Abstract
BACKGROUND
In pediatric patients with anxiety disorders, existing symptom inventories are either not freely available or require extensive time and effort to administer. We sought to evaluate a brief, self-report scale—the Generalized Anxiety Disorder 7-item Scale (GAD-7)—in adolescents with generalized anxiety disorder (GAD).
METHODS
The Pediatric Anxiety Rating Scale (PARS) and the GAD-7 were administered to youth with GAD (confirmed by structured interview). Relationships between the measures were assessed and sensitivity and specificity was determined with regard to a global symptom severity measure (Clinical Global-Impression-Severity).
RESULTS
In adolescents with GAD (N=40, mean age: 14.8±2.8), PARS and GAD-7 scores strongly correlated (R=0.65, p<0.001) and a main effect for symptom severity was observed (p<0.001). GAD-7 scores ≥11 and GAD-7 scores ≥17 represented the optimum specificity and sensitivity for the detection of moderate and severe anxiety, respectively.
CONCLUSIONS
The PARS and GAD-7 similarly reflect symptom severity and the GAD-7 is associated with acceptable specificity and sensitivity for detecting clinically-significant anxiety symptoms. GAD-7 scores may be used to assess anxiety symptoms and to differentiate between mild and moderate GAD in adolescents and may be more efficient than the PARS.
Keywords: GAD-7, PARS, generalized anxiety disorder, self-report, pediatric, anxiety
INTRODUCTION
Anxiety disorders are among the most prevalent psychiatric conditions in children and adolescents and, in the United States, affect 15% of youth.1 However, in the pediatric population, these disorders are often under-diagnosed and untreated.2 In adult patients, a number of instruments are available to screen for anxiety disorders and to track anxiety symptoms.3–5 These symptom inventories facilitate increased screening and the ability to monitor symptoms over time, in both clinical practice and in clinical research.6 However, in youth, fewer instruments are available for the tracking of symptoms and self-report rating scales7–9—with several exceptions—are under-utilized by clinicians in child and adolescent populations.10 Additionally, some scales that effectively assess anxiety symptoms in adults are difficult to use in pediatric patients. For example, the Hamilton Anxiety Rating Scale4 may over-represent somatic symptoms (e.g., autonomic, genitourinary, cardiovascular, sensory) that are less commonly reported in pediatric patients compared to adults11 and may not fully assess cognitive aspects of anxiety in youth.12
Clinician-rated instruments that have been systematically evaluated in youth include the Pediatric Anxiety Rating Scale9 which has been utilized as the primary outcome measure in the majority of psychopharmacologic treatment studies in youth with anxiety disorders.13–16 This clinician-administered scale includes a 50 item symptom checklist which encompasses social anxiety/performance anxiety (9 items), separation anxiety (10 items), generalized anxiety (8 items), specific phobia (4 items), physical/somatic symptoms (13 items) as well as “other” items (6 items), The score, however, is determined from 7 severity and impairment items that are rated on a 6-point scale, with higher scores representing more severe symptoms and impairment. The PARS has established, acceptable, convergent validity with 3 anxiety rating scales although lower correlation coefficients have been observed between the PARS and self-report measures (e.g., Multidimensional Anxiety Scale for Children [MASC, R=0.22, p<0.05], Screen for Child Anxiety Related Disorders-Child [SCARED-Child, R=0.32, p<0.001], SCARED-Parent, R=0.46, p<0.001) relative to clinician-administered scales (e.g., HAM-A, R=0.49, p<0.001).9 Additionally, cutoffs have been established for response and remission.17 However, this scale takes approximately 20 minutes to administer thus attenuating its practical utility in routine clinical practice, despite its excellent psychometric properties.9
In terms of self-report measures, the Screen for Child Anxiety Related Disorders (SCARED)7,18 is frequently used to screen for anxiety disorders, assess a number of anxiety symptoms and is validated in pediatric populations. This instrument requires approximately 10 minutes to administer, includes 41 items and assesses symptoms of (1) panic disorder (or significant somatic symptoms); (2) generalized anxiety disorder symptoms; (3) social anxiety disorder symptoms; (4) separation anxiety symptoms and (5) significant school avoidance.7 Finally, the Multidimensional Anxiety Scale for Children (MASC) is a self-report instrument that has been validated in youth 8–19 years of age and rates anxiety symptoms relative to aged norms. The MASC consists of 50 questions and is administered to both the pediatric patient and to his or her parent or caregiver.8 This instrument includes scales related to separation anxiety/phobias, GAD, social anxiety, obsessions and compulsions and assesses physical symptoms/harm avoidance.
