Abstract
Human carcinoembryonic antigen (CEA) is the prototypic member of a family of highly related cell surface glycoproteins that includes carcinoembryonic antigen‐related cell adhesion molecule 6 (CEACAM6) and others. CEACAM6 (formerly NCA), which belongs to the immunoglobulin superfamily, is a cell adhesion protein of the CEA family. It is normally expressed on the epithelial surfaces and on the surface of myeloid cells (CD66c). CEACAM6 is a multi‐functional glycoprotein that mediates homotypic binding with other CEA family members and heterotypic binding with integrin receptors. It functions by organizing tissue architecture and regulating different signal transduction, while aberrant expression leads to the development of human malignancies. It was first discovered in proliferating cells of adenomas and hyperplastic polyps in comparison to benign colonic tissue when overexpressed on the surface of various cell types in model systems. CEACAM6 functions as a pan‐inhibitor of cell differentiation and cell polarization, and it also causes distortion of tissue architecture. Moreover, overexpression of CEACAM6 modulates cancer progression through aberrant cell differentiation, anti‐apoptosis, cell growth and resistance to therapeutic agents. In addition, CEACAM6 overexpression in multiple malignancies promotes cell invasion and metastasis, thereby representing an acquired advantage of tumor cells directly responsible for an invasive phenotype. This review focuses on the findings supporting the mechanisms of actions linking the oncogenic potential of CEACAM6 to the onset of cancer progression and pathogenesis, especially in breast cancer, and to validating CEACAM6 as a target to pave the way towards the design of efficient therapeutic strategies against breast cancer.
Keywords: adhesion, cancer, CEACAM6, invasion and metastasis, review
1. INTRODUCTION
Human carcinoembryonic antigen (CEA) is part of a larger family of related glycoproteins made up of 2 separate branches: the CEA cellular adhesion molecules (CEACAM) and the pregnancy‐specific glycoprotein (PSG) subgroup.1 In addition, cell adhesion molecules (CAM) are predominantly transmembrane glycoproteins, involved in intercellular binding of adjacent cells to each other or to the extracellular matrix (ECM). They play a crucial role in a number of biological processes, including homeostasis, embryogenesis and development of neural tissue, inflammation, immune cell transmigration and immune response.2 Four major groups of CAM have been described: the immunoglobulin (Ig) superfamily, integrins, selectins and cadherins.3 The CEACAM are highly glycosylated transmembrane proteins of the Ig superfamily, located on chromosome 19q13.1‐13.2, and were described in 1972 (Figure 1).4, 5 They are involved in modulate cellular processes, such as neovascularization, shaping of tissue architecture, angiogenesis, T‐cell proliferation and cell–cell recognition, and regulation of insulin action, and have also been described as bacterial and viral pathogen receptors, such as Neisseria species, Escherichia coli and Salmonella.6 Moreover, CEACAM play an important role in tumor‐associated mechanisms.7 The CEACAM family consists of membrane‐linked glycoproteins anchored to the cell surface either by glycophosphatidyl‐inositol (GPI) anchor or a transmembrane domain, as well as secretory glycoproteins.8 The GPI‐anchored members include CEACAM7 (formerly CGM2), CEACAM8 (formerly CGM6), CEACAM5 and CEACAM6 (formerly NCA).9 CEACAM1 (formerly BGP), CEACAM3 (formerly CGM1), CEACAM4 (formerly CGM7), CEACAM19, CEACAM20 and CEACAM21 are all attached to the cell membrane through transmembrane domains.2 CEACAMs manifest their function as an intercellular adhesion molecule through GPI anchorage to the cell surface or transmembrane domains.8
Figure 1.

The carcinoembryonic antigen‐related cell adhesion molecule (CEACAM) gene superfamily.79 The CEACAM immunoglobulin‐like domain superfamily is a large family of proteins encoded by 12 independent genes and located on chromosome 19. The Ig domain consists generally of one IgV‐like N‐domain (red), except CEACAM 16, which has 2 N‐domains and IgC‐like domains identified as (A and B) shown in (blue). The membrane‐linked glycoproteins are anchored to the cell surface either by transmembrane domain (green zigzag lines), such as CEACAM5‐8, or anchored to the membrane by glycophosphatidyl‐inositol anchor (green arrowhead), including CEACAM1, 3, 4, 19, 20 and 21. CEACAM16 is a secreted version with no membrane anchorage. The CEACAM 19 and 20 cytoplasmic domain has immune‐receptor tyrosine‐based activator (ITAM) motifs (blue circles). Heavily N‐ Glycosylation sites in all family members are shown as black lollipops on the extracellular domains
Most of the research has been directed at CEACAM1 due to the homology identified in a number of mammals, making it more accessible as an in vivo model,10 as well as towards CEACAM5, the gastrointestinal oncofetal antigen first described in 1965.8 It is found in most carcinomas of the respiratory and genitourinary systems, in breast cancer, and in cancers of the gastrointestinal tract.11 In contrast, CEACAM6, also referred to as CD66c, is a multi‐functional glycoprotein that mediates homotypic binding with other CEA family members and heterotypic binding with integrin receptors.12 It functions by organizing tissue architecture and regulation of signal transduction, while aberrant expression leads to the development of human malignancies.12 It was first discovered in proliferating cells of adenomas and hyperplastic polyps in comparison to benign colonic tissue.13 Overexpression of CEACAM6 modulates cancer progression through aberrant cell differentiation, anti‐apoptosis, cell growth and resistance to therapeutic agents.14 CEACAM6 overexpression in multiple malignancies promotes cell invasion and metastasis, thereby representing an acquired advantage of tumor cells directly responsible for an invasive phenotype.15 In vitro studies have shown that antibodies directed against CEACAM6 on overexpressing cells inhibited cell migration, invasion and adhesion.15
This article will serve as a comprehensive review highlighting the role of CEACAM6 in various malignancies, identifying common and unique pathways suspected of playing a central role in the malignant process. Furthermore, targeting CEACAM6 with novel therapeutic approaches provides an opportunity to treat several human malignancies.
2. CARCINOEMBRYONIC ANTIGEN/CARCINOEMBRYONIC ANTIGEN‐RELATED CELL ADHESION MOLECULE FAMILY: CHROMOSOMAL LOCATION, EXPRESSION AND REGULATION
2.1. Chromosomal location of carcinoembryonic antigen/carcinoembryonic antigen‐related cell adhesion molecules
The human CEACAM gene family is composed of 29 genes/pseudogenes and gene‐like sequences that are clustered in human chromosome 19 (q13.2.).16 This large gene family can be divided into the CEA subgroup (n = 12, where 5 of them are pseudogenes), the PSG‐subgroup (n = l1) and the incomplete non‐expressed CGM (CEA gene family member) subgroup (n = 6).17, 18 These genes were arranged during evolution into 850‐kb distal clusters and 250‐kb proximal clusters in relation to the centromere, separated by 700 kb of genomic DNA containing a few unrelated genes.19 CEACAM4, CEACAM7, CEACAM5, CEACAM6 and CEACAM3 are closely clustered in the proximal cluster; CEACAM1, CEACAM8 and PSG/CGM genes are clustered in the distal cluster.16
Gene amplification is an essential mechanism of insertional mutagenesis, in addition to loss of control mechanisms, structural alterations, chromosome translocations and oncogene activation. The comparative genome hybridization analysis identified DNA copy number changes in all cancers.20 The region 19q13.2‐13.32, spanning 3.25 MB, is amplified in hepatocellular carcinoma as well as being amplified in other cancers such as follicular lymphoma (19q13), mantle cell lymphoma (19q13), respiratory tract small cell lung cancer (19q13.1), non‐small cell lung cancer (19qcen‐q13.3), hepatocellular carcinoma (19q13.1), breast carcinoma (19q13.1‐qter), and chondrosarcoma (19q13.2),21 whereas deletion of this locus occurs infrequently and was only observed in colon tumors.22 It is clear that the precise characterization of chromosomal amplicon areas will be of prognostic and therapeutic value to revolutionize clinical molecular genetics in oncology.
