Abstract
Circulating tumor cells (CTC) are newly discovered biomarkers of cancers. Although many systems detect CTC, a gold standard has not yet been established. We analyzed CTC in uterine cervical cancer patients using an advanced version of conditionally replicative adenovirus targeting telomerase‐positive cells, which was enabled to infect coxsackievirus‐adenovirus receptor‐negative cells and to reduce false‐positive signals in myeloid cells. Blood samples from cervical cancer patients were hemolyzed and infected with the virus and then labeled with fluorescent anti‐CD45 and anti‐pan cytokeratin antibodies. GFP (+)/CD45 (−) cells were isolated and subjected to whole‐genome amplification followed by polymerase chain reaction analysis of human papillomavirus (HPV) DNA. CTC were detected in 6 of 23 patients with cervical cancers (26.0%). Expression of CTC did not correlate with the stage of cancer or other clinicopathological factors. In 5 of the 6 CTC‐positive cases, the same subtype of HPV DNA as that of the corresponding primary lesion was detected, indicating that the CTC originated from HPV‐infected cancer cells. These CTC were all negative for cytokeratins. The CTC detected by our system were genetically confirmed. CTC derived from uterine cervical cancers had lost epithelial characteristics, indicating that epithelial marker‐dependent systems do not have the capacity to detect these cells in cervical cancer patients.
Keywords: cervical cancer, circulating tumor cell, cytokeratin, human papilloma virus, telomerase
Abbreviations
- CAR
coxsackievirus‐adenovirus receptor
- CTC
circulating tumor cell
- DTC
disseminated tumor cell
- EpCAM
epithelial cell adhesion molecule
- HPV
human papillomavirus
- hTERT
human telomerase reverse transcriptase
- NK cells
natural killer cells
- NSCLC
non‐small‐cell lung cancer
1. INTRODUCTION
Locally growing cancer cells need to intravasate to metastasize to distant organs. Difficulties have long been reported in identifying cancer cells in the bloodstream.1 The clinical relevance of CTC as a prognostic or surrogate marker of treatment response has already been established for several cancer types, such as breast, colorectal, and prostate cancer.2, 3, 4 We also previously showed the efficacy of CTC in gynecological cancers as a surrogate marker of treatment response.5 The most commonly used CTC detection method is CellSearch®, which mainly relies on epithelial markers, such as the EpCAM and cytokeratins.2, 6 The importance of this system is clear; however, the expression of epithelial markers decreases during epithelial‐to‐mesenchymal transition, which may be associated with cancer progression and intravasation.7 A previous study identified CTC with both epithelial and mesenchymal characteristics in patients with breast cancer, suggesting that epithelial characteristics are not essential for CTC.8 There are several approaches for the isolation of CTC independent of epithelial markers, such as a method that assesses the size of cells9 and a method based on dielectrophoresis.10 However, a gold standard has not yet been established.
Immortality is one of the crucial characteristics of malignant cells. Telomerase elongates chromosomal ends that shorten with every cell division, resulting in cell immortality.11 The expression of telomerase is strictly controlled in normal cells12; therefore, telomerase activity has potential as a marker of cancer cells. hTERT is the catalytic subunit of telomerase.13, 14 The expression of hTERT has been identified as a determinant of telomerase activity and is transcriptionally regulated by its promoter.15, 16 Telomerase‐specific replication‐selective adenoviruses have been constructed from adenovirus vectors by inserting the hTERT promoter upstream of E1A and E1B genes, thereby restricting their proliferation to telomerase‐active cells only. These adenoviruses have been applied to cancer virotherapy and visualization of cancer cells both in vitro and in vivo.17, 18, 19 The GFP‐expressing type of this virus (OBP‐401) has been used to detect CTC in patients with various cancers.5, 20, 21 CTC have been isolated and analyzed in patients with colorectal cancer using OBP‐401.22 However, the use of OBP‐401 to detect CTC has some limitations. It cannot infect CAR‐negative cells because it is based on adenovirus serotype 5, and false‐positive GFP signals caused by telomerase‐positive blood cells may affect examinations and clinical decisions. OBP‐1101, a modified adenoviral vector, was recently developed.23 In OBP‐1101, adenovirus 5 fibers were exchanged for type 35 fibers, which bind to CD46, to infect CAR‐negative cells, and complementary sequences of the blood cell‐specific microRNA miR142‐3p were inserted into the 3′‐UTR of E1 and GFP genes to prevent false‐positive signals in blood cells (Figure 1A).
Figure 1.