A decade ago, the GAD-7 was developed as a self-report screening tool for generalized anxiety symptoms in the primary care setting.3 The scale was developed to address the limited number of anxiety measures in clinical problems and to address the common issue of symptom ratings seldom being used in clinical practice because of “their length, proprietary nature, lack of usefulness as a diagnostic and severity measure, and requirement of clinician administration rather than patient self-report.”3 As such, the GAD-7 has been successfully disseminated in adult primary care and psychiatric clinics and has been systematically evaluated in US and international samples.19 The scale consists of 7 questions, requires approximately 1–2 minutes to administer and is sensitive to treatment-related changes in adults with generalized anxiety disorder. However, it has never been evaluated in adolescents with a primary diagnosis of generalized anxiety disorder. With this in mind, we sought to evaluate the GAD-7 in adolescents with GAD who participated in a double-blind, placebo-controlled treatment trial and to explore the relationship between the GAD-7 and the PARS with regard to one another and with regard to Clinical Global Impression-Severity (CGI-S) scores.20 We hypothesized that GAD-7 scores would correlate with the PARS scores; that GAD-7 scores would be reflective of symptomatic/functional burden and that the GAD-7 would be sensitive/specific for differentiating mild from moderate-severe disease severity.
METHODS
This study was approved by the Institutional Review Board at the University of Cincinnati and written, informed consent and assent were obtained from parents/guardians and from patients, respectively before inclusion in the study.
Subjects
Study participants were outpatient youth aged 12 through 17 years who met DSM-IV-TR criteria for GAD, assessed by unstructured and semi-structured assessments by a board-certified child and adolescent psychiatrist (JRS). The diagnosis of GAD was confirmed with the Anxiety Disorders Interview Schedule for DSM-IV (ADIS-IV).21 Demographic data including age, sex, and race/ethnicity were collected. Additionally, pubertal status was determined for 37 of the 40 patients using the Duke Self-Rated Tanner Scale (Self Report).22
Measures
To assess anxiety symptoms, the Pediatric Anxiety Rating Scale (PARS)9 and GAD-7 served as screening and primary outcome measures. As described above, the PARS is a 50-item clinician-rated checklist of anxiety symptoms in children/adolescents in addition to 7 dimensional questions related to anxiety symptom severity (i.e., number of symptoms, severity of symptom distress, behavioral avoidance, functional interference at home, and functional interference outside of home) that are rated on a 6-point scale (0 = none to 5 = extreme). Higher scores represent higher levels of distress and anxiety.
The GAD-7 consists of 7 questions based in part on the DSM-IV criteria for GAD and reflects the frequency of symptoms during the preceding 2-week period. The GAD-7 requires approximately 1–2 minutes to administer and for each symptom queried provides the following response options: “not at all,” “several days,” “over half the days” and “nearly every day” and these are scored, respectively, as 0, 1, 2 or 3.3
The CGI-S, a clinician-administered instrument, was administered by a board-certified child and adolescent psychiatrist with more than a decade of experience with the instrument. This scale, which is anchored with the question: “Considering your total clinical experience with this particular population, how mentally ill is the patient at this time?” is rated on a seven-point scale. Scores of 1 reflect patients who are “normal, not at all ill;” scores of 2 reflect patients who are “borderline mentally ill;” scores of 3 describe patients who are “mildly ill;” scores of 4 reflect patients who are “moderately ill;” scores of 5 reflect patients who are “markedly ill;” and scores of 6 and 7 are associated with the descriptions “severely ill” and “among the most extremely ill patients,” respectively.20 The rating—which is performed by a clinician—is based both on observed and reported symptoms, functional impairment and behavior over a 7 day period.
Statistical analysis
Relationships between the total GAD-7 score and total PARS score as well as relationships between individual items from the PARS and GAD-7 were evaluated with Spearman correlation coefficients. Additionally, the distribution of PARS scores and GAD-7 scores was evaluated descriptively with regard to CGI-S stratification (e.g., CGI-S ≤3, CGI-S 4–5, CGI-S ≥6) and was statistically evaluated with an analysis of variance (ANOVA), as previously utilized in the comparison of symptom rating scales in pediatric patients with affective disorders.23 Using receiver operating characteristic (ROC) methods24,25 as previously employed to evaluate the PARS in youth with anxiety (Caporino et al. 2013), a vector matrix list of predicted disease severity (based on CGI-S score ≥4 and CGI-S score ≥6 for moderate and severe illness) was created in addition to a data frame containing the true labels for CGI-S classification (ROCR, Predictions, version 1.0–7). Cutoff-parameterized performance curves were then generated to evaluate the sensitivity and specificity of the GAD-7 score with regard to the detection of moderate (CGI-S 4–5) and severe (i.e., CGI-S ≥6) severity anxiety symptoms. Finally, a confirmatory analysis was performed in which these cutoffs for GAD-7 scores were determined using optimal cutpoints methods.27 Specifically, to maximize sensitivity and specificity, for each cutpoint, the minimum sensitivity and specificity were determined, where mi represented the minimums across cutpoints. For the maximum of mi (M), the cutpoint that maximized both sensitivity and specificity (and corresponded to the cutpoint that yielded M) was selected, as previously described.28
All analyses were conducted in R (version 3.3.2, “Sincere Pumpkin Patch”). P-values <0.05 were considered statistically significant and, given the exploratory nature of these analyses, no correction for multiple comparisons was made.