2.2. Transcriptional regulation of carcinoembryonic antigen/carcinoembryonic antigen‐related cell adhesion molecule 6
Studies on transcriptional regulation of the CEACAM family genes have previously been performed with human CEACAMl and CEACAM6 genes.23 Research on the regulation of other CEACAM family genes in humans and other species is still scarce. The upstream promoter sequences of CEACAM1 and CEACAM6 genes lack the classical TATA and CCAAT boxes. TATNCCAAT‐less genes can generally be grouped into: (i) constitutively active house‐keeping genes with relatively G/C‐rich promoter regions, SPI sites and often multiple transcriptional start sites; or (ii) genes lacking G/C‐rich regions that have tightly clustered transcriptional start sites that are differentially or developmental regulated.24 However, in contrast to other TATNCCAAT‐less genes, CEACAM family genes possess features from both groups.23 They have G/C‐rich promoter regions and SPI sites, but they also have clusters of transcriptional start sites and are differentially expressed. CEACAM6 promoters show a sequence homology of 80% within the first 230 nucleotides upstream of the translational start site, where they could share the same transcriptional binding factors. The sequences farther upstream diverge significantly from each other.25
In TGF‐β signaling, CEACAM6 was defined as a major SMAD3‐mediated target gene. Moreover, HER2 expression was associated significantly with SMAD3 phosphorylation in only CEACAM6‐positive cancers.26 TGF‐β elaborated in the malignant tumor microenvironment (TME) binds to the type II receptor (T_RII), promoting hetero‐tetramerization with the type I receptor (T_RI) and increasing the phosphorylation of SMAD3 by activated T_RI. Phosphorylated SMAD3 forms a complex with Co‐Smad (SD4), which promotes translocation into the nucleus and activate expression of target genes, specifically CEACAM6.27 Hence, HER2 signaling through the TGF‐β pathway may regulate CEACAM6 expression.
Although genetics is concerned with gene expression as a function of DNA sequence, epigenetic alteration of gene expression can be stably modulated by different mechanisms. It has been reported that HDAC inhibitor (NaB) and DNA methyltransferase inhibitor (5‐AZA) upregulate CEA expression in different cancer cells.28 In colon carcinoma cells, NaB and 5‐AZA appear to have major effects in increasing the rate of transcription through epigenetic modifications of chromatin and DNA methylation, respectively.28 Furthermore, epigenetic analysis of CEACAM6 promoter in different primary breast cancers, identified downregulation of expression level by hypermethylation of CEACAM6 promoter among triple‐negative breast cancers specifically.29 Strongly suggesting that aberrant DNA hypermethylation of CEACAM6 promoter by overexpression of DNMT3b, DNA methyltransferase enzymes, and concurrent methylation‐dependent silencing might trigger breast cancer progression.
2.3. Expression of carcinoembryonic antigen/carcinoembryonic antigen‐related cell adhesion molecule 6
Each CEACAM family member has a distinct tissue‐specific expression pattern that varies during different stages of development. In healthy human adults, the expression of CEA is mostly found in columnar epithelial cells and mucus‐secreting cells of the colon.30 CEA can also be found at a lower level in secretory epithelial cells present in the stomach and small intestine, in epithelial cells of the prostate, in secretory epithelial and duct cells of the sweat glands, in transitional epithelial cells of the urinary bladder, as well as in squamous epithelial cells of the esophagus, tongue and cervix.13 Expression of CEA in lung, breast and pancreas is still controversial. The expression pattern of CEACAM6 is somewhat broader but generally similar to that of CEA with a few exceptions.30 CEACAM6 is not expressed in small intestine, but in duct cells of the breast and pancreas, in myeloid cells of the bone marrow and spleen, and in pneumocytes and bronchiole epithelial cells of the lung, as well as in many other tissues.13 Over the past 10 years, a tremendous amount of information regarding the expression patterns and potential of CEACAM6 to serve as a prognostic indicator as well as a therapeutic target has emerged.
3. STRUCTURE OF CARCINOEMBRYONIC ANTIGEN/CARCINOEMBRYONIC ANTIGEN‐RELATED CELL ADHESION MOLECULE 6
The ECM domains of CEACAM6 consist of an Ig variable‐like N‐terminal domain followed by 6 (AIB l, A2B2, A3B3), 3 (AIB l, A2) or 2 (AlB1) C2‐type Ig domains, respectively, as shown in Figure 2.31 CEACAM6 and CEACAM1 share striking homology at both the nucleotide and amino acid levels. Relative to the CEA family, CEACAM1 and CEACAM6 are 80/90% homologous, respectively, at the nucleotide level and 70/85% homologous, respectively, at the amino acid level.31 While CEACAM6 molecules are bound to the plasma membrane by GPI anchors, CEACAM1 contains a transmembrane domain followed by a cytoplasmic tail.32 This distinctive structural feature may explain the opposite roles in human cancer of CEACAM1 vs CEACAM6.
Figure 2.