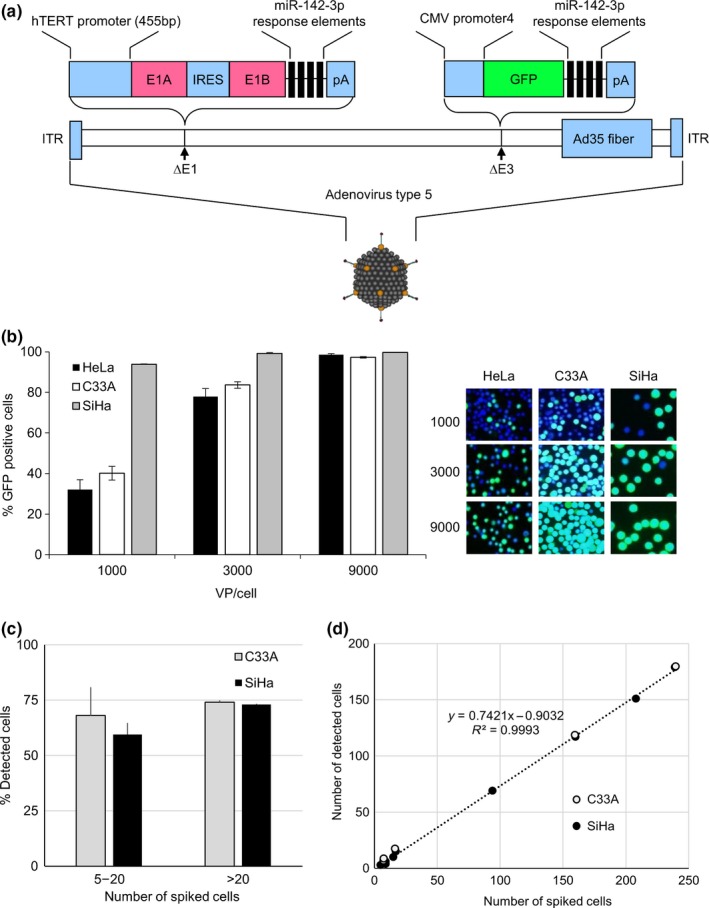
GFP expression in cervical cancer cells infected with OBP‐1101, an advanced telomerase‐specific replication‐selective adenovirus. A, Schematic structure of OBP‐1101. It replicates in cells in which the human telomerase reverse transcriptase (hTERT) promoter is active and expresses GFP. Adenoviral fibers are replaced with type 35 fibers to infect coxsackievirus‐adenovirus receptor‐negative cells. Response elements of miR‐142‐3p, a blood cell‐specific microRNA, are inserted into the 3′‐UTR of E1B and GFP genes to attenuate non‐specific GFP expression in blood cells. IRES, internal ribosomal entry site; ITR, internal terminal repeat; pA, bovine growth hormone polyadenylation signal. B, OBP‐1101 mediates the expression of GFP in uterine cervical cancer cell lines. Ratios of GFP‐positive cells and representative pictures are shown. Number in each picture indicates the viral concentration (viral particles/cell). Cells are also stained with DAPI. C, Cancer cell spike‐in model of circulating tumor cells. Various numbers of SiHa or C33A cells (ranging from 5 to 239) are spiked into blood from healthy volunteers. Five experiments were carried out for each cell type. Bars indicate standard errors. D, Linear relationship between the numbers of spiked cells and detected cells
In the present study, we focused on uterine cervical cancers because HPV genes are integrated into most cancer lesions and, thus, they have potential as good indicators of CTC. We applied the newly developed OBP‐1101 to detect CTC in patients with cervical cancers to verify its efficacy and accuracy. CTC were immunocytologically characterized as to whether they expressed cytokeratins as epithelial markers. The origin of CTC was confirmed using PCR amplification of HPV genes.
2. MATERIALS AND METHODS
2.1. Patients
Patients who were treated for primary or recurrent cervical cancers at Kanazawa University Hospital between August 2013 and March 2015 were eligible to participate in the present study. All experimental procedures, including blood sampling, processing, and analyses, were carried out after receiving approval from the Medical Ethics Committee of Kanazawa University Graduate School of Medical Science. All patients provided written informed consent.
2.2. Cell culture
Synovial sarcoma cell line SYO‐1 was a kind gift from Dr Akira Kawai (Department of Musculoskeletal Oncology, National Cancer Center Japan) and Dr Toshifumi Ozaki (Department of Orthopedic Surgery, Okayama University Graduate School of Medicine).24, 25 Human cervical cancer cell lines C33A, HeLa, SiHa, NSCLC cell line H1703 and SYO‐1 were cultured at 37°C under 5% CO2 in DMEM, supplemented with 10% heat‐inactivated FCS (Sigma‐Aldrich, St Louis, MO, USA), 100 μg/mL streptomycin, and 100 IU/mL penicillin.
2.3. Quantitative real‐time RT‐PCR analysis
Total RNA was extracted from frozen cervical cancer tissues using RNeasy Mini Kit (Qiagen, Venlo, Netherlands). cDNA was synthesized from total RNA using QuantiTect Reverse Transcription Kit (Qiagen). cDNA from 25 ng RNA was applied for real‐time PCR assay using ABI PRISM 7700 Sequence Detection System (Thermo Fisher Scientific, Waltham, MA, USA). Expressions of hTERT and GAPDH mRNA were quantified by Taqman Gene Expression Assays Hs00972650_m1 and Hs02758991_g1 respectively (Thermo Fisher Scientific). All assays were carried out in triplicate. Expression of hTERT was standardized by that of GAPDH in each sample.
2.4. Viruses
Viruses were propagated in HeLa‐S3 cells, purified by ion‐exchange chromatography and several steps of filtration, and stored at −80°C.
2.5. Cancer cells spike‐in model
SiHa, C33A, H1703 or SYO‐1 cells were harvested and labeled with PKH26 (Sigma‐Aldrich). The cell suspension was diluted to contain 5‐300 cells per 50 μL. The actual number of viable cells was determined by microscopic quantification of 4 out of 5 cell suspensions, treated with Trypan blue to exclude dead cells from being counted. The average of 4 cell counts was used as the number of spiked cells. The remaining cell suspension was spiked into 7.5 mL of blood from a healthy volunteer and applied as a positive control in the subsequent analysis. GFP (+)/PKH26 (+) cells were recognized as positive cells. The detection ratio was defined as positive cells/spiked cells.
2.6. Blood sample preparation
The schematic flow diagram is shown in Figure S1. Blood samples (7.5 mL) were drawn into citrate‐, phosphate‐, and dextrose‐containing tubes and incubated with lysis buffer containing ammonium chloride (NH4Cl) for 15 minutes twice to remove erythrocytes. After centrifugation, the cell pellets were resuspended in 1 mL DMEM with 10% FBS, mixed with 109 viral particles/mL OBP‐1101, and incubated at 37°C for 24 hours with gentle rotation. Following centrifugation, the cells were fixed with 2% paraformaldehyde. After washing with PBS, the cells were incubated with a 1/200‐diluted mouse anti‐human CD45 antibody (Life Technologies, Carlsbad, CA, USA). The cells were treated with 0.15% Triton X‐100 (Sigma‐Aldrich) and then incubated with a 1/200‐diluted anti‐pan cytokeratin (cytokeratins 4, 5, 6, 8, 10, 13, and 18) mouse antibody and anti‐cytokeratin 19 mouse antibody labeled with Alexa Fluor 555 using the Zenon Alexa Fluor 555 mouse IgG Labeling Kit (Life Technologies) and 1 μg/mL DAPI (Sigma‐Aldrich) on ice for 20 minutes. Expression of CD45 was detected by incubating the mixture with an Alexa Fluor 647 goat anti‐mouse IgG goat anti‐mouse secondary antibody (Life Technologies) for 20 minutes in the dark. Following washing with PBS and centrifugation, cells were resuspended in 1 mL PBS and divided into each well of a 96‐well plate.