RESULTS
Participants
Forty PARS and GAD-7 assessments were performed in adolescents with GAD (n=40) of whom the majority were female (n=31, 77.5%) and Caucasian (n=34, 85%). The mean age of patients was 14.8±2.8 years and co-occurring anxiety disorders were common (Table 1). The modal self-rated Tanner score was 4 (45% of participants). Additional demographic and clinical characteristics are reported in Table 1.
Table 1.
Demographic and clinical characteristics of adolescents with generalized anxiety disorder (GAD).
| Age, years, mean ± SD | 14.8 ± 2.8 |
|
| |
| Male gender, n (%) | 9 (22.5%) |
|
| |
| Race, n (%) | |
| White | 34 (85.0%) |
| Black or African American | 2 (5.0%) |
| Multiracial | 3 (7.5%) |
| Asian | 1 (2.5%) |
|
| |
| Ethnicity, n (%) | |
| Hispanic | 3 (7.5%) |
| Non-Hispanic | 37 (92.5%) |
|
| |
| Current medical state, n (%) | |
| GAD | 40 (100%) |
| Separation anxiety disorder | 9 (22.5%) |
| Social anxiety disorder | 19 (47.5%) |
| Specific phobia disorder | 7 (17.5%) |
| Panic disorder | 22 (55.0%) |
| ADHD | 8 (20.0%) |
|
| |
| PARS Score, mean ± SD | 17.0 ± 3.3 |
|
| |
| CGI-S Score, mean ± SD | 4.1 ± 1.2 |
|
| |
| GAD-7 Score, mean ± SD | 14.1 ± 4.3 |
|
| |
| Tanner Score, n (%)* | |
| Stage III | 5 (12.5%) |
| Stage IV | 18 (45%) |
| Stage V | 13 (32.5%) |
ADHD, Attention-Deficity/Hyperactivity Disorder; PARS, Pediatric Anxiety Rating Scale; CGI-S, Clinical Global-Impression-Severity; GAD-7, Generalized Anxiety Disorder 7-item;
data only collected for 37/40 patients
Relationship between the PARS and GAD-7
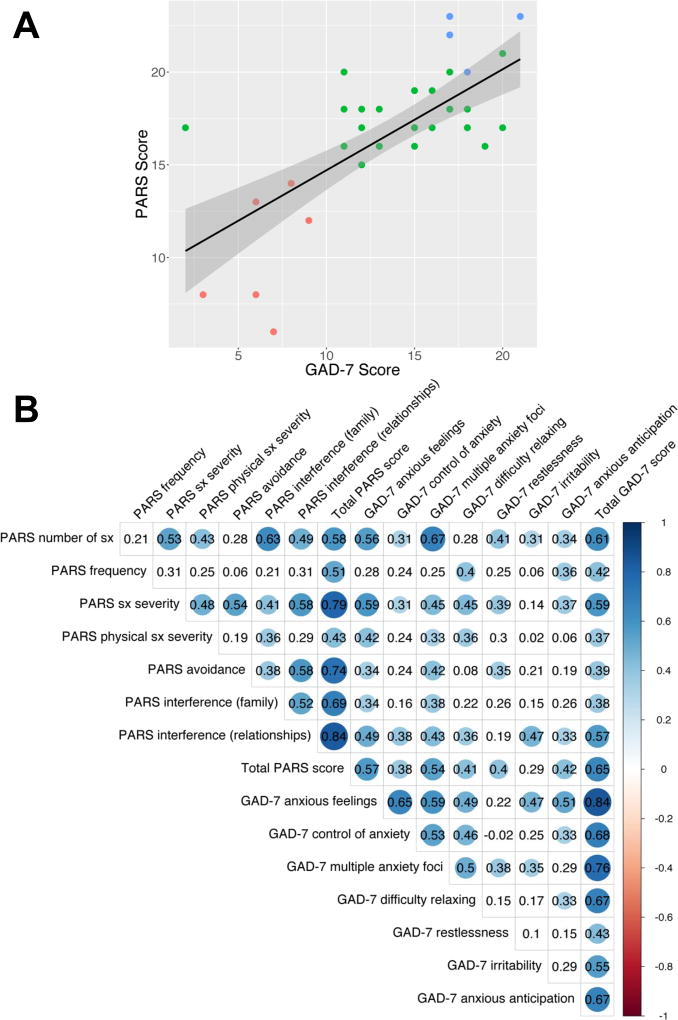
Total PARS scores were highly correlated with total GAD-7 score (n=40, R=0.65, p<0.05). Among the individual components of the GAD-7, all items except irritability exhibited statistically significant correlations with the total PARS score and among specific GAD-7 items, the strongest correlations were noted for GAD-7 items 1 and 2: “feeling nervous, anxious or on edge” (R=0.57, p<0.05) and “worrying too much about different things” (R=0.54, p<0.05). Additional correlation coefficients for significant and non-significant relationships are shown in Figure 2.
