Abstract
Background and Purpose
Since the long-term survival rate has improved in laryngeal cancer patients who receive radiotherapy, concerns about postradiation complications (including carotid atherosclerosis) have increased. We followed changes in the common carotid artery (CCA) after radiotherapy and identified the underlying risk factors.
Methods
Consecutive patients with laryngeal cancer who underwent radiotherapy between January 1999 and December 2009 and who had received computed tomography (CT) both pre- and postradiotherapy were enrolled. Changes in the wall thickness and in the vessel and lumen areas as well as the presence of calcification or atherosclerosis were investigated. Demographics and risk factors were compared between patients with and without atherosclerosis at follow-up CT.
Results
In total, 125 patients were enrolled. The wall thickness had increased and the lumen area had decreased several months after radiotherapy. These changes were not associated with vascular risk factors and were not progressive. Calcification and atherosclerosis were observed in 37 (29.6%) and 71 (56.8%) patients, respectively. Diabetes was associated with calcification (p=0.02). The prevalence of hyperlipidemia was higher in patients with atherosclerosis (28.2% vs. 11.1%, p=0.02) and for a longer period postradiation [62.7±32.1 vs. 40.0±24.2 months (mean±SD), p<0.001]. Atherosclerosis occurred mostly in the middle portion of the CCA (n=31, 24.6%), followed by the proximal CCA at the intrathoracic level (n=26, 20.6%) and the distal CCA (n=6, 4.8%). Positive remodeling was also observed, but this was less common in patients with calcification (p=0.02).
Conclusions
Various types of postradiation changes occur in the CCA and can be easily observed in postradiation CT. The prevalence and burden of postradiation atherosclerosis increased in a close relationship with baseline cholesterol levels and the time after radiotherapy. Postradiation atherosclerosis was observed at unusual sites of the CCA.
Keywords: radiotherapy, carotid artery, atherosclerosis, hypercholesterolemia
INTRODUCTION
Laryngeal cancer is one of the most common types of malignant tumor of the head and neck area.1 Radiotherapy is used in most of these patients,2 and treatments and survival rates for laryngeal cancer have improved recently. However, concerns about late complications related to radiotherapy have also increased.3,4 Moreover, radiotherapy of the neck area increases the risk of ischemic stroke due to its effects on the carotid arteries.5
Postradiotherapy changes in the common carotid artery (CCA) can be extensive and involve multiple vessels in the radiation field, and can cause late-phase hemodynamic or embolic infarctions.6 Changes that can occur in the CCA include the following: a combination of direct vessel-wall injury resulting in intimal proliferation, necrosis of the media and periarterial fibrosis around the adventitia,7 calcification of the intima and media, and increased endothelial permeability allowing monocyte invasion. The invaded monocytes differentiate into foam cells that scavenge serum cholesterol.8 However, there have been few long-term studies focusing on these diverse CCA changes after radiotherapy, and so the optimal management strategy for patients with extensive postradiation CCA changes has yet to be established.
Medications that prevent the progression of atherosclerosis have improved in recent decades, and the treatment strategies for carotid artery stenosis have changed accordingly.9,10 Since the disease course in carotid atherosclerosis can be modified by medical treatment,11 the early identification of highrisk patients is critical. We attempted to further characterize postradiotherapy changes in the CCA using computed tomography (CT) and identify potential therapeutic targets for preventing these changes.
METHODS
Patients and design
Patients with laryngeal cancer who began radiotherapy between January 1999 and December 2009 were consecutively reviewed from a prospectively obtained database. Patients who received CT of the neck area before and after radiotherapy were enrolled. Patients with nonatherosclerotic carotid disease (e.g., Takayasu's arteritis, giant-cell arteritis, or fibromuscular dysplasia) were excluded. Clinical factors, such as demographics, conventional risk factors for atherosclerosis, and medication use, were obtained from the patient medical records. The total cholesterol levels as measured at the initiation of radiotherapy were also assessed. The treatment of choice for each patient was discussed at a multidisciplinary team meeting involving an otorhinolaryngologist and oncologist, along with radiation oncologists and radiologists. All patients were treated with a curative intent.