2.7. In vitro fluorescence imaging and capturing of CTC
Fluorescence levels were assessed with excitation/emission at 485 nm/505 nm for GFP, 555 nm/580 nm for Alexa Fluor 555 and PKH26, 650 nm/665 nm for Alexa Fluor 647, and 358 nm/451 nm for DAPI using a IX73 fluorescent microscope (Olympus, Tokyo, Japan), and cells were photographed and counted by viewing the monitor. High‐resolution image acquisition was accomplished using an EPSON personal computer (SEIKO EPSON, Suwa, Japan). GFP (+)/CD45(−) cells were counted as CTC and were captured by automatically manipulated glass pipettes using the MMI CellEctor system (Tomy Digital Biology, Tokyo, Japan). Images were processed for contrast and brightness using Adobe Photoshop CS2 software (Adobe, San Jose, CA, USA) and were observed by individuals blinded to the clinical status of the patients.
2.8. Immunohistochemistry
Immunohistochemical analyses were carried out using 10% formalin‐fixed and paraffin‐embedded specimens of cervical cancers. Specimens were deparaffinized in xylene and graded alcohol solutions. Epitope retrieval was subsequently carried out; sections were heated for 10 minutes at 90°C in Antigen Retrieval Citra Solution (BioGenex, San Ramon, CA, USA). Endogenous peroxidase was then blocked by immersing the sections in 0.3% H2O2 methanol for 30 minutes. The sections were then stained with 1/100‐diluted anti‐human pan cytokeratin (cytokeratins 4, 5, 6, 8, 10, 13, and 18) mouse antibody (Santa Cruz Biotechnology, Dallas, TX, USA) at room temperature for 1 hour. The Envision Detection Kit (Dako, Glostrup, Denmark) and diaminobenzidine tetrahydrochloride were used to visualize immunoreactivity. Each section was also counterstained with a GM hematoxylin stain solution (Muto Pure Chemicals, Tokyo, Japan).
2.9. HPV gene detection and mutation analysis in cancer samples
Genomic DNA was extracted from frozen or 10% formalin‐fixed and paraffin‐embedded specimens of cervical cancers using Dexpat (Takara, Kusatsu, Japan). HPV genes of subtypes 16, 18, 31, 33, 35, 52, and 58 were detected using the Takara PCR Human Papillomavirus Typing Set (Takara). PCR was carried out to amplify DNA fragments within the ORF of E6 and E7 using the consensus primers pU‐1M and pU‐2R. Sequences of PCR primers and PCR conditions are listed in Table S1. The amplified products were analyzed using restriction enzyme AvaII digestion patterns for HPV typing or direct sequencing.26 PIK3CA exons 9 and 20 and KRAS exons 1 and 2 were also PCR amplified, and direct sequencing was done using the dideoxy chain termination method with the BigDye‐Terminator cycle sequencing kit (Perkin‐Elmer, Waltham, MA, USA). After ethanol precipitation, samples were analyzed using the ABI PRISM 3100 genetic analyzer (Thermo Fisher Scientific).
2.10. HPV gene detection from CTC
Genomic DNA was extracted and amplified from CTC using the GenomePlex® Single Cell Whole‐Genome Amplification Kit (Sigma‐Aldrich). DNA fragments within the ORF of E6 and E7 were then amplified using HPV‐type‐specific primers. Sequences of PCR primers and PCR conditions are listed in Table S1. Direct sequencing of PCR products was carried out as described previously.
2.11. Statistical analysis
Linear regression analysis was carried out to evaluate the spike‐in model experiments. To assess the significance of differences in the number of CTC, the chi‐squared test, Mann‐Whitney U test, t test, or Fisher's exact probability test was carried out. Survival analysis was done using the Kaplan‐Meier method. The log‐rank test was used to statistically compare the curves. All analyses were carried out using Microsoft Excel 2013. P‐value < .05 was considered to indicate statistical significance.
3. RESULTS
3.1. GFP expression in OBP‐1101‐infected uterine cervical cancer cell lines in vitro and in spike‐in models
We first evaluated the expression of GFP in the uterine cervical cancer cell lines HeLa, SiHa, and C33A using OBP‐1101 (Figure 1A). Cells expressing GFP were counted after infection for 24 hours. GFP expression ratios varied among the cell lines at a low viral concentration (1000 viral particles/cell). At higher viral concentrations, most of the cancer cells (98.6% for HeLa, 99.7% for SiHa, and 97.2% for C33A) expressed GFP irrespective of the cell type (Figure 1B). A cytotoxic effect was not observed within 24 hours of infection.
We next carried out a spike‐in model experiment to evaluate the efficiency of OBP‐1101 for detecting cancer cells in blood. Various numbers of SiHa and C33A cells (range, 5‐239) were spiked into 7.5 mL of blood from healthy volunteers. Spiked cells were pretreated with PKH26 to exhibit orange fluorescence. Unspiked blood was also treated as a negative control. After OBP‐1101 infection followed by anti‐CD45 antibody treatment, GFP (+)/PKH26 (+) cells were recognized as positive cells. On spiking a small number of cells (range, 5‐20), the mean percent detection (positive cells/spiked cells) was 59.4% ± 5.2% for SiHa and 68.0% ± 12.8% for C33A (Figure 1C). On spiking a larger number of cells (more than 20), the mean percent detection was 73.0% ± 0.29% for SiHa and 74.0% ± 0.89% for C33A. There was no statistical difference in the detection percentage across the range of cells or cell lines spiked into the blood. Linear regression analysis of the number of detected cancer cells against the number of spiked‐in cancer cells exhibited a slope of 0.74, an intercept of 0.90, and an R 2 value of 1.00 (Figure 1D). GFP (+)/CD45 (+) cells were considered as false‐positive cells originating from myeloid cells. The number of these cells varied from 1 to 13 (6.6 ± 6.00) in each 7.5‐mL unspiked negative control. In GFP (+) cells, the expressions of PKH26 and CD45 were completely independent of each other. We also carried out the same experiments using NSCLC cell line H1703 and synovial sarcoma cell line SYO‐1 as a model of cytokeratin‐negative mesenchymal tumor cells.25, 27 The detection rate was 16% for H1703 and 27% for SYO‐1, which was lower than that of cervical cancer cell lines (data not shown).
3.2. Detection of CTC in patients with cervical cancer using OBP‐1101
We analyzed blood samples collected from 23 patients with primary or recurrent cervical cancer. All samples were collected prior to treatment of the tumors. After infection for 24 hours, cells were treated with anti‐CD45 and anti‐pan cytokeratin antibodies. GFP (+)/CD45 (−) cells were counted as CTC and captured.