FIGURE 2. GAD-7 scores and Pediatric Anxiety Rating Scale (PARS) Scores.
GAD-7 scores significantly and positively correlated with PARS scores (R=0.65, p<0.001). Clinical Global Impression-Severity (CGI-S) scores of 1–3 are denoted by red dots, scores of 4–5 are represented by green dots and scores of 6 and 7 are shown as blue dots (A). Additionally, individual correlation coefficients between GAD-7 items and PARS items are shown in a heat map with cooler colors reflecting larger correlation coefficients. Colored circles represent p-values <0.05 while white boxes reflect non-significant correlations (B).
Relationship of CGI-S with GAD-7 and PARS
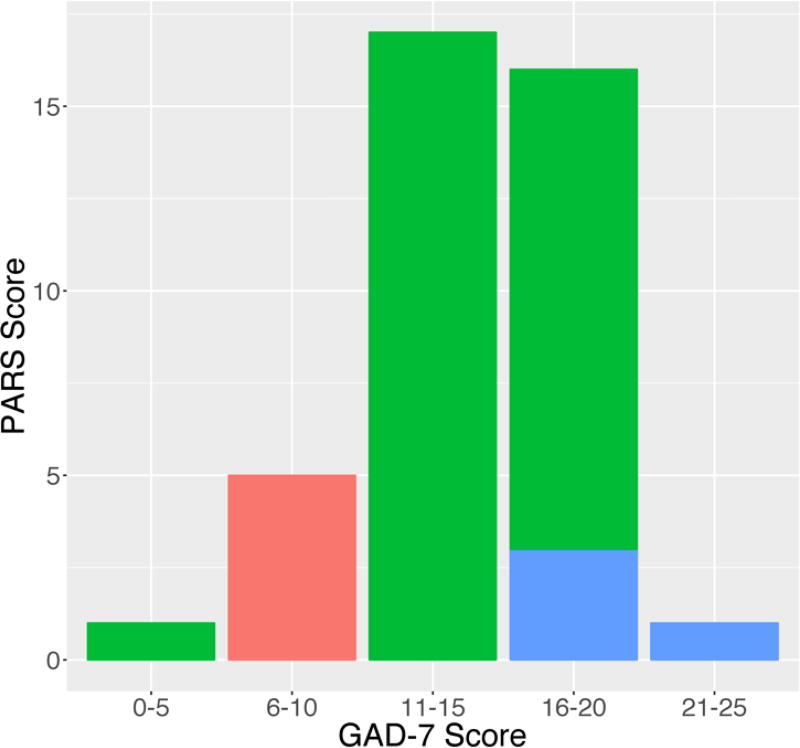
Based on the CGI-S scores, patients were categorized as shown in Table 2 and statistically significant effects of symptom severity for the three CGI-S groups were observed for GAD-7 (F2,37=26.83, p<0.001) and PARS (F2,37=126.50, p<0.001) scores (Figure 1).
Table 2.
PARS and GAD-7 scores of groups derived by CGI-S scores.
| CGI-S Group | n | PARS score Mean ± SD (range) |
GAD-7 score Mean ± SD (range) |
|---|---|---|---|
| 1, 2, 3 | 5 | 10.6 ± 3.4(6–14) | 7.2 ± 1.3 (6–9) |
| 4, 5 | 31 | 17.4 ± 1.5 (15–21) | 14.7 ± 3.6 (2–20) |
| 6, 7 | 4 | 22.0 ± 1.4 (20–23) | 18.3 ± 1.9 (17–21) |
|
| |||
| df | 1, 39 | 1, 39 | |
| F | 126.5 | 26.83 | |
| p | <0.001 | <0.001 | |
CGI-S, Clinical Global-Impression-Severity; PARS, Pediatric Anxiety Rating Scale; GAD-7, Generalized Anxiety Disorder 7-item;
FIGURE 1. GAD-7 scores and Clinical Global Impression-Severity (CGI-S) scores.
Frequency plots of GAD-7 scores with regard to Clinical Global Impression-Severity (CGI-S) scores in adolescents with GAD. Red bars indicate CGI-S scores of 1–3, green bars represent CGI-S scores of 4 and 5 and blue bars represent CGI-S scores of 6 and 7.