Radiotherapy was typically administered by three-dimensional conformal radiotherapy using four to nine radiation fields and two-dimensional image guidance in daily fractional doses, on 5 days per week for approximately 7 weeks. The cumulative radiation doses administered ranged from 27 to 82.6 Gy (2.0–2.25 Gy/fraction). The total dose and field of radiation were determined based on the staging of the laryngeal cancer by a radiation oncologist. The primary tumor site and neck region including the whole CCA area were included within the radiation field. The Institutional Review Board of our local center approved this study (approval no. AMC 2014-0770) and waived the requirement for written informed consent because of its retrospective design.
Imaging analysis
Laryngeal cancer was evaluated by contrast-enhanced CT using a Somatom Sensation 16 scanner (Siemens Medical Solution, Forchheim, Germany) or a LightSpeed QX/i scanner (GE Medical Systems, Milwaukee, WI, USA) with a reconstructed slice thickness of 3 mm or less. For contrast enhancement, 90 mL of iodinated contrast agent (Ultravist 300, Schering, Berlin, Germany) was injected intravenously at 3 mL/sec. CT was performed at least twice. The initial CT scan was performed within 3 months before the initiation of radiation therapy, and the follow-up CT scan, which was performed at least 6 months after initiating radiotherapy, was used to avoid hyperacute changes induced by radiation therapy. In cases with multiple follow-up CT scans, the last scan was used to evaluate postradiation changes.
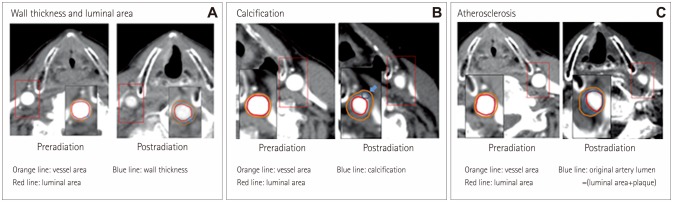
Postradiotherapy changes in the CCA were evaluated extensively using the following nine parameters: 1) wall thickness, 2) lumen area, 3) calcification, 4) atherosclerosis, 5) vessel area, 6) area of the original arterial lumen, 7) residual lumen area, 8) degree of stenosis, and 9) remodeling ratio. The wall thickness (Fig. 1A) was measured at baseline and follow-up from the posterior-medial aspect of the CCA in order optimize the visualization (blue line in Fig. 1A). The difference between baseline and follow-up was calculated, and the proportions of patients with any increase or decrease in wall thickness or lumen area (red circle in Fig. 1A) were determined. A scout film and the lower border of the thyroid cartilage were used as an indicator to find the identical location at follow-up (Fig. 1A). The presence of calcification (Fig. 1B) and atherosclerosis (Fig. 1C) were investigated throughout the right and left CCAs. Calcification was defined as a high-density lesion at the vessel wall confirmed in both non-contrast-enhanced and contrast-enhanced CT. Atherosclerosis was defined as a low-density lesion with an eccentric involvement of the vessel wall.12
Fig. 1. Measurements of pre- and postradiotherapy changes in the common carotid artery: vessel wall thickness, vessel area, and lumen area (A), calcification (B), and degree of stenosis (C).
In patients with atherosclerosis at follow-up, additional parameters were measured at the location of the greatest stenosis (Fig. 1C). The first slice was chosen based on a visual inspection, and the degree of stenosis was measured both there and in the two adjacent slices, with the slice with the greatest stenosis then used for subsequent measurements. The vessel area quantified as the outer contour of the CCA (orange line in Fig. 1C), the area of the original arterial lumen (including both the luminal area and atherosclerotic plaque, as indicated by the blue line in Fig. 1C), and the residual lumen area (the area filled with contrast, as indicated by the red line in Fig. 1C) were measured. These parameters were used to calculate the degree of stenosis [as 1-(luminal area/original artery lumen)] and the positive remodeling ratio, based on a modified version of a method applied to the coronary artery.13 All measurements were made at baseline and again at follow-up using an embedded program in the picture archiving and communication system of Asan Medical Center by a vascular neurologist who was blind to the clinical data. The wall thickness and luminal area were measured twice at baseline to validate the measurement, which indicated acceptable intrarater agreement (96.8% and 96.0% of measures within 2 SDs in a Bland-Altman plot of the wall thickness and lumen area, respectively).