CTC were identified in 6 of 23 samples (26.0%), and their numbers ranged from 1 to 4 (Table 1). Mean diameter of CTC (14.41 ± 1.64 μm) was significantly larger than that of the surrounding blood cells (8.85 ± 1.58 μm) under our fixation conditions. All CTC recognized in this study were larger than 12.0 μm. Mean number of GFP (+)/CD45 (+) cells which were recognized as false‐positive WBC was 4.57 ± 9.25. There was no difference between CTC (+) and CTC (−) cases (3.85 ± 5.33 and 5.75 ± 10.78, respectively). All CTC‐positive patients had squamous cell carcinoma. Although all patients with distal metastasis were positive for CTC, a statistical correlation was not observed between CTC‐positive rates and the clinicopathological characteristics (Table 2). The relationship between the existence of CTC and patient prognosis was evaluated using the Kaplan‐Meier method (Figure S2). There was no significant correlation between CTC and overall survival or progression‐free survival.
Table 1.
Circulating tumor cells (CTC) detected in patients with cervical cancers
| Patient no. | Stage | Site of metastasis | HPV subtype primary | HPV subtype CTC | No. CTC | No. contaminated WBC | hTERT mRNA expression | Prognosis |
|---|---|---|---|---|---|---|---|---|
| 2 | IIA | no metastasis | 16 | 16 | 2 | 0 | + | NED 40 mo |
| 4 | Recurrence | Lung | 33 | ND | 4 | 14 | NA | Progression 7 mo, DOD 27 mo |
| 5 | IB1 | no metastasis | 16 | 16 | 1 | 2 | + | NED 40 mo |
| 6 | IB1 | no metastasis | 16 | 16 | 3 | 1 | NA | NED 40 mo |
| 10 | Recurrence | Lung | 16a | 16 | 4 | 5 | NA | Progression 4 mo, DOD 5 mo |
| 16 | IB1 | no metastasis | 16 | 16 | 1 | 0 | + | NED 29 mo |
DOD, died of disease; HPV, human papillomavirus; hTERT, human telomerase reverse transcriptase; NA, not available; ND, not detected; NED, no evidence of disease; WBC, white blood cells.
The HPV subtype was identified using the AMPLICORE® human papilloma virus test (Roche, Basel, Switzerland).
Table 2.
Patient demographics and clinical characteristics
| N | CTC (+) | CTC (−) | Detection rate (%) | |
|---|---|---|---|---|
| Cervical cancer | 23 | 6 | 17 | 26.0 |
| Primary | 21 | 4 | 17 | 19.0 |
| Recurrence | 2 | 2 | 0 | 100 |
| FIGO stage I | 11 | 3 | 8 | 27.3 |
| FIGO stage II‐IV and recurrence | 12 | 3 | 9 | 25.0 |
| Hematogenous metastasis (−) | 21 | 4 | 17 | 19.0 |
| Hematogenous metastasis (+) | 2 | 2 | 0 | 100 |
| Squamous cell carcinoma | 19 | 6 | 13 | 31.6 |
| Others | 4 | 0 | 4 | 0.0 |
CTC, circulating tumor cell; FIGO, International Federation of Gynecology and Obstetrics.
3.3. Evaluation of epithelial cell markers in CTC and primary lesions
Immunocytological expression of epithelial markers, such as cytokeratins 4, 5, 6, 8, 10, 13, 18, and 19 on GFP (+)/CD45 (−) CTC was evaluated. None of the CTC (15 cells in 6 patients) expressed cytokeratins (Figure 2A). In contrast, the immunohistological expression of cytokeratins in the original lesions was confirmed in 5 patients whose clinical samples were available (Figure 2B).
Figure 2.
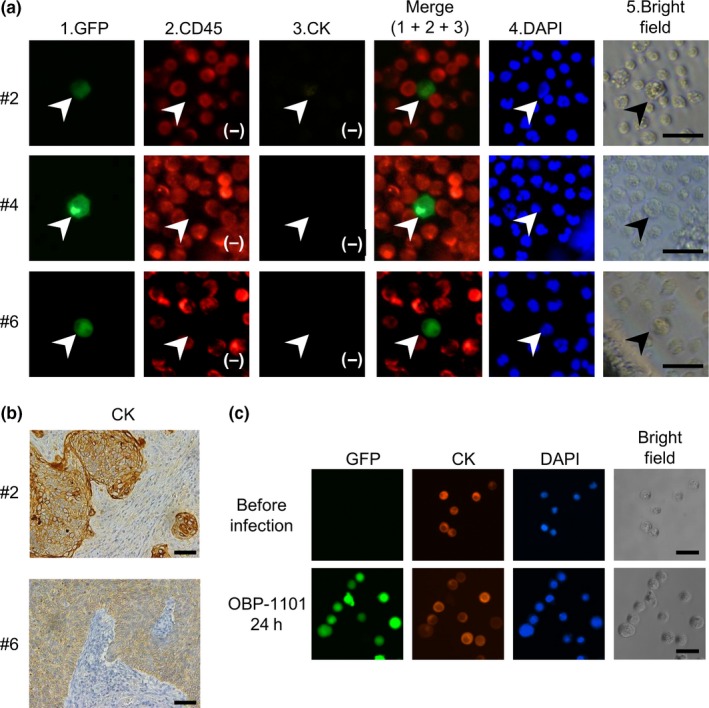
Expression of cytokeratins in circulating tumor cells (CTC) from cervical cancer patients. A, Representative pictures of CTC detected in blood samples from cervical cancer patients. Cells infected with OBP‐1101 express GFP. Cells that express GFP and are negative for CD45 (marked in red), a leukocyte common antigen, are counted as CTC (indicated by arrowhead). CTC are larger than the surrounding blood cells. All CTC are negative for cytokeratins (marked in orange). Bars, 30 μm. B, Immunohistochemical analysis of the expression of cytokeratins in cervical cancer specimens using an anti‐pan cytokeratin antibody (cytokeratins 4, 5, 6, 8, 10, 13, and 18). Original magnification, 200×. Bars, 100 μm. C, Immunocytochemical analysis of the expression of cytokeratins in an experimental model of CTC. HeLa human cervical cancer cells are infected with 109 viral particles/mL OBP‐1101 and incubated at 37°C for 24 h. HeLa cells express cytokeratins before and after infection with OBP‐1101. Bars, 50 μm
To exclude the artificial effects of viral infection and subsequent 24‐hour incubation under floating conditions on the expression profiles of epithelial cell markers, we examined changes in the expression of cytokeratins on human cervical cancer cell lines during sample preparation. Hela cells (1 × 106 cells) were mixed with 109 viral particles/mL OBP‐1101 and incubated at 37°C for 24 hours with gentle rotation. Immunocytochemical staining showed that the treatment did not affect the expression of cytokeratins in HeLa cells (Figure 2C).