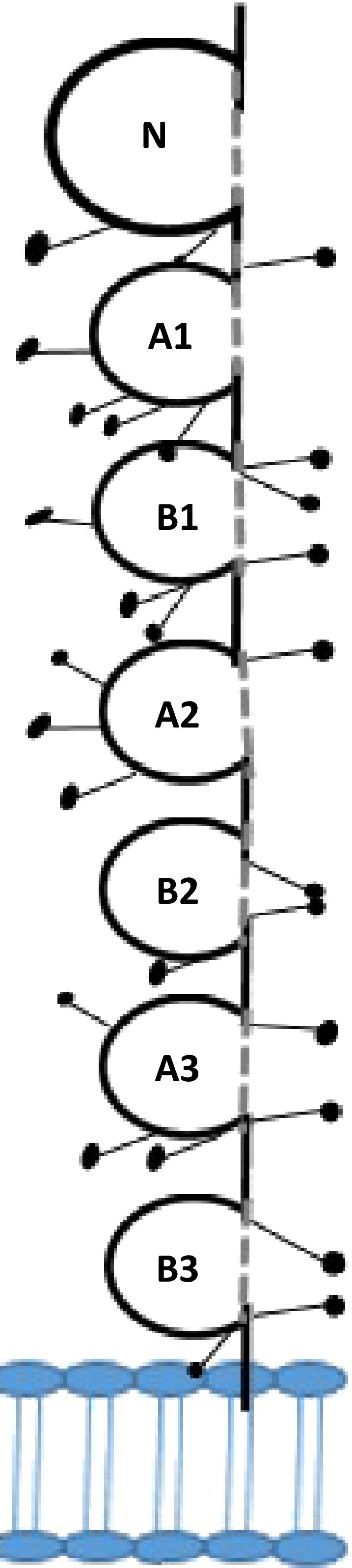
Model of a CEA molecule. CEA has been named in the CD system as CD66e. It consists of one IgV‐like N‐domain and 6 IgC‐like domains (A and B). Glycosylation sites are shown as lollipops. The glycophosphatidyl‐inositol linkage to the cell membrane is shown by an arrowhead
As mentioned above, CEACAM6 is bound to the plasma membrane through a GPI anchor. Such anchors are synthesized in the endoplasmic reticulum.33 The eukaryotic GPI anchor consists of a backbone of N‐acetyl‐glucosamine, 3 mannose residues, and ethanolamine bound to a phosphoinositol molecule covalently linked to a glycerolipid moiety located within the outer leaflet of the plasma membrane. CEACAM6 are likely attached to the GPI anchor through an amide linkage between their C‐terminus and the core phosphoethanolamine moiety of the anchor. There are variations of the core structure of the eukaryotic GPI anchor, such as palmitoylation of the inositol ring, number and length of lipid chains attached to glycerol, and modifications of mannose residues.34 GPI‐anchored proteins, such as CEA and CEACAM6, are excluded from the polarized epithelial cells and are only specifically targeted to apical membranes.35 Moreover, GPI‐anchored proteins are found in specific rafts or micro‐domains of the plasma membrane known as detergent insoluble glycolipid domains (DIG).36 Several members of the Src‐family of GTP‐binding proteins and protein tyrosine kinases involved in signal transduction pathways are often associated with the cytoplasmic side of DIG.36
4. FUNCTIONS OF CARCINOEMBRYONIC ANTIGEN/CARCINOEMBRYONIC ANTIGEN‐RELATED CELL ADHESION MOLECULE 6
Carcinoembryonic antigen is expressed on the apical surface of columnar epithelial cells and mucus‐secreting goblet cells of the human gastrointestinal tract, mainly in the upper one‐third of the colonic crypts. In addition, CEA is expressed in squamous epithelial cells of the cervix and tongue,13 in sweat glands37 and in epithelial cells of the prostate.38 CEACAM6 is expressed in epithelial cells of the colon,39 lung and stomach, and in granulocytes and monocytes as well.40
4.1. Physiological functions of carcinoembryonic antigen/carcinoembryonic antigen‐related cell adhesion molecule 6 in normal tissues
Cell adhesion molecules play a crucial role in a number of biological functions, including homeostasis, embryogenesis, development of neural tissue, inflammation, immune cell transmigration and immune response.3 Moreover, expression of CEA and CEACAM6, but not CEACAM1, inhibit colonocyte differentiation.41 CEA was also shown to inhibit neurogenic and adipogenic differentiation.42 Altogether, these results suggested that the differentiation block by CEA is possibly a result of clustering of multiple CEA molecules on the cell surface and activation of downstream signaling cascades.35 In addition, findings showed that opposite effects can be mediated by the 2 groups of CEACAM, as in cell differentiation. Hence, it is advantageous for the tumor cells to overexpress CEACAM6 and to downregulate CEACAM1, so that they can escape apoptosis as well.43
4.2. Physiological functions of carcinoembryonic antigen/carcinoembryonic antigen‐related cell adhesion molecule 6 in cancer
The human CEACAM family genes are deregulated in many human cancers. CEA is overexpressed in many gastrointestinal cancers, including colorectal, esophageal, gastric, pancreatic, hepatocellular and gallbladder carcinomas, breast cancer, lung cancer, the female reproductive tract cancer (ovarian, cervical and endometrial carcinomas), medullary thyroid carcinomas, urinary bladder cancer and prostate cancer.44 The question of the potential mechanism of CEA/CEACAM6 effects arises. There are at least 3 key physiological processes that function to maintain the homeostasis of a normal human epithelium: cell proliferation, cell differentiation and apoptosis.45 The disturbance of these processes, such as through an increase in cell proliferation or inhibition of cell differentiation and apoptosis, or a combination of these events, could contribute to malignant transformation of the epithelial cells. The forced overexpression of CEA/CEACAM6 on the cell surface of several cell lines inhibited cell differentiation without increasing the rate of cell proliferation.44 CEA/CEACAM6 are mostly expressed on the apical surface of the human colonial epithelium but are also expressed in other cell types.35 This normal pattern of expression is dramatically disrupted upon malignant transformation when CEA and CEACAM6 are overexpressed over the entire surface of the malignant cells whereas CEACAM1 is largely absent.46 CEACAM6 are overexpressed in more than 50% of all human adenocarcinomas. Based on these observations, the prevailing hypothesis is that CEACAM6 overexpression plays an oncogenic role.15 In contrast, transmembrane CEACAM1 was proposed to be a tumor suppressor protein because it is downregulated in many cancers and its forced expression in various cancer cell lines, inc1uding prostate, colon and breast, decreases proliferation and increases apoptosis.43 However, this generalization becomes blurred based on the following observations: (i) detection of its overexpression in various cancers;47 (ii) its contribution to tumor aggressiveness;48 (iii) the nature of its immune suppressive effects;49 and (iv) its pro‐angiogenic properties.50
The GPI‐anchored CEA/CEACAM6 have a variety of tumorigenic effects on cells cultured in vitro and in xenograft mouse models. Overexpression of CEA and CEACAM6 impedes myogenic, adipogenic, neurogenic and colonic differentiation programs,41 inhibits anoikis/apoptosis in colon and pancreatic cancer cells,51, 52 disrupts cell polarization and tissue architecture,41 increases chemoresistance53 and enhances liver metastasis.52
Although earlier studies examined the role of CEACAM6 in gastrointestinal malignancies such as colon and pancreas, numerous other carcinomas (e.g. breast, gastric, thyroid, B‐ALL and multiple myeloma) have been shown to overexpress CEACAM6, resulting in increased metastatic potential.
The fundamental role of CEACAM 6 in breast cancer progression will be discussed in detail below.