Statistical analysis
The proportion of patients with each change was investigated. The wall thickness and luminal area before and after radiotherapy were compared using paired-samples t-tests in each CCA. Factors associated with these changes were also studied. Patients were then divided into two groups: those with and without atherosclerosis at follow-up. Clinical factors and factors associated with radiotherapy were compared between these two groups. Multivariate analysis was performed to identify independent risk factors for the development of atherosclerosis. Known risk factors and factors that were potentially associated from the univariate analysis (p<0.10) were entered into a multivariate analysis. Since preexisting atherosclerosis may affect postradiation atherosclerosis,14 we additionally investigated the factors associated with newly developed atherosclerosis, after excluding those with atherosclerosis before radiotherapy.
A similar statistical analysis was carried out to identify factors associated with calcification. Correlations between the degree of stenosis and various factors were also investigated. The chi-square test, Student's t-test, and Mann-Whitney U test were used as appropriate. Pearson's correlation coefficient was used in the correlation analyses. A threshold of p<0.05 was used to define statistical significance. All statistical analyses were performed using SPSS for Windows (version 17.0, SPSS, Chicago, IL, USA).
RESULTS
Radiotherapy was applied to 152 laryngeal cancer patients during the study period. Eight of these cases did not undergo a CT scan of the neck prior to radiotherapy, and 19 did not undergo a follow-up CT scan at 6 months after the initiation of radiotherapy, and so a cohort of 125 patients was ultimately enrolled. The age of the enrolled patients at the initiation of radiotherapy was 61.5±9.7 years (mean±SD; range, 24–83 years), and only 3 (2.4%) of the 125 patients were female. The follow-up duration for CT scans of the neck after initiating radiotherapy was 4.35±2.55 years.
Changes in the CCA wall and lumen after radiotherapy
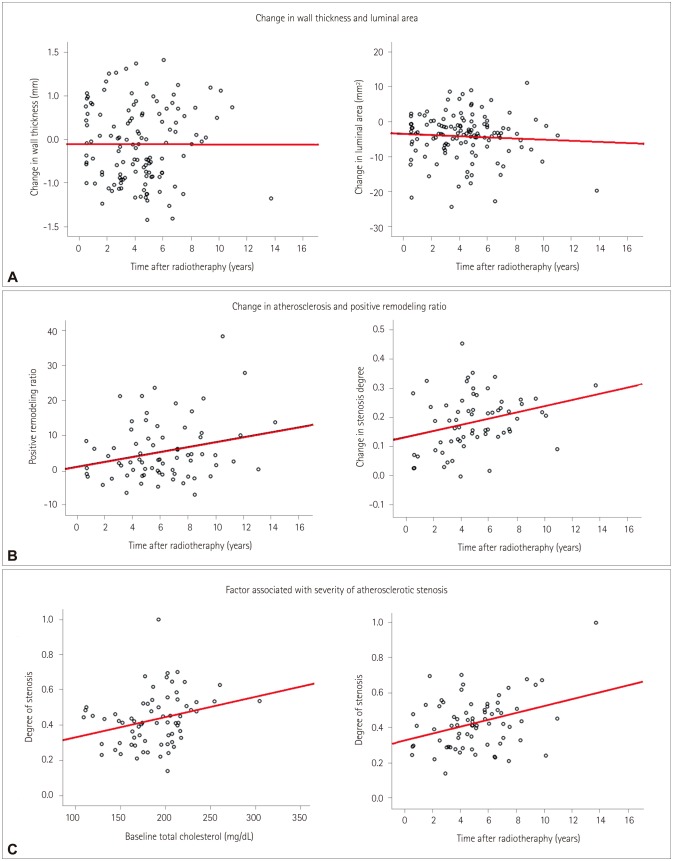
The wall thicknesses of the right and left CCAs were 1.06±0.31 mm and 1.12±0.33 mm, respectively, at baseline, and increased significantly to 1.47±0.61 mm and 1.60±0.57 mm after radiotherapy (p<0.001 for both CCAs). Based on differences between the initial and follow-up CT scans, the thickness of the CCA wall had increased in 104 (83.2%) patients in the right CCA and in 95 (76.0%) patients in the left CCA. Demographics, risk factors, and radiation doses were not associated with changes in the CCA wall thickness. The changes in the wall thicknesses of the right and left CCAs were found to be correlated with each other (r=0.476, p<0.001), but there was no significant association between the change in wall thickness and the time after radiotherapy (r=0.001, p=0.99) (Fig. 2A).