3.4. Evaluation of hTERT mRNA expression in primary lesions
Because the OBP‐1101 system relies on hTERT promoter activity in cancer cells, we examined the levels of hTERT mRNA expression in primary lesions in 13 patients (3 CTC‐positive and 10 CTC‐negative) whose frozen tissues were available for quantitative RT‐PCR. In 12 out of 13 patients, hTERT mRNA were detectable, and the only hTERT mRNA undetectable case was CTC‐negative. There was no significant difference in hTERT expression between CTC‐positive and ‐negative patients (Figure 3).
Figure 3.
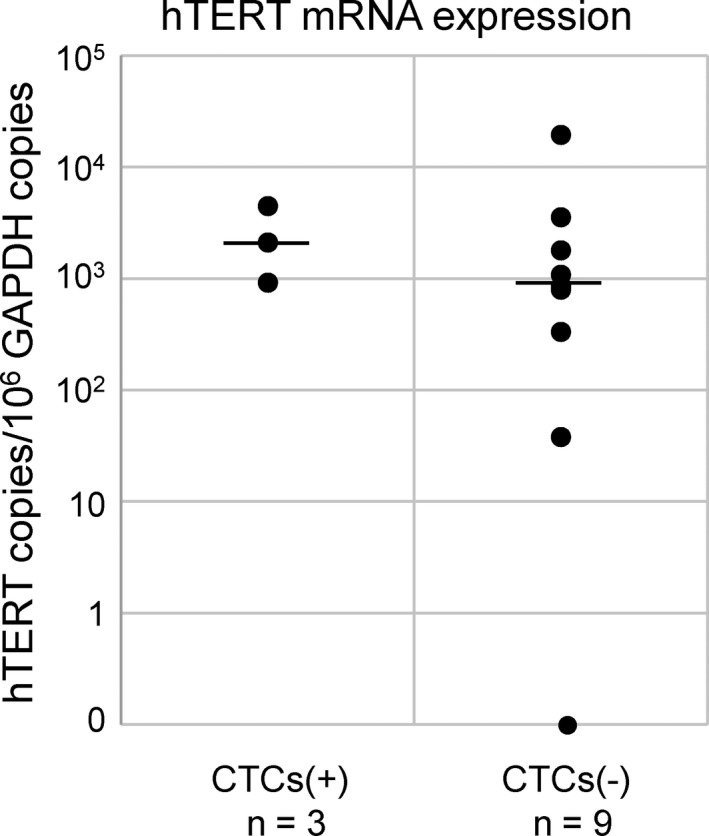
Human telomerase reverse transcriptase (hTERT) expression in cervical cancer tissues. hTERT mRNA expression was standardized with that of GAPDH (hTERT copies/106 GAPDH copies) in original cervical cancer tissues. All assays were carried out in triplicate. Horizontal bar indicates the median. CTC, circulating tumor cells
3.5. Detection of HPV genes in CTC
We confirmed the presence of HPV genes in the primary lesions of CTC‐positive patients. In 5 of 6 patients, HPV genes were amplified using consensus primers for the HPV subtypes 16, 18, 31, 33, 35, 52, and 58. The subtype of HPV was identified by the pattern of restriction enzyme AvaII digestion and direct sequencing of PCR products (Figure S3A). HPV types 16 and 33 DNA were detected in 4 patients and 1 patient, respectively (Table 1). In the remaining patient, the DNA of HPV type 16 had already been identified using the AMPLICORE® human papilloma virus test (Roche, Basel, Switzerland) in the initial diagnosis. We further investigated tumor‐specific mutations in primary lesions. Because these mutation “hot spots” were previously reported, PIK3CA exons 9 and 20 and KRAS exons 1 and 2 were also PCR amplified and directly sequenced. No mutations were found in the 5 patients whose genomic DNA from the primary lesion was available.
We then examined the presence of HPV genes in isolated CTC. We carried out HPV‐type‐specific PCR of the E6/E7 region after whole‐genome amplification (Figure S3B). The DNA sequences of PCR products were confirmed by direct sequencing. Identical types of HPV genes in the original lesions and CTC were successfully detected in 5 of 6 patients. However, we failed to detect the HPV gene in 1 patient whose CTC sample had been contaminated with 14 WBC (patient no. 4 in Table 1).
The existence of HPV genes in GFP (+)/CD45 (+) false‐positive cells isolated from healthy volunteers was also examined. The cells were treated in the same way as CTC, but the HPV gene was not amplified in any sample (data not shown).
4. DISCUSSION
In the present study, we analyzed CTC in patients with cervical cancers using a telomerase‐specific replication‐selective adenovirus vector, which expresses GFP in infected cells (OBP‐1101). The spike‐in model experiment exhibited high sensitivity (5 cells in 7.5 mL was sufficient for detection) and linearity (R 2 = 1.00) for both HPV‐positive and HPV‐negative cervical cancer cell lines, suggesting its utility for CTC detection in cervical cancer patients.
Although the authenticity of the CTC detected is an important issue, definitive markers for CTC have not yet been established. Detection of cancer‐specific mutations or exogenous DNA identical to that of the primary lesion indicated that CTC originated from cancer cells. Genetic mutations in cervical cancer have been reported most frequently in PIK3CA (29%‐37%) and KRAS (3%‐8.8%).28, 29, 30, 31, 32 We analyzed mutations in PIK3CA exons 9 and 20 and KRAS exons 1 and 2 in 5 patients whose genomic DNA from the primary lesion was available; however, no mutations were found. The rate of PIK3CA mutations in cervical cancer samples from Chinese individuals has been reported to be 12.3%, which is lower than the rate in other reports, suggesting that the ratio of PIK3CA mutations in cervical cancer is lower in Asia.28, 29, 30, 31, 32, 33 This may affect the present data. Owing to the relatively low ratio of somatic mutations, it appears problematic to prove that mutations are identical in primary lesions and CTC in cervical cancer.