4.3. Physiological functions of carcinoembryonic antigen‐related cell adhesion molecule 6 in breast cancer
Carcinoembryonic antigen‐related cell adhesion molecule 6 expression may represent an early event in the development of human epithelial malignancies. Breast cancer cells illustrate CEACAM6 better than normal breast tissue.54 As we have seen in precursor lesions for both colorectal cancer and pancreatic ductal adenocarcinoma (PDA), overexpression of CEACAM6 is predictive of progression to breast cancer.55 A retrospective analysis of 243 patient samples revealed that CEACAM6 overexpression correlated with tamoxifen resistance.56 In a multivariate analysis, CEACAM6 overexpression was an independent predictor of disease recurrence.57 Furthermore, siRNA‐mediated gene silencing of CEACAM6 in tamoxifen‐resistant MCF7 cell line derivatives reversed anchorage independence and re‐established endocrine sensitivity.57 Previous work revealed that the development of resistance to tamoxifen in breast tumor cells is related to augmented invasive potential58 as well as CEACAM6 overexpression.54 The relationship between CEACAM6 overexpression and acquired tamoxifen resistance leads to invasion and migration in MCF‐7.2A and MCF‐7.5C breast cancer cells.59 CEACAM6 is markedly overexpressed in estrogen‐diminished breast cancer cells. These in vitro data were further validated in human breast cancer as CEACAM6 is recurrently overexpressed in the setting of post‐adjuvant endocrine therapy (e.g. tamoxifen). In a cohort study of 840 invasive breast cancers and a separate validation cohort of 300 invasive breast cancers, CEACAM6 expression was found to be present in 37.1% (312/840 patients) cases.26 Interestingly, CEACAM6 expression was highest in HER2+ tumors (67%) and lowest in triple negative breast cancer (25%).60 The patients in this study were followed for a mean of 56.4 months, and 105 patients developed distant metastatic disease.26 Expression rates for CEACAM6 were highest for patients with bone metastasis (42%). The study reported that CEACAM6 adverse prognostic significance for OS was independent of traditional prognostic factors (age, tumor grade, size and Ki‐67 expression level). One unique feature of the signal transduction in HER2+ breast cancer cell lines and CEACAM6 expression is the interaction of the HER2 pathway and TGF‐β signaling.17 Overexpression of CEACAM6 results in alteration and reorganization of the ECM and activation of the TME playing a key role in tumor invasion.54 CEACAM6 signaling increases SRC activity, leading to increased IGF‐I secretion, with autocrine and paracrine stimulation of the IGF‐1R activating the PI3K/AKT pathway. Increased IGF‐I expression results in MMP‐2 elaboration, subsequently altering the ECM and promoting a malignant TME. Aggregation of CEACAM6 at the cell surface enhances anoikis resistance through activation of SRC in a caveolin‐1‐dependent manner, allowing for phosphorylation of its substrate focal adhesion kinase.17
In addition, TGF‐β signals through the TGF‐β receptors activate alternate pathways, such as AKT/PI3K.17 CEACAM6 expression also serves as an indicator of response to therapy in Her2+ breast cancer. CEACAM6 underexpression indicated trastuzumab responsive disease, whereas overexpression reflected trastuzumab‐resistant breast cancer.61 Further investigation is warranted to identify whether CEACAM6 overexpression in metastatic breast cancer patients also serves as a biomarker of response to gemcitabine, an approved third line therapy.61 CEACAM6 expression would then serve as an avenue to identify high‐risk cohorts of breast cancer patients whose therapy could be specifically tailored based on their underlying tumor biology, highlighting CEACAM6's role as both prognostic and underscoring its potential as a therapeutic target in epithelial carcinomas.
Based on the observations described above and the research work ongoing in our research lab, we generated the following dual hypothesis depicted in (Figure 3) below: (i) CEACAM6, beyond its normal functions, including intercellular adhesion, cell death regulation, and also its function as a general inhibitor of cell differentiation, might play a novel role in malignant transformation and tumor cell invasion/metastasis in breast cancer; and (ii) in addition to a better understanding of the mechanisms that underpin CEACAM6‐mediated tumor cell invasion, our recent research findings have the potential to validate CEACAM6 as a target to pave the way towards the design of efficient therapeutic strategies against breast cancer.
Figure 3.
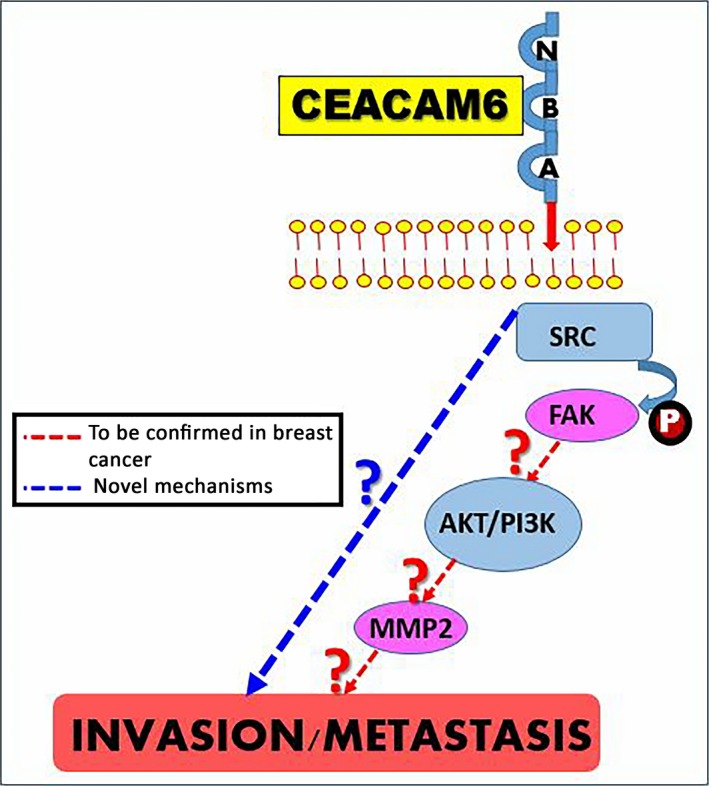
The novel predicted molecular mechanisms underlying carcinoembryonic antigen‐related cell adhesion molecule 6 (CEACAM6) promoting breast cancer progression
4.4. Physiological functions of carcinoembryonic antigen‐related cell adhesion molecule 6 in other cancers
Over the past 2 decades, physiological and pathological roles of this large gene family, mainly in cancer, infectious diseases, immunology and diabetes, have been gradually elucidated in different model systems.49 Although the growing knowledge on the functions of CEACAM has already been beneficial to clinical practice and therapy, further understanding of their mechanism of action in associated diseases and discovery of their participation in other diseases may have greater impact on both clinical and biological sciences in the future.
Carcinoembryonic antigen‐related cell adhesion molecule 6 is overexpressed primarily by colorectal, gastric and pancreatic cancers, with the vast majority of these tumors (>90%) expressing CEA.62 In addition, it is expressed by the majority of medullary thyroid cancers63 and more than 70% of non‐small cell lung cancers.64 Reports of expression on many other cancers exist. Overexpression of CEA colorectal cancer was described by inhibition of terminal differentiation, loss of cellular polarization, distortion of tissue architecture, and anoikis resistance,8 while in lung adenocarcinoma, it induces cell proliferation and anchorage‐independent growth.65, 66 In gastric cancer, CEACAM6 promotes lymph node metastasis, migration and invasion through increasing SRC phosphorylation.50 In contrast, in B‐cell acute lymphoblastic lymphoma, presumed enhancement of anoikis through increased caspase activation as well as upregulation of the AKT cell survival pathway.50 In addition, in multiple myeloma, CEACAM6 inactivation of CTL (cytotoxic lymphocytes), through binding and cross‐linking of CEACAM6 on plasma cells, resulted in T‐cell unresponsiveness immune evasion.51, 67 In addition, in pancreatic ductal adenocarcinoma, increased metastatic potential through increased IGF‐I resulted in expression of proteolytic MMP‐2 and MMP‐9, altering the ECM through SRC‐AKT signaling pathway.68 These findings correlate CEACAM6 overexpression with an increased metastatic phenotype through elevated SRC activity and matrix metalloproteinase 2 (MMP2) expression. Hence, CEACAM6 overexpression through activation of the SRC‐AKT signaling provides chemoresistance to gemcitabine in PDA cell lines.