Sensitivity and specificity
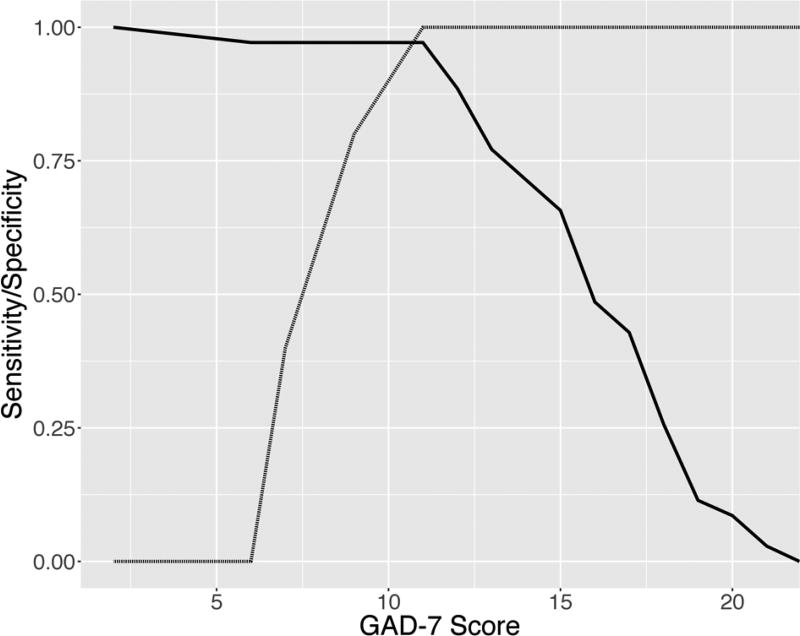
Figure 3 depicts the sensitivity and specificity of GAD-7 scores relative to the CGI-S classification for “at least moderate” (i.e., CGI-S ≥4) and “severe” (i.e., CGI-S ≥6) illness. A GAD-7 score of ≥11 represents the optimal compromise between sensitivity (97%) and specificity (100%) (AUC=0.971, 95% confidence interval: 0.915–1.027) for diagnosis of at least “moderate” anxiety. In other words, 100% of patients classified as less than “moderately ill” had a GAD-7 score <11 and 97% of patients with a GAD-7 score ≥11 were classified as at least “moderately ill.” A GAD-7 score of ≥17 represents the optimal compromise between sensitivity (100%) and specificity (69%) for diagnosis of “severe” severity (AUC=0.84, 95% confidence interval: 0.694–0.987). As such, 69% of patients classified as less than “severly ill” had a GAD-7 score <17 and 100% of patients with a GAD-7 score ≥17 were classified as “severly ill.” Confirmatory analyses using a maximum sensitivity and specificity approach (MaxSpSe) for GAD-7 scores ≥11 was associated with a positive predictive value of >99% and a negative predictive value of 0.833. For GAD-7 scores ≥17 were associated with a positive predictive value of 0.266 and a negative predictive value of >99%.
FIGURE 3. Sensitivity and specificity for the GAD-7 with regard to CGI-Severity Score ≤4 (moderate illness).
Solid and dashed lines represent sensitivity and specificity curves, respectively. A GAD-7 score of ≥11 represents the optimal compromise between sensitivity (97%) and specificity (100%) for classification of patients as at least “moderately ill.”
DISCUSSION
This is, to our knowledge, the first report of GAD-7 in pediatric patients with GAD and provides preliminary data supporting the potential use of the GAD-7 as a simple, self-rated questionnaire in clinical practice and for clinical research. Moreover, GAD-7 scores which may be rapidly obtained, strongly correlate with scores from the clinician-rated PARS, which is administered to both the parent and the child and requires approximately 30 minutes to administer.9
That some items on the GAD-7 are more weakly correlated with items on the PARS is important with regard to (1) the construction of the respective assessments and (2) the fact that the PARS represents an interview-based assessment whereas the GAD-7 is a self-report. In our sample, all PARS items significantly correlated with the total GAD-7 score; however, the PARS physical symptom item, the avoidance of anxiety-provoking situations and the interference with non-family relationships items exhibited relatively lower correlation coefficients. This is noteworthy in that the PARS assesses symptom severity, frequency and impairment whereas the GAD-7 assesses the frequency of GAD symptoms. While, in general, the frequency and disability of symptoms correlate, some patients may exhibit more discordance and this may be particularly relevant to pediatric patients. In this regard, family-related factors such as accommodation (i.e., the degree to which a family system shifts family or parental behaviors to reassure the child or to assist a patient with the avoidance of symptom triggers) uniquely contribute to symptom severity and symptom burden in pediatric patients with anxiety disorders29. Thus, factors such as accommodation may moderate the relative dissociation of item-level relationships between impairment items on the PARS and symptom frequency on the GAD-7.