HPV infection is associated with carcinogenesis in cervical cancer and is detectable in more than 95% of cervical cancer cases.34 We consequently adopted HPV genes as markers of cancer cells to evaluate the authenticity of CTC isolated in the present study. Several reports have carried out PCR amplification of HPV DNA or mRNA from peripheral blood cells to detect CTC in cervical cancer.35, 36, 37, 38, 39 In older studies, PBMC fractions were directly subjected to analyses.35, 36, 37 Existence of HPV DNA was associated with overall survival or poor prognosis in these studies. In a relatively recent report, PBMC fractions enriched with anti‐EpCAM antibody were used for HPV RT‐PCR.38 Out of a total of 13 cervical cancer patients, 3 were positive for HPV mRNA (23%). In another study, digital RT‐PCR analysis was carried out for HPV genes to estimate the presence of CTC.39 Of 10 cervical cancer patients, 3 were positive.
In contrast, the existence of episomal HPV in PBMC of healthy donors has been reported.40 The characteristics of HPV‐positive cells in peripheral blood was analyzed in HPV16‐infected males, which showed that these cells were CD45 (+) and CD20 (+) or CD56 (+), likely phenotypes of B cells and NK cells, respectively.41 The proportion of HPV‐positive cells ranged from 0.1% to 0.6% of PBMC. It is necessary to consider the existence of such cells when applying HPV DNA as the marker of cancer cells. Assessment of CD45 expression is thought to be a good approach for distinguishing CTC from HPV (+) WBC because these cells belong to the lymphoid lineage and have a relatively strong expression of CD45.42
This is the first study to directly analyze HPV genes in isolated CTC. In most cases, HPV genes identical to those in the original lesions were successfully detected in GFP (+)/CD45 (−) CTC, indicating that they originated from cancer cells in uterine cervical primary lesions. However, HPV genes were not detected in 1 sample contaminated by a large number of WBC (patient no. 4 in Table 1). Our CTC collection system is automated, and the procedure is simple and easy to manipulate, but it was sometimes difficult to separate the attached WBC from the target CTC, as observed in case no. 4. Although the reason for this currently remains unknown, WBC contamination may interfere with subsequent gene analysis of CTC.
Cell size is another candidate for discriminating cells. CTC (14.41 ± 1.64 μm) were significantly larger than surrounding blood cells (8.85 ± 1.58 μm) under our fixation conditions. All CTC were larger than 12.0 μm, and this can be considered a threshold.
Because the CTC detection system using OBP‐1101 depends on hTERT promoter activity, we examined hTERT mRNA expression in original lesions both in CTC‐positive and ‐negative patients. Except one CTC‐negative patient, all samples expressed hTERT mRNA, but the levels of hTERT mRNA expression were not significantly different in CTC‐positive and ‐negative patients, suggesting that the level of hTERT expression is not a simple determinant of CTC detection. One possible explanation of this result is that hTERT upregulation occurs at a single cell level, associating with intravasation of tumor cells as it is reported that hTERT promotes epithelial‐mesenchymal transition and invasion of cancer cells.43, 44 Other factors may also be involved in the detection of CTC, such as viral infectivity to CTC.
Emerging evidence suggests the presence of epithelial‐like CTC and mesenchymal‐like CTC.8 Cytokeratins are the proteins of keratin‐containing intermediate filaments that exist in the cytoskeleton of epithelial cells. Expressions of cytokeratins in primary lesions were confirmed by immunohistochemical analysis in 5 patients whose clinical samples were available. In contrast to the primary lesions, none of the CTC analyzed in the present study expressed cytokeratins. We confirmed that OBP‐1101 infection did not affect cytokeratin expression in vitro. We also examined a blood spike‐in model using H1703, a cytokeratin‐negative mesenchymal transformed NSCLC cell line, and SYO‐1, a synovial sarcoma cell line. The detection ratios were lower than that of cervical cancer cell lines. It may be a reflection of lower infectivity of virus indicated in in vitro experiments shown in former reports.24, 45 However, this result suggests that OBP‐1101 does not preferentially detect cytokeratin‐negative mesenchymal type cells. In relation to this result, both epithelial and mesenchymal‐like CTC were detected in a recent study on CTC in NSCLC patients using OBP‐1101.45 These results indicate that mesenchymal‐like CTC are present in uterine cervical cancers, and CTC detection methods dependent on epithelial markers have been overlooked in these cell populations. These epithelial marker‐negative CTC should be considered when investigating the mechanisms of the spread of cancer.
Several studies on CTC in cervical cancer have been carried out, including HPV‐PCR‐based studies (as discussed earlier) and other such studies.46, 47 However, these studies were relatively small. Some of them indicated a prognostic value or association with clinical findings; however, we did not find such associations in our data. There has been a relatively large‐scale study of DTC in bone marrow.48 Cytokeratin‐positive cells in bone marrow aspirations were considered to be DTC. Out of 228 cases of cervical cancer, 37 (16%) were DTC‐positive. DTC were associated with stage, nodal status, and lymphangiosis, but there was no association between DTC status and prognosis. The detection system in that study did not recognize cytokeratin‐negative tumor cells, which may have affected the results.
The number of CTC detected in this study was relatively small (2.5 ± 1.3 cells/7.5 mL). However, it is consistent with recent studies using OBP‐1101 in NSCLC (2.1 ± 1.8 and 2.3 ± 0.3).23, 45 To improve the sensitivity, increasing viral dose or extending reaction time may be useful. Further studies are necessary to determine optimal conditions for CTC detection.
In the present study, CTC were detected in 3 of 11 cases with stage IB disease who had no recurrence for at least 2 years, suggesting that existence of CTC is not directly associated with the presence of distal metastasis. Further characterization of CTC is necessary to distinguish CTC that associate with metastasis from those that do not.
There are limitations in the present study. First, because of the small number of patients, we could not reach a definitive result of clinical relevance of CTC in cervical cancer and could not carry out enough genetic analyses of CTC. Second, we did not examine the expression of mesenchymal markers in CTC, such as vimentin, which are necessary to show the character of CTC more clearly.