5. CONTRIBUTION OF CARCINOEMBRYONIC ANTIGEN/CARCINOEMBRYONIC ANTIGEN‐RELATED CELL ADHESION MOLECULE 6 IN CELL SIGNALING
The CEA family plays an active role in multiple signal transduction processes.69 Using transfected basophile leukemia cells as a model, it has been demonstrated that GPI‐bound CEA participates in a stimulatory signal transduction pathway.8
Many cell surface molecules, including receptors and adhesion molecules, can convey information from the milieu to the interior of the cells by means of signal transduction, so that the cells can respond to the changes in the environment. The CEACAM gene family of cell surface adhesion molecules is no exception. Nevertheless, Screaton et al35 suggested that the presence of CEA‐specific GPI anchors, which reside in specific membrane microdomains (lipid rafts), is essential for their cellular functions, at least as inhibitors of cell differentiation. Screaton et al further suggested that perturbation of integrin functions and integrin‐ECM interactions may be the underlying mechanism responsible for their inhibitory effect on anoikis.35 When CEACAM6 is cross‐linked in BxPC3 cells, anoikis resistance develops through activation of SRC in a caveolin‐1‐dependant manner.53 SRC was identified as a key player in tumor progression, providing oncogenic signals for mitogenesis, cell survival, angiogenesis, invasion and metastasis.70 Caveolin‐1 is an integral membrane protein found to co‐precipitate with SRC and is required for SRC kinases to activate integrin signaling.71 The co‐clustering of cell surface lipid raft‐associated CEACAM6 and integrin α5β1 leads to increased fibronectin binding to the cells and recruitment of downstream signaling elements, including ILK, Src family kinases, PBK and Akt/PKB.72 and, hence, it has been proposed that clustering could activate the integrin signaling pathway.11 A similar observation was made by another group involving CEACAM6 and integrin α7β3 in pancreatic adenocarcinoma cell lines.53 Altogether, the GPI‐anchored CEACAM can mediate cell signaling through modulation of integrin functions (Figure 4).
Figure 4.
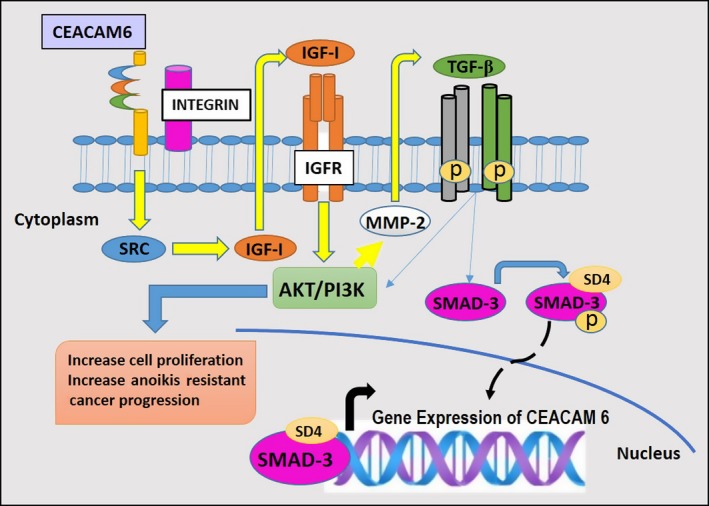
Carcinoembryonic antigen‐related cell adhesion molecule (CEACAM6) signaling pathways
6. CARCINOEMBRYONIC ANTIGEN‐RELATED CELL ADHESION MOLECULE 6 TARGETED CANCER THERAPIES
Combinations of surgery, chemotherapy and radiation therapy represent the conventional treatment for cancer. However, with the growing knowledge of cancer molecular biology, novel cancer therapies are focused on personalized tumor targets to minimize systemic toxicity and increase efficacy.73
In the present review, the role of CEACAM6 in various malignancies has been discussed, as well as the identification of common and unique pathways suspected to play a central role in the malignant process. Furthermore, targeting CEACAM6 provides an opportunity to treat several human malignancies, including the following potential approaches.
6.1. Anti‐carcinoembryonic antigen‐related cell adhesion molecule 6 single chain variable fragment therapeutic humanized
Mouse monoclonal antibody (Mab) 13‐1, targeting a unique epitope on human CEACAM6, leads to apoptosis of PDA cells expressing CEACAM6 with an IC50 independent of antibody cytotoxicity.74 In silico CDR grafting and antibody engineering generated single chain variable fragment (scFv) humanized anti‐CEACAM6 with a linker that could be PEGylated to enhanced plasma half‐life. The unique features of humanized anti‐CEACAM6 scFv include the ability to produce targeted tumor cell apoptosis and also represent an effective combination with standard chemotherapy at very low doses.74
6.2. Monoclonal antibodies
Utilizing GW‐39 human colonic cancer cells, the effects of 3 Mabs that target specific epitopes A1B1 domains (MN‐15) and the NH2‐terminal (MN‐3) present in CEACAM6, CEACAM5 and the A3B3 domain (MN‐14) were investigated.15 Although treatment with MN‐15 and MN‐3 resulted in an increase in survival, decreased adhesion to the ECM and abrogated metastatic potential, these Mabs were not efficacious with regard to tumor growth inhibition or regression.15 The availability of therapeutic monoclonal antibodies (unconjugated and conjugated) targeting CEACAM6 provides potential therapeutic opportunities likely to lead to a plethora of pre‐clinical and clinical activity in the next decade.
6.3. Antibody‐drug conjugates
A study of an antibody‐drug conjugate targeting CEACAM6 investigated the efficacy of the anti‐CEACAM6‐maytansinoid (DM1) immune‐conjugate in a murine xenograft model of PDA.75 The non‐conjugated Mab did not have anti‐tumor activity, and showed reversible and modest neutropenia. Further development of an antibody‐drug conjugate‐based therapeutic approach in PDA has yet to be pursued in early phase clinical trials.75
6.4. Immunotoxin‐based therapy
Targeting CEACAM6 in PDA using immunotherapeutic strategies has been classified as direct or indirect.76 Indirectly using the Mab (114) specific for CEACAM6 with no anti‐tumor activity mediated cross‐linking of CEACAM6, which resulted in cytoplasmic accumulation that increased efficacy of immunotherapy with saporin with caspase‐mediated apoptosis.76
6.5. Knockdown mediated gene silencing
Knockdown models in CEACAM6‐positive tumors identify key mechanistic pathways and potential targets involved in the development of the malignant phenotype.77 Mice CEACAM6‐specific siRNA studies diminished tumor proliferation significantly, with a decreased Ki‐67 level impairing angiogenesis and increased apoptosis. Furthermore, CEACAM6‐specific siRNA prevented metastasis and improved survival without toxicity. Knockdown models inform us of the critical role CEACAM6 plays in cancer development, further solidifying a focus on this molecule as a therapeutic target.77
7. SUMMARY AND PERSPECTIVE
The migration of cancerous cells from their primary location to distant sites is the cause of 90% of human cancer mortality.78 Dysregulated overexpression of CEACAM6 plays a role in several of the hallmarks of cancer, including uncontrolled proliferation, anoikis resistance, neoangiogenesis, immune evasion, invasion and metastasis. We believe that safely targeting CEACAM6 is most likely the key to changing the natural history of the tumor and tumor microenvironment to allow novel combination therapies to be developed for treating both solid and hematologic malignancies. We believe that safely targeting CEACAM6 is most likely to change the natural history of the tumor and TME and allow novel combination therapies to be developed for treating both solid and hematologic malignancies.