From a clinical trials standpoint, preliminary validation of an alternative scale for the assessment of GAD severity, which correlates with the PARS, may be helpful with regard to the development of inclusion criteria for clinical trials. In this regard, several recent studies in adults have noted that baseline score inflation, which may occur unintentionally, represents a significant contributor to placebo response, particularly given that patients with less severe symptomatology exhibit greater placebo responses30 and that lower severity has been found to be associated with higher placebo response, a phenomenon which appears to be increasing over the last 30 years for psychopharmacologic trials in pediatric patients with anxiety disorders.31 In studies of adults with anxiety and depressive disorders, computer-administered self-report data have recently been utilized32 and alternative rating scales—as opposed to scores on the primary outcome measure—have been used for inclusion criteria. Thus, GAD-7 scores could represent an alternative entry criterion for inclusion in clinical trials of adolescents with GAD, particularly given that they both correlate with the PARS and that they have good sensitivity and specificity for detecting the severity level of GAD.
With regard to the use of the GAD-7 or other structured symptom-based assessments as a guide for treatment interventions, it has been suggested that the “uptake” of research findings in the clinic is related to the ability of clinicians to relate the clinical progress of their individual patients to the outcomes in clinical trials.17 Thus if clinicians are able to relate GAD-7 scores—which are easily obtained in the clinic—to the PARS (i.e., the primary or secondary outcome in the majority of psychopharmacologic trials in youth with anxiety disorders),33 clinicians may be better able to apply clinical trials findings to their individual patients. Additionally, the American Academy of Child & Adolescent Psychiatry recommends “self-report measures for anxiety” in children >8 years of age as these instruments may “assist with screening and monitoring response to treatment”34 and thus, the GAD-7 may be easily implemented into clinical practice settings for the tracking of clinical response and, if subsequent studies confirm our findings related to the validity of the instrument with regard to the reflection of disease severity, scores could guide treatment decisions and planning. In this regard, it has been previously suggested that cut-offs may guide treatment planning in youth with anxiety disorders,17 a suggestion that is of particular importance in that clinicians may not always reliably detect treatment failure and that “formal methods of identifying” non-response have been recommended by some.35
LIMITATIONS
While this is the first study to evaluate the GAD-7 in adolescents, there are a number of important limitations. First, the sample size is small and all patients were evaluated at a single site, thus potentially limiting generalizability. Second, healthy comparison subjects are not included in this sample and therefore GAD-7 scores in healthy adolescents who ostensibly would have lower CGI-S ratings remains unexplored and will be important to evaluate prior to the GAD-7 being utilized as a screening test. Third, the participants were all individuals who—with their families—were “treatment-seeking” and recognized, to some extent, the impairment associated with their symptoms. Thus, it remains possible that greater insight in this treatment-seeking population created a more “accurate” reporting of their symptoms on the GAD-7 thus increasing the likelihood of detecting changes.
CLINICAL SIGNIFICANCE
The results described herein suggest that the GAD-7, a self-rating scale, may reflect symptom severity in adolescents with GAD and that GAD-7 scores are highly correlated with clinician-administered ratings of anxiety symptoms. Taken together, these preliminary findings raise the possibility that this publically available rating, which may be completed by adolescents in <2 min, could be utilized by clinicians to assess anxiety symptom severity in youth with GAD.
Acknowledgments
Dr. Strawn has received research support from Eli Lilly, Edgemont, Shire, Forest, Lundbeck, Neuronetics and from the National Institutes of Mental Health (NIMH). Dr. DelBello has received research support from Johnson & Johnson, Shire, Ortho-McNeil Janssen, Pfizer, Bristol Myers Squibb, Repligen, Somerset, Sumitomo, Thrasher Foundation, GlaxoSmithKline and NIH, and has served as a consultant for GlaxoSmithKline, Eli Lilly, France Foundation, Kappa Clinical, Pfizer, Medical Communications Media and Shering-Plough. Dr. Barzman has received support from Teva Pharmaceuticals, PCORI and from the Benderson Family and Cincinnati Children’s Hospital Medical Center. Dr. Gilman has received support from Eunice Kennedy Shriver National Institute of Child Health and Human Development.
This study was funded by the National Institute of Mental Health (NIMH, K23MH106037, JRS). The content of this paper is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Disclosures: The other authors report no biomedical conflicts of interest.