In conclusion, this is the first study that genetically confirmed isolated CTC in uterine cervical cancer. Our results indicate that mesenchymal‐like CTC are present in squamous cell carcinoma in the uterine cervix. Further studies of mesenchymal‐like CTC are needed to clarify the mechanisms responsible for distant metastasis through circulation.
CONFLICT OF INTEREST
Authors declare no conflicts of interest for this article.
Supporting information
ACKNOWLEDGMENTS
The authors appreciate Ms Nakajima and Ms Kawakita for their technical support. This work was supported by JSPS KAKENHI Grant Numbers 26462516 and 17K11270.
Takakura M, Matsumoto T, Nakamura M, et al. Detection of circulating tumor cells in cervical cancer using a conditionally replicative adenovirus targeting telomerase‐positive cells. Cancer Sci. 2018;109:231–240. https://doi.org/10.1111/cas.13449
Funding information
Japan Society for the Promotion of Science (Grant/Award Numbers: “17K11270”, “26462516”).
REFERENCES
- 1. Racila E, Euhus D, Weiss AJ, et al. Detection and characterization of carcinoma cells in the blood. Proc Natl Acad Sci USA. 1998;95:4589‐4594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cristofanilli M, Budd GT, Ellis MJ, et al. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N Engl J Med. 2004;351:781‐791. [DOI] [PubMed] [Google Scholar]
- 3. Cohen SJ, Punt CJ, Iannotti N, et al. Relationship of circulating tumor cells to tumor response, progression‐free survival, and overall survival in patients with metastatic colorectal cancer. J Clin Oncol. 2008;26:3213‐3221. [DOI] [PubMed] [Google Scholar]
- 4. de Bono JS, Scher HI, Montgomery RB, et al. Circulating tumor cells predict survival benefit from treatment in metastatic castration‐resistant prostate cancer. Clin Cancer Res. 2008;14:6302‐6309. [DOI] [PubMed] [Google Scholar]
- 5. Takakura M, Kyo S, Nakamura M, et al. Circulating tumour cells detected by a novel adenovirus‐mediated system may be a potent therapeutic marker in gynaecological cancers. Br J Cancer. 2012;107:448‐454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Miller MC, Doyle GV, Terstappen LW. Significance of circulating tumor cells detected by the cell search system in patients with metastatic breast colorectal and prostate cancer. J Oncol. 2010;2010:617421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gorges TM, Tinhofer I, Drosch M, et al. Circulating tumour cells escape from EpCAM‐based detection due to epithelial‐to‐mesenchymal transition. BMC Cancer. 2012;12:178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Konigsberg R, Obermayr E, Bises G, et al. Detection of EpCAM positive and negative circulating tumor cells in metastatic breast cancer patients. Acta Oncol. 2011;50:700‐710. [DOI] [PubMed] [Google Scholar]
- 9. Ma YC, Wang L, Yu FL. Recent advances and prospects in the isolation by size of epithelial tumor cells (ISET) methodology. Technol Cancer Res Treat. 2013;12:295‐309. [DOI] [PubMed] [Google Scholar]
- 10. Fabbri F, Carloni S, Zoli W, et al. Detection and recovery of circulating colon cancer cells using a dielectrophoresis‐based device: KRAS mutation status in pure CTCs. Cancer Lett. 2013;335:225‐231. [DOI] [PubMed] [Google Scholar]
- 11. Counter CM, Avilion AA, LeFeuvre CE, et al. Telomere shortening associated with chromosome instability is arrested in immortal cells which express telomerase activity. EMBO J. 1992;11:1921‐1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Takakura M, Kyo S, Kanaya T, Tanaka M, Inoue M. Expression of human telomerase subunits and correlation with telomerase activity in cervical cancer. Cancer Res. 1998;58:1558‐1561. [PubMed] [Google Scholar]
- 13. Meyerson M, Counter CM, Eaton EN, et al. hEST2, the putative human telomerase catalytic subunit gene, is up‐regulated in tumor cells and during immortalization. Cell. 1997;90:785‐795. [DOI] [PubMed] [Google Scholar]
- 14. Nakamura TM, Morin GB, Chapman KB, et al. Telomerase catalytic subunit homologs from fission yeast and human. Science. 1997;277:955‐959. [DOI] [PubMed] [Google Scholar]
- 15. Takakura M, Kyo S, Kanaya T, et al. Cloning of human telomerase catalytic subunit (hTERT) gene promoter and identification of proximal core promoter sequences essential for transcriptional activation in immortalized and cancer cells. Cancer Res. 1999;59:551‐557. [PubMed] [Google Scholar]
- 16. Takakura M, Kyo S, Sowa Y, et al. Telomerase activation by histone deacetylase inhibitor in normal cells. Nucleic Acids Res. 2001;29:3006‐3011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kishimoto H, Kojima T, Watanabe Y, et al. In vivo imaging of lymph node metastasis with telomerase‐specific replication‐selective adenovirus. Nat Med. 2006;12:1213‐1219. [DOI] [PubMed] [Google Scholar]
- 18. Takakura M, Nakamura M, Kyo S, et al. Intraperitoneal administration of telomerase‐specific oncolytic adenovirus sensitizes ovarian cancer cells to cisplatin and affects survival in a xenograft model with peritoneal dissemination. Cancer Gene Ther. 2010;17:11‐19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kojima T, Watanabe Y, Hashimoto Y, et al. In vivo biological purging for lymph node metastasis of human colorectal cancer by telomerase‐specific oncolytic virotherapy. Ann Surg. 2010;251:1079‐1086. [DOI] [PubMed] [Google Scholar]
- 20. Kojima T, Hashimoto Y, Watanabe Y, et al. A simple biological imaging system for detecting viable human circulating tumor cells. J Clin Invest. 2009;119:3172‐3181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ito H, Inoue H, Sando N, et al. Prognostic impact of detecting viable circulating tumour cells in gastric cancer patients using a telomerase‐specific viral agent: a prospective study. BMC Cancer. 2012;12:346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Shigeyasu K, Tazawa H, Hashimoto Y, et al. Fluorescence virus‐guided capturing system of human colorectal circulating tumour cells for non‐invasive companion diagnostics. Gut. 2015;64:627‐635. [DOI] [PubMed] [Google Scholar]