In conclusion, our review highlights the pathologic roles of CEACAM6 in the development of multiple human malignancies, warranting an ongoing focus to successfully transition this cell adhesion protein as a primary or adjunctive therapeutic target for anti‐cancer therapy in the clinic.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Rizeq B, Zakaria Z, Ouhtit A. Towards understanding the mechanisms of actions of carcinoembryonic antigen‐related cell adhesion molecule 6 in cancer progression. Cancer Sci. 2018;109:33–42. https://doi.org/10.1111/cas.13437
REFERENCES
- 1. Zhang Y, Zang M, Li J, et al. CEACAM6 promotes tumor migration, invasion, and metastasis in gastric cancer. Acta Biochim Biophys Sin. 2014;46:283‐290. [DOI] [PubMed] [Google Scholar]
- 2. Schäfer MK, Altevogt P. L1CAM malfunction in the nervous system and human carcinomas. Cell Mol Life Sci. 2010;67:2425‐2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. La Flamme SE, Kowalczyk AP. Cell Junctions: Adhesion. Development and Disease. Hoboken, NJ: John Wiley & Sons; 2008. [Google Scholar]
- 4. Von Kleist S, Chavanel G, Burtin P. Identification of an antigen from normal human tissue that crossreacts with the carcinoembryonic antigen. Proc Natl Acad Sci USA. 1972;69:2492‐2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Horst A, Wagener C. CEA‐related CAMs In: Cell Adhesion. Berlin: Springer; 2004:283‐341. [DOI] [PubMed] [Google Scholar]
- 6. Hauck CR, Agerer F, Muenzner P, Schmitter T. Cellular adhesion molecules as targets for bacterial infection. Eur J Cell Biol. 2006;85:235‐242. [DOI] [PubMed] [Google Scholar]
- 7. Kuespert K, Pils S, Hauck CR. CEACAMs: their role in physiology and pathophysiology. Curr Opin Cell Biol. 2006;18:565‐571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Stanners C, Chan CH. Recent advances in tumor biology of the GPI‐anchored carcinoembryonic antigen family members CEACAM5 and CEACAM6. Curr Oncol. 2007;14:70‐73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hammarstrom S, Olsen A, Teglund S, Baranov V. The nature and expression of the human CEA family In: Stanners CO, ed. Cell Adhesion and Communications Mediated by the CEA Family Basic and Clinical Perspectives (Vol. 5). Amsterdam, The Netherlands: Harwood Academic Publishers; 1998:1‐30. [Google Scholar]
- 10. Horst AK, Ito WD, Dabelstein J, et al. Carcinoembryonic antigen–related cell adhesion molecule 1 modulates vascular remodeling in vitro and in vivo. J Clin Invest. 2006;116:1596‐1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Camacho‐Leal P, Zhai AB, Stanners CP. A co‐clustering model involving α5β1 integrin for the biological effects of GPI‐anchored human carcinoembryonic antigen (CEA). J Cell Physiol. 2007;211:791‐802. [DOI] [PubMed] [Google Scholar]
- 12. Benchimol S, Fuks A, Jothy S, Beauchemin N, Shirota K, Stanners CP. Carcinoembryonic antigen, a human tumor marker, functions as an intercellular adhesion molecule. Cell. 1989;57:327‐334. [DOI] [PubMed] [Google Scholar]
- 13. Schölzel S, Zimmermann W, Schwarzkopf G, Grunert F, Rogaczewski B, Thompson J. Carcinoembryonic antigen family members CEACAM6 and CEACAM7 are differentially expressed in normal tissues and oppositely deregulated in hyperplastic colorectal polyps and early adenomas. Am J Pathol. 2000;156:595‐605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Blumenthal RD, Leon E, Hansen HJ, Goldenberg DM. Expression patterns of CEACAM5 and CEACAM6 in primary and metastatic cancers. BMC Cancer. 2007;7:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Blumenthal RD, Hansen HJ, Goldenberg DM. Inhibition of adhesion, invasion, and metastasis by antibodies targeting CEACAM6 (NCA‐90) and CEACAM5 (Carcinoembryonic Antigen). Cancer Res. 2005;65:8809‐8817. [DOI] [PubMed] [Google Scholar]
- 16. Kammerer R, Zimmermann W. Coevolution of activating and inhibitory receptors within mammalian carcinoembryonic antigen families. BMC Biol. 2010;8:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hammarström S, ed. The carcinoembryonic antigen (CEA) family: structures, suggested functions and expression in normal and malignant tissues. Semin Cancer Biol. 1999;9:67‐81. [DOI] [PubMed] [Google Scholar]
- 18. Frängsmyr L, Israelsson A, Teglund S, Matsunaga T, Hammarström S. Evolution of the Carcinoembryonic antigen family. Tumor Biol. 2000;21:63‐81. [DOI] [PubMed] [Google Scholar]
- 19. Zebhauser R, Kammerer R, Eisenried A, McLellan A, Moore T, Zimmermann W. Identification of a novel group of evolutionarily conserved members within the rapidly diverging murine Cea family. Genomics. 2005;86:566‐580. [DOI] [PubMed] [Google Scholar]
- 20. Beroukhim R, Mermel CH, Porter D, et al. The landscape of somatic copy‐number alteration across human cancers. Nature. 2010;463:899‐905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Knuutila S, Björkqvist A‐M, Autio K, et al. DNA copy number amplifications in human neoplasms: review of comparative genomic hybridization studies. Am J Pathol. 1998;152:1107. [PMC free article] [PubMed] [Google Scholar]
- 22. Smith DH, Christensen IJ, Jensen NF, et al. An explorative analysis of ERCC1‐19q13 copy number aberrations in a chemonaive stage III colorectal cancer cohort. BMC Cancer. 2013;13:489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Koops MD, Thompson J, Zimmermann W, Stanners CP. Transcriptional regulation of the non‐specific cross‐reacting antigen gene, a member of the carcinoembryonic antigen gene family up‐regulated in colorectal carcinomas. Eur J Biochem. 1998;253:778‐786. [DOI] [PubMed] [Google Scholar]
- 24. Smale ST, Baltimore D. The, “initiator” as a transcription control element. Cell. 1989;57:103‐113. [DOI] [PubMed] [Google Scholar]
- 25. Du X, Luka J, Stafford LJ, et al. Colon and pancreas cancer specific antigens and antibodies. Google Patents; 2011.