References
- 1.Kessler RC, Avenevoli S, Costello J, et al. Severity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication Adolescent Supplement. Arch Gen Psychiatry. 2012;69(4):381–389. doi: 10.1001/archgenpsychiatry.2011.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chavira DA, Stein MB, Bailey K, Stein MT. Child anxiety in primary care: Prevalent but untreated. Depress Anxiety. 2004;20:155–164. doi: 10.1002/da.20039. [DOI] [PubMed] [Google Scholar]
- 3.Spitzer RL, Kroenke K, Williams JBW, Löwe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166(10):1092–1097. doi: 10.1001/archinte.166.10.1092. [DOI] [PubMed] [Google Scholar]
- 4.Hamilton M. The assessment of anxiety states by rating. Br J Med Psychol. 1959;32(1):50–55. doi: 10.1111/j.2044-8341.1959.tb00467.x. [DOI] [PubMed] [Google Scholar]
- 5.Norman SB, Campbell-Sills L, Hitchcock CA, et al. Psychometrics of a brief measure of anxiety to detect severity and impairment: The overall anxiety severity and impairment scale (OASIS) J Psychiatr Res. 2011;45(2):262–268. doi: 10.1016/j.jpsychires.2010.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stein MB, Sareen J. Clinical Practice. Generalized Anxiety Disorder. N Engl J Med. 2015;373(21):2059–2068. doi: 10.1056/NEJMcp1502514. [DOI] [PubMed] [Google Scholar]
- 7.Birmaher B, Brent Da, Chiappetta L, Bridge J, Monga S, Baugher M. Psychometric properties of the Screen for Child Anxiety Related Emotional Disorders (SCARED): a replication study. J Am Acad Child Adolesc Psychiatry. 1999;38(10):1230–1236. doi: 10.1097/00004583-199910000-00011. [DOI] [PubMed] [Google Scholar]
- 8.March JS, Parker JD, Sullivan K, Stallings P, Conners CK. The Multidimensional Anxiety Scale for Children (MASC): factor structure, reliability, and validity. J Am Acad Child Adolesc Psychiatry. 1997;36(4):554–565. doi: 10.1097/00004583-199704000-00019. [DOI] [PubMed] [Google Scholar]
- 9.The Pediatric Anxiety Rating Scale (PARS): development and psychometric properties. J Am Acad Child Adolesc Psychiatry. 2002;41(9):1061–1069. doi: 10.1097/00004583-200209000-00006. [DOI] [PubMed] [Google Scholar]
- 10.Winters NC, Collett BR, Myers KM. Ten-year review of rating scales, VII: scales assessing functional impairment. J Am Acad Child Adolesc Psychiatry. 2005;44(4):309–338. 342. doi: 10.1097/01.chi.0000153230.57344.cd. [DOI] [PubMed] [Google Scholar]
- 11.Crawley SA, Caporino NE, Birmaher B, et al. Somatic complaints in anxious youth. Child Psychiatry Hum Dev. 2014;45(4):398–407. doi: 10.1007/s10578-013-0410-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kendall PC, Chansky TE. Considering cognition in anxiety-disordered children. J Anxiety Disord. 1991;5(2):167–185. doi: 10.1016/s0887-6185(97)00015-7. [DOI] [PubMed] [Google Scholar]
- 13.Walkup JT, Albano AM, Piacentini J, et al. Cognitive behavioral therapy, sertraline, or a combination in childhood anxiety. N Engl J Med. 2008;359:2753–2766. doi: 10.1056/NEJMoa0804633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Strawn JR, Prakash A, Zhang Q, et al. A Randomized, Placebo-Controlled Study of Duloxetine for the Treatment of Children and Adolescents With Generalized Anxiety Disorder. J Am Acad Child Adolesc Psychiatry. 2015 Jan;:1–11. doi: 10.1016/j.jaac.2015.01.008. [DOI] [PubMed] [Google Scholar]
- 15.Birmaher B, Axelson Da, Monk K, et al. Fluoxetine for the treatment of childhood anxiety disorders. J Am Acad Child Adolesc Psychiatry. 2003;42(4):415–423. doi: 10.1097/01.CHI.0000037049.04952.9F. [DOI] [PubMed] [Google Scholar]
- 16.Pine DS, Walkup JT, Labellarte MJ, et al. Fluvoxamine for the treatment of anxiety disorders in children and adolescents. N Engl J Med. 2001;344:1279–1285. doi: 10.1056/NEJM200104263441703. [DOI] [PubMed] [Google Scholar]
- 17.Caporino NE, Brodman DM, Kendall PC, et al. Defining Treatment Response and Remission in Child Anxiety: Signal Detection Analysis Using the Pediatric Anxiety Rating Scale. J Am Acad Child Adolesc Psychiatry. 52(1):57–67. doi: 10.1016/j.jaac.2012.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Birmaher B, Khetarpal S, Brent D, et al. The Screen for Child Anxiety Related Emotional Disorders (SCARED): scale construction and psychometric characteristics. J Am Acad Child Adolesc Psychiatry. 1997;36(4):545–553. doi: 10.1097/00004583-199704000-00018. [DOI] [PubMed] [Google Scholar]
