores were higher in MCI patients than in cognitively normal control subjects, with demented patients showing similar or lower scores than MCI patients. The authors supposed that the higher SCCs score in MCI patients than control may be a reflection of self-awareness for cognitive dysfunction, with the lower SCCs score of demented patients being due to their deterioration of awareness. Unlike AD, the subjective feeling of cognitive impairment in patients with PD seems to be maintained by their demented status, which suggests that the underlying pathophysiological mechanisms of SCCs in patients with PD may differ from that of AD.
SCCs are well known to be related to psychological problems, especially depression. The BDI score was strongly correlated with the CCI score in the present study, and so regression models were used to adjust for the BDI score. This adjustment reduced the strength of the correlation between cognitive performance and CCI score, while the correlations in many subtests including the K-MMSE and K-MoCA remained significant. Depression is a very common nonmotor symptom in patients with PD, and the incidence rates of both depression and cognitive impairment increase with the disease progression. The present data also revealed a strong correlation between BDI and CCI scores; however, further investigations are needed into the causal relationship between depression and SCCs.
The present results show that the CCI score is useful for discriminating dementia from normal cognition or MCI. A previous study using the CCI in patients with PD found that this metric had insufficient power to screen for PD-associated dementia (AUC=0.69).17 This discrepancy may have been due to differences in cognitive assessment methods and diagnostic criteria for dementia. That previous study divided patients into cognitively normal and impaired groups, while the present study classified subjects into cognitively normal, MCI, and dementia groups. The CCI score could be used to distinguish between demented and nondemented patients in present study, and so the heterogeneity in the cognitively impaired group of the previous study could have weakened the discriminant power of the CCI.
This study was subject to several limitations. First, the cross-sectional design meant that longitudinal changes in cognition could not be predicted, and so a future long-term study is needed to expand on the clinical implications of the CCI. Second, selection bias may have been present since this study involved a patient population from a tertiary referral hospital. Finally, this study did not include pathological data. Because AD pathology was also observed in patients with PD,23,24 it is possible that AD pathology was intermixed with PD pathology in the present subjects.
In summary, SCCs as measured by the CCI are strongly correlated with objective cognitive performance in patients with PD. In addition, the CCI can be used to screen for dementia in patients with PD.
Acknowledgements
This research was supported by the Original Technology Research Program for Brain Science through the National Research Foundation of Korea (NRF) funded by the Korean government (MSIP) (No. 2014M3C7A1064752).
Footnotes
Conflicts of Interest: The authors have no financial conflicts of interest.
Supplementary Materials
The online-only Data Supplement is available with this article at https://doi.org/10.3988/jcn.2018.14.1.16.
Neuropsychological performance of subjects classified according to cognitive status.
References
- 1.Schrag A, Jahanshahi M, Quinn N. What contributes to quality of life in patients with Parkinson's disease? J Neurol Neurosurg Psychiatry. 2000;69:308–312. doi: 10.1136/jnnp.69.3.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aarsland D, Bronnick K, Williams-Gray C, Weintraub D, Marder K, Kulisevsky J, et al. Mild cognitive impairment in Parkinson disease: a multicenter pooled analysis. Neurology. 2010;75:1062–1069. doi: 10.1212/WNL.0b013e3181f39d0e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Muslimovic D, Post B, Speelman JD, Schmand B. Cognitive profile of patients with newly diagnosed Parkinson disease. Neurology. 2005;65:1239–1245. doi: 10.1212/01.wnl.0000180516.69442.95. [DOI] [PubMed] [Google Scholar]
- 4.Hely MA, Reid WG, Adena MA, Halliday GM, Morris JG. The Sydney multicenter study of Parkinson's disease: the inevitability of dementia at 20 years. Mov Disord. 2008;23:837–844. doi: 10.1002/mds.21956. [DOI] [PubMed] [Google Scholar]
- 5.Lehrner J, Moser D, Klug S, Gleiß A, Auff E, Pirker W, et al. Subjective memory complaints, depressive symptoms and cognition in Parkinson's disease patients. Eur J Neurol. 2014;21:1276–1284.:e1277. doi: 10.1111/ene.12470. [DOI] [PubMed] [Google Scholar]