- 26. Tsang JY, Kwok YK, Chan KW, et al. Expression and clinical significance of carcinoembryonic antigen‐related cell adhesion molecule 6 in breast cancers. Breast Cancer Res Treat. 2013;142:311‐322. [DOI] [PubMed] [Google Scholar]
- 27. Jensen‐Jarolim E, Fazekas J, Singer J, et al. Crosstalk of carcinoembryonic antigen and transforming growth factor‐β via their receptors: comparing human and canine cancer. Cancer Immunol Immunother. 2015;64:531‐537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kim BM, Hong Y, Lee S, et al. Therapeutic implications for overcoming radiation resistance in cancer therapy. Int J Mol Sci. 2015;16:26880‐26913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Roll JD, Rivenbark AG, Sandhu R, et al. Dysregulation of the epigenome in triple‐negative breast cancers: basal‐like and claudin‐low breast cancers express aberrant DNA hypermethylation. Exp Mol Pathol. 2013;95:276‐287. [DOI] [PubMed] [Google Scholar]
- 30. Frängsmyr L, Baranov V, Hammarström S. Four carcinoembryonic antigen subfamily members, CEA, NCA, BGP and CGM2, selectively expressed in the normal human colonic epithelium, are integral components of the fuzzy coat. Tumor Biol. 1999;20:277‐292. [DOI] [PubMed] [Google Scholar]
- 31. Hefta LJ, Schrewe H, Thompson JA, Oikawa S, Nakazato H, Shively JE. Expression of Complementary DNA and genomic clones for carcinomebryonic antigen and nonspecific cross‐reacting antigen in Chinese hamster ovary and mouse fibroblast cells and characterization of the membrane‐expressed products. Cancer Res. 1990;50:2397‐2403. [PubMed] [Google Scholar]
- 32. Downie KJ. Live cell imaging of CEACAM1 dynamics and self‐association during bacterial binding. Master's thesis; 2013.
- 33. Fujihara Y, Ikawa M. GPI‐AP release in cellular, developmental, and reproductive biology. J Lipid Res. 2016;57:538‐545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. McConville MJ, Ferguson M. The structure, biosynthesis and function of glycosylated phosphatidylinositols in the parasitic protozoa and higher eukaryotes. Biochem J. 1993;294:305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Screaton RA, DeMarte L, Dráber P, Stanners CP. The specificity for the differentiation blocking activity of carcinoembryonic antigen resides in its glycophosphatidyl‐inositol anchor. J Cell Biol. 2000;150:613‐626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wang R, Bi J, Ampah KK, Ba X, Liu W, Zeng X. Lipid rafts control human melanoma cell migration by regulating focal adhesion disassembly. Biochim Biophys Acta. 2013;1833:3195‐3205. [DOI] [PubMed] [Google Scholar]
- 37. Metze D, Bhardwaj R, Amann U, et al. Glycoproteins of the carcinoembryonic antigen (CEA) family are expressed in sweat and sebaceous glands of human fetal and adult skin. J Invest Dermatol. 1996;106:64‐69. [DOI] [PubMed] [Google Scholar]
- 38. Thompson J. Molecular cloning and expression of carcinoembryonic antigen gene family members. Tumor Biol. 1995;16:10‐16. [DOI] [PubMed] [Google Scholar]
- 39. Grunert F, AbuHarfeil N, Schwarz K, Von Kleist S. Two CEA and three NCA species, although distinguishable by monoclonal antibodies, have nearly identical peptide patterns. Int J Cancer. 1985;36:357‐362. [PubMed] [Google Scholar]
- 40. Burtin P, Quan P, Sabine M. Nonspecific cross reacting antigen as a marker for human polymorphs, macrophages and monocytes. Nature. 1975;255:714‐716. [DOI] [PubMed] [Google Scholar]
- 41. Ilantzis C, Demarte L, Screaton RA, Stanners CP. Deregulated expression of the human tumor marker CEA and CEA family member CEACAM6 disrupts tissue architecture and blocks colonocyte differentiation. Neoplasia. 2002;4:151‐163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Stanners C, Fuks A. Properties of adhesion mediated by the human CEA family In: Stanners C, ed. Cell Adhesion and Communications Mediated by the CEA Family. Amsterdam, The Netherlands: Harwood Academic Publishers; 1998:57‐72. [Google Scholar]
- 43. Kirshner J, Chen C‐J, Liu P, Huang J, Shively JE. CEACAM1‐4S, a cell–cell adhesion molecule, mediates apoptosis and reverts mammary carcinoma cells to a normal morphogenic phenotype in a 3D culture. Proc Natl Acad Sci USA. 2003;100:521‐526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ordóñez NG. The immunohistochemical diagnosis of mesothelioma: a comparative study of epithelioid mesothelioma and lung adenocarcinoma. Am J Surg Pathol. 2003;27:1031‐1051. [DOI] [PubMed] [Google Scholar]
- 45. Esteban JM, Felder B, Ahn C, Simpson JF, Battifora H, Shively JE. Prognostic relevance of carcinoembryonic antigen and estrogen receptor status in breast cancer patients. Cancer. 1994;74:1575‐1583. [DOI] [PubMed] [Google Scholar]
- 46. Ilantzis C, Jothy S, Alpert LC, Draber P, Stanners CP. Cell‐surface levels of human carcinoembryonic antigen are inversely correlated with colonocyte differentiation in colon carcinogenesis. Lab Invest. 1997;76:703‐716. [PubMed] [Google Scholar]
- 47. Jantscheff P, Terracciano L, Lowy A, et al. Expression of CEACAM6 in resectable colorectal cancer: a factor of independent prognostic significance. J Clin Oncol. 2003;21:3638‐3646. [DOI] [PubMed] [Google Scholar]
- 48. Sienel W, Dango S, Woelfle U, et al. Elevated expression of carcinoembryonic antigen‐related cell adhesion molecule 1 promotes progression of non‐small cell lung cancer. Clin Cancer Res. 2003;9:2260‐2266. [PubMed] [Google Scholar]
- 49. Kammerer R, Riesenberg R, Weiler C, Lohrmann J, Schleypen J, Zimmermann W. The tumour suppressor gene CEACAM1 is completely but reversibly downregulated in renal cell carcinoma. J Pathol. 2004;204:258‐267. [DOI] [PubMed] [Google Scholar]
- 50. Wagener C, Ergün S. Angiogenic properties of the carcinoembryonic antigen‐related cell adhesion molecule 1. Exp Cell Res. 2000;261:19‐24. [DOI] [PubMed] [Google Scholar]
- 51. Ordoñez C, Screaton RA, Ilantzis C, Stanners CP. Human carcinoembryonic antigen functions as a general inhibitor of anoikis. Cancer Res. 2000;60:3419‐3424. [PubMed] [Google Scholar]
- 52. Duxbury M, Ito H, Zinner M, Ashley S, Whang E. CEACAM6 gene silencing impairs anoikis resistance and suppresses metastasis of pancreatic adenocarcinoma. J Surg Res. 2003;114:241. [Google Scholar]