Fig. 2. Factors affecting postradiation changes in the common carotid artery (CCA). Postradiation changes in the CCA according to the time after radiotherapy, including changes in the vessel wall thickness and lumen area (A) and the vessel area and degree of stenosis at the location of maximum stenosis resulting from atherosclerosis (B). Factors associated with the degree of stenosis (C).
The luminal areas of the right and left CCAs were 39.3±11.1 mm2 and 37.6±10.4 mm2, respectively, at baseline, and decreased significantly to 35.7±10.8 mm2 and 33.1±9.0 mm2 after radiotherapy (p<0.001 for both CCAs). The luminal area of the right CCA decreased in 91 (72.8%) patients, while that of the left CCA decreased in 97 (77.6%) patients. Demographics, risk factors, and radiation doses were not found to be associated with changes in the CCA lumen area. The changes in the right and left CCA lumen areas were also correlated with each other (r=0.523, p<0.001), but these changes were not correlated with the time after radiotherapy (r=−0.068, p= 0.45) (Fig. 2A).
Atherosclerosis and calcification after radiotherapy
Among the current 125 laryngeal cancer patients who received radiotherapy, 71 (57%) had atherosclerosis at the follow-up CT: 51 (71.8%) in bilateral CCAs, 18 in the left CCA, and 2 in the right CCA. While the luminal area decreased at the location of maximum stenosis, the vessel area increased and the remodeling ratio in the slice with the atherosclerotic plaque was 1.17±0.21. Finally, the positive remodeling ratio (r=0.247, p=0.04) and the degree of stenosis (r=0.289, p= 0.02) increased with time after radiotherapy (Fig. 2B).
There were no differences in the demographic characteristics or vascular risk factors between patients with and without atherosclerosis of the CCA, with the exception of patients with atherosclerosis at follow-up showing a higher incidence of hyperlipidemia (Table 1). Patients with atherosclerosis also demonstrated a higher baseline level of total cholesterol (181±38 mg/dL vs. 157±36 mg/dL, p=0.001) and a longer time after radiotherapy (62.7±32.1 months vs. 40.0±24.2 months, p<0.001). Based on multivariate analyses, the baseline levels of total cholesterol [odds ratio (OR)=1.024, 95% confidence interval (CI)=1.012–1.037, p=0.01] and the time after radiotherapy (OR=1.031, 95% CI=1.014–1.048, p<0.001) were both independently associated with the presence of atherosclerosis at follow-up.
Table 1. Differences between laryngeal cancer patients with and without common carotid artery atherosclerosis after radiation therapy.
| Radiation-induced atherosclerosis (+) (n=71) | Radiation-induced atherosclerosis (−) (n=54) | p | |
|---|---|---|---|
| Age (years) | 61±10 | 62±9 | 0.49 |
| Male | 70 (98.6) | 52 (96.3) | 0.50 |
| Hypertension | 26 (36.6) | 21 (38.9) | 0.80 |
| Diabetes | 9 (12.7) | 8 (14.8) | 0.73 |
| Hyperlipidemia | 20 (28.2) | 6 (11.1) | 0.02 |
| Smoking | 51 (71.8) | 37 (68.5) | 0.69 |
| Medication | |||
| Antiplatelet | 6 (8.5) | 6 (11.1) | 0.62 |
| ACE inhibitor | 6 (8.5) | 4 (7.4) | 0.83 |
| Calcium-channel blocker | 9 (12.7) | 11 (20.4) | 0.25 |
| Statin | 1 (1.4) | 3 (5.6) | 0.19 |
| Chemotherapy | 23 (32.4) | 24 (44.4) | 0.17 |
| Total cholesterol (mg/dL) | 181±38 | 157±36 | 0.001 |
| Time after radiotherapy (months) | 62.7±32.1 | 40.0±24.2 | <0.001 |
| Radiation dose (Gy) | 65.4±8.5 | 65.3±9.9 | 0.94 |
| Atherosclerosis before radiotherapy | 21 (29.6) | 0 (0.0) | <0.001 |
| Calcification before radiotherapy | 10 (14.1) | 11 (20.4) | 0.35 |
| Calcification at follow-up | 22 (31.0) | 14 (25.9) | 0.54 |
Data are n (%) or mean±SD values.
ACE: angiotensin-converting enzyme.