- 6.Mills KA, Mari Z, Pontone GM, Pantelyat A, Zhang A, Yoritomo N, et al. Cognitive impairment in Parkinson's disease: association between patient-reported and clinically measured outcomes. Parkinsonism Relat Disord. 2016;33:107–114. doi: 10.1016/j.parkreldis.2016.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sitek EJ, Sołtan W, Wieczorek D, Robowski P, SXławek J. Self-awareness of memory function in Parkinson's disease in relation to mood and symptom severity. Aging Ment Health. 2011;15:150–156. doi: 10.1080/13607863.2010.508773. [DOI] [PubMed] [Google Scholar]
- 8.Copeland JN, Lieberman A, Oravivattanakul S, Tröster AI. Accuracy of patient and care partner identification of cognitive impairments in Parkinson's disease-mild cognitive impairment. Mov Disord. 2016;31:693–698. doi: 10.1002/mds.26619. [DOI] [PubMed] [Google Scholar]
- 9.Castro PC, Aquino CC, Felício AC, Doná F, Medeiros LM, Silva SM, et al. Presence or absence of cognitive complaints in Parkinson's disease: mood disorder or anosognosia? Arq Neuropsiquiatr. 2016;74:439–444. doi: 10.1590/0004-282X20160060. [DOI] [PubMed] [Google Scholar]
- 10.Gibb WR, Lees AJ. The relevance of the Lewy body to the pathogenesis of idiopathic Parkinson's disease. J Neurol Neurosurg Psychiatry. 1988;51:745–752. doi: 10.1136/jnnp.51.6.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Litvan I, Goldman JG, Tröster AI, Schmand BA, Weintraub D, Petersen RC, et al. Diagnostic criteria for mild cognitive impairment in Parkinson's disease: Movement Disorder Society Task Force guidelines. Mov Disord. 2012;27:349–356. doi: 10.1002/mds.24893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kang YW, Jang SM, Na DL. Seoul Neuropsychological Screening Battery. 2nd ed. Seoul: Human Brain Research & Consulting Co.; 2012. [Google Scholar]
- 13.Hwang S, Kim J, Park G, Chey J, Hong S. Korean Wechsler Adult Intelligence Scale. 4th ed. Daegu: Korea Psychology Co. Ltd.; 2012. [Google Scholar]
- 14.Kim SG, Lee DY, Seo EH, Choo IH, Kim JW, Do YJ, et al. A Normative Study of an Executive Clock Drawing Task (CLOX) in Korean Elderly. J Korean Neuropsychiatr Assoc. 2009;48:437–446. [Google Scholar]
- 15.Kang Y, Na DL, Hahn S. A validity study on the Korean Mini-Mental State Examination (K-MMSE) in dementia patients. J Korean Neurol Assoc. 1997;15:300–308. [Google Scholar]
- 16.Kang YW, Park JS, Yu KH, Lee BC. A reliability, validity, and normative study of the Korean-Montreal Cognitive Assessment (K-MoCA) as an instrument for screening of Vascular Cognitive Impairment (VCI) Korean J Clin Psychol. 2009;28:549–562. [Google Scholar]
- 17.Dujardin K, Duhamel A, Delliaux M, Thomas-Antérion C, Destée A, Defebvre L. Cognitive complaints in Parkinson's disease: its relationship with objective cognitive decline. J Neurol. 2010;257:79–84. doi: 10.1007/s00415-009-5268-2. [DOI] [PubMed] [Google Scholar]
- 18.Dubois B, Burn D, Goetz C, Aarsland D, Brown RG, Broe GA, et al. Diagnostic procedures for Parkinson's disease dementia: recommendations from the movement disorder society task force. Mov Disord. 2007;22:2314–2324. doi: 10.1002/mds.21844. [DOI] [PubMed] [Google Scholar]
- 19.Rami L, Mollica MA, García-Sanchez C, Saldaña J, Sanchez B, Sala I, et al. The Subjective Cognitive Decline Questionnaire (SCD-Q): a validation study. J Alzheimers Dis. 2014;41:453–466. doi: 10.3233/JAD-132027. [DOI] [PubMed] [Google Scholar]
- 20.Rattanabannakit C, Risacher SL, Gao S, Lane KA, Brown SA, McDonald BC, et al. The Cognitive Change Index as a measure of self and informant perception of cognitive decline: relation to neuropsychological tests. J Alzheimers Dis. 2016;51:1145–1155. doi: 10.3233/JAD-150729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hong JY, Lee JE, Sohn YH, Lee PH. Neurocognitive and atrophic patterns in Parkinson's disease based on subjective memory complaints. J Neurol. 2012;259:1706–1712. doi: 10.1007/s00415-011-6404-3. [DOI] [PubMed] [Google Scholar]
- 22.Kehagia AA, Barker RA, Robbins TW. Neuropsychological and clinical heterogeneity of cognitive impairment and dementia in patients with Parkinson's disease. Lancet Neurol. 2010;9:1200–1213. doi: 10.1016/S1474-4422(10)70212-X. [DOI] [PubMed] [Google Scholar]
- 23.Foster ER, Campbell MC, Burack MA, Hartlein J, Flores HP, Cairns NJ, et al. Amyloid imaging of Lewy body-associated disorders. Mov Disord. 2010;25:2516–2523. doi: 10.1002/mds.23393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jellinger KA, Attems J. Prevalence and impact of vascular and Alzheimer pathologies in Lewy body disease. Acta Neuropathol. 2008;115:427–436. doi: 10.1007/s00401-008-0347-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Neuropsychological performance of subjects classified according to cognitive status.