- 53. Duxbury MS, Ito H, Ashley SW, Whang EE. CEACAM6 cross‐linking induces caveolin‐1‐dependent, Src‐mediated focal adhesion kinase phosphorylation in BxPC3 pancreatic adenocarcinoma cells. J Biol Chem. 2004;279:23176‐23182. [DOI] [PubMed] [Google Scholar]
- 54. Scott D, Parkes A, Ponchel F, Cummings M, Poola I, Speirs V. Changes in expression of steroid receptors, their downstream target genes and their associated co‐regulators during the sequential acquisition of tamoxifen resistance in vitro. Int J Oncol. 2007;31:557‐566. [DOI] [PubMed] [Google Scholar]
- 55. Poola I, Shokrani B, Bhatnagar R, DeWitty RL, Yue Q, Bonney G. Expression of carcinoembryonic antigen cell adhesion molecule 6 oncoprotein in atypical ductal hyperplastic tissues is associated with the development of invasive breast cancer. Clin Cancer Res. 2006;12:4773‐4783. [DOI] [PubMed] [Google Scholar]
- 56. Beauchemin N, Arabzadeh A. Carcinoembryonic antigen‐related cell adhesion molecules (CEACAMs) in cancer progression and metastasis. Cancer Metastasis Rev. 2013;32:643‐671. [DOI] [PubMed] [Google Scholar]
- 57. Maraqa L, Cummings M, Peter MB, et al. Carcinoembryonic antigen cell adhesion molecule 6 predicts breast cancer recurrence following adjuvant tamoxifen. Clin Cancer Res. 2008;14:405‐411. [DOI] [PubMed] [Google Scholar]
- 58. Hiscox S, Jiang WG, Obermeier K, et al. Tamoxifen resistance in MCF7 cells promotes EMT‐like behaviour and involves modulation of β‐catenin phosphorylation. Int J Cancer. 2006;118:290‐301. [DOI] [PubMed] [Google Scholar]
- 59. Lewis‐Wambi JS, Cunliffe HE, Kim HR, Willis AL, Jordan VC. Overexpression of CEACAM6 promotes migration and invasion of oestrogen‐deprived breast cancer cells. Eur J Cancer. 2008;44:1770‐1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Balk‐Møller E, Kim J, Hopkinson B, Timmermans‐Wielenga V, Petersen OW, Villadsen R. A marker of endocrine receptor‐positive cells, CEACAM6, is shared by two major classes of breast cancer: luminal and HER2‐enriched. Am J Pathol. 2014;184:1198‐1208. [DOI] [PubMed] [Google Scholar]
- 61. Khoury T, Kanehira K, Wang D, et al. Breast carcinoma with amplified HER2: a gene expression signature specific for trastuzumab resistance and poor prognosis. Mod Pathol. 2010;23:1364‐1378. [DOI] [PubMed] [Google Scholar]
- 62. Wagener C, Petzold P, Köhler W, Totović V. Binding of five monoclonal anti‐CEA antibodies with different epitope specifities to various carcinoma tissues. Int J Cancer. 1984;33:469‐475. [DOI] [PubMed] [Google Scholar]
- 63. Ameur N, Lacroix L, Roucan S, et al. Aggressive inherited and sporadic medullary thyroid carcinomas display similar oncogenic pathways. Endocr Relat Cancer. 2009;16:1261‐1272. [DOI] [PubMed] [Google Scholar]
- 64. Lee DS, Kim SJ, Kang JH, et al. Serum carcinoembryonic antigen levels and the risk of whole‐body metastatic potential in advanced non‐small cell lung cancer. J Cancer. 2014;5:663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Yoon HI, Park KH, Lee E‐J, et al. Overexpression of SOX2 is associated with better overall survival in squamous cell lung cancer patients treated with adjuvant radiotherapy. Cancer Res Treat. 2016;48:473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Karachaliou N, Rosell R, Viteri S. The role of SOX2 in small cell lung cancer, lung adenocarcinoma and squamous cell carcinoma of the lung. Transl Lung Cancer Res. 2013;2:172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Witzens‐Harig M, Hose D, Jünger S, et al. Tumor cells in multiple myeloma patients inhibit myeloma‐reactive T cells through carcinoembryonic antigen‐related cell adhesion molecule‐6. Blood. 2013;121:4493‐4503. [DOI] [PubMed] [Google Scholar]
- 68. Heinemann V, Reni M, Ychou M, Richel D, Macarulla T, Ducreux M. Tumour–stroma interactions in pancreatic ductal adenocarcinoma: rationale and current evidence for new therapeutic strategies. Cancer Treat Rev. 2014;40:118‐128. [DOI] [PubMed] [Google Scholar]
- 69. Dráaber P, Skubitz KM. Signal transduction mediated by the CEA family In: Stanners CO, ed. Cell Adhesion and Communications Mediated by the CEA Family Basic and Clinical Perspectives (Vol. 5). Amsterdam, The Netherlands: Harwood Academic Publishers; 1998:125. [Google Scholar]
- 70. Sen B, Johnson FM. Regulation of SRC family kinases in human cancers. J Signal Transduct. 2011;2011:865819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Zhang J, Kalyankrishna S, Wislez M, et al. SRC‐family kinases are activated in non‐small cell lung cancer and promote the survival of epidermal growth factor receptor‐dependent cell lines. Am J Pathol. 2007;170:366‐376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Ordonez C, Zhai AB, Camacho‐Leal P, Demarte L, Fan MM, Stanners CP. GPI‐anchored CEA family glycoproteins CEA and CEACAM6 mediate their biological effects through enhanced integrin α5β1‐fibronectin interaction. J Cell Physiol. 2007;210:757‐765. [DOI] [PubMed] [Google Scholar]
- 73. Cheng T‐M, Liu Y‐R, Chang C‐C, et al. Carcinoembryonic cell adhesion molecule 6 as a therapeutic target for breast cancer (1048.2). FASEB J. 2014;28(1 Suppl):1048.2. [Google Scholar]
- 74. Riley CJ, Engelhardt KP, Saldanha JW, et al. Design and activity of a murine and humanized anti‐CEACAM6 single‐chain variable fragment in the treatment of pancreatic cancer. Cancer Res. 2009;69:1933‐1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Strickland LA, Ross J, Williams S, et al. Preclinical evaluation of carcinoembryonic cell adhesion molecule (CEACAM) 6 as potential therapy target for pancreatic adenocarcinoma. J Pathol. 2009;218:380‐390. [DOI] [PubMed] [Google Scholar]
- 76. Duxbury MS, Ito H, Ashley SW, Whang EE. CEACAM6 as a novel target for indirect type 1 immunotoxin‐based therapy in pancreatic adenocarcinoma. Biochem Biophys Res Commun. 2004;317:837‐843. [DOI] [PubMed] [Google Scholar]
- 77. Duxbury MS, Matros E, Ito H, Zinner MJ, Ashley SW, Whang EE. Systemic siRNA‐mediated gene silencing: a new approach to targeted therapy of cancer. Ann Surg. 2004;240:667‐676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Seyfried TN, Huysentruyt LC. On the origin of cancer metastasis. Crit Rev Oncog. 2013;18:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Tchoupa AK, Schuhmacher T, Hauck CR. Signaling by epithelial members of the CEACAM family–mucosal docking sites for pathogenic bacteria. Cell Commun Signal. 2014;12:27. [DOI] [PMC free article] [PubMed] [Google Scholar]