Baseline CT revealed atherosclerosis in 21 patients. Another atherosclerotic lesion in addition to the initial atherosclerosis was identified in 2 of these patients, while the remaining 19 patients showed progression of preexisting atherosclerosis. After excluding these 21 patients with atherosclerosis at the initial CT, 50 (48.1%) of 104 patients demonstrated newly developed atherosclerosis. The baseline cholesterol level (OR=1.021, 95% CI=1.007–1.035, p=0.004) and time after radiotherapy (OR=1.036, 95% CI=1.017–1.055, p<0.001) were independently associated with newly developed atherosclerosis.
Among the 71 patients with CCA atherosclerosis after radiotherapy, the degree of stenosis showed a weak but significant correlation with the initial total cholesterol level (r= 0.274, p=0.02), and a moderate correlation with the time after radiotherapy (r=0.342, p=0.004) (Fig. 2C). However, the degree of stenosis was not associated with the patient's age. Atherosclerosis occurred mostly in the middle portion of the CCA (n=31, 24.6%), followed by the proximal CCA at the intrathoracic level (n=26, 20.6%) and the distal CCA (n=6, 4.8%).
Vascular calcification was observed in 37 (29.6%) of the 125 patients. The detection of vascular calcification at follow-up CT was associated with the presence of diabetes (24.3% vs. 9.1%, p=0.02) (Table 2). Neither the time after radiotherapy (55.5±31.0 months vs. 51.9±31.1 months, p=0.56) nor the presence of atherosclerosis (62.2% vs. 54.5%, p=0.43) differed between patients with and without vascular calcification. Among the 71 patients with atherosclerosis, the vessel area at the location of maximum stenosis changed less in patients with vascular calcification than in those without calcification (6.00±7.97 mm2 vs. 13.50±13.81 mm2, p=0.02), and the remodeling ratio was lower in patients with calcification (1.09±0.13 vs. 1.21±0.22, p=0.02) (Table 2). Based on the results of multivariate analysis and after adjusting for potential confounding factors, the presence of diabetes was independently associated with vascular calcification (OR=3.375, 95% CI=1.184–9.618, p=0.02). After excluding patients with prior calcification before radiotherapy, 17 (16.3%) of 104 patients demonstrated newly developed calcification. The presence of diabetes was independently associated with newly developed calcification (OR=4.884, 95% CI=1.303–18.307, p=0.019).
Table 2. Differences between the laryngeal cancer patients with and without common carotid artery calcification after radiation therapy.
| Radiation-induced calcification (+) (n=37) | Radiation-induced calcification (−) (n=88) | p | |
|---|---|---|---|
| Age (years) | 61.3±10.8 | 61.7±9.3 | 0.84 |
| Male | 36 (97.3) | 86 (97.7) | 0.66 |
| Hypertension | 16 (43.2) | 31 (35.2) | 0.40 |
| Diabetes | 9 (24.3) | 8 (9.1) | 0.02 |
| Hyperlipidemia | 7 (18.9) | 19 (21.6) | 0.74 |
| Smoking | 26 (70.3) | 62 (70.5) | 0.98 |
| Medication | |||
| Antiplatelet | 6 (16.2) | 6 (6.8) | 0.10 |
| ACE inhibitor | 2 (5.4) | 8 (9.1) | 0.49 |
| Calcium-channel blocker | 9 (24.3) | 11 (12.5) | 0.10 |
| Statin | 0 (0.0) | 4 (4.5) | 0.19 |
| Chemotherapy | 15 (40.5) | 32 (36.4) | 0.66 |
| Total cholesterol (mg/dL) | 166±40 | 177±38 | 0.17 |
| Time after radiotherapy (months) | 55.5±31.0 | 51.9±31.1 | 0.56 |
| Radiation dose (Gy) | 63.3±10.7 | 66.3± 8.2 | 0.14 |
| Calcification before radiotherapy | 20 (54.1) | 1 (1.1) | <0.001 |
| Atherosclerosis before radiotherapy | 9 (24.3) | 12 (13.6) | 0.15 |
| Degree of stenosis* | 0.42±0.13 | 0.43±0.16 | 0.75 |
| Remodeling ratio* | 1.09±0.13 | 1.12±0.22 | 0.02 |
Data are n (%) or mean±SD values.
*The degree of stenosis and remodeling ratio were calculated from 71 patients with atherosclerosis.
ACE: angiotensin-converting enzyme.