- 19.García-Campayo J, Zamorano E, Ruiz MA, et al. Cultural adaptation into Spanish of the generalized anxiety disorder-7 (GAD-7) scale as a screening tool. Health Qual Life Outcomes. 2010;8(1):8. doi: 10.1186/1477-7525-8-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guy W. CGI Clinical Global Impressions. ECDEU Assessment Manual. 1976:217–222. [Google Scholar]
- 21.Silverman WK, Saavedra LM, Pina AA. Test-retest reliability of anxiety symptoms and diagnoses with the Anxiety Disorders Interview Schedule for DSM-IV: child and parent versions. J Am Acad Child Adolesc Psychiatry. 2001;40(8):937–944. doi: 10.1097/00004583-200108000-00016. [DOI] [PubMed] [Google Scholar]
- 22.Duke PM, Litt IF, Gross RT. Adolescents’ self-assessment of sexual maturation. Pediatrics. 1980;66(6):918–920. [PubMed] [Google Scholar]
- 23.Patel NC, DelBello MP, Kowatch RA, Strakowski SM. Preliminary study of relationships among measures of depressive symptoms in adolescents with bipolar disorder. J Child Adolesc Psychopharmacol. 2006;16(3):327–335. doi: 10.1089/cap.2006.16.327. [DOI] [PubMed] [Google Scholar]
- 24.Swets JA. Measuring the accuracy of diagnostic systems. Science. 1988;240(4857):1285–1293. doi: 10.1126/science.3287615. [DOI] [PubMed] [Google Scholar]
- 25.Swets Ja, Dawes RM, Monahan J. Psychological Science Can Improve Diagnostic Decisions. Psychol Sci Public Interes. 2000;1(1):1–26. doi: 10.1111/1529-1006.001. [DOI] [PubMed] [Google Scholar]
- 26.Caporino NE, Brodman DM, Kendall PC, et al. Defining treatment response and remission in child anxiety: Signal detection analysis using the pediatric anxiety rating scale. J Am Acad Child Adolesc Psychiatry. 2013;52(1):57–67. doi: 10.1016/j.jaac.2012.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lopez-Raton Monica, Rodriguez-Alvarez Maria Xose, Cadarso-Suarez Carmen, Gude-Sampedro F. OptimalCutpoints : An R Package for Selecting Optimal Cutpoints in Diagnostic Tests. J Stat Softw. 2014;61(8) [Google Scholar]
- 28.Gallop RJ, Crits-Christoph P, Muenz LR, Tu XM. Determination and Interpretation of the Optimal Operating Point for ROC Curves Derived Through Generalized Linear Models. Underst Stat. 2003;2(4):219–242. [Google Scholar]
- 29.Settipani CA, Kendall PC. The Effect of Child Distress on Accommodation of Anxiety: Relations With Maternal Beliefs, Empathy, and Anxiety. J Clin Child Adolesc Psychol. 2015 Dec;:1–14. doi: 10.1080/15374416.2015.1094741. [DOI] [PubMed] [Google Scholar]
- 30.Mancini M, Wade AG, Perugi G, Lenox-Smith A, Schacht A. Impact of patient selection and study characteristics on signal detection in placebo-controlled trials with antidepressants. J Psychiatr Res. 2014;51(1):21–29. doi: 10.1016/j.jpsychires.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 31.Dobson ET, Strawn JR. Placebo Response in Pediatric Anxiety Disorders: Implications for Clinical Trials Design and Interpretation. J Child Adolesc Psychopharmocology. 2017 doi: 10.1089/cap.2015.0192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Byrom B, JC M. The value of computer-administered self-report data in central nervous system clinical trials. Curr Opin Drug Discov Devel. 2005;8(3):374–383. [PubMed] [Google Scholar]
- 33.Strawn JR, Welge JA, Wehry AM, Keeshin B, Rynn MA. Efficacy and tolerability of antidepressants in pediatric anxiety disorders: a systematic review and meta-analysis. Depress Anxiety. 2015;32(3):149–157. doi: 10.1002/da.22329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Connolly SD, Bernstein GA. Practice parameter for the assessment and treatment of children and adolescents with anxiety disorders. J Am Acad Child Adolesc Psychiatry. 2007;46(2):267–283. doi: 10.1097/01.chi.0000246070.23695.06. [DOI] [PubMed] [Google Scholar]
- 35.Hannan C, Lambert MJ, Harmon C, et al. A lab test and algorithms for identifying clients at risk for treatment failure. J Clin Psychol. 2005;61(2):155–163. doi: 10.1002/jclp.20108. [DOI] [PubMed] [Google Scholar]