- 23. Sakurai F, Narii N, Tomita K, et al. Efficient detection of human circulating tumor cells without significant production of false‐positive cells by a novel conditionally replicating adenovirus. Mol Ther Methods Clin Dev. 2016;3:16001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sasaki T, Tazawa H, Hasei J, et al. Preclinical evaluation of telomerase‐specific oncolytic virotherapy for human bone and soft tissue sarcomas. Clin Cancer Res. 2011;17:1828‐1838. [DOI] [PubMed] [Google Scholar]
- 25. Kawai A, Naito N, Yoshida A, et al. Establishment and characterization of a biphasic synovial sarcoma cell line, SYO‐1. Cancer Lett. 2004;204:105‐113. [DOI] [PubMed] [Google Scholar]
- 26. Fujinaga Y, Shimada M, Okazawa K, Fukushima M, Kato I, Fujinaga K. Simultaneous detection and typing of genital human papillomavirus DNA using the polymerase chain reaction. J Gen Virol. 1991;72(Pt 5):1039‐1044. [DOI] [PubMed] [Google Scholar]
- 27. Thomson S, Buck E, Petti F, et al. Epithelial to mesenchymal transition is a determinant of sensitivity of non‐small‐cell lung carcinoma cell lines and xenografts to epidermal growth factor receptor inhibition. Cancer Res. 2005;65:9455‐9462. [DOI] [PubMed] [Google Scholar]
- 28. Wright AA, Howitt BE, Myers AP, et al. Oncogenic mutations in cervical cancer: genomic differences between adenocarcinomas and squamous cell carcinomas of the cervix. Cancer. 2013;119:3776‐3783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Verlaat W, Snijders PJ, van Moorsel MI, et al. Somatic mutation in PIK3CA is a late event in cervical carcinogenesis. J Pathol Clin Res. 2015;1:207‐211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Millis SZ, Ikeda S, Reddy S, Gatalica Z, Kurzrock R. Landscape of phosphatidylinositol‐3‐kinase pathway alterations across 19784 diverse solid tumors. JAMA Oncol. 2016;2:1565‐1573. [DOI] [PubMed] [Google Scholar]
- 31. Lou H, Villagran G, Boland JF, et al. Genome analysis of Latin American cervical cancer: frequent activation of the PIK3CA pathway. Clin Cancer Res. 2015;21:5360‐5370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Spaans VM, Trietsch MD, Peters AA, et al. Precise classification of cervical carcinomas combined with somatic mutation profiling contributes to predicting disease outcome. PLoS ONE. 2015;10:e0133670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Xiang L, Li J, Jiang W, et al. Comprehensive analysis of targetable oncogenic mutations in Chinese cervical cancers. Oncotarget. 2015;6:4968‐4975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. zur Hausen H. Papillomaviruses in the causation of human cancers – a brief historical account. Virology. 2009;384:260‐265. [DOI] [PubMed] [Google Scholar]
- 35. Pao CC, Hor JJ, Yang FP, Lin CY, Tseng CJ. Detection of human papillomavirus mRNA and cervical cancer cells in peripheral blood of cervical cancer patients with metastasis. J Clin Oncol. 1997;15:1008‐1012. [DOI] [PubMed] [Google Scholar]
- 36. Tseng CJ, Pao CC, Lin JD, Soong YK, Hong JH, Hsueh S. Detection of human papillomavirus types 16 and 18 mRNA in peripheral blood of advanced cervical cancer patients and its association with prognosis. J Clin Oncol. 1999;17:1391‐1396. [DOI] [PubMed] [Google Scholar]
- 37. Scheungraber C, Muller B, Kohler C, et al. Detection of disseminated tumor cells in patients with cervical cancer. J Cancer Res Clin Oncol. 2002;128:329‐335. [DOI] [PubMed] [Google Scholar]
- 38. Weismann P, Weismanova E, Masak L, et al. The detection of circulating tumor cells expressing E6/E7 HR‐HPV oncogenes in peripheral blood in cervical cancer patients after radical hysterectomy. Neoplasma. 2009;56:230‐238. [DOI] [PubMed] [Google Scholar]
- 39. Pfitzner C, Schroder I, Scheungraber C, et al. Digital‐Direct‐RT‐PCR: a sensitive and specific method for quantification of CTC in patients with cervical carcinoma. Sci Rep. 2014;4:3970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bodaghi S, Wood LV, Roby G, Ryder C, Steinberg SM, Zheng ZM. Could human papillomaviruses be spread through blood? J Clin Microbiol. 2005;43:5428‐5434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Foresta C, Bertoldo A, Garolla A, et al. Human papillomavirus proteins are found in peripheral blood and semen Cd20+ and Cd56+ cells during HPV‐16 semen infection. BMC Infect Dis. 2013;13:593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hermiston ML, Xu Z, Weiss A. CD45: a critical regulator of signaling thresholds in immune cells. Annu Rev Immunol. 2003;21:107‐137. [DOI] [PubMed] [Google Scholar]
- 43. Liu Z, Li Q, Li K, et al. Telomerase reverse transcriptase promotes epithelial‐mesenchymal transition and stem cell‐like traits in cancer cells. Oncogene. 2013;32:4203‐4213. [DOI] [PubMed] [Google Scholar]
- 44. Qin Y, Tang B, Hu CJ, et al. An hTERT/ZEB1 complex directly regulates E‐cadherin to promote epithelial‐to‐mesenchymal transition (EMT) in colorectal cancer. Oncotarget. 2016;7:351‐361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Togo S, Katagiri N, Namba Y, et al. Sensitive detection of viable circulating tumor cells using a novel conditionally telomerase‐selective replicating adenovirus in non‐small cell lung cancer patients. Oncotarget. 2017;8:34884‐34895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Mitsuhashi A, Tanaka N, Suzuka K, Matsui H, Seki K, Sekiya S. Detection of epidermal growth factor receptor mRNA in peripheral blood of cervical cancer patients. Gynecol Oncol. 2003;89:480‐485. [DOI] [PubMed] [Google Scholar]
- 47. Kolostova K, Spicka J, Matkowski R, Bobek V. Isolation, primary culture, morphological and molecular characterization of circulating tumor cells in gynecological cancers. Am J Transl Res. 2015;7:1203‐1213. [PMC free article] [PubMed] [Google Scholar]
- 48. Walter CB, Taran FA, Wallwiener M, et al. Prevalence and prognostic value of disseminated tumor cells in primary endometrial, cervical and vulvar cancer patients. Future Oncol. 2014;10:41‐48. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