DISCUSSION
This study investigated the changes in the vessel wall, lumen, vascular calcification, and atherosclerosis of the CCA after radiotherapy in patients with laryngeal cancer. The CCA thickness increased and the lumen area decreased after radiotherapy. These changes occurred in the early phase of treatment and did not differ with the time after radiotherapy, but stenosis resulting from atherosclerosis was more severe in those with a longer time after radiotherapy. The occurrence of atherosclerosis was independently associated with the presence of hyperlipidemia, whilst more-severe atherosclerotic stenosis was associated with both the baseline cholesterol level and the time after radiotherapy.
Several distinct patterns of changes in the CCA after radiotherapy were observed on the follow-up CT scans. First, the vessel wall became concentrically thicker and the lumen area decreased. These changes were not associated with the time after radiotherapy and were observed consistently, even on CT scans performed within a year after radiotherapy. High-resolution magnetic resonance imaging (MRI) has revealed changes similar to vasculitis in the intracranial artery. 15 However, the pathologies that we observed were much more chronic since they were sustained without progression or regression, so they may represent periarterial fibrosis.16 Further pathological studies may be helpful for verifying the exact pathological changes observed after radiotherapy and understanding the underlying mechanisms. Second, vascular calcification was also observed in one-third of the patients who received radiotherapy, but its presence was not associated with the time after radiotherapy. Only diabetes, which is a well-known risk factor for vascular calcification,17 has been reported to be independently associated with vascular calcification after radiotherapy.18
Finally, atherosclerosis, a low-density lesion that is eccentrically located inside the lumen, was observable in a CT scan, after radiotherapy. However, atherosclerosis was found in the current analysis to be more closely associated with the postradiation time than were changes in wall thickness and calcification. Radiation induces endothelial dysfunction, which can increase the vascular permeability for plasma lipoproteins, resulting in progressive lipid accumulation in the vessel wall.19 Following radiotherapy, positive remodeling of the CCA was also observed in the present cohort. A particularly interesting finding was that positive remodeling was less frequent in the current patients with vascular calcification, which is consistent with the findings of previous studies that examined other vascular beds.20 The degree of atherosclerotic stenosis was also strongly correlated with the baseline total cholesterol levels in the current study. Our findings are thus consistent with previous reports of an association between baseline cholesterol levels and increases in the intima-media thickness (IMT) or plaque scores as measured by duplex sonography.21
Carotid duplex sonography is a noninvasive technique for detecting changes that occur after radiotherapy. However, the predictive value of the IMT for cardiovascular events remains controversial,22 and carotid duplex sonography can only explore a spatially limited range of the CCA. Compared to atherosclerosis typically mostly affecting the distal CCA near its bifurcation, radiation-induced atherosclerosis most frequently appears in the middle portion of the CCA followed by the proximal intrathoracic CCA, and atherosclerotic stenosis can be difficult to detect by duplex sonography in these locations. Compared to duplex sonography, neck CT usually includes the full length of the CCA, and even the intrathoracic level can be screened for postradiation changes. Moreover, CT is usually performed in laryngeal cancer patients to follow up the prognosis of cancer itself. Hence, neck CT and duplex sonography may be informative and complementary methodologies for detecting atherosclerotic changes after radiotherapy.
Statin treatment has been shown to be effective in stroke patients with atherosclerosis, and is now considered to be an essential component of carotid artery disease management.10,23 Some studies have suggested that radiation-induced atherosclerosis differs from typical atherosclerosis.24 However, the present findings indicate that cholesterol remains one of the most important risk factors for the development of postradiation atherosclerosis. Statins are thought to exert beneficial pleiotropic effects on radiation-induced tissue damage by reducing endothelial activation, limiting acute inflammatory responses, and protecting against delayed fibrotic tissue remodeling and cell death.25 Statins could therefore be considered as part of the therapeutic strategy for managing head and neck cancer patients treated with radiation therapy who are at a high risk of CCA atherosclerosis.
The present study was subject to some notable limitations. First, the changes observed using follow-up CT were not confirmed pathologically. Second, we used data from routine neck CT follow-ups, which may have a lower spatial resolution than CT angiography. Future studies utilizing the source images of CT angiography or high-resolution MRI may be helpful for confirming our findings. Third, this study had a retrospective design, the levels of cholesterol were not measured throughout the study period, and the radiation fields were not characterized in detail. These features make it difficult to draw conclusions about the importance of lipid management throughout the postradiation period or identifying a less-atherogenic method of radiation therapy. However, our findings may help in the future identification of patients at a high risk of postradiation atherosclerosis.
In conclusion, various types of postradiation changes can be observed on CT scans of laryngeal cancer patients. Among these changes, atherosclerosis develops in patients with hypercholesterolemia and the degree of stenosis is associated with the baseline cholesterol level and the time after radiotherapy. Furthermore, radiation-associated atherosclerosis is located at unusual sites such as the middle portion of the CCA and the intrathoracic CCA. CT may be effective in complementing carotid sonography to detect atherosclerosis in these locations. Identifying patients who are at a high risk of developing postradiotherapy atherosclerosis and detecting such cases in the early stages of atherosclerosis with postradiation CT may therefore be important.
Acknowledgements
This study was supported by a grant from the Korea Healthcare Technology R&D Project, Ministry of Health and Welfare Republic of Korea (HI10C2020 and HI14C1731).
Footnotes
Conflicts of Interest: The authors have no financial conflicts of interest.
References
- 1.Pfister DG, Laurie SA, Weinstein GS, Mendenhall WM, Adelstein DJ, et al. American Society of Clinical Oncology Clinical Practice Guideline for the use of larynx-preservation strategies in the treatment of laryngeal cancer. J Clin Oncol. 2006;24:3693–3704. doi: 10.1200/JCO.2006.07.4559. [DOI] [PubMed] [Google Scholar]
- 2.Forastiere AA, Goepfert H, Maor M, Pajak TF, Weber R, Morrison W, et al. Concurrent chemotherapy and radiotherapy for organ preservation in advanced laryngeal cancer. N Engl J Med. 2003;349:2091–2098. doi: 10.1056/NEJMoa031317. [DOI] [PubMed] [Google Scholar]
- 3.Carmody BJ, Arora S, Avena R, Curry KM, Simpkins J, Cosby K, et al. Accelerated carotid artery disease after high-dose head and neck radiotherapy: is there a role for routine carotid duplex surveillance? J Vasc Surg. 1999;30:1045–1051. doi: 10.1016/s0741-5214(99)70042-x. [DOI] [PubMed] [Google Scholar]
- 4.Kim K, Lee JH. Risk factors and biomarkers of ischemic stroke in cancer patients. J Stroke. 2014;16:91–96. doi: 10.5853/jos.2014.16.2.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Plummer C, Henderson RD, O'Sullivan JD, Read SJ. Ischemic stroke and transient ischemic attack after head and neck radiotherapy: a review. Stroke. 2011;42:2410–2418. doi: 10.1161/STROKEAHA.111.615203. [DOI] [PubMed] [Google Scholar]
- 6.Weintraub NL, Jones WK, Manka D. Understanding radiation-induced vascular disease. J Am Coll Cardiol. 2010;55:1237–1239. doi: 10.1016/j.jacc.2009.11.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dorresteijn LD, Kappelle AC, Scholz NM, Munneke M, Scholma JT, Balm AJ, et al. Increased carotid wall thickening after radiotherapy on the neck. Eur J Cancer. 2005;41:1026–1230. doi: 10.1016/j.ejca.2005.01.020. [DOI] [PubMed] [Google Scholar]
- 8.Gujral DM, Shah BN, Chahal NS, Senior R, Harrington KJ, Nutting CM. Clinical features of radiation-induced carotid atherosclerosis. Clin Oncol (R Coll Radiol) 2014;26:94–102. doi: 10.1016/j.clon.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 9.Amarenco P, Bogousslavsky J, Callahan A, 3rd, Goldstein LB, Hennerici M, Rudolph AE, et al. High-dose atorvastatin after stroke or transient ischemic attack. N Engl J Med. 2006;355:549–559. doi: 10.1056/NEJMoa061894. [DOI] [PubMed] [Google Scholar]
- 10.Paraskevas KI, Hamilton G, Mikhailidis DP. Statins: an essential component in the management of carotid artery disease. J Vasc Surg. 2007;46:373–386. doi: 10.1016/j.jvs.2007.03.035. [DOI] [PubMed] [Google Scholar]
- 11.Yamagami H, Sakaguchi M, Furukado S, Hoshi T, Abe Y, Hougaku H, et al. Statin therapy increases carotid plaque echogenicity in hypercholesterolemic patients. Ultrasound Med Biol. 2008;34:1353–1359. doi: 10.1016/j.ultrasmedbio.2008.01.019. [DOI] [PubMed] [Google Scholar]
- 12.Oliver TB, Lammie GA, Wright AR, Wardlaw J, Patel SG, Peek R, et al. Atherosclerotic plaque at the carotid bifurcation: CT angiographic appearance with histopathologic correlation. AJNR Am J Neuroradiol. 1999;20:897–901. [PMC free article] [PubMed] [Google Scholar]
- 13.Hardie AD, Kramer CM, Raghavan P, Baskurt E, Nandalur KR. The impact of expansive arterial remodeling on clinical presentation in carotid artery disease: a multidetector CT angiography study. AJNR Am J Neuroradiol. 2007;28:1067–1070. doi: 10.3174/ajnr.A0508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chang YJ, Chang TC, Lee TH, Ryu SJ. Predictors of carotid artery stenosis after radiotherapy for head and neck cancers. J Vasc Surg. 2009;50:280–285. doi: 10.1016/j.jvs.2009.01.033. [DOI] [PubMed] [Google Scholar]
- 15.Li M, Wu SW, Xu WH. High-resolution MRI of radiation-induced intracranial vasculopathy. Neurology. 2015;84:631. doi: 10.1212/WNL.0000000000001223. [DOI] [PubMed] [Google Scholar]
- 16.Virmani R, Farb A, Carter AJ, Jones RM. Pathology of radiation-induced coronary artery disease in human and pig. Cardiovasc Radiat Med. 1999;1:98–101. doi: 10.1016/s1522-1865(98)00010-9. [DOI] [PubMed] [Google Scholar]
- 17.Wu XH, Chen XY, Wang LJ, Wong KS. Intracranial artery calcification and its clinical significance. J Clin Neurol. 2016;12:253–261. doi: 10.3988/jcn.2016.12.3.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Demer LL, Tintut Y. Vascular calcification: pathobiology of a multifaceted disease. Circulation. 2008;117:2938–2948. doi: 10.1161/CIRCULATIONAHA.107.743161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gujral DM, Chahal N, Senior R, Harrington KJ, Nutting CM. Radiation-induced carotid artery atherosclerosis. Radiother Oncol. 2014;110:31–38. doi: 10.1016/j.radonc.2013.08.009. [DOI] [PubMed] [Google Scholar]
- 20.Higashikuni Y, Tanabe K, Yamamoto H, Aoki J, Nakazawa G, Onuma Y, et al. Relationship between coronary artery remodeling and plaque composition in culprit lesions: an intravascular ultrasound radiofrequency analysis. Circ J. 2007;71:654–660. doi: 10.1253/circj.71.654. [DOI] [PubMed] [Google Scholar]
- 21.Pereira EB, Gemignani T, Sposito AC, Matos-Souza JR, Nadruz W., Jr Low-density lipoprotein cholesterol and radiotherapy-induced carotid atherosclerosis in subjects with head and neck cancer. Radiat Oncol. 2014;9:134. doi: 10.1186/1748-717X-9-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wilbers J, Hoebers FJ, Boogerd W, van Werkhoven ED, Nowee ME, Hart G, et al. Prospective cohort study of carotid intima-media thickness after irradiation. J Stroke Cerebrovasc Dis. 2014;23:2701–2707. doi: 10.1016/j.jstrokecerebrovasdis.2014.06.009. [DOI] [PubMed] [Google Scholar]
- 23.Moon GJ, Kim SJ, Cho YH, Ryoo S, Bang OY. Antioxidant effects of statins in patients with atherosclerotic cerebrovascular disease. J Clin Neurol. 2014;10:140–147. doi: 10.3988/jcn.2014.10.2.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Santoro F, Ferraretti A, Centola A, Cuculo A, Totaro A, Cocco D, et al. Early clinical presentation of diffuse, severe, multi-district atherosclerosis after radiation therapy for Hodgkin lymphoma. Int J Cardiol. 2013;165:373–374. doi: 10.1016/j.ijcard.2012.08.027. [DOI] [PubMed] [Google Scholar]
- 25.Gaugler MH, Vereycken-Holler V, Squiban C, Vandamme M, Vozenin-Brotons MC, Benderitter M. Pravastatin limits endothelial activation after irradiation and decreases the resulting inflammatory and thrombotic responses. Radiat Res. 2005;163:479–487. doi: 10.1667/rr3302. [DOI] [PubMed] [Google Scholar]